Abstract
Objective
The aim of this study was to determine the correlation between human adrenocortical carcinoma and the proteins involved in tumor angiogenesis, and to evaluate the angiogenic status of adrenocortical carcinoma.
Methods
The expression of signal transducer and activator of transcription 3 and insulin-like growth factor 2 as well as microvessel density was measured in a series of tissue samples from 44 human sporadic adrenocortical tumors by immunohistochemistry. These specimens were classified as adenomas (n = 20) and carcinomas (n = 24) according to the histological criteria defined by Weiss.
Results
A total of 19 of 24 (79.17 %) malignant cases showed positive staining for signal transducer and activator of transcription 3 and 4 of 20 (20.00 %) benign cases showed positive, the difference of signal transducer and activator of transcription 3 expression between adrenocortical adenomas and adrenocortical carcinomas was statistically significant (P < 0.001). Similarly, insulin-like growth factor 2 staining was seen in 70.83 % (17/24) of the malignant cases versus 25.00 % (5/20) of the benign, the difference of insulin-like growth factor 2 expression among two groups was statistically significant (P = 0.002). Malignant cases showed higher microvessel density compared to benign tumors (84.70 ± 12.44 vs 21.05 ± 8.07, P < 0.001). Signal transducer and activator of transcription 3 and insulin-like growth factor 2 expression were positively correlated with microvessel density in all specimens (r_s = 0.832, P < 0.001; r_s = 0.703, P = 0.001).
Conclusions
This study has confirmed that adrenocortical carcinoma overexpress signal transducer and activator of transcription 3 and insulin-like growth factor 2; these results suggest that angiogenesis of human adrenocortical carcinoma may be mediated by these proteins and they could represent selective targets for the molecularly targeted treatments of adrenocortical carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unlike adrenal incidentalomas with a prevalence of >4 %, adrenocortical carcinoma (ACC) is a rare but typically aggressive malignancy with an estimated annual incidence of 0.7–2.0 cases per million population [1, 2]. Radical surgery resection remains the only potentially curative option for ACC until recently; however, about one-third of patients initially present with distant metastases [3, 4]. Even after seemingly complete removal of tumor, the recurrence occur in approximately 60–80 % of ACC patients, and the recurrence ratio of patients in stage I, II and III was 27, 46 and 63 % respectively within 2 years [5]. Although the combination of cytotoxic drug and mitotane is recommended to be the first-line therapy in advanced ACCs, the overall prognosis is still very limited [6]. It is obvious that management of ACC requires a multidisciplinary approach; however, no effective treatment has been developed yet.
The incomplete understanding of molecular features of ACC might be one of the reasons for this situation of unsatisfied clinical efficacy and dismal prognosis, although recent gene expression studies have significantly improved our knowledge. Signal transducer and activator of transcription 3 (STAT3) is one of the six members of the family of transcription factors, which regulates a complex spectrum of gene expression that mediate survival, proliferation, invasion, and angiogenesis [7]. Constitutively, activated STAT3 is routinely observed in a variety of malignant tumors, playing a pivotal transcriptional role in cancer cell proliferation, differentiation, and survival by up-regulating the expression of downstream genes [8].
Angiogenesis, the formation of new blood vessels from the pre-existing vasculature, is a complex multistage process regulated by a number of signal transduction pathways [9]. STAT3 has been proposed to be a critical multifunctional mediator which regulates many aspects of angiogenesis at the transcriptional level. Moreover, insulin-like growth factor 2 (IGF2) is a member of the insulin-like growth factor (IGF) family which has growth-promoting as well as differentiating functions in the adrenal gland. Accumulating evidence also suggests that IGF2 promote neovascularization in vitro and in vivo systems. Microvessel density (MVD) is considered as golden standard in evaluating tumor angiogenesis [10]. To quantify the angiogenic status, markers of endothelial cells, such as Factor VIII, CD31, and CD34 have been used.
