Abstract
Malignant gliomas are the most common primary brain tumors in adults. Prognosis remains dismal despite aggressive treatment with surgical resection, radiation, and chemotherapy. The use of advanced sequencing technologies and large-scale gene expression studies has provided an in-depth description of the distinct molecular and genetic alterations in glioblastoma inspiring interest in the development of targeted therapies. Unfortunately, despite trials with several agents and different pathways, targeted therapies have currently failed to improve the overall survival of glioblastoma patients. New therapies examining novel targets and innovative drug combinations are presently under investigation that will hopefully prove more beneficial.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Malignant glioma
- Targeted therapy
- The cancer genome atlas
- Retinoblastoma pathway
- p53 pathway
- Receptor tyrosine kinase pathway
Targeting Aberrant Signaling Pathways
Malignant gliomas are the most common primary brain tumor found in adults [1]. Regretfully, prognosis for these tumors remains dismal despite aggressive treatment with surgical resection, radiation, and chemotherapy. Treatment of glioblastoma patients with the current standard of care consisting of maximal resection, 6 weeks of concurrent chemoradiation with daily temozolomide followed by 6–12 cycles of adjuvant temozolomide, results in a median overall survival of only approximately 16 months [2]. Malignant gliomas have proven to be among the most difficult cancers to treat due to their genetic heterogeneity, elaborate overlapping signaling pathways, and difficulties in delivering drugs across the blood–brain barrier [3]. Recent in-depth description of the distinct molecular and genetic alterations in glioblastomas, using advanced sequencing technologies and large-scale gene expression studies, has inspired interest in the development of targeted therapies. Targeted therapies work by the inhibition of the deregulated cell signaling pathways in cancer cells by small molecules or antibodies, whereas traditional cytotoxic chemotherapies operate by impeding DNA synthesis or cell metabolism. This chapter will explore these aberrant signaling pathways in malignant gliomas and the results of the clinical trials of therapeutics targeting them.
The Cancer Genome Atlas
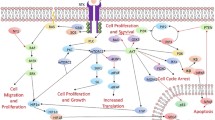
The Cancer Genome Atlas (TCGA), a collaboration between the National Cancer Institute (NCI) and National Human Genome Research Institute (NHGRI), was undertaken to generate comprehensive, multidimensional maps of the major genomic changes in several types of cancer. One of the first cancers studied by the TCGA was glioblastoma, and the analysis characterized a decidedly interrelated network of aberrations. It identified three key pathways: the retinoblastoma (RB) and p53 tumor suppressor pathways, and the receptor tyrosine kinases (RTKs) signaling pathway [4]. For glioblastoma patients with sequencing data, the frequencies of somatic alterations were 78, 87, and 88%, respectively, in each of these pathways (Fig. 9.1). Of further note, 74% of glioblastoma samples contained abnormalities in all three pathways [4].
Critical signaling pathways altered in malignant gliomas. Primary sequence alterations and significant copy number changes for the components of the a | RTK/RAS/PI3K, b | p53, and c | Rb signaling pathways are shown. Red indicates activating genetic alterations. Conversely, blue indicates inactivating alterations. For each altered component of a particular pathway, the nature of the alteration and the percentage of tumors affected are indicated. Boxes contain the final percentages of glioblastomas with alterations in at least one known component gene of the designated pathway. Abbreviation: RTK receptor tyrosine kinase
Retinoblastoma Tumor Suppressor Pathway
The RB protein is a tumor suppressor protein that is dysfunctional in several cancer types [5]. It is encoded by the RB gene, which is located at chromosome 13q14.1-q14.2. Normally, the RB protein prevents unwarranted cell growth by inhibiting cell cycle progression until a cell is set to undergo mitosis. When ready for cell division, the RB protein is then phosphorylated by cyclin D, cyclin-dependent kinase 4 (CDK4), and cyclin-dependent kinase 6 (CDK6) inactivating the protein and allowing for cell cycle progression [5]. Most commonly, homozygous deletion of cyclin-dependent kinase inhibitor 2A (CDKN2A) can produce loss of p16INK4a and a suppressor of CDK4, leading to dysregulation of RB signaling [5–7]. Mutations in retinoblastoma protein 1 (RB1) and CDK4 amplification can also trigger dysfunction of the RB signaling pathway. A CDK4 inhibitor, PD-0332991 (palbociclib isethionate), has been examined in two phase I trials leading to a phase II trial in recurrent RB-positive glioblastoma with results not yet reported [8–10].
p53 Tumor Suppressor Pathway
The tumor protein p53 (p53) gene is the most frequently mutated gene in human cancer and performs a critical function in preventing cancer formation [11]. It is located at chromosome 17p13.1 and reacts to DNA injury and toxic pressures by producing cell cycle arrest and apoptosis [12, 13]. Loss of p53 pathway function can be due to p53 mutation/deletion itself or by interferences in other genes that regulate p53 function such as murine double minute (MDM2/4) and the tumor suppressor protein alternate reading frame (ARF) [14–16].
The use of an intratumoral injection of a p53-containing adenovirus vector to increase wild-type p53 expression in tumor cells has been attempted in a phase 1 study in recurrent glioma, but it did not appear to achieve systemic viral dissemination [17]. Phase I trials of wild-type Ad5CMV-p53 gene therapy and recombinant adenovirus p53 (SCH-58500) in combination with surgery in recurrent malignant gliomas have been completed, but the results have yet to be published [18, 19].
