Abstract
Dysregulation of insulin/insulin-like growth factor (IGF) pathways is a major feature of both the metabolic syndrome (MetS) and cancer. This chapter explains the molecular events linking MetS to carcinogenesis, thereby focusing on the insulin/IGF signaling. Specific differences in receptor expression, ligand affinity, and substrate activation enabling differential signaling of insulin and IGFs are summarized.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Insulin
- Insulin-like growth factor
- IGF1
- IGF2
- Insulin receptor
- IR
- IGF receptor
- IGF1R
- Hyperinsulinemia
- Metabolic syndrome
- Insulin resistance
- Warburg effect
4.1 Introduction
Both the metabolic syndrome (MetS) and cancer constitute a growing health problem worldwide. In the last decades, MetS as a risk factor for cancer has become apparent [1]. The MetS is a cluster of risk factors for both cardiovascular disease and type 2 diabetes and includes glucose intolerance or insulin resistance together with two or more of the following components: raised arterial pressure, raised plasma triglyceride and/or low HDL-C, central obesity, and microalbuminuria. Jaggers and colleagues demonstrated in a study with more than 30,000 patients that the MetS is associated with an increased risk of all-cause cancer mortality in men [2]. Also other studies reported that the individual components of the MetS independently increase the risk for the development of certain cancer types [3,4,5]. For example, MetS was described to be associated with increased incidences of colorectal and prostate cancer, and with the recurrence of breast cancer [6,7,8]. A meta-analysis reported an association of MetS with liver, colorectal, bladder, endometrial, pancreatic, and breast cancers [9].
The mechanisms linking MetS and cancer risk are not completely understood. MetS may be only concomitant with other cancer risk factors, such as decreased physical activity, consumption of high calorie foods, high dietary fat intake, low-fiber intake, and oxidative stress [9]. Still, adiposity, in particular visceral obesity, results in a chronic inflammatory state, in which adipocytes and infiltrating immune cells create a pro-tumorigenic environment by producing inflammatory cytokines and chemokines [10]. The obesity-driven altered balance between proinflammatory and antiinflammatory cytokines influences insulin sensitivity [11]. Increased concentrations of inflammatory cytokines suppress insulin signal transduction, which, in turn, promotes inflammation [12, 13]. Chronic inflammation is commonly known to promote tumorigenesis [14].
Also other symptoms of MetS have been linked to insulin resistance and type 2 diabetes, i.e., high blood pressure and hypertriglyceridemia [15]. Insulin resistance can predict microalbuminuria [16].
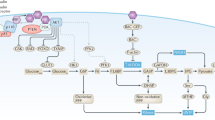
This chapter focuses on the link between type 2 diabetes and cancer, thereby omitting other symptoms of MetS. Especially alterations in the insulin metabolism seem to increase cancer risk [17,18,19]. Patients with type 2 diabetes were reported to show increased cancer risk, which may be caused by hyperinsulinemia, elevated IGF1, or potentially both factors [20]. While normal cells often show little responsiveness toward insulin and IGF-dependent growth stimulation, tumor cells highly express both insulin and IGF1 receptors [20] (Fig. 4.1). Insulin resistance is characterized by a defective classical metabolic signaling. At the same time, altered signaling is induced due to increased levels of insulin, IGFs, and other factors as discussed below. Low insulin, IGF1, and IGF2 levels appear to protect from tumorigenesis [21].
Cancer promoting insulin/IGF signaling during insulin resistance. In normal cells of insulin target tissues, high glucose and insulin levels lead to glucose uptake and metabolic actions such as glucogen synthesis and lipid synthesis. Nonclassical insulin target tissues lack mechanisms which regulate mitogenic actions of insulin. In insulin resistance, increased systemic levels of insulin and glucose induce hepatic IGF1 production, which can lead to tumorigenesis due to the growth and survival-promoting effects of IGF1, especially in nonclassical insulin target organs. Elevated insulin and glucose levels can elicit an elevated generation of reactive oxygen species (ROS), which induce DNA damage, thereby facilitating tumor initiation
Noteworthy, insulin induces the generation of reactive oxygen species (ROS) [22] (Fig. 4.1). Also hyperglycemia is known to increase oxidative stress [23], leading to increased DNA damage in diabetic individuals compared to healthy subjects [24] (Fig. 4.1). ROS can lead to downregulation of the tumor suppressor phosphatase and tensin homolog (PTEN) [25], a process known to promote insulin signaling. ROS generation, in general, is regarded as a hallmark of inflammation and can lead to carcinogenesis due to DNA damage [26].
Insulin/insulin-like growth factor (IGF) signaling is mediated by binding of insulin or IGFs to insulin and/or IGF receptors. IGF levels can be regulated by IGF-binding proteins (IGFBPs), which can inhibit and potentiate IGF actions by ligand binding. High circulating insulin levels decrease levels of IGFBP1 and IGFBP2, thereby increasing the bioavailability of IGF1 and concomitant changes in the cellular environment facilitating tumor formation (Fig. 4.1). In insulin resistance, nonclassical insulin target tissues which express insulin receptors are exposed to the elevated plasma levels of insulin, triglycerides, free fatty acids, and glucose [27] (Fig. 4.1). In contrast to classical insulin target tissues, such as skeletal muscle, adipose tissue, and liver, these tissues may lack a specific mechanism regulating the mitogenic actions of insulin [27]. Additional changes in signaling pathways may be induced by the increased availability of energy substrates, such as glucose, triglycerides, and free fatty acids, which also ensure energy substrates for already transformed cells [27]. High insulin levels as found in insulin resistance enhance growth hormone (GH) receptor signaling and hepatic IGF1 production [28], both of which can contribute to carcinogenesis. Concordantly, in vitro, animal, and human epidemiological studies demonstrate that despite suppressed classical metabolic insulin signaling, high concentrations of insulin and insulin-like growth factors (IGFs) promote cancer development by acting through the insulin/IGF axis [29] (Fig. 4.1).
