Abstract
Diastolic dysfunction is frequent in elderly subjects and in patients with left ventricular hypertrophy, vascular disease and diabetes mellitus. Patients with diastolic dysfunction demonstrate a reduced exercise capacity and might suffer from congestive heart failure (CHF). Presence of symptoms of CHF in the setting of a normal systolic function is referred to as heart failure with normal ejection fraction (HFNEF) or, if evidence of an impaired diastolic function is observed, as diastolic heart failure (DHF). Reduced exercise capacity in diastolic dysfunction results from a number of pathophysiological alterations such as slowed myocardial relaxation, reduced myocardial distensibility, elevated filling pressures, and reduced ventricular suction forces. These alterations limit the increase of ventricular diastolic filling and cardiac output during exercise and lead to pulmonary congestion. In healthy subjects, exercise training can enhance diastolic function and exercise capacity and prevent deterioration of diastolic function in the course of aging. In patients with diastolic dysfunction, exercise capacity can be enhanced by exercise training and pharmacological treatment, whereas improvement of diastolic function can only be observed in few patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The importance of diastole for cardiac function in terms of cardiac output and exercise capacity has been elucidated in the past few years. Isolated diastolic dysfunction is frequent in elderly subjects and in patients with left ventricular hypertrophy, diabetes, and vascular disease. Diastolic dysfunction with preserved systolic function has been identified as a cause for exertional dyspnea and exercise intolerance, and nearly half of the patients with new onset of congestive heart failure (CHF) demonstrate isolated diastolic dysfunction [1]. Thus, diastolic dysfunction represents a pathologic entity with high prevalence and morbidity which needs early dedicated treatment in order to prevent progression to severer grades. It is well known that exercise intolerance due to cardiac dysfunction can be ameliorated by exercise training. This accounts for systolic as well as for diastolic impairment of cardiac function.
The purpose of this article is to thoroughly review the literature in order to provide an overview of the role of diastole for cardiac function at exercise in normal subjects and the physiologic alterations that limit cardiac output and exercise capacity in patients with diastolic dysfunction. Furthermore, the possibilities of exercise training and pharmacological interventions for the treatment of patients with diastolic dysfunction are described and recommendations for exercise training are given.
Definition of diastolic dysfunction
Diastole is referred to as the phase of the cardiac cycle during which myocardial fibers relax and return to their non-contracted length and geometry, and during which left ventricular filling takes place. Left ventricular pressure during diastole takes a bidirectional course. Onset, velocity, and extent of myocardial relaxation and left ventricular untwisting determine the drop of left ventricular pressure during early diastole, whereas the time course of left ventricular filling and myocardial elastic properties (distensibility and stiffness) are the determinants of the reincrease of left ventricular pressure during late diastole.
To establish the diagnosis of diastolic dysfunction, evidence of a slow left ventricular relaxation, an abnormal left ventricular filling, a reduced diastolic distensibility, or an increased left ventricular stiffness is required [2]. Parameters that are used to evaluate diastolic function are usually obtained at rest. However, there is evidence that diastolic dysfunction can be present at exercise in patients with normal diastolic function at rest [3, 4]. The finding of diastolic dysfunction by abnormal mechanical properties of the left ventricle in diastole does not imply the presence of clinical symptoms. Patients with clinical symptoms that are related to an impaired diastolic function might be classified as patients with diastolic heart failure (DHF).
Definition of diastolic heart failure
Different propositions have been made to establish the diagnosis of DHF. The criteria proposed by the European Study Group on Diastolic Heart Failure comprise (a) signs or symptoms of CHF; (b) a normal or only mildly abnormal left ventricular systolic function, generally defined as an ejection fraction (EF) ≥ 45%; and (c) an abnormal diastolic function [2]. This definition has been challenged by a study that examined diastolic function by simultaneous cardiac catheterization and echocardiography in 63 patients with a history of CHF and echocardiographic evidence of normal systolic function (EF ≥ 50%) and LV hypertrophy [5]. It could be shown that all 63 patients showed at least one abnormal parameter of diastolic function. The authors concluded that measurements of diastolic function are not necessary to make the diagnosis of DHF in patients with symptoms and signs of CHF and a normal systolic function. The term DHF is not used uniformly throughout literature. Often, the same clinical syndrome is referred to as heart failure with normal ejection fraction (HFNEF) of which the definition rather emphasizes the presence of symptoms of CHF and a normal systolic function and neglects the evidence of an abnormal diastolic function. However, in patients with HFNEF, a slow myocardial relaxation and an increased left ventricular stiffness were observed as evidence for an impaired diastolic dysfunction [6]. Since it does not seem of importance for the issue that is addressed in this manuscript whether DHF and HFNEF are different entities or synonyms for one syndrome, the term DHF will be used throughout the manuscript for both.
Diagnosis of diastolic dysfunction
Since extensive review of the modalities to diagnose an impaired diastolic function has been provided elsewhere [7–9], only a brief synopsis is given.
Invasive measurement of intracardiac pressures
An increase of the time constant τ of the left ventricular pressure decay [10] with a value > 48 ms [9], a left ventricular end-diastolic pressure (LVEDP) > 16 mmHg [11], and a mean pulmonary capillary wedge pressure (PCWP) > 12 mmHg [12] are indicative of an impaired diastolic function. Prolongation of τ is suggestive for a slow myocardial relaxation, whereas elevated values for LVEDP and PCWP indicate a reduced myocardial distensibility. An increase in muscle stiffness can be identified by an increase of the slope of the pressure-volume relationship (dP/dV) in the pressure-volume plot.