SGT-53 is a nanocomplex of cationic liposome encapsulating a normal human wild-type p53 DNA sequence in a plasmid backbone exhibited to supply the p53 cDNA to the tumor cells with the goal of the p53 cDNA sequence to restore wild-type p53 function in the apoptotic pathway [20]. SGT-53 has shown to prolong survival in a mouse model and is currently undergoing investigation in a phase II trial in recurrent glioblastoma [20, 21]. An MDM2 inhibitor, JNJ-26854165, was examined in a phase I study in refractory solid tumors but has yet to be examined in brain tumor patients exclusively [22, 23]. MK-1775, a Wee1 kinase inhibitor, has been shown to radiosensitize p53-defective human tumor cells and is currently under investigation in a multicenter phase I trial in newly diagnosed or recurrent glioblastoma [24, 25].
Receptor Tyrosine Kinase Signaling Pathway
Receptor tyrosine kinases (RTKs) are high-affinity cell surface receptors and primary mediators of signal transduction events shown to have an essential function in the growth and progression of many cancers [26]. Twenty different RTK classes have been identified, and members of this family include the vascular endothelial growth factor receptor (VEGFR), epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR), and hepatocyte growth factor receptor (MET). The receptor tyrosine kinase signaling pathway has been the most extensively studied pathway in malignant gliomas to date (Table 9.1).
Vascular Endothelial Growth Factor Receptor (VEGFR)
Vascular endothelial growth factor (VEGF) is a significant component implicated in the formation of new blood vessels which is a distinguishing feature of glioblastoma [27]. VEGF binding to its receptors VEGFR-1 and VEGFR-2 leads to phosphorylation of tyrosine kinase and initiation of downstream signaling pathways including phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/protein kinase B (Akt/PBK) and Ras/mitogen-activated protein kinases (MAPK) [28].
Bevacizumab, a monoclonal antibody that targets the VEGF-A ligand, was granted accelerated approval by the Food and Drug Administration for use as a single agent in recurrent glioblastoma in 2009 [29]. Approval was granted based on the results of two phase II clinical trials that demonstrated durable objective imaging responses based on independent radiologic review with stable or decreasing corticosteroid use [29–31]. Subsequently, two phase III clinical trials (RTOG 0825 and AVAglio) were performed examining the addition of bevacizumab or placebo to the current standard of care regimen of concurrent chemoradiation with temozolomide followed by adjuvant temozolomide in newly diagnosed glioblastoma [32, 33]. Both studies found that the addition of bevacizumab improved progression-free but not overall survival [32, 33]. While the addition of irinotecan to bevacizumab was not beneficial in the early studies of bevacizumab, a subsequent phase II study appeared to suggest that the combination of lomustine and bevacizumab may prolong overall survival compared to either treatment administered alone [30, 31, 34]. Disappointingly, the preliminary report of the results of an EORTC phase III study comparing lomustine alone versus lomustine and bevacizumab in reccurent glioblastoma failed to demonstrate an improvement in overall survival with the combination treatment [35]. Phase II trials of recurrent glioblastoma examining the addition of fotemustine or carboplatin to bevacizumab also did not demonstrate any increased survival benefit with the addition of these cytotoxic therapies [36, 37]. Despite the improvement in progression-free survival and increased imaging response rate, bevacizumab has yet to improve overall survival in either the upfront or recurrent setting.
Other VEGF inhibitors have failed to even match the limited success of bevacizumab. Cediranib, an oral pan-VEGF receptor tyrosine kinase inhibitor, demonstrated a 6-month progression-free survival of 25.8% and partial radiographic responses in 56.7% of patients in a phase II study of patients with recurrent glioblastoma [38]. However, a phase III randomized trial in recurrent glioblastoma comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone failed to show any improvement with cediranib either as monotherapy or in combination with lomustine versus lomustine alone [39]. Pazopanib, a multikinase inhibitor of c-Kit, FGFR, PDGFR, and VEGFR, also did not show any prolongation of progression-free survival in a phase II study in recurrent glioblastoma [40]. A subsequent phase I/II examining pazopanib in combination with lapatinib (an EGFR inhibitor) in relapsed malignant glioma patients had limited antitumor activity leading to early termination of the study [41]. Sorafenib, an oral VEGFR-2, Raf, PDGFR, c-Kit, and Flt-3 inhibitor, was used in combination with temozolomide for initial adjuvant therapy in a phase II study for patients with glioblastoma but failed to improve the efficacy when compared to historical controls [42]. Additionally, sorafenib has been examined in several other phase II studies in recurrent glioblastoma in combination with temozolomide, bevacizumab, erlotinib, and temsirolimus of which no combination resulted in a prolongation of survival [43–46]. Nintedanib, an inhibitor that targets VEGFR 1-3, FGFR 1-3, and PDGFR-α/β, was studied in a phase II study that was terminated early following a preplanned futility analysis [47]. Vandetanib, an inhibitor of VEFGR-2 and EGFR, failed to display any significant activity in a phase I/II trial of patients with recurrent malignant glioma [48]. In addition, a phase I/II study of vandetanib plus sirolimus (an mTor inhibitor) in adults with recurrent glioblastoma failed to display benefit when compared to historical controls [49]. Sunitinib, an inhibitor of VEGFR-2, PDGFR-α, and KIT, similarly did not show any improvement in survival either as a monotherapy or in combination with irinotecan in phase I or phase II studies in recurrent glioma [50–53]. An inhibitor of VEGF and placental growth factor, aflibercept, also had minimal evidence of single-agent activity in unselected patients with recurrent malignant glioma [54]. Vatalanib, an inhibitor of VEGFR, PDGFR, and c-Kit, was examined alone in newly diagnosed glioblastoma patients and with imatinib and hydroxyurea at time of tumor recurrence in two phase I studies [55, 56]. However, a planned randomized phase II trial was terminated at its initiation (after completion of its phase I component) because of industry decision [57]. Cabozantinib, an inhibitor of VEGFR-2, MET, and RET, was used in a phase II study whose final results are yet to be published [58].
