Abstract
Objective
To determine the usefulness of FDG PET/CT scanning in the management and staging of myeloma and to assess its strengths and limitations.
Design
FDG PET/CT scans and all other available imaging studies were reviewed retrospectively from 16 consecutive patients by two experienced musculoskeletal radiologists and two nuclear medicine physicians working in consensus.
Patients
The 16 patients had undergone a total of 19 FDG PET/CT scans. Radiographs were available in all cases, including 13 skeletal surveys; 25 CT scans (16 chest, three abdominal, four pelvic, one spine, one neck) and 22 MR imaging studies (17 spine, three pelvic, two extremity) also were reviewed. Patients’ records were examined for relevant clinical information. All focal areas of abnormal FDG uptake were correlated with the other imaging studies to determine clinical significance. FDG PET/CT scans also were reviewed to see if small lesions shown on the other imaging studies could be identified in retrospect.
Results
The 12 men and four women had an average age of 58 years (range 30–69 years). All 16 patients had an established diagnosis of multiple myeloma, with average duration of disease, from time of initial diagnosis to review, of 30 months (range 6 months to 11+ years). The FDG PET/CT scans revealed a total of 104 sites (90 in bone, 14 soft tissue) that were suspicious for neoplastic activity based on a standardized uptake value (SUV) greater than 2.5. Fifty-seven of these sites (55%) were new or previously undetected. The other imaging studies (X-ray, CT, MR) and clinical information confirmed the other 47 areas but also revealed 133 other small skeletal lesions. Six of these 133 additional lesions showed mild FDG uptake on re-review of the PET/CT scans. The FDG PET/CT findings led to management changes in 9/16 patients. MR imaging revealed five cases of diffuse bone involvement (four spine, one scapula) that were not evident by FDG PET/CT.
Conclusion
FDG PET/CT scans are useful for the management and staging of myeloma. However, if PET/CT were the sole imaging study done, it would miss many additional small lytic skeletal lesions and could miss diffuse spine involvement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multiple myeloma is the most common primary bone neoplasm, accounting for approximately 1% of all malignancies and 10–20% of all hematologic malignancies [1]. The unchecked proliferation of plasma cells results both in local mass effect and systemic effects, due to overproduction of monoclonal protein. In addition, plasma cell tumors located in bone induce a local paraneoplastic process that results in the stimulation of osteoclasts and suppression of osteoblasts, eventually giving rise to the radiographic and CT findings of “punched out” osseous lesions in flat and long bones [2]. Diagnosis is made on the basis of elevated monoclonal serum and/or urine protein, both of which are also used to monitor the progress of disease, and plasma cell infiltration of the bone marrow [3].
Determination of the total number of lesions is critical for staging purposes, as the treatment and prognosis is different for different types of myeloma, depending on precise staging. In the past, evaluation of the extent of osseous disease has relied primarily on the conventional radiographic skeletal survey (CRSS). The new Durie/Salmon PLUS staging system [4] is a refinement of the original 1975 Durie/Salmon system [5], which utilized the CRSS as its gold standard for skeletal involvement. Findings from MR imaging and FDG PET scanning have been added to the new staging system to provide the additional information necessary for more precise staging. The exact number of focal bone lesions for each stage also has been defined now: stage 1, 0–4 bone lesions; stage 2, 5–20 bone lesions; stage 3, >20 bone lesions. A few papers [6–9] have discussed the use of FDG PET or PET/CT in myeloma patients, but they have consisted of small series or mixed patient populations, and none has presented a detailed comparison of the findings between the various imaging modalities and their respective limitations. We compared FDG PET/CT findings, in patients with a known diagnosis of multiple myeloma, with those of other imaging modalities (CRSS, CT, and MR) to determine the utility of FDG PET/CT in patients’ treatment management and staging and to determine the limitations of FDG PET/CT imaging.
