Abstract
Purpose of Review
This review provides an overview of the principal stages of wound healing, the populations of endogenous and therapeutic stem cells, applications of stem cells in specific types of wounds, and current approaches of stem cell delivery for tissue regeneration.
Recent Findings
New uses of progenitor stem cells have been developed for the treatment of wounds. Stem cells improve wound healing through both local and paracrine effects. Stem cell populations of therapeutic utility include embryonic stem cells, induced pluripotent stem cells, adult bone marrow and adipose-derived mesenchymal stem cells, as well as stem cells from skin, cord blood, and extra fetal tissue. Induced pluripotent stem cells mitigate many of the ethical and immunogenic concerns related to use of embryonically derived stem cells.
Summary
Skin, the largest organ in the human body, serves as a protective barrier for mammals. Both aging and disease contribute to loss of skin barrier function, which can result in consequences such as chronic wounds. Recent advances in many types of stem cell therapy may revolutionize treatment of difficult wounds. Optimal techniques for obtaining and delivering stem cells are still being refined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Structure and Development of Skin
The skin is composed of three layers, the epidermis, dermis, and hypodermis, which serve to protect the body from pathogens, irradiation, dehydration, and other physical stressors, as well as functioning as a thermoregulatory and sensory organ [1, 2]. The skin barrier may become damaged through a number of means, including burns, ischemia or hypoxia, trauma, poor nutritional or immune status, and infections or inflammatory processes. Wound healing is also impaired in many common chronic conditions, including diabetes, renal disease, vascular insufficiency, and poor mobility resulting in pressure-induced ulcers [3]. To repair damaged skin, the body utilizes progenitor stem cells for regeneration [4].
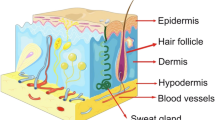
The epidermis, a multilayered epithelium, extends upward from the basement membrane, a fibrous extracellular matrix which houses progenitor stem cells that continuously self-renew and differentiate into keratinocytes. Keratinocytes, which constitute approximately 90% of epidermal cells, undergo terminal differentiation and maturation to ultimately form the outermost cornified layer of the skin, which confers its main barrier property [1, 4,5,6]. The dermis, connective tissue with an extensive extracellular matrix, is the thickest layer of the skin, accounting for its mechanical properties, providing strength and elasticity through fibroblasts that secrete precursors to collagen and elastin and contributing to the skin’s regenerative properties by housing vascular endothelial cells and accessory organs (sweat glands, sebaceous glands, and hair follicles), which also serve as nuclei for development of progenitor stem cells. Finally, the hypodermis, composed primarily of adipose cells, functions as insulation and cushion between the skin, muscle, and bone [4, 7].
Stages of Wound Healing
A basic understanding of the stages of normal wound healing is required to understand mechanisms by which stem cell therapies may improve wound healing; as such, stages of wound healing are reviewed here. Cutaneous tissue repair follows a complex sequential process of hemostasis, inflammation, proliferation, and maturation (remodeling), respectively [8,9,10,11,12,13,14,15,16]. To achieve these phases, numerous cytokines, growth factors, and cell types are required, including lymphocytes, neutrophils, macrophages, keratinocytes, fibroblasts, and endothelial cells [9, 17]. Various stem cells secrete cytokines and growth factors, accelerating or inducing phases of wound healing (Table 1). The earliest stage of wound healing, hemostasis, occurs rapidly after skin trauma via vascular constriction, fibrin clot formation, and secondary release of proinflammatory cytokines and growth factors from surrounding tissue, including platelet-derived growth factor (PDGF), transforming growth factor (TGF)-β, and epidermal growth factor (EGF). Together, these mediators and fibrin clots establish a transient wound matrix that serves as protection against fluid loss and pathogens, as well as a means for inflammatory cells to migrate (chemotaxis) to the area of injury [1, 4, 9, 10, 17].
The inflammatory (exudative) phase, which lasts approximately 4 days, is defined by localized erythema and edema due to involvement of neutrophils, macrophages, and lymphocytes. Neutrophils and lymphocytes defend against microbes, foreign or cellular debris to prevent infection, while macrophages aid in the disposal of necrotic tissue [4, 17]. These inflammatory cells also secrete growth factors (fibroblast growth factor [FGF], vascular endothelial growth factor [VEGF], TGF-β and -α), cytokines (IL-1α, IL-1β, IL-6, TNF-α), and prostaglandins to promote angiogenesis and recruitment of vascular endothelial cells, fibroblasts, and keratinocytes, ultimately forming granulation tissue [9, 18].
Granulation tissue marks transition into the proliferative stage, a 2–3-week period dedicated to re-epithelialization, marked by the differentiation of endothelial cells into mesenchymal components, production of collagen III, glycosaminoglycans, and proteoglycans, as well as continued removal of microbes and debris by macrophages [9]. To maintain successful wound healing, Kanji et al. note that keratinocytes and fibroblasts require a continuous paracrine loop of bidirectional communication [11, 13, 18]. Similarly, angiogenesis is critical for wound healing, which requires ample levels of oxygen and other nutrients. Epidermal stem cells have been shown to aid in wound healing through keratinocyte production, repair of accessory organs, and remodeling of damaged stroma [9].