Epidermal Growth Factor Receptor (EGFR)
The epidermal growth factor receptor is located on chromosome 7p12 and is a member of the ErbB family of receptor tyrosine kinases [59]. Overexpression of EGFR is one of the most common signaling mutations in GBM and is thought to occur in around 50% of glioblastomas [60]. Glioblastomas with EGFR overexpression have been demonstrated to potentially be more radioresistant [61]. Additionally, EGFR amplification is often associated with the expression of a constitutively active, ligand-independent mutant form of the receptor called EGFRvIII generated by an in-frame deletion of exons 2–7 [61, 62]. EGFRvIII expression may be an independent prognostic factor for poor survival [63].
Unfortunately, EGFR inhibitors in malignant glioma trials have likewise been disappointing. Gefitinib, an EGFR inhibitor approved to treat non-small-cell lung cancer in 2003, failed to show any benefit when added to the treatment in newly diagnosed glioblastoma patients [64, 65]. Additionally, gefitinib in the treatment of recurrent disease has shown minimal activity alone or in combination with mammalian target of rapamycin (mTOR) inhibitors such as sirolimus or everolimus [66–69]. Another EGFR inhibitor, Erlotinib, also has shown minimal efficacy against newly diagnosed or recurrent glioblastoma [70–76]. Furthermore, several phase II studies in recurrent glioblastoma examining the combination of erlotinib with carboplatin, sorafenib, bevacizumab, or sirolimus have failed to demonstrate significant antitumor activity [45, 77–80]. Cetuximab, another EGFR inhibitor used for the treatment of metastatic colorectal cancer, metastatic non-small-cell lung cancer, and head and neck cancer, regrettably has failed to show benefit in recurrent glioblastoma when used alone or in combination with bevacizumab and irinotecan [81, 82]. Lapatinib, the first dual inhibitor of EGFR and human epidermal growth factor receptor 2 (HER2) tyrosine kinases, also did not show significant activity in recurrent glioblastoma patients [83]. Additionally, lapatinib was studied in a phase I study with temodar and a phase I/II study with pazopanib in recurrent malignant gliomas [84, 85]. The phase II study of lapatinib and pazopanib revealed limited antitumor activity of this combination leading to early study termination [84]. AEE788, another inhibitor of EGFR, HER2, and VEGFR2, was associated with unacceptable toxicity and minimal activity for the treatment of recurrent glioblastoma in a phase I trial [86].
Afatinib, an irreversible covalent inhibitor of the EGFR and HER2, is approved for first-line treatment of patients with EGFR mutation-positive non-small-cell lung carcinoma [87]. Afatinib is currently under investigation in a phase I/II trial in recurrent malignant glioma. Additionally, a phase I trial of afatinib in newly diagnosed glioblastoma patients with radiotherapy alone in patients with an unmethylated MGMT promotor or radiotherapy and temozolomide in patients with a methylated MGMT promotor is ongoing [88, 89]. Dacomitinib is another selective and irreversible inhibitor of EGFR studied in two phase II trials in recurrent glioblastoma with one of the trials limited to only patients with EGFR gene amplification and/or EGFRvIII mutation [90, 91]. Another EGFR inhibitor, nimotuzumab, has received orphan drug status in the USA and EU for glioma. A phase I/II trial in high-grade glioma with nimotuzumab showed an excellent safety profile and significant survival benefit in combination with irradiation, but unfortunately, a subsequent phase III trial of nimotuzumab in newly diagnosed glioblastoma was negative [92, 93].
Platelet-Derived Growth Factor Receptor (PDGFR)
Platelet-derived growth factor receptors (PDGFRs) are cell surface receptors for members of the PDGF family and signal through the alpha and beta PDGF receptor tyrosine kinases [94]. The PDGFR alpha (PDGFRA) gene is located on chromosome 7p22 and amplified in approximately 13% of glioblastomas [4, 95]. PDGFRA can be overexpressed, amplified, mutated, or truncated in gliomas, with PDGFRA point mutations being observed exclusively in glioblastomas [96].
Imatinib mesylate is an inhibitor of the PDGFR, Bcr-Abl, and c-Kit tyrosine kinases that have been found to beneficial in the treatment of chronic myelogenous leukemia b and in gastrointestinal stromal tumors [97–99]. However, imatinib alone displayed only minimal activity in recurrent malignant gliomas and in newly diagnosed glioblastoma [100, 101]. Subsequent studies looking at imatinib with the addition of hydroxyurea in recurrent malignant gliomas also failed to show clinically meaningful antitumor activity [102–105]. As discussed previously, imatinib with hydroxyurea was also examined in combination with vatalanib [56].
Dasatinib, an inhibitor of PDGFR, Src, Bcr-Abl, c-Kit, and EphA2 receptors, was studied in a phase 2 trial in target-selected patients (activation or overexpression of ≥2 putative dasatinib targets) with recurrent glioblastoma and was found ineffective with no radiographic responses [106]. Additional, phase II studies with dasatinib in combination with bevacizumab in recurrent glioblastoma and in newly diagnosed glioblastoma with chemoradiation have not yet reported results [107, 108]. A phase I multicenter trial of dasatinib in combination with CCNU was found to have substantial hematological toxicities leading to suboptimal exposure to both agents [109]. Another phase I study of dasatinib in combination with erlotinib was better tolerated [110].
Furthermore, Tandutinib, a small molecule inhibitor of PDGFR, fms-like tyrosine kinase receptor-3 (FLT3), and c-Kit, has been examined alone or with bevacizumab in recurrent glioblastoma with results awaiting publication [111, 112].