Echocardiography
Doppler flow measurements can reveal a prolonged isovolumetric relaxation time (IVRT) as a measure of a slow relaxation. A ratio of peak mitral flow velocity during early (E) and late (A) diastolic filling < 1 and a deceleration time of the early flow velocity (DT) > 220 ms [13] represent evidence of a slow early left ventricular filling. A ratio of systolic (S) and diastolic (D) pulmonary vein peak flow velocity < 1 indicates an elevated left atrial pressure resulting in a reincrease of the early mitral flow velocity and thus normalizing the E/A-ratio. Therefore, Doppler analysis of the pulmonary vein flow allows to distinguish between a normal mitral valve flow pattern and a “pseudonormal” pattern with elevated left atrial pressure [8]. A reduction of the A-wave velocity due to an increased myocardial stiffness results in an abnormally high increase of the E/A-ratio > 2. This “restrictive filling pattern” is associated with a poor prognosis [14]. The mitral anular velocity during early diastolic filling (E′) correlates closely with LV relaxation kinetics and with age, whereas E from transmitral flow is dependent on LV filling pressure, on age, and on LV relaxation kinetics. Therefore, the ratio of E/E′ eliminates the influence of age and left ventricular relaxation kinetics and can be interpreted as the ratio of LV-filling pressure and early left ventricular filling. An E/E′-ratio > 15 indicates diastolic dysfunction with high LV-filling pressures whereas an E/E′-ratio < 8 excludes DHF [9, 15].
Biomarkers
The degree of diastolic dysfunction is closely related to the plasma level of natriuretic peptides such as atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP). Increasing values of BNP could be found with increasing degree of diastolic dysfunction by means of Doppler measurements of transmitral flow [16, 17]. However, due to a low specificity and multiple conditions with presence of elevated BNP levels, such as pulmonary disease [18], pulmonary embolism [19], renal dysfunction [20], and sepsis [21], BNP does not serve as a screening tool to detect diastolic dysfunction but is rather recommended to exclude DHF in patients with exertional dyspnea [9].
Normal diastolic function in exercise
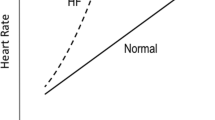
During physical exercise, cardiac output can increase several times over the value at rest [22]. The increase in cardiac output is achieved by an increase in heart rate and stroke volume [23]. Increases in heart rate result in a shortening of the time for diastolic filling, and increases in stroke volume demand that higher volumes of blood be shifted from the left atrium to the left ventricle during that time period. In fact, acceleration of diastolic left ventricular filling has been observed in healthy subjects and greater increases of diastolic filling rate were found in endurance trained athletes compared to normal subjects [24]. Acceleration of diastolic filling during exercise in normal subjects is provided by a shortened myocardial relaxation [25] due to an increased calcium uptake by the sarcoplasmic reticular [26], an increased elastic recoil after a more complete systolic contraction, a higher myocardial untwisting rate, and a faster intraventricular pressure decay in early diastole [27]. Also, sympathetic stimulation causes a downward shift of the early diastolic portion of the left ventricular pressure loop [28]. These mechanisms create a suction force in the left ventricle resulting in an increase of the transmitral pressure gradient [29], enhancement of transmitral flow, and acceleration of left ventricular filling without elevation of left atrial pressures.
Diastolic dysfunction in exercise
In diastolic dysfunction, the mechanical properties of the left ventricle are altered to an extent that diastolic filling is delayed, slowed, shortened, or associated with elevated left-ventricular pressures. The ability to enhance transmitral flow and accelerate diastolic filling at exercise is therefore reduced.
A number of studies compared the response of diastolic transmitral flow to resistance exercise in patients with diastolic dysfunction and in normal subjects. In these studies, resistance exercise led to an increase of late diastolic filling velocity of 6 ± 5% [30] and the late proportion of diastolic filling [31] and caused a drop of early diastolic flow velocity and of the early proportion of total diastolic filling of 16 ± 4% [32] in patients compared to normals. In diabetic patients without diastolic dysfunction at rest a greater increase of the ratio of late to early diastolic flow velocity was shown compared to normal subjects (0.29 ± 0.2 vs. 0.09 ± 0.07) suggesting that latent diastolic dysfunction can be unmasked by resistance exercise [33].
Other studies examined the diastolic transmitral flow pattern at aerobic exercise. In a group of patients with hypertension and diastolic dysfunction at rest with a reduced E/A ratio, the transmitral flow pattern became pseudonormal with increase of the E/A-ratio at maximal exercise indicating an exercise induced rise in atrial pressure[34]. Another study group showed an increase of the E/A-ratio up to 2.4 ± 1.5 with a S/D-ratio < 1 and a smaller increase of flow propagation velocity after exercise in patient with CHF compared to normals suggesting an increase of diastolic filling pressures and reduced suction forces [35]. In one study an elevated E/E′-ratio at rest in patients with exertional dyspnea was a good predictor of a short exercise duration in a symptom-limited exercise test compared to patients with a normal E/E′-ratio (426 vs. 625 s) [36]. Since no differences of transmitral flow velocities could be found between patients with elevated and normal E/E′-ratio, left ventricular filling pressures might reveal diastolic dysfunction earlier than the transmitral flow pattern alone.
Diastolic dysfunction is accompanied by neurohumoral activation which might be triggered or intensified by exercise. In patients with normal left ventricular function at rest but elevated filling pressures at exercise as measured by cardiac catheterization, elevated NT-proBNP levels were found compared to controls with normal filling pressures at exercise (median 145. 2 pg/ml [range 69.7–273.4] vs. 38.3 pg/ml [range 22.1–64.7]) [37]. Also, an elevated E/E′-ratio is associated with increased BNP levels after exercise in patients with diastolic dysfunction [38] or suspected DHF [39]. Exercise activates the renin-angiotensin-aldosteron system and causes increased plasma levels of angiotensin II (AT II)[40–42]. AT II prolongs the time constant τ of myocardial relaxation mediated through activation of AT1 receptors. In CHF, prolongation of τ is even stronger and myocardial contractility is depressed by AT II [43]. Elevated plasma levels of AT II could, therefore, play a role for exercise intolerance in patients with DHF and might be a target for pharmacological treatment.