Materials and methods
This study was approved by our hospital review board. Informed consent was not deemed to be necessary prior to PET/CT. F-18 FDG PET/CT scans and all other available imaging studies from 16 consecutive myeloma patients were reviewed retrospectively by teams of two experienced musculoskeletal radiologists and two nuclear medicine physicians working in consensus. Cases were identified using our nuclear medicine database and were collected over a 12-month period. The PET/CT examination indications were for worsening or new bone pain in spite of stable or decreasing immunoglobulin levels, for suspected relapse in allogeneic transplant patients, for follow-up of patients with non-secretory disease, for evaluation of newly detected soft tissue masses or for a combination of these reasons. The reviewing physicians had access to the diagnoses and findings of all imaging studies. Patients were selected for inclusion in the study if they had a known diagnosis of multiple myeloma, an FDG PET/CT scan, and at least one study utilizing one of the other modalities within 6 months of the date of the FDG PET/CT scan. The 16 patients had a total of 19 PET/CT scans. Radiographs were available in all cases, including 13 skeletal surveys. There were 25 diagnostic CT scans (16 chest, three abdominal, four pelvic, one spine, one neck), and 22 MR imaging studies (17 spine, three pelvic, two extremity) that also were reviewed. Patients’ records were examined for relevant clinical information. Staging was based on the new Durie/Salmon PLUS system. All focal areas of abnormal FDG uptake were correlated with the other imaging studies to determine clinical significance. A standardized uptake value (SUV) ≥2.5 was considered to be abnormal and to indicate a site of active disease. FDG PET/CT scans also were reviewed to see if additional lesions shown on the other imaging studies could be identified in retrospect.
FDG PET/CT scanning was performed on Siemens Exact 47 or Philips Gemini systems, with FDG-18 supplied by outside vendors. In the instances where the studies were performed on the Philips Gemini, simultaneous CT scans were acquired using the integral four-slice scanner. Either localization or diagnostic quality images were acquired utilizing approximately 80 keV or 140 keV, respectively. PET/CT images were reconstructed using a general purpose body filter; dedicated bone images using high pass algorithms were not obtained. Attenuation correction was performed using the integral germanium or cesium rod source, respectively. Dedicated CT scans were performed on Philips MX-8000 16-slice scanners, using a 140 keV technique. When dedicated bone CT studies were ordered, images were reconstructed using a high spatial frequency (bone) algorithm; in all other cases a general purpose body algorithm was employed. MR images were acquired on 1.5 T systems (Philips and Siemens). Pulse sequences for spine studies typically included sagittal T1-weighted, fast or turbo-spin echo (FSE) proton density, FSE T2-weighted and fast short-tau inversion recovery (STIR), as well as axial T2-weighted sequences. Pulse sequences for pelvic studies consisted of T1-weighted and STIR in the axial and coronal planes. Pulse sequences for limb lesions consisted of axial T1-weighted, proton density (PD) and T2-weighted with either sagittal or coronal T1proton density and STIR. Intravenous gadolinium agents were not used routinely.
A patient’s imaging stage was determined using the criteria of the new Durie/Salmon PLUS system as follows: stage I, 0–4 focal lesions or mild diffuse spine disease on MR imaging (mild spine disease is not defined); stage II, 5–20 focal lesions or moderate diffuse spine disease on MR imaging (defined as vertebral body signal brighter than the adjacent intervertebral disc with T1-weighting); stage III, >20 focal lesions or severe diffuse spine disease on MR imaging (defined as vertebral body signal intensity equal to or less than that of adjacent intervertebral discs with T1-weighting).
The available biopsy results in the clinical information system at our institution were reviewed. Data included reports from evaluation of prepared slides from biopsies performed at our institution and at outside hospitals.
