Abstract
Various experimental or clinical studies in the literature have demonstrated the benefits of stem cells, especially mesenchymal stem cells. This chapter will focus on existing stem cell technologies and their applications in the treatment of wound healing. To understand mechanisms of stem cells in wound healing, we will go over the structure of most commonly wounded tissues, namely, skin, which consist of epithelia and connective tissue. Then we will explain wound healing process with three stages as inflammation, proliferation, and remodeling and the roles of tissue components during wound healing. Treatment of wounds is directly related with the cause, location, and degree of the wound. Therefore, we will describe common wound types with causes under two categories as acute and chronic. We will explain stem cell types which play an important role in tissue regeneration and wound healing in adult life owing to their high competency and self-renewal features. Here, we will give examples of various studies on various wound types with or without underlying diseases. Finally, we will describe recently developed methods such as the use of cells' own products, exosomes, and demonstrate the results of exosome therapies on chronic wounds with most recent examples on human trials.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Stem cells are capable to form an organism starting from a fertilized egg and have the ability of unlimited proliferation, self-renewal, and differentiation into the cell types of target tissues they were transferred. One of the most important aspects of stem cells is differentiation which plays the key role in development of multicellular organisms. Differentiation is defined as the overall set of changes of cell phenotype with the effect of cytokines, growth factors, extracellular matrix (ECM) proteins, and intercellular signaling pathways. Stem cell transplantation is more accurately defined as cell therapy is the use of stem cells or their products, derived from their patient’s own tissues or from another donor for the treatment of tissue damage, various diseases, or loss of function. Cell therapies provide hope for many cases we cannot adequately treat with conventional medical methods so far. Along with their differentiation ability, stem cells have immunoregulatory, anti-inflammatory, antiapoptotic, anti-scarring, and neovascularization induction functions which are used for joint problems, some neurological and autoimmune diseases, as well as for the treatment of muscular-neurodegenerative disorders. More recently some nanoparticles secreted by stem cells such as exosomes or secretomes were shown to induce different pathways such as transdifferentiation when transferred into tissue microenvironment.
Use of stem cells in wound healing is among the numerous medical methods where the cell therapies have benefits. Wounds can be acquired during surgical incisions or accidents or may occur due to various conditions like aging, burns, or diseases such as diabetes. Healing of the wound is a highly complex process, requires cooperation of various cell types from different tissues such as epithelia, connective tissue, and blood cells. Coagulation, inflammation, and anti-inflammatory processes follow each other with the continuous degradation and formation of ECM. Growth factors, cytokines, and ECM components play important roles during wound healing, and interruption of these steps may cause nonhealing chronic wounds. This chapter will focus on existing stem cell technologies and their application in the treatment of wound healing along with recently developed methods such as use of stem cells' own products, exosomes. To understand mechanisms of stem cells, we will briefly go over the structure of most commonly wounded tissues, namely, skin, which consist of epithelia and connective tissue. We will explain the roles of tissue components during wound healing, describe common wound types with causes, and finally explain the results of stem cell therapies on wounds with most recent examples on human trials.
2 Histology of Common Wound Areas
Wounds generally occur externally through the skin, although some wounds such as ulcers may develop internally. Other limited numbers of wound types may happen in cartilage tissues, tendons, or ligaments (Paz and West 2014). Almost all internal or external wounds occur in the epithelia and underlying connective tissue (Marieb 1995). Deep wounds may reach other tissues beneath the connective tissue layer such as muscle, deeper blood vessels, and bone, which are also surrounded by connective tissue layers. Serious bone fractures create wounds that may reach the skin, made by the bone itself (Ertekin et al. 2005). Therefore, knowledge of the histologic structure of epithelia, underlying connective tissue, and, more specifically, skin, plays a key role in cellular therapies of both acute and chronic wounds (Metcalfe and Ferguson 2007).
2.1 Histology of Skin
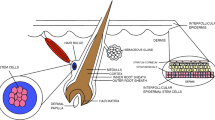
Skin and its derivatives (hair, nails, glands) are the largest organ of the human body. It covers the external surface of and constitutes up to 15–20% of the total body mass. Other than its barrier function, skin has immunologic, sensory, and endocrine functions, provides excretion via various glands, and helps maintain homeostasis (Weinzweig 1999). Skin consists of two main layers, the epidermis layer, formed of epithelia, and the dermis layer, which is formed by connective tissue (Vaccari et al. 2005).
2.1.1 Epidermis
The epidermis is the upper, epithelial part of the skin, composed of keratinized stratified squamous epithelium. Epidermal cells called keratinocytes are arranged as five layers, distinct by their shapes and characteristics although they are from the same origin. Proliferation starts from the stratum basale , the deepest of the five layers of epidermis, which lies on the basal lamina. Keratinocytes at this layer continuously proliferate, and daughter cells move upward and change shape and cellular features to maintain their functions (Singer et al. 2013).
Wound regeneration requires the stratum basale layer for the formation of other parts of the epidermis (Blumberg et al. 2012). Proliferation remarkably increases with the wound formation, and basal cells begin migration across the wound surface within 8–18 h of wound formation. The speed of migration may be as high as 0.5 mm/day.
The upper layers provide a barrier function to water, light, bacteria, and other particles. Keratin filaments begin accumulating as cells and they move upward and finally lose their nuclei and organelles and become filled with keratin at the topmost layers.
The epidermis also contains other cell types such as melanocytes, Langerhans, and Merkel’s cells. Langerhans cells are associated with the immune system and function as the antigen-presenting cells of the skin. Merkel’s cells are sensory cells responsible for cutaneous sensation; therefore, they are mostly accumulated at the fingertips. Free nerve endings are also intersparsed between keratinocytes of epidermis to receive various sensory modalities such as heat, cold, and fine touch. Melanocytes are dendritic cells located among the keratinocytes of the stratum basale but extend their cytoplasmic processes to the upper parts of the epidermis. The main function of melanocytes is producing the pigment, melanin, which protects the organism against the damaging effects of ultraviolet radiation (Pawlina 2016; Norton et al. 2003). Newly formed skin at the wound area cannot perform its all duties such as sensory, immunologic, or (UV) barrier functions, without these cell types, and shows discoloration, which causes unwanted cosmetic problems. Therefore, appropriate addition of these cell types among keratinocytes must be considered in cellular therapy methods.
2.1.2 Dermis
The dermis is the connective tissue part of the skin, which nourishes the avascular epidermis via its numerous small blood vessels and increases the thickness of the skin for mechanical support. Fibroblasts are the main cell type of connective tissue, which conduct the main functions such as extracellular matrix (ECM) production and tissue regeneration. The ECM primarily consists of type I collagen fibers along with less present collagen types such as type III (Pawlina 2016). Type I collagen is always the most important component; however, the thinner but stronger type III collagen becomes more prominent during wound healing and plays key roles. Synthesis and posttranslational modifications of collagen fibrils depend on various systemic and local factors such as oxygen support; adequate nutrition, especially with vitamin C; and the local micro-environment (Charles Brunicardi et al. 2005).