The final stage is maturation, or remodeling, in which type III collagen is replaced by more permanent type I collagen. In addition, fibroblasts differentiate into myofibroblasts which then coordinate wound contraction by producing α-smooth muscle actin (α-SMA) and crosslinking it with type I collagen. Finally, granulation tissue growth terminates, and regression of capillaries ensues. Together, these processes occur over approximately 12 months and results in avascular and acellular scar [1, 4, 9, 10, 17]. Fully healed scar may only regain 70–80% of its original tensile strength. On occasion, problems with wound repair processes may lead to hypertrophic or keloidal scarring, chronic wounds, and numerous other skin pathologies.
Report
Role of Epidermal Stem Cells in Physiologic Wound Healing
Wound healing is the result of a complex cascade of cell-signaling events between immune-modulating cells through overlapping phases. Among these immune-modulating cells are populations of epidermal stem cells localized to hair follicles (HF), the interfollicular epidermis (IFE), and eccrine sweat and sebaceous glands (Fig. 1) [1, 19,20,21,22]. Dermal sheath stem cells and mesenchymal stem cells are present through all layers of the skin. Epidermal stem cells coordinate tissue regeneration through both local and paracrine effects. Chu et al. demonstrated that epidermal stem cells transition between two life cycles: a slow phase during which they remain quiescent and an active phase, following skin injury, in which they contribute to re-epithelization [23].
Locally, cells within the basal membrane of the epidermis maintain continual mitotic activity until they detach and migrate towards the skin surface, during which process they undergo terminal differentiation to become mature keratinocytes. Multipotent mesenchymal stromal cells (MSCs) aid in type I and III collagen production, thus forming the connective tissue matrix necessary for wound healing [24, 25]. Within sebaceous glands, a thin layer of progenitor basaloid cells and sebocytes collectively maintain production of sebum, which empties into hair follicles to lubricate and waterproof the skin. Multipotent cutaneous stem cells of the hair follicle are localized to the bulge of the outer root sheath, which is contiguous with the epidermis. Research by Ito et al. demonstrated de novo production of hair follicles after wounding in genetically normal adult mice, as well as an increased production of hair follicles with Wnt pathway overexpression. This production of hair follicles was decreased with inhibition of Wnt [26]. Paralleling work by Shi et al. and Whyte et al., this suggests that sites of cutaneous injury revert to an embryonic phenotype to amplify regeneration and reduce scarring [27, 28]. Both mesenchymal stem cells and melanocyte precursors frequently localize to the bulge region of hair follicles—an ideal environment for cell proliferation due to the highly innervated, vascularized, and protected nature of follicular structures. Wong et al. also described dermally derived MSCs of two distinct populations, one localized to the dermal papilla of hair follicles and another identified as perivascular stem cells [29].
Efficient wound healing response is partially dependent on paracrine signaling, for which MSCs are key players. MSCs are defined by their ability to self-renew and produce multiple cell lineages, including osteoblasts, adipocytes, chondrocytes, tenocytes, and myocytes. In skin healing, epidermally derived MSCs can express a fibroblast-like morphology and a “secretome,” a collection of protein which includes several growth factors and cytokines. These signaling proteins modulate the inflammatory response to promote accelerated cell migration and neovascularization. They also function as survival factors on neighboring differentiated cells to promote epithelialization, increase granulation tissue formation, and inhibit scar formation [2, 13, 30, 31, 32•]. As MSCs are free of many of the ethical concerns surrounding use of embryonic stem cells, they have become attractive modalities for research (Fig. 2).
Categories of Stem Cells and Utility in Wound Healing
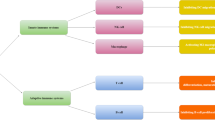
Endogenous stem cells, being multipotent and self-renewing, hold significant therapeutic potential in wound healing, particularly through secretion of pro-regenerative cytokines and ability to modulate cell-signaling pathways. Unlike tissue-engineered skin substitutes, stem cells allow for continually adaptive tissue properties to best support wound healing. Several studies have shown stem cell lineages that are active throughout all four phases of wound healing [4, 33]. However, due to the multipotent nature of stem cells, risks associated with this therapeutic modality include concerns of potential immunogenicity and tumorigenicity. In this section, we provide an overview of seven stem cell populations and their applications in wound healing: mesenchymal stem cells derived from skin, MSC from bone marrow, MSC from adipose tissue, stem cells derived from cord blood, stem cells derived from extra fetal tissue, embryonic stem cells, and induced pluripotent stem cells (Fig. 3) [4, 24, 33]. It should be noted that at this time all of these therapies are in research stages and are not yet commercially available.
