Abstract
The aim of this study was to investigate the expression patterns of IGF2 and IMP3 in osteosarcoma as well as its relationship with angiogenesis in the tumor. IGF2 and IMP3 expression was detected by immunohistochemical staining in the serial sections of the osteosarcoma. The impacts of IGF2 and IMP3 expression patterns on tumor angiogenesis were evaluated by statistics. The IGF2 and IMP3 staining had different expression patterns in different osteosarcoma. Twelve out of the sixty-four cases of conventional osteosarcoma showed nuclear staining patterns, and twenty-nine showed cytoplasmic staining of IGF2 and IMP3 simultaneously. On the other hand, fourteen cases showed nuclear IGF2 staining but cytoplasmic IMP3 expression, and nine cases showed nuclear IMP3 staining and cytoplasmic IGF2 expression. Twenty-eight out of forty-seven cases of parosteal osteosarcoma showed nuclear IGF2 and IMP3 expression, nine showed cytoplasmic IGF2 and IMP3 expression simultaneously. Seven out of forty-seven cases of parosteal osteosarcoma expressed IGF2 with nuclear staining but expressed IMP3 with cytoplasmic staining. Meanwhile, three cases expressed IGF2 with cytoplasmic staining but expressed IMP3 with nuclear staining. Similar to the parosteal osteosarcoma, the periosteal osteosarcoma expressed IGF2 and IMP3 mainly with nuclear staining simultaneously, forty out of fifty-five cases of periosteal osteosarcoma did that. Five out of fifty-five cases expressed IGF2 and IMP3 with cytoplasmic staining at the same time. Four cases showed nuclear IGF2 staining and cytoplasmic IMP3 staining. In the parosteal and periosteal osteosarcoma, there was no significant difference in IGF and IMP3 expression patterns (P = 0.216). However, compared with conventional osteosarcoma, the parosteal and periosteal osteosarcoma showed significant difference in IMP3 and IGF2 expression (P = 0.016, P = 0.023). IGF2 and IMP3 expression patterns were positive correlation in the different osteosarcoma (r = 0.1021, P = 0.032). The Microvessel density (MVD) in osteosarcoma with IGF2 and IMP3 cytoplasmic staining was more than that with nuclear expression of IGF2 and IMP3, and the difference was significant (P = 0.024). Moreover, the conventional osteosarcoma with cytoplasmic IGF and IMP3 showed more MVD than parosteal and periosteal osteosarcoma with cytoplasmic IGF and IMP3, and the difference was significant (P = 0.035). IGF2 and IMP3 had different expression patterns, which might be associated with angiogenesis. However, cytoplasmic and nuclear expression of IGF2 and IMP3 might play different roles in the angiogenesis of osteosarcoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metastasis is a major cause of cancer mortality and represents the invasive nature of malignant tumor. Cancer cells metastasize by hematogenous and lymphogenous pathways. In breast carcinoma, lymphatic metastasis occurs in the early stages of cancer development, and malignant cells can be further spread from the lymphatic system to the circulatory system (Fidler 2003). Recent studies demonstrate that peritumoral and intratumoral lymphatic vessels are critical structures for lymphatic metastasis (Beasley et al. 2002; Dadras et al. 2005). Blood vessels, lymphatic vessels in the adult remain quiescent under normal physiological conditions. The switch to a lymphangiogenic phenotype in tumors is likely to require up-regulation of expression of lymphangiogenic factors and down-regulation of expression of lymphangiogenic inhibitors (Alitalo and Carmeliet 2002). Genomic instability of tumor cells can lead to expression of multiple angiogenic and lymphangiogenic factors (Fokas 2010; Cao 2005). Members of the insulin-like growth factor (IGF) family are frequently expressed in many types of tumors associated with malignant progression and poor prognosis (Cullen et al. 1990; Long et al. 1998; Reinmuth et al. 2002; Ritter et al. 2002; Tanno et al. 2001; Xie et al. 1999). The IGF system consists of two ligands (IGF-1 and IGF-2), two transmembrane receptors (IGF-1R and IGF-2R), and several IGF binding proteins (IGFBPs). The IGFBPs may modulate the biological activity of IGFs (Clemmons 1997). There is increasing evidence that IGF-1 might contribute to cancer development by regulating cell proliferation, differentiation, and cell death (Baserga et al. 1997; Flossmann-Kast et al. 1998; Jenkins and Bustin 2004). Both IGF-1 and IGF-2 induce angiogenesis in several in vitro and in vivo systems (Che et al. 2002; Kern et al. 1989; Lee et al. 2000; Shigematsu et al. 1999).