4.2 IGF1 in Cancer
IGF1, i.e., circulating IGF1, is produced throughout life mainly in the liver under GH stimulation. A small amount of autocrine IGF1 is also produced in peripheral tissues and can be controlled by other factors released from surrounding cells. Cancer epidemiological studies have focused mainly on circulating total IGF1 and its major binding protein, IGFBP3. Circulating IGF1 is associated positively with the risk of breast, colorectal, prostate, and lung cancer, whereas total IGFBP3 concentrations are negatively associated with cancer risk [30,31,32]. In acromegaly patients, typically showing hypersecretion of GH, elevated levels of total IGF1, and hyperinsulinemia, the risk of colorectal cancer was increased [33]. In the healthy state, 99% of circulating IGF1 is bound by IGFBPs [34]. It is believed that free circulating IGF1 levels better reflect IGF1 bioactivity than total IGF1 levels [35]. Free circulating IGF1 has also been correlated to an increased risk of breast cancer, but independent of total IGF1 levels. In contrast to total IGF1 levels, free IGF1 was not related to tumor development in prostate cancer [36]. In addition to a hyperinsulinemia-induced increase in circulating levels of IGF1, prostate cancer cells in rodents were suggested to upregulate their intrinsic IGF1 production, thereby enabling independence from growth-promoting, circulating IGF1 [37]. In contrast, knockout mice with liver-specific IGF1 deficiency had decreased growth and metastasis of transplanted colonic adenocarcinomas and mammary tumors [38,39,40]. Administration of IGF1 abrogated the protective effect of IGF1 deficiency on tumor progression and resulted in neovascularization due to vascular endothelial growth factor (VEGF) induction [38, 40]. Angiogenesis is further promoted by IGF1-induced expression of hypoxia-inducible factor 1α (HIF1α) [41, 42]. Moreover, IGF1-induced metastatic tumor spread was suggested to be related to the relocation of integrins to the edge of migrating cells and the extension of lamellipodia [43, 44].
4.3 IGF2 in Cancer
IGF2 is expressed in the embryonic and neonatal state and its expression strongly drops after birth. IGF2 was reported to be reexpressed in several cancer types [45,46,47,48,49,50,51], defining IGF2 as an oncofetal protein [52]. Tumors take advantage of the proliferative [53, 54] and antiapoptotic properties of IGF2 by increasing IGF2 expression in tumor cells [55]. IGF2 expression was associated with the tumor grade in hepatocellular carcinoma [56, 57]. Furthermore, IGF2 expression was observed to correlate with tumor grade and lymph node metastasis in breast cancer [58]. In adrenocortical carcinoma and osteosarcoma, IGF2 expression was described to correlate with microvessel density [59, 60], to influence taxol resistance, and to be linked to a shortened disease-free survival [61]. Igf2 transgenic mice are more susceptible to diverse malignancies [62]. Mouse models of colon cancer showing overexpression of IGF2 had a doubled tumor incidence in the presence of the adenomatous polyposis coli gene mutation [63]. Also enhanced sensitivity to IGF2 signaling led to elevated expression of proliferation-related genes and enhanced tumor development [64].
4.4 Insulin and IGF Signaling and Its Implication in Carcinogenesis
The insulin/IGF signaling network impresses through its complexity. In the following section, we point out important links between insulin/IGF signaling and carcinogenesis.
4.4.1 Insulin Receptors, IGF Receptors, and Hybrid Receptors
The three ligands insulin, IGF1, and IGF2 can act via five different receptors, namely, insulin receptors (IR) A and B, IGF1 receptor (IGF1R), and two hybrid receptors IRA/IGF1R and IRB/IGF1R. Insulin displays highest affinity for the two IRs, whereas IGF1 and 2 rather bind to the IGF1R and the hybrid receptors. IRB/IGF1R is exclusively bound by IGF1 but not by IGF2 (Fig. 4.2). The activation of the respective receptor by the different ligands can induce distinct downstream effects. Interestingly, binding of IGF2 to IRA results in a different gene expression pattern compared to binding of insulin [65], which is of relevance for tumors showing elevated IGF2 expression. However, the exact mechanisms of the different consequences of ligand binding to the insulin/IGF receptors are still unknown.
Binding affinities of IR and IGF1R receptor ligands. Insulin preferentially binds to insulin receptors IRA and IRB. IGF1 rather activates the hybrid receptors and IGF1R. IGF1R and the hybrid receptor variant IRA/IGF1R are also bound by IGF2. IRA, the hybrid receptors, and IGF1R tend to a more mitogenic signaling, whereas IRB rather activates metabolic pathways. In cancer IRA, IGF1R, and the hybrid receptors are overexpressed, resulting in a mitogenic signaling
The different receptors mediate their effects through recruitment, phosphorylation, and finally activation of insulin receptor substrates (IRS), Src homology 2 domain containing transforming protein (SHC), and Janus kinase (JAK) 1/2, leading to an activation of phosphoinositide 3-kinase (PI3K), protein kinase B (PKB/AKT), and mammalian target of rapamycin (mTOR), mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK), or JAK/signal transducer and activator of transcription (STAT). Although all five receptors share the same signaling pathways, it is known that IRA and IGF1R favor mitogenic actions, whereas IRB rather induces metabolic effects (Fig. 4.2) [66,67,68]. Insulin resistance is caused by defects in the metabolic signaling pathways, favoring a mitogenic and growth-promoting signaling [27]. Concordantly, insulin induces transcription of a set of genes involved in metabolism, whereas insulin-like ligands increase expression of mitogenic genes [69]. Thus, differential expression of the respective receptors or their ligands in cancer, as well as in development, can implicate distinct consequences, i.e., metabolic and/or mitogenic or growth-related signaling. For example, overexpression of IGF2 in tumor cells also leads to increased mitogenic signaling via IRA [70].
IGF2 can also interact with a sixth receptor, IGF2R, which degrades IGF2 protein and therefore decreases IGF2 bioavailability. Thus, inhibitory IGF2R is often mutated or downregulated in cancer [71, 72].
