Abstract
While uncomplicated type 2 diabetes mellitus (T2DM) is already associated with an impaired exercise capacity, the presence of other comorbidities appears to further worsen exercise capacity in T2DM. Common diabetic comorbidities such as hypertension, arterial stiffness, cardiovascular disease, systolic dysfunction, diastolic dysfunction, pulmonary disease, and diabetic nephropathy are all associated with worse exercise capacity in T2DM. Benefits of exercise training programs for those with T2DM and certain comorbidities (e.g., hypertension, increased arterial stiffness, or post-myocardial infarction) have been shown to include improved exercise capacity. Exercise has also been shown to improve other complications and comorbidities of diabetes including neuropathy, fatty liver, obstructive sleep apnea, and nephropathy, but the independent effect of these benefits on exercise capacity has not been defined. Further study is warranted to determine the specific benefits and risks of exercise training in subpopulations of T2DM such as those with T2DM and either congestive heart failure or microvascular complications of diabetes.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Diabetic neuropathy
- Diabetic nephropathy
- Hypertension
- Arterial stiffness
- Heart failure
- Macrovascular disease
- Cardiac autonomic dysfunction
- Fatty liver
- Retinopathy
- Pulmonary function
Introduction
People with type 2 diabetes mellitus (T2DM), even when uncomplicated, have been shown to have decreased exercise capacity as compared with age- and weight-matched nondiabetic subjects as detailed in Chap. 1 of this book. Since the prevalence of comorbidities is relatively high in the diabetic population (Table 18.1), the impact of comorbidities on exercise performance is of major concern. Since exercise has a central therapeutic role in diabetes, it is important to recognize how differences in exercise physiology in the presence of diabetic comorbidities may impact upon exercise recommendations to this population. This chapter will describe the exercise abnormalities correlated with the common diabetic comorbidities of hypertension, arterial stiffness, congestive heart failure (CHF, including systolic and diastolic dysfunction), as well as macrovascular and microvascular disease. Certainly, many people with diabetes with complications will have more than one of these entities simultaneously, but the changes in exercise physiology attendant to these comorbidities will be addressed individually where possible. This chapter will also discuss the available data on the particular benefits of exercise training with each comorbidity when those data are available. The focus will be on exercise pathophysiology in subjects with T2DM with type 1 diabetes mellitus (T1DM) included as well, although the data in T1DM are more limited.
As context for this chapter’s discussion of diabetic comorbidities impact upon exercise impairment, it is useful to quickly review the exercise abnormalities in uncomplicated diabetes. Subjects with T1DM have been shown in two small studies to have no exercise impairment (as assessed by maximal exercise capacity) in comparison with similarly active nondiabetic controls matched for age, sex, and body weight [1, 2]. However, more recently, Nadeau et al. did find impaired functional exercise capacity in T1DM compared to similarly matched controls [3].Exercise impairment has incontrovertibly been shown in subjects with uncomplicated T2DM and will be discussed briefly.
Despite a lack of apparent microvascular or macrovascular complications , subjects with T2DM have approximately 20% worse maximal exercise capacity as compared with control subjects [4,5,6,7,8]. This impairment appears to be caused by slowed oxygen delivery to working muscles of both “central” (cardiac) and “peripheral” (exercising muscle) origins. The peripheral causes of decreased exercise capacity may include endothelial dysfunction (precluding appropriate vasodilation to increase perfusion of exercising muscle), impaired microvascular distribution of flow, decreased oxygen diffusion, and/or decreased oxygen extraction [9]. The central causes may include endothelial dysfunction (precluding appropriate vasodilation of coronary arteries in response to increased myocardial workload [9], decreased cardiac output during exercise [9], and exercise-associated impaired left ventricular function [10, 11]). Exercise-associated impaired left ventricular function (also termed “diabetic cardiomyopathy”) is not present in all subjects with T2DM but is relatively common and will be discussed separately in the “Congestive Heart Failure” section of this chapter.
Hypertension and Arterial Stiffness
Hypertension and arterial stiffness both reflect similar vascular pathophysiology relating to increased peripheral vascular resistance and/or cardiac output. Both may play important roles in altering usual exercise physiology. It is important to consider how these pathophysiologic factors may impact on exercise, since the prevalence of hypertension in subjects with T2DM ranges from 39% to 71% [12,13,14] and arterial stiffness has been shown to be 13% higher in subjects with T2DM than in those without [15]. This section will review how hypertension and arterial stiffness impair exercise performance in diabetes, the methods by which routine exercise training can remediate these deficits, and the attendant benefits to exercise in diabetics beyond improving exercise performance.
Effects of Hypertension on Exercise Performance in T2DM
The addition of hypertension to diabetes has been shown to decrease exercise capacity. One small Austrian study showed a significantly decreased maximal oxygen consumption (VO2max) in eight subjects with T2DM and hypertension as compared with six normotensive T2DM subjects, eight nondiabetic hypertensive subjects, and eight age-, sex-, and BMI-matched controls (p < 0.01 vs. normotensive T2DM, nondiabetic hypertensives, and controls) [16]. Babalola et al. showed a tendency toward a lower exercise time in diabetic hypertensives (289 ± 110 s) as compared to diabetic subjects without hypertension (321 ± 119 s), hypertensives who did not have diabetes (309 ± 73 s), and healthy controls (490 ± 156 s) using a modified Bruce protocol treadmill test [17]. This study lacked sample size to differentiate between the diabetic normotensive and diabetic hypertensive groups. However, there was a statistically significant difference in exercise duration between the four groups (p < 0.05), and a rank-order trend suggested the worst exercise capacity (as measured by maximal exercise time) was in the diabetic hypertensive group.
Effects of Hypertension on Exaggerated Sympathetic Nervous System Response to Exercise in T2DM
The greater response of the sympathetic nervous system to exercise in people with diabetes and comorbid hypertension is of interest for three reasons. Firstly, it is known that the sympathetic nervous system is already more active in resting subjects with diabetes or hypertension than in nondiabetic or non-hypertensive subjects [18,19,20,21]. This raises the question as to how that elevated baseline activity will impact sympathetic activity with exercise. Secondly, it is known that exercise induces an increase in sympathetic nervous system activity and catecholamine release in all subjects but that during exercise there are feedback mechanisms which further mediate sympathoadrenal activity levels [22]. Since catecholamines induce lipolysis, the insulin resistance-induced impairment of lipolysis in adipocytes is one such diabetic maladaptation which may result in positive feedback to the sympathoadrenal axis during exercise [23]. The existence of greater sympathetic activation with exercise in subjects with both diabetes and hypertension encourages the investigation of other possible contributors to this positive feedback. Thirdly, it is of clinical interest to know that catecholamine levels become higher with exercise in diabetic hypertensive than in diabetic normotensive individuals due to a more robust sympathoadrenal response. Future research may explore to what degree the exaggerated sympathoadrenal response to exercise in diabetes is a beneficial compensatory adaptation or a maladaptive response due to abnormal metabolic and circulatory factors.
Sympathoadrenal overactivity has been demonstrated in subjects with T2DM and comorbid hypertension as expressed by the increased release of catecholamines with exercise. One study looked at differences in exercise-induced catecholamine response between four groups: T2DM with hypertension, T2DM without hypertension, hypertension without T2DM, and control subjects [16]. Each subject performed a stationary cycling exercise for 15 min with 5-min incremental workload steps of 25%, 50%, and 75% of individually measured VO2max. Blood pressure and plasma catecholamine measurements were obtained 10 min prior to exercise and then at each 5-min workload and at timed intervals during recovery. This study showed greater exercise-induced unconjugated normetanephrine levels in the hypertensive T2DM subjects as compared with their age-, sex-, and BMI-matched controls (2156 ± 373 pg/ml/min vs. 1133 ± 180 pg/ml/min, p = 0.04) with no change in the normotensive T2DM or nondiabetic hypertensive subjects as compared with controls. At baseline, unconjugated metanephrines were lower in hypertensive T2DM and normotensive T2DM subjects (p = 0.03 and 0.04, respectively) than in their respective controls. Although there was a lower VO2max in the T2DM hypertensives than in the other groups (p < 0.01 vs. normotensive T2DM, nondiabetic hypertensives, and controls), no tests of correlation were performed between the unconjugated metanephrine levels and exercise capacity. The authors of this study concluded that the excessive response of plasma unconjugated normetanephrines may serve as a marker of exaggerated sympathoadrenal function in hypertensive T2DM [16]. Previous studies have found that subjects with excessive sympathoadrenal activity had elevated noradrenaline levels during exercise testing but not at baseline [24, 25]. It is not yet certain if the elevated catecholamine response to exercise is due to sympathoadrenal overactivity or to a greater catecholamine response requirement to maintain cardiac output and glucose homeostasis with exercise. Again, this suggests further research is warranted into the mechanisms of exaggerated exercise-induced sympathetic outflow as well as whether this greater sympathetic activity is beneficial or only maladaptive.
Arterial Stiffness in the Presence of Hypertension and T2DM
Increased arterial stiffness (also termed “decreased elasticity” or “decreased vascular compliance ”) is a ubiquitous endpoint of many disease processes. Not only diabetes but also arterial hypertension, hyperlipidemia, congestive heart failure, and chronic uremia have all been shown to lead to decreased elasticity in large arteries [26]. However, arterial stiffness may be particularly pronounced in T2DM. Given that arterial stiffness is a newer physiologic measure as yet without well-defined reference normal levels, the prevalence of arterial stiffness in T2DM is uncertain. However, one epidemiologic study showed a 13% increase in arterial stiffness (as measured by pulse pressure/stroke volume) in T2DM subjects as compared with controls [15]. In addition, measures of arterial stiffness have been shown to correlate with CVD risk and death, supporting arterial stiffness as a clinically important complication/comorbidity of diabetes [27].
Increased arterial stiffness results from three general types of changes to arterial structure and function [26]. Structural arterial changes include smooth muscle cell hypertrophy, increased collagen matrix deposits, and abnormal proteoglycan metabolism [26]. Functional abnormalities such as endothelial dysfunction, vascular insulin resistance, and abnormal vasa vasorum microcirculation also increase arterial wall stiffness [26, 28]. Finally, increased permeability of vessel walls leads to disruption of the interstitial matrix [26]. Thus, arterial stiffness results from a combination of structural and functional processes.
The degree of arterial stiffness observed is determined by the timing and magnitude of reflected waves from the peripheral vasculature as well as the cardiac output and central arterial vascular resistance [29, 30]. Noninvasive measurements of arterial stiffness include pulse pressure, pulse pressure/stroke volume, augmentation index (AI), pulse wave velocity (PWV), and ultrasound stiffness index β. For each of these metrics, higher measurements indicate greater stiffness. Like hypertension, increased arterial stiffness is related to increased vascular resistance but is felt to reflect central aortic blood pressure as opposed to the peripheral blood pressure measured with a sphygmomanometer [31]. Though a paucity of data exist to compare the utility of lowering arterial stiffness vs. treating blood pressure with regard to morbidity, the large ASCOT-CAFÉ randomized controlled trial illustrated that improved arterial stiffness between groups correlated with better cardiovascular outcomes (decreased cardiovascular events/procedures and/or decreased renal impairment) despite equivalent blood pressures between the amlodipine and atenolol-based regimens [32]. This illustrates that although arterial stiffness is related to hypertension, vascular compliance may have additional physiologic relevance beyond hypertension. The next section will review the implications of arterial stiffness upon exercise performance in subjects with T2DM.
Effects of Arterial Stiffness on Exercise Performance in T2DM
Arterial stiffness has been shown to be associated with low physical activity and reduced exercise capacity in adults with and without T2DM. In a study of 65 subjects with early uncomplicated T2DM and 65 controls, arterial stiffness, measured as carotid femoral PWV, correlated more tightly with low physical activity than with presence or absence of T2DM [33]. The negative correlation between several measures of arterial stiffness and exercise capacity has been reported in several studies (reviewed in [34]). The cause and effect relationship between exercise capacity/physical activity and arterial stiffness remains unclear, but some studies have found arterial stiffness-related defects that could worsen exercise capacity and tolerance. Increased arterial stiffness in diabetes is associated with abnormalities in the vascular circulation with exercise in subjects with T1DM and T2DM. Arterial stiffness (as measured by ultrasound with stiffness “β”) independently predicted decreased peripheral circulation to the foot during exercise (as measured by the well-validated transcutaneous oxygen tension index (TcPO2 index, [35,36,37]) in Japanese subjects with T2DM and normal peripheral circulation (ABI >0.9) [38]. In another study, arterial stiffness (measured as radial artery AI) correlated with echocardiographic measures of diastolic dysfunction in 213 high-risk individuals without CVD [39]. Consistent with this was the increased risk of incident congestive heart failure in individuals with higher carotid femoral PWV in 2539 participants in the Framingham study [40]. Furthermore, subjects with T1DM maintained a higher peripheral vascular resistance during cycle ergometry as compared with control subjects (p < 0.01) with an associated greater rise in diastolic blood pressure (p < 0.01, T1DM vs. controls) [41]. Other studies have also confirmed an exaggerated diastolic blood pressure rise with exercise in T1DM subjects vs. controls [42, 43]. In T1DM adolescents with increased arterial stiffness, elevated diastolic blood pressure with exercise and endothelial dysfunction (as measured by impaired forearm vasodilator response to brachial ischemia) were present and correlated with diabetes duration and glycemic control [42]. Overall, these studies support, but do not prove, the hypothesis that increased central arterial stiffness in T2DM, with resulting higher exercise afterload and a mechanistically related ventricular stiffness and diastolic dysfunction, leads to impaired cardiac output during exercise, thus directly contributing to reduced maximum exercise capacity in T2DM.
Benefits of Exercise Training in Persons with Diabetes Mellitus and Hypertension or Arterial Stiffness
Aerobic exercise has repeatedly been shown to lower blood pressure in nondiabetic hypertensive individuals by an average of 5–6 (systolic) and/or 4–5 (diastolic) mm Hg [44, 45]. Even lower-intensity exercise such as regular walking has been shown to lower blood pressure by 3 (systolic) and/or 2 (diastolic) mm Hg [46]. Less information is present on benefits in subjects with diabetes and comorbid hypertension (reviewed in [47]), but the available data will be reviewed briefly.
Several randomized control trials of exercise training in human subjects with both diabetes mellitus and hypertension have now been performed. Of note, some are confounded by concomitant weight loss. The trial with the highest percent of comorbid hypertension compared an “intensively treated” group with uncontrolled T2DM (n = 36, HbA1c >8%) vs. a comparable T2DM control group (n = 36) receiving “usual care” [48]. Over 85% of both study groups had comorbid hypertension with a similar degree of hypertensive control at baseline. The exercise intervention consisted of a recommended aerobic exercise bicycling regimen as well as resistance exercises with elastic exercise bands, three to five times per week for 45–55 min at 50–80% of maximal heart rate with adjustments over the course of the study to maintain this intensity level. Over 12 months in subjects with T2DM, weekly exercise levels increased 2.5-fold in the intervention group (from 7.5 to 19.7 METs) with no significant change in the control group. There was no increase in the use of antihypertensive agents from baseline in either the intervention or control groups, but there was a significant 12-month improvement in the intervention group’s mean blood pressure from 144/85 to 130/76 (p < 0.005). Interestingly, although this intervention also included diet, the intervention group did not significantly lose weight. In addition, over the 6 months following this intervention, the exercise level worsened significantly in the intervention group (from 19.7 to 9.1 METs), and the accompanying increased systolic blood pressure and weight in that group correlated negatively to amount of time spent on exercise (r = 0.43 for systolic blood pressure, r = 0.363 for weight, both p < 0.05) confirming the relationship between increased exercise and improved blood pressure. The largest randomized controlled studies that looked at effect of lifestyle (diet plus exercise) interventions on blood pressure in T2DM were the Look AHEAD [49] and the Italian Diabetes and Exercise Study (IDES) [50]. In Look AHEAD 5145 individuals with T2DM (75% on antihypertensive medications) were randomized to an intensive lifestyle intervention (ILI) group versus a diabetes support and education (DSE) group. The ILI included calorie restriction and increased physical activity with a goal of 175 min per week of moderate-intensity physical activity. After 1 year of follow-up, blood pressure was significantly improved in the ILI compared to the DSE group (−6.8/−3.0 vs. −2.8/−1.8, p < 0.001). However, confounding this result is the fact that this group also lost 8.6% of their body weight compared to 0.7% in the DSE group. In contrast, the IDES emphasized exercise alone. Six hundred six subjects with T2DM and metabolic syndrome were randomized to twice-weekly supervised aerobic plus resistance training plus counseling versus counseling alone. At baseline, BP was ~140/85 in both groups, and >60% of the cohort was on antihypertensive medication. At 1 year, the exercise group had significantly greater improvement in BP than the control group (difference in delta BP −4.2/−1.7; p = 0.002 for SBP, 0.03 for DBP). BMI also decreased in the intervention group, but the decline, though significant, was small (2.9%). Three smaller RTCs similarly found improved BP in individuals with T2DM (68%, 50%, and 52% with apparent comorbid HTN, respectively) in the exercise group compared to a control group [51,52,53]. Weight decreased significantly in one [52] but not in the other two. In contrast, three other small RTCs did not find a benefit of an exercise intervention on blood pressure in T2DM individuals [54,55,56]. One study [56] compared calorie restriction with or without aerobic exercise in 29 individuals with T2DM and was likely simply underpowered in that a similar decrease in SBP was noted in the diet plus exercise group (n = 13) but did not reach statistical significance (p = 0.09). Another implemented a 2-year supervised endurance plus resistance training intervention in 50 men with T2DM and found no change in either weight or SBP [54]. Finally, Sigal et al. compared 22 weeks of aerobic, resistance, combined, and no-exercise control groups (~60 per group) and found no change in BP in any of the groups despite a significant weight loss in only the aerobic exercise group [55]. In summary, there is evidence that exercise can improve blood pressure in T2DM, but this conclusion is [1] not universally supported by the literature and [2] confounded by the concomitant weight loss in some of the studies.