IGF-1 and IGF-2 induce lymphangiogenesis detected with LYVE-1, a specific marker for lymphatic endothelium. Interestingly, IGF-1-induced lymphangiogenesis can not be inhibited by a soluble VEGFR-3, indicating that the VEGF-3-signaling pathway is not required for IGF-induced lymphangiogenesis. IGF-1 and IGF-2 significantly stimulated proliferation and migration of primary lymphatic endothelial cells In vitro. IGF-1 and IGF-2 induced phosphorylation of intracellular signaling components, such as Akt, Src, and extracellular signal-regulated kinase in lymphatic endothelial cells. IGFs might act as direct lymphangiogenic factors, although any indirect roles in the induction of lymphangiogenesis cannot be excluded. Because members of the IGF ligand and receptor families are widely expressed in various types of solid tumors suggesting that these factors are likely to contribute to lymphatic metastasis (Bjorndahl et al. 2005).
The insulin-like growth factors I and II (IGF-I, IGF-II), their receptors, and high affinity binding proteins (IGFBPs) represent a family of cellular modulators that play indispensable roles in the development and differentiation of cells and tissues including the skeleton. There is an alteration of the insulin-like growth factor axis during in vitro differentiation of the human osteosarcoma cell line HOS 58. The human osteosarcoma cell line HOS 58 cells with low cell density at early time points of the in vitro differentiation show a low expression of IGF-I and –II, also lowly synthesize IGFBP-2, -3, -4, and -5, but highly expressed both the type I and II IGF receptors. During the in vitro differentiation, IGF-I and -II expressions increased continuously in parallel with an upregulation of IGFBP-2, -3, -4, and -5 whereas the IGF-I receptor and IGF-II/M6P receptor mRNA were downregulated. These data are indicative for a role of the IGF axis during the in vitro differentiation of HOS 58 cells (Viereck et al. 2007). The physiological ligands of IGF-IR–IGF-I and IGF-II–are potent mitogens for diverse cancer cell lines in vitro (Hermanto et al. 2000; Guo et al. 1995; Kappel et al. 1994; Steller et al. 1996). High levels of IGF-I and IGF-II expression have been noted in tumors and associated stromal cells and may stimulate cancer cell growth in an autocrine or paracrine manner (Khandwala et al. 2000; Quinn et al. 1996). IGF2 is involved in the mitogenic action of androgens on human osteoblastic cells. Androgens may sensitize the osteoblastic cells to show an enhanced response to FGF and IGF-II, possibly by changing the receptor binding of mitogenic growth factors (Kasperk et al. 1990). Gene hypomethylation and hypermethylation can lead to a loss of genetic imprinting in malignant tumors. The mechanism responsible for overexpression of the imprinted insulin-like growth factor II (IGF2) gene remains unknown in osteosarcoma. Methylation analysis has revealed that the methylation patterns of the different methylation region of IGF2 are not uniform, regardless of IGF2-P3 expression. Elevated IGF2-P3 expression in osteosarcoma tissues is due to P3 promoter hypomethylation, which represents an early event in the progression of osteosarcoma (Li et al. 2009). Insulin-like growth factor-II mRNA binding protein 3 (IMP3) is one of the RNA binding proteins involved in mRNA localization and translational control. It is expressed during embryogenesis, as well as in some malignant tumors. Recent studies indicate its potential application as a prognostic factor or as a therapeutic target in cancer. IMP3 expression is correlated with tumor metastasis independent of survival, tumor site, histologic type, age, and gender suggesting that IMP3 could be used as an independent prognostic factor for osteosarcoma (Do et al. 2008). BRCA1 and BRCA2 mutation carriers have a lifetime breast cancer risk of 40–80%, the additional IGF signaling genes have been identified as risk modifiers for breast cancer development in BRCA carriers (Neuhausen et al. 2011). The human IGF2-P3 and IGF2-P4 promoters are highly active in bladder carcinoma, while existing at a nearly undetectable level in the surrounding normal tissue. A double promoter DTA-expressing vector, selected from the cancer-specific promoters IGF2-P3 and IGF2-P4, has been created, carrying on a single construct two separate genes expressing diphtheria toxin A-fragment (DTA). The double promoter vector P4-DTA-P3-DTA shows enhanced anti-cancer activity relative to single promoter expression vectors carrying either gene alone suggesting that bladder tumors may be successfully treated by intravesical instillation of the double promoter vector (Amit et al. 2011). IGF2 expression was low in normal and dysplasia tissue but was increased 1.97-fold in both squamous cell carcinoma and adenocarcinoma in oesophagium (Chava et al. 2011).