Human STAT3, IGF2 and MVD were found to be up-regulated in tissue specimens derived from various tumors, and encouraging results of anti-angiogenic substances were published showing significant prolongation of survival and good tolerance in advanced cancer. However, the preliminary results of anti-angiogenic therapy in ACC have been largely disappointing. In 10 patients, no response was found with a combination of the anti-VEGF antibody bevacicumab plus capecitabine given as salvage treatment [11]. Expression of STAT3 and IGF2 and their association with angiogenesis in ACC have not been comprehensively studied until recently. The aim of this study was to examine the impact on the oncogenetic process through investigating the expression of STAT3 and IGF2, as well as quantitatively examined MVD in our collection of adrenocortical adenomas (ACAs) and ACCs, further seeking the molecularly targeted treatments directed at inhibiting the angiogenesis of ACC.
Materials and methods
Patients and tissue samples
Approved by institutional ethics review board, pathology specimens and medical records of 44 sporadic adrenocortical tumor (ACT) patients who underwent adrenalectomy at Ruijin Hospital between October 1986 and March 2010 were reviewed from our database. The pre-operative diagnosis was based on the clinical history, symptoms, signs, endocrine evaluation, imagine examination (e.g., MRI, CT), and the pathological diagnosis of ACC was based on Weiss’s criteria with its score ≥3 [12]. Formalin-fixed, paraffin-embedded tumor tissues collected from surgical specimens from 44 patients were classified as ACAs (N = 20) and ACCs (N = 24). Besides clinical diagnosis, histopathologic slides were classified by two pathologists independently and no discrepancy exists between them.
Immunohistochemistry
Specimens were fixed in 10 % neutral buffered formalin, embedded in paraffin, and cut into serial sections at a thickness of 3 μm. Paraffin-embedded tissues were dewaxed in xylene, rehydrated by serial concentrations of ethanol, and then rinsed in phosphate buffer solution (PBS) followed by treatment with 3 % H2O2 to refrain endogenous peroxidase. After being heated in a microwave at 750 W for 15 min to repair the tissue antigen, the sections were incubated with 10 % normal goat serum at room temperature for 10 min to block non-specific reactions. Sections were incubated with polyclonal rabbit antihuman STAT3 antibody (Abcam, USA) diluted to 1:150 for 12 h at 4 °C. The slides were followed by a PBS wash and incubated by anti-mouse EnVisionTM kit (DAKO, USA) for 30 min at room temperature. After a PBS wash, the sections were developed in diaminobenzidine (DAB) substrate. The sections were then counter-stained in hematoxylin for 2 min and then dehydrated in ethanol and xylene before being mounted. Sections were re-prepared by EnVision immunohistochemical staining. IGF2 was polyclonal rabbit anti-human antibody (Abcam, USA), diluted to 1:250. CD34 was monoclonal mouse anti-human antibody (Novocastra, USA), diluted to 1:200. The positive controls were gastric carcinoma, pancreatic carcinoma and colon carcinoma with positive expressions of STAT3, IGF2, and CD34. PBS instead of primary antibodies was as negative control.
Evaluation of immunohistochemical results
Positive STAT3 or IGF2 staining was characterized by purple-brown granules located diffusely in the cell cytoplasm. Lack of any obvious purple-brown or brown-red pigmentation in the cytoplasm of tumor cell was considered negative. For quantitative analyses of expression, five representative high-power fields were chosen. Staining was scored according to the percent of positive-staining cells, including 0 (<5 %); 1 (5–29 %); 2 (30–50 %); 3 (>50 %), and as intensity, including 0 (no); 1 (weak); 2 (moderate); 3 (strong) staining. Positive or negative expression was determined according to the combination of these two variables. A total score of greater than 3 was considered positive and a total score of 3 or less was considered negative, as previously reported [13]. The results were scored by two independent pathologists who were blinded to the subtype of the tumors. A single microvessel was defined as any brown or brownish yellow CD34-immunostained endothelial cells. We evaluated MVD by following the method mentioned by Weider [10]: high vascular density area was selected under low power objective and counted the number of vascular stained by CD34 in three visual fields under high power microscope (400×), and average value was regarded as the MVD value of the tumor.
Statistical analysis
SPSS for Windows, version 15.0 (SPSS Inc, Chicago, IL) was used for all analyses. The Chi square test was used to compare STAT3 and IGF2 expressions in ACAs and ACCs. The average size of primary tumor of the two groups was also examined. MVD result was expressed as mean ± SD, and Wilcoxon combined test was used to compare MVD expression between two groups. Spearman rank correlation analysis was used to determine the relationship between STAT3, IGF2 expression, and MVD. Statistical significance was taken at the P < 0.05 level.