Results
The 12 men and four women had an average age of 58 years (range 30–69 years). At the time of review all 16 patients had an established diagnosis of multiple myeloma (eight IgG, two IgA, three light chain, and three non-secretory), with an average duration of disease, from time of initial diagnosis to review, of 30 months (range 6 months to 11+ years). One patient had monoclonal gammopathy of undetermined significance (MGUS) initially with subsequent development of IgG myeloma (Table 1). Eleven patients had known active disease; four were thought to be in remission after receiving transplants. The FDG PET/CT scans revealed a total of 104 abnormal sites (90 in bone, 14 in soft tissue) in 11 patients, that were suspicious for neoplastic activity based on SUVs greater than 2.5 (range 2.5–8.7). Of these 104 sites, 57 (55%) represented new or previously undetected disease in eight of the patients. Conventional radiographs had been done in all eight patients that failed to identify 56 of these 57 sites. Computed tomography had been done in three patients that failed to identify nine of the sites that were within the field of view. All but one of the 104 sites were felt to represent areas of myelomatous involvement. The one site not suspected to be a focus of myeloma was subsequently proven to represent a previously occult bronchogenic carcinoma. FDG PET/CT findings were normal in the other five patients. The other imaging studies (CRSS, CT, MR) confirmed the other 47 sites as areas of myelomatous disease but also revealed 133 other predominantly small focal lytic skeletal lesions (87 were 10 mm or less, 40 were 11–20 mm, six were >20 mm). These 133 lesions were primarily detected by CT (96/133) but also by CRSS (22/133) and MR imaging (15/133) or after more than one examination (Table 2). All 133 lesions were felt to be typical for myeloma. Six of these 133 additional lesions showed mild FDG uptake on re-review of the PET/CT scans, with SUVs ranging from 2.2 to 2.4. MR imaging also revealed four cases of diffuse spine involvement (three moderate, one mild) and one scapula with diffuse involvement. None of these MR cases was felt to be positive by PET/CT, even in retrospect.
The PET/CT findings led to treatment management changes in 56% (9/16) of patients. Since this is a retrospective study, management changes were based on the best information available at the time the studies were interpreted. Unusual or new sites of involvement were biopsied and proven to represent myelomatous disease in five patients or were established by the subsequent clinical course or by follow-up imaging studies in the other patients. After the presence of new disease had been established, chemotherapeutic regimens were altered or begun again in seven patients; one patient had local radiotherapy for nodal disease and one patient underwent surgical resection for his newly diagnosed bronchogenic carcinoma.
Discussion
Solitary plasmacytomas of bone (SPBs) are treated usually with external beam radiation only; there is no proven benefit for chemotherapy, even though the morbidity and mortality rates of the established regimens are minimal [10]. The survival of these patients is greater than 50% at 10 years [10] if they do not progress to multiple myeloma. All patients with symptomatic multiple myeloma are treated differently and have a poorer prognosis, with 5-year survival rates of 10–40% and median survival rates of 36–48 months, depending upon stage [11]. Treatment regimens differ, depending primarily on the patient’s age and disease staging.
Given that there are different treatment and prognostic implications at the various stages of this disease, accurate staging is critical. The new Durie/Salmon PLUS staging system incorporates advanced imaging studies (MR, FDG PET/CT) for accurate staging and lists a specific number of focal lesions for each stage. Therefore, the detection of one additional lesion can change a patient’s imaging stage from stage 1 to stage 2 (four versus five lesions) or from stage 2 to stage 3 (20 versus 21 lesions). Although our sample size was not large (n=19 FDG PET/CTs), the results demonstrate that FDG PET/CT and the other imaging modalities are complementary. When they are read together there is increased detection of skeletal and soft tissue lesions. Our sample size was limited by insurance reimbursement issues and a reluctance of clinicians to refer patients for FDG PET/CT because of payment concerns.
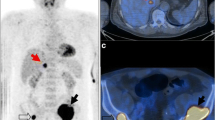
In our study there were 57 instances where FDG PET/CT revealed unsuspected soft tissue or osseous lesions that were not evident on the other imaging studies. These findings led to treatment management changes in 56% of our cases and an upstaging of disease in 37.5% of our patients (Fig. 1). This is similar to the 31% upstaging of disease that influenced subsequent management in the series of patients reported by Bredella et al. [6]. FDG PET/CT is uniquely suited to detecting foci of active extramedullary disease, for detecting disease in patients with non-secretory myeloma and in post-transplantation patients (Fig. 2). We found 14 sites of extramedullary disease in 11 patients, including involvement of lung, pleura, lymph nodes and spleen. Splenic involvement by myeloma is rare. Although the presence of extramedullary disease is said to be a grave prognostic indicator [8], only two of our 11 patients with extramedullary involvement had died at the time of this report.