Conventional osteosarcoma is a primary intramedullary high grade malignant tumour in which the neoplastic cells produce osteoid. Conventional osteosarcoma is universally fatal and pulmonary metastases are the most frequent site. Parosteal osteosarcoma is a low grade osteosarcoma which arises on the surface of bone. Prognosis is excellent with 91% overall survival at 5 years. Periosteal osteosarcoma, is an intermediate grade chondroblastic osteosarcoma arising on the surface of bone periosteal osteosarcoma is associated with a better prognosis than conventional osteosarcoma (Fletcher et al. 2002). The invasion ability of neuroblastoma cells decreased as IMP3 knock-down by using RNA interference in vitro, and high expression of IMP3 in neuroblastoma may contribute to the undifferentiated phenotype and invasive behaviors, leading to a poor prognosis (Chen et al. 2011). In the present study, the parosteal and periosteal osteosarcoma have better prognosis than the conventional osteosarcoma, but the reasons remains unknown except for the histological differentiation (Fletcher et al. 2002). So we want to know the expression and impacts of IMP3 and IGF2 on the different osteosarcoma. However, IGF2 and IMP3 have not been studied in osteosarcoma and the roles they play in tumourigenesis remain unknown. Here we examined the expression of IGF2 and IMP3 as well as MVD via immunohistochemical staining thereby investigated their impacts on angiogenesis in osteosarcoma.
Materials and methods
Antibodies
The rabbit polyclonal anti-IGF2 antibody (Abcam ab9574, dilution 1:100), and rabbit anti-human polyclonal antibody CD31 (ab28364, dilution 1:50) were all purchased from Abcam plc. (Cambridge, UK). The rabbit polyclonal anti-IMP3 antibody was purchased from PTG (PTG; 14642-1-AP, Chicago, dilution 1:300).
Patients
The study included 166 patients, including 64 patients with conventional osteosarcoma, 47 patients with parosteal osteosarcoma and 55 patients with periosteal osteosarcoma who all underwent primary surgical resection between 2002 and 2009 at Qilu Hospital of Shandong University.
Immunohistochemistry (IHC) staining
Immunohistochemistry was performed on 4 μm-thick, routinely processed paraffin sections in series. IGF2 was detected with a rabbit polyclonal anti-IGF2 antibody (Abcam ab9574, dilution 1:100), IMP3 was examined with a rabbit polyclonal andti-IMP3 antibody (PTG; 14642-1-AP, Chicago, dilution 1:300). Sections were dewaxed, and endogenous peroxidase was blocked by immersing the slides in a 3% solution of hydrogen peroxide in methanol for 10 min. This was followed by a step of antigen retrieval. The slides were immersed in 0.01 mol/L citrate buffer solution (pH 6.0) and placed in a microwave oven for 25 min. Following a wash in 0.01 mol/L phosphate-buffered saline (PBS, pH 7.4), the sections were covered with normal serum in a humidity chamber for 30 min at room temperature. Excess serum was rinsed off with 0.01 mol/L PBS, and the sections were incubated with the primary antibody in a humidity chamber for 45 min at room temperature. Then, sections were rinsed with PBS before being incubated with the biotinylated second antibody in a humidity chamber for 40 min at 37°C. After rinsing with PBS, the streptavidin–peroxidase complex reagent was added. Slides were incubated for 45 min at room temperature, then washed in 0.01 mol/L PBS, and covered with 3,3-diaminobenzidine tetrahydrochloride solution for 15 min under a microscope. Sections were then immersed in running tap water, counterstained with hematoxylin for 1 min, followed by tap water bath, immersion in a series of alcohol baths of increasing concentrations, and xylene, then covered with coverslips. Negative controls were performed, in which the primary antibody was omitted. Evaluation of IGF2 and IMP3 staining was performed independently by three pathologists. Slides with equivocal evaluation were reevaluated, and a consensus was reached. For each sample, at least 3,000 carcinoma cells were evaluated for the immunohistochemical staining. We examined the sections at 200× magnification, and the carcinoma cells with cytoplasmic or nuclear staining was determined.