4.4.2 Insulin Receptor Substrates
Autophosphorylation of the five signaling receptors mentioned above leads to the recruitment of different proteins, mainly IRS1, IRS2, and SHC, resulting in PI3K or MAPK pathway activation. Although IRS1 and IRS2 share biological effects, they exert tissue-specific roles [73]. PI3K can be activated by both IRS1 and IRS2. Besides antiapoptotic signaling, the PI3K/AKT pathway regulates metabolic pathways in tumors which promote aerobic glycolysis, a hallmark of cancer [74, 75]. Cancer cells depend rather on glycolysis than oxidative phosphorylation for energy production, even in high oxygen states, a phenomenon called the “Warburg” effect [76]. IRS2 signaling preferentially regulates tumor cell metabolism, i.e., aerobic glycolysis by inhibition of GSK-3β [77]. In line with this finding, aerobic glycolysis is diminished in IRS2 knockout cells compared to IRS1 knockout cells. Moreover, IRS2 may be required for glucose transporter (GLUT) 1 to localize to the cell surface where it can facilitate glucose uptake [78].
MAPK signaling seems to be preferentially induced by IRS1 (Fig. 4.3) [79]. Indeed, several studies suggest that IRS1 distinctly mediates the insulin/IGF1-induced mitogenic effects, whereas IRS2 appears to be more involved in generating the metabolic responses of insulin [80,81,82,83] and the migration-promoting potential of IGF1 (Fig. 4.3) [84]. However, metabolic stress induces specific phosphorylations of IRS1, which aggravate insulin resistance [85]. Specific responses were suggested to be altered by integrins differentially regulating IRS1 and IRS2 expression (Fig. 4.3) [86]. While IRS2 promotes aggressive tumor behavior, IRS1 may negatively regulate tumor progression, although IRS1 and IRS2 may play redundant roles in tumor initiation and primary tumor growth [78]. However, IRS1 was described to elevate growth and migration in breast cancer cells [87]. Different activation of and by IRS1 and IRS2 may be also due to the structural differences, since they share only 14 conserved sites of 21 and 23 phosphorylation sites of IRS1 and IRS2, respectively [88].
Preferential signaling of the two insulin receptor substrates supporting tumorigenesis. IRS1 preferentially activates MAPK signaling, leading to a mitogenic signaling, thereby increasing proliferation and growth. IRS2 inhibits GSK-3βs by PI3K phosphorylation, leading to an increased aerobic glycolysis, which is essential in tumor metabolism
4.4.3 PI3K-Related Signaling
The PI3K/AKT pathway is the major signaling network involved in insulin/IGF signaling (Fig. 4.4). PI3K plays a central role in cancer promoting cancer cell growth, survival, motility, and metabolism [89]. By induction of several activating factors, as well as by repression of different inhibitory factors, a constitutively activated pro-survival signaling is achieved. One of these inhibitory factors is PTEN, which usually counters cell growth and cell cycle progression by inhibiting PI3K-induced PIP3 phosphorylation. PTEN displays one of the most commonly mutated tumor suppressor genes in human cancer. Loss of PTEN results in increased signaling of IGF2 through IGF1R and IRA in breast cancer cells [90]. PIP3 activates AKT, resulting in activation of the key metabolic regulator mTOR and thereby initiating ribosomal protein synthesis and mitosis through 4E–BP1 (Fig. 4.4). Deletion of the mTOR target S6K1 in mice was shown to result in hyperinsulinemia and glucose intolerance [91]. These mTOR-induced mechanisms all favor tumor growth; thus, dysregulated mTOR signaling has been linked to numerous human cancers [92,93,94]. Loss of PTEN leads to constitutively activated mTOR [95]. mTOR regulation is controlled not only by PTEN but also by the tumor suppressor gene products tuberous sclerosis (TSC) 1, TSC2, and AMP-activated protein kinase (AMPK). AMPK interacts with both TSC2 and mTOR and thus directly and indirectly inhibits the activation of mTOR (Fig. 4.4) [96]. In colorectal cancer, frameshift mutations in the AMPK-encoding gene were observed [97]. mTOR itself was also shown to be mutated in several types of cancer [98,99,100].
Antiapoptotic insulin/IGF signaling via AKT is realized by initiating phosphorylation of the Bcl-2 family member BAD, followed by Bcl-XL leading to inhibition of apoptosis (Fig. 4.4). Moreover, multiple transcription factors, such as cAMP response element-binding protein (CREB), nuclear factor (NF)-κB, and p53, which are involved in the transcription of genes encoding apoptotic mediators, are regulated by IGFs [101]. Akt hyperactivation in cancer not only contributes to the inhibition of apoptosis but is also coupled with metabolic alterations in cancer cells, including aerobic glycolysis [102].
4.4.4 MAPK-Related Signaling
Besides PI3K activation, insulin or IGF stimulation has been shown to increase interaction with SHC [103]. SHC initiates the MAPK pathway, which represents a key promoter of cell proliferation, tumor development, tumor growth [104], as well as in the maintenance and progression of several tumors [105, 106]. The MAPK pathway involves activation of Ras, which can activate both JNK and MEK/ERK pathways (Fig. 4.4). The Ras/Raf cascade is frequently elevated in cancer, either growth factor dependently or independently, e.g., due to mutations [107, 108].
Noteworthy, ERK signaling is also implicated in metabolic alterations, such as insulin resistance. Chronic activation of ERK induces severe insulin resistance by inhibiting expressions of both GLUT4 and insulin-signaling proteins [109]. Targeting the MEK/ERK cascade normalized hyperglycemia and hyperlipidemia and improved insulin sensitivity, as well as glucose tolerance in diabetic mice [110]. Thus, the MAPK pathway displays a second important insulin/IGF-mediated pathway linking insulin resistance to cancer.
Conclusion
Insulin/IGF signaling is of particular importance in carcinogenesis, especially when tumor development is the consequence of chronic metabolic diseases. Insulin/IGF signaling mediates its effects through different signaling cascades. Not surprisingly, tumor cells activate multiple signaling pathways at once to achieve growth, protection against apoptosis, metastasis, metabolic alterations, and other features being a characteristic for cancer. Here, the activation of the insulin/IGF axis offers the advantage of activating several pathways at once for tumor development and progression. As a result from the extensive basic research, several therapeutic approaches targeting the insulin/IGF axis in cancer are currently under investigation and reviewed in detail elsewhere [111,112,113].
References
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart J-C, James WPT, Loria CM, Smith SC. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. PMID: 19805654. doi:10.1161/circulationaha.109.192644.