Diastolic dysfunction is associated with a number of other alterations at exercise. It is correlated with an increase of pulmonary blood volume at exercise [44] and a reduced coronary flow velocity reserve at peak dose dobutamine [45]. Furthermore, the submaximal ventilation equivalent for carbon dioxide is higher, whereas peak oxygen consumption and tidal volume are lower in patients with diastolic dysfunction compared to normal subjects [46]. Also, patients with diastolic dysfunction report greater dyspnea at submaximal exercise than controls. Inspiratory muscle weakness and a breathing pattern with rapid shallow breaths were found compared to the controls which might account for the perception of dyspnea [47].
Certain clinical conditions are associated with a normal diastolic function at rest but an impaired diastolic function during or after exercise. Evidence of an exercise induced impairment of diastolic function caused by myocardial ischemia was found in patients with coronary artery disease [48] and angina pectoris [49]. Exercise induced diastolic dysfunction could also be found in patients with type 1 diabetes mellitus [4] and in patients with hyperlipidemia and a normal stress ECG [3]. It was hypothesized that exercise induced diastolic dysfunction in these patients might be an early sign of subclinical myocardial ischemia. However, impairment of diastolic dysfunction by exercise is not only limited to the time of exercise. In a number of studies persistence of exercise induced diastolic dysfunction was described 30 min [50, 51], 1 h [52], and up to 2 days [53] after stress testing in patients with coronary artery disease. The incidence and magnitude of diastolic impairment was determined by the severity of the exercise induced ischemia [52] suggesting that ischemia results in myocardial stunning which can persist even a long time after resolution of the ischemic condition.
Diastolic dysfunction and exercise capacity
The enhancement of diastolic left ventricular filling is a crucial determinant for the increase of cardiac output at exercise. Impairment of diastolic function can result in the inability to increase cardiac output adequately and can limit exercise capacity. Hypertensive rats developing left ventricular hypertrophy with normal systolic function but abnormal diastolic function can lose more than 25% of their initial exercise capacity suggesting that exercise intolerance was caused by diastolic dysfunction [54]. Similar observations are made in humans. Patients with isolated diastolic dysfunction due to type 2 diabetes [55] and arterial hypertension [56] demonstrate maximal workloads that are 1.9 and 2.5 metabolic equivalents (METs), respectively, lower than matched controls without diastolic dysfunction. In another study, maximal workloads were more than 80 watts and maximal specific oxygen uptake 41% lower in patients with diastolic dysfunction than in normals [46] (Table 1). Also, a negative correlation exists between exercise capacity and left ventricular stiffness in hypertensive patients [57] and an index of left ventricular filling pressure at exercise (duration of diastolic reverse pulmonary vein flow and mitral flow at atrial contraction) in patients with acromegaly [58].
In patients with symptoms of congestion due to DHF, reduction of exercise capacity is even more severe. The extent of exercise intolerance in DHF is even comparable to patients with systolic heart failure [59]. Comparative studies of patients with DHF and matched controls revealed a reduction of maximal specific oxygen uptake between 38 and 29% and a reduction of maximal workload between 31% and 50% [59–63] (Table 2). Therefore, reduction of exercise capacity represents a major clinical problem in patients with diastolic dysfunction and, more pronounced, in DHF. Hence, reversal of exercise intolerance constitutes a main target for treatment in these patients.
Pathological left ventricular filling pressures are consequence of diastolic dysfunction. The importance of elevated filling pressures for the limitation of exercise capacity is well known for patients with impaired systolic function [64]. More recent results claim a relation between filling pressure and exercise capacity also for patients with diastolic dysfunction. A study with a larger number of patients found that a reduced exercise capacity was even better related to elevated left ventricular filling pressures at rest than to an impaired myocardial relaxation. In that study, an E/E′-ratio ≥ 10 was the best predictor of a low maximal metabolic equivalent [65]. In another study, E/E′ was the most powerful predictor not only of exercise capacity but also of reduced peak oxygen uptake. An E/E′-ratio > 7.5 was able to predict a reduction of peak oxygen uptake ≤ 14 ml/min per kg with high sensitivity and specificity [66].
Since diastolic dysfunction and elevated filling pressures are associated with elevated levels of natriuretic peptides, the level of BNP in relation to exercise capacity was investigated in patients with exertional dyspnea and isolated diastolic dysfunction. It could be shown that patients with BNP ≥ 50 pg/ml had a lower peak oxygen uptake and anaerobic threshold than patients with BNP < 50 pg/ml [67]. Therefore, the degree of neurohumeral activation by diastolic dysfunction seems to be correlated to the severity of exercise intolerance.
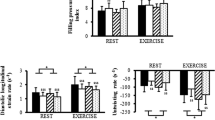
However, reduced exercise capacity in patients with diastolic dysfunction does not seem to be merely related to diastolic function itself. Comparing matched groups of patients with diastolic dysfunction and DHF, the latter showed a more profound reduction of exercise capacity, a smaller increase in heart rate and cardiac output, and less systemic vasodilation despite a similar rise in end-diastolic volume, stroke volume, and contractility [60]. Other studies found a greater intraventricular conduction delay (IVCD) in patients with diastolic dysfunction and increase of aortic stiffness in patients with DHF compared to normals. Exacerbation of dyssynchrony after exercise was noted in the group of patients with DHF which was positively correlated with post-exercise NT-pro BNP [68]. Therefore, exercise intolerance in DHF can be caused by alterations that might be associated with diastolic dysfunction but are not related to the mechanical myocardial properties determining diastole.
