Abstract
The success of wound healing is impaired in several medical conditions resulting in increased morbidity. The current available therapeutic options frequently fail to promote full tissue regeneration, and stem cell-based therapies have emerged as promising alternatives. Mesenchymal stem/stromal cells (MSCs) have gained relevance within this context not only due to their ability to promote healing through engraftment and further differentiation, but also because of their paracrine effects. Recently, it has been suggested that the secretion of exosomes may be a dominant mechanism by which MSCs exert their healing function, thus granting them a potential new role as active players in cell-free-based therapies. This chapter focuses on the recent advances on MSC-based therapies for the treatment of cutaneous wounds, namely on their mechanisms of action and the strategies adopted to improve their therapeutic efficacy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Mesenchymal stem cells
- Wound healing
- Skin regeneration
- Homing
- Recruitment
- Paracrine factors
- Secretome
- Exosomes
- MSC priming
- 3D cultures

The success of wound healing is impaired in several medical conditions resulting in increased morbidity. The current available therapeutic options frequently fail to promote full tissue regeneration, and stem cell-based therapies have emerged as promising alternatives. Mesenchymal stem/stromal cells (MSCs) have gained relevance within this context not only due to their ability to promote healing through engraftment and further differentiation, but also because of their paracrine effects. Recently, it has been suggested that the secretion of exosomes may be a dominant mechanism by which MSCs exert their healing function, thus granting them a potential new role as active players in cell-free-based therapies. This chapter focuses on the recent advances on MSC-based therapies for the treatment of cutaneous wounds, namely on their mechanisms of action and the strategies adopted to improve their therapeutic efficacy.
13.1 Introduction
Chronic wounds affect 2% of the general population in the Western world, having tremendous social and economic impacts [1, 2]. They are mostly associated to medical conditions such as diabetes, vascular and autoimmune diseases, being responsible for a decreasing quality of life of the affected individuals, and frequently leading to major complications, namely amputations and even, early death [1]. The lack of effective therapies is one of the causes potentiating the chronic wound burden, making the search for alternative therapeutic strategies an issue of utmost importance. Supported by recently accumulated evidence, mesenchymal stem/stromal cells (MSCs) have increasingly become valid candidates to be applied in cell-based therapies due to their beneficial effects on tissue regeneration [3]. The potential roles and applications of MSCs for the treatment of cutaneous wounds will be the focus of this chapter.
The main function of the skin is to form an effective barrier against the external environment, conferring protection and participating in the defence against pathogens, in the regulation of body temperature and in the prevention of dehydration [4]. Cutaneous wound healing is, therefore, a crucial factor for the survival of organisms and the loss of integrity of this barrier, either as a result of injury or a disease, must be rapidly and efficiently treated [4].
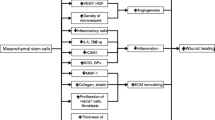
Wound healing is a dynamic biological event mediated by a complex set of growth factors secreted by a variety of cell types, controlling events like clotting, inflammation, cellular migration, proliferation, extracellular matrix (ECM) deposition, angiogenesis, vasculogenesis and ultimately, leading to remodelling of the mature scar tissue [4]. There are three classic stages of wound repair (◘ Fig. 13.1) that, although distinct, do overlap: i) the inflammatory stage, ii) new tissue formation/proliferation stage and iii) the remodelling phase [4]. Inflammation is the first stage of wound repair, occurring immediately after tissue injury. In this process, haemostasis is achieved, initially by the formation of a platelet plug, followed by the formation of a fibrin matrix. In turn, platelets embedded in the recently formed fibrin clot recruit leucocytes and macrophages [5]. Neutrophils eliminate contaminating microorganisms and secrete pro-inflammatory cytokines that activate local fibroblasts and keratinocytes [4, 5]. On the other hand, macrophages are responsible for initiating the granulation tissue deposition by releasing platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF), which then leads to a feedback loop of continued secretion of several other growth factors that are crucial to the healing process, including transforming growth factor α and β (TGF-α, TGF-β), interleukin-1 (IL)-1 and insulin-like growth factor (IGF) [6]. Afterwards, the crosstalk between keratinocytes and fibroblasts becomes critical during the tissue formation/proliferation phase. Through paracrine signalling, keratinocytes release IL-1 thus inducing fibroblasts to secrete keratinocyte growth factor (KGF), fibroblast growth factor-7 (FGF-7), IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF) and hepatocyte growth factor (HGF). In parallel, the keratinocyte proliferation culminates in wound re-epithelialisation [5]. In the final stages, and with the contribution of bone marrow-derived mesenchymal stem cells (BM-MSCs), fibroblasts replace the fibrin matrix by granulation tissue, which is associated to recently formed capillaries [5, 7]. Ultimately, the interaction between myofibroblasts and fibroblasts leads to the production of the ECM, mainly in form of collagen. This newly formed ECM turns into a mature scar in the final remodelling stage [4,5,6]. Concomitantly at this stage, an almost acellular matrix enriched in type III collagen is left behind and further replaced mostly by type I collagen fibres that reinforce the repaired tissue, a process mediated by matrix metalloproteinases (MMPs) [5, 6].
Being a very complex process, numerous factors can impact the different healing phases. The factors that influence wound healing can be divided in local and systemic [8]. Local factors are the ones capable of affecting the wound characteristics, such as oxygenation/hypoxia, infection, foreign body and venous sufficiency, while systemic factors are those related to the individual’s healing ability, which can be affected by physiologic or medical conditions such as age, hormones, stress, obesity, medication, nutrition, among others [8]. Diabetic patients, for instance, are unable to respond to signalling pathways of normal wound healing, thus compromising their function [9]. Chronic wounds usually fail to progress beyond the first stage of wound healing, leading to a continuous inflammatory condition characterized by excessive levels of pro-inflammatory cytokines, reactive oxygen species (ROS) and senescent cells. The maintenance of this inflammatory state also leads to excessive proteolysis promoted by MMP, that unlike in acute wounds, are not controlled by their inhibitors causing degradation of ECM, growth factors and their receptors [9, 10]. In turn, fibroblasts present reduced levels of TGF-β receptors in ulcerated tissues promoting their senescence and correlated weakened migratory capacity even in the presence of the motogenic stimulant TGF-β [11]. This event along with ECM degradation leads to a positive feedback loop that amplifies inflammation due to inflammatory cell recruitment, which again hampers the wound healing progression to the proliferative/new tissue formation stage [9]. In addition, chronic wounds contain senescent cell populations of keratinocytes, endothelial cells and macrophages, probably due to oxidative stress, DNA damage-related cell cycle arrest or abnormal metabolic changes. Chronic wounds are also subject to persistent infections and in most cases the stem cell niche is compromised or even dysfunctional [9].
