Abstract
During incremental exercise tests, chronotropic incompetence (CI), which is the inability of the heart rate (HR) to rise in proportion to an increase in metabolic demand, is often observed in patients with type 2 diabetes mellitus (T2DM). Despite the fact that CI is associated with exercise intolerance and elevated risks of development of cardiovascular disease and premature death, this clinical anomaly is often ignored or overlooked by clinicians and physiologists. CI is, however, a significant clinical abnormality that deserves further attention, examination and treatment. The aetiology of CI in T2DM remains poorly understood and is complex. Certain T2DM-related co-morbidities or physiological anomalies may contribute to development of CI, such as altered blood catecholamine and/or potassium levels during exercise, structural myocardial abnormalities, ventricular and/or arterial stiffness, impaired baroreflex sensitivity and cardiovascular autonomic neuropathy. Clinicians should thus be aware of the potential presence of yet undetected anomalies or diseases in T2DM patients who experience CI during exercise testing. However, an effective treatment for CI in T2DM is yet to be developed. Exercise training programmes seem to be the only potentially effective and feasible interventions for partial restoration of the chronotropic response in T2DM, but it remains poorly understood how these interventions lead to restoration of the chronotropic response. Studies are thus warranted to elucidate the aetiology of CI and develop an effective treatment for CI in T2DM. In particular, the impact of (different) exercise interventions on CI in T2DM deserves greater attention in future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Chronotropic incompetence (CI) is often overlooked by clinicians and physiologists, despite its association with exercise intolerance and risks of development of cardiovascular disease and premature death. |
Certain co-morbidities or physiological anomalies related to type 2 diabetes mellitus (T2DM) may contribute to development of CI. However, the aetiology of CI in this population remains poorly understood and is complex. |
Studies are warranted to elucidate the aetiology of CI and to develop an effective treatment for CI in T2DM. |
1 Introduction
During the second half of the 20th century, the global prevalence of type 2 diabetes mellitus (T2DM) increased substantially [1]. Recent data indicate that about 285 million individuals worldwide have been diagnosed with diabetes, 90 % of whom suffer from T2DM [2]. It is anticipated that this prevalence will increase to 439 million people worldwide by 2030, which represents 7.7 % of the global population [2].
T2DM is associated with development of hypertension, dyslipidaemia, retinopathy, nephropathy and cardiovascular disease. Moreover, disturbed lipolysis, a lowered resting metabolic rate and exercise intolerance are often present in T2DM [3–5]. As a result, T2DM is a disease that ultimately leads to a reduced life expectancy (by ~4.3 years) and a significant socio-economic burden [6–8].
Traditionally, the three cornerstones of therapy for T2DM are glucose-lowering medication, dietary intervention and exercise training [3]. The clinical benefits of exercise training in T2DM are well documented: it reduces systemic inflammation and leads to improvements in glycaemic control, insulin sensitivity, the lipid profile, body composition, quality of life, endothelial function, cardiac function and arterial compliance [1, 3, 4, 9–12]. It thus follows that exercise therapy should be considered as a cornerstone in the treatment of T2DM. The current recommendation for exercise training in T2DM is an exercise volume of at least 150 minutes per week, which should be spread throughout the week, and it is also recommended that the exercises should be of the endurance type at a moderate intensity (50–75 % of peak oxygen uptake) [13, 14]. The addition of strength training exercises (three sets of 8–10 repetitions at a weight that cannot be lifted more than 8–10 times) is also recommended [1].
Prior to initiation of an exercise training intervention, T2DM patients are advised to undergo a cardiopulmonary exercise test [5] to verify the absence of cardiovascular abnormalities (myocardial ischaemia, arrhythmias or hypertension) or pulmonary abnormalities [15]. In addition, such exercise tests are very useful to examine exercise tolerance and to properly determine training modalities (intensity, frequency, volume and type) [5].
One commonly observed anomaly during exercise testing in patients with T2DM is a suppressed peak heart rate (HR), commonly mentioned in the literature as chronotropic incompetence (CI) [16, 17]. Unfortunately, even though a suppressed peak HR during exercise signifies a worse prognosis, is related to development of exercise intolerance and could even be a marker for other (subclinical) diseases or abnormalities, many clinicians and physiologists do not notice CI in their evaluation, or even ignore it. Therefore, clinicians should be stimulated to systematically verify the presence or absence of CI [18]. The aim of this review is to stimulate clinicians and physiologists to assess the chronotropic response to exercise in T2DM more often in clinical practice, to offer a valid methodology for the detection of CI, and to provide insights into interventions for the treatment of CI. Finally, the need for studies on the aetiology of CI in T2DM and the impacts of different interventions are highlighted.
2 Chronotropic Incompetence
CI is defined as the inability of HR to rise in proportion to an increase in activity or metabolic demand. During physical exercise, a sufficient increase in HR is necessary to augment cardiac output and increase perfusion of working muscles. CI is therefore a significant contributor to exercise intolerance and reduced quality of life [18]. This clinical anomaly is frequently seen in patients with (yet undiagnosed) cardiovascular disease. Moreover, CI is an independent predictor of major cardiovascular events and premature death [18]. It thus follows that CI should be considered a significant clinical abnormality that deserves further attention, examination and treatment.
2.1 Prevalence and Assessment Methodology
Because of the absence of standardized criteria and consistent methodology for determining CI, and depending on the population examined, various prevalence rates of CI (9–89 %) have been reported in the current literature [19–22]. A recent study showed that CI was present in ~42 % of male T2DM patients, as opposed to ~6 % of their healthy counterparts [17]. With the exception of the latter study, the data on CI prevalence in T2DM are limited. Therefore, CI should be assessed more often in clinical practice and in experimental studies, in order to determine the prevalence and severity of CI in large cohorts of T2DM patients. From these data, associations between CI severity and patient characteristics can be studied, as well as the prognostic impact of CI severity. For example, Hansen and Dendale observed a greater likelihood of CI in T2DM patients with a greater waist circumference [17]. The authors hypothesized that visceral fat accumulation could be an important contributor to development of CI in T2DM patients. As a result, it could thus be proposed that interventions that lead to decrements in visceral fat mass could (partly) restore the chronotropic response during exercise in T2DM patients. This hypothesis is discussed in Sect. 4.3 of the present review.