PI3K/AKT/mTOR Pathway
Along with targeting cell surface receptors, there has been a significant effort undertaken on inhibiting downstream survival signaling pathways stimulated by these receptors. The PI3K/Akt/mTOR pathway can be crucial in controlling cellular functions regulating cellular proliferation, apoptosis, cell invasion, and mobility. Activation of phosphatidylinositide 3-kinase (PI3K) complex is regulated by several growth factors in conjunction with their receptors, the most frequent of which is the amplification of EGFR [113, 114]. PI3K activation phosphorylates and activates Akt (protein kinase B) a serine/threonine-specific protein kinase [115]. Akt next activates mTOR (mammalian target of rapamycin), another serine/threonine protein kinase that controls cell growth and proliferation via the regulation of protein synthesis and transcription [116]. It is comprised of two parts, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), operating as both a downstream effector and upstream regulator of PI3K [117].
PX-866, an oral PI3K inhibitor, in a recent phase II study had a low overall response rate and failed to improve progression-free survival in patients with recurrent glioblastoma [118]. BKM-120, another oral inhibitor of PI3 kinase, is currently under investigation in combination with standard of care for newly diagnosed glioblastoma and as monotherapy in recurrent glioblastoma [119, 120]. Additionally, a phase II study in recurrent glioblastoma examining the combination of BKM-120 and bevacizumab is underway [121]. XL-147 (a potent inhibitor of PI3K) and XL-765 (a dual PI3K and mTOR inhibitor) are currently being investigated as monotherapy in recurrent glioblastoma [122]. XL-765 has also been studied in combination with temozolomide in malignant glioma patients with no results published yet [123]. Pictilisib (a potent inhibitor of PI3K) and BEZ235 (a dual ATP-competitive PI3K and mTOR inhibitor) are also currently undergoing investigation in a phase II study of recurrent glioblastoma [124].
Enzastaurin is a protein kinase C and phosphoinositide-3 kinase/Akt inhibitor that failed to improve survival in newly diagnosed patients with and without O(6)-methylguanine-DNA-methyltransferase (MGMT) promotor methylation in combination with standard of care and as a monotherapy in recurrent glioblastoma [125–128]. Perifosine, an Akt inhibitor and a PI3K inhibitor, is currently being examined in recurrent malignant gliomas alone or in combination with temsirolimus [129, 130]. Ipatasertib, a highly selective pan-Akt inhibitor targeting Akt1/2/3, is also present in a phase II study of recurrent glioblastoma [131]. Nelfinavir, a protease inhibitor interfering with Akt activity, has been given neo-adjuvantly and concomitant to chemoradiotherapy with temozolomide in a phase I/II study of patients with newly diagnosed glioblastoma [132].
Several mTOR inhibitors have been developed over the past few years. mTOR is inhibited by these agents forming a complex with FK-binding protein-12 (FKBP-12) which joins to mTOR, blocking its stimulation and constraining tumor cell proliferation [133]. Everolimus, an mTOR inhibitor, has been shown to cause a marked reduction in the volume of subependymal giant cell astrocytomas and seizure frequency in patients with tuberous sclerosis [134]. Unfortunately, everolimus has not displayed durable responses as a monotherapy or when combined with gefitinib in patients with recurrent glioblastoma [69, 135]. Moreover, the addition of everolimus to standard of care in newly diagnosed glioblastoma patients did not translate into an appreciable survival benefit [136]. Furthermore, a phase II study in newly diagnosed glioblastoma patient of concurrent radiation therapy, temozolomide, and bevacizumab followed by bevacizumab/everolimus as first-line treatment failed to improve survival compared to historical controls [137].
Temsirolimus, an mTOR inhibitor approved for the treatment of renal cell carcinoma, likewise has shown limited benefit in recurrent glioblastoma as a monotherapy or in combination with sorafenib [46, 138, 139]. When temsirolimus was used in a phase I study in addition to standard of care in newly diagnosed glioblastoma, an increased risk of infection was noted [140].
Rapamycin (sirolimus), another mTOR inhibitor, has also failed to show benefit in recurrent glioblastoma when used in combination with vandetanib, erlotinib, or gefitinib as discussed earlier [49, 68, 79, 80]. Additionally, a phase II trial of rapamycin in combination with bevacizumab in recurrent glioblastoma patients was stopped early due to lack of response [141].
New mTOR-specific inhibitors are in development which can block activity of both mTOR complexes. AZD8055, one of these dual mTORC1/mTORC2 inhibitors, is currently in a phase I trial in adults with recurrent gliomas [142].
The PTEN (phosphatase and tensin homologue) gene, located on chromosome 10, is a tumor suppressor gene that negatively controls the PI3K/AKT/PKB pathway by preventing Akt signaling via the reduction of intracellular levels of phosphatidylinositol-3,4,5-triphosphate [143]. PTEN mutations have been reported in up to 40% of glioblastoma [4, 144]. In addition to inhibiting the Akt pathway, PTEN has also demonstrated the ability to enable the degradation of activated EGFR leading to the extinction of EGFR signaling [145]. Instigating expression of functional PTEN has been suggested as a potential future therapeutic approach in glioblastoma.
RAS/MAPK Pathway
The RAS/MAPK is another downstream survival signaling pathway stimulated by RTKs such as EGFR and PDGFR. Ras (rat sarcoma) gene mutations are present in a diverse group of tumor types with varying incidence [146]. Mutations in one of the three Ras genes (H-Ras, N-Ras, or K-Ras) in humans transform these genes to operating oncogenes [147]. Ras proteins have critical functions in regulating the activity of vital signaling pathways that control normal cellular proliferation. Activation and deactivation of Ras are regulated by cycling between its binding with the active guanosine triphosphate (GTP) and inactive guanosine diphosphate (GDP) forms [148]. Activated Ras results in activation of a serine/threonine kinase named Raf (rapidly accelerated fibrosarcoma). Raf subsequently phosphorylates and activates a kinase enzyme MEK (mitogen/extracellular signal-regulated Kinase) which in turn then phosphorylates and activates MAPK (mitogen-activated protein kinases).