The traditional and currently available therapeutic approaches for the treatment of (chronic) wounds consist in debridement [12], application of wound dressings [13], negative pressure and hyperbaric oxygen [12] or the administration of growth factors. However, current research advances in such solutions have been restricted to amelioration of patient care [14]. More curative approaches have resorted to the application of stem cells to improve the healing process, thus raising the hopes for a complete wound resolution.
13.2 Mesenchymal Stem Cells in Wound Healing: Sources and Mechanisms
MSCs have gained interest within the scientific community for they represent promising alternatives to the repair and regeneration of damaged tissues. Unlike the initial postulates, supported by strong plasticity data, the way MSCs exert their regenerative capacity is not so much by their ability for multipotent differentiation, but instead through the secretion of bioactive molecules, which renders them potent inducers of pro-healing mechanisms [15,16,17].
MSCs are a subset of multipotent, less committed postnatal stem cells, characterized according to The International Society for Cellular Therapy (ISCT) criteria [18], as being able to adhere to a plastic surface, of undergoing trilineage differentiation (into osteoblasts, adipocytes and chondroblasts) and expressing specific surface proteins. MSCs present several advantages over other stem cell types. As opposed to embryonic stem cells (ESCs) that require the sacrifice of the embryo for collection, MSCs’ procurement is easy and obtained from non-controversial tissue sources at a relatively low cost. The use of MSCs also overcomes the safety concerns related to genome stability of ESCs and induced pluripotent stem cells (iPSCs) that have hindered their clinical applications. Moreover, cultivation of MSCs does not require the use of feeder layers or high concentrations of serum as ESCs do. Finally, although MSCs are not an infinite cell source, they have an extraordinary replicative capacity in vitro, which is of extreme relevance, due to the large amounts of cells needed for cell-based therapies [15].
Despite the fact that the perivascular niche is thought to be a common stem cell microenvironment for resident MSC-like populations [19], MSCs can be isolated from a number of neonatal tissues, including amnion, placenta [20, 21], foetal blood, liver [22] and umbilical cord blood (UCB) [23] and tissue/stroma (UC) [24, 25]; as well as from adult tissues, such as bone marrow, thymus, brain, liver, lung, kidney, aorta, muscle, spleen [19] and adipose tissue (AT) [26]. These cells display many common characteristics suggesting that all MSC populations share a similar ontogeny. However, MSCs may present variations in morphology, proliferation potential, growth rates, differentiation capacity as well as in their regenerative potential, including wound healing capacities [27]. In fact, one of the main advantages of using human neonatal MSCs is the fact that they are isolated from a tissue containing a more primitive MSC population expressing the pluripotency gene markers Oct-4, Nanog and Sox2, which are present in ESCs. Epigenetic modifications are also altered in neonatal tissues. Indeed, the aging of these cells is one of the hypothesis to explain the differences in MSC regenerative potential and may be associated to DNA and mitochondrial damages [28]. Several comparisons using MSCs from distinct origins but with similar number of passages have been reported as indicating differences in regenerative potential [24, 29].
The MSCs derived from bone marrow (BM-MSCs) were the first to be isolated, being, therefore, the most investigated. BM-MSCs present increased differentiation potential to osteogenic and chondrogenic lineages [29], when compared with MSCs from other sources, namely adipose tissue or umbilical cord blood [30]. In turn, AT-MSCs have been reported to better contribute to the formation of capillary networks, whereas pericytes genesis has been shown to be potentiated by UCB-MSCs [31]. UC-MSCs [24, 25] and BM-MSCs [24] have demonstrated to promote early motogenic effects on keratinocytes and fibroblasts, respectively. In contrast to UC-MSC along with UCB-MSCs, AT-MSCs and BM-MSCs have been implicated in improving vasculogenesis, whereas amniotic membrane-derived MSCs have not [31].
Therefore, the regenerative potential of MSCs may depend on their tissue of origin, which is supported by the stem cell niche theory that postulates that cell fate is the result of the impact of stem cell niche [32]. As such, the tissue source from which MSCs are isolated is an important factor that not only conditions the healing potential, but also must be taken into consideration to better define the therapeutic strategy for further clinical applications.
13.2.1 Homing of Mesenchymal Stem Cells
In stem cell science, the term “homing” refers to the stem cells’ ability to migrate to a specific destination, or “niche”, within a given organism. In the case of MSCs, their ability to respond to chemotactic cues and migrate towards injured sites is recognised. Therein they may differentiate or influence the wound environment by secreting regenerative paracrine factors [33, 34]. MSC homing is also characterized by MSC arrest within vessels and subsequent passage/transmigration through the endothelium [34]. In terms of clinical applications, this property allows a less invasive and systemic administration of MSCs that are then guided by cytokine gradients and other cues originating from tissue damage loci.
Evidence of MSC homing in homeostatic conditions has proven to be difficult to obtain, having produced some controversial results as reported in the literature [34]. However, despite the failure to isolate circulating MSCs [35], Wang et al. have shown that higher amounts of MSCs are isolated from injured mice [36], being this fact associated with increased levels of VEGF and G-CSF in peripheral blood.
The mechanism by which MSCs home to specific damaged tissues is barely understood, but it is thought to resemble the leukocyte homing cascade, due to the presence of a number of similar cell adhesion and cytokine receptors at the surface of MSCs [34] (◘ Figs. 13.2 and 13.3). After injury, the systemic secretion of P- and E-selectins as well as VCAM-1 mobilizes leukocytes. Other chemokines such as SDF-1, IL-8, CCL2, and CXCL10 are also secreted and recognized by MSCs via specific receptors [37]. The SDF-1/CXCR4 axis has been shown to be significantly up-regulated in bone marrow and in ischemic tissues [34], being well known for their roles in hematopoietic stem and immune cell recruitment [38], as well as in MSC mobilization in experimental models of brain injury and heart myocardial infarction [39, 40]. SDF-1 acts as a signal for the retention of BM-MSCs at the bone marrow, and the G-CSF used clinically to mobilize stem cells from the BM into the peripheral circulation is thought to work by triggering the degradation of SDF-1 (◘ Fig. 13.2) [38]. Indeed, Miranda et al. have hypothesized that G-CSF could be involved in BM-MSC recruitment to injured tissues, which is known to be important for promoting tissue regeneration [24].