Soft tissue findings. a A 52-year-old woman with IgG lambda myeloma. Whole-body F-18 FDG PET shows multiple FDG-avid foci along the pleural surface of the right lung and one in the superior mediastinum. b A 50-year-old man with non-secretory myeloma. F-18 FDG PET/CT shows diffuse involvement of the spleen that was an unknown site of disease. c A 50-year-old man with IgG kappa myeloma. F-18 FDG PET/CT shows avid uptake in a left lung nodule (SUV 3.6). This was biopsy proven bronchogenic carcinoma
Five of our patients had non-secretory disease at the time of initial diagnosis. Two had normal FDG PET/CT results, one had stable findings, and two had new abnormalities detected, including one with involvement of the spleen. Two of these patients have subsequently developed secretory disease. Patients with non-secretory disease are difficult to follow by the usual laboratory parameters, and FDG PET/CT has a significant role in the assessment of disease activity in these patients [8].
Three of our patients who had undergone allogeneic transplantation and who had been in remission were found to have new areas of disease activity on their PET/CT examinations. As mentioned above, for patients with initial non-secretory disease, PET/CT is very useful in the post-transplantation patient because such patients become non-secretors after transplantation and the usual laboratory parameters cannot be assessed.
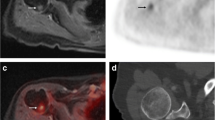
FDG PET/CT examinations do not have 100% sensitivity or specificity. Previous studies have reported false positive FDG PET findings due to infection, inflammation, post-surgical change and hemangioma [6, 8, 9]. We did not have any false positive cases in our series. There were many false negatives. Findings on the other imaging studies in our cases resulted in focused scrutiny of FDG PET/CT studies, revealing additional sites of disease. False negatives were primarily due to small lesion size (Fig. 3a–d) and the use of an arbitrary SUV of 2.5. Our results reflect snapshots in time of the state of the art of clinical imaging systems and techniques. Although CT is more sensitive for small skeletal lesions than conventional radiographs, the CT component of some of our studies was performed with a four-slice scanner using a low kilo-electronvolt technique, and the PET/CT studies were not reconstructed with bone algorithms. Later studies were performed with a 16-slice scanner module that is the equivalent of our dedicated CT scanners. Many of the small lesions that we noted on CT were not FDG avid on PET when a SUV ≥2.5 was used as a threshold. This arbitrary threshold value may need to be modified for small lesions in myeloma patients. Six patients in our series had lesions on FDG PET/CT identified in retrospect that ranged in size from 9 mm to 34 mm, with SUVs that ranged from 2.2 to 2.4. The clinical importance of such lesions has not been established, but it should be noted that volume averaging artifact causes FDG PET scanning to underestimate (or possibly miss completely) the glycolytic hyperactivity of a small lesion as its size approaches the threshold of detection (approximately 6 mm with current equipment). The larger lesions we found with the other imaging modalities that were not FDG avid most likely represented areas of treated, inactive disease. As the technology and technique evolves, and its use is optimized for the task at hand, sensitivity and specificity of FDG PET/CT is likely to improve.
False negatives. a,b A 66-year-old man with IgG kappa myeloma. a Fused FDG/PET CT images through the sternum show no area of avid FDG uptake. b Image from diagnostic chest CT shows 19 mm lytic lesion in left side of sternum with erosion of anterior cortex and infiltration into anterior chest wall soft tissues. c,d A 50-year-old man with non-secretory myeloma. c Fused FDG PET/CT images through the upper sacrum snow no area of avid FDG uptake. d Three consecutive images from diagnostic CT examination show a 15 mm lytic lesion in the right sacrum, adjacent to the S2 neural foramen. e,f A 68-year-old man with IgG kappa myeloma. e FDG PET/CT images through the spine read as no areas of abnormal FDG uptake. f Sagittal MR images taken 2 days before PET/CT show diffuse abnormal vertebral involvement from C2 through L5
There are also data to suggest that FDG PET/CT is useful for assessing response to treatment [6, 8, 9], although some [7] prefer other scintigraphic techniques employing Tc-99m sestamibi (MIBI). Currently, the MR imaging bone marrow index [12] is probably the best method for evaluating treatment response for known spine involvement, although long-term follow up of patients with FDG PET/CT or MIBI may change the imaging approach. Our study showed another limitation of FDG PET/CT in that four patients with diffuse spinal involvement and one with diffuse scapular involvement by MR imaging were FDG negative (Fig. 3e,f). A combination of imaging examinations seems to be needed for a complete follow-up evaluation.