Another fiber type is the elastic fibers, which are responsible of the elasticity of the skin. Glycosaminoglycans (GAGs) are made of protein and carbohydrate components and perform many functions of the ECM such as entangling water molecules, thereby creating a highly liquid-like environment. Other important functions of the ECM are the formation of a 3D environment for cell migration, facilitating diffusion of nutrients, and helping tissue regeneration and development via attached growth factors and cytokines (Pawlina 2016; Norton et al. 2003). The most important GAGs are dermatan sulfate and chondroitin sulfate, which are synthesized by fibroblasts at increasing amounts in the 3 months following an injury. GAGs attach proteins to form proteoglycans. Collagens and proteoglycans actively interact, and proteoglycans become entrapped within collagen fibers because the amount of collagen increases with time, especially at the last step of wound healing (Xue and Jackson 2015).
The epidermis overlays and sticks to the dermis via the basement membrane. Additionally, wavelike protrusions called dermal papillae, which contain numerous capillaries to nourish the avascular epidermis layer, increase the attachment. The dermis is also formed by two layers, the papillary and reticular layers. The superficial papillary layer is composed of loose connective tissue with abundant cell types, collagen type I and III, and elastic fibers. The reticular layer contains less cells, changes in thickness throughout the body, but always remains thicker than the upper papillary layer. Type I collagen and elastic fibers are also thicker and present as irregular bundles in this layer. However, these bundles form regular lines called Langer’s lines in regions of tension. Langer’s lines are especially important for skin surgeries; incisions made parallel to Langer’s lines leave the least scar marks while healing. Adipose tissue layers, smooth muscle, and sometimes striated muscle can be found beneath the reticular layer. Encapsulated nerve endings such as Pacinian corpuscles are also present within the dermis, which detect pressure, Meissner’s corpuscles, which are sensitive to light touch, and Ruffini’s corpuscles, which detect stretching of the skin.
The hypodermis, also known as the subcutaneous fascia in anatomy, is the adipose tissue-containing part of the skin, which lies underneath of dermis layer. The main function of the hypodermis is mechanical protection and heat isolation. Adipose tissue within the hypodermis also functions as energy storage units. Therefore, the hypodermis thickens with nourishment or in cold climates. The hypodermis also participates in hormonal regulation via its adipose tissue-secreted factors .
The skin also contains epidermal appendages such as nails, hair follicles, sweat glands, and sebaceous glands, which produce an oily substance called sebum that is thought to have bacteriostatic, barrier, and pheromone functions. Hair follicles cover almost the entire body. Even though their roots may be in very deep regions of the dermis, they are continuous parts of the epidermis. Epidermal stem cells residing within the root sheath of hairs are responsible for hair growth (Pawlina 2016; Norton et al. 2003). These stem cells may form new epidermis in some cases if the epidermal layer is destroyed by trauma or removed during surgery, while being protected due to their deeper location. However, extensive wounds such as third-degree burns may destroy the dermis and epidermis completely and require grafting for healing. Other than hair transplant surgeries, hair follicle stem cells are also important for their clinical implication in skin regeneration (Singer et al. 2013; Leirósa et al. 2014).
Skin thickness varies from 1 to 5 mm. The skin on the palms of the hands and soles of the feet are hairless and have thicker epidermis and are therefore called thick skin, whereas other parts of the skin have thinner epidermal layers and contain hair follicles (Pawlina 2016). Superficial wounds of the epithelium heal without scar formation, but wounds of the dermis may require surgical intervention or other medical treatments to avoid scar formation (Weinzweig 1999).
2.2 Histology of Other Epithelia
Although most injuries harm the body from outside to inside, some acute or chronic wounds can occur within the body going outward. Usually the first affected tissue is again the epithelia, even though the injury may have occurred within a very large organ like the stomach, which could result from swallowing sharp materials or as tiny as inside blood capillaries (Ertekin et al. 2005). Epithelia cover all open surfaces, canals, glands, and ducts of the body. Similar to skin, various epithelia of the body contain unipotent stem cells, which reside in the basal compartment and constantly regenerate epithelia. The height and lamination of epithelia differ depending on the organ, which can be as thin as a single thin layer of squamous cells like alveolar epithelia of lungs, or multiple layers of cells of various shapes, such as can be seen within the oral cavity, bladder, vagina, or anus. The shape of the cells at the top layer gives epithelia its names, e.g., columnar, cuboidal, or squamous epithelia, along with being simple with only one layer or stratified with many layers. Most of the digestive tract is formed by simple columnar epithelia, whereas the skin and oral cavity are formed by stratified squamous epithelia. The regeneration rate of these various epithelia also differs according to organ and function. Epithelia of the stomach and intestines continuously regenerate via their stem cells, which reside in the deeper parts of gastric pits or intestinal villi; the regeneration rate of glandular epithelia in other parts of the body is generally much slower.
Like the skin, internal epithelia require a connective tissue support that connects it to the deeper layers of the organ and also provides nourishment. The underlying connective tissue of epithelia called lamina propria, is similar to the dermis of the skin, except being thinner. The amount of vascular support effects the regeneration rate of epithelia, as well as the lamina propria. Poorly oxygenated tissues such as cartilage regenerate much more slowly than epithelial wounds because they receive less nutrition (Pawlina 2016; Norton et al. 2003). Vascularization becomes higher than normal during wound regeneration, which decreases to the normal levels at the last step of wound healing (Blumberg et al. 2012). The lamina propria lies on top of various tissues according to the region and function, such as smooth or stratified muscle, glands, cartilage, or bone. Each tissue has its own regeneration system. However, mesenchymal stem cells (MSC) and pericytes play a major role along with tissue-specific unipotent stem cells like the osteoprogenitor cells of bone (Pawlina 2016).
3 Wound Healing Process
Wound is defined as the disruption of tissue integrity, which consists of a series of cellular and biochemical events, starts with trauma, and ends with new tissue formation. These events overlap each other and follow certain steps. These steps of wound healing are defined as:
-
1.
Hemostasis and inflammation
-
2.
Proliferation
-
3.
Maturation (remodeling)
Although these stages are sequential, there is no distinct border between them, and various stages can be observed simultaneously in different regions of the wound area (Ertekin et al. 2005; Norton et al. 2003).
3.1 Hemostasis and Inflammation
Clean surgical incisions and many other wounds begin the healing process with naturally occurring blood clotting. Platelets of damaged blood vessels make direct contact with the ECM at the injured site. Contact of platelets with subendothelial collagen causes platelets to aggregate and degranulate and starts the coagulation cascade. The clot contains an important fiber called fibrin, along with blood cells. This structure closes the wound surface, protects against infection, and helps subsequent inflammatory and healing processes by providing a 3D ultrastructure for movement of migrating cells. Increased capillary permeability, local release of chemoattractants such as prostaglandins, complement factors, interleukin 1 (IL-1), transforming growth factor-beta (TGFβ) , and tumor necrosis factor-alpha (TNFα) induce neutrophil growth. Bacterial products also serve as chemoattractants if the wound site is infected (Duque and Descoteaux 2014).