The SDF-1/CXCR4 axis and the role of G-CSF after tissue injury. In a non-injury scenario, SDF-1 acts as a signal for the retention of BM-MSCs at the bone marrow. Conversely, in an injury scenario, cytokines, chemokines, and growth factors, like G-CSF, are released at the injured tissue, triggering the degradation of SDF-1 and consequent homing of MSC from the BM to sites of injury via the circulation
13.2.2 Mesenchymal Stem Cell Paracrine Mechanisms
Increasing amount of evidences shows that MSCs possess the ability to induce tissue regeneration without local engraftment or differentiation leading to a paradigm shift in regenerative medicine [41]. Emerging data suggest that stem cells can be considered as a reservoir of trophic factors, including growth factors and cytokines as IL-1, GM-CSF, IL-7, IL-8, IL-10, IL-11, SDF-1 or micro and nanovesicles, that are released (secretome) when needed to modulate and repair surrounding damaged tissues [42]. It has been demonstrated that trophic factors can have many roles such as regulation of inflammatory reactions, immunomodulation, anti-apoptotic and pro-angiogenic, just to mention a few (◘ Fig. 13.3) [42,43,44]. Moreover, instead of transplantation of MSCs, the administration of their secretome can overcome many of the safety concerns regarding transplantation of viable replicating cells [45]. Understanding the cell secretome has thus attracted much attention.
MSC-mediated immune regulation is the result of the cumulative action displayed by several anti-inflammatory molecules [46], and its effects occur in a localized environment and not systemically [47]. The immunomodulatory effects of MSCs are quite relevant for their application as novel therapeutics since MSCs were shown to be able to inhibit the proliferation of CD4+ and CD8+ T cells, B cells, and natural killer (NK) cells [24, 48]. The mechanisms by which MSCs exert effects of immunomodulation are related to the suppression of pro-inflammatory behaviour of T and B cells, macrophages, dendritic cells and neutrophils [37]. These effects are mediated by the MSC secretion of growth factors such as TGF-β, HGF, IL-10, PGE2 and NO [49], IL-6 [50] and CCL2 [51], among others [37, 49]. In fact, their therapeutic role in a variety of inflammatory autoimmune diseases, such as rheumatoid arthritis [27, 44], is currently under wide pre-clinical and clinical investigation [52].
13.3 Mesenchymal Stem Cells in Skin Regeneration/Cutaneous Wound Healing
13.3.1 Endogenous MSCs
Apart from the role of stem cell niches existing in all tissues, including the skin, understanding the contribution of other endogenous stem cells to the regenerative process is a challenging quest and has even led to controversy.
Within the skin context, some authors have shown that skin-specific MSC niches, located at either the hair follicle bulge [53] or the dermal sheath [54], play differential roles in wound healing: while the former migrate to induce keratinocyte function, the latter differentiate into fibroblasts to further help on ECM deposition. After damage, epidermal repair is dependent on the migration of hair follicle bulge stem cells that through transient proliferation induce re-epithelialization [53]. The mechanism by which dermal sheath stem cells assist in wound repair is not related to migratory activity. Instead, the cells around the hair follicle assume a wound healing or myofibroblast phenotype, being involved in dermal repair [54].
On the other hand, endogenous BM-MSCs could act in the different phases of the wound healing process [24]. MSCs can induce the recruitment of endothelial cells through the secretion of VEGF and the modulation of inflammatory and immune responses through tumour necrosis factor-α (TNF-α) regulation and natural killer (NK) cell elimination. Afterwards, the proliferation of epidermal cells at the wound margins and further vasculogenesis allow the granulation tissue deposition. Finally, in later phases, BM-MSCs can affect in scar deposition by regulating interleukins and secreting prostaglandin E2 (PGE2), which further reduces collagen production [50].
13.3.2 Exogenous MSCs
Several reports support that chronic wound patients are deficient and defective in stem cells, as is also seen in in vivo healing-related disease models [55]. Hence, to overcome impaired wound healing, these patients may require a direct and active therapy through “exogenous” MSC administration whose mechanism of action can be due to cellular engraftment or through a paracrine mechanism.
13.3.2.1 Cellular Engraftment
Liu et al. have recently reported that systemic UC-MSCs, delivered intravenously, migrate to a wound site and remarkably reduce the amount of inflammatory infiltrated cells and signals while locally stimulating IL-10 and TNF-stimulated gene-6 (TSG-6). They also observed higher levels of collagen deposition as well as improved revascularization and VEGF secretion [56].
However, local application of MSCs at the wound site has also shown therapeutic benefits [57], avoiding cell loss within capillary networks. Locally applied UCB-MSCs improved the healing process by differentiating into keratinocytes as seen by the expression of keratin 19 or pan-keratin antigen [58]. On the other hand, Kong et al. have described that placenta-derived MSCs transplanted into the wound site have engrafted into the recipient’s vasculature. Engraftment without apparent differentiation resulted in enhanced microvessel density and ultimately led to improved wound healing in diabetic rats, probably due to the secretion of VEGF, HGF, FGF-2, TGF-β and IGF-1 [59]. Similar findings were reported by Hong et al. who found that AT-MSCs delivered topically engrafted and promoted a higher number of CD31 positive cells at the wound site with no signs of MSC differentiation. Although not exploring in depth the signalling pathways behind such findings, the paracrine secretion of pro-healing trophic factors by AT-MSCs, implicated in endothelial cell and macrophage recruitment in vivo, has been anticipated [60]. Finally, Shin et al. have reported the beneficial effects of locally applied BM-MSCs on both, normal and impaired healing, as a result of MSC engraftment [61]. In this model, the recruitment of higher numbers of autologous CD90 and CD166 positive cells (MSCs) to the wound site was observed, as a result of the increased production of Wnt3a, VEGF and platelet-derived growth factor receptor α (PDGFR-α) [61].