The most commonly used criterion for diagnosing CI is when HR is not able to reach a given percentage (80–85 %) of the age-predicted maximal HR (220 bpm − age) during maximal endurance exercise testing [23, 24]. However, a major disadvantage of this method is the fact that on an individual basis, the maximal HR during exercise testing can vary considerably, even in healthy subjects [25, 26]. Another technique for determining CI is via the HR reserve method, which is defined as the change in HR from rest to peak endurance exercise, minus HR at rest [18]. Thus, the adjusted HR reserve (the change in HR from rest to peak exercise, minus resting HR) divided by the difference between the resting HR (measurement of the radial pulse during a 1-min recording, after a 5-min rest in the supine position [18, 27]) and the age-predicted maximal HR is commonly used to establish CI [28]. From this calculation, the most widely used criterion for CI is the inability to reach ≥80 % of HR reserve during maximal endurance exercise testing on a bike or treadmill [18].
To diagnose CI correctly, subjects have to perform an exercise test until maximal exercise effort is achieved [18]. To quantify the level of exertion during exercise testing, ratings of perceived exertion (Borg RPE) can be used. However, because of the altered motivation of T2DM patients with regard to exercise (many T2DM patients lack the motivation to exercise), this criterion for maximal effort during exercise testing should be used with great caution. Therefore, persons with T2DM report greater Borg RPE during exercise than non-diabetic controls while being exposed to identical absolute work rates and physiological loads [29]. The respiratory gas exchange ratio (RER; volume of carbon dioxide output/volume of oxygen uptake [VO2]) is the most objective measure of exercise effort. RER is a continuous variable with a range from <0.85 at rest to >1.20 during exhaustive exercise [18]. RER values should be >1.10 at peak exercise to obtain the reliable exercise test results needed for determination of CI. Exercise tests with continuous gas exchange monitoring are thus advised. It follows that facilities that are not equipped with ergospirometers are severely limited in their ability to diagnose CI in patients with T2DM.
In clinical practice, physicians and physiologists are often confronted with patients who are not able or willing to execute a maximal cardiopulmonary exercise test (patients with kinesiophobia, lack of motivation and/or orthopaedic limitations). Therefore, in these specific cases, clinicians need to be able to diagnose CI from a submaximal exercise test. In this regard, the metabolic–chronotropic relationship (MCR; the chronotropic index), which is calculated from the ratio of HR reserve to metabolic reserve (the relationship between HR and VO2) during submaximal exercise, is used. Using the MCR to evaluate CI could sometimes be more feasible because maximal exercise tests are not necessary [30]. In healthy subjects, the achieved percentage of HR during exercise equals the percentage of metabolic reserve (mobilization of glycogen, a switch of substrate oxidation from fat to carbohydrates and the capacity to increase oxidative metabolism of energy-providing substrates). Hereby, it is possible to state that for a single HR at any point during endurance exercise (HRstage), this HR can be determined as being (in)consistent with normal chronotropic function. By using the Wilkoff equation, it is possible to calculate the CI index [30]:
In order to use this formula (where metabolic equivalents [METs] = VO2 in mL kg−1 min−1/3.5) to evaluate CI, the following parameters should be recorded during a dynamic exercise test (Bruce protocol, RAMP exercise protocol): age, resting HR (HRrest), age-predicted maximal HR (APMHR) = 220 bpm − age, age-predicted HR reserve (= APMHR − HRrest), maximally achieved HR during exercise (HRmax), oxygen consumption (VO2 = mL kg−1 min−1) and RER.
Here, METsstage equals VO2 (mL/kg/min)/3.5 at the respective stage, and METspeak equals VO2 (mL/kg/min)/3.5 under maximum stress [31].
This formula then provides a CI index, where the cut-off value is set at 0.80. Thus, a patient with an MCR of ≤0.80 can be diagnosed as having CI [18, 30].
2.2 Prognosis and Clinical Consequences of CI in T2DM
An important determinant of quality of life is the ability to perform physical work with maximal comfort throughout the day. Because CI leads to a suppressed (increase in) cardiac output, perfusion of working muscles will be compromised. This can lead to severe or symptomatic exercise intolerance [6, 18, 31, 32]. Subsequently, T2DM patients who experience CI during exercise will be very likely to suffer from exercise intolerance, a reduced quality of life and reduced motivation to exercise. Because of the reduced motivation to exercise, these patients become or stay sedentary, which further leads to worsening of their cardiovascular disease risk factors and hence their prognosis. In this way, a vicious cycle can develop. It should therefore be of no surprise that CI has frequently been associated with increased risks of major cardiovascular events and premature death [17, 33–35]. For example, in one study, patients with CI were at a 2.5-fold greater risk of premature death and experienced a greater need for cardiac transplantation and ventricular assist device placement [33]. Moreover, the worse the degree of CI during exercise testing, the greater the risk of adverse cardiovascular events becomes [34].
It is thus evident that CI is associated with major and pervasive medical risks in T2DM, although the prognosis is variable and is dependent on the underlying aetiology of CI [5, 15]. CI could thus indicate the presence of other, yet undiagnosed, diseases or medical anomalies. The aetiology of CI in T2DM patients should therefore be determined, as the treatment for CI may vary accordingly [34].
Moreover, CI may induce diverse practical complications during diagnosis and rehabilitation of T2DM patients. For example, during assessment of cardiac function (for example, stress echocardiography) or during medical imaging (for example, cardiac positron emission tomography), a given HR is often aimed at. Because of CI, the cardiac metabolic demand at certain HR thresholds may be higher than anticipated in T2DM patients. This may thus complicate the interpretation of such diagnostic tests. Furthermore, in rehabilitation programmes, exercise intensity is often based on a theoretically given HR. In T2DM patients with CI, there is a greater likelihood that too high a target HR will be prescribed during exercise training. This complicates participation in exercise interventions. It is thus important that clinicians and physiologists take CI into account in T2DM patients when executing cardiac function tests and/or prescribing exercise training intensities [7, 14, 16].
3 Aetiology of Chronotropic Incompetence
3.1 Chronotropic Response During Exercise in Healthy Individuals
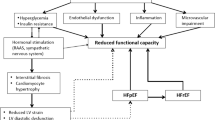
Before discussing anomalies in HR response during exercise in T2DM patients and exploring the aetiology of CI, the cascades and mechanisms leading to increments in HR during exercise should be discussed in healthy subjects first (see Fig. 1).
An increase in HR with dynamic exercise reflects the ability of both sympathetic and vagal activity to respond adequately to an increase in metabolic demand.