The impact of training exercise on arterial stiffness has also been examined (reviewed in [57]). Several studies in adults without T2DM but with metabolic syndrome [58, 59] or end-stage renal disease [60] have demonstrated reduced arterial stiffness after an exercise intervention. In metabolic syndrome both aerobic [59] and resistance [58] training have been found to improve arterial stiffness. In a cohort of 23 human subjects with T2DM, 3 weeks of moderate exercise training for all subjects resulted in lessened arterial stiffness (as measured by ultrasound stiffness index β) at the carotid (p = 0.020) and femoral (p < 0.001) arteries [61]. In this study, improved insulin resistance resulting from exercise training correlated with decreased arterial stiffness at the carotid (p = 0.040) and femoral artery (p = 0.016). Another study randomized 35 women with T2DM to an aerobic exercise group (AEG) or a control group [62]. The AEG completed a 12 week intervention of accelerometer-confirmed 60 min of aerobic exercise three times a week. Arterial stiffness (AI) improved significantly in the AEG, and the percent change in AI correlated with the increase in physical activity energy expenditure, but interestingly not with insulin sensitivity. In contrast, despite increasing VO2max, another small crossover human study did not show an improvement in arterial stiffness or blood pressure after 8 weeks of bicycle exercise training (thrice-weekly at 60% maximum heart rate) in five men and women with T2DM and isolated systolic hypertension [63].
In summary, hypertension and arterial stiffness are related abnormal pathophysiological processes which are prevalent in diabetes. Arterial stiffness is a much newer physiologic measurement than hypertension, and so the clinical consequences of its presence and treatment are generally less well known than that of hypertension. Comorbid hypertension has been shown to impair exercise capacity and increase catecholamine release with exercise in subjects with T2DM. Arterial stiffness has been correlated with impaired diastolic function and decreased peripheral muscle perfusion during exercise in T2DM persons with normal peripheral circulation as well as increased peripheral vascular resistance with exercise in T1DM. In the majority of studies done to date, both hypertension and arterial stiffness are at least partially remediable with exercise training. The benefits of lowering blood pressure and arterial stiffness in diabetic hypertensive subjects and lack of harmful side effects with appropriate prescreening of subjects are encouraging enough to recommend exercise routinely to patients with diabetes mellitus and comorbid hypertension.
Congestive Heart Failure (CHF)
It is well established that cardiac function is impaired even early in diabetes with a predominance of diastolic dysfunction that may play a role in exercise impairment in diabetes [64]. This section is divided into two parts focused on the roles in exercise impairment and response to exercise of diastolic and systolic dysfunction, respectively.
Effects of Impaired Diastolic Dysfunction on Exercise Performance in T2DM
In 1972, Rubler et al. described four diabetic subjects with congestive heart failure (CHF) despite normal coronary arteries and no convincing etiology for their cardiomyopathy [65]. Further recognition of “diabetic cardiomyopathy ” followed, with prevalence rate estimates of diastolic dysfunction in diabetic subjects ranging from 30% in studies using conventional echocardiography [66,67,68] to 52–70% with more detailed Doppler echocardiograms using Valsalva maneuvers and pulmonary venous recordings [69, 70]. Since then many studies have confirmed this high prevalence of diastolic dysfunction even in early uncomplicated, asymptomatic well-controlled T2DM without hypertension or evidence of coronary artery disease [71,72,73,74,75,76,77,78]. Since its discovery four decades ago, greater understanding has developed as to the characteristics and causes of diabetic cardiomyopathy, though it is still incompletely understood. Current theory holds that diabetic cardiomyopathy is caused by multiple factors including hyperglycemia-induced myocardial fibrosis, metabolic disturbances related to insulin resistance, chronically increased oxidative stress, endothelial dysfunction, coronary microvascular dysfunction, cardiac autonomic dysfunction, advanced glycation end products, activated protein kinase C-β, microRNAs, mitochondrial dysfunction, and possibly more (reviewed in [77, 79,80,81,82,83]). Physiologically, diastolic dysfunction is a cardinal feature of the diabetic cardiomyopathy [84, 85].
Diastolic dysfunction is usually asymptomatic unless accompanied by other comorbidities [84]. In the presence of comorbid hypertension or myocardial ischemia, clinical features of CHF may develop from diastolic dysfunction despite the maintenance of a normal ejection fraction [85]. Several studies have shown that asymptomatic subjects with T2DM and diastolic dysfunction still remain at higher risk to develop CHF [84, 85] and also appear to have lower exercise capacity than diabetic subjects without diastolic dysfunction [73, 75, 76].
Considerable evidence links diastolic dysfunction to impaired exercise capacity in populations without diabetes (reviewed in [86, 87]). Four studies to date have correlated diastolic dysfunction with exercise impairment specifically in diabetes, while one did not. Poirier et al. showed worse maximal exercise treadmill performance in men with well-controlled uncomplicated T2DM and diastolic dysfunction (n = 10) as compared with age, weight, and clinically matched T2DM controls without diastolic dysfunction (n = 9) [10]. In this study, the diabetic subjects with resting diastolic dysfunction had a decreased duration of exercise time on a modified Bruce protocol (662 vs. 803 s, p < 0.02) and decreased metabolic equivalents (“METs ”) of 9.5 vs. 11.4 METs (p < 0.02). A correlation was also seen between the Em/Am ratio (echocardiographic marker of diastolic dysfunction as defined by the ratio of early to late mitral valve filling velocities) and exercise duration (r = 0.64, p = 0.004) and METs (r = 0.66, p = 0.003). In another study, a group of both T1DM and T2DM subjects (69.6% T2DM) performed symptom-limited Bruce protocol exercise tests. In this study exercise performance in METs was lower in the diabetic subjects with diastolic dysfunction vs. those without diastolic dysfunction (8.56 vs. 10.32 METs, p < 0.05) [76]. Irace et al. performed ergometer exercise stress tests in 38 subjects with T2DM and compared the presence of diastolic dysfunction in the subjects with a symptom-limited stress test (prior to reaching maximal predicted heart rate) vs. the subjects who completed ergometer tests to maximal predicted heart rate [73]. The 24 T2DM subjects with symptom-limited ergometer exercise stress tests had a correlation between decreased diastolic function and exercise duration. However, no significant correlation between diastolic dysfunction and exercise duration was found in the 14 subjects with T2DM who were able to complete ergometer exercise stress tests to maximal predicted heart rate. In comparing subjects with T2DM and normal exercise capacity (n = 52) or abnormal exercise capacity (n = 118), Fang et al. showed that preserved diastolic function (as defined by maximal early mitral valve filling velocity = E m) was correlated with better maximal exercise treadmill capacity (r = 0.43, p < 0.001) and remained an independent predictor of exercise capacity after multivariate analysis (p < 0.05) [88]. Finally and in contrast, Gurdal et al. studied 43 individuals with T2DM and 20 healthy controls. They found that the significantly reduced exercise capacity in T2DM was independent of diastolic function [89]. In summary, most studies do find a correlation between diastolic dysfunction and decreased exercise capacity in T2DM.
Though diastolic dysfunction is certainly a cardinal feature of “diabetic cardiomyopathy ”, some evidence is mounting that a subclinical depression of systolic function may also be present in some diabetic individuals. Despite maintaining categorically “normal” systolic function, subjects with T2DM have been shown to have significantly lower cardiac ejection fractions as compared to nondiabetic subjects. Sasso et al. found subjects with well-controlled, recent onset T2DM (3.9-year mean duration of diabetes) have lower ejection fractions both at rest (57% vs. 67%, p < 0.001) and during exercise (64% vs. 72%, P < 0.001) than age-, gender-, and body mass index-matched control subjects [90]. Among the T2DM subjects, greater insulin sensitivity was correlated with higher rest and exercise ejection fractions (r = 0.59, p < 0.004 for rest, r = 0.58, p < 0.005 for exercise). Conversely, a study by Willemson et al. in subjects with overt congestive heart failure with or without diabetes found that despite similar systolic function, the subjects with diabetes had worse exercise capacity. They proposed that the differential impairment in exercise capacity in diabetes was a result of diastolic dysfunction which in turn correlated with levels of advanced glycation end products [91].
Although exercise training is generally accepted to improve diastolic function in populations without T2DM (reviewed in [86]), the literature is more conflicted in diabetic cardiomyopathy (reviewed in [92]). Several studies have now examined the impact of exercise training in diabetes upon diastolic dysfunction with mixed results. In addition, comparison of these studies is difficult in light of the considerable methodological differences between them in exercise intervention, assessment of diastolic function, and statistical analysis.
Two small studies found completely negative results for exercise benefits on diastolic function. Sacre et al. studied a 6-month exercise intervention (combined aerobic and resistance training for ≥150 min moderate or ≥90 min vigorous intensity per week) versus standard care in 49 individuals with T2DM and diastolic dysfunction. They found improvement in exercise capacity but not in diastolic function [93]. Similarly, another randomized controlled trial of 42 men with T2DM in which the intervention group underwent supervised training four times a week for 12 months found no change in diastolic function (myocardial diastolic tissue velocity) despite improved BP, VO2max, HbA1c, and strength [53]. Another very small study found no improvement at rest, but improved cardiac output response to exercise suggesting that exercise may improve diastolic function response to stress, but not resting diastolic function [94].
In apparent contrast, Brassard et al. demonstrated normalization of diastolic dysfunction (E/A ratio) in 5 of 11 subjects with well-controlled T2DM and varying degrees of diastolic dysfunction after a 3-month aerobic exercise intervention [95]. A control group did not improve. Of note, the five subjects in whom diastolic function normalized were five of the six subjects with the mildest impairment in diastolic function. The authors also do not present a comparison of pre- and post-intervention averages for the exercise group raising the question of whether this difference reached statistical significance. In the largest study, 223 patients with T2DM were randomized to a usual care group versus a 3-year intervention group that started with supervised exercise and lifestyle and diet advice and then progressed to telephone-guided supervision [96]. Diastolic function was assessed at baseline and at 3 years, and diastolic dysfunction was defined based on E/Em ratio and/or deceleration time. In the intention to treat analysis, the between-group difference in prevalence of diastolic dysfunction at the 3 years did not achieve statistical significance (60% for usual care vs. 48% in the intervention group, p = 0.10). However, in a subgroup analysis of only those subjects who completed the full 3-year study, a significantly increased OR for diastolic dysfunction was found only in the usual care group (OR 2.46, p = 0.034). Interestingly diastolic function did not correlate with exercise capacity. Similarly the ABCD study randomized 100 patients with T2DM to a multi-intervention arm versus standard care and reported a neutral effect of the intervention on cardiac function, including diastolic function [97]. Again, though there was no statistically significant difference, the data showed a trend to increased E/A ratio at 2 years in the intervention group (p = 0.082). Finally, a recent study investigated high-intensity intermittent training (HIIT) in T2DM [98]. Twenty-eight subjects were randomized to 12 weeks of HIIT vs. standard care . This study found increased early diastolic filling (E) with stable late diastolic filling (A) suggesting improved E/A ratio though this data analysis is not provided. Another large RCT is currently underway in Australia that may shed more light on the controversial effect of exercise training on diabetic diastolic dysfunction [99]. However, results to date suggest that exercise may improve diastolic function in T2DM, particularly in those with mild impairment, and that this benefit may be dependent on duration and intensity of exercise.
Impairment of Exercise Performance in Diabetes Mellitus with Comorbid CHF Due to Systolic Cardiomyopathy
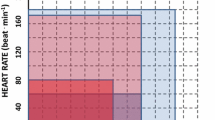
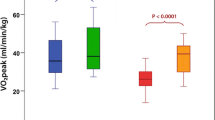
Congestive heart failure due to systolic cardiomyopathy has an estimated prevalence of 11.8% in T2DM [100]. Several studies have shown that diabetes in conjunction with systolic cardiomyopathy (T2DM-CHF) leads to worse exercise performance even when compared to subjects with CHF due to systolic cardiomyopathy alone. In 20 subjects with tightly controlled T2DM (HbA1c <7%) and moderate CHF symptoms, peak exercise performance yielded a VO2max nearly 20% less than nondiabetic age- and gender-matched subjects with moderate CHF (LVEF <40%) [101]. Multivariate linear regression further determined that the strongest predictor of VO2max in the DM-CHF subjects was alveolar-capillary membrane conductance (which determines the diffusing capacity of the lung (DLCO) along with pulmonary capillary blood volume). The authors suggested that the T2DM-CHF subjects may have a pulmonary angiopathy which allows leakage across the alveolar-capillary membrane as exercise raises the capillary pulmonary pressure. More on the pulmonary complications of diabetes is included in a later section of this chapter. Tibb et al. found a 30% reduction in VO2max in 78 subjects with systolic cardiomyopathy (defined by LVEF <40%) and comorbid T2DM as compared with 78 similarly sedentary age- and gender-matched controls (Fig. 18.1) [102]. Ingle et al. showed that 6-min walking distances are impaired in subjects with T2DM-CHF as compared with age- and gender-matched nondiabetic CHF patients (238 vs. 296 m, p = 0.005) [103]. In both the Tibb and Ingle studies, there was a higher prevalence of cardiovascular disease (CVD) in the T2DM subjects, but the Ingle study performed a sub-analysis matching only subjects with CVD, and the walking distance remained statistically impaired (231 vs. 283 m, p = 0.001). Prevalence of angiotensin-converting enzyme inhibitor, angiotensin-II receptor blocker, and beta-blocker usage between groups was analyzed in all three studies, and no differences were observed. In summary, subjects with T2DM and CHF from systolic cardiomyopathy have a greater exercise impairment than nondiabetic subjects with systolic cardiomyopathy alone; however, the reasons for this difference are not fully understood .
Individual peak oxygen uptake (VO2peak) in diabetic (DM) and nondiabetic (N-DM) patients with chronic heart failure due to left ventricular systolic dysfunction. Mean VO2peak is significantly lower in DM than in N-DM patients (Reprinted from Tibb et al. [102], with permission from American College of Cardiology Foundation)
Theoretically, insulin may improve exercise tolerance in DM-CHF subjects by increasing the ejection fraction. Insulin has been shown to have a direct inotropic effect on the myocardium in animals [104] and to increase resting left ventricular ejection fraction in nondiabetic human subjects (54% vs. 47%, p < 0.01) [105]. When an insulin-dextrose infusion was administered to T2DM and nondiabetic subjects (both groups with preserved systolic function), the left ventricular ejection fraction rose both at baseline and with exercise in T2DM [90]. In the nondiabetic subjects given insulin-dextrose, the LVEF rose with exercise but not at baseline. The exact physiologic mechanisms whereby insulin is able to increase LVEF without provoking hypoglycemia are still uncertain.
One study has shown that insulin administration may improve maximal exercise capacity in T2DM-CHF subjects by mechanisms other than increased LVEF. Guazzi postulated that insulin administration may improve exercise capacity in T2DM-CHF subjects in part by ameliorating pulmonary angiopathy and therefore looked at the impact of insulin therapy on VO2max and alveolar-capillary membrane diffusing capacity (DLCO) in T2DM-CHF subjects [106]. Using a parallel crossover design with subjects acting as their own controls, they found administration of insulin improved VO2max by 13.5% (p < 0.01) and improved ventilatory efficiency (slope of ventilation/carbon dioxide production decreased by 18%, p < 0.01). Changes in both VO2max and ventilatory efficiency after insulin administration correlated strongly with a better alveolar-capillary membrane diffusing capacity (r = 0.67, p = 0.002 for VO2max and DLCO, r = −0.73, p < 0.001 for ventilatory efficiency and DLCO). These changes were present both 1 h and 6 h after a 60-min insulin infusion but had resolved within 24 h after the insulin. The changes from insulin were not due to glycemic changes (dextrose counter-infusions maintained glucose homeostasis) or from a change in ejection fraction in these subjects. In summary, insulin therapy has been shown to improve exercise capacity in subjects with T2DM and CHF from systolic cardiomyopathy, at least in part by improving pulmonary angiopathy and seemingly without any changes in glycemic control or ejection fraction. However, it is unlikely that insulin would be utilized clinically to increase exercise capacity alone, as the risk of hypoglycemia creates an unfavorable risk-benefit ratio .