For cutaneous wound healing, the most well-studied stem cell populations are of mesenchymal origin. Mesenchyme, derived from embryonic mesoderm, is able to differentiate into either connective tissue or hematopoietic cells, whereas MSCs or multipotent stromal progenitor cells may only differentiate into connective tissue constituents. Per definitions by the International Society of Cellular Therapy, MSCs express CD73, CD90, and CD105, which allow them to adhere to a plastic surface, and lack hematopoietic markers CD14, CD34, CD45, CD11b/CD79, and CD19/HLA-DR [34]. MSCs preferentially reside in bone marrow and adipose tissue but may also be found in the skin. They also localize to synovial fluid, traumatized muscle, corneal stroma, and fetal tissue. MSCs aid epithelialization through paracrine effects rather than direct structural contributions to wound sites [35,36,37,38]. MSCs contribute to extracellular matrix formation, angiogenesis, and cell differentiation by secreting matrix metalloproteinase-9 (MMP), IL-6, IL-10, TNF-a, VEGF, EGF, IGF, and Ang-1. MSCs modulate immune cells via regulation of migration and proliferation and reduce scar formation through prostaglandin E2 secretion [2, 31, 32•, 39, 40]. Of MSCs affecting the inflammatory phase, bone marrow-derived stem cells (BM-MSCs) appear to be the major contributor. BM-MSCs produce higher amounts of procollagen, growth factors, and angiogenic factors compared to dermal fibroblasts [4].
In a randomized control trial, Chen et al. utilized both topical and injectable MSC-conditioned medium, with cells derived from bone marrow and adipose tissue, on full-thickness wounds in mice which demonstrated accelerated wound closure and improved tensile strength [41]. Wu et al. obtained similar results with diabetic wounds and excisional wounds, respectively [42]. Falanga et al. showed improved wound healing in both acute surgical and chronic lower extremity wounds with topical fibrin polymer spray mixed with BM-MSCs [43]. Findings were attributed to increased neovascularization, attenuation of the inflammatory response, and differentiation of wound cells (keratinocytes, endothelial cells, and pericytes) by MSCs. However, large wounds may be impractical to treat with this modality as they require substantial quantities of MSC culture.
Adipose-derived mesenchymal stem cells (ASCs) are another promising population of stem cells for wound healing, due to their multipotent nature, allowing them to differentiate into bone, fat, cartilage, and muscle tissue. These cells localize to the stromal-vascular portion of enzymatically digested fat [44•]. ASCs contribute to accelerated wound healing and scar reduction by stimulating angiogenesis and subcutaneous tissue production [45]. Adipose tissue may be extracted with minimal donor morbidity from solid tissue specimens, through excision, or in liposuction aspirates. Several studies have shown no malignant transformation from ASC transplantation within at least 120 days, and the procedure is generally well-tolerated. In comparison to bone marrow aspiration, it is relatively easy to obtain high yields of ASCs from subcutaneous tissue or lipoaspirate. Due to all these benefits, ASCs have become a useful modality for treatment of acute wounds, such as burns, as well as refractory wounds, such as diabetic foot ulcers [2, 46, 47].
Recent studies have also shown accelerated wound healing with MSCs from both umbilical cord blood (UCB) and extra-embryonic fetal tissue. As with ASCs, UCB stem cells are an attractive treatment modality due to easy accessibility of large numbers of stem cells [33]. UCB stem cells express CD24+ surface markers which accelerate wound healing through inhibition of several matrix metalloproteinases, causing decreased inflammatory response, as well as promoting neovascularization and collagen production. UCB stem cells have been utilized for refractory wounds with good success in large traumatic wounds and in diabetic ulcers. Multipotent MSCs may also be derived from extra-embryonic fetal tissue and have been cultured from amniotic fluid, Wharton’s jelly, and placental tissue [48,49,50]. These sources, however, are considered less favorable due to risk of immune-mediated rejection, transmission of genetic diseases, and potential for malignant transformation.
Embryonic stem cells (ESCs) are also a less favorable source due to ethical considerations. These pluripotent cells originate in human blastocysts and possess the ability to differentiate into any germ layer: ectoderm, endoderm, or mesoderm. They have been used to successfully foster keratinocytes and subsequently rebuild sections of epidermis [51]. Because of their pluripotency, they hold significant potential in both wound healing and constructing bioengineered skin substitutes [11]. However, when compared with commercially available skin substitutes, such as xenografts or hydrocolloid dressings, ESCs have shown increased costs and few advantages. Their robust pluripotent capacity also results in increased risk of immunogenicity and tumorigenicity [4, 52]. Finally, debate persists regarding ethically acceptable use of cells harvested from human embryos, largely centered on the metaphysical and controversial questions of precisely when an embryo becomes a person and whether embryos that would otherwise be discarded are ethically appropriate to use for research purposes. Fortunately, advances in procurement of adult stem cells and the development of induced pluripotent stem cells have reduced the acuity of this debate, as acceptable alternatives not fraught with the ethical concerns surrounding embryonic stem cells are now available [53].