The next phase, inflammation, starts around 24 h after injury. During the inflammatory step, dead tissues are cleared along with fibrin, followed by the deposition of new extracellular matrix (ECM) molecules. Neutrophils and monocytes infiltrate into the injured area at the beginning of inflammation, which peaks around the first to second day after injury. These leukocytes are the main source of TNFα, one of the key factors for angiogenesis and collagen synthesis; however, leukocytes do not involve in collagen synthesis or contribute toward mechanical strength. Later at this step, migrated monocytes differentiate into macrophages, which play a key role in successful healing. Macrophages replace neutrophils, reaching maximum numbers around 48–96 h after injury, increase the removal rate of dead tissues, and remain until the end of the healing process. Other functions of macrophages are cell proliferation regulation, matrix production, and angiogenesis through secreted mediators such as vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), epithelial growth factor (EGF), and TGFβ (Norton et al. 2003; Nandy and Mukhopadhyay 2011).
T lymphocytes are present in lower numbers than macrophages in the wound area and peak around one week after injury. They mediate transition from the inflammatory phase to the proliferation phase. The amount and strength of collagen in a wound decrease with the increase of T lymphocytes. The selective decrease of CD8+ cells increases wound healing; the decrease of CD4+ cells has no effect (Barbul et al. 1989).
3.2 Proliferation
Proliferation is the second step of wound healing, which starts between the 4th and 12th days. Fibroblasts and endothelial cells join the healing process, attracted mainly via PDGF and VEGF, respectively. After they reach the wound area, fibroblasts must proliferate and become activated in order to execute their primary function of matrix production. Cytokines and growth factors secreted by macrophages induce fibroblast activation. Fibroblasts isolated from wounds have been shown to synthesize more collagen than controls, proliferate less, and perform active matrix contraction. Endothelial cells of the damaged capillaries also start to proliferate, migrate, and contribute to angiogenesis under the influence of various cytokines and growth factors such as TNFα, TGFβ, and VEGF. Wounds appear to be in reddish color at this stage and are called granulation tissue due to the numerous newly formed capillaries. These vessels accelerate healing until the end of the process, and then redundant vessels disappear, leaving tissue with a normal vascularization rate.
3.3 Contraction of Wound
All wounds contract with time in order to bring wound sites together. Granulation tissue contains mainly fibroblasts, myofibroblasts, and various other connective tissue cells along with numerous newly formed small blood vessels. TGFβ stimulates the differentiation of fibroblasts into myofibroblasts, which have a key role in wound gap closure with their contractile abilities provided by alpha-smooth muscle actin molecules (α-SMA) within their cytoplasm. Fibroblasts and myofibroblasts recognize the stress lines of the wound and localize on this lines. Myofibroblasts apply steady force along the gap, shorten current ECM fibers, and slowly pull the sides of the wound together. Meanwhile, fibroblasts and myofibroblasts produce new collagen and other ECM molecules.
After one week, inflammatory cells , fibroblasts, and myofibroblasts undergo apoptosis, which leaves scar tissue containing mostly connective tissue fibers and few cells. New-forming epidermis covers the surface of the scar during normal healing of small wounds. However some pathologic conditions, such as hypertrophic scar or keloid formation, connective tissue-forming cells cannot completely undergo apoptosis and remain at the wound area. Hypertrophic scars have excessive connective tissue formation with little or no epithelial cover. These types of scars form white, protruding scar marks; however, the borders remain within the wound area. Keloids, on the other hand, are scar tissues that go beyond the wound borders of initial scar with no reduction in connective tissue formation. Another difference in keloids is the absence of myofibroblasts. Persistent myofibroblast activity also causes continuous pulling of tissue, which may cause fibromatosis like in Dupuytren’s disease (Pawlina 2016). Hypertrophic scars are not related with skin pigmentation; however, keloid formation is more common in the African-American population. Scar formation risk is also higher in individuals with darker skin than lighter-skinned people (Ertekin et al. 2005; Weinzweig 1999).
3.4 Maturation and Remodeling
Maturation and remodeling of wound tissue start at the fibroblastic stage and are related with the rearranging of the previously synthesized collagen fibers. Matrix metalloproteinases are responsible for the breakdown of collagen, and net amount of collagen is balanced between collagenolysis and new collagen formation. During the remodeling phase, degradation of the ECM decreases, and the balance shifts in favor of production. However, deposition of large amounts of collagen also causes relatively acellular scar tissue formation. EMC deposition in the wound area follows certain steps: fibronectin and type III collagen constitute the early matrix, followed by glycosaminoglycans, proteoglycans, and, finally, type I collagen (Ghatak et al. 2015). The amount of collagen plateaus a few weeks after wound formation, but the tensile strength continues to rise for a few more mouths. However, the maximum rate of tensile strength of a healed wound remains at 80% of the pre-scar tissue. Remodeling of scar tissue continues its maturation for 6 to 12 more months (Weinzweig 1999).
3.5 Epithelization
While rebuilding the integrity of the connective tissue, the outlining epithelial barrier must also be completed. Incisional wounds re-epithelize within 48 h if the wound sides are stitched together; however, major epidermal and dermal loses take much longer to heal. Regeneration of the epidermal layer is characterized by the migration of neighboring epithelial cells. The epidermis at the rim of the wound area thickens following wound formation, some keratinocytes at the stratum basale lose cellular adhesion with the neighbor cells and basal membrane, enlarge, and migrate along the temporary wound matrix, while others rapidly proliferate without leaving their position. This proliferation continues until the defect has been closed. After closure, shapes of proliferating cells become columnar again, and epidermal layers reform. The last step of epithelization is keratin deposition (Weinzweig 1999; Pawlina 2016; Norton et al. 2003) Fig. 10.1.
Illustration of wound healing stages; (A) hemostasis and inflammation, (B) proliferation, and (C) maturation (remodeling). (a) During the first step of wound healing, blood clot fills the wound gap and entraps leaked erythrocytes. Leucocytes migrate to initiate inflammation, while epithelial cells of the adjacent stratum basale layer start to proliferate. (b) Macrophages phagocyte fibrin, dead cells, and bacteria, while proliferated fibroblasts produce new collagen bundles. (c) Epithelia reform with all layers, and underlying dermis matures into the functioning connective tissue
4 Wound Types
Wounds can be classified under two categories as acute and chronic. Acute wounds heal on time more regularly at cellular level, and anatomic and functional integrity is better maintained. However, healing of chronic wounds takes consderably more time than acute wounds; therefore, anatomic and functional amelioration cannot be properly repaired (Arya et al. 2014).