Several of the studies discussed above regarding effects of exercise on diastolic dysfunction also addressed systolic function and found no benefit of exercise for systolic dysfunction [93, 94, 96, 97]. However, in the HIIT intervention study, both stroke volume and EF improved significantly, suggesting that high-intensity exercise may also have the potential to improve systolic function [98].
Macrovascular Disease
The incidence of CVD in subjects with diabetes mellitus (both type 1 and type 2) is two to three times increased over that of the general population [107, 108], and mortality following acute myocardial infarction (MI) in individuals with diabetes is double than that of nondiabetic controls similar in age [109, 110]. Importantly, it has been shown that post-MI subjects with greater peak VO2max levels achieved through cardiac rehabilitation have lower cardiovascular mortality and morbidity [111, 112]. In addition, inadequate physical activity has been linked to increased mortality, largely through T2DM and CVD, in many epidemiological studies [113,114,115,116]. However, it has become clear that the development of macrovascular disease in T2DM is a nearly lifelong process, beginning well before the development of overt diabetes [117]. Furthermore, large-scale studies of interventions in diabetes have all been multitargeted, including combinations of diet, weight loss, glycemic control, and exercise. Thus, the exploration of specific effects of macrovascular disease on exercise performance and of exercise interventions on macrovascular disease has been challenging. This section will first present a selection of specific studies and otherwise summarize the current view of the interaction between exercise physiology and active macrovascular disease in the context of diabetes. More detailed information in this area is available in several recent systematic reviews [108, 117, 118]. The section will conclude with a discussion of two recent large RCTs of lifestyle intervention and their implications for the role of exercise in prevention of macrovascular disease. Current recommendations for exercise in macrovascular disease are summarized in Table 18.2.
Impairment of Exercise Performance and Response to Exercise in Diabetes Mellitus with Comorbid CVD
In general studies of exercise capacity post MI, with or without cardiac rehabilitation (CR), have continued the trend of lower exercise capacity in diabetes compared to the population without diabetes although the results are somewhat mixed. In post-MI populations without cardiac rehabilitation Izawa et al. found that the maximal exercise capacity was impaired in 30 post-MI T2DM subjects as opposed to 41 nondiabetic controls (22.6 mL/min/kg vs. 26.1 mL/min/kg, p < 0.01) despite similar resting ejection fractions between groups [119]. However, another study found no difference in exercise capacity without CR between 59 post-MI subjects with T2DM and 36 post-MI nondiabetic controls (20.2 mL/min/kg vs. 22.4 mL/min/kg, p = NS) [120]. Results in the first study implicated an impaired chronotropic response to exercise, possibly due to beta-adrenergic desensitization [121] which correlated with impaired VO2max in post-MI subjects with diabetes as compared with the nondiabetic post-MI controls. This chronotropic response to exercise has also been shown by Colucci et al. to inversely correspond to impaired VO2max in nondiabetic subjects with systolic cardiomyopathy [121]. Apart from lowering VO2max, an inhibited chronotropic response has been shown elsewhere to predict cardiovascular events within a T2DM cohort [122]. In contrast, a study of 225 patients, most without diabetes, found the most significant predictor of impaired exercise capacity 1 month after MI was impaired diastolic function, suggesting that mediators of impaired exercise function after MI may differ between diabetic and nondiabetic populations [123].
Recent studies have also explored the ability of exercise to improve exercise capacity in CVD. Kim et al. found lower VO2peak in 12 patients with T2DM than in 25 without both pre- and post-cardiac rehabilitation, with neither group showing a significant improvement with CR [124]. A cohort of 59 T2DM subjects and 36 well-matched (including for baseline exercise capacity) nondiabetic subjects were followed after a CR program performed for indications of acute MI or unstable angina in the month prior to enrollment [120]. Both groups compliantly participated in a 2-month CR program consisting of three 1-h moderate exercise training sessions per week. The T2DM group did show improvement with the CR program, including an increased VO2max of 13% from study entry, but their improvement was drastically attenuated as compared with the nondiabetic subjects. Despite no difference between groups VO2max at study entry, at completion of the study the nondiabetic subjects had a higher VO2max (28.8 vs. 22.6, P < 0.001), peak workload (139 W vs. 120 W, p = 0.009), and longer duration of exercise (13.7 vs. 11.8 min, p – 0.017) than the subjects with T2DM. Linear regression was performed to determine predictors of change in VO2max in the T2DM group and showed the change in VO2max was independently associated with fasting blood glucose (p = 0.001) These results have been supported by two other larger studies. One study of 370 subjects with T2DM compared to 942 without who completed a 36 session CR. Both groups improved there exercise capacity, but the group with T2DM had lower exercise capacity at baseline and significantly less improvement with CR [125]. A study of >7000 T2DM and >1500 non DM subjects found lower adherence to CR in the T2DM group, as well as a smaller increase in exercise capacity at 1-year post-CR [126]. The DARE study found that glycemic control was an important predictor of improvement in peak VO2 after CR [127]. In another study of exercise training in subjects with T2DM and known CVD with or without prior MI, an exercise intervention improved peak VO2 only in the subgroup of patients without a prior MI suggesting that the extent of macrovascular disease may impact the ability of exercise to improve functional capacity [128]. Exercise rehabilitation has also been shown to improve mortality in post-MI patients by 20%, but benefits may be attenuated in T2DM subjects whose exercise training response appears inhibited [129, 130]. To our knowledge, no studies to date have compared mortality after CR in post-MI nondiabetic subjects vs. those with T2DM.
In summary, data now strongly suggest that diabetic subjects post-MI have an impaired maximal exercise capacity prior to CR as compared to similar nondiabetic post-MI individuals. Differences may be mediated by an impaired chronotropic response to exercise. Patients with T2DM appear to be less likely to participate in CR, but even in those who do, post-MI subjects with T2DM show less improvement in exercise capacity as compared to those without T2DM. Fasting glucose levels and overall glycemic control were the best predictors of improved exercise capacity after CR in T2DM post-MI subjects. In general, response to exercise interventions may have greater benefit in patients with earlier, milder macrovascular disease, i.e., before an actual ischemic event. More study is warranted to determine the impact of exercise training on outcomes such as mortality and cardiovascular morbidity in subjects with T2DM and comorbid CVD.
Impairment of Exercise Performance in Diabetes Mellitus with Comorbid Peripheral Arterial Disease (PAD )
T2DM is a strong risk factor for the development of PAD. Prevalence of PAD based on ankle-brachial index in T2DM ranges from 15% to 30% [131, 132]. The cumulative incidence of PAD was 11% over 18 years following T2DM diagnosis in the UK Prospective Diabetes Study (UKPDS) cohort [133]. In the UKPDS study, a multivariate model examined the relative contributions of different risks for PAD in this diabetic cohort, and the strongest predictors were cardiovascular disease and current smoking which both ascribed threefold odds of PAD. Lesser but distinct risk was ascribed to worse glycemic control, higher systolic blood pressure, and lower high-density lipoprotein levels.
There are conflicting results in the small studies to date comparing exercise capacity in subjects with PAD and comorbid T2DM to nondiabetic PAD subjects [134,135,136,137]. Oka et al. found a decreased maximal walking distance (279 m vs. 461 m, p = 0.01) and decreased distance to onset of claudication (127 m vs. 187 m, p = 0.01) in patients with T2DM and PAD as compared with PAD alone [137]. Both groups were well matched for ankle brachial index (ABI), cholesterol, and systolic blood pressure levels and had similar prevalence of known CVD. Similarly, Dolan et al. showed T2DM subjects with PAD had a shorter 6-min walking distance (1040 vs. 1168 ft, p < 0.001) and slower walking velocity 0.83 vs. 0.90 m/s, p < 0.001) despite age adjustment between groups and similar baseline ABI and physical activity levels [134]. Unfortunately, there was a greater BMI in the subjects with T2DM as compared to the subjects without in this study. A multivariate linear regression model in this study found DM-associated neuropathy, greater exertional leg symptomatology, and greater comorbid CVD to be predictive of the worsened exercise capacity in the T2DM group. However, in subjects with a comparable BMI, ABI, and blood pressure levels, Katzel et al. found no difference in either age-adjusted VO2max or onset of claudication time between 47 diabetic and 72 nondiabetic subjects with PAD (1.16 in diabetics vs. 1.12 L/min in nondiabetics) [136]. Green et al. furthered the concept of BMI as an explanatory variable of exercise performance [135]. In their study, there was a significant difference in maximal exercise time between 12 T2DM PAD subjects and 12 age- and gender-matched leaner nondiabetic PAD subjects, but no difference between maximal walking time between the 12 T2DM PAD subjects and 7 nondiabetic subjects matched for BMI (median 845 s T2DM, 915 s “heavy” nondiabetics, 1448 s “leaner” nondiabetics). No difference was found between the three groups for pain-free exercise time, maximum cycling time, or VO2max, although trends toward significance were seen in the latter two parameters for “leaner” nondiabetics vs. both T2DM and “heavy” nondiabetics . Maximal walking time was significantly negatively correlated with BMI (r = −0.38, p < 0.05) as well as with the VO2 time constant, tau (r = −0.49, p > 0.05). The time constant, tau, reflects the rapidity with which VO2 responds to exercise and was significantly worse in T2DM subjects as compared to both the “heavy” and “lean” nondiabetic groups (p < 0.05, 71 s (T2DM) vs. 38 s (“heavy”) vs. 37 s (“lean”), respectively). The longer tau in T2DM and its inverse correlation with maximal walking time suggest the greater time for working muscles to receive steady-state oxygen distribution may decrease walking time in T2DM separately from BMI. A significant limitation of this study is the greater female distribution in the “heavy” control group as compared to both the T2DM and “lean” control groups, which may have lowered the exercise capacity in the “heavy” control group. Thus, current limited evidence suggests a greater BMI and longer VO2 time constant, tau, may play a role in the impaired maximal exercise times for T2DM subjects with PAD found in some studies .
The optimal form of exercise for subjects with T2DM and symptomatic PAD is a supervised exercise rehabilitation program with therapeutic modality of walking to near-maximal claudication pain over 6 months [138, 139]. It has been recommended that subjects with PAD and comorbid conditions that limit weight-bearing exercise consider low-impact activities such as stationary bicycling or aquatic exercise, although improvements in walking may be less [13, 140].
Data are lacking on the impact of exercise training on exercise capacity in subjects with diabetes and comorbid PAD; only limited subgroup analyses have been made to date. A systematic review and meta-analysis of exercise intervention studies in PAD identified 18 studies, 12 of which were confirmed to include subjects with diabetes (19–43%). Comparison of studies that included at least 25 % of subjects with diabetes to those that did not found greater improvements in maximum walking distance in the studies that did not, suggesting that subjects with PAD and diabetes benefited less than those without diabetes. Pain-free walking distance and 6-min walking distance were equally improved in both subsets of studies [141]. Sanderson et al. studied 42 subjects with PAD, 33% of whom had diabetes, and randomized the subjects (stratified for age, gender, and diabetes) to 6 weeks of treadmill exercise training at 80% of subject’s VO2max (n = 13), 6 weeks of bicycle exercise training at 80% of subject’s VO2max (n = 15), or no-exercise therapy [140]. Both the treadmill exercise training and cycling training regimens improved VO2max in this study. A subgroup analysis showed more severe pain in the symptomatic limb was the only baseline characteristic to differentiate “exercise responders” who increased their mean cycling or walking times from the entire sample; therefore, diabetes did not appear to play a role in the likelihood of a subject to respond. Ekroth et al. showed a mean 234% improved walking distance after 4–6 months of training in PAD subjects that was independent of the presence of diabetes [142]. Gardner et al. performed two studies with subjects with intermittent claudication. In one with 43% of subjects with T2DM, maximum walking time and VO2peak were improved with either a supervised intervention or a home-based exercise program compared to usual care [143]. A later larger study from the same group (23% T2DM) compared usual care with a supervised exercise program and again found improved maximum walking time and VO2peak within 2 months of intervention [144]. McDermott et al. similarly performed two studies. In the first, 156 subjects with PAD (~43% T2DM) were randomized to usual care versus supervised treadmill exercise versus lower extremity resistance training. VO2peak was not reported, but maximum walking time improved with both interventions (more with the treadmill intervention), and 6-min walking distance improved with the treadmill intervention, but not with the resistance training [145]. In the second study from this group, 194 PAD patients (32% T2DM) were randomized to a home-based behavioral walking intervention versus an attention control condition. VO2peak was again not reported, but the walking intervention significantly improved 6-min walking time and maximum walking distance, including in the subgroup with T2DM [146].
Beyond these subgroup analyses, we are not aware of any studies designed to differentiate the response to exercise training in subjects with PAD and comorbid DM as compared to nondiabetic PAD controls. In summary it appears that exercise does improve PAD in T2DM, but definitive studies in T2DM alone are lacking .
The Role of Exercise in Prevention of Cardiovascular Outcomes
Many observational studies have linked increased habitual physical activity with improved cardiovascular outcomes in diabetes. Because of the extensive confounding of these observational studies by other uncontrolled variables, they will not be discussed here. This section will focus on the few interventional studies that have explored the effect of exercise-inclusive lifestyle interventions on hard clinical CVD outcomes.
Two large long-term multicenter randomized controlled trials have explored the utility of a lifestyle intervention that includes exercise in the prevention of CVD, the Chinese Da Qing study and Look AHEAD and found interestingly contrasting results. Da Qing enrolled 577 adults with impaired fasting glucose, but not diabetes, and randomized them to a control group versus one of three 6-year intervention groups: diet, exercise, or both. After 23 years of follow-up, the intervention groups combined had reduced CVD mortality (HR 0.59, 95%CI 0.36–0.96, p − 0.033), as well as decreased incident diabetes (HR 0.55, 95%CI 0.40–0.76, p = 0.001) [147]. A subgroup analysis demonstrated that the majority of the deaths that occurred were in individuals who subsequently developed diabetes [148]. Unfortunately the study was not large enough to allow separate analyses of the three intervention groups, and it remains impossible to say whether exercise was a key intervention component in the CVD mortality reduction.
In contrast the Look AHEAD study recruited over 5000 overweight or obese patients with T2DM to a participate in a randomized controlled study of an intervention with the primary goal of weight loss [149]. The primary outcome was a composite cardiovascular outcome including death from cardiovascular cause, nonfatal MI or stroke, and angina-related hospitalization. The intervention included caloric restriction and nonsupervised increased physical activity compared to a control group that received diabetes support and education. The study was intended to continue for 13.5 years but was stopped early at 9.6 years for futility as the event rate in the control group was much lower than anticipated and there was virtually no signal for a difference in the intervention group. It is, however, a gross overinterpretation of the study to say that exercise does not provide CVD benefit in T2DM for several reasons: (1) It does not appear that physical activity was monitored in the intervention group; (2) in fact, exercise capacity was measured only in the first 4 years. Exercise capacity increased markedly over the first year but then declined to nearly the level of the control group by year 4 raising the likelihood that compliance with the increased physical activity advice was poor; (3) weight, waist circumference, and HbA1c all rebounded dramatically after the first year again suggesting poor compliance with the overall intervention; and (4) despite this there is a strong trend to benefit in the subgroup of individuals who did not have CVD at baseline (HR 0.86, 95% CI 0.72–1.02) [150].
In addition one smaller study combined an observational component with an exercise intervention in a subgroup of the observational cohort [151]. The study recruited 539 CVD patients with T2DM and 507 without T2DM. All completed a validated questionnaire to measure leisure-time physical activity (LTPA). Of these 143 and 148, respectively, were willing and appropriate for inclusion in a 2-year exercise intervention. The observational analysis confirmed the benefits of higher LTPA in decreasing CVD events with and without T2DM, with the groups that reported <thrice-weekly LTPA having at least a twofold increase in CVD events over the 2-year follow-up compared to the group that reported LTPA at least three times a week. This difference persisted after adjusting for participation in the exercise intervention. In contrast the 2-year exercise intervention had no effect on CVD event rates over the 2 years.