13.3.2.2 Paracrine Activity
As previously mentioned, the paracrine activity of MSCs alone for the treatment of cutaneous wounds has also been directly evaluated by many authors who chose to apply MSC secretome, instead of physical cells, in their experimental setups (◘ Table 13.1). Media that have been conditioned by MSCs (CM) contain MSC-secreted growth factors, cytokines and chemokines, including IL-8, IL-6, HGF, FGF-2, TGF-β, IGF-1, TSG-6, tumour necrosis factor receptor 1 (TNFR1), VEGF, granulocyte colony-stimulating factor (G-CSF), KGF and epidermal growth factor (EGF), that play important roles in the different phases of normal wound healing [24, 25, 56, 62].
Yew et al. demonstrated that CM from BM-MSCs accelerated wound closure with increased re-epithelialization, cell infiltration, granulation formation and angiogenesis, by enhancing epithelial and endothelial migration, which was related to the observed high levels of IL-6 mediated through the activation of p38 MAPK. In turn, in vitro studies using dermal fibroblasts have revealed that the presence of CM promoted fibroblast activation, proliferation and migration, further enhancing the healing of the wounds [24, 25, 62, 63].
In addition, the application of CM from amniotic fluid (AF)-derived MSCs led to accelerated wound repair in vivo by dermal fibroblasts through the TGF-β/SMAD2 pathway [62]. During the healing process, TGF-β is involved in inflammation, angiogenesis, re-epithelialization and granulation tissue deposition, being dependent on the activation of SMAD2 which further stimulates ECM production by fibroblasts promoting healing. Along with this new tissue formation, stromal cell-derived factor 1 (SDF-1) production by exogenously administered MSCs acts as a positive feedback by stimulating the endogenous MSC migration that will also secrete cytokines; overall increasing the crosstalk with adjacent cells including keratinocytes. In fact, cell-free lysates prepared from BM-MSCs have proved to be effective in inducing faster wound resolution by increasing expression of SDF-1 and CXCL-5, which are strong keratinocyte stimulators [64]. Moreover, MSC CM has also been implicated in the treatment of wounds characteristic of diabetic conditions where keratinocyte migration and proliferation are impaired. Such as shown in vitro by the treatment with CM derived from MSCs that overcame the effects of hyperglycemia by decreasing ROS overproduction, and allowing keratinocyte motility, which can be translated as an alternative therapeutic approach to ameliorate the poor wound healing conditions prompted by diabetes [65].
Finally, in a relevant comparative study, Miranda et al. have demonstrated that either CM obtained from UC-MSCs or BM-MSCs have the capacity to accelerate wound closure in vivo. However, the motogenic activity promoted by the CM from UC-MSC was significantly higher for human keratinocytes, in opposition to the effect seen with CM produced by BM-MSCs, which preferentially induced fibroblast migration. Accordingly, a comparative quantification of key factors with vital importance in the consecutive stages of wound healing revealed very different secretome profiles between the two MSCs. The relatively higher UC-MSC expression of EGF, FGF-2, and KGF strongly supported the early induction of keratinocyte migration and function necessary to trigger the later remodelling stages, where fibroblasts, triggered by IL-6, play a major role in ECM production. Concomitantly, the newly discovered UC-MSC-specific expression of G-CSF has revealed additional capacities for the CM derived from UC-MSCs to mobilize other healing-related cells (including CD34−/CD45− precursors – MSCs). These results were noteworthy since they underpinned i) different paracrine activities between MSCs derived from different tissue sources and ii) the viability of using (complementary) CMs rather than physical cells to promote skin regeneration [27].
13.3.3 The Role of Exosomes Derived from MSCs
Recent studies have uncovered that therapeutically valuable paracrine factors secreted by MSCs are often contained in small secreted lipid vesicles (40–100 nm) termed exosomes [66,67,68]. Exosomes have been found to cause alterations in biological pathways by playing key roles in normal physiology as well as in different pathological conditions [66, 69]. Indeed, they are released into the extracellular space shuttling several molecules, including proteins (e.g. growth factors, cytokines and receptors, as CXCR4) and nucleic acids (mRNA, miRNA) to neighbouring cells [70]. Because they are extracellular vesicles (EVs), their cargo is protected from adverse conditions, namely differential temperatures and pH environment variations [70].
The role of MSC-derived exosomes as well as their mechanisms of action is currently under investigation, introducing another dimension to the way MSCs can be applied in regenerative medicine. Within the context of wound healing, MSC-derived exosomes have been linked to the processes of re-epithelialization [71, 72] and angiogenesis [72, 73]. ◘ Table 13.2 reviews the works where MSC-derived exosomes have been implicated in the mechanisms related to wound healing.
Zhang et al. have shown that exosomes isolated from human iPSC-derived MSCs (iMSCs) promoted fibroblast secretion of collagen and elastin, and also angiogenesis in vitro, albeit without exploring mechanisms of action [72].
On the other hand, an in vitro study conducted by Shabbir et al. demonstrated that BM-MSC-derived exosomes improved the growth and migration of fibroblasts isolated from normal and chronic wounds and also induced angiogenesis by activating AKT, ERK 1/2, and STAT3 pathways. These authors found that BM-MSC exosomes carried STAT3, which is reported to promote gene expression related to growth factor production, such as HGF, IGF1, NGF, and SDF-1. Similarly, the AKT and ERK1/2 pathways were also reported to be activated in keratinocytes and dermal fibroblasts in vitro after incubation with exosomes from UC-MSCs [71] and iMSCs [74], respectively. Moreover, exosome-mediated delivery of Wnt4 signalling led to the enhancement of the healing process in a rat skin burn model by inducing β-Catenin, a dual function protein, involved in regulation and coordination of cell–cell adhesion and transcription regulation [71].
In turn, exosomes from AT-MSCs induced the expression of N-cadherin, cyclin-1, proliferating cell nuclear antigen (PCNA) and collagen I and III, associated with migration and proliferation of fibroblasts. The result was an improved collagen synthesis phenotype of these cells, which promoted a faster resolution of soft-tissue wounds [75]. Accordingly, Zhang et al. have also reported increased expression of CK19, PCNA, collagen I (compared to collagen III) in wounds treated with UC-MSC exosomes in vivo [71].