4.1 Acute Wounds
An acute wound can be formed during a surgery procedure which are done under sterile conditions, neat, and more likely to heal. However, most wounds are not that cleanly formed, cover more surface, and fail to heal properly, proportional with the deepness and surface area of the wound. Burns, occurred by heat or other causes like chemicals or electricity, usually fall into this category and carry a serious, even life-threatening risk if not medically tended due to the fluid loss or infection. We will briefly discuss these wound types with their treatment methods before explaining the outcomes of stem cell treatment on acute wounds.
4.1.1 Cuts and Surgical Incisions
Cuts that may occur under surgery, called incisions, are usually made with a sterile scalpel, cautery pen, or laser which forms neat wounds. Cuts with sharp objects like knife or glass may also cause to formation of neat wounds. Damage done by scalpels and lasers is minimal; however, despite their convenience, incisions made with cautery pens which can be categorized under burns cause more damage to the tissues (Ertekin et al. 2005; Charles Brunicardi et al. 2005).
Medical treatment of cuts universally made via stitching which brings the two sides of the wound together minimalizes the gap, which should be filled with inflammatory tissue if not stitched therefore skips the pulling process of myofibroblasts and causes faster and effective healing. Different stitching methods and techniques exist which are selected by the surgeon according to wound type, location, and shape (Weinzweig 1999).
4.1.2 Burns
Various causes such as heat, cold, electricity, acids, bases, and various other chemicals, such as phosphorus compounds, may cause burns. Therefore, the reaction of the body varies along with treatment methods (Weinzweig 1999).
Burns are dynamic and invasive wounds that can cause maximum tissue loss due to necrosis; therefore, they are not considered as ordinary wounds. Lesser but serious damage such as ischemia may occur outside regions of necrosis. Although the exact mechanism of ischemia transition to necrosis remains unknown, it is probably caused by disruption of the cell cycle due to oxidative stress or infection (Cavanagh et al. 2012).
Cells can tolerate relatively high temperatures; however, cellular changes overwhelm repair mechanisms over 45 °C due to protein denaturation, even though cells do not have a static response and react differently to temperature increases. The depth and surface area of burns also affect wound healing. Burns covering more than 15–20% of the body surface affect other non-burnt areas as well as underlying organs, as such they are considered systemic injuries or disease. The standard classification of burns is well known as first- to third-degree burns. First-degree burns affect the epidermis, and second-degree burns reach the dermis layer. These types of burns are painful due to the affected nerve endings and generally leave a permanent scar. Third-degree burns reach beneath the skin. Patients do not feel pain because of the complete destruction of nerve endings but lose all sensory stimulation from these areas.
Burns wound tissues in two steps. The first step is the immediate cell damage and coagulation necrosis caused by heat. The second step is delayed progressive cell damage caused by ischemia, which occurs 24–48 h after the initial damage. These two steps can be observed in the burnt area. The necrotic area is covered by an ischemic (stasis) zone. Stasis zone also becomes necrotic during delayed damage step. Another third area, called hyperemia zone, can also be observed around the stasis zone which is characterized by inflammation and vasodilatation. The hyperemia zone heals faster, within 7–10 days. If delayed damage of the ischemia zone can be prevented via pharmaceutical agents or another method such as stem cell treatment. Necrosis and deepening of progressive burns can be prevented and can be healed faster. Burns smaller than 3–5 mm heal within 3–4 weeks, but larger burns require grafting. Chemical burns can form through external contact (Ertekin et al. 2005) or accidental ingestion of harmful substances such as alkali cleaning agents and cause epithelial burns in digestive tract, especially at esophagus epithelia. Chemical burns may be classified under four groups as acid, alkali, phosphorus burns, and chemical injections, which require slightly different treatment methods (Norton et al. 2003).
4.1.3 Grafting Methods
Grafting techniques , used in burns or other wound types, include transplantation of a part of a patient’s own skin, taken from another appropriate area of the body or artificial grafts. However, grafting is not 100% successful and may cause the formation of two scarred areas instead of having just one original wounded site. On the other hand, artificial grafts, or artificial skin, are quite useful when donor skin areas are small or need to be decreased. Artificial grafts are matrices that constitute GAGs and collagen fibers, which function as dermis and therefore create a 3D network for wound healing while protecting the wound area from heat and fluid loss and infections via additional materials such as silver. Epithelization of artificially grafted areas can be enhanced by grafting thinner epidermal grafts of a patient’s own tissue, which is considerably smaller than full skin grafts. Artificial grafts can be obtained as allografts and xenografts (Ertekin et al. 2005; Norton et al. 2003).
4.2 Chronic Wounds
Wounds that do not heal within 3 months are called chronic wounds. They can be classified under three categories as diabetic wounds, decubitus ulcers, and wounds caused by venous hypertension.
Evidence for the treatment of chronic ulcers in the elderly is still limited. Dressings containing calcium alginate may shorten healing period of pressure ulcers in the elderly; however, other dressings in this age group are not studied sufficiently (Madec et al. 2009a).
4.3 Diabetic Wounds
Diabetes mellitus (DM) is a complex, chronic metabolic disorder which affects almost all age groups. Diabetes mellitus affects several organs, including the muscle, skin, heart, brain, and kidneys, and requires continuous medical care beyond glycemic control due to prolonged and uncontrolled diabetes may lead various complications which are divided generally into microvascular complications and macrovascular complications due to damages to small blood vessels and arteries, respectively.
Patients with diabetes are prone to the development of chronic wounds, especially diabetic foot ulcers (DFUs) , due to the deficiencies in either peripheral tissue homing and engraftment of bone marrow or endothelial progenitor cells. Additionally, diabetes impairs wound healing at various steps. During the proliferation step, macrophage number and activation decrease which results in the reduced lymphatic vessel formation. Diabetes mellitus also affects signaling mechanism, responsible for coordinating/regulating angiogenesis and vasculogenesis. DFU is considered as a major source of morbidity and a primer cause of hospitalization in diabetic patients. DFUs are caused by neuropathic, ischemic, or combined neuroischemic abnormalities. The most common pathway to develop foot problems in diabetic patients is autonomic and peripheral sensorimotor neuropathy that causes foot deformities, high foot pressure, and gait instability, which increases the risks of developing ulcers. Primary management goal for DFU is to obtain wound closure as fast as possible. Proper education of the patient and blood sugar control are the initial techniques for DFU therapies. Most common therapy method is debridement, which is the removal of necrotic tissues as well as foreign and infected materials from the wound. Different kinds of debridement exist such as surgical, enzymatic, autolytic, mechanical, and biological (use of maggots of the green bottle fly). Debridement decreases in the possibility of limb amputation; however, all debridement methods have their own drawbacks. Dressings are highly important in the treatment of DFU, which vary according to the classification and degree of DFU. Other advanced therapies such as hyperbaric oxygen treatment, negative pressure, or artificial skin grafts are also in use for DFU (Arya et al. 2014; Yazdanpanah et al. 2015). We will discuss in detail the use of stem cells in DFU treatment with examples from both literature and our studies.