Results
Tumor characteristics and clinical features of 44 patients are summarized in Table 1. Twenty patients of ACA, consisting of 3 Cushing’s syndrome, 7 aldosteronomas and 10 non-functional adenomas, are 12 females and 8 males, ranging from 28 to 59 years of age (mean age of 44.95 years at presentation). Twenty-four ACCs, consisting of 13 females and 11 males, had a mean age of 58.21 at presentation (range 38–74). Fourteen patients of ACC have endocrine symptoms and signs that indicated excess secretion of cortisols (in 7 patients), cortisols, and androgens concomitantly (in 6 patients), or estrogens (in 1 male patient). Eighteen patients had a left adrenal neoplasm, 26 had a right adrenal tumor, while no one had bilateral tumors. The mean primary tumor diameter of malignant cases was larger than benign lesions. The mean post-surgery follow-up of patients was for 151.5 months (range, 102–264 months) and 47.2 months (range, 6–113 months) respectively.
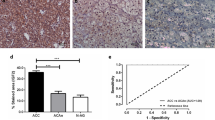
Positive staining for STAT3 was observed in 20.00 % (4/20) of the benign group and 79.17 % (19/24) of the ACC group. The difference of STAT3 expression between ACA and ACC was statistically significant (P < 0.001). IGF2 staining was seen in 25.00 % (5/20) of the benign versus 70.83 % (17/24) of the malignant cases. The difference of IGF2 expression between benign and malignant PCCs was statistically significant (P = 0.002). The mean value of MVD was 84.70 ± 12.44/field in ACC group and 21.05 ± 8.07/field in ACAs, statistically significance was observed between them (P < 0.001).
Sixteen out of 24 ACCs (66.67 %) were stained positive for both STAT3 and IGF2, while only 3 out of 20 ACAs (15.00 %) were positive. Four of ACCs (16.67 %) stained negative by both STAT3 and IGF2, compared to 70.00 % of benign tumors (n = 14). The mean value of MVD was 88.40 ± 13.61/field in sections which was positive for both STAT3 and IGF2, and 14.40 ± 7.24 in sections which was negative for both STAT3 and IGF2, statistical significance was observed between them (P < 0.001). STAT3 and IGF2 expression in benign and malignant adrenal neoplasm was positively correlated with MVD (r_s = 0.832, P < 0.001; r_s = 0.703, P = 0.001) (Figs. 1, 2, 3).
Discussion
ACC is a rare malignancy characterized by dismal prognosis and lacking efficacious therapeutic regimens. The poor prognosis may be attributable to the fact that many ACCs are not detected until they are at advanced stage. For patients without disseminated disease, aggressive surgical excision should be the mainstay of treatment of ACC. For those patients not amenable to surgery, chemotherapy including mitotane alone or in combination with cytotoxic drugs should be considered. Results have been published for the phase III clinical of etoposide, doxorubicin, cisplatin, and mitotane (EDP/M) versus streptozocin and mitotane (Sz/M) in advanced ACC. Preliminary results released that EDP/M is superior to Sz/M in terms of progression-free survival and thus should be considered the standard first-line treatment for patients presenting with stage III or IV ACC [14]. Despite the treatment, the 5-year survival rate is less than 15 % among patients with metastatic disease [15]. The overall prognosis is limited indicating the need for improved therapies directed at potential molecular targets.
The molecular pathogenesis of ACC, particularly in tumor angiogenesis and various signaling pathways, has attracted growing attention during the last decade. Preclinical investigations and clinical trials of emerging targeted therapy (e.g., small-molecule tyrosine kinase inhibitors, antiangiogenic compounds) have been initiated to seek more rational treatment of choice in advanced ACCs; however, the first preliminary results of these new drugs have been largely disappointing [16]. In the present study, immunohistochemistry analysis was performed for comparison of expression of STAT3 and IGF2 between ACA and ACC group, to clarify their usefulness as candidate molecular targets and determine if they correlate with angiogenesis in ACC.