The formation of a scar tissue after skin damage, caused by myofibroblast aggregations, is particularly important since it compromises the regeneration process leading to cutaneous tissue with native elastic properties. The contribution of UC-MSCs to scar tissue deposition remains vague, but Fang et al. have found that specific exosomal miRNAs including miR-21, −23a, −125b, and − 145 were essential to the myofibroblast suppression and anti-scarring functions through inhibition of α-smooth muscle actin (α-SMA). The suppression of α-SMA, which in turn is associated to TGF-β/SMAD2 pathway, resulted in reduced scar formation and myofibroblast accumulation [76].
Uncovering the mechanisms underlying the therapeutic effects associated to the use of MSC-derived exosomes for the repair of cutaneous wound is still a challenge. Nevertheless, exosomes seem to act throughout the full wound healing process: in the initial stages, by improving re-epithelization and angiogenesis by activation of AKT and STAT3 signalling; and in the final stages, by decreasing scar tissue deposition by miRNA-mediated pathways.
13.4 Priming MSCs: Strategies for Improving Cutaneous Wound Healing
MSC response to different environments implies different outcomes of their behaviour. As such, strategies to improve MSC therapeutic efficacy, both in cell-based and cell-free-based therapies, have been attempted [77].
13.4.1 Preconditioning Strategies
Although MSCs constitutively produce several growth factors, these cells are able to reprogram their set of paracrine factors as a response to local stimuli, e.g. when administered in a wounded site.
For example, the presence of hypoxia is a typical feature of damaged tissues. Low levels of oxygen lead to hypoxia-inducible factor 1α (HIF-1α) activation, further inducing the secretion of VEGF and SDF-1. It has been shown that CM from BM-MSCs grown under hypoxic conditions further promoted cutaneous wound healing, being capable of accelerating wound closure in vivo when compared to CM from BM-MSCs cultured under normoxia conditions [78]. Similarly, the CM from hypoxic-treated AF-derived-MSCs presented significantly higher levels of VEGF and TGF-β in vitro as compared to the corresponding normoxic CM [79].
A prolonged inflammatory state is also a characteristic of the chronic wound environment that may be circumvented by MSCs. The exposure of AT-MSCs to inflammation-inducing agents, namely lipopolysaccharide (LPS) and TNF-α, has shown to improve its therapeutic efficacy by inducing skin flap survival in a diabetic rat model [80]. MSC response to such a stimulus was found to be mediated by DNA hydroxymethylation and the microRNAs miR146a, miR150 and miR155. In addition, MSC secretory capacity has been investigated within this context, where exosomes derived from LPS-primed UC-MSCs showed to enhance wound healing in diabetic rats by mediating macrophage polarization through let-7b via TLR4/NF-κB/STAT3/AKT regulatory signalling, therefore reducing inflammation within the wounded sites [81].
13.4.2 Tissue Engineering: Three-Dimensional Cultures
The multicellular 3D structure of spheroids, reached by the ability of MSCs to self-assemble in ex vivo 3D culture systems, constitutes a very efficient strategy to recreate the properties of a more physiological environment [25, 82]. Among others, 3D spheroid properties include greater cell-to-cell communication and cell-to-ECM interactions, enhanced cell viability, improved cell morphology/phenotype, stimulation of angiogenesis and anti-inflammatory modulation [25, 82]. The fact that cell proliferation and metabolism are rather different between 2D and 3D culture systems has also an important effect upon the overall cellular activity, leading to large differences in the secretion of relevant paracrine factors.
There are reports addressing the advantage of the microenvironment originated by 3D architecture for promoting wound healing. Hsu et al. have combined AT-MSC self-assembled spheroids with chitosan-hyaluronan membranes, a condition that enhanced expression of cytokine genes, namely CCL2, VEGF, FGF-1 and of migration-associated genes, such as CXCR4 and MMP-1. In a rat dorsal skin model, the wounds where AT-MSC spheroids were applied revealed faster wound closure and angiogenesis enhancement [83].
Similar observations have been described by Kwon et al. where again, CM obtained from AT-MSC 3D cultures presented higher concentrations of HGF, VEGF, FGF-2 and SDF-1, which suggests an accelerated wound closure triggered by paracrine mechanisms [84].
Regarding the potential of UC-MSC-derived CM, Santos et al. reported that dynamic spheroid 3D culturing resulted in a distinct secretome profile. Several key trophic factors involved in early and late stages of wound healing, namely HGF, TGF-β1, G-CSF, VEGF, FGF-2 and IL-6 were found to increase up to 80-fold when compared to CM of UC-MSCs cultured in 2D monolayers. In fact, in an in vivo excisional rat model, the treatment of wounds with CM from 3D cultured UC-MSCs resulted in a faster wound closure and in the fully regeneration of a more mature tissue phenotype [25].
13.4.3 Genetic Manipulation
Genetic manipulation of MSCs to express growth factors and cytokines that enhance skin regeneration has also been attempted, namely to promote angiogenesis [85], suppress inflammation [86] or to induce cell migration and function [87]. Li et al. have reported that overexpression of angiopoietin-1 in BM-MSCs-induced epidermal and dermal regeneration as well as angiogenesis in vivo, suggesting a direct contribution of angiopoietin-1 in this process [88]. Similarly, Xia et al. have demonstrated shortened healing time of radiation-wound injury when treated with BM-MSCs overexpressing VEGF and β-defensin-3. These authors showed a significant improvement in granulation tissue formation with collagen deposition and skin appendage regeneration [85]. Following the same rationale, but trying to reduce inflammatory signals, Qi et al. administered transfected BM-MSCs with TSG-6 to mouse excisional wounds, demonstrating that these cells suppressed TNF-α secretion by macrophages which led to accelerated wound healing with markedly reduced tissue fibrosis [86].
Along with the MSCs manipulated to express growth factors, chemokine receptor overexpression has also demonstrated therapeutic benefits in wounds of irradiated mice. Herein, the migration of CXCR4-overexpressing BM-MSCs was enhanced through a specific SDF-1-expression-dependent manner culminating in faster skin wound resolution [87]. Taking advantage of SDF-1 biological functions, besides inducing survival and growth of CXCR4-expressing cells [89], Nakamura et al., using a rat excisional wound model, have reported that SDF-1-engineered MSCs improved dermal fibroblast migration and new capillary vessel formation, translating into wound size decrease [90].