Before exercise, feed-forward control by brain centres (= central command) causes vagal withdrawal and allows sympathetic activity to dominate and produce cardiac acceleration, causing an increase in HR [36, 37]. Therefore, the earliest increase in HR, immediately before initiation of an exercise test, is mainly dependent on the patient’s anticipation of exercise. This response can vary considerably and should be taken into account during an attempt to assess resting HR, because this may not be the ideal setting for doing so.
At the onset of exercise (within the first 20 s, up to an HR of 100 beats/min), a rapid HR increase is noticed, which is mainly attributable to withdrawal of tonic vagal activity. This vagal tone withdrawal causes very rapid changes in HR and hence cardiac output. After about 30 s of incremental exercise, or when the HR exceeds 100 beats/min, the HR change is taken over by other mechanisms [38].
As the intensity of exercise increases, a further increment in sympathetic stimulation from the central nervous system emerges, together with an increase in circulatory catecholamine levels. Because of reduced parasympathetic tone and increased sympathetic tone, the stimulation of the sinus node increases, which leads to an increase in HR. Thus, the chronotropic response to endurance exercise reflects the vagal and sympathetic outflow to the sinus node [38].
Another mechanism contributing to an adequate HR increase during endurance exercise is the baroreflex, which is activated by stimulation of the baroreceptors. These receptors, which are located in the arterial vessel wall, influence and modulate the activity of the brainstem cardiovascular and respiratory centres through connections with the central nervous system. The arterial blood pressure is constantly regulated by these baroreceptors, which are sensitive to stretching of the vessel wall. Under normal physiological conditions, firing of these baroreceptors exerts a tonic inhibitory action on sympathetic outflow from the medulla. Hypotension, on the other hand, leads to disinhibition of the medulla centres, resulting in increased sympathetic and decreased parasympathetic outflow. Under the influence of these autonomic changes, peripheral vasoconstriction occurs, leading to tachycardia and thus restoration of arterial blood pressure [39]. Thus, the baroreflex contributes to a chronotropic response to endurance exercise through modulation of blood pressure and HR.
The baroreflex also interacts with another mechanism responsible for the regulation of HR and blood pressure—namely, the chemoreflex. This reflex controls the regulation of ventilatory responses to changes in arterial oxygen and carbon dioxide content. Both peripheral and central chemoreceptors influence neural circulatory control, particularly in situations involving pronounced changes in arterial oxygen and/or carbon dioxide (e.g. during endurance exercise), as these receptors are sensitive to hypoxia and hypercapnia [40].
Activation of the chemoreflex causes an increase in sympathetic activation, HR, blood pressure and ventilation. Activation of these mechanisms is seen during endurance exercise, where HR, blood pressure and ventilation ought to increase proportionally to an increase in activity or metabolic demand [40].
Other reflex mechanisms contributing to changes in HR during exercise are the mechanoreflex and the metaboreflex. The muscle mechanoreflex is activated by stimulation of mechanoreceptors (e.g. by a tendon stretch), which are located in the group III and IV sensory fibres of exercising muscles. Activation of mechanoreceptors induces an increase in muscle sympathetic activity, blood pressure and HR, and also reduces cardiac vagal activity. Metaboreceptors, which share the same location as mechanoreceptors, evoke activation of the metaboreflex, which leads to cardiovascular responses during exercise. These receptors are activated by accumulation of certain metabolites (lactic acid, bradykinin, potassium) in contracting skeletal muscles. This leads to increases in blood pressure, cardiac output and vascular resistance, thus contributing to normal HR responses during exercise [37, 41].
All of the above-mentioned reflex mechanisms contribute to a collective increase in sympathetic tone, which leads to increased synthesis/release of circulatory catecholamines (epinephrine, norepinephrine). These catecholamines activate the sinus node and cardiomyocytes to increase HR and stroke volume [42–44].
3.2 Impaired Chronotropic Response in T2DM
In T2DM patients, a disturbed HR response to exercise is often present [16, 17], though the underlying mechanisms of CI in T2DM are not fully understood. T2DM is, however, associated with certain co-morbidities that may contribute to development of CI. These co-morbidities are altered catecholamine and/or potassium levels during exercise, structural myocardial alterations, ventricular and/or arterial stiffness, impaired baroreflex sensitivity (BRS) and cardiovascular autonomic neuropathy (CAN). The associations between these clinical anomalies and CI in T2DM, and their mechanisms, are described in detail in Sects. 3.2.1–3.2.5. It must be mentioned, however, that we can only partly explain the aetiology of CI in T2DM, because of its complexity and because of the lack of data/studies.
3.2.1 Arterial Stiffness and Altered Baroreceptor/Chemoreflex Sensitivity
In T2DM patients, myocardial fibrosis and microvascular disease are often present [16]. It is believed that these myocardial and microvascular disturbances in T2DM are induced by hyperglycaemia, accumulation of advanced glycation end products (AGEs), a disturbed lipid profile (lowered blood high-density lipoprotein levels and elevated low-density lipoprotein levels), long-term hypertension and (mainly visceral) obesity. The presence of these co-morbidities can lead to chronic low-degree inflammation and stimulation of the renin–angiotensin–aldosterone system, from which interstitial and perivascular fibrosis emerges [16]. The latter eventually leads to increased arterial/ventricular stiffness and diastolic dysfunction in T2DM patients.
Coherent with the previously discussed influences of altered baroreceptor sensitivity, increased arterial stiffness, as in atherosclerosis, may attenuate the HR response during exercise in T2DM patients [39, 45, 46]. Given that the baroreceptors are ‘stretch’ receptors, decreased compliance of the vessel wall would lead to decreased stretching of these receptors during exercise, causing an attenuated HR response [39, 45, 47–52]. Consistent with these findings, Fukuma et al. [46] showed that reduced baroreflex function is associated with CI during exercise. In that study, the chronotropic index was lower in patients with depressed BRS than in those with normal BRS. These data indicate that impaired BRS may alter sympathetic and parasympathetic nervous system activity and thus attenuate the HR increment during exercise. In this regard, atherosclerosis seems to play a pivotal role in development of this reduced baroreflex activity in T2DM patients.