Evaluation of MVD in IGF2 and IMP3 staining areas
Microvessel density (MVD) was assessed in the IGF2-positive and IMP3-positive tumor areas. Vessel counts were performed under light microscope based on staining of CD31. Five areas of maximal MVD was identified by screening (magnification 40×). The number of vessels was counted within a counting grid at 400× magnification (40 × objective and 10 × ocular). For the blood vessels counts, any stained endothelial cell or cell cluster separated from another microvessel structure was considered as a countable microvessel. But a lumen was not necessary for a structure to be counted as a microvessel. The number of vessels were expressed as the mean value of counted microvessel in five evaluated grids in areas of maximum vessel density. Data were shown as the mean ± SD (Table 2).
Statistical analysis
Statistical analysis was performed using the SPSS 11.0 software package for Windows. Measures of central tendency and dispersion were determined. The Mann–Whitney test were used to analyze the data. Differences were considered significant at P < 0.05 level.
Results
Immunohistochemical staining of IGF2 and IMP3 in osteosarcoma
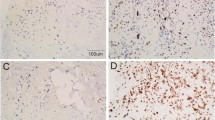
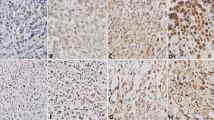
The IGF2 and IMP3 staining had different expression patterns in different osteosarcoma. In the 64 cases of conventional osteosarcoma, twelve cases showed same nuclear staining patterns (Fig. 1aA, B), and twenty-nine cases showed same cytoplasmic staining of IGF2 and IMP3 (Fig. 1aC, D). On the other hand, fourteen cases showed nuclear IGF2 staining but cytoplasmic IMP3 expression (Fig. 1aE, F), and 9 cases showed nuclear IMP3 staining and cytoplasmic IGF2 expresison (Fig. 1aG, H) (Table 1). Twenty-eight out of forty-seven cases of parosteal osteosarcoma showed nuclear IGF2 and IMP3 expression (Fig. 1bA, B), nine showed cytoplasmic IGF2 and IMP3 expression simultaneously (Fig. 1bC, D). Seven out of forty-seven cases of parosteal osteosarcoma expressed IGF2 with nuclear staining but expressed IMP3 with cytoplalsmic staining (Fig. 1bE, F). Meanwhile, three cases expressed IGF2 with cytoplasimic staining but expressed IMP3 with nuclear staining (Fig. 1bG, H). Similar to the parosteal osteosarcoma, the periosteal osteosarcoma expressed IGF2 and IMP3 mainly with nuclear staining simultaneously, forty out of fifty-five cases of preiosteal osteosarcoma did that (Fig. 1cA, B). Five out of fifty-five cases expressed IGF2 and IMP3 with cytoplasmic staining at the same time (Fig. 1cC, D). Four cases showed nuclear IGF2 staining and cytoplasmic IMP3 staining (Fig. 1cE, F). Also there were six cases showed cytoplasmic IGF2 staining and nuclear IMP3 staining (Fig. 1cG, H) (Table 1). In the parosteal and periosteal osteosarcoma, there was no significant difference in IGF and IMP3 expression patterns (P = 0.216). However, compared with conventional osteosarcoma, the parosteal and periosteal osteosarcoma showed significant difference in IMP3 and IGF2 expression (P = 0.016, P = 0.023).
IGF2 and IMP3 have different distribution in conventional, parosteal and periosteal osteosarcoma. A, B show IGF2 and IMP3 nuclear expression pattern, C, D show IGF2 and IMP3 cytoplasmic expression pattern simultaneously, E, F show IGF2 nuclear but IMP3 cytoplasmic expression; G, H show IGF2 cytoplasmic but IMP3 nuclear expression
The relationship of IGF2 and IMP3 expression in osteosarcoma
IGF2 and IMP3 were mainly expressed with cytoplasmic staining in conventional osteosarcoma simultaneously. However, parosteal and periosteal osteosarcoma mainly showed IGF2 and IMP3 expression with nuclear staining. Therefore, IGF2 and IMP3 expression patterns were positive correlation in the different osteosarcoma (r = 0.1021, P = 0.032).