Treatments to improve exercise capacity in diastolic dysfunction
Since a reduced exercise capacity is frequent in patients with diastolic dysfunction and represents a major limitation of their quality of life, numerous studies have investigated the possibilities to improve exercise capacity in those patients. Interventions that have been examined comprise exercise training and pharmacological treatment.
Exercise training
It is well known that exercise training improves diastolic function in healthy subjects. Trained subjects demonstrate a higher increase of peak diastolic filling rate during exercise compared to untrained subjects [69]. Especially, endurance training shows beneficial effects on diastolic function. An interventional study with 6 months of intensive aerobic endurance training in young and elderly subjects demonstrated improvement of diastolic function at rest and exercise. Remarkably, in both groups improvement of the early part of diastolic filling was achieved, whereas only in the group of elderly subjects reduction of the initially elevated atrial filling rate could be shown [70]. A number of observational studies compared diastolic function in highly trained endurance athletes and in normal subjects. At rest, despite an increased left ventricular mass in endurance athletes, early to late diastolic filling ratio was either unchanged [71] or even slightly increased [72]. During exercise, endurance athletes demonstrate an up to 71% higher ventricular filling rate at corresponding heart rates compared to normal subjects resulting in a higher stroke volume [24]. Also, enhanced peak early diastolic filling [71, 73], an increased early to late diastolic filling ratio [71], and greater peak lengthening rate, filling volume, and filling fraction of the left ventricle during the first 100 ms of diastole [74] were found in endurance athletes. In addition, it could be shown that diastolic filling dynamics in endurance-trained elderly subjects are more similar to younger subjects than to untrained elderly subjects [75, 76], which provides evidence that endurance training not only improves diastolic function in healthy subjects but also prevents deterioration of diastolic function in the course of aging.
However, the effect of exercise training on diastolic function and exercise capacity in patients with preexisting diastolic dysfunction has only been examined in few studies (Table 3). Patients with diastolic dysfunction show an improvement of exercise tolerance by training but the effects of training on diastolic function are less clear. In a study with patients with coronary artery disease who underwent a rehabilitation program with regular exercise training for at least 8 weeks, only the subgroup of patients with an abnormal left ventricular relaxation pattern showed significant improvement of diastolic function [77]. Another study in patients with type 2 diabetes mellitus and diastolic dysfunction who performed exercise training 4 times weekly for 12 months could not find a change in tissue Doppler indices of diastolic function but a significant improvement of maximal specific oxygen uptake of 8% [78]. Also, a recent study of patients with diastolic dysfunction and exercise intolerance who underwent a 16-week aerobic training program 3 times per week demonstrated a significant increase of exercise capacity of 19% and of peak oxygen uptake of 30% but no change of diastolic function [79]. Therefore, the gain in exercise capacity seems to be related rather to peripheral mechanisms, such as improvement of muscular aerobic metabolism, muscular mass, vasculature, and coordination. Only a small study of type 2 diabetics with diastolic dysfunction found an improvement of diastolic function after 3 months of exercise [80]. Therefore, despite improvement of exercise capacity and evidence for the beneficial effect of exercise training on diastolic function in healthy subjects, the subgroups of patients with diastolic dysfunction who respond to exercise training with improvement of diastolic function are not yet identified.
Pharmacological treatment
The effect of pharmacological treatment on exercise tolerance and diastolic function in patients with impaired diastole has been evaluated in a number of studies (Table 3). In patients with diastolic dysfunction by means of Doppler echocardiography and a hypertensive response to exercise, increased exercise tolerance and improved quality of life was found after only 2 weeks of treatment with losartan [81]. Similar effects on the blood pressure response were found with short-term verapamil and long-term hydrochlorotiazide compared to candesartan [82] and losartan [83], respectively, but improvement of exercise tolerance and quality of life was only found in the patients treated with candesartan and losartan. It was hypothesized that increase of AT II during exercise might be responsible for a slowed left ventricular relaxation and can be antagonized by AT II antagonism. This would explain the beneficial effect of AT II antagonists already after short-term treatment. AT II increase during exercise is not suppressed by ACE-inhibition [84] which might lead to the assumption that ACE-inhibition could be less effective in improving exercise tolerance in patients with diastolic dysfunction than AT II antagonism. However, in a study comparing diuretics alone and the combination of diuretics with ramipril and irbesartan, respectively, no significant advantage regarding exercise capacity and quality of life was found for any of the treatment arms [85]. Only indices of left ventricular long axis motion and BNP-levels were slightly improved under ramipril and irbesartan compared to diuretics alone. In a small study improvement of resting and post-exercise diastolic function could be observed in a group of 11 patients with borderline hypertension after 24 weeks of ACE-blockade [86]. Unfortunately, no data on exercise performance were reported in that study. Therefore, other mechanisms than mere AT II antagonism such as reduction of afterload or myocardial fibrosis might be the mode of action for all inhibitors of the renin-angiotensin-aldosteron-system. Consequently, it could be shown that treatment with aldosterone receptor antagonists improved E/A ratio and deceleration time in elderly patients with diastolic dysfunction [87]. In another study, increased exercise tolerance in patients with isolated diastolic dysfunction could be achieved by treatment with an aldosteron antagonist but no significant change of the indices of diastolic function could be observed and the improvement of exercise tolerance was attributed to an enhancement of systolic function [88]. ß-blockers have also shown to improve exercise capacity and E/A ratio in patients with diastolic dysfunction. However, no change of left ventricular filling pressures at rest and during exercise could be achieved [89]. Therefore, similar to exercise training, pharmacological treatment of patients with diastolic dysfunction enhances exercise capacity but improvement of diastolic function is limited and occurs only in few patients.