The baroreflex has the potential to affect sympathetic nervous system activity both directly and indirectly during exercise. In addition, the baroreflex also interacts with the chemoreflex, which is an important mechanism in regulating sympathetic activity. As the chemoreflex induces an increase in sympathetic activity, HR, blood pressure and ventilation, it is presumable that impairment of this reflex causes an attenuated HR increment during dynamic exercise. Disturbance of chemoreflex sensitivity could be caused by habituation of the sympathetic nervous system via persistent sympathetic excitation [46], as seen in T2DM patients. This altered chemoreflex sensitivity would then complicate a normal HR increase during exercise.
3.2.2 Ventricular Stiffness and Structural Myocardial Alterations
The role of increased ventricular stiffness and diastolic dysfunction (caused by T2DM) in development of CI is uncertain. It is hypothesized that contraction of a stiffened ventricle may stimulate ventricular mechanoreceptors with greater magnitude, as opposed to contraction of a non-stiffened ventricle. The augmented stimulation of ventricle mechanoreceptors gives way to a greater increase in vagal activation, which counteracts a sympathetically mediated increase in HR during exercise [47]. Via this mechanism, an increase in HR during exercise becomes suppressed. If this hypothesis holds true, CI during exercise testing could indicate the presence of ventricular stiffness as well in T2DM patients.
In addition, it could also be argued that a hypertensive response to exercise develops in T2DM patients because of structural changes in the myocardium (myocardial fibrosis and/or ventricular stiffening) that result in an impaired HR response to exercise. For example, diastolic dysfunction is often present in T2DM patients, possibly resulting from diabetic cardiomyopathy induced by long-term hyperglycaemia. Diastolic dysfunction in T2DM entails delayed myocardial relaxation, impaired left ventricular (LV) filling and increased myocardial stiffness [53]. These structural and biomechanical changes could then result in an inability to increase the stroke volume commensurately with the degree of effort, causing inadequate HR responses to exercise.
Furthermore, Poanta et al. [54] described a correlation between LV diastolic dysfunction and cardiac dysautonomia in T2DM patients. In that study, patients with restricted LV filling showed significant lower HR variability (a marker of cardiac autonomic function) than patients with non-restrictive LV filling. Hence, because of the correlation with cardiac autonomic dysfunction, diastolic dysfunction may contribute to a disturbed HR response during exercise.
Vandergoten et al. [51] described additional factors contributing to development of CI, such as ischemia (due to coronary artery disease) and LV dysfunction. Oliveira et al. [52] found an association between the prevalence of CI and ventricular wall motion abnormalities. It is speculated that myocardial ischemia (which often leads to ventricular wall abnormalities) during exercise could lead to development of CI in T2DM patients. Consistent with this, silent myocardial ischemia is often detected in T2DM patients during maximal exercise testing. It thus follows that T2DM patients with CI during exercise testing should have an examination of myocardial perfusion to rule out (silent) myocardial ischemia.
3.2.3 Blood Potassium Levels During Exercise
T2DM is associated with suppressed blood potassium levels during exercise. This abnormality is linked to hypertension during exercise and cardiac arrhythmias. As a result, altered blood potassium levels during exercise could, at least in part, explain CI in T2DM patients [55].
Under normal circumstances, physical stress entails an increase in plasma catecholamine levels, leading to an increase in HR and potassium release from contracting muscles. Since potassium is considered an important stimulator of HR during exercise, the release and re-uptake of potassium by contracting muscles could play an important role in the regulation of cardiac activity during physical exercise [56]. Release of potassium into the circulation is primarily caused by contracting muscles during exercise, whereas potassium re-uptake is mediated by β-adrenergic receptors to prevent exercise hyperkalaemia.
In obesity, lower circulatory potassium levels are seen during exercise, which result in a less prompt cardiovascular response to higher workloads and thus a smaller increase in HR. These lower plasma potassium levels in obesity could be due to type 2 muscle fibre hypertrophy in obesity, which is associated with higher Na+–K+ pump activity during exercise. The higher Na+–K+ pump activity then leads to greater K+ re-uptake from the circulation and thus a reduced plasma potassium level [56].
As T2DM is often associated with obesity and CI has been detected in T2DM patients mainly with an increased waist circumference [17], it is possible that CI is partly due to altered muscular potassium handling [57].
Salvadori et al. [56] reported that insulin resistance is related to abnormal regulation of muscle K+ uptake and metabolism during exercise. Such abnormalities in K+ uptake during dynamic exercise could possibly lead to a disturbed cardiovascular response to higher workloads. It thus seems likely that the blood potassium content plays an important role in the HR response to exercise and could therefore contribute to an incompetent chronotropic response in T2DM patients.
3.2.4 Blood Catecholamine Levels During Exercise
An adequate increase in plasma catecholamine levels is imperative during exercise, to achieve a sufficient rise in HR in response to an increase in metabolic demand. However, Mittendorfer et al. [58] reported a blunted rise in blood catecholamine levels during exercise in obese patients. Unfortunately, the mechanism responsible for an attenuated catecholamine response during exercise in obese men presently remains unknown [58]. Because obesity is often associated with development of insulin resistance and T2DM, it is plausible that smaller changes in blood catecholamine levels occur during exercise testing in T2DM patients. A blunted catecholamine release prevents HR from increasing concomitantly with the increase in metabolic demand. Therefore, disturbed catecholamine responses could be partly responsible for the presence of CI in T2DM patients during dynamic exercise. CI during exercise testing in T2DM patients could thus alert clinicians to suspect endocrine hormone disturbances during exercise in these patients.
3.2.5 Cardiovascular Autonomic Neuropathy
T2DM patients are at an elevated risk of developing CAN [59, 60]. In diabetes, CAN is ultimately the result of worsened long-term glycaemic control and interactions among factors such as diabetes duration, age-related neuronal attrition and disturbed blood pressure (hypertension).
Hyperglycaemia plays a key role in development and progression of CAN. Initially, CAN is characterized by increased sympathetic activity with an increased resting HR [16, 60], followed by damage to nerves of the autonomic nervous system in the longer term (mainly parasympathetic fibres, such as the vagus nerve) [61, 62]. This leads to a predominance of sympathetic nervous system activity and thus a compromised sympathovagal balance during exercise, which may lead to development of CI. The sympathetic predominance in CAN leads to disturbances in blood pressure and HR, possibly arising from post-synaptic desensitization of the β-adrenergic receptor pathways in the sinoatrial node. This complication arises from frequent/chronic activation of sympathetic nerves, which leads to down-regulation of β-adrenergic receptors in the sinus node. This ultimately results in post-synaptic desensitization [63–66]. As the HR response to exercise is determined by the extent of sympathetic drive to the heart and the ability of β-adrenergic receptors in the sinoatrial node to respond to circulating catecholamines, such desensitization could then result in a disturbed HR regulation/response during exercise [64, 67]. Thus, hyperglycaemia in T2DM patients induces CAN, leading to sympathetic predominance, ultimately contributing to development of CI by post-synaptic desensitization of the β-adrenergic receptor pathways in the sinoatrial node. CI during exercise testing could thus be an indicator of CAN in T2DM patients.