The impact of IGF2 and IMP3 on angiogenesis in osteosarcoma
The results revealed that the nuclear expression of IGF2 and IMP3 might inhibit angiogenesis (Fig. 2A) and cytoplasmic IGF2 and IMP3 expression might promote angiogenesis (Fig. 2B) regardless of the histological classification (Table 2). In the conventional, parosteal and periosteal osteosarcoma, the cytoplasmic IGF2 and IMP3 promoted angiogenesis, and the cases with nuclear expression showed less MVD, the difference was significant (P = 0.024) (Table 2). Interestingly, the cytoplasmic IGF and IMP3 promoted angiogenesis more actively in conventional than in parosteal and periosteal osteosarcoma (Fig. 3A, B, C), and the difference of MVD was significant (P = 0.035) (Table 2), but the mechanism remained unknown. The nuclear expression and mixed expression of IGF2 and IMP3 showed no significant difference in angiogenesis (P = 0.120, P = 0.087, P = 0.235) (Table 2) regardless of the histological classification.
Discussion
IGF2 and IMP3 might contribute to the prognosis or invasive behaviors. But the detailed mechanism remain unknown. Overexpression of IGF2 in cell lines and tumors occurs with high frequency and may result from loss of genomic imprinting of the IGF2 gene (Yaginuma et al. 1997). In addition, highly metastatic cancer cells have been shown to possess higher expression of IGF2 and IGF-1R than tumor cells that are less prone to metastasize (Guerra et al. 1996). Study has revealed that IGF-1 and IGFBP-3 serum levels are significantly lower in patients with osteosarcoma, but IGF2 serum levels significantly higher than in healthy controls. Increased IGF2 levels are associated with a decreased disease-free survival. After tumor excision, both IGF1 and IGF2 values returned to normal level. The patients all expressed high mRNA level of IGF2 and higher than IGF1. IGF2 rather than IGF1 is the predominant growth factor produced by osteosarcoma cells (Avnet et al. 2009). We have examined the IGF2 and IMP3 (Insulin-like growth factor II mRNA-binding protein 3) in three subtype of osteosarcoma, the immunostaining showed that conventional osteosarcoma expressed with cytoplasmic staining, but parosteal and periosteal osteosarcoma with nuclear staining. According to the three subtype have different prognosis, there should be some impacts of IGF2 and IMP3 with different expression patterns on the osteosarcoma. Under physiologic conditions, IGF2 is abundantly stored in the bone matrix (Bautista et al. 1991), and during fetal development, IGF2 expression is higher than in the postnatal life (Baker et al. 1993). In most tissues, IGF2 is maternally imprinted, but this imprinting is usually lost in cancer (Ohlaaon et al. 1993; Rainier et al. 1993; Ogawa et al. 1993), leading to IGF2 biallelic production that provides an increased growth-promoting signaling. In osteosarcoma cell line MG-63 cells, transcriptional mechanisms are involved in the IGF1-induced increase of IGFBP-3, and GH-R is very low at the plasma membrane. The dowstream GH-signaling cascade is fully functional, and the endogenous IGFBP-3 gene is respond to IGF1 but not hGH in human MG-63 osteosarcoma cells (Rosato et al. 2001). IGFBP6 and IMP3 gene of osteosarcoma cells are up-regulated after decitabine treatment (Al-Romaih et al. 2007). The subpopulations of human osteosarcoma cell line (SaOS2) expressing high and low levels of alkaline phosphatase (ALP). Dihydrotestosterone (DHT) increased specific ALP activity and type-I procollagen peptide secretion in both the subpopulations. DHT pretreatment enhanced the mitogenic action of basic fibroblast growth factor (bFGF) and insulinlike growth factor 2 (IGF2) only in the subpopulation expressing high levels of ALP. The enhanced mitogenic effect of IGF2 in the subpopulation expressing high ALP after DHT pretreatment is related with increased IGF2-receptor mRNA levels suggesting