5 Stem Cells
Stem cells can be classified according to their source or differentiation potential. Stem cells are acquired from early-stage embryos called embryonic stem cells (ESCs) , whereas adult stem cells (ASCs) can be obtained from adult tissues, as well as later-stage embryos or fetuses (Ozturk and Karagoz 2015). ESCs, which are acquired from later-stage embryos, generally from the inner cell mass of blastocysts, are characterized by their ability to form colonies when cultured, superior proliferation ability, and, most importantly, in vitro and in vivo differentiation into all types of cells of three germ layers. They can form chimeric organisms when transferred into early-stage embryos (not applicable for humans) and can form embryonic bodies when cultured in appropriate conditions. In contrast, ASCs have a limited differentiation capacity but still can self-renew (Maehr et al. 2009; Melton and Cowan 2009; Draper et al. 2007).
Another classification of stem cells is based on their differentiation ability, which is called potency. Most potent cells are called totipotent stem cells, including zygote and first blastomeres, which can differentiate into all types of cells present within the body and also placenta. Pluripotent stem cells (PSCs) , like ESCs, can differentiate into all cell types except placental cells. PSCs can be acquired more conveniently by reprogramming somatic cells back to the embryonic stage via viruses (Takahashi and Yamanaka 2006) or other methods (Polo et al. 2010). Different types of pluripotent cells also exist such as embryonic carcinoma cells and embryonic germ cells. Cells generated via reprogramming are called induced pluripotent stem cells (iPSCs) and do not carry the ethical problems of ESCs because they do not require human embryos (Balbach et al. 2009).
Pluripotent human stem cells are characterized through their surface markers, stage-specific embryonic antigen-3 (SSEA-3) , SSEA-4, and keratan sulfate proteoglycans TRA-1-60 and TRA-1-81, which are also useful for sorting these cells. Pluripotent stem cells also express key pluripotency genes; Oct4 and Nanog (Draper et al. 2007; Harrison et al. 2011). A limited but increasing number of clinical trials can be found for PSC; however, a less potent but more easily accessible stem cell type, multipotent stem cells, has been used for a quite more number of clinical trials (Yolanda et al. 2014).
Multipotent stem cells are more specialized stem cells that can still differentiate into various cell types such as neural, adipogenic, osteogenic, and chondrogenic cells, as well as blood cells. Hematopoietic stem cells (HSC) reside within bone marrow and continuously produce all types of blood cells. MSCs are the most common multipotent stem cell type within the body, except bone marrow (Gupta et al. 2016 and Xue et al. 2013). Other than differentiation into various cell types, MSCs play key roles in the regeneration of adult tissues and have functions such as immunoregulation, angiogenesis, and epithelialization augmentation. MSCs have been shown to accelerate wound closure and correct chronic wound inflammation, as such they carry great promise in wound healing (Khosrotehrani 2013; Duscher et al. 2016; Lee et al. 2016). Though stem cells are known for their ability to home damaged areas within the body and differentiate into required cell types, only a small percentage of these survive in the host organism. However, latest research emphasizes the paracrine effects of MSCs , which will be detailed later in this chapter (Jayaraman et al. 2013).
6 Stem Cell Therapies on Acute Wounds
Shumakov et al. were the first to use mesenchymal bone marrow-derived stem cells (BM-MSC) in burn wound healing and compared them to embryonic fibroblasts on rats (Ghieh et al. 2015). Where BM-MSCs were applied, wounds showed decreased cell infiltration of the wound and an accelerated formation of new vessels and granulation tissue in comparison with embryonic fibroblasts and controls. Another study done by Rasulov et al. on rats also showed the superiority of stem cells in burn wound healing (Ozturk and Karagoz 2015). In another study, the application of MSC on burns reduced cell infiltration, improved neoangiogenesis, and reduced the formation of granulation tissue (van Zuijlen et al. 2015).
7 Stem Cell Therapies on Chronic Wounds
Various experimental or clinical studies in the literature have demonstrated the benefits of stem cells, especially MSCs. Here we will give examples of various studies on various wound types with or without underlying diseases.
7.1 Stem Cell Therapies for Ischemic Wounds
Ischemia, related to the various diseases such as Buerger’s, may cause the occlusion of arteries at the extremities and conclusively, ischemia and necrosis of related tissues. Untreated ischemia may cause chronic wounds in these patients, which are difficult to heal due to low nourishment and cellular support. Stem cell therapies have shown clinical improvement in these disease in both double-blinded randomized studies (Shumakov et al. 2003), phase I (Lu et al. 2011a) or phase II clinical trials (Bura et al. 2014).
In a study study included 15 male patients with critical limb ischemia (CLI) Rutherford’s class II-4, III-5, or III-6 and patients with ischemic resting pain in one limb with/without nonhealing ulcers and necrotic foot. Adipose tissue-derived mesenchymal stem cells (ATMSCs) were isolated from adipose tissue of patients with thromboangiitis obliterans (TAO) , otherwise known as Buerger’s disease (B-ATMSC), patients with diabetes (D-ATMSC), and healthy donors (control ATMSC). In a colony-forming unit assay, the stromal vascular fraction of patients with TAO and diabetes yielded lesser colonies than those of healthy donors. D-ATMSCs showed lower proliferation ability than B-ATMSCs and control ATMSCs but showed similar angiogenic factor expression to control ATMSCs and B-ATMSCs. Multiple intramuscular ATMSC injections caused no complications during the follow-up period (mean follow-up time: 6 months). Clinical improvement occurred in 66.7% of patients. Five patients required a minor amputation during follow-up, and all amputation sites healed completely. At 6 months, significant improvement was noted on pain rating scales and in claudication walking distance. Digital subtraction angiography before and 6 months after ATMSC implantation showed formation of numerous vascular collateral networks across affected arteries (Lee et al. 2012).
7.2 Stem Cell Therapies for Diabetic Wounds
Diabetes is a common medical problem in modern communities. Diabetic foot ulcers (DFUs) are the significant complications of diabetes, affect the life quality, and can be life-threatening. DFUs are a chronic significant health problem which presents with nonhealing wounds on foot (Fritschi 2001; Sener and Albeniz 2015). Various studies indicated that the prevalence of diabetic foot ulcer is around 2% (Besse et al. 2011). About 15% of diabetic patients have a tendency of developing diabetic foot ulcer throughout their life. Therefore, clinical studies carry great importance in fighting against diabetes. MSC has been used to produce insulin-releasing cells, fighting against autoimmunity, to provide islet compatibility and their living, and in treatment of diabetic ulcers and arm and leg ischemia (Karnieli et al. 2007; Madec et al. 2009b; Fiorina et al. 2009; Ding et al. 2009; Berman et al. 2010; Lu et al. 2011b). Studies have also shown that MSCs may also repair the glomerular cells which are damaged due to acute renal failure (Morigi et al. 2004; Black and Woodbury 2001; Jiang et al. 2010). These findings show that clinically, the MSCs may be used in treatment of diabetic foot ulcers.