Ras gene mutations have been found to only rarely occur in glioblastoma [4]. However, activation of Ras can occur by mechanisms that do not involve mutations in Ras. The neurofibromin 1 (NF-1) gene located on chromosomal segment 17q11.2 is a negative regulator of Ras, and loss of NF-1 may activate Ras [149]. NF-1 mutations have been reported in up to 18% of patients with glioblastoma [4].
Ras is posttranscriptionally modified by farnesyltransferase, and in vitro studies of glioblastoma with farnesyltransferase inhibitors have shown reduced cellular proliferation as well as the ability to trigger cell cycle arrest and induce apoptosis [150]. Tipifarnib is a potent and selective inhibitor of farnesyltransferase that has been examined in a phase I trial in newly diagnosed glioblastoma plus radiation therapy with and without temozolomide [151]. Unfortunately, a phase II trial of tipifarnib as a treatment for recurrent malignant glioma did not show benefit in 6-month progression-free survival compared to historical controls [152]. Lonafarnib, another farnesyltransferase inhibitor, has shown the ability to inhibit cell growth in preclinical studies [153, 154]. Two phase I studies have examined lonafarnib in combination with temozolomide, with one study in patients with malignant glioma after radiation and the other in patients with recurrent glioblastoma [155, 156].
Gene Expression-Based Molecular Classification of GBM into Subtypes
Following The Cancer Genome Atlas Network cataloging recurrent genomic abnormalities in glioblastoma, they subsequently defined four subtypes of glioblastoma (proneural, neural, classical, and mesenchymal) based on gene expression-based molecular classification [157]. Additionally, alterations and gene expression of EGFR, NF1, and PDGFRA/IDH1 were found to distinctly delineate the classical, mesenchymal, and proneural subtypes, respectively [157]. These discoveries support the supposition that certain molecular-targeted therapies may potentially be most effective against a segment of glioblastomas. Results from a retrospective analysis of AVAglio (a randomized, placebo-controlled phase III trial examining the addition of bevacizumab to radiotherapy plus temozolomide in newly diagnosed glioblastoma) suggest that patients with IDH1 wild-type proneural glioblastoma may derive an overall survival benefit from first-line bevacizumab treatment [158]. This finding, however, still remains to be independently validated in future studies.
Furthermore, the classification of glioblastoma into unique subtypes based on genomic expression implies that there is a propensity for particular aberrations to group together. This theoretically could enable particular combinations of molecularly targeted agents to be more successful in certain subtypes.
Discussion
Large-scale gene expression studies have recently provided an in-depth description of the distinct molecular and genetic alterations in glioblastomas. This scientific progress has spurred an interest in the development of targeted therapies for these signaling pathways. Unfortunately, despite trying several agents and different pathways, targeted therapies have currently failed to improve the overall survival of glioblastoma patients. New therapies examining novel targets and innovative combinations are presently under investigation (Table 9.2).
Several possible explanations have been proposed on why early clinical results of molecularly targeted agents in malignant glioma have been so disappointing. These include the significant intratumoral heterogeneity, overlapping/redundant signaling pathways, use of molecular data from initial tumor resection as entry criteria in trials of recurrent disease, poor drug delivery to the brain, and unclear pharmacodynamic effects of drugs on tumor tissue.
Tumor heterogeneity poses a significant challenge with glioblastoma being renowned for its intratumoral heterogeneity. Due to the heterogenous nature of these tumors, it is possible that we may be inhibiting a distinct group of cells susceptible to that specific targeted pathway yet still permitting the proliferation of another group of cells whose development is independent of that pathway. A recent study has demonstrated that glioblastoma subtype classifiers can variably be expressed even across individual cells within a tumor [159].
Additionally, these tumors appear to have the intrinsic ability to respond to the inhibition of one pathway by upregulating another different pathway making a single agent unsuccessful in stopping tumor progression. For example, the use of EGFR inhibitors has demonstrated the lack of ability to change downstream targets like Akt and may even upregulate the activity of the PI3K/Akt pathway [160, 161]. Moreover, many of the mutations that we are currently targeting may be essential only for the early development of the tumor and subsequently are superseded by secondary pathways of tumor growth.
Another potential reason for the lack of success with these agents may be that entry into targeted therapy trials for recurrent disease is often based upon molecular characteristics from initial resection due to surgical resections at tumor recurrence not being routinely performed. However, it is possible that these targeted mutations may be altered at time of recurrence compared to initial diagnosis. A recent study in glioblastoma found that of the tumors expressing EGFRvIII at initial diagnosis, approximately one-half loses their EGFRvIII expression at tumor recurrence [162].
Additionally, with surgery at tumor recurrence being difficult, it is often challenging to determine how well the drugs are crossing the blood–brain barrier. Furthermore, even if the agent crosses into the brain, it is often uncertain whether the drug is inhibiting its intended target and having its envisioned effect without pathological confirmation.
Numerous ways to improve the success of targeted therapies in malignant glioma have been propositioned and are currently underway. These include the creation of more advanced preclinical animal models, development of more potent inhibitors that can affect multiple pathways, trials with a combination of drugs designed uniquely for each individual, identification of predictive molecular biomarkers, and novel adaptive trial designs.
Despite the discouraging results to date and the above challenges, the use of targeted therapies remains a promising approach that continues to be explored in malignant glioma and will hopefully someday prove more beneficial for this patient population in desperate need of more effective treatments.
References
Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507.
Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96.
Tanaka S, Louis DN, Curry WT, Batchelor TT, Dietrich J. Diagnostic and therapeutic avenues for glioblastoma: no longer a dead end? Nature Rev Clin Oncol. 2013 Jan;10:14–26. Doi:10.1038/nrclinonc.2012.20.
Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–8. Doi:10.1038/nature07385.
Murphree AL, Benedict WF. Retinoblastoma: clues to human oncogenesis. Science. 1984;223(4640):1028–33. Doi:10.1126/science.6320372.