Recommendations for exercise training in diastolic dysfunction
To date no guideline or consensus document explicitly addresses the prescription of exercise training in patients with isolated diastolic dysfunction. However, since there is growing evidence of the beneficial effects of exercise training in patients with diastolic dysfunction, exercise prescription is likely to be suited to maintain or regain quality of life for these patients. However, patients with isolated diastolic function are rather of higher age and show a considerable prevalence of relevant comorbidities. Therefore, care must be taken to exclude patients with contraindications for exercise training (Table 4).
Recommendations for exercise prescription in patients with CHF and other cardiovascular diseases have been published in the past [90, 91]. As long as no specific statements for patients with diastolic dysfunction are available, these recommendations represent the best evidence for beneficial effects in these patients. In brief, aerobic exercise is the training of choice since it demonstrates the best balance between beneficial cardiovascular, muscular, metabolic, and respiratory effects and associated risks such as cardiovascular or orthopedic events. It should be performed 3–5 times per week and should comprise a warm-up period of 10–15 min, an exercise time of 20–30 min, and a cool-down period. Exercise intensity can be low at 40–60% of peak VO2 [90] or moderate at 70–80% of peak VO2 [91]. Heart rate should be between 50% and 70% of maximal heart rate.
Resistance training also exerts beneficial effects on the musculo-skeletal system, on the glucose- and lipid-metabolism, and on the quality of life in CHF patients. Recently, the updated AHA statement for resistance exercise [92] pointed out the safety of resistance training in patients with cardiovascular disease, if performed correctly and at submaximal level. Resistance training should be performed 2–3 times per week with 8–10 different exercises. For each exercise a set of 8–12 repetitions with 70–80% of the maximal power is recommended for patients <50 years of age. Patients >50 years of age should perform sets of 10–15 repetitions at reduced levels of resistance.
Summary
Diastolic dysfunction can be caused by an abnormal myocardial relaxation or impaired left ventricular filling dynamics. Patients with diastolic dysfunction can be asymptomatic or show clinical signs of DHF. However, diastolic dysfunction is associated with a reduced exercise capacity due to a slowed myocardial relaxation, reduced left ventricular suction forces, and elevated ventricular filling pressures. Exercise training and pharmacological treatment can enhance exercise capacity in patients with diastolic dysfunction, but the beneficial effect on diastolic function is small and is only observed in few patients.
References
Senni M, Tribouilloy CM, Rodeheffer RJ et al (1998) Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation 98(21):2282–2289
European Study Group on Diastolic Heart Failure (1998) How to diagnose diastolic heart failure. Eur Heart J 19(7):990–1003. doi:10.1053/euhj.1998.1057
Salmasi AM, Frost P, Dancy M (2004) Impaired left ventricular diastolic function during isometric exercise in asymptomatic patients with hyperlipidaemia. Int J Cardiol 95(2–3):275–280. doi:10.1016/j.ijcard.2003.06.005
Jermendy G, Khoor S, Koltai MZ et al (1990) Left ventricular diastolic dysfunction in type 1 (insulin-dependent) diabetic patients during dynamic exercise. Cardiology 77(1):9–16
Zile MR, Gaasch WH, Carroll JD et al (2001) Heart failure with a normal ejection fraction: is measurement of diastolic function necessary to make the diagnosis of diastolic heart failure? Circulation 104(7):779–782. doi:10.1161/hc3201.094226
Zile MR, Baicu CF, Gaasch WH (2004) Diastolic heart failure—abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 350(19):1953–1959. doi:10.1056/NEJMoa032566
Zile MR, Brutsaert DL (2002) New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation 105(11):1387–1393. doi:10.1161/hc1102.105289
Galderisi M (2005) Diastolic dysfunction and diastolic heart failure: diagnostic, prognostic and therapeutic aspects. Cardiovasc Ultrasound 3:9. doi:10.1186/1476-7120-3-9
Paulus WJ, Tschope C, Sanderson JE et al (2007) How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28(20):2539–2550. doi:10.1093/eurheartj/ehm037
Hirota Y (1980) A clinical study of left ventricular relaxation. Circulation 62(4):756–763
Yamakado T, Takagi E, Okubo S et al (1997) Effects of aging on left ventricular relaxation in humans. Analysis of left ventricular isovolumic pressure decay. Circulation 95(4):917–923
Little WC, Downes TR (1990) Clinical evaluation of left ventricular diastolic performance. Prog Cardiovasc Dis 32(4):273–290. doi:10.1016/0033-0620(90)90017-V
Cohen GI, Pietrolungo JF, Thomas JD et al (1996) A practical guide to assessment of ventricular diastolic function using Doppler echocardiography. J Am Coll Cardiol 27(7):1753–1760. doi:10.1016/0735-1097(96)00088-5
Moller JE, Sondergaard E, Poulsen SH et al (2000) Pseudonormal and restrictive filling patterns predict left ventricular dilation and cardiac death after a first myocardial infarction: a serial color M-mode Doppler echocardiographic study. J Am Coll Cardiol 36(6):1841–1846. doi:10.1016/S0735-1097(00)00965-7