Li XY et al. transplanted human cord blood mesenchymal stem cells (hCB-MSC) to patients diagnosed as having type 2 diabetes and diabetic foot ulcer and evaluated the Tregs/Th17/Th1 cell distribution. hCB-MSC was injected directly into quadriceps muscle in diabetic foot ulcer patients, and the rates of Treg/Th17, Treg/Th1, and Th17/Th1 cells were calculated in flow cytometry. In addition, their correlation with various cytokines (FoxP3, IL-17, INF-γ, C-RP, TNFα, and VEGF) was evaluated. There was a significant increase in the rates of CD4+CD25hiFoxP3+ Treg/Th17 and CD4+CD25hiFoxP3+ Treg/Th1 cells 4 months after transplant; the rates of Th17/Th1 cells did not change (Li et al. 2013). This data is supportive for Treg T cells in the initiation and proceeding of T2D (Lau et al. 2009a).
Plasticity of MSC is the basic feature of stem cells for use in treatment of disorders. hCB-MSC has differentiated into epidermal cells within the microenvironment of skin injuries. Also several existing bioactive factors, including cytokines and chemokines, can transform stem cells into skin cells in the wound region. Thus, both methods may enhance the recovery rate of injury and the quality. Conversely, MSC are reported to inhibit wound inflammatory reaction. MSCs have been reported to regulate IL-10, TGF-B1, IL-6, and TSP-1 constructs via paracrine pathway in the chemically burned cornea model, which does not result in healing enhancement.
Type 2 diabetes mellitus (T2DM) is one of the most complex and common types of diabetes. The most frequently linked factors to insulin resistance that progress to T2DM include obesity, aging, cell dysfunction, tissue lipid accumulation, oxidative stress, endoplasmic reticulum stress (ER stress) in cells, tissue inflammation, and physical inactivity (Akash et al. 2013). In a diabetes study, Li et al. transplanted hCB-MSCs into patients with T2DM who had diabetic foot ulcers and evaluated the distribution of Tregs/Th17/Th1 cells. hCB-MSCs were injected directly into the patients’ quadriceps femoris muscle (2 × 106 cells per point). Proportions of Treg/Th17, Treg/Th1, and Th17/Th1cells were measured using flow cytometry, and their correlations with various cytokines (FoxP3, IL-17, INF-g, C-RP, TNF-a, and VEGF) were analyzed. While the proportions of CD4 + CD25hiFoxP3+ Treg/Th17 and CD4 + CD25hiFoxP3+ Treg/Th1 cells displayed a significant increase at 4 weeks posttransplantation, the percentage of Th17/Th1 cells did not change. These data suggest a role for Treg cells in the initiation and progression of T2DM (Li et al. 2014).
Xue et al. transplanted human BM-MSCs carrying yellow fluorescent protein into a mouse burn model to enhance healing with 30 mice in each group. BM-MSCs carry minimal to no immunogenetic markers, which is their advantage. Obvious wound healing was observed in 14 days, whereas controls took approximately 25 days to reach the same stage. Allogenic MSC increased wound healing as well as blood vessel intensity and angiogenesis (Metcalfe and Ferguson 2007).
Oskouei et al. showed that cardiac stem cells (C-kit, SCA1, anbg2 positive) had ameliorating effects and reduced scar formation, which was enhanced when they were used with BM-MSC (Stem Cells Transl Med 2012).
Singer et al. used BM-MSCs in a totally necrotic mice burn model with 48 animals in their experimental group. Burns of the 54 animals of control group completely necrotized, and 29 animals in the experimental group survived. Tissue amount and angiogenesis increased 20% in the area of necrosis. The authors also tested the effects of MSCs on tail biopsy wounds of diabetic mice and observed a near-complete regain of physical abilities (Lau et al. 2009b).
Zhao et al. isolated MSCs from human cord blood, which helped treatment of foot ulcers of rats (Lau et al. 2009b; Reed et al. 2000). hCB-MSCs were injected through left femoral artery into streptozotocin-induced diabetic rats. The foot ulcers of the rats significantly reduced when evaluated on the 7th and 14th days of the experiment. The number of inflammatory cells and development of new blood vessels on the third day, granulation on the seventh day, and increase in epithelization on the 14th day were significantly higher than in the controls. This study showed that MSCs could target and localize at wounded sites and increase ECM production and via cytokeratin 19-induced epithelization (Zhao et al. 2014).
7.3 Stem Cell Therapies for Nondiabetic Chronic Wounds
Chronic wounds may also occur due to the continued pressure at patients with movement disabilities, who are hospitalized for very long periods, or had inadequate medical care. This type of wounds, known as bed sores, are called decubitus ulcers. Decubitus ulcers are often seen at elder patients whom tissues regenerate very slowly and paralyzed or comatose patients. Several studies (Leung 2007; Game et al. 2012) were published, and one patented product also exists for the treatment of decubitus ulcers (Van Koppen and Hartmann 2015) which show clinical improvement of patients’ chronic wounds.
7.4 Grafts Used in Combination with Stem Cells
The most recent in vivo studies reported the power of dermal autologous micrografts in ameliorating the healing of venous, diabetic, pressure, and post-traumatic ulcers. Various wounds are completely healed after a few weeks of treatment meanwhile increased the quality of life for the patients. In vitro final analyses showed that these micrografts express MSC markers such as CD34, CD73, CD90, and CD105 and are able to form a viable and proliferative biocomplex with collagen sponge. Finally, the sites of ulcers displayed varying expressions of several cytokines such as epidermal growth factors, insulin-like growth factors, platelet-derived growth factors and their receptors, and tumor necrosis factor-β, each one is different than normal tissues (De Francesco et al. 2016).
7.5 Exosome Applications in Wound Healing
Exosomes are nano-sized vesicles, usually between 30 and 100 nm in diameter. They are secreted by many cell types. Exosomes contain various cell components such as proteins, mRNA, and microRNAs, which are packed with a membrane which also contains specific receptors and markers. Exosomes are transported at the extracellular matrix where they incorporate to target cells and therefore deliver their contents into target cells (Shabbir et al. 2015; Zhang et al. 2015a).
Zhang et al. showed that human hCB-MSC-derived exosomes accelerated re-epithelization and enhanced proliferation and migration of keratinocytes via Wnt4 pathway on experimental acute burn (Zhang et al. 2015b).
We are currently using hCB-MSC-derived exosomes in our GMP standard laboratory for the treatment of various diseases such as muscular dystrophies, neurodegenerative diseases, and chronic wounds. Figure 10.2a shows that the diabetic foot ulcer of an 86-year-old female patient. Exosomes are applied into connective tissue peripheral to the wound area (Fig. 10.2b). Exactly 5 months later, Fig. 10.2c shows complete healing of wound which otherwise could progress to amputation of foot. Our unpublished data also showed anti-inflammatory and antibacterial effects of MSC -derived exosomes in chronic wounds. We believe that the exosome treatment will be a future therapy method in many diseases.