In summary these two large studies, as well several smaller studies described above, suggest that failure of exercise to provide CVD benefit may be a case of “too little, too late” in diabetes where macrovascular damage may already be quite advanced by the time of diagnosis. Studies and subgroup analyses in earlier prediabetes, those with T2DM but no evidence of CVD, and those that explore longer-term physical activity habits do show, or at least hint at, reduction in macrovascular disease with exercise.
Cardiac Autonomic Dysfunction
Cardiac autonomic neuropathy (CAN) may be a specific neuropathy and therefore could be considered together with microvascular neuropathies. However, in light of evidence for a significant role of CAN in exercise intolerance, diabetic cardiomyopathy, and CVD risk, it is briefly discussed separately in this section. Two separate aspects of CAN may have significant roles in T2DM: (1) impaired resting cardiac sympathetic/parasympathetic balance and (2) impaired chronotropic response to exercise. Both appear to be associated with CVD risk, have been linked to cardiac maladaptation to exercise, and are improved with exercise.
Reduced heart rate variability (HRV), the simplest and most commonly used measure of CAN, has repeatedly been shown to correlate with CVD risk in T2DM [152,153,154,155,156]. In addition, the DIAD study found that CAN was one of the independent predictors of silent ischemia in T2DM [157]. More detailed analysis of HRV includes the isolation of high-frequency (HF) variation reflective of parasympathetic/vagal input . Decline in parasympathetic/vagal input and/or increase in sympathetic input at rest is thought to contribute to the increased CVD risk associated with CAN. The other significant measure of CAN is the response of cardiac autonomic modulation or chronotropic response to stress, usually exercise. This is typically measured as the increase in heart rate with exercise [158] and reflects the desirable increase in sympathetic and decrease in parasympathetic stimulation with stress, usually exercise. Impairment in this response, known as chronotropic incompetence (CI), is another measure of CAN that has also been associated with poor CVD prognosis [158].
No studies have directly addressed the relationship between CAN and reduced exercise capacity in diabetes. However, a few studies have linked CAN to other measures that may impact exercise tolerance and exercise capacity. A recent study recruited 83 patients with T2DM without CVD and performed sophisticated “state-of-the-art” measures of autonomic function, as well as 82Rb-PET/CT and 123I-MIBG measurement of cardiac perfusion and sympathetic responsiveness thereof [159]. Eleven percent met ADA criteria for CAN, but nearly half of the patients had at least one measure consistent with some degree of CAN. In the full cohort, multiple measures of CAN correlated significantly with impaired cardiac flow reserve. Although exercise tolerance was not tested in this study, it is likely that this impairment in increased blood flow with sympathetic activation, as well as the CI described above would contribute to increased perceived exertion and hence exercise intolerance in T2DM. CAN has also been correlated with diastolic dysfunction [160]. As described above, diastolic dysfunction may also contribute to decreased exercise capacity in T2DM. In fact, Baldi argues that CAN is a major contributor to the diabetic cardiac dysfunction described earlier and implicated in impaired exercise capacity in diabetes [64].
The literature on response of CAN to exercise in T2DM is limited, complicated by the multiple measures used to assess CAN, and mixed in results. Multiple studies (reviewed in [161]) have found improved cardiac autonomic function with exercise in individuals without CAN at baseline. However, few studies have looked at effects in individuals with CAN at baseline. One small study in T1DM demonstrated improved HRV after a 12-week supervised intervention in early CAN but no change in severe CAN [162]. A similar study in T2DM of a 6-month supervised exercise intervention found improved HRV with increased HF variation indicating increased parasympathetic tone [163]. In contrast, Sacre et al. randomized 49 subjects with T2DM to a 6-month exercise intervention versus usual care and found no changes in CAN despite improved VO2peak and lowered resting heart rate [93].
The response of CI to exercise has been demonstrated in CHF and CVD but has not been studied directly in diabetes. One study found an increase in peak HR with a walking intervention in T2DM suggesting that exercise may improve CI in T2DM as well [164]. Another study used glucose ingestion as a metabolic stress that also stimulates cardiac sympathetic tone in a manner similar to exercise [165]. Fifty-nine obese subjects (23 with T2DM, 36 without) were recruited to this observational study of a 16-week moderate-intensity aerobic exercise program. Multiple measures of cardiac autonomic modulation including HRV, blood pressure variability, and baroreflex sensitivity were performed before and after glucose ingestion and before and after the intervention. These demonstrated increased sympathetic cardiac modulation and decreased vagal modulation with glucose ingestion after the intervention, indirectly suggesting that the exercise intervention improved CI.
A recent study explored the impact of an exercise intervention on a new index of CAN, heart rate recovery [166]. In this study 42 subjects with T2DM and abnormal heart rate recovery (about a third of the screened subjects with T2DM) were randomized to usual care versus an intervention that combined resistance and moderate-intensity aerobic training for 12 weeks. The intervention group was found to have significantly lower resting heart rate and greater improvement in heart rate recovery than the control group.
In summary, CAN likely plays a significant role in the cardiac dysfunction in diabetes and hence in the decreased exercise capacity in diabetes. A beneficial role of exercise is supported by limited studies, but more studies are needed addressing direct effects of exercise on CAN.
Microvascular Disease
Impaired Exercise Capacity from Microvascular Complications in Diabetes Mellitus
Microvascular complications of T2DM include nephropathy, neuropathy, and retinopathy, and all of these have an increasing incidence with greater duration of T2DM. The prevalence of nephropathy and retinopathy in T2DM have been reported to range from 7% to 30% [167] and from 3% to 27% [168], respectively. Given their occurrence later in the course of diabetes, microvascular complications are present at a more advanced stage of diabetic pathophysiology. As such, it is reasonable to consider they may be explicitly associated with increased exercise impairment but also present simultaneously with other abnormalities that impair exercise capacity (e.g., nephropathy in the form of microalbuminuria has been linked with the presence of diastolic dysfunction [169]). This section will review the evidence in the literature that microvascular complications are correlated with exercise impairment and that exercise can improve or slow progression of many of these complications and review current guidelines for exercising with complications (Table 18.2).
Diabetic Nephropathy Decreases Exercise Capacity
Diabetic nephropathy has been shown to adversely affect exercise capacity in both T1DM and T2DM subjects. Jensen et al. found a 25–30% reduction in maximal exercise capacity when comparing normoalbuminuric T1DM subjects and T1DM subjects with either microalbuminuria (30–300 mg/day) or macroalbuminuria (>300 mg/day) [170]. In an earlier non-exercise-related study by this group, resting left ventricular function was also found to be impaired in T1DM subjects with microalbuminuria and macroalbuminuria as evidenced by greater left ventricular end-diastolic volume (p < 0.05), lower stroke volumes (p < 0.05), and a trend toward decreased cardiac output (p = 0.10 for macroalbuminuric subjects and p < 0.05 for microalbuminuric subjects) [171]. More recently, Bjornstad et al. demonstrated decreased VO2peak in adolescents with T1DM relative to controls [172]. These adolescents exhibited only the presumed earliest sign of renal disease, hyperfiltration, with an elevated eGFR relative to controls. Peak VO2 was strongly inversely correlated with eGFR (the degree of hyperfiltration), but not with HbA1c, LDL, insulin resistance, or blood pressure.
In T2DM, the Strong Heart Study showed a correlation between the severity of microalbuminuria and the degree of diastolic dysfunction [169]. Lau et al. also showed a decrement in maximal exercise capacity in T2DM subjects with microalbuminuria (30–300 mg/day of microalbumin) as compared with normoalbuminuric T2DM subjects (p = 0.015) and nondiabetic control subjects (p < 0.001) [173]. The authors hypothesized pulmonary microangiopathy and diastolic dysfunction may partially explain this exercise decrement, as their subjects had worsened gas exchange with exercise (p = 0.019 for group trend between control, T2DM, and T2DM with nephropathy for minute ventilation/carbon dioxide production) and a greater frequency of diastolic dysfunction that normoalbuminuric T2DM subjects (p = 0.013). Thus, diabetic nephropathy was clearly correlated with exercise impairment [170], and comorbid diastolic dysfunction [169, 171, 173] as well as pulmonary angiopathy [173] may partially explain this impairment.
Other studies that were not limited to subjects with diabetes have further strengthened the association of chronic kidney disease with impaired exercise performance, including in diabetes. One study of 136 patients with moderate chronic kidney disease (eGFR 40 ± 9, 38% with diabetes) found markedly impaired peak VO2 and heart rate response that were more prevalent in the diabetes subgroup and were independently associated with aortic stiffness, impaired left ventricular function, and increased left ventricular afterload [174]. Another study of 39 CKD patients (11 with diabetes) found a nonsignificant (p = 0.099) reduction in VO2max in the group with diabetes compared to those without [175]. They also demonstrated that VO2max was independently inversely related to hsCRP levels independent of diabetes status, implicating inflammation as a contributory mechanism in CKD-associated exercise impairment .
Effects of End-Stage Renal Disease on Exercise Performance in T2DM
End-stage renal disease (ESRD ) from T2DM has been shown to occur in only 0.8% of a cohort of T2DM patients followed for 10 years; however, incidence does continue to increase with time. Accordingly, diabetic nephropathy was the single most common cause of new-onset ESRD in the United States in 2002 (45% of incident dialysis patients). Given the multiple comorbidities associated with ESRD [176], it is understandable that it would correspond to even greater decreased exercise capacity than non-ESRD nephropathy. In both diabetic and nondiabetic subjects with ESRD on dialysis, maximal exercise capacity has been shown to be about 60% that of age-matched control subjects [177, 178]. Moderate anemia (hematocrit <30%) has been shown to lower VO2max, and is improved with erythropoietin administration [179]. However, other factors which depress exercise capacity are felt to be numerous and have not yet been specified [178, 180]. More intensive hemodialysis sessions (five to six nocturnal sessions/week lasting 6–8 h per session) led to significant improvements in VO2max 3–6 months after the transition from thrice-weekly conventional hemodialysis [181]. Also, 1 month after renal transplant, VO2max showed improvement to nearly that expected for sedentary age-matched subjects [180, 182]. These improvements in VO2max either from more intensive hemodialysis or after renal transplant occurred despite the absence of any exercise training or significant improvements in anemia in these studies [180,181,182]. Such findings further suggest that as yet undefined factors related to ESRD significantly depress exercise capacity in both diabetic and nondiabetic subjects with ESRD.
Benefits of Exercise Training in Chronic Renal Disease
Exercise training-specific studies have not been done in the population with diabetes and comorbid renal failure. However, extensive literature exists on the benefits and safety of exercise training in the renal failure population independent of diabetes status. Since diabetes is well represented in this population, these studies are likely to be relevant to diabetes-related renal disease, and a few will be briefly discussed here. Watson et al. implemented a progressive resistance training program in CKD stage 3b/4 patients [183]. The intervention was safe and well tolerated and resulted in improved strength and endurance walking time. However, they note that this outpatient program was offered to over 400 patients and accepted by only 38 (15% with diabetes vs. 27% in the age- and BMI-matched control group), suggesting that this type of outpatient supervised strategy, while beneficial, is impractical, perhaps especially in the diabetes population. In a randomized, controlled trial of 16 weeks of aerobic exercise training in 25 patients with stage 3 CKD (40% diabetes), aerobic capacity, endothelin 1 levels, and QOL measures, but not aortic stiffness, were significantly improved [184]. No diabetes subgroup analyses were reported. Another small study randomized 20 stage 3/4 CKD patients to standard care versus 12 months of supervised resistance and aerobic training [185]. Unfortunately none of the intervention group had diabetic nephropathy, and the small study was confounded by significant between-group differences. However, the intervention group showed promising results with improved eGFR, PWV, VO2peak, and waist circumference (versus worsening in the control group). The Look AHEAD study in T2DM had weight loss as its primary goal, but the intervention did include exercise. The rate of incident CKD in this large study population was significantly decreased in the intervention group (by 31%), and multivariate analysis implicated reductions in weight, A1c, and BP as significant mediators [149]. More studies in the diabetic CKD population are clearly needed given the debilitating effects of renal disease on functional capacity and the encouraging results from small intervention studies in the nondiabetic CKD population .
Limited Data on Exercise Capacity Association with Retinopathy or Neuropathy
Diabetic retinopathy has also been associated with reductions in exercise capacity in T2DM subjects. Despite adjusting for known predictors of exercise capacity such as age and duration of diabetes in a regression analysis, the VO2max in ABCD trial subjects with T2DM was independently reduced by the presence of diabetic nephropathy (p = 0.04) and retinopathy (p = 0.026) [186]. Other studies have not explicitly looked at the relationship between diabetic retinopathy and exercise capacity or the causes of this abnormality. To our knowledge, no studies have explored any potential associations between diabetic neuropathy and exercise capacity.
Hazards of Exercise Training with Diabetic Microvascular Complications
Although exercise training is highly beneficial to most participants, the presence of microvascular complications raises some safety considerations. Diabetic retinopathy may lead to adverse outcomes with vigorous exercise. Diabetic subjects with active proliferative diabetic retinopathy (PDR ) are at higher risk for vitreous hemorrhage or retinal detachment [187]. Subjects with PDR or moderate to severe nonproliferative retinopathy are recommended to avoid strenuous exercise, Valsalva maneuvers, and jarring activities per the most recent ADA position statements (Table 18.2) [188, 189]. No studies have looked specifically at the impact of exercise training on the remediation of retinopathy in humans.
Recent ADA position statements on diabetes and exercise have relaxed the recommended limitations on exercise for peripheral neuropathy. Current recommendations refer to the increased risk of skin breakdown and infection with peripheral neuropathy and recommend proper footwear and daily examination of the feet for lesions with weight-bearing exercise. However, the recommendation to avoid weight-bearing exercise has now been limited to individuals with foot injuries or open sores [188, 189]. The ADA suggests nonweight-bearing exercises such as swimming, bicycling, or rowing for these patients. This relaxation of exercise limitation for peripheral neuropathy was driven by recent studies demonstrating tolerance and safety of exercise interventions in peripheral neuropathy. In some cases, these studies also found improvement in neuropathy or in side effects of neuropathy (e.g., balance and gait issues) with the exercise interventions (reviewed in [190,191,192]). Streckman et al. performed a systemic review and identified 11 studies of exercise interventions in diabetic neuropathy [192]. Of these nine demonstrated improvement in side effects of neuropathy or neuropathy itself, while one did not (one did not report on intergroup differences). One study compared a weight-bearing intervention to a nonweight-bearing intervention and found no difference in the number of foot lesions occurring during the intervention [193]. A larger Italian study [194] randomized 78 diabetic participants without peripheral neuropathy to usual care versus 4 h/week of observed treadmill walking addressing a role in prevention of neuropathy. Treadmill walkers improved vibration detection and nerve conduction indices and were significantly less likely to develop neuropathy over the 4 years of the study. Most significantly, a 2012 study using a 10-week aerobic exercise plus strength training intervention in individuals with T2DM and peripheral neuropathy demonstrated improvement in maximum pain, overall symptom score, and intraepidermal nerve fiber branching [195]. No foot ulcerations, delayed healing of biopsy sites, or infections were noted during the study.
ADA guidelines for exercise with autonomic neuropathy note that autonomic neuropathy can increase the risk for exercise-induced injury and recommend that all individuals with autonomic neuropathy undergo cardiac investigation before beginning more-intense-than-usual physical activity [188, 189].
Some experts have discouraged strenuous physical activity in subjects with diabetic nephropathy given the propensity for exaggerated blood pressure elevations with high-intensity exercise and proteinuria [196,197,198,199,200] associated with acute exercise-induced blood pressure excursions [187]. Results have been mixed on whether microalbuminuria increases to a significant degree in subjects without baseline nephropathy [200,201,202]. However, the most recent ADA guidelines state that there is no evidence that vigorous intensity exercise increases the rate of progression of diabetic kidney disease and place no specific exercise restrictions on this population. In fact, a prior recommendation of exercise stress testing prior to an aerobic exercise program in previously sedentary individuals with diabetic kidney disease given their significant prevalence of CVD has been removed. The recommendations now simply state that high-risk (including individuals with significant nephropathy), previously sedentary individuals should be encouraged to start with short periods of low-intensity exercise and slowly increase frequency and intensity [47, 188, 189].
Separate from safety considerations, high-intensity exercise may be precluded by pain or early fatigue from comorbid diabetic neuropathy [203, 204], musculoskeletal pain/osteoarthritis [205,206,207], renal osteodystrophy [208], or myopathy [208] and generally in subjects with end-stage renal disease [208, 209].