4 Therapy for CI in T2DM
Considering the significant impact of CI during exercise on the prognosis and health of T2DM patients, it is imperative that an effective therapy for this clinical anomaly is developed for T2DM patients. However, to develop an effective treatment for a symptom or disease, its aetiology needs to be known. Unfortunately, this has not yet been established for CI. Therefore, the examined treatments for CI are only partly effective, at best. It is currently speculated that CI during exercise in T2DM patients could be partly remedied or modulated through four different types of interventions: implantation of cardiac devices, medication, diet or exercise. The possible effects of these therapies on CI in T2DM patients are discussed in Sects 4.1–4.4.
4.1 Cardiac Devices
In theory, to treat cardiac sinus node dysfunction, implantation of a pacemaker may be suggested. Therefore, it could be hypothesized that T2DM patients with CI during exercise testing are candidates for pacemaker implantation.
Rate-responsive pacing, as a pacemaker-based therapy to modulate severe CI and to improve maximal exercise capacity, has been used in previous studies and in other populations [33, 68]. Rate-adaptive pacing has been shown to enhance functional capacity in patients with an inadequate chronotropic response [18] by restoring cardiac output changes during exercise. At first glance, pacemaker implantation may seem a logical choice for the treatment of CI in T2DM patients. It remains questionable, however, whether pacemaker implantation should be executed in T2DM patients with CI during exercise. First, pacemaker implantation is a surgical procedure, which is associated with certain complications and is relatively expensive. It therefore remains uncertain whether the clinical benefits of this therapy (reductions in mortality and morbidity, if present) would be greater than the costs for patients and the community (surgery and follow-up consultations). Second, the aetiology of CI is not targeted by pacemaker implantation. It thus follows that the underlying cause of CI (for example, atherosclerosis, CAN, ventricular stiffness or myocardial ischemia) remains untreated and could be of potential danger to the patient in the long term. Third, when CI could be improved by other non-surgical interventions (see Sects. 4.2–4.4) as well, implantation of a pacemaker becomes significantly less appealing, because of its invasive and expensive character. It therefore seems unlikely that pacemaker implantation will become the (gold) standard treatment for CI in T2DM.
4.2 Medication
In patients with CI, it could be questioned whether administration of HR-lowering drugs is appropriate and/or whether HR-increasing drugs should be prescribed.
Intake of β-blockers leads to a reduction in resting HR and exercise peak HR, and may thus result in pharmacologically induced CI during exercise, or may worsen already present CI [18, 65]. In patients with T2DM, β-blockers are, however, often prescribed to treat hypertension. Thus, it is questioned whether β-blocker treatment should be stopped in T2DM patients with CI, and whether β-blockers should be replaced by other blood pressure-lowering medication (such as calcium channel antagonists or angiotensin-converting enzyme [ACE] inhibitors). However, this should be considered with great caution and determined for each patient individually. In T2DM patients experiencing heart failure or arrhythmias, replacement of β-blockers by other anti-hypertensive drugs is not debatable. If, instead, β-blockers are prescribed to T2DM patients who experience hypertension only, it could be argued that these should be replaced by other blood pressure-lowering medication, thus preventing pharmacologically induced CI during exercise.
Nevertheless, Witte et al. [65] observed that although the prevalence of CI was greater in patients taking β-blockers, patients experiencing CI during exercise who did not take β-blockers had a higher mortality rate than patients who did take β-blockers. Therefore, to prevent premature death in patients with T2DM and hypertension, intake of β-blockers should be continued.
Besides the withdrawal of HR-lowering medications, it may also be argued that T2DM patients with CI should receive HR-increasing medication. Munagala et al. [69] reported the impact of administration of atropine, which is an anticholinergic agent causing an increase in HR, in patients with CI during treadmill stress testing (TMST). Intake of atropine led to an increase in resting HR by ~10 bpm. This effect persisted throughout the entire exercise test and during the recovery period. Therefore, patients were able to reach a significantly higher HR through intake of atropine. However, whether atropine would improve exercise capacity remains uncertain [69–71]. Moreover, further studies are warranted to verify whether long-term intake of atropine is medically safe and feasible for treatment of CI in T2DM patients during exercise [70]. For example, it could be argued that long-term intake of atropine would lead to elevated blood pressure, resulting in (worsening of) hypertension in T2DM patients [69–71] and a worse prognosis for those patients. It thus seems doubtful that long-term atropine administration is an intelligent choice in the treatment of CI in T2DM.
4.3 Dietary Intake Restriction Intervention in T2DM Patients with CI
Dietary intake restriction interventions in T2DM patients lead to improvements in glycaemic control, insulin sensitivity, the lipid profile, endothelial dysfunction, LV diastolic function and body composition [72–76]. Because some of these parameters are believed to be related to development of CI, it should thus be examined whether such an intervention will also lead to improved HR responses to exercise in T2DM patients with CI.
Poirier et al. [77] described a correlation between body weight and autonomic cardiac modulation. Cardiac autonomic dysfunction, caused by an imbalance between sympathetic and parasympathetic activity, could contribute to development of CI. Because sympathetic predominance leads to sympathetic desensitization, altered HR responses during exercise emerge. Moreover, Poirier et al. [77] reported that weight gain caused a decrement in parasympathetic tone and thus an increased HR at rest and during exercise. Conversely, weight loss was associated with significant improvements in autonomic cardiac modulation through enhancement of parasympathetic modulation, which clinically translates into a decrease in resting HR.
However, as promising as these results seem to be, information regarding changes in peak HR during dietary interventions is sparse. Most studies that have investigated the impact of dietary intake restriction focused on body composition. Even when exercise tolerance is discussed in these studies, peak HR during exercise testing is often not mentioned. In two studies by Brinkworth et al. [71, 78], caloric intake restriction did not translate into an increase in peak HR during exercise testing, despite a significant reduction in adipose tissue mass. However, further studies are warranted to study the impact of dietary intake restriction on peak HR during exercise testing. It is therefore concluded that it remains undecided whether caloric intake restriction interventions should be initiated to treat CI in T2DM.