Jaggers JR, Sui X, Hooker SP, LaMonte MJ, Matthews CE, Hand GA, Blair SN. Metabolic syndrome and risk of cancer mortality in men. Eur J Cancer. 2009;45(10):1831–8. PMID: 19250819. doi:10.1016/j.ejca.2009.01.031.
Nicolucci A. Epidemiological aspects of neoplasms in diabetes. Acta Diabetol. 2010;47(2):87–95. PMID: 20376506. doi:10.1007/s00592-010-0187-3.
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. PMID: 18280327. doi:10.1016/S0140-6736(08)60269-X.
Jafri H, Alsheikh-Ali AA, Karas RH. Baseline and on-treatment high-density lipoprotein cholesterol and the risk of cancer in randomized controlled trials of lipid-altering therapy. J Am Coll Cardiol. 2010;55(25):2846–54. PMID: 20579542. doi:10.1016/j.jacc.2009.12.069.
Chiu HM, Lin JT, Shun CT, Liang JT, Lee YC, Huang SP, Wu MS. Association of metabolic syndrome with proximal and synchronous colorectal neoplasm. Clin Gastroenterol Hepatol. 2007;5(2):221–9. PMID: 16931168. doi:10.1016/j.cgh.2006.06.022.
Laukkanen JA, Laaksonen DE, Niskanen L, Pukkala E, Hakkarainen A, Salonen JT. Metabolic syndrome and the risk of prostate cancer in Finnish men: a population-based study. Cancer Epidemiol Biomarkers Prev. 2004;13(10):1646–50. PMID: 15466982.
Pasanisi P, Berrino F, De Petris M, Venturelli E, Mastroianni A, Panico S. Metabolic syndrome as a prognostic factor for breast cancer recurrences. Int J Cancer. 2006;119(1):236–8. PMID: 16450399. doi:10.1002/ijc.21812.
Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35(11):2402–11. PMID: 23640372. doi:10.2337/dc12-0336.
Harvey AE, Lashinger LM, Hursting SD. The growing challenge of obesity and cancer: an inflammatory issue. Ann N Y Acad Sci. 2011;1229(1):45–52. PMID: 21793838. doi:10.1111/j.1749-6632.2011.06096.x.
Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121(6):2111–7. PMID: 21633179. doi:10.1172/JCI57132.
Adabimohazab R, Garfinkel A, Milam EC, Frosch O, Mangone A, Convit A. Does inflammation mediate the association between obesity and insulin resistance? Inflammation. 2016;39(3):994–1003. PMID: 26956471. doi:10.1007/s10753-016-0329-z.
Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25(1):4–7. PMID: 14698276. doi:10.1016/j.it.2003.10.013.
Kamp DW, Shacter E, Weitzman SA. Chronic inflammation and cancer: the role of the mitochondria. Oncology (Williston Park). 2011;25(5):400–10, 413. PMID: 21710835.
DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53(7):1270–87. PMID: 20361178. doi:10.1007/s00125-010-1684-1.
Hsu C-C, Chang H-Y, Huang M-C, Hwang S-J, Yang Y-C, Tai T-Y, Yang H-J, Chang C-T, Chang C-J, Li Y-S, Shin S-J, Kuo KN. Association between insulin resistance and development of microalbuminuria in type 2 diabetes: a prospective cohort study. Diabetes Care. 2011;34(4):982–7. PMID: 21335369. doi:10.2337/dc10-1718.
Nilsen TIL, Vatten LJ. Prospective study of colorectal cancer risk and physical activity, diabetes, blood glucose and BMI: exploring the hyperinsulinaemia hypothesis. Br J Cancer. 2001;84(3):417–22. PMID: 11161410. doi:10.1054/bjoc.2000.1582.
Trevisan M, Liu J, Muti P, Misciagna G, Menotti A, Fucci F. Markers of insulin resistance and colorectal cancer mortality. Cancer Epidemiology Biomarkers Prev. 2001;10(9):937–41. PMID: 11535544.
Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293(2):194–202. PMID: 15644546. doi:10.1001/jama.293.2.194.
Taubes G. Unraveling the obesity-cancer connection. Science. 2012;335(6064):28–32. PMID: 22223787. doi:10.1126/science.335.6064.28.
Gallagher EJ, LeRoith D. Minireview: IGF, Insulin, and Cancer. Endocrinology. 2011;152(7):2546–51. PMID: 21540285. doi:10.1210/en.2011-0231.
Goldstein BJ, Mahadev K, Wu X, Zhu L, Motoshima H. Role of insulin-induced reactive oxygen species in the insulin signaling pathway. Antioxid Redox Signal. 2005;7(7-8):1021–31. PMID: 15998257. doi:10.1089/ars.2005.7.1021.
Paolisso G, Giugliano D. Oxidative stress and insulin action: is there a relationship? Diabetologia. 1996;39(3):357–63. PMID: 8721784. doi:10.1007/s001250050454.
Dandona P, Thusu K, Cook S, Snyder B, Makowski J, Armstrong D, Nicotera T. Oxidative damage to DNA in diabetes mellitus. Lancet. 1996;347(8999):444–5. PMID: 8618487. doi:10.1016/S0140-6736(96)90013-6.
Lee S-R, Yang K-S, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277(23):20336–42. PMID: 11916965. doi: 10.1074/jbc.M111899200.
Block K, Gorin Y. Aiding and abetting roles of NOX oxidases in cellular transformation. Nat Rev Cancer. 2012;12(9):627. PMID: 22918415. doi:10.1038/nrc3339.
Gunter MJ, Leitzmann MF. Obesity and colorectal cancer: epidemiology, mechanisms and candidate genes. J Nutr Biochem. 2006;17(3):145–56. PMID: 16426829. doi:10.1016/j.jnutbio.2005.06.011.
Baxter RC, Bryson JM, Turtle JR. Somatogenic receptors of rat liver: regulation by insulin. Endocrinology. 1980;107(4):1176–81. PMID: 6250795. doi:10.1210/endo-107-4-1176.
Godsland Ian F. Insulin resistance and hyperinsulinaema in the development and progression of cancer. Clin Sci (Lond). 2010;118(5):315–32. PMID: 19922415. doi:10.1042/cs20090399.
Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: the role of the insulin–IGF axis. Trends Endocrinol Metab. 2006;17(8):328–36. PMID: 16956771. doi:10.1016/j.tem.2006.08.006.
Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363(9418):1346–53. PMID: 15110491. doi:10.1016/S0140-6736(04)16044-3.
Morris JK, George LM, Wu T, Wald NJ. Insulin-like growth factors and cancer: no role in screening. Evidence from the BUPA study and meta-analysis of prospective epidemiological studies. Br J Cancer. 2006;95(1):112–7. PMID: 16804529. doi:10.1038/sj.bjc.6603200.
Renehan AG, O’Connell J, O’Halloran D, Shanahan F, Potten CS, O'Dwyer ST, Shalet SM. Acromegaly and colorectal cancer: a comprehensive review of epidemiology, biological mechanisms, and clinical implications. Horm Metab Res. 2003;35(11-12):712–25. PMID: 14710350. doi:10.1055/s-2004-814150.
Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16(1):3–34. PMID: 7758431. doi:10.1210/edrv-16-1-3.
Janssen JAMJL, Lely AJ, Lamberts SWJ. Circulating free insulin-like growth-factor-I (IGF-I) levels should also be measured to estimate the IGF-I bioactivity. J Endocrinol Invest. 2014;26(6):588–94. PMID: 12952376. doi:10.1007/bf03345225.
Janssen JAMJL, Wildhagen MF, Ito K, Blijenberg BG, Van Schaik RHN, Roobol MJ, Pols HAP, Lamberts SWJ, Schröder FH. Circulating free insulin-like growth factor (IGF)-I, total IGF-I, and IGF binding protein-3 levels do not predict the future risk to develop prostate cancer: Results of a case-control study involving 201 patients within a population-based screening with a 4-year interval. J Clin Endocrinol Metab. 2004;89(9):4391–6. PMID: 15356036. doi:10.1210/jc.2004-0232.
Wang YZ, Wong YC. Sex hormone-induced prostatic carcinogenesis in the noble rat: the role of insulin-like growth factor-1 (IGF-1) and vascular endothelial growth factor (VEGF) in the development of prostate cancer. Prostate. 1998;35(3):165–77. PMID: 9582085. doi:10.1002/(SICI)1097-0045(19980515)35:3<165::AID-PROS2>3.0.CO;2-G.
Dunn SE, Kari FW, French J, Leininger JR, Travlos G, Wilson R, Barrett JC. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53- deficient mice. Cancer Res. 1997;57(21):4667–72. PMID: 9354418.
Wu Y, Cui K, Miyoshi K, Hennighausen L, Green JE, Setser J, LeRoith D, Yakar S. Reduced circulating insulin-like growth factor I levels delay the onset of chemically and genetically induced mammary tumors. Cancer Res. 2003;63(15):4384–8. PMID: 11861378.
Wu Y, Yakar S, Zhao L, Hennighausen L, LeRoith D. Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res. 2002;62(4):1030–5. PMID: 11861378.
Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem. 2002;277(41):38205–11. PMID: 12149254. doi:10.1074/jbc.M203781200.
Héron-Milhavet L, LeRoith D. Insulin-like growth factor I induces MDM2-dependent degradation of p53 via the p38 MAPK pathway in response to DNA damage. J Biol Chem. 2002;277(18):15600–6. PMID: 11877395. doi:10.1074/jbc.M111142200.
Canonici A, Steelant W, Rigot V, Khomitch-Baud A, Boutaghou-Cherid H, Bruyneel E, Van Roy F, Garrouste F, Pommier G, André F. Insulin-like growth factor-I receptor, E-cadherin and αv integrin form a dynamic complex under the control of α-catenin. Int J Cancer. 2008;122(3):572–82. PMID: 17955485. doi:10.1002/ijc.23164.
Meyer GE, Shelden E, Kim B, Feldman EL. Insulin-like growth factor I stimulates motility in human neuroblastoma cells. Oncogene. 2001;20(51):7542–50. PMID: 11709726. doi:10.1038/sj.onc.1204927.
Cariani E, Lasserre C, Seurin D, Hamelin B, Kemeny F, Franco D, Czech MP, Ullrich A, Brechot C. Differential expression of insulin-like growth factor II mRNA in human primary liver cancers, benign liver tumors, and liver cirrhosis. Cancer Res. 1988;48(23):6844–9. PMID: 3180092.
Cheng YW, Idrees K, Shattock R, Khan SA, Zeng Z, Brennan CW, Paty P, Barany F. Loss of imprinting and marked gene elevation are 2 forms of aberrant IGF2 expression in colorectal cancer. Int J Cancer. 2010;127(3):568–77. PMID: 19957330. doi:10.1002/ijc.25086.
Faria AM, Almeida MQ. Differences in the molecular mechanisms of adrenocortical tumorigenesis between children and adults. Mol Cell Endocrinol. 2012;351(1):52–7. PMID: 22019901. doi:10.1016/j.mce.2011.09.040.
Lu ZL, Luo DZ, Wen JM. Expression and significance of tumor-related genes in HCC. World J Gastroenterol. 2005;11(25):3850–4. PMID: 15991281.
Vasiljevic A, Champier J, Figarella-Branger D, Wierinckx A, Jouvet A, Fèvre-Montange M. Molecular characterization of central neurocytomas: potential markers for tumor typing and progression. Neuropathology. 2013;33(2):149–61. PMID: 22816789. doi:10.1109/MED.2013.6608886.
Wu MS, Wang HP, Lin CC, Sheu JC, Shun CT, Lee WJ, Lin JT. Loss of imprinting and overexpression of IGF2 gene in gastric adenocarcinoma. Cancer Lett. 1997;120(1):9–14. PMID: 9570380. doi:10.1016/S0304-3835(97)00279-6.
Zhao R, DeCoteau JF, Geyer CR, Gao M, Cui H, Casson AG. Loss of imprinting of the insulin-like growth factor II (IGF2) gene in esophageal normal and adenocarcinoma tissues. Carcinogenesis. 2009;30(12):2117–22. PMID: 19843644. doi:10.1093/carcin/bgp254.
Takeda S, Kondo M, Kumada T, Koshikawa T, Ueda R, Nishio M, Osada H, Suzuki H, Nagatake M, Washimi O, Takagi K, Takahashi T, Nakao A, Takahashi T. Allelic-expression imbalance of the insulin-like growth factor 2 gene in hepatocellular carcinomas and underlying disease. Oncogene. 1996;12(7):1589–92. PMID: 8622877.