Nevertheless, and despite the promising results, the strategies involving the use of genetically modified MSCs for the treatment of cutaneous wounds need to consider further safety issues, e.g. those related to the viral vectors used for gene transfection, before being considered for clinical application.
13.5 Conclusion
Adapting the MSC therapeutic potential to the essential re-establishment of trophic factor, protease and metabolically competent cell equilibrium, needed to overcome impaired wound healing, constitutes an ambitious goal.
Overall, MSCs have proven to be able to influence every stage of the cutaneous wound healing process through two distinct mechanisms: provide replacement units for perished cells in tissues, or secrete trophic factors to their surroundings, thus influencing the endogenous regeneration potential [41]. The manipulation of MSCs for improving their therapeutic performance, through preconditioning, tissue engineering strategies or genetic modification, has also revealed to be beneficial. Yet, the establishment of reliable standard operational procedures for MSC-based therapies to be applied in clinical settings is still hard to achieve. The need for a better elucidation on MSC therapeutic mechanisms, to improve differentiation between different MSCs from different tissue sources, for better and consistent priming conditions, and for the best strategy of administration, are important questions whose answers will facilitate the final translation to the clinic.
Take-Home Message
-
Impaired wound repair constitutes a severe health problem with great economic impact, due to the lack of effective therapies.
-
MSC-based therapies have gained relevance as an alternative and effective treatment for cutaneous wounds.
-
MSCs are a subset of stem cells that can be isolated from different tissue sources, which directly impacts on their therapeutic outcome.
-
The therapeutic mechanisms of MSCs involve their proliferation and differentiation after engraftment as well as their paracrine action.
-
Both endogenous and administered exogenous MSCs and/or their secretome have shown to be important players in granting MSCs therapeutic value for wound healing.
-
Exosomes derived from MSCs represent an important cell-free-based alternative therapy.
-
Strategies to improve the MSC therapeutic outcomes include preconditioning insults, culture conditions modulation or genetic manipulation of the MSCs.
References
Heublein H, Bader A, Giri S. Preclinical and clinical evidence for stem cell therapies as treatment for diabetic wounds. Drug Discov Today. 2015;20(6):703–17.
Guest JF, Vowden K, Vowden P. The health economic burden that acute and chronic wounds impose on an average clinical commissioning group/health board in the UK. J Wound Care. 2017;26(6):292–303.
Domaszewska-Szostek A, Krzyżanowska M, Siemionow M. Cell-based therapies for chronic wounds tested in clinical studies. Ann Plast Surg. 2019;83(6):e96–e109.
Lau K, Paus R, Tiede S, Day P, Bayat A. Exploring the role of stem cells in cutaneous wound healing. Exp Dermatol. 2009;18(11):921–33.
Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–21.
Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–46.
Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180(4):2581–7.
Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–29.
Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care. 2015;4(9):560–82.
Nunan R, Harding KG, Martin P. Clinical challenges of chronic wounds: searching for an optimal animal model to recapitulate their complexity. Dis Model Mech. 2014;7(11):1205–13.
Pastar I, Stojadinovic O. Attenuation of the transforming growth Factor β–signaling pathway in chronic venous ulcers. Mol Med. 2010;16(3–4):92–101.
Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599–610.
Jones V, Grey JE, Harding KG. Wound dressings. BMJ. 2006;332(7544):777–80.
Liu Y, Dulchavsky DS, Gao X, Kwon D, Chopp M, Dulchavsky S, et al. Wound repair by bone marrow stromal cells through growth factor production. J Surg Res. 2006;136(2):336–41.
Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev. 2006;2(2):155–62.
Gunawardena TNA, Rahman MT, Abdullah BJJ, Abu Kasim NH. Conditioned media derived from mesenchymal stem cell cultures: the next generation for regenerative medicine. J Tissue Eng Regen Med. 2019;13(4):569–86.
Harrell CR, Fellabaum C, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Molecular mechanisms responsible for therapeutic potential of mesenchymal stem cell-derived Secretome. Cell. 2019;8(5):467.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7.
da Silva Meirelles L. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(11):2204–13.
In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GMJS, Claas FHJ, Fibbe WE, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004;22(7):1338–1345.
Antonucci I, Stuppia L, Kaneko Y, Yu S, Tajiri N, Bae EC, et al. Amniotic fluid as a rich source of mesenchymal stromal cells for transplantation therapy. Cell Transplant. 2011;20(6):789–96.
Campagnoli C. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98(8):2396–402.
Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109(1):235–42.
Miranda JP, Filipe E, Fernandes AS, Almeida JM, Martins JP, De La Fuente A, et al. The human umbilical cord tissue-derived MSC population UCX ® promotes early Motogenic effects on keratinocytes and fibroblasts and G-CSF-mediated mobilization of BM-MSCs when transplanted In vivo. Cell Transplant. 2015;24(5):865–77.
Santos JM, Camões SP, Filipe E, Cipriano M, Barcia RN, Filipe M, et al. Three-dimensional spheroid cell culture of umbilical cord tissue-derived mesenchymal stromal cells leads to enhanced paracrine induction of wound healing. Stem Cell Res Ther. 2015;6:90.
Zuk PA. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–95.
Santos JM, Bárcia RN, Simões SI, Gaspar MM, Calado S, Água-Doce A, et al. The role of human umbilical cord tissue-derived mesenchymal stromal cells (UCX®) in the treatment of inflammatory arthritis. J Transl Med. 2013;11(1):18.
Rohban R, Pieber TR. Mesenchymal stem and progenitor cells in regeneration: tissue specificity and regenerative potential. Stem Cells Int. 2017;2017:1–16.
Shafiee A, Seyedjafari E, Soleimani M, Ahmadbeigi N, Dinarvand P, Ghaemi N. A comparison between osteogenic differentiation of human unrestricted somatic stem cells and mesenchymal stem cells from bone marrow and adipose tissue. Biotechnol Lett. 2011;33(6):1257–64.
Ardeshirylajimi A, Mossahebi-Mohammadi M, Vakilian S, Langroudi L, Seyedjafari E, Atashi A, et al. Comparison of osteogenic differentiation potential of human adult stem cells loaded on bioceramic-coated electrospun poly (L-lactide) nanofibres. Cell Prolif. 2015;48(1):47–58.