Ohgaki H, Kleihues P. Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci. 2009;100:2235–41.
Lin F, de Gooijer MC, Hanekamp D, Brandsma D, Beijnen JH, van Tellingen O. Targeting core (mutated) pathways of high-grade gliomas: challenges of intrinsic resistance and drug efflux. CNS Oncol. 2013;2(3):271–88. Doi:10.2217/cns.13.15.
Schwartz GK, LoRusso PM, Dickson MA, et al. Phase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (schedule 2/1). Br J Cancer. 2011;104(12):1862–8.
Flaherty KT, LoRusso PM, Demichele A, et al. Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin Cancer Res. 2012;18(2):568–76.
ClinicalTrials.gov. A Study of PD 0332991 in Patients With Recurrent Rb Positive Glioblastoma (PD0332991). Identifier: NCT01227434. http://clinicaltrials.gov/show/NCT01227434.
Surget S, Khoury MP, Bourdon JC. Uncovering the role of p53 splice variants in human malignancy: a clinical perspective. Onco Targets Ther. 2013;7:57–68. Doi:10.2147/OTT.S53876.
McBride OW, Merry D, Givol D. The gene for human p53 cellular tumor antigen is located on chromosome 17 short arm (17p13). Proc Natl Acad Sci USA. 1986;83(1):130–4. Doi:10.1073/pnas.83.1.130.
Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–83.
Reifenberger G, Liu L, Ichimura K, Schmidt EE, Collins VP. Amplification and overexpression of the MDM2 gene in a subset of human malignant gliomas without p53 mutations. Cancer Res. 1993;53:2736–9.
Riemenschneider MJ, Buschges R, Wolter M, et al. Amplification and overexpression of the MDM4 (MDMX) gene from 1q32 in a subset of malignant gliomas without TP53 mutation or MDM2 amplification. Cancer Res. 1999;59:6091–6.
Kamijo T, Weber JD, Zambetti G, Zindy F, Roussel MF, Sherr CJ. Functional and physical interactions of the ARF tumor suppressor with p53 and Mdm2. Proc Natl Acad Sci USA. 1998;95:8292–7.
Lang FF, Bruner JM, Fuller GN, Aldape K, Prados MD, Chang S, Berger MS, McDermott MW, Kunwar SM, Junck LR, Chandler W, Zwiebel JA, Kaplan RS, Yung WK. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol. 2003;21:2508–18.
ClinicalTrials.gov. Gene Therapy in Treating Patients With Recurrent Malignant Gliomas Identifier: NCT00004041. https://clinicaltrials.gov/ct2/show/NCT00004041?term=NCT00004041&rank=1.
ClinicalTrials.gov. Gene Therapy in Treating Patients With Recurrent or Progressive Brain Tumors Identifier: NCT00004080. https://clinicaltrials.gov/ct2/show/NCT00004080?term=NCT00004080&rank=1.
Kim SS, Rait A, Kim E, Pirollo KF, Chang EH. A tumor-targeting p53 nanodelivery system limits chemoresistance to temozolomide prolonging survival in a mouse model of glioblastoma multiforme. Nanomedicine. 2015;11(2):301–11. Doi:10.1016/j.nano.2014.09.005 Epub 2014 Sep 18.
ClinicalTrials.gov. Phase II Study of Combined Temozolomide and SGT-53 for Treatment of Recurrent Glioblastoma Identifier: NCT02340156. https://clinicaltrials.gov/ct2/show/NCT02340156?term=sgt-53&rank=1.
Yuan Y, Liao YM, Hsueh CT, Mirshahidi HR. Novel targeted therapeutics: inhibitors of MDM2, ALK and PARP. J Hematol Oncol. 2011;4:16. Doi:10.1186/1756-8722-4-16.
Tabernero J, et al. Phase I pharmacokinetic (PK) and pharmacodynamic (PD) study of HDM-2 antagonist JNJ-26854165 in patients with advanced refractory solid tumors. J Clin Oncol (Meet Abs). 2009;27(15S):3514.
Bridges KA, Hirai H, Buser CA, Brooks C, Liu H, Buchholz TA, Molkentine JM, Mason KA, Meyn RE. MK-1775, a novel Wee1 kinase inhibitor, radiosensitizes p53-defective human tumor cells. Clin Cancer Res. 2011;17(17):5638–48. Doi:10.1158/1078-0432.CCR-11-0650 Epub 2011 Jul 28.
ClinicalTrials.gov. WEE1 Inhibitor MK-1775, Radiation Therapy, and Temozolomide in Treating Patients With Newly Diagnosed or Recurrent Glioblastoma Multiforme. Identifier: NCT01849146. https://clinicaltrials.gov/ct2/show/study/NCT01849146?term=wee1+glioblastoma&rank=1&show_desc=Y#desc.
Zwick E, Bange J, Ullrich A. Receptor tyrosine kinase signalling as a target for cancer intervention strategies. Endocr. Relat Cancer. 2001;8(3):161–73. Doi:10.1677/erc.0.0080161.
Plate KH, Breier G, Weich HA, et al. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–8.
Rini BI, Small EJ. Biology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinoma. J Clin Oncol. 2005;23(5):1028–43.
Cohen MH, Shen YL, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14:1131–8.
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–40. Doi:10.1200/JCO.2008.19.8721 Epub 2009 Aug 31.
Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–5.
Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ Jr, Mehta MP. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. Doi:10.1056/NEJMoa1308573.
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–22. Doi:10.1056/NEJMoa1308345.
Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC, Buter J, Honkoop AH, Boerman D, de Vos FY, Dinjens WN, Enting RH, Taphoorn MJ, van den Berkmortel FW, Jansen RL, Brandsma D, Bromberg JE, van Heuvel I, Vernhout RM, van der Holt B, van den Bent MJ. Single agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–53. Doi:10.1016/S1470-2045(14)70314-6 Epub 2014 Jul 15.