Hartmann W, Koch A, Brune H, Waha A, Schüller U, Dani I, Denkhaus D, Langmann W, Bode U, Wiestler OD, Schilling K, Pietsch T. Insulin-like growth factor II is involved in the proliferation control of medulloblastoma and its cerebellar precursor cells. Am J Pathol. 2005;166(4):1153–62. PMID: 15793295.
Pacher M, Seewald MJ, Mikula M, Oehler S, Mogg M, Vinatzer U, Eger A, Schweifer N, Varecka R, Sommergruber W, Mikulits W, Schreiber M. Impact of constitutive IGF1/IGF2 stimulation on the transcriptional program of human breast cancer cells. Carcinogenesis. 2007;28(1):49–59. PMID: 16774935. doi:10.1093/carcin/bgl091.
Eichenmüller M, Hemmerlein B, Von Schweinitz D, Kappler R. Betulinic acid induces apoptosis and inhibits hedgehog signalling in rhabdomyosarcoma. Br J Cancer. 2010;103(1):43–51. PMID: 20517313. doi:10.1038/sj.bjc.6605715.
Abou-Alfa GK, Capanu M, O'Reilly EM, Ma J, Chou JF, Gansukh B, Shia J, Kalin M, Katz S, Abad L, Reidy-Lagunes DL, Kelsen DP, Chen HX, Saltz LB. A phase II study of cixutumumab (IMC-A12, NSC742460) in advanced hepatocellular carcinoma. J Hepatol. 2014;60(2):319–24. PMID: 24045151. doi:10.1016/j.jhep.2013.09.008.
Tovar V, Alsinet C, Villanueva A, Hoshida Y, Chiang DY, Solé M, Thung S, Moyano S, Toffanin S, Mínguez B, Cabellos L, Peix J, Schwartz M, Mazzaferro V, Bruix J, Llovet JM. IGF activation in a molecular subclass of hepatocellular carcinoma and pre-clinical efficacy of IGF-1R blockage. J Hepatol. 2010;52(4):550–9. PMID: 20206398. doi:10.1016/j.jhep.2010.01.015.
Qiu J, Yang R, Rao Y, Du Y, Kalembo FW. Risk factors for breast cancer and expression of insulin-like growth factor-2 (IGF-2) in women with breast cancer in Wuhan city, China. PLoS One. 2012;7(5):e36497. PMID: 22662119. doi:10.1371/journal.pone.0036497.
Chen P, Wang SJ, Wang HB, Ren P, Wang XQ, Liu WG, Gu WL, Li DQ, Zhang TG, Zhou CJ. The distribution of IGF2 and IMP3 in osteosarcoma and its relationship with angiogenesis. J Mol Histol. 2012;43(1):63–70. PMID: 22042095. doi:10.1007/s10735-011-9370-2.
Zhu Y, Xu Y, Chen D, Zhang C, Rui W, Zhao J, Zhu Q, Wu Y, Shen Z, Wang W, Ning G, Wang X. Expression of STAT3 and IGF2 in adrenocortical carcinoma and its relationship with angiogenesis. Clin Transl Oncol. 2013;16(7):644–9. PMID: 24178245. doi:10.1007/s12094-013-1130-1.
Huang GS, Brouwer-Visser J, Ramirez MJ, Kim CH, Hebert TM, Lin J, Arias-Pulido H, Qualls CR, Prossnitz ER, Goldberg GL, Smith HO, Horwitz SB. Insulin-like growth factor 2 expression modulates taxol resistance and is a candidate biomarker for reduced disease-free survival in ovarian cancer. Clin Cancer Res. 2010;16(11):2999–3010. PMID: 20404007. doi:10.1158/1078-0432.CCR-09-3233.
Rogler CE, Yang D, Rossetti L, Donohoe J, Alt E, Chang CJ, Rosenfeld R, Neely K, Hintz R. Altered body composition and increased frequency of diverse malignancies in insulin-like growth factor-II transgenic mice. J Biol Chem. 1994;269(19):13779–84. PMID:7514593.
Sakatani T, Kaneda A, Iacobuzio-Donahue CA, Carter MG, De Boom WS, Okano H, Ko MSH, Ohlsson R, Longo DL, Feinberg AP. Loss of imprinting of Igf2 alters intestinal maturation and tumorigenesis in mice. Science. 2005;307(5717):1976–8. PMID:15731405. doi:10.1126/science.1108080.
Kaneda A, Wang CJ, Cheong R, Timp W, Onyango P, Wen B, Iacobuzio-Donahue CA, Ohlsson R, Andraos R, Pearson MA, Sharov AA, Longo DL, Ko MSH, Levchenko A, Feinberg AP. Enhanced sensitivity to IGF-II signaling links loss of imprinting of IGF2 to increased cell proliferation and tumor risk. Proc Natl Acad Sci U S A. 2007;104(52):20926–31. doi:10.1073/pnas.0710359105.
Pandini G, Conte E, Medico E, Sciacca L, Vigneri R, Belfiore A. IGF-II binding to insulin receptor isoform A induces a partially different gene expression profile from insulin binding. Ann N Y Acad Sci. 2004;1028:450–6. PMID:18087038. doi:10.1196/annals.1322.053.
Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30(6):586–623. PMID:19752219. doi:10.1210/er.2008-0047.
LeRoith D, Werner H, Beitner-Johnson D, ACT R. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16(2):143–63. PMID:7540132. doi:10.1210/edrv-16-2-143.
Yakar S, Pennisi P, Kim CH, Zhao H, Toyoshima Y, Gavrilova O, LeRoith D. Studies involving the GH-IGF axis: lessons from IGF-I and IGF-I receptor gene targeting mouse models. J Endocrinol Invest. 2005;28(5 Suppl):19–22. PMID:16114270.
ter Braak B, Wink S, Koedoot E, Pont C, Siezen C, van der Laan JW, van de Water B. Alternative signaling network activation through different insulin receptor family members caused by pro-mitogenic antidiabetic insulin analogues in human mammary epithelial cells. Breast Cancer Res. 2015;17(1):97. PMID:26187749. doi:10.1186/s13058-015-0600-5.