Pulmonary Function
Considerable evidence now exists that T2DM is also accompanied by impaired pulmonary function which may also play a role in exercise impairment. Similar evidence exists in T1DM, but this discussion will focus on T2DM. While past studies measuring pulmonary function tests in diabetes had yielded somewhat mixed results (reviewed in [210]), more recent studies have consistently demonstrated restrictive lung disease in diabetes relative to controls, while others have suggested additional obstructive and alveolar defects . In one cross-sectional study of pulmonary center patients with pulmonary function tests (PFTs) and no specific pulmonary diagnosis, patients with T2DM (n = 560) had decreased FVC, FEV1, and DLCO (p < 0.0001) compared to those without diabetes (n = 3604) after adjustment for age, sex, race, height, BMI, smoking, and heart failure [211, 212]. Similarly, a retrospective analysis of 62 diabetic and 27 obese nondiabetic patients admitted to an endocrinology service who also underwent pulmonary function testing found that FVC, FEV1, DLCO, and multiple other measures of pulmonary volume and function were significantly lower in the patients with T2DM compared to the obese nondiabetic group [213]. Furthermore, Kinney et al. studied a population of smokers with or without COPD and with and without T2DM. They found that across COPD stages, those with T2D had reduced FEV1 and FVC, as well as reduced 6 min walk and quality of life [214].
While these studies were admittedly confounded by apparent existing pulmonary issues (known smoking and/or COPD or some clinical indication for PFTs), other studies have measured PFTs either prospectively or as routine health care and found similar results. For instance, in a large Korean study after exclusion of all individuals with known pulmonary or neuromuscular disease or heart failure, over 35,000 adults who had PFTs as part of their routine health care were studied [215]. The prevalence of restrictive lung disease was more than doubled in those with T2DM compared to those with normal fasting glucose (18% vs. 8%). Obstructive ventilatory dysfunction was less prevalent overall but was also increased in T2DM. Multivariate analyses implicated insulin resistance (HOMA-IR, fasting insulin, and triglycerides) in the increased restrictive lung disease, while the obstructive lung disease appeared to be largely related to age blood pressure, and to glycemic control in the T2DM population. Similarly, the Berlin Aging Study II (BASE II) of about 700 individuals with adequately performed PFTs found the FEV1 and FVC were decreased in the T2DM population [216]. However, in contrast to the Korean study, restrictive lung disease in this study was largely related to central adiposity and muscle mass. Finally, several smaller but controlled and population-matched studies have found similar results linking T2DM to restrictive lung pathology, apparently independent of obesity. For instance, Aparna (2013) compared 40 subjects with T2DM to 40 age-, sex-, and BMI-matched controls and found that FVC, FEV1, and peak expiratory flow rate were significantly decreased in the subjects with T2DM [217]. FEV1/FVC% was significantly increased, again consistent with a primarily restrictive defect in T2DM. Shah et al. similarly studied 60 adult males with T2DM versus 60 obese adult males without T2DM and found decreased FEV1 and FVC, but no difference in FEV1/FVC [218]. In this study, the pulmonary measures showed no correlation with HbA1c or duration of T2DM.
Several studies demonstrating pulmonary defects in T2DM have further explored associations to shed light on causation. Overall these studies have suggested that the restrictive pulmonary disease component is independent of glycemic control, consistent with the Shah study above [219, 220]. In addition, a review of pulmonary disease in metabolic syndrome [219] presents data from several studies demonstrating restrictive lung disease associated with all components of met abolic syndrome and concluding that insulin resistance, and not hyperglycemia, is the underlying mechanism. A 2013 study in India supported and added to this conclusion [220]. In this cross-sectional study of 30 T2DM patients and 30 age-, weight-, and sex-matched nondiabetic subjects, FVC, FEV1, PEFR, PIF, FIVC, TLC, DLCO, and DLCO/VA were significantly reduced in patients with T2DM. Within the T2DM population, DLCO and DLCO/VA (but not FVC and FEV1) were reduced in the subpopulation with HbA1c >7. They conclude that T2DM, independent of glycemic control, is associated with a restrictive pulmonary abnormality, while poor glycemic control then contributes an additional alveolar diffusion defect. This is consistent with other studies implicating microvascular disease in the alveolar diffusion defects in T2DM [213, 221]. However, other studies have also shown a correlation of the restrictive lung disease with other complications of diabetes typically associated with poor glycemic control, including nephropathy [222] and CAN [223]. Shafiee et al. demonstrated a worsening of restrictive pulmonary disease across three subgroups of T2DM patient with increasing evidence of nephropathy (no nephropathy vs. microalbuminuria vs. macroalbuminuria) [222]. Similarly in a study in adolescents with T1DM, restrictive pulmonary disease was found only in those with CAN [223]. In contrast, Kaminski et al. studied pulmonary disease in T2DM adults with and without CAN and found that PFTs and max expiratory pressure were similar, but inspiratory pressure (reflecting respiratory muscle strength) was decreased in subjects with CAN and correlated negatively with resting HR as a measure of sympathetic/parasympathetic ratio in CAN [224].
Overall, these studies suggest that several forms of pulmonary disease are prevalent in T2DM as complications/comorbidities of T2DM. These include (1) a prominent restrictive component that is likely at least in part related to insulin resistance and independent of glycemic control, (2) an alveolar diffusion defect that is likely an example of diabetic microvascular disease with a large contribution from hyperglycemia, and (3) lesser contributions from inspiratory muscle weakness and possibly obstructive pulmonary disease that are less well studied in T2DM.
Effects of Pulmonary Disease on Exercise Performance in T2DM
The effects of T2DM pulmonary disease on exercise performance and of exercise training on T2DM pulmonary disease have not been well studied. However, limited studies suggest that restrictive pulmonary disease does contribute to decreased exercise capacity in T2DM and that some forms of exercise training may improve restrictive pulmonary disease. Kitahara et al. measured exercise capacity and pulmonary function in 31 male patients with uncomplicated T2DM and no evidence of cardiopulmonary disease (including no frankly abnormal PFTs). They found that the percentage of predicted maximal oxygen uptake (%VO2max) correlated significantly with percentage of predicted FEV1 (%FEV1) and that a mild reduction in %FEV1 was associated with measurably impaired exercise capacity [225]. Conversely, very limited studies have suggested that exercise training may improve pulmonary function. Inspiratory muscle training has been shown to dramatically improve (>2×) max inspiratory pressure [226]. However, this training failed to have any effect on PFTs or on functional exercise capacity (peak VO2).
In contrast, arm swing exercise in T2DM did improve FVC, FEV1, and maximal voluntary ventilation, as well as HbA1c, a low-density lipoprotein, malondialdehyde, oxidized glutathione, and the percent body fat [227]. However, overall exercise capacity was not tested in this study .
In summary, there is strong evidence for multiple forms of pulmonary disease in both T1DM and T2DM, complications that are not widely recognized in diabetes. Relatively limited evidence suggests that pulmonary complications may contribute to the exercise defects in diabetes. Very limited evidence exists to support benefits of exercise in specifically treating pulmonary complications of diabetes. Further studies are needed to explore methods and benefits of specifically targeting treatment of pulmonary disease in diabetes.
Special Cases
Exercise Impairment with Diabetes Mellitus and Atrial Fibrillation
Subjects with T2DM have been shown to develop atrial fibrillation more often than nondiabetics [228, 229]. The prevalence of comorbid diabetes was recently found to be 23% in a trial of elderly subjects with atrial fibrillation [230]. Physiologically, these two diseases may be linked as cardiovascular abnormalities predict the development of atrial fibrillation [228] and diabetes confers a significant risk of cardiovascular morbidity [231,232,233].
One small trial compared the VO2max before and after direct current cardioversion to establish sinus rhythm in subjects with atrial fibrillation without comorbidity (“lone atrial fibrillation”), atrial fibrillation and hypertension, or atrial fibrillation and diabetes [234]. This study found no improvement in VO2max or subject-measured effort of exercise (Borg scale) in subjects with diabetes and atrial fibrillation after cardioversion, despite an improvement in VO2max and subject-measured effort of exercise in lone atrial fibrillation and, to a lesser degree, in subjects with hypertension and atrial fibrillation. The authors theorized the lack of improvement in diabetes and atrial fibrillation corresponded to the lack of improved endothelial function, as this had improved in both the hypertensive and lone atrial fibrillation groups.
Exercise and Fatty Liver in Type 2 Diabetes
A recent analysis was performed of >200,000 Korean subjects with T2DM who were part of an occupational health screening program that included ultrasound assessment for fatty liver and completion of a validated physical activity form [235]. The results demonstrated that exercise ≥5 times a week was associated with an OR of 0.86 for incident fatty liver and 1.4 for resolution of preexisting fatty liver (p < 0.001 for both). Although more was better, a significant improvement in both outcomes was also noted with any increase in physical activity and with one to two bouts of exercise per week. These results support the findings of other recent smaller studies demonstrating benefits of exercise for fatty liver [98, 236, 237]. Though the associated exercise interventions also improved exercise capacity, no studies have specifically linked fatty liver with reduced exercise capacity.
Exercise and Obstructive Sleep Apnea
Obstructive sleep apnea (OSA ) is a common comorbidity in T2DM that is also improved by exercise interventions. A recent systematic review and meta-analysis of eight articles demonstrated that exercise as a sole intervention decreased the apnea hypopnea index, BMI, and Epworth sleepiness scale [238]. Again, no studies have demonstrated that OSA itself independently contributes to exercise impairment in diabetes.
Summary
There is a high prevalence of hypertension, arterial stiffness, vascular disease, and diastolic and systolic dysfunction which deleteriously impact exercise capacity in diabetes. Hypertension and arterial stiffness may both be improved in diabetes by exercise training programs and exercise should be recommended. Subjects who have suffered an MI and have T2DM have been shown to rehabilitate to a lesser degree than nondiabetic post-MI subjects. Data on the impact of exercise training for diabetic individuals with diastolic dysfunction, systolic cardiomyopathy, and PAD are only available from subgroup analyses or nondiabetic populations. Since it is recognized that mortality is generally lower in the diabetic population with better exercise capacity, exercise training to raise the exercise capacity is worthwhile, at least in theory, in diabetic individuals with all comorbid conditions. However, more studies are needed to explicitly clarify the benefits of exercise training in the diabetic with diastolic or systolic dysfunction, CVD (without recent MI), or PAD.
The presence of diabetic nephropathy has been consistently associated with decreased exercise capacity, while possible associations between exercise capacity and either diabetic retinopathy or diabetic neuropathy are understudied. Existing diabetic retinopathy, nephropathy, or neuropathy may pose safety concerns to the diabetic individual planning to institute a new exercise regimen more intense than brisk walking. Given the lack of randomized trial data, the recommendations given in the ADA position statement should be followed with regard to exercise precautions in the diabetic person with microvascular disease. More study in the area of safety and efficacy of exercise training in the diabetic person with microvascular disease is also warranted.
In summary, exercise training is important to treat the metabolic and cardiovascular abnormalities associated with T2DM. Clinicians should work to insure their diabetic patients may exercise safely to achieve these goals.
References
Rowland TW, Martha PM Jr, Reiter EO, Cunningham LN. The influence of diabetes mellitus on cardiovascular function in children and adolescents. Int J Sports Med. 1992;13(5):431–5. Epub 1992/07/11.
Veves A, Saouaf R, Donaghue VM, Mullooly CA, Kistler JA, Giurini JM, et al. Aerobic exercise capacity remains normal despite impaired endothelial function in the micro- and macrocirculation of physically active IDDM patients. Diabetes. 1997;46(11):1846–52. Epub 1997/11/14.
Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 2010;95(2):513–21. Epub 2009/11/17.
Kjaer M, Hollenbeck CB, Frey-Hewitt B, Galbo H, Haskell W, Reaven GM. Glucoregulation and hormonal responses to maximal exercise in non-insulin-dependent diabetes. J Appl Physiol. 1990;68(5):2067–74.
Regensteiner JG, Bauer TA, Reusch JE, Brandenburg SL, Sippel JM, Vogelsong AM, et al. Abnormal oxygen uptake kinetic responses in women with type II diabetes mellitus. J Appl Physiol. 1998;85(1):310–7.
Regensteiner JG, Sippel J, McFarling ET, Wolfel EE, Hiatt WR. Effects of non-insulin-dependent diabetes on oxygen consumption during treadmill exercise. Med Sci Sports Exerc. 1995;27(6):875–81.
Reusch JE, Bridenstine M, Regensteiner JG. Type 2 diabetes mellitus and exercise impairment. Rev Endocr Metab Disord. 2013;14(1):77–86. Epub 2013/01/10.
Schneider SH, Khachadurian AK, Amorosa LF, Clemow L, Ruderman NB. Ten-year experience with an exercise-based outpatient life-style modification program in the treatment of diabetes mellitus. Diabetes Care. 1992;15(11):1800–10. Epub 1992/11/01.
Regensteiner JG. Type 2 diabetes mellitus and cardiovascular exercise performance. Rev Endocr Metab Disord. 2004;5(3):269–76. Epub 2004/06/24.
Poirier P, Garneau C, Bogaty P, Nadeau A, Marois L, Brochu C, et al. Impact of left ventricular diastolic dysfunction on maximal treadmill performance in normotensive subjects with well-controlled type 2 diabetes mellitus. Am J Cardiol. 2000;85(4):473–7. Epub 2000/03/23.
Regensteiner JG, Bauer TA, Reusch JE, Quaife RA, Chen MY, Smith SC, et al. Cardiac dysfunction during exercise in uncomplicated type 2 diabetes. Med Sci Sports Exerc. 2009;41(5):977–84. Epub 2009/04/07.
Hypertension in Diabetes Study (HDS): I. Prevalence of hypertension in newly presenting type 2 diabetic patients and the association with risk factors for cardiovascular and diabetic complications. J Hypertens. 1993;11(3):309–17. Epub 1993/03/01.
Albright A, Franz M, Hornsby G, Kriska A, Marrero D, Ullrich I, et al. American College of Sports Medicine position stand. Exercise and type 2 diabetes. Med Sci Sports Exerc. 2000;32(7):1345–60. Epub 2000/07/27.
Geiss LS, Rolka DB, Engelgau MM. Elevated blood pressure among U.S. adults with diabetes, 1988–1994. Am J Prev Med. 2002;22(1):42–8. Epub 2002/01/05.
Devereux RB, Roman MJ, Paranicas M, O’Grady MJ, Lee ET, Welty TK, et al. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101(19):2271–6. Epub 2000/05/16.
Raber W, Raffesberg W, Waldhausl W, Gasic S, Roden M. Exercise induces excessive normetanephrine responses in hypertensive diabetic patients. Eur J Clin Investig. 2003;33(6):480–7. Epub 2003/06/11.
Babalola RO, Ajayi AA. A cross-sectional study of echocardiographic indices, treadmill exercise capacity and microvascular complications in Nigerian patients with hypertension associated with diabetes mellitus. Diabet Med J Br Diabet Assoc. 1992;9(10):899–903. Epub 1992/12/01.
Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13(6 Pt 2):99S–105S. Epub 2000/08/02.
Esler M, Rumantir M, Kaye D, Lambert G. The sympathetic neurobiology of essential hypertension: disparate influences of obesity, stress, and noradrenaline transporter dysfunction? Am J Hypertens. 2001;14(6 Pt 2):139S–46S. Epub 2001/06/20.
Esler M, Rumantir M, Wiesner G, Kaye D, Hastings J, Lambert G. Sympathetic nervous system and insulin resistance: from obesity to diabetes. Am J Hypertens. 2001;14(11 Pt 2):304S–9S. Epub 2001/11/28.
Johnson RJ, Rodriguez-Iturbe B, Kang DH, Feig DI, Herrera-Acosta J. A unifying pathway for essential hypertension. Am J Hypertens. 2005;18(3):431–40. Epub 2005/03/31.
Christensen NJ, Galbo H. Sympathetic nervous activity during exercise. Annu Rev Physiol. 1983;45:139–53. Epub 1983/01/01.
Sullivan L. Obesity, diabetes mellitus and physical activity – metabolic responses to physical training in adipose and muscle tissues. Ann Clin Res. 1982;14(Suppl 34):51–62. Epub 1982/01/01.
Goldstein DS. Plasma norepinephrine during stress in essential hypertension. Hypertension. 1981;3(5):551–6. Epub 1981/09/01.
Goldstein DS. Plasma catecholamines and essential hypertension. An analytical review. Hypertension. 1983;5(1):86–99. Epub 1983/01/01.
Et-Taouil K, Safar M, Plante GE. Mechanisms and consequences of large artery rigidity. Can J Physiol Pharmacol. 2003;81(3):205–11. Epub 2003/05/08.
Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63(7):636–46. Epub 2013/11/19.
Mather KJ, Steinberg HO, Baron AD. Insulin resistance in the vasculature. J Clin Invest. 2013;123(3):1003–4. Epub 2013/03/05.