Induced pluripotent stem cells (iPSC) are a relatively recently engineered type of multipotent and hybrid stem cell population. They originate from adult somatic cells (such as keratinocytes, fibroblasts, lymphocytes, or hepatocytes) that are de-differentiated through application of a cocktail of transcription factors, such as Oct-3, Oct-4, Sox2, c-Myc, and KLF4 [48, 54, 55]. Accordingly, iPSCs may be obtained from adult tissue and cultured indefinitely and, thus, may be an invaluable source for regenerative medicine. When a patient’s somatic cells are harvested, reprogrammed, and replaced as iPSCs, little to no immunogenicity has been found [55]. However, some potential for genetic instability and tumorigenicity has been observed, though ongoing work attempts to improve these risks. Use of iPSCs in wounds rats has demonstrated increased angiogenesis and collagen production [56]. iPSCs have been successfully utilized for treatment of wounds from recessive dystrophic epidermolysis bullosa [57, 58]. As iPSCs are derived from adult cells, they present less ethical controversy than stem cells derived from umbilical cord blood, extra-embryonic fetal tissue, or embryonic stem cells.
Special Cases: Chronic Wounds, Burns, and Corneal Ulcerations
Cutaneous wound healing involves a complex interplay of several cell populations and signaling molecules through sequential phases of repair. This well-orchestrated process may be impaired due to local or systemic factors, such as age, systemic diseases, continued trauma, ischemia, pressure, or exposure to substances such as tobacco [17]. Chronic wounds, though still incompletely understood, arise from an inability to meet the biologic demands of cutaneous tissue repair. Inadequate neovascularization is often a primary characteristic of chronic wounds, and a well-vascularized dermal wound bed appears essential for viability of treatment with stem cells or keratinocytes [59]. Human cells require adequate nutrients and oxygen to thrive, whereas microbes can proliferate in anaerobic conditions and immune response is compromised without sufficient oxygen. Cutaneous wounds typically require oxygen tension of at least 20 mmHg to heal, but chronic wounds often demonstrate oxygen tension of less than 5 mmHg [10]. Hypoxia immediately following traumatic injury stimulates angiogenesis via the release of cytokines and growth factors, but sustained healing thereafter requires restoration of adequate oxygenation [17]. Overwhelming or chronic inflammation also hinders wound healing by dysregulating cell signaling within the wound. For example, inflammation deregulates protease and fibroblast proliferation, decreasing collagen formation, increasing ECM deposition, and ultimately inducing hypertrophic scars.
Historically, treatments for chronic wounds have been limited to optimizing local wound health through debridement and dressing changes, surgical intervention, antibiotics, or use of compression and pressure-relieving devices. Improvement of systemic factors, such as obesity, hyperglycemia, venous stasis, decreased cardiac output, or smoking, has also been helpful in healing of chronic wounds [60,61,62]. More recently, stem cell therapies have shown promising results in the treatment of chronic wounds. As detailed previously, MSCs stimulate angiogenesis and modulate the immune response, anti-microbial properties, and structural integrity of wounds [63, 64]. In chronic wounds in rats, application of MSCs causes an increase in IL-10, an anti-inflammatory cytokine, and LL-37, an antimicrobial peptide [65,66,67].
MSCs have shown similar promise in the treatment of burn wounds, with decreased inflammation and subsequently faster healing, and decreased fibrosis, contraction, and scar formation [42, 67]. Commonly, full-thickness burns are repaired with full- or split-thickness skin grafts. However, these treatments are limited by availability of suitable skin grafts, and the risks of infection, fluid loss, and graft loss. MSCs have been used to rebuild both dermal and epidermal layers in full-thickness burns [68]. Stimulation of MSCs in hair bulbs of scalp burns can lead to re-epithelialization of skin layers and return of functioning of hair follicles and sebaceous glands [69, 70]. Stem cells within burns are induced by human alpha defensin 5, CXCL12, and CXCL4 pathways [71]. Substantial hair and epithelial growth in burn wounds has been obtained through use of progenitor cells and cytokines in amniotic fluid [72]. Intravenous injection of umbilical cord blood in rats has demonstrated increases in IL-10, VEGF, and healing of severe burns [65]. BM-MSCs were first used in human burns in 2004 and showed signs of faster healing and angiogenesis in extensive burns. In subsequent rat studies, BM-MSCs also caused decreased formation of granulation tissue in burn wounds [73]. Addition of BM-MSCs to skin treated with bleomycin demonstrated decreased fibrosis during healing [42]. Finally, autologous adipose-derived MSCs appear to accelerate healing and result in decreased pain and necrosis in burns [46, 74].
Corneal wounds have also been treated with stem cell therapy. Corneal opacity, often due to infection, burns, or trauma, is a common cause of blindness. Blindness ensues when the extent of injury outweighs the regenerative capacity of native corneal epithelial stem cells [75]. Autologous limbal stem cells (corneal MSCs) have been utilized in several trials of corneal injuries from burns and trauma, with substantial benefits in revascularization, re-epithelialization, decreased irritation, and improved vision in human and animal trials [72, 76].