Angiogenesis involves endothelial cell proliferation, selective degradation of the basement membrane and the surrounding extracellular matrix, endothelial cell migration, and the formation of a tubular structure [17]. Not surprisingly, angiogenesis is related to tumor growth and is regulated by the balance between angiogenic and angiostatic factors. Vascular endothelial growth factor (VEGF) has been clearly identified as one of the most important mediators of angiogenesis, and we found higher expression levels of the VEGF protein and its receptor as detected by immunohistochemistry in ACCs compared with benign one’s in a previous study [18]. Recently, increasing studies have shown that STAT3, as a critical multifunctional mediator, participates in regulating tumor angiogenesis through modulating VEGF expression at the transcriptional level [9].
STAT3 is one of the six members of the family of transcription factors. It was discovered almost 15 years ago as a DNA-binding protein which has now been associated with inflammation, cellular transformation, survival, proliferation, invasion, angiogenesis, and metastasis of cancer. STAT3 is constitutively activated in multiple human cancers including ovarian, breast, and prostate, as well as leukemia and lymphoma, playing a pivotal transcriptional role in cancer cell progression, differentiation, and survival by interacting directly with HSP90 and up-regulating the expression of downstream genes [8]. To our knowledge, however, there has been no further study on STAT3 expression in ACC, and we found that positive staining for STAT3 was observed in 20.00 % of ACA and 79.17 % of ACC. Our findings on ACC are in line with the previously reported other different tumor entities, suggesting that overexpression of STAT3 may be associated with the malignant phenotype. Given the significant expression difference in STAT3 between ACCs and ACAs, we presumed that elevated levels of STAT3 were associated with increased ACC cell proliferation, survival and metastasis.
MVD has been regarded as a golden standard that assess significant angiogenesis in tumors. The microvessel count in tumor was made by highlighting the tumor-derived vascular endothelial cells using monoclonal antibodies CD31, CD34, VIII factor, etc. We adopted CD34 immunostaining to assess MVD in our study and calculated the mean value of the CD34 positive vessel counts in the three selected hot spots per section. Our results demonstrated that expression of MVD was higher in ACCs than ACAs (84.70 ± 12.44 vs 21.05 ± 8.07, P < 0.001). In addition, our studies have indicated that STAT3 expression is directly correlated with MVD (r_s = 0.832, P < 0.001), which confirmed the important role of STAT3 in angiogenesis.
The molecular mechanisms of tumorigenesis of the adrenal cortex are to be found in a multistep process [19]. The insulin-like growth factor system has growth-promoting as well as differentiating functions in the adrenal gland, which is comprised of two peptide ligands (IGF1 and IGF2), two IGF receptors (IGF1R and IGF2R), and six high-affinity binding proteins (IGF binding proteins 1–6) [20]. Overexpression of IGF2 has been reported in a number of tumor types and appears of significant prognostic value in various malignant tumors, including ACC. Higher IGF2 expression levels are associated with more malignant phenotype and a higher risk for ACC recurrence [21]. Our results are in line with previous studies that demonstrate ACCs showed increased IGF2 expression (70.83 %, 17/24) in comparison to that observed in adenomas (25.00 %, 5/20).
There are few researches on the relationship between IGF2 expression and the degree of angiogenesis which is expressed as MVD. Piecewicz et al [22] demonstrated that both IGF1 and IGF2 induces angiogenesis by promote embryonic stem cell differentiation into endothelial cells acting through the IGF1R pathway. Besides, IGF2 directly induces angiogenesis by stimulating migration and morphological differentiation of endothelial cells, suggesting that IGF2 may play a crucial role in the progression of tumorigenesis by promoting the deleterious neovascularization [23]. In this study, we observed that IGF2 expression also showed the positive correlation with MVD count in ACCs that was, the stronger the expression of IGF2, the higher the MVD (r_s = 0.703, P = 0.001). Moreover, the MVD significantly upregulated in both STAT3 and IGF2 positive tumors. The ability of STAT3 and IGF2 to promote angiogenesis and its involvement in tumorigenesis make it to be a promising target for therapy of ACC.
In conclusion, our data show that STAT3 and IGF2 expressions are higher in ACCs than ACAs. We find a statistically significant correlation among STAT3 and IGF2, and MVD. However, further studies will be required to understand the molecular mechanism of these two proteins involved in ACC progression and prognosis, which may lead to further development of new approaches targeting STAT3 for effective tumor management.