ClinicalTrials.gov. Bevacizumab and Lomustine for Recurrent GBM. Identifier: NCT01290939. https://clinicaltrials.gov/ct2/show/NCT01290939?term=ccnu+and+bevacizumab+and+ccnu&rank=4.
Soffietti R1, Trevisan E, Bertero L, Cassoni P, Morra I, Fabrini MG, Pasqualetti F, Lolli I, Castiglione A, Ciccone G, Rudà R. Bevacizumab and fotemustine for recurrent glioblastoma: a phase II study of AINO (Italian Association of Neuro-Oncology). J Neurooncol. 2014 Feb;116(3):533–41. Doi:10.1007/s11060-013-1317-x Epub 2013 Dec 1.
Field KM, Simes J, Nowak AK, Cher L, Wheeler H, Hovey EJ, Brown CS, Barnes EH, Sawkins K, Livingstone A, Freilich R, Phal PM, Fitt G, CABARET/COGNO investigators, Rosenthal MA. Randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma. Neuro Oncol. 2015 Nov;17(11):1504–13. Doi:10.1093/neuonc/nov104 Epub 2015 Jun 30.
Batchelor TT, Duda DG, di Tomaso E, Ancukiewicz M, Plotkin SR, Gerstner E, Eichler AF, Drappatz J, Hochberg FH, Benner T, Louis DN, Cohen KS, Chea H, Exarhopoulos A, Loeffler JS, Moses MA, Ivy P, Sorensen AG, Wen PY, Jain RK. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28(17):2817–23. Doi:10.1200/JCO.2009.26.3988 Epub 2010 May 10.
Batchelor TT, Mulholland P, Neyns B, Nabors LB, Campone M, Wick A, Mason W, Mikkelsen T, Phuphanich S, Ashby LS, Degroot J, Gattamaneni R, Cher L, Rosenthal M, Payer F, Jürgensmeier JM, Jain RK, Sorensen AG, Xu J, Liu Q, van den Bent M. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–8. Doi:10.1200/JCO.2012.47.2464 Epub 2013 Aug 12.
Iwamoto FM, Lamborn KR, Robins HI, Mehta MP, Chang SM, Butowski NA, Deangelis LM, Abrey LE, Zhang WT, Prados MD, Fine HA. Phase II trial of pazopanib (GW786034), an oral multi-targeted angiogenesis inhibitor, for adults with recurrent glioblastoma (North American Brain Tumor Consortium Study 06-02). Neuro Oncol. 2010;12(8):855–61. Doi:10.1093/neuonc/noq025 Epub 2010 Mar 3.
Reardon DA, Groves MD, Wen PY, Nabors L, Mikkelsen T, Rosenfeld S, Raizer J, Barriuso J, McLendon RE, Suttle AB, Ma B, Curtis CM, Dar MM, de Bono J. A phase I/II trial of pazopanib in combination with lapatinib in adult patients with relapsed malignant glioma. Clin Cancer Res. 2013;19(4):900–8. Doi:10.1158/1078-0432.CCR-12-1707 Epub 2013 Jan 30.
Hainsworth JD, Ervin T, Friedman E, Priego V, Murphy PB, Clark BL, Lamar RE. Concurrent radiotherapy and temozolomide followed by temozolomide and sorafenib in the first-line treatment of patients with glioblastoma multiforme. Cancer. 2010;116(15):3663–9. Doi:10.1002/cncr.25275.
Reardon DA, Vredenburgh JJ, Desjardins A, Peters K, Gururangan S, Sampson JH, Marcello J, Herndon JE 2nd, McLendon RE, Janney D, Friedman AH, Bigner DD, Friedman HS. Effect of CYP3A-inducing anti-epileptics on sorafenib exposure: results of a phase II study of sorafenib plus daily temozolomide in adults with recurrent glioblastoma. J Neurooncol. 2011;101(1):57–66. Doi:10.1007/s11060-010-0217-6 Epub 2010 May 5.
Galanis E, Anderson SK, Lafky JM, Uhm JH, Giannini C, Kumar SK, Kimlinger TK, Northfelt DW, Flynn PJ, Jaeckle KA, Kaufmann TJ, Buckner JC. Phase II study of bevacizumab in combination with sorafenib in recurrent glioblastoma (N0776): a north central cancer treatment group trial. Clin Cancer Res. 2013;19(17):4816–23. Doi:10.1158/1078-0432.CCR-13-0708 Epub 2013 Jul 5.
Peereboom DM, Ahluwalia MS, Ye X, Supko JG, Hilderbrand SL, Phuphanich S, Nabors LB, Rosenfeld MR, Mikkelsen T, Grossman SA. New Approaches to Brain Tumor Therapy Consortium. NABTT 0502: a phase II and pharmacokinetic study of erlotinib and sorafenib for patients with progressive or recurrent glioblastoma multiforme. Neuro Oncol. 2013;15(4):490–6. Doi:10.1093/neuonc/nos322 Epub 2013 Jan 17.
Lee EQ, Kuhn J, Lamborn KR, Abrey L, DeAngelis LM, Lieberman F, Robins HI, Chang SM, Yung WK, Drappatz J, Mehta MP, Levin VA, Aldape K, Dancey JE, Wright JJ, Prados MD, Cloughesy TF, Gilbert MR, Wen PY. Phase I/II study of sorafenib in combination with temsirolimus for recurrent glioblastoma or gliosarcoma: North American Brain Tumor Consortium study 05-02. Neuro Oncol. 2012;14(12):1511–8. Doi:10.1093/neuonc/nos264 Epub 2012 Oct 24.
Muhic A, Poulsen HS, Sorensen M, Grunnet K, Lassen U. Phase II open-label study of nintedanib in patients with recurrent glioblastoma multiforme. J Neurooncol. 2013;111(2):205–12. Doi:10.1007/s11060-012-1009-y Epub 2012 Nov 27.