Delivery Approaches of Stem Cells for Wound Healing
In attempting to deliver stem cells to wounds, several approaches have been used. Systemic administration is appealing for extensive wounds, but somewhat limited by specificity of tissue targeting and difficulties with proportionately high cell loss. Concomitant administration of other factors may help improve targeting: for instance, early clinical trials in bone healing have shown that addition of intermittent parathyroid hormone therapy to MSC therapy speeds repair of rib fractures [77].
In general, local delivery approaches for stem cells have been preferred due to ease of targeting. However, inflammation within wounds makes them difficult environments for single cells to engraft and survive. Trials involving injection of stem cells into wounds have shown durable engraftment rates as low as 0%, likely due to shear injury to fragile cells during application [78]. Topical sprays have been used to apply stem cells, but they do not provide any protection to cells either and do not allow for fine control of cell spacing [43].
Delivery of stem cells within bioscaffolds has become the most popular and marketable technique. Numerous products exist, consisting of both naturally derived and synthetic molecules, with stem cells seeded within these matrices. These provide a framework for stem cells and thus grant structural protection; they also appear to maintain stem cells in a pre-differentiated state for longer, extending expression of genes unique to stem cells [79]. Several decellularized bioscaffold allogenic skin substitutes are currently commercially available, though none of these products contain living stem cells at this time [80,81,82,83,84,85]. Though all of these products appear to accelerate wound healing, problems with wound contracture or hypopigmentation due to loss of melanocytes are common following healing. In experimental settings, similar bioscaffolds have been used for delivery of living stem cells and appear to confer improved cellular survival and engraftment [68, 78, 79, 84].
More recently, devices such as the CelluTome™ have been used to update and standardize the traditional technique of “pinch grafting” [86]. Epidermal micrografts containing epidermal MSCs are obtained from normal skin with minimal donor site morbidity and are then minced and distributed over wounds, sometimes in combination with a hydrogel. The transplanted cells proliferate and expand to heal the wound [87•].
Conclusion
Wound healing occurs physiologically through a straightforward sequence, but this process may often become derailed due to age, disease, local factors, or other causes. Stem cell therapy in many forms has emerged as a promising approach for these difficult wounds (Fig. 2). Growth factors and cytokines released by stem cells introduced to wounds promote healing through improved angiogenesis and immune modulation. No consensus has yet been reached on the optimal types of stem cell or delivery methods for various types of wounds, but much translational work has already been done with direct benefit to patients. Research in this aspect of regenerative medicine continues to actively progress. Further studies are likely to build on the findings discussed in this review, refining techniques to harvest and deliver stem cells to optimize engraftment and wound healing. These advances may revolutionize the treatment of problematic wounds.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Li Y, Zhang J, Yue J, Gou X, Wu X. Epidermal stem cells in skin wound healing. Adv Wound Care. 2017;6(9):297–307. https://doi.org/10.1089/wound.2017.0728.
Lee DE, Ayoub N, Agrawal DK. Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res Ther. 2016;7(1):37. https://doi.org/10.1186/s13287-016-0303-6.
Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6:265. https://doi.org/10.1126/scitranslmed.3009337.
Chen M, Przyborowski M, Berthiaume F. Stem cells for skin tissue engineering and wound healing. Crit Rev Biomed Eng. 2009;37(4–5):399–421. https://doi.org/10.1615/critrevbiomedeng.v37.i4-5.50.
McGrath JA, Eady RAJ, Pope FM. Anatomy and organization of human skin. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook’s textbook of dermatology. 7th ed. Hoboken: Blackwell Publishing; 2004. p. 4190. https://doi.org/10.1002/9780470750520.ch3. ISBN 978-0-632-06429-8. Retrieved 2010-06-01.
Bouwstra JA, Ponec M. The skin barrier in healthy and diseased state. Biochim Biophys Acta Biomembr. 2006;1758(12):2080–95. https://doi.org/10.1016/j.bbamem.2006.06.021.
Harvey C. Wound healing. Orthop Nurs. 2005;24(2):143–57.
Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–21.
Gonzalez ACDO, Costa TF, Andrade ZDA, Medrado ARAP. Wound healing—a literature review. An Bras Dermatol. 2016;91(5):614–20. https://doi.org/10.1590/abd1806-4841.20164741.
Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599–610. https://doi.org/10.1007/s12325-017-0478-y.
Kanji S, Das H. Advances of stem cell therapeutics in cutaneous wound healing and regeneration. Mediat Inflamm. 2017;2017:1–14. https://doi.org/10.1155/2017/5217967.
Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40(11):1291–9. https://doi.org/10.1038/ng.239.
Cerqueira MT, Pirraco RP, Marques AP. Stem cells in skin wound healing: are we there yet? Adv Wound Care. 2016;5(4):164–75. https://doi.org/10.1089/wound.2014.0607.
Bergen TV, Velde SVD, Vandewalle E, Moons L, Stalmans I. Improving patient outcomes following glaucoma surgery: state of the art and future perspectives. Clin Ophthalmol. 2014:857. https://doi.org/10.2147/opth.s48745.