4.4 Exercise Therapy
Exercise training leads to improvements in glycaemic control, insulin sensitivity, the lipid profile, body composition, endothelial function, LV diastolic function and physical fitness, and less arterial stiffness and systematic inflammation in T2DM patients [1, 4, 10]. Because many of these parameters and factors are associated with development of CI, it may be hypothesized that exercise training could contribute to prevention or treatment of CI in T2DM.
The impact of exercise training interventions on the chronotropic response to exercise has been investigated in other populations—but only infrequently in T2DM patients, unfortunately.
In heart failure patients, exercise training leads to increases in peak HR during exercise testing, cardiac output and VO2 peak [18]. In line with these observations, Miossi et al. [79] described improvements in chronotropic responses during exercise as the result of a 3-month exercise training programme (resistance and endurance training) in patients with systemic lupus erythematosus. Keteyian et al. [35] showed that exercise training for a duration of 24 weeks resulted in an increase in peak HR and HR reserve during exercise testing, as well as partial reversal of CI, in patients with heart failure. Adams et al. [80] found improvements in the chronotropic index after a 12-week exercise training programme (3×/week; 50–80 % HR reserve) in patients with cardiovascular disease. Finally, Brubaker and Kitzman [32] reported improvements in the chronotropic response (increases in peak HR and cardiac output) and exercise capacity with endurance exercise training in patients with heart failure. Other authors [18] have mentioned that exercise training leads to favourable changes in chronotropic function, as indicated by a decrease in resting HR and submaximal exercise HR and a faster decline in post-exercise HR. It thus seems fair to conclude that exercise training intervention is effective to improve chronotropic responses to exercise, at least in patients with heart failure or other diseases.
Improvements in chronotropic responses to exercise through exercise training are believed to be related to alterations in the balance between sympathetic and parasympathetic tone at rest and during exercise. Moreover, exercise training positively affects baroreceptor sensitivity and HR variability [18]. It is thus speculated that improved chronotropic responses during exercise testing, as a result of endurance exercise training, are related to increases in β-adrenergic receptor sensitivity and improved nervous system parasympathetic–sympathetic balance [18, 32, 35, 79, 80]. In addition, improvements in chronotropic responses during exercise testing seem to correlate with improvements in VO2 peak and cardiac output [18, 32, 35, 79, 80].
Although the impact of exercise training on the chronotropic response during exercise has been studied sporadically in T2DM, the few available data seem promising [81]. Morton et al. [81] showed that T2DM patients were able to increase peak HR during exercise testing when following a 7-week walking programme in a randomised controlled trial. The increase in peak HR during exercise testing was believed to be related to improvements in myocardial functional capacity. In that study, improvements in LV ejection fraction, end-diastolic volume and stroke volume were observed. These improvements could eventually lead to better chronotropic responses to exercise in T2DM.
As promising as these results are, the impact of exercise therapy on chronotropic responses during exercise testing in T2DM patients needs to be studied more frequently. In this regard, also, the impact of different selections of training modalities (for example, different intensities, exercise and programme durations, exercise volumes and exercise types) on the chronotropic response to exercise should be examined in T2DM patients.
5 Conclusion
CI is often present in T2DM patients and is an independent predictor of major cardiovascular events and premature death. CI in T2DM is probably due to altered blood catecholamine and/or potassium levels during exercise, structural myocardial alterations, ventricular and/or arterial stiffness, impaired BRS and CAN. Clinicians should thus suspect other, yet undiagnosed, anomalies that deserve further attention in T2DM patients who experience CI during exercise testing. According to the current literature, exercise training is the only potentially clinically effective and feasible treatment for CI in T2DM. Further research regarding the aetiology and treatment of CI in T2DM is warranted.
References
Praet SF, van Loon LJ. Exercise therapy in type 2 diabetes. Acta Diabetol. 2009;46(4):263–78.
Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol. 2012;8(4):228–36.
Madden KM. Evidence for the benefit of exercise therapy in patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2013;6:233–9.
Reusch JE, Bridenstine M, Regensteiner JG. Type 2 diabetes mellitus and exercise impairment. Rev Endocr Metab Disord. 2013;14(1):77–86.
Stewart KJ. Exercise training: can it improve cardiovascular health in patients with type 2 diabetes? Br J Sports Med. 2004;38(3):250–2.
Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31–40.
Portero McLellan KC, Wyne K, Villagomez ET, et al. Therapeutic interventions to reduce the risk of progression from prediabetes to type 2 diabetes mellitus. Ther Clin Risk Manag. 2014;10:173–88.
Price HC, Clarke PM, Gray AM, et al. Life expectancy in individuals with type 2 diabetes: implications for annuities. Med Decis Making. 2010;30(3):409–14.
van Dijk JW, Tummers K, Stehouwer CD, et al. Exercise therapy in type 2 diabetes: is daily exercise required to optimize glycemic control? Diabetes Care. 2012;35(5):948–54.
Stewart KJ. Exercise training and the cardiovascular consequences of type 2 diabetes and hypertension: plausible mechanisms for improving cardiovascular health. JAMA. 2002;288(13):1622–31.
McAuley PA, Myers JN, Abella JP, et al. Exercise capacity and body mass as predictors of mortality among male veterans with type 2 diabetes. Diabetes Care. 2007;30(6):1539–43.
Nylen ES, Kokkinos P, Myers J, et al. Prognostic effect of exercise capacity on mortality in older adults with diabetes mellitus. J Am Geriatr Soc. 2010;58(10):1850–4.
Hansen D, Dendale P, Jonkers RA, et al. Continuous low- to moderate-intensity exercise training is as effective as moderate- to high-intensity exercise training at lowering blood HbA(1c) in obese type 2 diabetes patients. Diabetologia. 2009;52(9):1789–97.
Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33(12):e147–67.
Chipkin SR, Klugh SA, Chasan-Taber L. Exercise and diabetes. Cardiol Clin. 2001;19(3):489–505.
Jellis CL, Stanton T, Leano R, et al. Usefulness of at rest and exercise hemodynamics to detect subclinical myocardial disease in type 2 diabetes mellitus. Am J Cardiol. 2011;107(4):615–21.
Hansen D, Dendale P. Modifiable predictors of chronotropic incompetence in male patients with type 2 diabetes. J Cardiopulm Rehabil Prev. 2014;34(3):202–7.