Mitchell GF, Lacourciere Y, Ouellet JP, Izzo JL Jr, Neutel J, Kerwin LJ, et al. Determinants of elevated pulse pressure in middle-aged and older subjects with uncomplicated systolic hypertension: the role of proximal aortic diameter and the aortic pressure-flow relationship. Circulation. 2003;108(13):1592–8. Epub 2003/09/17.
Nichols W, O’Rourke M. Blood flow in arteries: theoretical, experimental and clinical principles. London: Arnold Publishers Ltd; 2005.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72. Epub 2003/05/16.
Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113(9):1213–25. Epub 2006/02/16.
Funck KL, Laugesen E, Hoyem P, Fleischer J, Cichosz SL, Christiansen JS, et al. Low physical activity is associated with increased arterial stiffness in patients recently diagnosed with type 2 diabetes. Am J Hypertens. 2016;29(7):882–8. Epub 2015/12/31.
Kingwell BA. Large artery stiffness: implications for exercise capacity and cardiovascular risk. Proc Aust Physiol Pharmacol Soc. 2001;32(1):156–61.
Franzeck UK, Talke P, Bernstein EF, Golbranson FL, Fronek A. Transcutaneous PO2 measurements in health and peripheral arterial occlusive disease. Surgery. 1982;91(2):156–63. Epub 1982/02/01.
Rooke TW, Osmundson PJ. The influence of age, sex, smoking, and diabetes on lower limb transcutaneous oxygen tension in patients with arterial occlusive disease. Arch Intern Med. 1990;150(1):129–32. Epub 1990/01/01.
Wyss CR, Matsen FA 3rd, Simmons CW, Burgess EM. Transcutaneous oxygen tension measurements on limbs of diabetic and nondiabetic patients with peripheral vascular disease. Surgery. 1984;95(3):339–46. Epub 1984/03/01.
Kizu A, Koyama H, Tanaka S, Maeno T, Komatsu M, Fukumoto S, et al. Arterial wall stiffness is associated with peripheral circulation in patients with type 2 diabetes. Atherosclerosis. 2003;170(1):87–91. Epub 2003/09/06.
Kim G, Kim JH, Moon KW, Yoo KD, Kim CM, Moon D, et al. The relationships between the arterial stiffness index measured at the radial artery and left ventricular diastolic dysfunction in asymptomatic high risk patients without atherosclerotic cardiovascular disease. Int Heart J. 2016;57(1):73–9. Epub 2016/01/09.
Tsao CW, Lyass A, Larson MG, Levy D, Hamburg NM, Vita JA, et al. Relation of central arterial stiffness to incident heart failure in the community. J Am Heart Assoc. 2015;4(11). Epub 2015/11/26.
Matthys D, Craen M, De Wolf D, Vande Walle J, Verhaaren H. Reduced decrease of peripheral vascular resistance during exercise in young type I diabetic patients. Diabetes Care. 1996;19(11):1286–8. Epub 1996/11/01.
Newkumet KM, Goble MM, Young RB, Kaplowitz PB, Schieken RM. Altered blood pressure reactivity in adolescent diabetics. Pediatrics. 1994;93(4):616–21. Epub 1994/04/01.
Rubler S, Arvan SB. Exercise testing in young asymptomatic diabetic patients. Angiology. 1976;27(9):539–48. Epub 1976/09/01.
Kelley GA, Kelley KA, Tran ZV. Aerobic exercise and resting blood pressure: a meta-analytic review of randomized, controlled trials. Prev Cardiol. 2001;4(2):73–80. Epub 2002/02/06.
Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2002;136(7):493–503. Epub 2002/04/03.
Kelley GA, Kelley KS, Tran ZV. Walking and resting blood pressure in adults: a meta-analysis. Prev Med. 2001;33(2 Pt 1):120–7. Epub 2001/08/09.
Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33(12):e147–67. Epub 2010/12/01.
Menard J, Payette H, Baillargeon JP, Maheux P, Lepage S, Tessier D, et al. Efficacy of intensive multitherapy for patients with type 2 diabetes mellitus: a randomized controlled trial. CMAJ Can Med Assoc J: J Assoc Med Can. 2005;173(12):1457–66. Epub 2005/11/19.
Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–83. Epub 2007/03/17.
Balducci S, Zanuso S, Nicolucci A, De Feo P, Cavallo S, Cardelli P, et al. Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: a randomized controlled trial: the Italian Diabetes and Exercise Study (IDES). Arch Intern Med. 2010;170(20):1794–803. Epub 2010/11/10.
Kadoglou NP, Iliadis F, Angelopoulou N, Perrea D, Ampatzidis G, Liapis CD, et al. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Cardiovasc Prev Rehabil Off J Eur Soc Cardiol Work Group Epidemiol Prev Card Rehabil Exerc Physiol. 2007;14(6):837–43. Epub 2007/11/29.
Kim SH, Lee SJ, Kang ES, Kang S, Hur KY, Lee HJ, et al. Effects of lifestyle modification on metabolic parameters and carotid intima-media thickness in patients with type 2 diabetes mellitus. Metab Clin Exp. 2006;55(8):1053–9. Epub 2006/07/15.
Loimaala A, Groundstroem K, Rinne M, Nenonen A, Huhtala H, Vuori I. Exercise training does not improve myocardial diastolic tissue velocities in type 2 diabetes. Cardiovasc Ultrasound. 2007;5:32. Epub 2007/09/28.
Loimaala A, Groundstroem K, Rinne M, Nenonen A, Huhtala H, Parkkari J, et al. Effect of long-term endurance and strength training on metabolic control and arterial elasticity in patients with type 2 diabetes mellitus. Am J Cardiol. 2009;103(7):972–7. Epub 2009/03/31.
Sigal RJ, Kenny GP, Boule NG, Wells GA, Prud’homme D, Fortier M, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(6):357–69. Epub 2007/09/19.
Wycherley TP, Brinkworth GD, Noakes M, Buckley JD, Clifton PM. Effect of caloric restriction with and without exercise training on oxidative stress and endothelial function in obese subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(11):1062–73. Epub 2008/04/26.
Way KL, Keating SE, Baker MK, Chuter VH, Johnson NA. The effect of exercise on vascular function and stiffness in type 2 diabetes: a systematic review and meta-analysis. Curr Diabetes Rev. 2015. Epub 2015/08/19.
DeVallance E, Fournier S, Lemaster K, Moore C, Asano S, Bonner D, et al. The effects of resistance exercise training on arterial stiffness in metabolic syndrome. Eur J Appl Physiol. 2016;116(5):899–910. Epub 2016/03/05.
Donley DA, Fournier SB, Reger BL, DeVallance E, Bonner DE, Olfert IM, et al. Aerobic exercise training reduces arterial stiffness in metabolic syndrome. J Appl Physiol (1985). 2014;116(11):1396–404. Epub 2014/04/20.
Mustata S, Chan C, Lai V, Miller JA. Impact of an exercise program on arterial stiffness and insulin resistance in hemodialysis patients. J Am Soc Nephrol JASN. 2004;15(10):2713–8. Epub 2004/10/07.
Yokoyama H, Emoto M, Fujiwara S, Motoyama K, Morioka T, Koyama H, et al. Short-term aerobic exercise improves arterial stiffness in type 2 diabetes. Diabetes Res Clin Pract. 2004;65(2):85–93. Epub 2004/06/30.
Jung JY, Min KW, Ahn HJ, Kwon HR, Lee JH, Park KS, et al. Arterial stiffness by aerobic exercise is related with aerobic capacity, physical activity energy expenditure and total fat but not with insulin sensitivity in obese female patients with type 2 diabetes. Diabetes Metab J. 2014;38(6):439–48. Epub 2014/12/30.
Ferrier KE, Waddell TK, Gatzka CD, Cameron JD, Dart AM, Kingwell BA. Aerobic exercise training does not modify large-artery compliance in isolated systolic hypertension. Hypertension. 2001;38(2):222–6. Epub 2001/08/18.
Baldi JC, Wilson GA, Wilson LC, Wilkins GT, Lamberts RR. The type 2 diabetic heart: its role in exercise intolerance and the challenge to find effective exercise interventions. Sports Med. 2016;46(11):1605–17. Epub 2016/04/24.
Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30(6):595–602. Epub 1972/11/08.
Beljic T, Miric M. Improved metabolic control does not reverse left ventricular filling abnormalities in newly diagnosed non-insulin-dependent diabetes patients. Acta Diabetol. 1994;31(3):147–50. Epub 1994/09/01.
Di Bonito P, Cuomo S, Moio N, Sibilio G, Sabatini D, Quattrin S, et al. Diastolic dysfunction in patients with non-insulin-dependent diabetes mellitus of short duration. Diabet Med J Br Diabet Assoc. 1996;13(4):321–4. Epub 1996/04/01.
Nicolino A, Longobardi G, Furgi G, Rossi M, Zoccolillo N, Ferrara N, et al. Left ventricular diastolic filling in diabetes mellitus with and without hypertension. Am J Hypertens. 1995;8(4 Pt 1):382–9. Epub 1995/04/01.
Poirier P, Bogaty P, Garneau C, Marois L, Dumesnil JG. Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes: importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care. 2001;24(1):5–10. Epub 2001/02/24.
Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202. Epub 2003/01/09.
Boyer JK, Thanigaraj S, Schechtman KB, Perez JE. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol. 2004;93(7):870–5. Epub 2004/03/31.
Fontes-Carvalho R, Ladeiras-Lopes R, Bettencourt P, Leite-Moreira A, Azevedo A. Diastolic dysfunction in the diabetic continuum: association with insulin resistance, metabolic syndrome and type 2 diabetes. Cardiovasc Diabetol. 2015;14:4. Epub 2015/01/15.
Irace L, Iarussi D, Guadagno I, De Rimini ML, Lucca P, Spadaro P, et al. Left ventricular function and exercise tolerance in patients with type II diabetes mellitus. Clin Cardiol. 1998;21(8):567–71. Epub 1998/08/14.
Patil VC, Patil HV, Shah KB, Vasani JD, Shetty P. Diastolic dysfunction in asymptomatic type 2 diabetes mellitus with normal systolic function. J Cardiovasc Dis Res. 2011;2(4):213–22. Epub 2011/12/03.
Salmasi AM, Rawlins S, Dancy M. Left ventricular hypertrophy and preclinical impaired glucose tolerance and diabetes mellitus contribute to abnormal left ventricular diastolic function in hypertensive patients. Blood Press Monit. 2005;10(5):231–8. Epub 2005/10/06.
Saraiva RM, Duarte DM, Duarte MP, Martins AF, Poltronieri AV, Ferreira ME, et al. Tissue Doppler imaging identifies asymptomatic normotensive diabetics with diastolic dysfunction and reduced exercise tolerance. Echocardiography. 2005;22(7):561–70. Epub 2005/08/03.
Schilling JD, Mann DL. Diabetic cardiomyopathy: bench to bedside. Heart Fail Clin. 2012;8(4):619–31. Epub 2012/09/25.
Zahiti BF, Gorani DR, Gashi FB, Gjoka SB, Zahiti LB, Haxhiu BS, et al. Left ventricular diastolic dysfunction in asymptomatic type 2 diabetic patients: detection and evaluation by tissue Doppler imaging. Acta Inform Med AIM J Soc Med Inform Bosnia Herzegovina Cas Drustva Med Inform BiH. 2013;21(2):120–3. Epub 2013/09/17.
Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57(4):660–71. Epub 2014/01/31.
Felicio JS, Koury CC, Carvalho CT, Neto JF, Mileo KB, Arbage TP, et al. Present insights on cardiomyopathy in diabetic patients. Curr Diabetes Rev. 2015. Epub 2015/09/15.
Miki T, Yuda S, Kouzu H, Miura T. Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev. 2013;18(2):149–66. Epub 2012/03/29.
Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res. 2016;118(11):1808–29. Epub 2016/05/28.
Westermeier F, Riquelme JA, Pavez M, Garrido V, Diaz A, Verdejo HE, et al. New molecular insights of insulin in diabetic cardiomyopathy. Front Physiol. 2016;7:125. Epub 2016/05/06.
Bell DS. Diabetic cardiomyopathy. Diabetes Care. 2003;26(10):2949–51. Epub 2003/09/30.
Trost S, LeWinter M. Diabetic cardiomyopathy. Curr Treat Options Cardiovasc Med. 2001;3(6):481–92. Epub 2001/11/07.
Barmeyer A, Mullerleile K, Mortensen K, Meinertz T. Diastolic dysfunction in exercise and its role for exercise capacity. Heart Fail Rev. 2009;14(2):125–34. Epub 2008/09/02.
Kosmala W, Jellis CL, Marwick TH. Exercise limitation associated with asymptomatic left ventricular impairment: analogy with stage B heart failure. J Am Coll Cardiol. 2015;65(3):257–66. Epub 2014/12/24.
Fang ZY, Sharman J, Prins JB, Marwick TH. Determinants of exercise capacity in patients with type 2 diabetes. Diabetes Care. 2005;28(7):1643–8. Epub 2005/06/29.
Gurdal A, Kasikcioglu E, Yakal S, Bugra Z. Impact of diabetes and diastolic dysfunction on exercise capacity in normotensive patients without coronary artery disease. Diab Vasc Dis Res. 2015;12(3):181–8. Epub 2015/02/12.
Sasso FC, Carbonara O, Cozzolino D, Rambaldi P, Mansi L, Torella D, et al. Effects of insulin-glucose infusion on left ventricular function at rest and during dynamic exercise in healthy subjects and noninsulin dependent diabetic patients: a radionuclide ventriculographic study. J Am Coll Cardiol. 2000;36(1):219–26. Epub 2000/07/18.
Willemsen S, Hartog JW, Hummel YM, van Ruijven MH, van der Horst IC, van Veldhuisen DJ, et al. Tissue advanced glycation end products are associated with diastolic function and aerobic exercise capacity in diabetic heart failure patients. Eur J Heart Fail. 2011;13(1):76–82. Epub 2010/09/24.
Johnson EJ, Dieter BP, Marsh SA. Evidence for distinct effects of exercise in different cardiac hypertrophic disorders. Life Sci. 2015;123:100–6. Epub 2015/01/31.
Sacre JW, Jellis CL, Jenkins C, Haluska BA, Baumert M, Coombes JS, et al. A six-month exercise intervention in subclinical diabetic heart disease: effects on exercise capacity, autonomic and myocardial function. Metab Clin Exp. 2014;63(9):1104–14. Epub 2014/07/07.
Fournier SB, Donley DA, Bonner DE, Devallance E, Olfert IM, Chantler PD. Improved arterial-ventricular coupling in metabolic syndrome after exercise training: a pilot study. Med Sci Sports Exerc. 2015;47(1):2–11. Epub 2014/05/30.
Brassard P, Legault S, Garneau C, Bogaty P, Dumesnil JG, Poirier P. Normalization of diastolic dysfunction in type 2 diabetics after exercise training. Med Sci Sports Exerc. 2007;39(11):1896–901. Epub 2007/11/08.
Hare JL, Hordern MD, Leano R, Stanton T, Prins JB, Marwick TH. Application of an exercise intervention on the evolution of diastolic dysfunction in patients with diabetes mellitus: efficacy and effectiveness. Circ Heart Fail. 2011;4(4):441–9. Epub 2011/05/18.
Ofstad AP, Johansen OE, Gullestad L, Birkeland KI, Orvik E, Fagerland MW, et al. Neutral impact on systolic and diastolic cardiac function of 2 years of intensified multi-intervention in type 2 diabetes: the randomized controlled Asker and Baerum Cardiovascular Diabetes (ABCD) study. Am Heart J. 2014;168(3):280–8 e2. Epub 2014/09/01.
Cassidy S, Thoma C, Hallsworth K, Parikh J, Hollingsworth KG, Taylor R, et al. High intensity intermittent exercise improves cardiac structure and function and reduces liver fat in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2016;59(1):56–66. Epub 2015/09/10.
Asrar UL, Haq M, Wong C, Levinger I, Srivastava PM, Sbaraglia M, Toia D, et al. Effect of exercise training on left ventricular remodeling in diabetic patients with diastolic dysfunction: rationale and design. Clin Med Insights Cardiol. 2014;8:23–8. Epub 2014/03/22.
Nichols GA, Hillier TA, Erbey JR, Brown JB. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care. 2001;24(9):1614–9. Epub 2001/08/28.
Guazzi M, Brambilla R, Pontone G, Agostoni P, Guazzi MD. Effect of non-insulin-dependent diabetes mellitus on pulmonary function and exercise tolerance in chronic congestive heart failure. Am J Cardiol. 2002;89(2):191–7. Epub 2002/01/17.
Tibb AS, Ennezat PV, Chen JA, Haider A, Gundewar S, Cotarlan V, et al. Diabetes lowers aerobic capacity in heart failure. J Am Coll Cardiol. 2005;46(5):930–1. Epub 2005/09/06.