Ommen SR, Nishimura RA, Appleton CP et al (2000) Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 102(15):1788–1794
Lubien E, DeMaria A, Krishnaswamy P et al (2002) Utility of B-natriuretic peptide in detecting diastolic dysfunction: comparison with Doppler velocity recordings. Circulation 105(5):595–601. doi:10.1161/hc0502.103010
Dudek D, Rzeszutko L, Petkow Dimitrow P et al (2001) Circulating N-terminal brain natriuretic peptide precursor and endothelin levels in patients with syndrome X and left bundle branch block with preserved systolic function. Int J Cardiol 79(1):25–30. doi:10.1016/S0167-5273(01)00400-4
Ando T, Ogawa K, Yamaki K et al (1996) Plasma concentrations of atrial, brain, and C-type natriuretic peptides and endothelin-1 in patients with chronic respiratory diseases. Chest 110(2):462–468. doi:10.1378/chest.110.2.462
Tulevski II, Hirsch A, Sanson BJ et al (2001) Increased brain natriuretic peptide as a marker for right ventricular dysfunction in acute pulmonary embolism. Thromb Haemost 86(5):1193–1196
Tsutamoto T, Wada A, Sakai H et al (2006) Relationship between renal function and plasma brain natriuretic peptide in patients with heart failure. J Am Coll Cardiol 47(3):582–586. doi:10.1016/j.jacc.2005.10.038
Jones AE, Kline JA (2003) Elevated brain natriuretic peptide in septic patients without heart failure. Ann Emerg Med 42(5):714–715. doi:10.1016/S0196-0644(03)00622-X
Bevegard S, Holmgren A, Jonsson B (1963) Circulatory studies in well trained athletes at rest and during heavy exercise. With special reference to stroke volume and the influence of body position. Acta Physiologica Scandinavica 57:26–50
Mc Ardle WD, Katch FI, Katch VL (1994) Essentials of exercise physiology. Lea Febiger, Philadelphia, pp 257–259
Gledhill N, Cox D, Jamnik R (1994) Endurance athletes’ stroke volume does not plateau: major advantage is diastolic function. Med Sci Sports Exerc 26(9):1116–1121. doi:10.1249/00005768-199409000-00008
Janssen PM, Periasamy M (2007) Determinants of frequency-dependent contraction and relaxation of mammalian myocardium. J Mol Cell Cardiol 43(5):523–531. doi:10.1016/j.yjmcc.2007.08.012
Tate CA, Taffet GE, Hudson EK et al (1990) Enhanced calcium uptake of cardiac sarcoplasmic reticulum in exercise-trained old rats. Am J Physiol 258(2 Pt 2):H431–H435
Notomi Y, Popovic ZB, Yamada H et al (2007) Ventricular untwisting: a temporal link between left ventricular relaxation and suction. Am J Physiol: Heart Circ Physiol (Nov):21
Cheng CP, Igarashi Y, Little WC (1992) Mechanism of augmented rate of left ventricular filling during exercise. Circ Res 70(1):9–19
Yellin EL, Nicolic SD, Frater RWM (1994) Diastolic suction and the dynamics of left ventricular filling. In: Gassch WH, LeWinter MM (eds) Left ventricular diastolic dysfunction and heart failure. Lea Febiger, Philadelphia, pp 89–102
Tavli T, Cin VG, Tavli V et al (1995) The use of the handgrip maneuver to identify left ventricular diastolic function abnormalities by Doppler echocardiography in patients with coronary artery disease. Jpn Heart J 36(1):23–28
Pu M (1991) Influence of isometric exercise on left ventricular diastolic function in the normal subjects and in patients with hypertension and coronary heart disease. Zhonghua Xin Xue Guan Bing Za Zhi 19(5):311–313, 332
Borgia MC, Pellicelli AM, Medici F et al (1998) Left ventricular filling in young patients affected by insulin-dependent diabetes mellitus: a stress Doppler echocardiographic study. Panminerva Med 40(3):204–209
Tarumi N, Iwasaka T, Takahashi N et al (1993) Left ventricular diastolic filling properties in diabetic patients during isometric exercise. Cardiology 83(5–6):316–323
Nair VM, Tekin UN, Khan IA et al (2000) Worsening of left ventricular diastolic dysfunction during exercise causes decreased exercise tolerance in hypertension. Clin Cardiol 23(9):660–664
Rovner A, Greenberg NL, Thomas JD et al (2005) Relationship of diastolic intraventricular pressure gradients and aerobic capacity in patients with diastolic heart failure. Am J Physiol: Heart Circ Physiol 289(5):H2081–H2088. doi:10.1152/ajpheart.00951.2004
Ha JW, Oh JK, Pellikka PA et al (2005) Diastolic stress echocardiography: a novel noninvasive diagnostic test for diastolic dysfunction using supine bicycle exercise Doppler echocardiography. J Am Soc Echocardiogr 18(1):63–68. doi:10.1016/j.echo.2004.08.033
Tschope C, Kasner M, Westermann D et al (2005) Elevated NT-ProBNP levels in patients with increased left ventricular filling pressure during exercise despite preserved systolic function. J Card Fail 11(5, Suppl):S28–S33. doi:10.1016/j.cardfail.2005.04.013
Fukuta H, Little WC (2007) Elevated left ventricular filling pressure after maximal exercise predicts increased plasma B-type natriuretic peptide levels in patients with impaired relaxation pattern of diastolic filling. J Am Soc Echocardiogr 20(7):832–837. doi:10.1016/j.echo.2007.01.004
Mottram PM, Haluska BA, Marwick TH (2004) Response of B-type natriuretic peptide to exercise in hypertensive patients with suspected diastolic heart failure: correlation with cardiac function, hemodynamics, and workload. Am Heart J 148(2):365–370. doi:10.1016/j.ahj.2004.02.012
Kotchen TA, Hartley LH, Rice TW et al (1971) Renin, norepinephrine, and epinephrine responses to graded exercise. J Appl Physiol 31(2):178–184
Wade CE, Claybaugh JR (1980) Plasma renin activity, vasopressin concentration, and urinary excretory responses to exercise in men. J Appl Physiol 49(6):930–936