Brubaker PH, Kitzman DW. Chronotropic incompetence: causes, consequences, and management. Circulation. 2011;123(9):1010–20.
Corbelli R, Masterson M, Wilkoff BL. Chronotropic response to exercise in patients with atrial fibrillation. Pacing Clin Electrophysiol. 1990;13(2):179–87.
Coyne JC, Rohrbaugh MJ, Shoham V, et al. Prognostic importance of marital quality for survival of congestive heart failure. Am J Cardiol. 2001;88(5):526–9.
Gwinn N, Leman R, Kratz J, et al. Chronotropic incompetence: a common and progressive finding in pacemaker patients. Am Heart J. 1992;123(5):1216–9.
Lamas GA, Knight JD, Sweeney MO, et al. Impact of rate-modulated pacing on quality of life and exercise capacity—evidence from the Advanced Elements of Pacing Randomized Controlled Trial (ADEPT). Heart Rhythm. 2007;4(9):1125–32.
Dresing TJ, Blackstone EH, Pashkow FJ et al. Usefulness of impaired chronotropic response to exercise as a predictor of mortality, independent of the severity of coronary artery disease. Am J Cardiol. 2000;86(6):602–9.
Elhendy A, van Domburg RT, van Bax JJ, et al. The functional significance of chronotropic incompetence during dobutamine stress test. Heart. 1999;81(4):398–403.
Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37(1):153–6.
Nes BM, Janszky I, Wisloff U, et al. Age-predicted maximal heart rate in healthy subjects: the HUNT Fitness Study. Scand J Med Sci Sports. 2013;23(6):697–704.
Diaz A, Bourassa MG, Guertin MC, et al. Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J. 2005;26(10):967–74.
Okin PM, Lauer MS, Kligfield P. Chronotropic response to exercise: improved performance of ST-segment depression criteria after adjustment for heart rate reserve. Circulation. 1996;94(12):3226–31.
Huebschmann AG, Reis EN, Emsermann C, et al. Women with type 2 diabetes perceive harder effort during exercise than nondiabetic women. Appl Physiol Nutr Metab. 2009;34(5):851–7.
Wilkoff BL, Miller RE. Exercise testing for chronotropic assessment. Cardiol Clin. 1992;10(4):705–17.
Melzer C, Witte J, Reibis R, et al. Predictors of chronotropic incompetence in the pacemaker patient population. Europace. 2006;8(1):70–5.
Brubaker PH, Kitzman DW. Prevalence and management of chronotropic incompetence in heart failure. Curr Cardiol Rep. 2007;9(3):229–35.
Sims DB, Mignatti A, Colombo PC, et al. Rate responsive pacing using cardiac resynchronization therapy in patients with chronotropic incompetence and chronic heart failure. Europace. 2011;13(10):1459–63.
Lauer MS. Chronotropic incompetence: ready for prime time. J Am CollCardiol. 2004;44(2):431–2.
Keteyian SJ, Brawner CA, Schairer JR, et al. Effects of exercise training on chronotropic incompetence in patients with heart failure. Am Heart J. 1999;138(2 Pt 1):233–40.
Matsukawa K. Central command: control of cardiac sympathetic and vagal efferent nerve activity and the arterial baroreflex during spontaneous motor behaviour in animals. Exp Physiol. 2012;97(1):20–8.
Nobrega AC, O’Leary D, Silva BM, et al. Neural regulation of cardiovascular response to exercise: role of central command and peripheral afferents. Biomed Res Int. 2014;2014:478965.
Kiviniemi AM, Tulppo MP, Hautala AJ, et al. Long-term outcome of patients with chronotropic incompetence after an acute myocardial infarction. Ann Med. 2011;43(1):33–9.
De Sutter J, Van de Veire N, Elegeert I. Chronotropic incompetence: are the carotid arteries to blame? Eur Heart J. 2006;27(8):897–8.
Narkiewicz K, Pesek CA, van de Borne PJ, et al. Enhanced sympathetic and ventilatory responses to central chemoreflex activation in heart failure. Circulation. 1999;100(3):262–7.
Choi HM, Stebbins CL, Lee OT, et al. Augmentation of the exercise pressor reflex in prehypertension: roles of the muscle metaboreflex and mechanoreflex. Appl Physiol Nutr Metab. 2013;38(2):209–15.
Schmid A, Huonker M, Barturen JM, et al. Catecholamines, heart rate, and oxygen uptake during exercise in persons with spinal cord injury. J Appl Physiol (1985). 1998;85(2):635–41.
Peinado AB, Rojo JJ, Calderon FJ, et al. Responses to increasing exercise upon reaching the anaerobic threshold, and their control by the central nervous system. BMC Sports Sci Med Rehabil. 2014;6:17.
Tota B, Cerra MC, Gattuso A. Catecholamines, cardiac natriuretic peptides and chromogranin A: evolution and physiopathology of a ‘whip-brake’ system of the endocrine heart. J Exp Biol. 2010;213(Pt 18):3081–103.
Jae SY, Fernhall B, Heffernan KS, et al. Chronotropic response to exercise testing is associated with carotid atherosclerosis in healthy middle-aged men. Eur Heart J. 2006;27(8):954–9.
Fukuma N, Oikawa K, Aisu N, et al. Impaired baroreflex as a cause of chronotropic incompetence during exercise via autonomic mechanism in patients with heart disease. Int J Cardiol. 2004;97(3):503–8.
Savonen KP, Lakka TA, Laukkanen JA, et al. Usefulness of chronotropic incompetence in response to exercise as a predictor of myocardial infarction in middle-aged men without cardiovascular disease. Am J Cardiol. 2008;101(7):992–8.
Phan TT, Shivu GN, Abozguia K, et al. Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3(1):29–34.
Camm AJ, Fei L. Chronotropic incompetence—part II: clinical implications. Clin Cardiol. 1996;19(6):503–8.
Meine M, Achtelik M, Hexamer M, et al. Assessment of the chronotropic response at the anaerobic threshold: an objective measure of chronotropic function. Pacing Clin Electrophysiol. 2000;23(10 Pt 1):1457–67.
Vandergoten P, Vijgen J, Timmermans P, et al. Chronotropic incompetence: a case report. Congest Heart Fail. 2001;7(4):202–4.