Beitz JM. Pharmacologic impact (aka “breaking bad”) of medications on wound healing and wound development: a literature-based overview. Advances in pediatrics. https://www.ncbi.nlm.nih.gov/pubmed/28355136. Published March 2017. Accessed 6 June 2018.
Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Perspective article: growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585–601. https://doi.org/10.1111/j.1524-475x.2008.00410.x.
Guo S, Dipietro L. Factors affecting wound healing. J Dent Res. 2010;89(3):219–29. https://doi.org/10.1177/0022034509359125.
Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–70.
Smola H, Thiekotter G, Fusenig NE. Mutual induction of growth factor gene expression by epidermal-dermal cell interaction. J Cell Biol. 1993;122(2):417–29.
Kumar A, Mohanty S, Gupta S, Paulkhurana SM. Stem cells of the hair follicular tissue: application in cell based therapy for vitiligo. Hair Ther Transplant. 2015;05(01). https://doi.org/10.4172/2167-0951.1000132.
Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327(5971):1385–9. https://doi.org/10.1126/science.1184733.
Woo W-M, Oro AE. SnapShot: hair follicle stem cells. Cell. 2011;146(2):334–334.e2. https://doi.org/10.1016/j.cell.2011.07.001.
Chu G-Y, Chen Y-F, Chen H-Y, Chan M-H, Gau C-S, Weng S-M. Stem cell therapy on skin: mechanisms, recent advances and drug reviewing issues. J Food Drug Anal. 2018;26(1):14–20. https://doi.org/10.1016/j.jfda.2017.10.004.
Opalenik SR, Davidson JM. Fibroblast differentiation of bone marrow-derived cells during wound repair. FASEB J. 2005;19(11):1561–3. https://doi.org/10.1096/fj.04-2978fje.
Fathke C. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells. 2004;22(5):812–22. https://doi.org/10.1634/stemcells.22-5-812.
Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447(7142):316–20. https://doi.org/10.1038/nature05766.
Shi Y, Shu B, Yang R, et al. Wnt and Notch signaling pathway involved in wound healing by targeting c-Myc and Hes1 separately. Stem Cell Res Ther. 2015;6(1):120. https://doi.org/10.1186/s13287-015-0103-4.
Whyte JL, Smith AA, Liu B, Manzano WR, Evans ND, Dhamdhere GR, et al. Augmenting endogenous Wnt signaling improves skin wound healing. PLoS One. 2013;8(10):e76883. https://doi.org/10.1371/journal.pone.0076883.
Wong VW, Levi B, Rajadas J, Longaker MT, Gurtner GC. Stem cell niches for skin regeneration. Int J Biomater. 2012;2012:1–8. https://doi.org/10.1155/2012/926059.
Agrawal GK, Jwa N-S, Lebrun M-H, Job D, Rakwal R. Plant secretome: unlocking secrets of the secreted proteins. Proteomics. 2010;10(4):799–827. https://doi.org/10.1002/pmic.200900514.
Baraniak PR, Mcdevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5(1):121–43. https://doi.org/10.2217/rme.09.74.
• Denu RA, Nemcek S, Bloom DD, et al. Fibroblasts and mesenchymal stromal/stem cells are phenotypically indistinguishable. Acta Haematol. 2016;136(2):85–97. https://doi.org/10.1159/000445096 This study demonstrates indistinguishable cell surface makers and morphology between fibroblasts and mesenchymal stem cells. This supports our understanding that MSCs parallel fibroblasts in their wound healing capacity: both cell types secrete extracellular matrix and suppress the inflammatory cascade.
Duscher D, Barrera J, Wong VW, Maan ZN, Whittam AJ, Januszyk M, et al. Stem cells in wound healing: the future of regenerative medicine? A mini-review. Gerontology. 2015;62(2):216–25. https://doi.org/10.1159/000381877.
Dominici M, Blanc KL, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. https://doi.org/10.1080/14653240600855905.
Gorskaya YF, Fridenshtein AY, Kulagina NN. Precursor cells of fibroblasts detected by in vitro cloning of cells from hematopoietic organs of normal and irradiated mice. Bull Exp Biol Med. 1976;81(5):765–8. https://doi.org/10.1007/bf00797159.
Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5(5):362–9. https://doi.org/10.1080/14653240310003026.
Meliga E, Strem BM, Duckers HJ, Serruys PW. Adipose-derived cells. Cell Transplant. 2007;16(9):963–70. https://doi.org/10.3727/096368907783338190.
Jones EA, English A, Henshaw K, Kinsey SE, Markham AF, Emery P, et al. Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis Rheum. 2004;50(3):817–27. https://doi.org/10.1002/art.20203.
Rezvani HR, Ali N, Nissen LJ, Harfouche G, de Verneuil H, Taïeb A, et al. HIF-1a in epidermis: oxygen sensing, cutaneous angiogenesis, cancer, and non-cancer disorders. J Investig Dermatol. 2011;131:1793–805.
Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013;31(1):285–316. https://doi.org/10.1146/annurev-immunol-032712-095919.
Chen L, Tredget EE, Wu PYG, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3(4):e1886. https://doi.org/10.1371/journal.pone.0001886.
Wu Y, Zhao RC, Tredget EE. Concise review: bone marrow-derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells. 2010;28:905–15.
Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, et al. Autologous bone marrow derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299–312.
• Summa PGD, Schiraldi L, Cherubino M, et al. Adipose derived stem cells reduce fibrosis and promote nerve regeneration in rats. The Anatomical Record. 2018. https://doi.org/10.1002/ar.23841 This study illustrates the therapeutic potential of adipose derived stem cells by demonstrating reduced scar formation and increased nerve regeneration.
Altman AM, Matthias N, Yan Y, et al. Dermal matrix as a carrier for in vivo delivery of human adipose-derived stem cells. Biomaterials. 2008;29(10):1431–42.
Riccobono D, Agay D, Scherthan H, Forcheron F, Vivier M, Ballester B, et al. Application of adipocyte-derived stem cells in treatment of cutaneous radiation syndrome. Health Phys. 2012;103(2):120–6.
Alexaki V-I, Simantiraki D, Panayiotopoulou M, Rasouli O, Venihaki M, Castana O, et al. Adipose tissue-derived mesenchymal cells support skin reepithelialization through secretion of KGF-1 and PDGF-BB: comparison with dermal fibroblasts. Cell Transplant. 2012;21(11):2441–54. https://doi.org/10.3727/096368912x637064.
Anker PSI’t. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102(4):1548–9. https://doi.org/10.1182/blood-2003-04-1291.
Alviano F, Fossati V, Marchionni C, Arpinati M, Bonsi L, Franchina M, et al. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol. 2007;7:11.
Kim W-S, Park B-S, Sung J-H, Yang JM, Park SB, Kwak SJ, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48(1):15–24. https://doi.org/10.1016/j.jdermsci.2007.05.018.
Guenou H, Nissan X, Larcher F, Feteira J, Lemaitre G, Saidani M, et al. Human embryonic stem-cell derivatives for full reconstruction of the pluristratified epidermis: a preclinical study. Lancet. 2009;374(9703):1745–53. https://doi.org/10.1016/s0140-6736(09)61496-3.
Douglas CW. Embryonic stem cell transplantation: potential applicability in cell replacement therapy and regenerative medicine. Front Biosci. 2007;12(8–12):4525. https://doi.org/10.2741/2407.
Lo B, Parham L. Ethical issues in stem cell research. Endocr Rev. 2009;30(3):204–13. https://doi.org/10.1210/er.2008-0031.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76.
Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–84.
Tsai S-Y, Clavel C, Kim S, Ang YS, Grisanti L, Lee DF, et al. Oct4 and Klf4 reprogram dermal papilla cells into induced pluripotent stem cells. In: Stem Cells; 2009. https://doi.org/10.1002/stem.281.
Sebastiano V, Zhen HH, Haddad B, Bashkirova E, Melo SP, Wang P, et al. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6(264):264ra163. https://doi.org/10.1126/scitranslmed.3009540.
Umegaki-Arao N, Pasmooij AMG, Itoh M, Cerise JE, Guo Z, Levy B, et al. Induced pluripotent stem cells from human revertant keratinocytes for the treatment of epidermolysis bullosa. Sci Transl Med. 2014;6(264):264ra164. https://doi.org/10.1126/scitranslmed.3009342.
Macneil S. Progress and opportunities for tissue-engineered skin. Nature. 2007;445(7130):874–80. https://doi.org/10.1038/nature05664.
Werdin F, Tenenhaus M, Rennekampff H-O. Chronic wound care. Lancet. 2008;372(9653):1860–2. https://doi.org/10.1016/s0140-6736(08)61793-6.
Steed DL, Attinger C, Colaizzi T, Crossland M, Franz M, Harkless L, et al. Guidelines for the treatment of diabetic ulcers. Wound Repair Regen. 2006;14(6):680–92.
Robson MC, Barbul A. Guidelines for the best care of chronic wounds. Wound Repair Regen. 2006;14(6):647–8.
Krasnodembskaya A, Song Y, Fang X, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28(12):2229–38.
Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2):141–50. https://doi.org/10.1016/j.stem.2007.11.014.
Liu L, Yu Y, Hou Y, et al. Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats. PLoS One. 9(2):e88348, 2014.
Németh K, Leelahavanichkul A, Yuen PST, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2008;15(1):42–9. https://doi.org/10.1038/nm.1905.
Benbernou N, Esnault S, Shin HCK, Fekkar H, Guenounou M. Differential regulation of IFN-gamma, IL-10 and inducible nitric oxide synthase in human T cells by cyclic AMP-dependent signal transduction pathway. Immunology. 1997;91(3):361–8. https://doi.org/10.1046/j.1365-2567.1997.00260.x.