Convertino VA, Keil LC, Greenleaf JE (1983) Plasma volume, renin, and vasopressin responses to graded exercise after training. J Appl Physiol 54(2):508–514
Cheng CP, Suzuki M, Ohte N et al (1996) Altered ventricular and myocyte response to angiotensin II in pacing-induced heart failure. Circ Res 78(5):880–892
Alexopoulos D, Machac J, Arora RR et al (1989) Exercise-induced pulmonary blood volume changes and diastolic dysfunction of the aged heart. Clin Cardiol 12(4):209–213
Galderisi M, Cicala S, De Simone L et al (2001) Impact of myocardial diastolic dysfunction on coronary flow reserve in hypertensive patients with left ventricular hypertrophy. Italian Heart J 2(9):677–684
Arruda AL, Pellikka PA, Olson TP et al (2007) Exercise capacity, breathing pattern, and gas exchange during exercise for patients with isolated diastolic dysfunction. J Am Soc Echocardiogr 20(7):838–846. doi:10.1016/j.echo.2006.12.006
Lavietes MH, Gerula CM, Fless KG et al (2004) Inspiratory muscle weakness in diastolic dysfunction. Chest 126(3):838–844. doi:10.1378/chest.126.3.838
Reduto LA, Wickemeyer WJ, Young JB et al (1981) Left ventricular diastolic performance at rest and during exercise in patients with coronary artery disease. Assessment with first-pass radionuclide angiography. Circulation 63(6):1228–1237
Manolas J (1990) Value of the handgrip apex cardiography test for detection of early diastolic ventricular dysfunction in patients with angina pectoris. Zeitschrift fur Kardiologie 79(12):825–830
Sakamoto K, Nakamura T, Zen K et al (2004) Identification of exercise-induced left ventricular systolic and diastolic dysfunction using gated SPECT in patients with coronary artery disease. J Nucl Cardiol 11(2):152–158. doi:10.1016/j.nuclcard.2003.12.007
Barnes E, Baker CS, Dutka DP et al (2000) Prolonged left ventricular dysfunction occurs in patients with coronary artery disease after both dobutamine and exercise induced myocardial ischaemia. Heart (British Cardiac Society) 83(3):283–289. doi:10.1136/heart.83.3.283
Paul AK, Kusuoka H, Hasegawa S et al (2002) Prolonged diastolic dysfunction following exercise induced ischaemia: a gated myocardial perfusion SPECT study. Nucl Med Commun 23(11):1129–1136. doi:10.1097/00006231-200211000-00014
Fragasso G, Benti R, Sciammarella M et al (1991) Symptom-limited exercise testing causes sustained diastolic dysfunction in patients with coronary disease and low effort tolerance. J Am Coll Cardiol 17(6):1251–1255
Guazzi M, Brenner DA, Apstein CS et al (2001) Exercise intolerance in rats with hypertensive heart disease is associated with impaired diastolic relaxation. Hypertension 37(2):204–208
Poirier P, Garneau C, Bogaty P et al (2000) Impact of left ventricular diastolic dysfunction on maximal treadmill performance in normotensive subjects with well-controlled type 2 diabetes mellitus. Am J Cardiol 85(4):473–477. doi:10.1016/S0002-9149(99)00774-2
Dekleva M, Celic V, Kostic N et al (2007) Left ventricular diastolic dysfunction is related to oxidative stress and exercise capacity in hypertensive patients with preserved systolic function. Cardiology 108(1):62–70. doi:10.1159/000095883
Dumont CA, Monserrat L, Peteiro J et al (2007) Relation of left ventricular chamber stiffness at rest to exercise capacity in hypertrophic cardiomyopathy. Am J Cardiol 99(10):1454–1457. doi:10.1016/j.amjcard.2006.12.077
Spinelli L, Petretta M, Verderame G et al (2003) Left ventricular diastolic function and cardiac performance during exercise in patients with acromegaly. J Clin Endocrinol Metab 88(9):4105–4109. doi:10.1210/jc.2003-030462
Brubaker PH, Marburger CT, Morgan TM et al (2003) Exercise responses of elderly patients with diastolic versus systolic heart failure. Med Sci Sports Exerc 35(9):1477–1485. doi:10.1249/01.MSS.0000084416.71232.EA
Borlaug BA, Melenovsky V, Russell SD et al (2006) Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation 114(20):2138–2147. doi:10.1161/CIRCULATIONAHA.106.632745
Witte KK, Nikitin NP, Cleland JG et al (2006) Excessive breathlessness in patients with diastolic heart failure. Heart (British Cardiac Society) 92(10):1425–1429. doi:10.1136/hrt.2005.081521
Hundley WG, Kitzman DW, Morgan TM et al (2001) Cardiac cycle-dependent changes in aortic distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol 38(3):796–802. doi:10.1016/S0735-1097(01)01447-4
Kitzman DW, Little WC, Brubaker PH et al (2002) Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. J Am Med Assoc 288(17):2144–2150. doi:10.1001/jama.288.17.2144
Yuasa F, Sumimoto T, Takeuchi M et al (1995) Effects of left ventricular diastolic dysfunction on exercise capacity three to six weeks after acute myocardial infarction in men. Am J Cardiol 75(1):14–17. doi:10.1016/S0002-9149(99)80518-9
Skaluba SJ, Litwin SE (2004) Mechanisms of exercise intolerance: insights from tissue Doppler imaging. Circulation 109(8):972–977. doi:10.1161/01.CIR.0000117405.74491.D2
Terzi S, Sayar N, Bilsel T et al (2007) Tissue Doppler imaging adds incremental value in predicting exercise capacity in patients with congestive heart failure. Heart Vessels 22(4):237–244. doi:10.1007/s00380-006-0961-x
Eroglu S, Yildirir A, Bozbas H et al (2007) Brain natriuretic peptide levels and cardiac functional capacity in patients with dyspnea and isolated diastolic dysfunction. Int Heart J 48(1):97–106. doi:10.1536/ihj.48.97