Rohban R, Etchart N, Pieber TR. Vasculogenesis potential of stem cells from various human tissues. BioRxiv. 2016;1–22.
Lin H. The stem-cell niche theory: lessons from flies. Nat Rev Genet. 2002;3(12):931–40.
da Silva Meirelles L, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20(5–6):419–27.
Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4(3):206–16.
He Q, Wan C, Li G. Concise review: multipotent mesenchymal stromal cells in blood. Stem Cells. 2007;25(1):69–77.
Wang C-H, Cherng W-J, Yang N-I, Kuo L-T, Hsu C-M, Yeh H-I, et al. Late-outgrowth endothelial cells attenuate intimal hyperplasia contributed by mesenchymal stem cells after vascular injury. Arterioscler Thromb Vasc Biol. 2007;28(1):54–60.
Barminko J, Gray A, Maguire T, Schloss R, Yarmush ML. Mesenchymal stem cell therapy. In: Chase LG, Vemuri MC, editors. Stem cell biology and regenerative medicine. Totowa: Humana Press; 2013. p. 15–39.
Bollag WB, Hill WD. CXCR4 in epidermal keratinocytes: crosstalk within the skin. J Invest Dermatol. 2013;133(11):2505–8.
Yu J, Li M, Qu Z, Yan D, Li D, Ruan Q. SDF-1/CXCR4-mediated migration of transplanted bone marrow stromal cells towards areas of heart myocardial infarction via activation of PI3K/Akt. J Cardiovasc Pharmacol. 2010;55(5):1.
Wang Y, Deng Y, Zhou G-Q. SDF-1α/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res. 2008;1195:104–12.
Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–84.
El Omar R, Beroud J, Stoltz J-F, Menu P, Velot E, Decot V. Umbilical cord mesenchymal stem cells: the new gold standard for mesenchymal stem cell-based therapies? Tissue Eng Part B Rev. 2014;20(5):523–44.
Marfia G, Navone SE, Di Vito C, Ughi N, Tabano S, Miozzo M, et al. Mesenchymal stem cells: potential for therapy and treatment of chronic non-healing skin wounds. Organogenesis. 2015;11(4):183–206.
Miranda JP, Camões SP, Gaspar MM, Rodrigues JS, Carvalheiro M, Bárcia RN, et al. The Secretome derived from 3D-cultured umbilical cord tissue MSCs counteracts manifestations typifying rheumatoid arthritis. Front Immunol. 2019;10:18.
Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6(4):481–92.
Ghannam S, Bouffi C, Djouad F, Jorgensen C, Noël D. Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res Ther. 2010;1(1):2.
Chapel A, Bertho JM, Bensidhoum M, Fouillard L, Young RG, Frick J, et al. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med. 2003;5(12):1028–38.
Bárcia RN, Santos JM, Filipe M, Teixeira M, Martins JP, Almeida J, et al. What makes umbilical cord tissue-derived mesenchymal stromal cells superior Immunomodulators when compared to bone marrow derived mesenchymal stromal cells? Stem Cells Int. 2015;2015:583984.
Soleymaninejadian E, Pramanik K, Samadian E. Immunomodulatory properties of mesenchymal stem cells: cytokines and factors. Am J Reprod Immunol. 2012;67(1):1–8.
Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–22.
Rafei M, Hsieh J, Fortier S, Li M, Yuan S, Birman E, et al. Mesenchymal stromal cell-derived CCL2 suppresses plasma cell immunoglobulin production via STAT3 inactivation and PAX5 induction. Blood. 2008;112(13):4991–8.
Wang L-T, Ting C-H, Yen M-L, Liu K-J, Sytwu H-K, Wu KK, et al. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials. J Biomed Sci. 2016;23(1):76.
Levy V, Lindon C, Zheng Y, Harfe BD, Morgan B. a. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21(7):1358–66.
Jahoda CA, Reynolds AJ. Hair follicle dermal sheath cells: unsung participants in wound healing. Lancet. 2001;358(9291):1445–8.
Rodriguez-Menocal L, Salgado M, Ford D, Van Badiavas E. Stimulation of skin and wound fibroblast migration by mesenchymal stem cells derived from normal donors and chronic wound patients. Stem Cells Transl Med. 2012;1(3):221–9.
Liu L, Yu Y, Hou Y, Chai J, Duan H, Chu W, et al. Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats. PLoS One. 2014;9(2):e88348.
Basiouny HS, Salama NM, Mohamed Z, Maadawi E, Farag EA. Effect of bone marrow derived mesenchymal stem cells on healing of induced full-thickness skin wounds in albino rat. Int J Stem Cells. 2013;6(1):12–25.
Luo G, Cheng W, He W, Wang X, Tan J, Fitzgerald M, et al. Promotion of cutaneous wound healing by local application of mesenchymal stem cells derived from human umbilical cord blood. Wound Repair Regen. 2010;18(5):506–13.
Kong P, Xie X, Li F, Liu Y, Lu Y. Placenta mesenchymal stem cell accelerates wound healing by enhancing angiogenesis in diabetic Goto-Kakizaki (GK) rats. Biochem Biophys Res Commun. 2013;438(2):410–9.
Hong SJ, Jia S-X, Xie P, Xu W, Leung KP, Mustoe TA, et al. Topically delivered adipose derived stem cells show an activated-fibroblast phenotype and enhance granulation tissue formation in skin wounds. PLoS One. 2013;8(1):e55640.
Shin L, Peterson D. a. Human mesenchymal stem cell grafts enhance normal and impaired wound healing by recruiting existing endogenous tissue stem/progenitor cells. Stem Cells Transl Med. 2013;2(1):33–42.
Yoon BS, Moon J-H, Jun EK, Kim J, Maeng I, Kim JS, et al. Secretory profiles and wound healing effects of human amniotic fluid–derived mesenchymal stem cells. Stem Cells Dev. 2010;19(6):887–902.
Arno AI, Amini-Nik S, Blit PH, Al-Shehab M, Belo C, Herer E, et al. Human Wharton’s jelly mesenchymal stem cells promote skin wound healing through paracrine signaling. Stem Cell Res Ther. 2014;5(1):28.
Mishra PJ. Cell-free derivatives from mesenchymal stem cells are effective in wound therapy. World J Stem Cells. 2012;4(5):35.