Sciacca L, Costantino A, Pandini G, Mineo R, Frasca F, Scalia P, Sbraccia P, Goldfine ID, Vigneri R, Belfiore A. Insulin receptor activation by IGF-II in breast cancers: evidence for a new autocrine/paracrine mechanism. Oncogene. 1999;18(15):2471–9. PMID:10229198. doi:10.1038/sj.onc.1202600.
Kreiling JL, Montgomery MA, Wheeler JR, Kopanic JL, Connelly CM, Zavorka ME, Allison JL, MacDonald RG. Dominant-negative effect of truncated mannose 6-phosphate/insulin-like growth factor II receptor species in cancer. FEBS J. 2012;279(15):2695–713. PMID: 22681933. doi:10.1111/j.1742-4658.2012.08652.x.
Oates AJ, Schumaker LM, Jenkins SB, Pearce AA, DaCosta SA, Arun B, Ellis MJC. The mannose 6-phosphate/insulin-like growth factor 2 receptor (M6P/IGF2R), a putative breast tumor suppressor gene. Breast Cancer Res Treat. 1998;47(3):269–81. PMID: 9516081. doi:10.1023/A:1005959218524.
Kido Y, Burks DJ, Withers D, Bruning JC, Kahn CR, White MF, Accili D. Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J Clin Invest. 2000;105(2):199–205. PMID:10642598. doi:10.1172/JCI7917.
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7(1):11–20.PMID:18177721. doi:10.1016/j.cmet.2007.10.002.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. PMID:21376230. doi:10.1016/j.cell.2011.02.013.
Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14. PMID:13298683. doi:10.1126/science.123.3191.309.
Landis J, Shaw LM. Insulin receptor substrate 2-mediated phosphatidylinositol 3-kinase signaling selectively inhibits glycogen synthase kinase 3β to regulate aerobic glycolysis. J Biol Chem. 2014;289(26):18603–13. PMID:24811175. doi:10.1074/jbc.M114.564070.
Shaw LM. The insulin receptor substrate (IRS) proteins. Cell Cycle. 2011;10(11):1750–6. PMID:21597332. doi:10.4161/cc.10.11.15824.
Byron SA, Horwitz KB, Richer JK, Lange CA, Zhang X, Yee D. Insulin receptor substrates mediate distinct biological responses to insulin-like growth factor receptor activation in breast cancer cells. Br J Cancer. 2006;95(9):1220–8. PMID:17043687. doi:10.1038/sj.bjc.6603354.
Brüning JC, Winnay J, Cheatham B, Kahn CR. Differential signaling by insulin receptor substrate 1 (IRS-1) and IRS-2 in IRS-1-deficient cells. Mol Cell Biol. 1997;17(3):1513–21.PMID:9032279.
Miele C, Caruso M, Calleja V, Auricchio R, Oriente F, Formisano P, Condorelli G, Cafieri A, Sawka-Verhelle D, Van Obberghen E, Beguinot F. Differential role of insulin receptor substrate (IRS)-1 and IRS-2 in L6 skeletal muscle cells expressing the Arg1152 → Gln insulin receptor. J Biol Chem. 1999;274(5):3094–102. PMID:9915848. doi:10.1074/jbc.274.5.3094.
Myers MG, Wang LM, Sun XJ, Zhang Y, Yenush L, Schlessinger J, Pierce JH, White MF. Role of IRS-1-GRB-2 complexes in insulin signaling. Mol Cell Biol. 1994;14(6):3577–87. PMID:8196603. doi:10.1128/mcb.14.6.3577.
Rose DW, Saltiel AR, Majumdar M, Decker SJ, Olefsky JM. Insulin receptor substrate 1 is required for insulin-mediated mitogenic signal transduction. Proc Natl Acad Sci U S A. 1994;91(2):797–801. PMID:8290602. doi:10.1073/pnas.91.2.797.
Jackson JG, Zhang X, Yoneda T, Yee D. Regulation of breast cancer cell motility by insulin receptor substrate-2 (IRS-2) in metastatic variants of human breast cancer cell lines. Oncogene. 2001;20(50):7318–25. PMID:11704861. doi:10.1038/sj.onc.1204920.
Hançer NJ, Qiu W, Cherella C, Li Y, Copps KD, White MF. Insulin and metabolic stress stimulate multisite serine/threonine phosphorylation of insulin receptor substrate 1 and inhibit tyrosine phosphorylation. J Biol Chem. 2014;289(18):12467–84. PMID:24652289. doi:10.1074/jbc.M114.554162.
Lebrun P, Baron V, Hauck CR, Schlaepfer DD, Van Obberghen E. Cell adhesion and focal adhesion kinase regulate insulin receptor substrate-1 expression. J Biol Chem. 2000;275(49):38371–7. PMID:10967115. doi:10.1074/jbc.M006162200.
Meyer K, Albaugh B, Schoenike B, Roopra A. Type 1 insulin-like growth factor receptor/insulin receptor substrate 1 signaling confers pathogenic activity on breast tumor cells lacking REST. Mol Cell Biol. 2015;35(17):2991–3004. PMID:26100015. doi:10.1128/mcb.01149-14.
Sun XJ, Wang L-M, Zhang Y, Yenush L, Myers Jr MG, Glasheen E, Lane WS, Pierce JH, White MF. Role of IRS-2 in insulin and cytokine signalling. Nature. 1995;377(6545):173–7.PMID: 7675087. doi: 10.1038/377173a0.
Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28(6):1075–83. PMID:20085938. doi:10.1200/jco.2009.25.3641.
Perks CM, Vernon EG, Rosendahl AH, Tonge D, Holly JMP. IGF-II and IGFBP-2 differentially regulate PTEN in human breast cancer cells. Oncogene. 2007;26(40):5966–72. PMID: 17369847. doi: 10.1038/sj.onc.1210397 .
Soliman GA. The mammalian target of rapamycin signaling network and gene regulation. Curr Opin Lipidol. 2005;16(3):317–23. PMID: 15891393. doi:10.1097/01.mol.0000169352.35642.06.
Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, Shi EY, Stumpf CR, Christensen C, Bonham MJ, Wang S, Ren P, Martin M, Jessen K, Feldman ME, Weissman JS, Shokat KM, Rommel C, Ruggero D. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485(7396):55–61. PMID: 22367541. doi:10.1038/nature10912.
Sabatini DM. mTOR and cancer: Insights into a complex relationship. Nat Rev Cancer. 2006;6(9):729–34. PMID: 16915295. doi:10.1038/nrc1974.
Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35. PMID: 21157483. doi:10.1038/nrm3025.
Reiling JH, Sabatini DM. Stress and mTORture signaling. Oncogene. 2006;25(48):6373–83. PMID: 17041623. doi:10.1038/sj.onc.1209889.
Nellist M, Burgers PC, van den Ouweland AMW, Halley DJJ, Luider TM. Phosphorylation and binding partner analysis of the TSC1–TSC2 complex. Biochem Biophys Res Commun. 2005;333(3):818–26. PMID: 15963462. doi:10.1016/j.bbrc.2005.05.175.
Choi MR, An CH, Yoo NJ, Lee SH. Frameshift mutations of PRKAG1 gene encoding an AMPK gamma subunit in colorectal cancers. J Gastrointest Liver Dis. 2014;23(3):343–5. PMID: 25267969. doi:10.1543/jgld.2014.1121.
Ghosh AP, Marshall CB, Coric T, Shim EH, Kirkman R, Ballestas ME, Ikura M, Bjornsti MA, Sudarshan S. Point mutations of the mTOR-RHEB pathway in renal cell carcinoma. Oncotarget. 2015;6(20):17895–10. PMID: 26255626. doi:10.18632/oncotarget.4963.
Lim JS, Lee JH. Brain somatic mutations in MTOR leading to focal cortical dysplasia. BMB Rep. 2016;49(2):71–2. PMID: 26779999. doi:10.5483/BMBRep.2016.49.2.010.
Nakashima M, Saitsu H, Takei N, Tohyama J, Kato M, Kitaura H, Shiina M, Shirozu H, Masuda H, Watanabe K, Ohba C, Tsurusaki Y, Miyake N, Zheng Y, Sato T, Takebayashi H, Ogata K, Kameyama S, Kakita A, Matsumoto N. Somatic Mutations in the MTOR gene cause focal cortical dysplasia type IIb. Ann Neurol. 2015;78(3):375–86. PMID: 26018084. doi:10.1002/ana.24444.
Braun S, Bitton-Worms K, LeRoith D. The link between the metabolic syndrome and cancer. Int J Biol Sci. 2011;7(7):1003–15. PMID: 21912508.
Robey RB, Hay N. Is Akt the “Warburg kinase”?—Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol. 2009;19(1):25–31. PMID: 19130886. doi:10.1016/j.semcancer.2008.11.010.
Novosyadlyy R, Vijayakumar A, Lann D, Fierz Y, Kurshan N, LeRoith D. Physical and functional interaction between polyoma virus middle T antigen and insulin and IGF-I receptors is required for oncogene activation and tumour initiation. Oncogene. 2009;28(39):3477–86.PMID: 19617901. doi:10.1038/onc.2009.209.
Gallagher EJ, LeRoith D. The proliferating role of insulin and insulin-like growth factors in cancer. Trends Endocrinol Metab. 2010;21(10):610–8. PMID: 20663687. doi:10.1016/j.tem.2010.06.007.
Neuzillet C, Tijeras-Raballand A, De Mestier L, Cros J, Faivre S, Raymond E. MEK in cancer and cancer therapy. Pharmacol Ther. 2014;141(2):160–71. PMID: 24121058. doi:10.1016/j.pharmthera.2013.10.001.
Yumoto K, Eber MR, Berry JE, Taichman RS, Shiozawa Y. Molecular pathways: niches in metastatic dormancy. Clin Cancer Res. 2014;20(13):3384–9. PMID: 24756372. doi:10.1158/1078-0432.CCR-13-0897.
Caunt CJ, Sale MJ, Smith PD, Cook SJ. MEK1 and MEK2 inhibitors and cancer therapy: the long and winding road. Nat Rev Cancer. 2015;15(10):577–92. PMID: 26399658. doi:10.1038/nrc4000.
Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer. 2011;11(11):761–74. PMID: 21993244. doi:10.1038/nrc3106.
Fujishiro M, Gotoh Y, Katagiri H, Sakoda H, Ogihara T, Anai M, Onishi Y, Ono H, Abe M, Shojima N, Fukushima Y, Kikuchi M, Oka Y, Asano T. Three mitogen-activated protein kinases inhibit insulin signaling by different mechanisms in 3T3-L1 adipocytes. Mol Endocrinol. 2003;17(3):487–97. PMID: 12554784. doi:10.1210/me.2002-0131.
Ozaki KI, Awazu M, Tamiya M, Iwasaki Y, Harada A, Kugisaki S, Tanimura S, Kohno M. Targeting the ERK signaling pathway as a potential treatment for insulin resistance and type 2 diabetes. Am J Physiol Endocrinol Metab. 2016;310(8):E643-E651. PMID: 26860984. doi:10.1152/ajpendo.00445.2015.
Iams WT, Lovly CM. Molecular pathways: clinical applications and future direction of insulin-like growth factor-1 receptor pathway blockade. Clin Cancer Res. 2015;21(19):4270–7.PMID: 26429980. doi:10.1158/1078-0432.ccr-14-2518.
Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12(3):159. PMID: 22337149. doi:10.1038/nrc3215.
Singh P, Alex JM, Bast F. Insulin receptor (IR) and insulin-like growth factor receptor 1 (IGF-1R) signaling systems: novel treatment strategies for cancer. Med Oncol. 2014;31(1):805. PMID: 24338270. doi:10.1007/s12032-013-0805-3.
Acknowledgements
The authors’ work was funded, in part, by the Else Kröner-Fresenius-Stiftung (2012_A250).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Kessler, S.M., Kiemer, A.K. (2017). Insulin Signaling Linking Metabolism and Malignancy. In: Haybaeck, J. (eds) Mechanisms of Molecular Carcinogenesis – Volume 1. Springer, Cham. https://doi.org/10.1007/978-3-319-53659-0_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-53659-0_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-53657-6
Online ISBN: 978-3-319-53659-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)