Natesan S, Zamora DO, Wrice NL, Baer DG, Christy RJ. Bilayer hydrogel with autologous stem cells derived from debrided human burn skin for improved skin regeneration. J Burn Care Res. 2013;34(1):18–30.
Watt FM, Lo Celso C, Silva-Vargas V. Epidermal stem cells: an update. Curr Opin Genet Dev. 2006;16:518–24.
Yang Y, Zhang W, Li Y, Fang G, Zhang K. Scalded skin of rat treated by using fibrin glue combined with allogeneic bone marrow mesenchymal.
Hu C, Yong X, Li C, Lü M, Liu D, Chen L, et al. CXCL12/CXCR4 axis promotes mesenchymal stem cell mobilization to burn wounds and contributes to wound repair. J Surg Res. 2013;183(1):427–34. https://doi.org/10.1016/j.jss.2013.01.019.
Basu S, Ali H, Sangwan VS. Clinical outcomes of repeat autologous cultivated limbal epithelial transplantation for ocular surface burns. Am J Ophthalmol. 2012;153(4). https://doi.org/10.1016/j.ajo.2011.09.016.40.
Rasulov MF, Vasilenko VT, Zaidenov VA, Onishchenko NA. Cell transplantation inhibits inflammatory reaction and stimulates repair processes in burn wound. Bull Exp Biol Med. 2006;142(1):112–5. https://doi.org/10.1007/s10517-006-0306-x.
Collawn SS, Banerjee NS, de la Torre J, Vasconez L, Chow LT. Adipose-derived stromal cells accelerate wound healing in an organotypic raft culture model. Ann Plast Surg. 2012;68(5):501–4. https://doi.org/10.1097/SAP.0b013e31823b69fc.
Shortt AJ, Secker GA, Notara MD, Limb GA, Khaw PT, Tuft SJ, et al. Transplantation of ex vivo cultured limbal epithelial stem cells: a review of techniques and clinical results. Surv Ophthalmol. 2007;52(5):483–502. https://doi.org/10.1016/j.survophthal.2007.06.013.
Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000;44(5):415–25.
Cohn Yakubovich D, Sheyn D, Bez M, Schary Y, Yalon E, Sirhan A, et al. Systemic administration of mesenchymal stem cells combined with parathyroid hormone therapy synergistically regenerates multiple rib fractures. Stem Cell Res Ther. 2017;8:51. https://doi.org/10.1186/s13287-017-0502-9.
Garg RK, Rennert RC, Duscher D, Sorkin M, Kosaraju R, Auerbach LJ, et al. Capillary force seeding of hydrogels for adipose-derived stem cell delivery in wounds. Stem Cells Transl Med. 2014;3(9):1079–89. https://doi.org/10.5966/sctm.2014-0007.
Rustad KC, Wong VW, Sorkin M, Glotzbach JP, Major MR, Rajadas J, et al. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials. 2012;33(1):80–90. https://doi.org/10.1016/j.biomaterials.2011.09.041.
Stem cell basics. Sigma-Aldrich. https://www.sigmaaldrich.com/technical-documents/articles/biology/what-are-stem-cells.html. Accessed 5 June 2018.
Little M-T, Storb R. History of haematopoietic stem-cell transplantation. Nature News. https://www.nature.com/articles/nrc748. Published March 1, 2002. Accessed 5 June 2018.
Markers & methods to verify mesenchymal stem cell identity, potency, & quality. R&D Systems. https://www.rndsystems.com/resources/articles/markers-and-methods-verify-mesenchymal-stem-cell-identity-potency-and-quality. Accessed 5 June 2018.
Boston Children's Hospital. Boston Children’s Hospital. http://stemcell.childrenshospital.org/about-stem-cells/history/. Accessed 5 June 2018.
Bello YM, Falabella AF, Eaglstein WH. Tissue-engineered skin. Am J Clin Dermatol. 2001;2(5):305–13. https://doi.org/10.2165/00128071-200102050-00005.
Marston WA, Hanft J, Norwood P, Pollak R. The efficacy and safety of dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26(6):1701–5. https://doi.org/10.2337/diacare.26.6.1701.
Wheeland RG. The technique and current status of pinch grafting. J Dermatol Surg Oncol. 1987;13(8):873–81. https://doi.org/10.1111/j.1524-4725.1987.tb00564.x.
• Osborne SN, Schmidt MA, Harper JR. An automated and minimally invasive tool for generating autologous viable epidermal micrografts. Adv Skin Wound Care. 2016;29(2):57–64. https://doi.org/10.1097/01.ASW.0000476072.88818.aa This study outlines a novel, minimally invasive epidermal harvesting tool that aids in rapid wound healing and reduced scarring at both donor and recipient graft sites.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Wound Care and Healing
Rights and permissions
About this article
Cite this article
Aronson, A., Laageide, L. & Powers, J. Use of Stem Cells in Wound Healing. Curr Derm Rep 7, 278–286 (2018). https://doi.org/10.1007/s13671-018-0233-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13671-018-0233-x