Ingle L, Reddy P, Clark AL, Cleland JG. Diabetes lowers six-minute walk test performance in heart failure. J Am Coll Cardiol. 2006;47(9):1909–10. Epub 2006/05/10.
Lee JC, Downing SE. Effects of insulin on cardiac muscle contraction and responsiveness to norepinephrine. Am J Phys. 1976;230(5):1360–5. Epub 1976/05/01.
Fisher BM, Gillen G, Dargie HJ, Inglis GC, Frier BM. The effects of insulin-induced hypoglycaemia on cardiovascular function in normal man: studies using radionuclide ventriculography. Diabetologia. 1987;30(11):841–5. Epub 1987/11/01.
Guazzi M, Tumminello G, Matturri M, Guazzi MD. Insulin ameliorates exercise ventilatory efficiency and oxygen uptake in patients with heart failure-type 2 diabetes comorbidity. J Am Coll Cardiol. 2003;42(6):1044–50. Epub 2003/09/19.
Coch RW, Green JB. Current cardiovascular outcomes trials in type 2 diabetes: perspectives and insight. Nutr Metab Cardiovasc Dis NMCD. 2016. Epub 2016/07/06.
Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus – mechanisms, management, and clinical considerations. Circulation. 2016;133(24):2459–502. Epub 2016/06/15.
Granger CB, Califf RM, Young S, Candela R, Samaha J, Worley S, et al. Outcome of patients with diabetes mellitus and acute myocardial infarction treated with thrombolytic agents. The Thrombolysis and Angioplasty in Myocardial Infarction (TAMI) Study Group. J Am Coll Cardiol. 1993;21(4):920–5. Epub 1993/03/15.
Rytter L, Troelsen S, Beck-Nielsen H. Prevalence and mortality of acute myocardial infarction in patients with diabetes. Diabetes Care. 1985;8(3):230–4. Epub 1985/05/01.
Kavanagh T, Mertens DJ, Hamm LF, Beyene J, Kennedy J, Corey P, et al. Prediction of long-term prognosis in 12 169 men referred for cardiac rehabilitation. Circulation. 2002;106(6):666–71. Epub 2002/08/07.
Vanhees L, Fagard R, Thijs L, Amery A. Prognostic value of training-induced change in peak exercise capacity in patients with myocardial infarcts and patients with coronary bypass surgery. Am J Cardiol. 1995;76(14):1014–9. Epub 1995/11/15.
Church TS, LaMonte MJ, Barlow CE, Blair SN. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med. 2005;165(18):2114–20.
Koivula RW, Tornberg AB, Franks PW. Exercise and diabetes-related cardiovascular disease: systematic review of published evidence from observational studies and clinical trials. Curr Diab rep. 2013;13(3):372–80. Epub 2013/03/16.
Lyerly GW, Sui X, Lavie CJ, Church TS, Hand GA, Blair SN. The association between cardiorespiratory fitness and risk of all-cause mortality among women with impaired fasting glucose or undiagnosed diabetes mellitus. Mayo Clin Proc. 2009;84(9):780–6. Epub 2009/09/02.
Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med. 2000;132(8):605–11. Epub 2000/04/15.
Staimez LR, Weber MB, Gregg EW. The role of lifestyle change for prevention of cardiovascular disease in diabetes. Curr Atheroscler rep. 2014;16(12):460. Epub 2014/11/05.
Lin X, Zhang X, Guo J, Roberts CK, McKenzie S, Wu WC, et al. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2015;4(7):1–28. Epub 2015/06/28.
Izawa K, Tanabe K, Omiya K, Yamada S, Yokoyama Y, Ishiguro T, et al. Impaired chronotropic response to exercise in acute myocardial infarction patients with type 2 diabetes mellitus. Jpn Heart J. 2003;44(2):187–99. Epub 2003/04/30.
Verges B, Patois-Verges B, Cohen M, Lucas B, Galland-Jos C, Casillas JM. Effects of cardiac rehabilitation on exercise capacity in type 2 diabetic patients with coronary artery disease. Diabet Med J Br Diabet Assoc. 2004;21(8):889–95. Epub 2004/07/24.
Colucci WS, Ribeiro JP, Rocco MB, Quigg RJ, Creager MA, Marsh JD, et al. Impaired chronotropic response to exercise in patients with congestive heart failure. Role of postsynaptic beta-adrenergic desensitization. Circulation. 1989;80(2):314–23. Epub 1989/08/01.
Endo A, Kinugawa T, Ogino K, Kato M, Hamada T, Osaki S, et al. Cardiac and plasma catecholamine responses to exercise in patients with type 2 diabetes: prognostic implications for cardiac-cerebrovascular events. Am J med Sci. 2000;320(1):24–30. Epub 2000/07/26.
Fontes-Carvalho R, Sampaio F, Teixeira M, Rocha-Goncalves F, Gama V, Azevedo A, et al. Left ventricular diastolic dysfunction and E/E′ ratio as the strongest echocardiographic predictors of reduced exercise capacity after acute myocardial infarction. Clin Cardiol. 2015;38(4):222–9. Epub 2015/02/25.
Kim HJ, Joo MC, Noh SE, Kim JH. Long-term outcomes of cardiac rehabilitation in diabetic and non-diabetic patients with myocardial infarction. Ann Rehabil Med. 2015;39(6):853–62. Epub 2016/01/23.
St Clair M, Mehta H, Sacrinty M, Johnson D, Robinson K. Effects of cardiac rehabilitation in diabetic patients: both cardiac and noncardiac factors determine improvement in exercise capacity. Clin Cardiol. 2014;37(4):233–8. Epub 2014/01/24.
Armstrong MJ, Martin BJ, Arena R, Hauer TL, Austford LD, Stone JA, et al. Patients with diabetes in cardiac rehabilitation: attendance and exercise capacity. Med Sci Sports Exerc. 2014;46(5):845–50. Epub 2013/10/16.
Verges B, Patois-Verges B, Iliou MC, Simoneau-Robin I, Bertrand JH, Feige JM, et al. Influence of glycemic control on gain in VO2 peak, in patients with type 2 diabetes enrolled in cardiac rehabilitation after an acute coronary syndrome. The prospective DARE study. BMC Cardiovasc Disord. 2015;15:64. Epub 2015/07/15.
Byrkjeland R, Njerve IU, Anderssen S, Arnesen H, Seljeflot I, Solheim S. Effects of exercise training on HbA1c and VO2peak in patients with type 2 diabetes and coronary artery disease: a randomised clinical trial. Diab Vasc Dis Res. 2015;12(5):325–33. Epub 2015/06/21.
O’Connor GT, Buring JE, Yusuf S, Goldhaber SZ, Olmstead EM, Paffenbarger RS Jr, et al. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation. 1989;80(2):234–44. Epub 1989/08/01.
Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. JAMA. 1988;260(7):945–50. Epub 1988/08/19.
Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006;47(5):921–9. Epub 2006/03/07.
Mukherjee D. Peripheral and cerebrovascular atherosclerotic disease in diabetes mellitus. Best Pract Res Clin Endocrinol Metab. 2009;23(3):335–45. Epub 2009/06/13.
Adler AI, Stevens RJ, Neil A, Stratton IM, Boulton AJ, Holman RR. UKPDS 59: hyperglycemia and other potentially modifiable risk factors for peripheral vascular disease in type 2 diabetes. Diabetes Care. 2002;25(5):894–9. Epub 2002/04/30.
Dolan NC, Liu K, Criqui MH, Greenland P, Guralnik JM, Chan C, et al. Peripheral artery disease, diabetes, and reduced lower extremity functioning. Diabetes Care. 2002;25(1):113–20. Epub 2002/01/05.
Green S, Askew CD, Walker PJ. Effect of type 2 diabetes mellitus on exercise intolerance and the physiological responses to exercise in peripheral arterial disease. Diabetologia. 2007;50(4):859–66. Epub 2007/01/24.
Katzel LI, Sorkin JD, Powell CC, Gardner AW. Comorbidities and exercise capacity in older patients with intermittent claudication. Vasc Med. 2001;6(3):157–62. Epub 2002/01/16.
Oka RK, Sanders MG. The impact of type 2 diabetes and peripheral arterial disease on quality of life. J Vasc Nurs Off Publ Soc Peripher Vasc Nurs. 2005;23(2):61–6. quiz 7–8. Epub 2005/08/17.
Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA. 1995;274(12):975–80. Epub 1995/09/27.
Parmenter BJ, Dieberg G, Smart NA. Exercise training for management of peripheral arterial disease: a systematic review and meta-analysis. Sports Med. 2015;45(2):231–44. Epub 2014/09/19.
Sanderson B, Askew C, Stewart I, Walker P, Gibbs H, Green S. Short-term effects of cycle and treadmill training on exercise tolerance in peripheral arterial disease. J Vasc Surg. 2006;44(1):119–27. Epub 2006/07/11.
Lyu X, Li S, Peng S, Cai H, Liu G, Ran X. Intensive walking exercise for lower extremity peripheral arterial disease: a systematic review and meta-analysis. J Diabetes. 2016;8(3):363–77. Epub 2015/05/06. meta.
Ekroth R, Dahllof AG, Gundevall B, Holm J, Schersten T. Physical training of patients with intermittent claudication: indications, methods, and results. Surgery. 1978;84(5):640–3. Epub 1978/11/01.
Gardner AW, Parker DE, Montgomery PS, Scott KJ, Blevins SM. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication: a randomized controlled trial. Circulation. 2011;123(5):491–8. Epub 2011/01/26.
Gardner AW, Montgomery PS, Parker DE. Optimal exercise program length for patients with claudication. J Vasc Surg. 2012;55(5):1346–54. Epub 2012/03/31.
McDermott MM, Ades P, Guralnik JM, Dyer A, Ferrucci L, Liu K, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009;301(2):165–74. Epub 2009/01/15.
McDermott MM, Liu K, Guralnik JM, Criqui MH, Spring B, Tian L, et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA. 2013;310(1):57–65. Epub 2013/07/04.
Li G, Zhang P, Wang J, An Y, Gong Q, Gregg EW, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol. 2014;2(6):474–80. Epub 2014/04/16.
Gong Q, Zhang P, Wang J, An Y, Gregg EW, Li H, et al. Changes in mortality in people with IGT before and after the onset of diabetes during the 23-year follow-up of the Da Qing Diabetes Prevention Study. Diabetes Care. 2016;39:1550–5. Epub 2016/07/15.
Effect of a long-term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes: a secondary analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2014;2(10):801–9. Epub 2014/08/16.
Steinberg H, Jacovino C, Kitabchi AE. Look inside Look AHEAD: why the glass is more than half-full. Curr Diab rep. 2014;14(7):500. Epub 2014/05/28.
Karjalainen JJ, Kiviniemi AM, Hautala AJ, Piira OP, Lepojarvi ES, Perkiomaki JS, et al. Effects of physical activity and exercise training on cardiovascular risk in coronary artery disease patients with and without type 2 diabetes. Diabetes Care. 2015;38(4):706–15. Epub 2015/01/17.
Astrup AS, Nielsen FS, Rossing P, Ali S, Kastrup J, Smidt UM, et al. Predictors of mortality in patients with type 2 diabetes with or without diabetic nephropathy: a follow-up study. J Hypertens. 2007;25(12):2479–85. Epub 2007/11/07.
Gerritsen J, Dekker JM, TenVoorde BJ, Kostense PJ, Heine RJ, Bouter LM, et al. Impaired autonomic function is associated with increased mortality, especially in subjects with diabetes, hypertension, or a history of cardiovascular disease: the Hoorn Study. Diabetes Care. 2001;24(10):1793–8. Epub 2001/09/28.
Gottsater A, Ahlgren AR, Taimour S, Sundkvist G. Decreased heart rate variability may predict the progression of carotid atherosclerosis in type 2 diabetes. Clin Auton Res Off J Clin Auton Res Soc. 2006;16(3):228–34. Epub 2006/06/10.
Routledge FS, Campbell TS, McFetridge-Durdle JA, Bacon SL. Improvements in heart rate variability with exercise therapy. Can J Cardiol. 2010;26(6):303–12. Epub 2010/06/16.
Vinik AI, Erbas T, Casellini CM. Diabetic cardiac autonomic neuropathy, inflammation and cardiovascular disease. J Diabetes Inv. 2013;4(1):4–18. Epub 2013/04/04.
Young LH, Wackers FJ, Chyun DA, Davey JA, Barrett EJ, Taillefer R, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009;301(15):1547–55. Epub 2009/04/16.
Keytsman C, Dendale P, Hansen D. Chronotropic incompetence during exercise in type 2 diabetes: aetiology, assessment methodology, prognostic impact and therapy. Sports Med. 2015;45(7):985–95. Epub 2015/04/04.
von Scholten BJ, Hansen CS, Hasbak P, Kjaer A, Rossing P, Hansen TW. Cardiac autonomic function is associated with the coronary microcirculatory function in type 2 diabetic patients. Diabetes. 2016;65:3129–38. Epub 2016/06/30.
Sacre JW, Franjic B, Jellis CL, Jenkins C, Coombes JS, Marwick TH. Association of cardiac autonomic neuropathy with subclinical myocardial dysfunction in type 2 diabetes. JACC Cardiovasc Imaging. 2010;3(12):1207–15. Epub 2010/12/18.
Voulgari C, Pagoni S, Vinik A, Poirier P. Exercise improves cardiac autonomic function in obesity and diabetes. Metab Clin Exp. 2013;62(5):609–21. Epub 2012/10/23.
Howorka K, Pumprla J, Haber P, Koller-Strametz J, Mondrzyk J, Schabmann A. Effects of physical training on heart rate variability in diabetic patients with various degrees of cardiovascular autonomic neuropathy. Cardiovasc Res. 1997;34(1):206–14. Epub 1997/04/01.
Pagkalos M, Koutlianos N, Kouidi E, Pagkalos E, Mandroukas K, Deligiannis A. Heart rate variability modifications following exercise training in type 2 diabetic patients with definite cardiac autonomic neuropathy. Br J Sports Med. 2008;42(1):47–54. Epub 2007/05/29.
Morton RD, West DJ, Stephens JW, Bain SC, Bracken RM. Heart rate prescribed walking training improves cardiorespiratory fitness but not glycaemic control in people with type 2 diabetes. J Sports Sci. 2010;28(1):93–9. Epub 2010/04/15.
Goulopoulou S, Baynard T, Franklin RM, Fernhall B, Carhart R Jr, Weinstock R, et al. Exercise training improves cardiovascular autonomic modulation in response to glucose ingestion in obese adults with and without type 2 diabetes mellitus. Metab Clin Exp. 2010;59(6):901–10. Epub 2009/12/18.
Liu Y, Liu SX, Zheng F, Cai Y, Xie KL, Zhang WL. Cardiovascular autonomic neuropathy in patients with type 2 diabetes. J Diabetes Inv. 2016;7(4):615–21. Epub 2016/05/18.
Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003;63(1):225–32. Epub 2002/12/11.
Brown JB, Pedula KL, Summers KH. Diabetic retinopathy: contemporary prevalence in a well-controlled population. Diabetes Care. 2003;26(9):2637–42. Epub 2003/08/28.
Liu JE, Robbins DC, Palmieri V, Bella JN, Roman MJ, Fabsitz R, et al. Association of albuminuria with systolic and diastolic left ventricular dysfunction in type 2 diabetes: the Strong Heart Study. J Am Coll Cardiol. 2003;41(11):2022–8. Epub 2003/06/12.
Jensen T, Richter EA, Feldt-Rasmussen B, Kelbaek H, Deckert T. Impaired aerobic work capacity in insulin dependent diabetics with increased urinary albumin excretion. Br Med J (Clin Res Ed). 1988;296(6633):1352–4. Epub 1988/05/14.
Kelbaek H, Jensen T, Feldt-Rasmussen B, Christensen NJ, Richter EA, Deckert T, et al. Impaired left-ventricular function in insulin-dependent diabetic patients with increased urinary albumin excretion. Scand J Clin Lab Invest. 1991;51(5):467–73. Epub 1991/09/01.
Bjornstad P, Cree-Green M, Baumgartner A, Maahs DM, Cherney DZ, Pyle L, et al. Renal function is associated with peak exercise capacity in adolescents with type 1 diabetes. Diabetes Care. 2015;38(1):126–31. Epub 2014/11/22.
Lau AC, Lo MK, Leung GT, Choi FP, Yam LY, Wasserman K. Altered exercise gas exchange as related to microalbuminuria in type 2 diabetic patients. Chest. 2004;125(4):1292–8. Epub 2004/04/14.
Howden EJ, Weston K, Leano R, Sharman JE, Marwick TH, Isbel NM, et al. Cardiorespiratory fitness and cardiovascular burden in chronic kidney disease. J Sci Med Sports/Sports Med Aust. 2015;18(4):492–7. Epub 2014/08/16.