8 Future Projections
Wound healing is a highly complex process with a combination of steps, which vary according to the cause and the amount of damage. Other factors such as age, malnutrition, diabetes, infection, kidney failure, hepatitis, chemotherapy, radiotherapy, and smoking negatively affect the healing process. Clinical trials with stem cells, conducted after many successful animal experiments have produced positive results, and therefore they are increasing in numbers. MSCs appear to be the best stem cell source for both availability and effectiveness. On the other hand, MSC-derived exosomes emerge as an alternative source in wound treatment. The development of new techniques for both acute and chronic wounds has shown that cell therapies are becoming the treatment method of the future. Various methods such as 3D-printed materials and robotic surgery methods that use stem cells may be the wound treatment methods of the next decade.
References
Akash MS, Rehman K, Chen S (2013) Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J Cell Biochem 114(3):525–531
Arya AK, Tripathi R, Kumar S, Tripathi K (2014) Recent advances on the association of apoptosis in chronic non healing diabetic wound. World J Diabetes 5(6):756–762
Balbach ST, Cavaleri FM, Gentile L, Araúzo-Bravo MJ, Schöler HR, Crosetto N, Boiani M (2009) Observing and manipulating pluripotency in normal and cloned mouse embryos. In: Baharvand H (ed) Trends in stem cell biology and technology. Humana Press, New York, p 101
Barbul A, Breslin JR, Woodyard JP, Wasserkrug HL, Efron G (1989) The effect of in vivo T helper and T suppressor lymphocyte depletion on wound healing. Ann Surg 209:479–483
Berman DM, Willman MA, Han D et al (2010) Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes 59:2558–2568
Besse JL, Leemrijse T, Deleu PA (2011) Diabetic foot: the orthopedic surgery angle. Orthop Traumatol Surg Res 97:314–329
Black IB, Woodbury D (2001) Adult rat and human bone marrow stromal stem cells differentiate into neurons. Blood Cells Mol Dis 27:632–636
Blumberg SN, Berger A, Hwang L, Pastar I, Warren SM, Chen W (2012) The role of stem cells in the treatment of diabetic foot ulcers. Diabetes Res Clin Pract 96(1):1–9
Bura A, Planat-Benard V, Bourin P, Silvestre JS, Gross F, Grolleau JL, Saint-Lebese B, Peyrafitte JA, Fleury S, Gadelorge M, Taurand M, Dupuis-Coronas S, Leobon B, Casteilla L (2014) Phase I trial: the use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy 16(2):245–257
Cavanagh P, Attinger C, Abbas Z, Bal A, Rojas N, Xu ZR (2012) Cost of treating diabetic foot ulcers in five different countries. Diabetes Metab Res Rev 28(Suppl 1):107–111
Charles Brunicardi F, Andersen DK, Billiar TR, Dunn DL, Hunter JG, Matthews JB, Raphael E (2005). Pollock, Schwartz’s principles of surgery, Chapter 9. Wound healing
De Francesco F, Graziano A, Trovato L, Ceccarelli G, Romano M, Marcarelli M, Cusella De Angelis GM, Cillo U, Riccio M, Ferraro GA (2016) A regenerative approach with dermal micrografts in the treatment of chronic ulcers. Stem Cell Rev 13(1):139–148
Ding Y, Xu D, Feng G, Bushell A, Muschel RJ, Wood KJ (2009) Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes 58:1797–1806
Draper JS, Seguin CA, Andrews PW (2007) Phenotypic analyses of human embryonic stem cells. In: Sullivan S, Cowan CA, Eggan K (eds) Human embryonic stem cells: the practical handbook. Wiley, Cambridge, MA, pp 93–106
Duque GA, Descoteaux A (2014) Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol 5:491
Duscher D, Barrera J, Wong VW, Maan ZN, Whittam AJ, Januszyk M, Gurtner GC (2016) Stem cells in wound healing: the future of regenerative medicine? Mini-Rev Gerontol 62(2):216–225
Ertekin C, Taviloğlu K, Güloğlu R, Kurtoğlu M (2005) İstanbul Medikal Yayıncılık, İstanbul. Travma pp 488–501
Fiorina P, Jurewicz M, Augello A et al (2009) Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol 183:993–1004
Fritschi C (2001) Preventive care of the diabetic foot. Nurs Clin North Am 36:303–320
Game FL, Hinchliffe RJ, Apelqvist J, Armstrong DG, Bakker K, Hartemann A, Löndahl M, Price PE, Jeffcoate WJ (2012) A systematic review of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev 28(Suppl 1):119–141
Ghatak S, Maytin EV, Mack JA, Hascall VC, Atanelishvili I, Rodriguez RM, Markwald RR, Misra S (2015) Roles of proteoglycans and glycosaminoglycans in wound healing and fibrosis. Int J Cell Biol http://dx.doi.org/10.1155/2015/834893
Ghieh F, Jurjus R, Ibrahim A, Geagea AG, Daouk H, El Baba B, Chams S, Matar M, Zein W, Jurjus A (2015) The use of stem cells in burn wound healing: a review. Biomed Res Int 2015:1–9
Gupta PK, Krishna M, Chullikana A, Desai S, Murugesan R, Dutta S, Sarkar U, Raju R, Dhar A, Parakh R, Jeyaseelan L, Viswanathan P, Vellotare PK, Seetharam RN, Thej C, Rengasamy M, Balasubramanian S, Majumdar AS (2016) Administration of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells in critical limb ischemia due to Buerger’s disease: phase II study report suggests clinical efficacy. Stem Cells Transl Med. pii: sctm.2016–0237
Harrison NJ, Barbaric I, Andrews PW (2011) Human embryonic stem cell characterization: similarities and differences between cell lines and sources. In: Bongso A, Lee EH (eds) Stem cells: from bench to bedside, 2nd edn. World Scientific, Singapore, pp 1–22
Jayaraman P, Nathan P, Vasanthan P, Musa S, Govindasamy V (2013) Stem cells conditioned medium: a new approach to skin wound healing management. Cell Biol Int 37(10):1122–1128
Jiang J, Lv Z, Gu Y, Li J, Xu L, Xu W et al (2010) Adult rat mesenchymal stem cells differentiate into neuronal-like phenotype and express a variety of neuro-regulatory molecules in vitro. Neurosci Res 66:46–52
Karnieli O, Izhar-Prato Y, Bulvik S, Efrat S (2007) Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells 25:2837–2844
Khosrotehrani K (2013) Mesenchymal stem cell therapy in skin: why and what for? Exp Dermatol 22(5):307–310
Lau TW, Lam FF, Lau KM, Chan YW, Lee KM, Sahota DS et al (2009) Pharmacological investigation on the wound healing effects of Radix Rehmanniae in an animal model of diabetic foot ulcer. J Ethnopharmacol 123:155–162
Lee HC, An SG, Lee HW, Park JS, Cha KS, Hong TJ, Park JH, Lee SY, Kim SP, Kim YD, Chung SW, Bae YC, Shin YB, Kim JI, Jung JS (2012) Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circ J 76(7):1750–1760. Epub 2012 Apr 12