Oliveira JL, Goes TJ, Santana TA, et al. Chronotropic incompetence and a higher frequency of myocardial ischemia in exercise echocardiography. Cardiovasc Ultrasound. 2007;5:38.
Freire CM, Moura AL, Barbosa MM, et al. Left ventricle diastolic dysfunction in diabetes: an update. Arq Bras EndocrinolMetabol. 2007;51(2):168–75.
Poanta L, Porojan M, Dumitrascu DL. Heart rate variability and diastolic dysfunction in patients with type 2 diabetes mellitus. Acta Diabetol. 2011;48(3):191–6.
Kitabchi AE, Wall BM. Management of diabetic ketoacidosis. Am Fam Physician. 1999;60(2):455–64.
Salvadori A, Fanari P, Giacomotti E, et al. Kinetics of catecholamines and potassium, and heart rate during exercise testing in obese subjects: heart rate regulation in obesity during exercise. Eur J Nutr. 2003;42(4):181–7.
Charalambous BM, Webster DJ, Mir MA. Elevated skeletal muscle sodium-potassium-ATPase in human obesity. Clin Chim Acta. 1984;141(2–3):189–95.
Mittendorfer B, Fields DA, Klein S. Excess body fat in men decreases plasma fatty acid availability and oxidation during endurance exercise. Am J Physiol Endocrinol Metab. 2004;286(3):E354–62.
Manzella D, Paolisso G. Cardiac autonomic activity and type II diabetes mellitus. Clin Sci (Lond). 2005;108(2):93–9.
Voulgari C, Pagoni S, Vinik A, et al. Exercise improves cardiac autonomic function in obesity and diabetes. Metabolism. 2013;62(5):609–21.
Schonauer M, Thomas A, Morbach S, et al. Cardiac autonomic diabetic neuropathy. Diab Vasc Dis Res. 2008;5(4):336–44.
Pop-Busui R. Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes Care. 2010;33(2):434–41.
Hirsh BJ, Mignatti A, Garan AR, et al. Effect of beta-blocker cessation on chronotropic incompetence and exercise tolerance in patients with advanced heart failure. Circ Heart Fail. 2012;5(5):560–5.
Colucci WS, Ribeiro JP, Rocco MB, et al. Impaired chronotropic response to exercise in patients with congestive heart failure: role of postsynaptic beta-adrenergic desensitization. Circulation. 1989;80(2):314–23.
Witte KK, Cleland JG, Clark AL. Chronic heart failure, chronotropic incompetence, and the effects of beta blockade. Heart. 2006;92(4):481–6.
Clark AL, Coats AJ. Chronotropic incompetence in chronic heart failure. Int J Cardiol. 1995;49(3):225–31.
Kawasaki T, Kaimoto S, Sakatani T, et al. Chronotropic incompetence and autonomic dysfunction in patients without structural heart disease. Europace. 2010;12(4):561–6.
Gentlesk PJ, Markwood TT, Atwood JE. Chronotropic incompetence in a young adult: case report and literature review. Chest. 2004;125(1):297–301.
Munagala VK, Guduguntla V, Kasravi B, et al. Use of atropine in patients with chronotropic incompetence and poor exercise capacity during treadmill stress testing. Am Heart J. 2003;145(6):1046–50.
Ghaffari S, Sohrabi B. Effect of intravenous atropine on treadmill stress test results in patients with poor exercise capacity or chronotropic incompetence. Saudi Med J. 2006;27(2):165–9.
Brinkworth GD, Noakes M, Buckley JD, et al. Weight loss improves heart rate recovery in overweight and obese men with features of the metabolic syndrome. Am Heart J. 2006;152(4):693–6.
Gannon MC, Nuttall FQ. Effect of a high-protein, low-carbohydrate diet on blood glucose control in people with type 2 diabetes. Diabetes. 2004;53(9):2375–82.
Boden G, Sargrad K, Homko C, et al. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med. 2005;142(6):403–11.
Rock CL, Flatt SW, Pakiz B, et al. Weight loss, glycemic control, and cardiovascular disease risk factors in response to differential diet composition in a weight loss program in type 2 diabetes: a randomized controlled trial. Diabetes Care. 2014;37(6):1573–80.
Jenkins DJ, Wong JM, Kendall CW, et al. Effect of a 6-month vegan low-carbohydrate (‘Eco-Atkins’) diet on cardiovascular risk factors and body weight in hyperlipidaemic adults: a randomised controlled trial. BMJ Open. 2014;4(2):e003505.
Wong CY, Byrne NM, O’Moore-Sullivan T, et al. Effect of weight loss due to lifestyle intervention on subclinical cardiovascular dysfunction in obesity (body mass index >30 kg/m2). Am J Cardiol. 2006;98(12):1593–8.
Poirier P, Hernandez TL, Weil KM, et al. Impact of diet-induced weight loss on the cardiac autonomic nervous system in severe obesity. Obes Res. 2003;11(9):1040–7.
Brinkworth GD, Noakes M, Clifton PM, et al. Effects of a low carbohydrate weight loss diet on exercise capacity and tolerance in obese subjects. Obesity (Silver Spring). 2009;17(10):1916–23.
Miossi R, Benatti FB, Luciade de Sa PA, et al. Using exercise training to counterbalance chronotropic incompetence and delayed heart rate recovery in systemic lupus erythematosus: a randomized trial. Arthritis Care Res (Hoboken). 2012;64(8):1159–66.
Adams BJ, Carr JG, Ozonoff A, et al. Effect of exercise training in supervised cardiac rehabilitation programs on prognostic variables from the exercise tolerance test. Am J Cardiol. 2008;101(10):1403–7.
Morton RD, West DJ, Stephens JW, et al. Heart rate prescribed walking training improves cardiorespiratory fitness but not glycaemic control in people with type 2 diabetes. J Sports Sci. 2010;28(1):93–9.
Acknowledgments
We thank Dr Inez Wens (Rehabilitation Research Center [REVAL], BIOMED, Faculty of Medicine and Life Sciences, Hasselt University, Diepenbeek, Belgium) for support and time-saving advice regarding certain aspects of this article. No conflicts of interest are reported. No sources of funding were used in the preparation of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keytsman, C., Dendale, P. & Hansen, D. Chronotropic Incompetence During Exercise in Type 2 Diabetes: Aetiology, Assessment Methodology, Prognostic Impact and Therapy. Sports Med 45, 985–995 (2015). https://doi.org/10.1007/s40279-015-0328-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-015-0328-5