References
Bovio S, Cataldi A, Reimondo G, Sperone P, Novello S, Berruti A. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. 2006;29:298–302.
Golden SH, Robinson KA, Saldanha I, Anton B, Ladenson PW. Clinical review: Prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94:1853–78.
Allolio B, Fassnacht M. Clinical review: Adrenocortical carcinoma: clinical update. J Clin Endocrinol Metab. 2006;91:2027–37.
Fassnacht M, Libe R, Kroiss M, Allolio B. Adrenocortical carcinoma: a clinician’s update. Nat Rev Endocrinol. 2011;7:323–35.
Abiven G, Coste J, Groussin L, Anract P, Tissier F, Legmann P, et al. Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J Clin Endocrinol Metab. 2006;91:2650–5.
Schteingart DE, Doherty GM, Gauger PG, Giordano TJ, Hammer GD, Korobkin M, et al. Management of patients with adrenal cancer: recommendations of an international consensus conference. Endocr Relat Cancer. 2005;12:667–80.
Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109:1139–42.
Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–88.
Chen Z, Han ZC. STAT3: a critical transcription activator in angiogenesis. Med Res Rev. 2008;28:185–200.
Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol. 1995;147:9–19.
Wortmann S, Quinkler M, Ritter C, Kroiss M, Johanssen S, Hahner S, et al. Bevacizumab plus capecitabine as a salvage therapy in advanced adrenocortical carcinoma. Eur J Endocrinol. 2010;162:349–56.
Kouyama R, Hiraishi K, Sugiyama T, Izumiyama H, Yoshimoto T, Akashi T, et al. Clinicopathological features, biochemical and molecular markers in 5 patients with adrenocortical carcinoma. Endocr J. 2011;58:527–34.
Zhu Y, He HC, Yuan F, Zhang J, Rui WB, Zhao JP, et al. Heparanase-1 and cyclooxygenase-2: prognostic indicators of malignancy in pheochromocytomas. Endocrine. 2010;38:93–9.
Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366:2189–97.
Fassnacht M, Johanssen S, Quinkler M, Bucsky P, Willenberg HS, Beuschlein F, et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a revised TNM Classification. Cancer. 2009;115:243–50.
Kirschner LS. The next generation of therapies for adrenocortical cancers. Trends Endocrinol Metab. 2012;23:343–50.
Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med. 2003;3:643–51.
Xu YZ, Zhu Y, Shen ZJ, Sheng JY, He HC, Ma G, et al. Significance of heparanase-1 and vascular endothelial growth factor in adrenocortical carcinoma angiogenesis: potential for therapy. Endocrine. 2011;40:445–51.
Doghman M, Axelson M, Lalli E. Potent inhibitory effect of the cyclolignan picropodophyllin (PPP) on human adrenocortical carcinoma cells proliferation. Am J Cancer Res. 2011;1:356–61.
Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47.
Gicquel C, Bertagna X, Gaston V, Coste J, Louvel A, Baudin E, et al. Molecular markers and long-term recurrences in a large cohort of patients with sporadic adrenocortical tumors. Cancer Res. 2001;61:6762–7.
Piecewicz SM, Pandey A, Roy B, Xiang SH, Zetter BR, Sengupta S. Insulin-like growth factors promote vasculogenesis in embryonic stem cells. PLoS ONE. 2012;7:e32191.
Lee OH, Bae SK, Bae MH, Lee YM, Moon EJ, Cha HJ, et al. Identification of angiogenic properties of insulin-like growth factor II in in vitro angiogenesis models. Br J Cancer. 2000;82:385–91.
Acknowledgments
This study was supported by the grants from the National Natural Science Foundation of China (No. 81272936) and Shanghai Municipal Natural Science Foundation (No. 134119a2700).
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. Zhu and Y. Xu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhu, Y., Xu, Y., Chen, D. et al. Expression of STAT3 and IGF2 in adrenocortical carcinoma and its relationship with angiogenesis. Clin Transl Oncol 16, 644–649 (2014). https://doi.org/10.1007/s12094-013-1130-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-013-1130-1