that androgens exert their osteoanabolic action (Fidler 2003) by stimulating differentiated functions of osteoblastic cells with a high and a low ALP phenotype, and (Beasley et al. 2002) via increased growth factor receptor expression and thereby strengthening mitogenic growth factor responses only in the subpopulation expressing high ALP (Kasperk et al. 1996). Since IGF2 and bFGF could be overexpressed in osteosarcoma, the IGF and FGF aslo were reported associated with angiogenesis in some tumors (Alitalo and Carmeliet 2002; Cao 2005; Cullen et al. 1990; Reinmuth et al. 2002; Tanno et al. 2001; Che et al. 2002; Lee et al. 2000; Shigematsu et al. 1999), then in osteosarcoma, different expression patterns of IGF2 and IMP3 should impact the angiogenesis. Our study showed that IGF2 and IMP3 had different expression patterns in conventional, parosteal and periosteal osteosarcoma. The cytoplasmic IGF2 and IMP3 are expressed in three osteosarcoma subtype with MVD increasing. Meanwhile, the nuclear IGF2 and IMP3 expressed in three osteosarcoma subtype with MVD decreasing. In addition, the conventional osteosarcoma also showed more MVD than parosteal and periosteal osteosarcoma. Vessel metastasis have been regarded as the main way of sarcoma metastasis. So our results might be the key that conventional sarcoma has poor prognosis. The mechanism of different impacts of IGF2 and IMP3 on MVD might be that nuclear expression of IGF2 and IMP3 should play a relatively normal role in osteosarcoma, but cytoplasmic expression should be aberrant, and have abnormal impacts on the tumor, that angiogenesis animated might be the exhibition. In our next study, the mechnism of IGF2 and IMP3 interaction should be lucubrated. We thought that IGF2 and IMP3 inhibition should be promising in tumor vascular targeting therapy.
References
Alitalo K, Carmeliet P (2002) Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell 1(3):219–227
Al-Romaih K, Somers GR, Bayani J (2007) Modulation by decitabine of gene expression and growth of osteosarcoma U2OS cells in vitro and in xenografts: identification of apoptotic genes as targets for demethylation. Cancer Cell Int 7:14
Amit D, Tamir S, Birman T, Gofrit ON, Hochberg A (2011) Development of targeted therapy for bladder cancer mediated by a double promoter plasmid expressing diphtheria toxin under the control of IGF2–P3 and IGF2–P4 regulatory sequences. Int J Clin Exp Med 4(2):91–102
Avnet S, Sciacca L, Salerno M, Gancitano G, Cassarino MF, Longhi A, Zakikhani M, Carboni JM, Gottardis M, Giunti A, Pollak M, Vigneri R, Nicola B (2009) Insulin receptor isoform A and insulin-like growth factor II as additional treatment targets in human osteosarcoma. Cancer Res 69(6):2443–2452
Baker J, Liu JP, Robertson EJ, Efstratiadis A (1993) Role of insulin-like growth factors in embryonic and postnatal growth. Cell 75:73–82
Baserga R, Hongo A, Rubini M, Prisco M, Valentinis B (1997) The IGF-I receptor in cell growth, transformation and apoptosis. Biochim Biophys Acta 1332(3):F105–F126
Bautista CM, Baylink DJ, Mohan S (1991) Isolation of a novel insulin-like growth factor (IGF) binding protein from human bone: a potential candidate for fixing IGFII in human bone. Biochem Biophys Res Commun 176:756–763
Beasley NJ, Prevo R, Banerji S et al (2002) Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res 62(5):1315–1320
Bjorndahl M, Renhai Cao L, Nissen J, Clasper S, Johnson LA, Xue Y, Zhou Z, Jackson D, Hansen AJ, Cao Y (2005) Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. PNAS 102(43):15593–15598
Cao Y (2005) Opinion: emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nat Rev Cancer 5(9):735–743
Chava S, Mohan V, Shetty PJ, et al. (2011) Immunohistochemical evaluation of p53, FHIT, and IGF2 gene expression in esophageal cancer. Dis Esophagus. 10 June 2011. doi:10.1111/j.1442-2050.2011.01213.x. [Epub ahead of print]