Shiraishi FG, Stringuetta Belik F, Oliveira ESVR, Martin LC, Hueb JC, Goncalves Rde S, et al. Inflammation, diabetes, and chronic kidney disease: role of aerobic capacity. Exp Diabetes Res. 2012;2012:750286. Epub 2012/05/09.
Zoccali C, Mallamaci F, Tripepi G. Traditional and emerging cardiovascular risk factors in end-stage renal disease. Kidney Int Suppl. 2003;85:S105–10. Epub 2003/05/20.
Johansen KL. Physical functioning and exercise capacity in patients on dialysis. Adv Ren Replace Ther. 1999;6(2):141–8. Epub 1999/05/07.
Painter P, Moore G, Carlson L, Paul S, Myll J, Phillips W, et al. Effects of exercise training plus normalization of hematocrit on exercise capacity and health-related quality of life. Am J Kidney Dis Off J Natl Kidney Found. 2002;39(2):257–65. Epub 2002/02/13.
Mayer G, Thum J, Cada EM, Stummvoll HK, Graf H. Working capacity is increased following recombinant human erythropoietin treatment. Kidney Int. 1988;34(4):525–8. Epub 1988/10/01.
Painter P, Hanson P, Messer-Rehak D, Zimmerman SW, Glass NR. Exercise tolerance changes following renal transplantation. Am J Kidney Dis Off J Natl Kidney Found. 1987;10(6):452–6. Epub 1987/12/01.
Chan CT, Notarius CF, Merlocco AC, Floras JS. Improvement in exercise duration and capacity after conversion to nocturnal home haemodialysis. Nephrol Dial Transplant Off Publ Eur Dial Transplant Assoc Eur Ren Assoc. 2007;22(11):3285–91. Epub 2007/06/28.
Painter P, Messer-Rehak D, Hanson P, Zimmerman SW, Glass NR. Exercise capacity in hemodialysis, CAPD, and renal transplant patients. Nephron. 1986;42(1):47–51. Epub 1986/01/01.
Watson EL, Greening NJ, Viana JL, Aulakh J, Bodicoat DH, Barratt J, et al. Progressive resistance exercise training in CKD: a feasibility study. Am J Kidney Dis Off J Natl Kidney Found. 2015;66(2):249–57. Epub 2014/12/24.
Headley S, Germain M, Wood R, Joubert J, Milch C, Evans E, et al. Short-term aerobic exercise and vascular function in CKD stage 3: a randomized controlled trial. Am J Kidney Dis Off J Natl Kidney Found. 2014;64(2):222–9. Epub 2014/04/30.
Greenwood SA, Koufaki P, Mercer TH, MacLaughlin HL, Rush R, Lindup H, et al. Effect of exercise training on estimated GFR, vascular health, and cardiorespiratory fitness in patients with CKD: a pilot randomized controlled trial. Am J Kidney Dis Off J Natl Kidney Found. 2015;65(3):425–34. Epub 2014/09/23.
Estacio RO, Regensteiner JG, Wolfel EE, Jeffers B, Dickenson M, Schrier RW. The association between diabetic complications and exercise capacity in NIDDM patients. Diabetes Care. 1998;21(2):291–5. Epub 1998/04/16.
Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(6):1433–8. Epub 2006/05/30.
Standards of medical care in diabetes-2016: summary of revisions. Diabetes Care. 2016;39(Suppl 1):S4–5. Epub 2015/12/24.
Professional practice committee for the standards of medical care in diabetes-2016. Diabetes Care. 2016;39(Suppl 1):S107–8. Epub 2015/12/24.
Singleton JR, Marcus RL, Jackson JE, M KL, Graham TE, Smith AG. Exercise increases cutaneous nerve density in diabetic patients without neuropathy. Ann Clin Transl Neurol. 2014;1(10):844–9. Epub 2014/12/11.
Singleton JR, Smith AG, Marcus RL. Exercise as therapy for diabetic and prediabetic neuropathy. Curr Diab rep. 2015;15(12):120. Epub 2015/11/06.
Streckmann F, Zopf EM, Lehmann HC, May K, Rizza J, Zimmer P, et al. Exercise intervention studies in patients with peripheral neuropathy: a systematic review. Sports Med. 2014;44(9):1289–304. Epub 2014/06/15.
Mueller MJ, Tuttle LJ, Lemaster JW, Strube MJ, McGill JB, Hastings MK, et al. Weight-bearing versus nonweight-bearing exercise for persons with diabetes and peripheral neuropathy: a randomized controlled trial. Arch Phys Med Rehabil. 2013;94(5):829–38. Epub 2013/01/02.
Balducci S, Iacobellis G, Parisi L, Di Biase N, Calandriello E, Leonetti F, et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complicat. 2006;20(4):216–23. Epub 2006/06/27.
Kluding PM, Pasnoor M, Singh R, Jernigan S, Farmer K, Rucker J, et al. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complicat. 2012;26(5):424–9. Epub 2012/06/22.
Hoogenberg K, Dullaart RP. Abnormal plasma noradrenaline response and exercise induced albuminuria in type 1 (insulin-dependent) diabetes mellitus. Scand J Clin Lab Invest. 1992;52(8):803–11. Epub 1992/12/01.
Poulsen PL, Ebbehoj E, Mogensen CE. Lisinopril reduces albuminuria during exercise in low grade microalbuminuric type 1 diabetic patients: a double blind randomized study. J Intern Med. 2001;249(5):433–40. Epub 2001/05/15.
Romanelli G, Giustina A, Cravarezza P, Caldonazzo A, Agabiti-Rosei E, Giustina G. Albuminuria induced by exercise in hypertensive type I and type II diabetic patients: a randomised, double-blind study on the effects of acute administration of captopril and nifedipine. J Hum Hypertens. 1991;5(3):167–73. Epub 1991/06/01.
Tuominen JA, Ebeling P, Koivisto VA. Long-term lisinopril therapy reduces exercise-induced albuminuria in normoalbuminuric normotensive IDDM patients. Diabetes Care. 1998;21(8):1345–8. Epub 1998/08/14.
Viberti GC, Jarrett RJ, McCartney M, Keen H. Increased glomerular permeability to albumin induced by exercise in diabetic subjects. Diabetologia. 1978;14(5):293–300. Epub 1978/05/01.
Huttunen NP, Kaar M, Puukka R, Akerblom HK. Exercise-induced proteinuria in children and adolescents with type 1 (insulin dependent) diabetes. Diabetologia. 1981;21(5):495–7. Epub 1981/11/01.
Lane JT, Ford TC, Larson LR, Chambers WA, Lane PH. Acute effects of different intensities of exercise in normoalbuminuric/normotensive patients with type 1 diabetes. Diabetes Care. 2004;27(1):28–32. Epub 2003/12/25.
Cohen JA, Jeffers BW, Faldut D, Marcoux M, Schrier RW. Risks for sensorimotor peripheral neuropathy and autonomic neuropathy in non-insulin-dependent diabetes mellitus (NIDDM). Muscle Nerve. 1998;21(1):72–80. Epub 1998/01/14.
Parving HH, Hommel E, Mathiesen E, Skott P, Edsberg B, Bahnsen M, et al. Prevalence of microalbuminuria, arterial hypertension, retinopathy and neuropathy in patients with insulin dependent diabetes. Br Med J (Clin Res Ed). 1988;296(6616):156–60. Epub 1988/01/16.
Kart-Koseoglu H, Yucel AE, Niron EA, Koseoglu H, Isiklar I, Ozdemir FN. Osteoarthritis in hemodialysis patients: relationships with bone mineral density and other clinical and laboratory parameters. Rheumatol Int. 2005;25(4):270–5. Epub 2004/03/05.
Kay J, Bardin T. Osteoarticular disorders of renal origin: disease-related and iatrogenic. Baillieres Best Pract Res Clin Rheumatol. 2000;14(2):285–305. Epub 2000/08/05.
Naidich JB, Mossey RT, McHeffey-Atkinson B, Karmel MI, Bluestone PA, Mailloux LU, et al. Spondyloarthropathy from long-term hemodialysis. Radiology. 1988;167(3):761–4. Epub 1988/06/01.
Evans N, Forsyth E. End-stage renal disease in people with type 2 diabetes: systemic manifestations and exercise implications. Phys Ther. 2004;84(5):454–63. Epub 2004/04/29.
Davison SN. Pain in hemodialysis patients: prevalence, cause, severity, and management. Am J Kidney Dis Off J Natl Kidney Found. 2003;42(6):1239–47. Epub 2003/12/05.
Klein OL, Krishnan JA, Glick S, Smith LJ. Systematic review of the association between lung function and type 2 diabetes mellitus. Diabet Med J Br Diabet Assoc. 2010;27(9):977–87. Epub 2010/08/21.
Klein OL, Jones M, Lee J, Collard HR, Smith LJ. Reduced lung diffusion capacity in type 2 diabetes is independent of heart failure. Diabetes Res Clin Pract. 2012;96(3):e73–5. Epub 2012/03/23.
Klein OL, Kalhan R, Williams MV, Tipping M, Lee J, Peng J, et al. Lung spirometry parameters and diffusion capacity are decreased in patients with type 2 diabetes. Diabet Med J Br Diabet Assoc. 2012;29(2):212–9. Epub 2011/07/28.
Fontaine-Delaruelle C, Viart-Ferber C, Luyton C, Couraud S. Lung function in patients with diabetes mellitus. Rev Pneumol Clin. 2016;72(1):10–6. Epub 2015/07/22. Fonction pulmonaire du patient diabetique.
Kinney GL, Black-Shinn JL, Wan ES, Make B, Regan E, Lutz S, et al. Pulmonary function reduction in diabetes with and without chronic obstructive pulmonary disease. Diabetes Care. 2014;37(2):389–95. Epub 2013/09/13.
Kim HK, Kim CH, Jung YJ, Bae SJ, Choe J, Park JY, et al. Association of restrictive ventilatory dysfunction with insulin resistance and type 2 diabetes in Koreans. Exp Clin Endocrinol Diabetes Off J Ger Soc Endocrinol Ger Diabetes Assoc. 2011;119(1):47–52. Epub 2011/01/20.
Buchmann N, Norman K, Steinhagen-Thiessen E, Demuth I, Eckardt R. Lung function in elderly subjects with metabolic syndrome and type II diabetes: data from the Berlin Aging Study II. Z Gerontol Geriatr. 2015. Epub 2015/10/29. Lungenfunktion bei alteren Probanden mit metabolischem Syndrom und Typ II Diabetes : Ergebnisse der Berliner Altersstudie II.
Aparna. Pulmonary function tests in type 2 diabetics and non-diabetic people -a comparative study. J Clin Diagn Res JCDR. 2013;7(8):1606–8. Epub 2013/10/03.
Shah SH, Sonawane P, Nahar P, Vaidya S, Salvi S. Pulmonary function tests in type 2 diabetes mellitus and their association with glycemic control and duration of the disease. Lung India Off Organ Indian Chest Soc. 2013;30(2):108–12. Epub 2013/06/07.
Baffi CW, Wood L, Winnica D, Strollo PJ Jr, Gladwin MT, Que LG, et al. Metabolic syndrome and the lung. Chest. 2016;149(6):1525–34. Epub 2016/02/03.
Anandhalakshmi S, Manikandan, Ganeshkumar, Ramachandran. Alveolar gas exchange and pulmonary functions in patients with Typ2 II diabetes. J Clin Diagn Res. 2013;7(9):1874–7.
Hsia CC, Raskin P. The diabetic lung: relevance of alveolar microangiopathy for the use of inhaled insulin. Am J Med. 2005;118(3):205–11. Epub 2005/03/05.
Shafiee G, Khamseh ME, Rezaei N, Aghili R, Malek M. Alteration of pulmonary function in diabetic nephropathy. J Diabetes Metab Disord. 2013;12(1):15. Epub 2013/04/27.
Durdik P, Vojtkova J, Michnova Z, Turcan T, Sujanska A, Kuchta M, et al. Pulmonary function tests in type 1 diabetes adolescents with diabetic cardiovascular autonomic neuropathy. J Diabetes Complicat. 2016;30(1):79–84. Epub 2015/11/26.
Kaminski DM, Schaan BD, da Silva AM, Soares PP, Plentz RD, Dall’Ago P. Inspiratory muscle weakness is associated with autonomic cardiovascular dysfunction in patients with type 2 diabetes mellitus. Clin Auton Res Off J Clin Auton Res Soc. 2011;21(1):29–35. Epub 2010/11/06.
Kitahara Y, Hattori N, Yokoyama A, Yamane K, Sekikawa K, Inamizu T, et al. The influence of lung function on exercise capacity in patients with type 2 diabetes. Hiroshima J Med Sci. 2010;59(1):7–13. Epub 2010/06/04.
Correa AP, Ribeiro JP, Balzan FM, Mundstock L, Ferlin EL, Moraes RS. Inspiratory muscle training in type 2 diabetes with inspiratory muscle weakness. Med Sci Sports Exerc. 2011;43(7):1135–41. Epub 2011/01/05.
Tunkamnerdthai O, Auvichayapat P, Donsom M, Leelayuwat N. Improvement of pulmonary function with arm swing exercise in patients with type 2 diabetes. J Phys Ther Sci. 2015;27(3):649–54. Epub 2015/05/02.
Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med. 1982;306(17):1018–22. Epub 1982/04/29.
Strongin LG, Korneva KG, Panova EI. Disturbances of cardiac rhythm and metabolic control in patients with type-2 diabetes. Kardiologiia. 2005;45(11):46–9. Epub 2005/12/15.
Douketis JD, Arneklev K, Goldhaber SZ, Spandorfer J, Halperin F, Horrow J. Comparison of bleeding in patients with nonvalvular atrial fibrillation treated with ximelagatran or warfarin: assessment of incidence, case-fatality rate, time course and sites of bleeding, and risk factors for bleeding. Arch Intern Med. 2006;166(8):853–9. Epub 2006/04/26.
Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–34. Epub 1998/07/23.
Kannel WB, McGee DL. Diabetes and cardiovascular risk factors: the Framingham study. Circulation. 1979;59(1):8–13. Epub 1979/01/01.
Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16(2):434–44. Epub 1993/02/01.
Guazzi M, Belletti S, Bianco E, Lenatti L, Guazzi MD. Endothelial dysfunction and exercise performance in lone atrial fibrillation or associated with hypertension or diabetes: different results with cardioversion. Am J Physiol Heart Circ Physiol. 2006;291(2):H921–8. Epub 2006/02/08.
Sung KC, Ryu S, Lee JY, Kim JY, Wild SH, Byrne CD. Development of new fatty liver, or resolution of existing fatty liver, over 5 years of follow up: effect of exercise. J Hepatol. 2016;65:791–7. Epub 2016/06/04.
Cuthbertson DJ, Shojaee-Moradie F, Sprung VS, Jones H, Pugh CJ, Richardson P, et al. Dissociation between exercise-induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non-alcoholic fatty liver disease. Clin Sci (Lond). 2016;130(2):93–104. Epub 2015/10/02.
Keating SE, Hackett DA, Parker HM, O’Connor HT, Gerofi JA, Sainsbury A, et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol. 2015;63(1):174–82. Epub 2015/04/13.
Aiello KD, Caughey WG, Nelluri B, Sharma A, Mookadam F, Mookadam M. Effect of exercise training on sleep apnea: a systematic review and meta-analysis. Respir Med. 2016;116:85–92. Epub 2016/06/15.
Albright AL, Mahan JD, Ward KM, Sherman WM, Roehrig KL, Kirby TE. Diabetic nephropathy in an aerobically trained rat model of diabetes. Med Sci Sports Exerc. 1995;27(9):1270–7. Epub 1995/09/01.
Anand DV, Lim E, Hopkins D, Corder R, Shaw LJ, Sharp P, et al. Risk stratification in uncomplicated type 2 diabetes: prospective evaluation of the combined use of coronary artery calcium imaging and selective myocardial perfusion scintigraphy. Eur Heart J. 2006;27(6):713–21. Epub 2006/02/25.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Schauer, I.E., Huebschmann, A.G., Regensteiner, J.G. (2018). Diabetes Mellitus and Exercise Physiology in the Presence of Diabetic Comorbidities. In: Reusch, MD, J., Regensteiner, PhD, MA, BA, J., Stewart, Ed.D., FAHA, MAACVPR, FACSM , K., Veves, MD, DSc, A. (eds) Diabetes and Exercise. Contemporary Diabetes. Humana Press, Cham. https://doi.org/10.1007/978-3-319-61013-9_18
Download citation
DOI: https://doi.org/10.1007/978-3-319-61013-9_18
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-61011-5
Online ISBN: 978-3-319-61013-9
eBook Packages: MedicineMedicine (R0)