Li M, Zhao Y, Hao H, Dai H, Han Q, Tong C, et al. Mesenchymal stem cell–conditioned medium improves the proliferation and migration of keratinocytes in a diabetes-like microenvironment. Int J Low Extrem Wounds. 2015;14(1):73–86.
Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214–22.
Shi Y, Shi H, Nomi A, Lei-lei Z, Zhang B, Qian H. Mesenchymal stem cell–derived extracellular vesicles: a new impetus of promoting angiogenesis in tissue regeneration. Cytotherapy. 2019;21(5):497–508.
Wu P, Zhang B, Shi H, Qian H, Xu W. MSC-exosome: a novel cell-free therapy for cutaneous regeneration. Cytotherapy. 2018;20(3):291–301.
Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22(6):845–54.
Marquez-Curtis LA, Gul-Uludag H, Xu P, Chen J, Janowska-Wieczorek A. CXCR4 transfection of cord blood mesenchymal stromal cells with the use of cationic liposome enhances their migration toward stromal cell-derived factor-1. Cytotherapy. 2013;15(7):840–9.
Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33(7):2158–68.
Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13(1):49.
Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Van Badiavas E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 2015;24(14):1635–47.
Kim S, Lee SK, Kim H, Kim TM. Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. Int J Mol Sci. 2018;19(10):pii: E3119.
Hu L, Wang J, Zhou X, Xiong Z, Zhao J, Yu R, et al. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep. 2016;6(1):32993.
Fang S, Xu C, Zhang Y, Xue C, Yang C, Bi H, et al. Umbilical cord-derived mesenchymal stem cell-derived Exosomal MicroRNAs suppress Myofibroblast differentiation by inhibiting the transforming growth factor-β/SMAD2 pathway during wound healing. Stem Cells Transl Med. 2016;5(10):1425–39.
Noronha Nc NDC, Mizukami A, Caliári-Oliveira C, Cominal JG, Rocha JLM, Covas DT, et al. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther. 2019;10(1):1–21.
Chen L, Xu Y, Zhao J, Zhang Z, Yang R, Xie J, et al. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS One. 2014;9(4):e96161.
Jun E, Zhang Q, Yoon B, Moon J-H, Lee G, Park G, et al. Hypoxic conditioned medium from human amniotic fluid-derived mesenchymal stem cells accelerates skin wound healing through TGF-β/SMAD2 and PI3K/Akt pathways. Int J Mol Sci. 2014;15(1):605–28.
Liu G-Y, Liu Y, Lu Y, Qin Y-R, Di G-H, Lei Y-H, et al. Short-term memory of danger signals or environmental stimuli in mesenchymal stem cells: implications for therapeutic potential. Cell Mol Immunol. 2016;13(3):369–78.
Ti D, Hao H, Tong C, Liu J, Dong L, Zheng J, et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13(1):308.
Anton D, Burckel H, Josset E, Noel G. Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci. 2015;16(3):5517–27.
Hsu S, Hsieh P-S. Self-assembled adult adipose-derived stem cell spheroids combined with biomaterials promote wound healing in a rat skin repair model. Wound Repair Regen. 2015;23(1):57–64.
Kwon SH, Bhang SH, Jang H-K, Rhim T, Kim B-S. Conditioned medium of adipose-derived stromal cell culture in three-dimensional bioreactors for enhanced wound healing. J Surg Res. 2015;194(1):8–17.
Xia Z, Zhang C, Zeng Y, Wang T, Ai G. Transplantation of BMSCs expressing hVEGF 165/hBD3 promotes wound healing in rats with combined radiation-wound injury. Int Wound J. 2014;11(3):293–303.
Qi Y, Jiang D, Sindrilaru A, Stegemann A, Schatz S, Treiber N, et al. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin wounds. J Invest Dermatol. 2014;134(2):526–37.
Yang D, Sun S, Wang Z, Zhu P, Yang Z, Zhang B. Stromal cell-derived factor-1 receptor CXCR4-overexpressing bone marrow mesenchymal stem cells accelerate wound healing by migrating into skin injury areas. Cell Reprogram. 2013;15(3):206–15.
Li Y, Zheng L, Xu X, Song L, Li Y, Li W, et al. Mesenchymal stem cells modified with angiopoietin-1 gene promote wound healing. Stem Cell Res Ther. 2013;4(5):113.
Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005;105(10):3793–801.
Nakamura Y, Ishikawa H, Kawai K, Tabata Y, Suzuki S. Enhanced wound healing by topical administration of mesenchymal stem cells transfected with stromal cell-derived factor-1. Biomaterials. 2013;34(37):9393–400.
Yew T-L, Hung Y-T, Li H-Y, Chen H-W, Chen L-L, Tsai K-S, et al. Enhancement of wound healing by human multipotent stromal cell conditioned medium: the paracrine factors and p38 MAPK activation. Cell Transplant. 2011;20(5):693–706.
Shohara R, Yamamoto A, Takikawa S, Iwase A, Hibi H, Kikkawa F, et al. Mesenchymal stromal cells of human umbilical cord Wharton’s jelly accelerate wound healing by paracrine mechanisms. Cytotherapy. 2012;14(10):1171–81.
Fong C-Y, Tam K, Cheyyatraivendran S, Gan S-U, Gauthaman K, Armugam A, et al. Human Wharton’s jelly stem cells and its conditioned medium enhance healing of excisional and diabetic wounds. J Cell Biochem. 2014;115(2):290–302.
Zhang B, Wu X, Zhang X, Sun Y, Yan Y, Shi H, et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl Med. 2015;4(5):513–22.
Wang X, Jiao Y, Pan Y, Zhang L, Gong H, Qi Y, et al. Fetal dermal mesenchymal stem cell-derived exosomes accelerate cutaneous wound healing by activating notch signaling. Stem Cells Int. 2019;2019:1–11.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Camões, S.P., Santos, J.M., Carvalho, F., Miranda, J.P. (2020). Mesenchymal Stem Cells for Cutaneous Wound Healing. In: Rodrigues, G., Roelen, B.A.J. (eds) Concepts and Applications of Stem Cell Biology. Learning Materials in Biosciences. Springer, Cham. https://doi.org/10.1007/978-3-030-43939-2_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-43939-2_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-43938-5
Online ISBN: 978-3-030-43939-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)