Che W, Lerner-Marmarosh N, Huang Q, Osawa M, Ohta S, Yoshizumi M, Glassman M, Lee JD, Yan C, Berk BC, Abe J (2002) Insulin-like growth factor-1 enhances inflammatory responses in endothelial cells: role of Gab1 and MEKK3 in TNF-alpha-induced c-Jun and NF-kappaB activation and adhesion molecule expression. Circ Res 90(11):1222–1230
Chen ST, Jeng YM, Chang CC, Chang HH, Huang MC, Juan HF, Hsu CH, Lee H, Liao YF, Lee YL, Hsu WM, Lai HS (2011) Insulin-like growth factor II mRNA-binding protein 3 expression predicts unfavorable prognosis in patients with neuroblastoma. Cancer Sci. 14 September 2011. doi:10.1111/j.1349-7006.2011.02100.x. [Epub ahead of print]
Clemmons DR (1997) Insulin-like growth factor binding proteins and their role in controlling IGF actions. Cytokine Growth Factor Rev 8(1):45–62
Cullen KJ, Yee D, Sly WS, Perdue J, Hampton B, Lippman ME, Rosen N (1990) Insulin-like growth factor receptor expression and function in human breast cancer. Cancer Res 50(1):48–53
Dadras SS, Lange-Asschenfeldt B, Velasco P, Nguyen L, Vora A, Muzikansky A, Jahnke K, Hauschild A, Hirakawa S, Mihm MC, Detmar M (2005) Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol 18(9):1232–1242
Do SI, Kim YW, Park HR, Park YK (2008) Expression of insulin-like growth factor-II mRNA binding protein 3 (IMP3) in osteosarcoma. Oncol Res 17(6):269–272
Fidler IJ (2003) The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3(6):453–458
Fletcher CDM, Unni KK, Mertens F (eds) (2002) World health organization classification of tumours. Pathology and genetics of tumours of soft tissue and bone. IARC Press, Lyon
Flossmann-Kast BB, Jehle PM, Hoeflich A, Adler G, Lutz MP (1998) Src stimulates insulin-like growth factor I (IGF-I)-dependent cell proliferation by increasing IGF-I receptor number in human pancreatic carcinoma cells. Cancer Res 58(16):3551–3554
Fokas E, Kamlah F, Hänze J, Engenhart-Cabillic R, Rose F, An HX (2010) EphA2 blockade enhances the anti-endothelial effect of radiation and inhibits irradiated tumor cell-induced migration of endothelial cells. Thorac Cancer 1:153–162
Guerra FK, Eijan AM, Puricelli L, Alonso DF, de Kier Joffe EB, Kornblihgtt AR, Charreau EH, Elizalde PV (1996) Varying patterns of expression of insulin-like growth factors I and II and their receptors in murine mammary adenocarcinomas of different metastasizing ability. Int J Cancer 65:812–820
Guo YS, Jin GF Jr, Townsend CM, Zhang T, Sheng HM, Beauchamp RD, Thompson JC (1995) Insulin-like growth factor II expression in carcinoma in colon cell lines: implications for autocrine actions. J Am Coll Surg 181:145–154
Hermanto U, Zong CS, Wang LH (2000) Inhibition of mitogen-activated protein kinase kinase selectively inhibits cell proliferation in human breast cancer cells displaying enhanced insulin-like growth factor I-mediated mitogen-activated protein kinase activation. Cell Growth Differ 11:655–664
Jenkins PJ, Bustin SA (2004) Evidence for a link between IGF-I and cancer. Eur J Endocrinol 151(Suppl 1):S17–S22
Kappel CC, Velez-Yanguas MC, Hirschfeld S, Helman LJ (1994) Human osteosarcoma cell lines are dependent on insulin-like growth factor I for in vitro growth. Cancer Res 54:2803–2807
Kasperk C, Fitzsimmons R, Strong D, Mohan S, Jennings J, Wergedal J, Baylink D (1990) Studies of the mechanism by which androgens enhance mitogenesis and differentiation in bone cells. J Clin Endocrinol Metab 71(5):1322–1329
Kasperk CH, Faehling K, Bo¨rcso¨k I, Ziegler R (1996) Effects of androgens on subpopulations of the human osteosarcoma cell line SaOS2. Calcif Tissue Int 58:376–382
Kern PA, Svoboda ME, Eckel RH, Van Wyk JJ (1989) Insulinlike growth factor action and production in adipocytes and endothelial cells from human adipose tissue. Diabetes 38(6):710–717
Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE (2000) The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev 21:215–244