One limitation of this study, and those previously reported on this subject, is the lack of a gold standard to determine active lesions. Under the old Durie/Salmon system all bone lesions evident on conventional radiographs were considered to be significant. The key question on follow-up imaging now is: which lesions are still active? One must use all the imaging information available in an individual patient to decide this, since it is not practicable to biopsy every lesion. Conventional radiographs or CT may show a stable or enlarging size for active lesions. Bright signal intensity on T2-weighted MR images does not guarantee that a lesion is active, and lack of enhancement after intravenous administration of gadolinium does not guarantee that the lesion is inactive [13]. FDG PET can be falsely positive or negative for a number of reasons, as discussed above.
Conclusion
Adding FDG PET/CT to the examination of myeloma patients increases the sensitivity for the detection of soft tissue masses and additional osseous foci, with important implications in the staging of the disease and management of treatment in these patients. FDG PET imaging has limitations, and studies must be correlated carefully with those of other imaging modalities.
References
Dispenzieri A, Kyle RA. Multiple myeloma: clinical features and indications for therapy. Best Pract Res Clin Haematol 2005;18:553–568
Mulligan M. Imaging techniques used in the diagnosis, staging, and follow-up of patients with myeloma. Acta Radiol 2005;46:716–724
International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 2003;121:749–757
Durie B, Kyle R, Belch A, et al. Myeloma management guidelines: a consensus report from the Scientific Advisors of the International Myeloma Foundation. Hematol J 2003;4:379–398
Durie B. Salmon S. A clinical staging system for multiple myeloma. Cancer 1975;36:842–854
Bredella MA, Steinbach L, Caputo G, Segall G, Hawkins R. Value of FDG PET in the assessment of patients with multiple myeloma. AJR Am J Roentgenol 2005;184:1199–1204
Mileshkin L, Blum R, Seymour JF, Patrikeos A, Hicks RJ, Prince HM. A comparison of fluorine-18 fluoro-deoxyglucose PET and technetium-99 m sestamibi in assessing patients with multiple myeloma. Eur J Haematol 2004;72:32–37
Durie BGM, Waxman AD, D’Agnolo A, Williams CM. Whole-body 18F-FDG PET identifies high-risk myeloma. J Nucl Med 2002;43:1457–1463
Jadvar H, Conti PS. Diagnostic utility of FDG PET in multiple myeloma. Skeletal Radiol 2003;31:690–694
Mendenhall WM, Mendenhall CM, Mendenhall NP. Solitary plasmacytoma of bone and soft tissue. Am J Otolaryngol 2003;24:395–399
Greipp PR, San Miguel J, Durie BGM, et al. International staging system for multiple myeloma. J Clin Oncol 2005;23:3412–3420
Lecouvet F, Dechambre S, Malghem J, Ferrant A, Vande Berg B, Maldague B. Bone marrow transplantation in patients with multiple myeloma: prognostic significance of MR imaging. AJR Am J Roentgenol 2001;176:91–96
Lecouvet F, De Nayer P, Garbar C, et al. Treated plasma cell lesions of bone with MRI signs of response to treatment: unexpected pathological findings. Skeletal Radiol 1998;27:692–695
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Breyer, R.J., Mulligan, M.E., Smith, S.E. et al. Comparison of imaging with FDG PET/CT with other imaging modalities in myeloma. Skeletal Radiol 35, 632–640 (2006). https://doi.org/10.1007/s00256-006-0127-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-006-0127-z