Wang YC, Hwang JJ, Lai LP et al (2007) Coexistence and exercise exacerbation of intraleft ventricular contractile dyssynchrony in hypertensive patients with diastolic heart failure. Am Heart J 154(2):278–284. doi:10.1016/j.ahj.2007.04.008
Brandao MU, Wajngarten M, Rondon E et al (1993) Left ventricular function during dynamic exercise in untrained and moderately trained subjects. J Appl Physiol 75(5):1989–1995
Levy WC, Cerqueira MD, Abrass IB et al (1993) Endurance exercise training augments diastolic filling at rest and during exercise in healthy young and older men. Circulation 88(1):116–126
Nixon JV, Wright AR, Porter TR et al (1991) Effects of exercise on left ventricular diastolic performance in trained athletes. Am J Cardiol 68(9):945–949. doi:10.1016/0002-9149(91)90414-G
Douglas PS, O’Toole ML, Hiller WD et al (1986) Left ventricular structure and function by echocardiography in ultraendurance athletes. Am J Cardiol 58(9):805–809. doi:10.1016/0002-9149(86)90358-9
Di Bello V, Santoro G, Talarico L et al (1996) Left ventricular function during exercise in athletes and in sedentary men. Med Sci Sports Exerc 28(2):190–196. doi:10.1097/00005768-199602000-00006
Matsuda M, Sugishita Y, Koseki S et al (1983) Effect of exercise on left ventricular diastolic filling in athletes and nonathletes. J Appl Physiol 55(2):323–328
Forman DE, Manning WJ, Hauser R et al (1992) Enhanced left ventricular diastolic filling associated with long-term endurance training. J Gerontol 47(2):M56–M58
Takemoto KA, Bernstein L, Lopez JF et al (1992) Abnormalities of diastolic filling of the left ventricle associated with aging are less pronounced in exercise-trained individuals. Am Heart J 124(1):143–148. doi:10.1016/0002-8703(92)90932-L
Yu CM, Li LS, Lam MF et al (2004) Effect of a cardiac rehabilitation program on left ventricular diastolic function and its relationship to exercise capacity in patients with coronary heart disease: experience from a randomized, controlled study. Am Heart J 147(5):e24. doi:10.1016/j.ahj.2003.12.004
Loimaala A, Groundstroem K, Rinne M et al (2007) Exercise training does not improve myocardial diastolic tissue velocities in Type 2 diabetes. Cardiovasc Ultrasound 5:32. doi:10.1186/1476-7120-5-32
Smart N, Haluska B, Jeffriess L et al (2007) Exercise training in systolic and diastolic dysfunction: effects on cardiac function, functional capacity, and quality of life. Am Heart J 153(4):530–536. doi:10.1016/j.ahj.2007.01.004
Brassard P, Legault S, Garneau C et al (2007) Normalization of diastolic dysfunction in type 2 diabetics after exercise training. Med Sci Sports Exerc 39(11):1896–1901
Warner JG Jr, Metzger DC, Kitzman DW et al (1999) Losartan improves exercise tolerance in patients with diastolic dysfunction and a hypertensive response to exercise. J Am Coll Cardiol 33(6):1567–1572. doi:10.1016/S0735-1097(99)00048-0
Little WC, Wesley-Farrington DJ, Hoyle J et al (2004) Effect of candesartan and verapamil on exercise tolerance in diastolic dysfunction. J Cardiovasc Pharmacol 43(2):288–293. doi:10.1097/00005344-200402000-00019
Little WC, Zile MR, Klein A et al (2006) Effect of losartan and hydrochlorothiazide on exercise tolerance in exertional hypertension and left ventricular diastolic dysfunction. Am J Cardiol 98(3):383–385. doi:10.1016/j.amjcard.2006.01.106
Aldigier JC, Huang H, Dalmay F et al (1993) Angiotensin-converting enzyme inhibition does not suppress plasma angiotensin II increase during exercise in humans. J Cardiovasc Pharmacol 21(2):289–295
Yip GW, Wang M, Wang T et al (2008) The Hong Kong diastolic heart failure study: a randomized control trial of diuretics, Irbesartan and Ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection fraction. Heart (British Cardiac Society) (Jan):20
Kapuku GK, Seto S, Mori H et al (1993) Reversal of diastolic dysfunction in borderline hypertension by long-term medical treatment. Longitudinal evaluation by pulsed Doppler echocardiography. Am J Hypertens 6(7 Pt 1):547–553
Roongsritong C, Sutthiwan P, Bradley J et al (2005) Spironolactone improves diastolic function in the elderly. Clin Cardiol 28(10):484–487. doi:10.1002/clc.4960281008
Mottram PM, Haluska B, Leano R et al (2004) Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation 110(5):558–565. doi:10.1161/01.CIR.0000138680.89536.A9
Nodari S, Metra M, Dei Cas L (2003) Beta-blocker treatment of patients with diastolic heart failure and arterial hypertension. A prospective, randomized, comparison of the long-term effects of atenolol vs. nebivolol. Eur J Heart Fail 5(5):621–627. doi:10.1016/S1388-9842(03)00054-0
Fletcher BJ, Balady GJ, Amsterdam EA et al (2001) Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation 104(14):1694–1740. doi:10.1161/hc3901.095960
Pina IL, Apstein CS, Balady GJ et al (2003) Exercise and heart failure: a statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation 107(4):1210–1225. doi:10.1161/01.CIR.0000055013.92097.40
Williams MA, Haskell WL, Ades PA et al (2007) Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation 116(5):572–584. doi:10.1161/CIRCULATIONAHA.107.185214
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barmeyer, A., Müllerleile, K., Mortensen, K. et al. Diastolic dysfunction in exercise and its role for exercise capacity. Heart Fail Rev 14, 125–134 (2009). https://doi.org/10.1007/s10741-008-9105-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-008-9105-y