Lee OH, Bae SK, Bae MH, Lee YM, Moon EJ, Cha HJ, Kwon YG, Kim KW (2000) Identification of angiogenic properties of insulin-like growth factor II in in vitro angiogenesis models. Br J Cancer 82(2):385–391
Li Y, Meng G, Huang L, Guo QN (2009) Hypomethylation of the P3 promoter is associated with up-regulation of IGF2 expression in human osteosarcoma. Hum Pathol 40(10):1441–1447
Long L, Rubin R, Brodt P (1998) Enhanced invasion and liver colonization by lung carcinoma cells overexpressing the type 1 insulin-like growth factor receptor. Exp Cell Res 238(1):116–121
Neuhausen SL, Brummel S, Ding YC, et al. (2011) Genetic variation in IGF2 and HTRA1 and breast cancer risk among BRCA1 and BRCA2 carriers. Cancer Epidemiol Biomarkers Prev. 26 July 2011. [Epub ahead of print]
Ogawa O, Eccles MR, Szeto J et al (1993) Relaxation of insulin-like growth factor II gene imprinting implicated in Wilms’ tumour. Nature 362:749–751
Ohlaaon R, Nystrom A, Pfeifer-Ohlsson S et al (1993) IGF2 is parentally imprinted during human embryogenesis and in the Beckwith-Wiedemann sindrome. Nat Genet 4:94–97
Quinn KA, Treston AM, Unsworth EJ, Miller MJ, Vos M, Grimley C, Battey J, Mulshine JL, Cuttitta F (1996) Insulin-like growth factor expression in human cancer cell lines. J Biol Chem 271:11477–11483
Rainier S, Johnson LA, Dobry CJ, Ping AJ, Grundy PE, Feinberg AP (1993) Relaxation of imprinted genes in human cancer. Nature 362:747–749
Reinmuth N, Fan F, Liu W, Parikh AA, Stoeltzing O, Jung YD, Bucana CD, Radinsky R, Gallick GE, Ellis LM (2002) Impact of insulin-like growth factor receptor-I function on angiogenesis, growth, and metastasis of colon cancer. Lab Invest 82(10):1377–1389
Ritter MR, Dorrell MI, Edmonds J, Friedlander SF, Friedlander M (2002) Insulin-like growth factor 2 and potential regulators of hemangioma growth and involution identified by large-scale expression analysis. Proc Natl Acad Sci USA 99(11):7455–7460
Rosato R, Gerland K, Jammes H, Bataille-Simoneau N, Segovia B, Mercier L, Groyer A (2001) The IGFBP-3 mRNA and protein levels are IGF-I-dependent and GH-independent in MG-63 human osteosarcoma cells. Mol Cell Endocrinol 175(1–2):15–27
Shigematsu S, Yamauchi K, Nakajima K, Iijima S, Aizawa T, Hashizume K (1999) IGF-1 regulates migration and angiogenesis of human endothelial cells. Endocr J 46(Suppl):S59–S62
Steller MA, Delgado CH, Bartels CJ, Woodworth CD, Zou Z (1996) Overexpression of the insulin-like growth factor I receptor and autocrine stimulation in human cervical cancer cells. Cancer Res 56:1761–1765
Tanno S, Tanno S, Mitsuuchi Y, Altomare DA, Xiao GH, Testa JR (2001) AKT activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res 61(2):589–593
Viereck V, Siggelkow H, Pannem R, Braulke T, Scharf JG, Kübler B (2007) Alteration of the insulin-like growth factor axis during in vitro differentiation of the human osteosarcoma cell line HOS 58. J Cell Biochem 102(1):28–40
Xie Y, Skytting B, Nilsson G, Brodin B, Larsson O (1999) Expression of insulin-like growth factor-1 receptor in synovial sarcoma: association with an aggressive phenotype. Cancer Res 59(15):3588–3591
Yaginuma Y, Nishiwaki K, Kitamura S, Hayashi H, Sengoku K, Ishikawa M (1997) Relaxation of insulin-like growth factor-II gene imprinting in human gynecologic tumors. Oncology (Basel) 54:502–507
Acknowledgments
This work was supported by Jinan Science and Technology Bureau: independent innovation projects of university and institutes Stationed in jinan city(No. 201102060).
Author information
Authors and Affiliations
Corresponding author
Additional information
Peng Chen and Shao-jin Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, P., Wang, Sj., Wang, Hb. et al. The distribution of IGF2 and IMP3 in osteosarcoma and its relationship with angiogenesis. J Mol Hist 43, 63–70 (2012). https://doi.org/10.1007/s10735-011-9370-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-011-9370-2