Lee DE, Ayoub N, Agrawal DK (2016) Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res Ther 7:37
Leirósa GJ, Kusinskya AG, Dragob H, Bossib S, Sturlab F, Castellanosa FL, Stellac IY, Balañáa ME (2014) Dermal papilla cells improve the wound healing process and generate hair Bud-like structures in grafted skin substitutes using hair follicle stem cells. Stem Cells Trans Med 3(10):1209–1219
Leung PC (2007) Diabetic foot ulcers – a comprehensive review. Surgeon 5(4):219–231
Li XY, Zheng ZH, Li XY, Guo J, Zhang Y, Li H, Wang YW, Ren J, Wu ZB (2013) Treatment of foot disease in patients with type 2 diabetes mellitus using human umbilical cord blood mesenchymal stem cells: response and correction of immunological anomalies. Curr Pharm Des 19(27):4893–4899
Li XY, Zheng ZH, Guo J, Zhang Y, Li H, Wang YW, Ren J, Wu ZB (2014) Treatment of foot disease in patients with type 2 diabetes mellitus using human umbilical cord blood mesenchymal stem cells: response and correction of immunological anomalies. Curr Pharm Des 19(27):4893–4899
Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S, Xu J, Wu Q, Zhang Z, Xie B, Chen S (2011a) Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract 92(1):26–36
Madec AM, Mallone R, Afonso G et al (2009) Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia 52:1391–1399
Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, Leibel RL, Melton DA (2009) Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci U S A 106:15768–15773
Marieb EN (1995) Human anatomy and physiology. Hodder & Stoughton, London, p 103
Melton DA, Cowan C (2009) “Stemness”: definitions, criteria and standards. In: Lanza R, Gaerhart J, Hogan B, Melton D, Thomson J, Wilmut I (eds) Essentials of stem cell biology, 2nd edn. Academic Press, San Diego, pp 23–29
Metcalfe AD, Ferguson MWJ (2007) Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface 4(14):413–437
Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M et al (2004) Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol 15:1794–1804
Nandy D, Mukhopadhyay D (2011) Growth factor mediated signaling in pancreatic pathogenesis. Cancers Basel 3(1):841–871
Norton JA, Bollinger RR, Chang AE, Lowry SF, Mulvihill SJ, Pass HI, Thompson RW (2003) Essential practice of surgery: basic science and clinical evidence. In: Lorenz HP, Longaker MT (eds) Chapter 7: Wounds: biology, pathology and management. Springer, New York, pp 89–94
Oskouei BN et al (2012) Increased potency of cardiac stem cells compared with bone marrow mesenchymal stem cells in cardiac repair. Stem Cells Transl Med 1(2):116–124
Ozturk S, Karagoz H (2015) Experimental stem cell therapies on burn wound: do source, dose, timing and method matter? Burns 41:1133
Pawlina W Histology: a text and atlas: with correlated cell and molecular biology. 7th Ed. . Philadelphia Wolters Kluwer. China. Int. Ed. 2016. p. 488–516.
Paz JC, West MP. (2014) Acute care handbook for phsical therapists, 4th edn. Saunders, Philadelphia, pp 297–299.
Polo JM, Liu S, Figueroa MA, Kulalert W, Eminli S, Tan KY, Apostolou E, Stadtfeld M, Li Y, Shioda T, Natesan S, Wagers AJ, Melnick A, Evans T, Hochedlinger K (2010) Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol 28:848–855
Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, Gadbois TM et al (2000) A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism 49:1390–1394
Sener LT, Albeniz I (2015) Challenge of mesenchymal stem cells against diabetic foot ulcer. Curr Stem Cell Res Ther 10:530–534
Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Van Badiavas E (2015) Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev 24(14):1635–1647
Shumakov VI, Onishchenko NA, Rasulov MF, Krasheninnikov ME, Zaidenov VA (2003) Mesenchymal bone marrow stem cells more effectively stimulate regeneration of deep burn wounds than embryonic fibroblasts. Bull Exp Biol Med 136(2):192–195
Singer DD, Singer AJ, Gordon C, Brink P (2013) The effects of rat mesenchymal stem cells on injury progression in a rat model. Acad Emerg Med 20(4):398–402
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676
Vaccari DA, Strom PF, Alleman JE (2005) Environmental biology for engineers and scientists. Wiley-Interscience, Hoboken
Van Koppen CJ, Hartmann RW (2015) Advances in the treatment of chronic wounds: a patent review. Expert Opin Ther Pat 25(8):931–937
van Zuijlen PPM, Gardien KLM, Jaspers MEH, Bos EJ, Baas DC, van Trier AJM, Middelkoop E (2015) Tissue engineering in burn scar reconstruction. Burns Trauma 3:18
Jeffrey Weinzweig (1999) Plastic surgery secrets. Hanley & Belfus Inc. 156053219X, 9781560532194
Xue M, Jackson CJ (2015) Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care (New Rochelle) 4(3):119–136
Xue L, Xu YB, Xie JL, Tang JM, Shu B, Chen L, Qi SH, Liu XS (2013) Effects of human bone marrow mesenchymal stem cells on burn injury healing in a mouse model. Int J Clin Exp Pathol 6(7):1327–1336
Yazdanpanah L, Nasiri M, Adarvishi S (2015) Literature review on the management of diabetic foot ulcer. World J Diabetes 6(1):37–53
Yolanda MM, Maria AV, Amaia FG, Marcos PB, Silvia PL et al (2014) Adult stem cell therapy in chronic wound healing. J Stem Cell Res Ther 4:162
Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, Xie Z, Zhang C, Wang Y (2015a) Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med 13:49
Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, Shi H, Wu L, Zhu W, Qian H, Xu W (2015b) HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells 33:2158–2168
Zhao QS, Xia N, Zhao N, Li M, Bi CL, Zhu Q, Qiao GF, Cheng ZF (2014) Localization of human mesenchymal stem cells from umbilical cord blood and their role in repair of diabetic foot ulcer in rat. Int J Biol Sci 10(1):80–89
Acknowledgements
Authors thank Prof. Dr. Murat AKSOY from Liv Hospital, Istanbul, for his sharing of patient photographs.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Şener, L.T., Darici, H., Albeniz, I., Karaöz, E. (2017). Wound Treatment by Stem Cells. In: Pham, P. (eds) Pancreas, Kidney and Skin Regeneration. Stem Cells in Clinical Applications. Springer, Cham. https://doi.org/10.1007/978-3-319-55687-1_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-55687-1_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-55686-4
Online ISBN: 978-3-319-55687-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)