Kreisl TN, McNeill KA, Sul J, Iwamoto FM, Shih J, Fine HA. A phase I/II trial of vandetanib for patients with recurrent malignant glioma. Neuro Oncol. 2012;14(12):1519–26. Doi:10.1093/neuonc/nos265 Epub 2012 Oct 25.
Chheda MG, Wen PY, Hochberg FH, Chi AS, Drappatz J, Eichler AF, Yang D, Beroukhim R, Norden AD, Gerstner ER, Betensky RA, Batchelor TT. Vandetanib plus sirolimus in adults with recurrent glioblastoma: results of a phase I and dose expansion cohort study. J Neurooncol. 2015 Feb;121(3):627–34. Doi:10.1007/s11060-014-1680-2 Epub 2014 Dec 13.
Neyns B, Sadones J, Chaskis C, Dujardin M, Everaert H, Lv S, Duerinck J, Tynninen O, Nupponen N, Michotte A, De Greve J. Phase II study of sunitinib malate in patients with recurrent high-grade glioma. J Neurooncol. 2011;103(3):491–501. Doi:10.1007/s11060-010-0402-7 Epub 2010 Sep 25.
Pan E, Yu D, Yue B, Potthast L, Chowdhary S, Smith P, Chamberlain M. A prospective phase II single-institution trial of sunitinib for recurrent malignant glioma. J Neurooncol. 2012;110(1):111–8. Doi:10.1007/s11060-012-0943-z Epub 2012 Jul 26.
Kreisl TN, Smith P, Sul J, Salgado C, Iwamoto FM, Shih JH, Fine HA. Continuous daily sunitinib for recurrent glioblastoma. J Neurooncol. 2013;111(1):41–8. Doi:10.1007/s11060-012-0988-z Epub 2012 Oct 20.
Reardon DA, Vredenburgh JJ, Coan A, Desjardins A, Peters KB, Gururangan S, Sathornsumetee S, Rich JN, Herndon JE, Friedman HS. Phase I study of sunitinib and irinotecan for patients with recurrent malignant glioma. J Neurooncol. 2011;105(3):621–7. Doi:10.1007/s11060-011-0631-4 Epub 2011 Jul 9.
de Groot JF1, Lamborn KR, Chang SM, Gilbert MR, Cloughesy TF, Aldape K, Yao J, Jackson EF, Lieberman F, Robins HI, Mehta MP, Lassman AB, Deangelis LM, Yung WK, Chen A, Prados MD, Wen PY. Phase II study of aflibercept in recurrent malignant glioma: a North American Brain Tumor Consortium study. J Clin Oncol. 2011 Jul 1;29(19):2689–95. Doi:10.1200/JCO.2010.34.1636 Epub 2011 May 23.
Gerstner ER, Eichler AF, Plotkin SR, Drappatz J, Doyle CL, Xu L, Duda DG, Wen PY, Jain RK, Batchelor TT. Phase I trial with biomarker studies of vatalanib (PTK787) in patients with newly diagnosed glioblastoma treated with enzyme inducing anti-epileptic drugs and standard radiation and temozolomide. J Neurooncol. 2011;103(2):325–32. Doi:10.1007/s11060-010-0390-7 Epub 2010 Sep 7.
Reardon DA, Egorin MJ, Desjardins A, Vredenburgh JJ, Beumer JH, Lagattuta TF, Gururangan S, Herndon JE 2nd, Salvado AJ, Friedman HS. Phase I pharmacokinetic study of the vascular endothelial growth factor receptor tyrosine kinase inhibitor vatalanib (PTK787) plus imatinib and hydroxyurea for malignant glioma. Cancer. 2009;115(10):2188–98. Doi:10.1002/cncr.24213.
Brandes AA, Stupp R, Hau P, Lacombe D, Gorlia T, Tosoni A, Mirimanoff RO, Kros JM, van den Bent MJ. EORTC study 26041-22041: phase I/II study on concomitant and adjuvant temozolomide (TMZ) and radiotherapy (RT) with PTK787/ZK222584 (PTK/ZK) in newly diagnosed glioblastoma. Eur J Cancer. 2010;46(2):348–54. Doi:10.1016/j.ejca.2009.10.029 Epub 2009 Nov 27.
Wen PY, Prados M, Schiff D, Reardon DA, Cloughesy T, Mikkelsen T, Batchelor T, Drappatz J, Chamberlain MC, De Groot JF. Phase II study of XL184 (BMS 907351), an inhibitor of MET, VEGFR2, and RET, in patients (pts) with progressive glioblastoma (GB) [abstract]. J Clin Oncol. 2010;28(15 suppl):181s.
Normanno N, De Luca A, Bianco C, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16.
Ekstrand AJ, James CD, Cavenee WK, et al. Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991;51:2164–72.
Barker FG 2nd, SimmonsML Chang SM, et al. EGFR overexpression and radiation response in glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001;51:410–8.
Pelloski CE, Ballman KV, Furth AF, et al. Epidermal growth factor receptor variant III status defines clinically distinct subtypes of glioblastoma. J Clin Oncol. 2007;25:2288–94.
Shinojima N, Tada K, Shiraishi S, Kamiryo T, Kochi M, Nakamura H, Makino K, Saya H, Hirano H, Kuratsu J, Oka K, Ishimaru Y, Ushio Y. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63:6962–70.
Uhm JH, Ballman KV, Wu W, et al. Phase II evaluation of gefitinib in patients with newly diagnosed grade 4 astrocytoma: Mayo/North Central Cancer Treatment Group Study N0074. Int J Radiat Oncol Biol Phys. 2011;80(2):347–53.
Chakravarti A, Wang M, Robins HI, et al. RTOG 0211: a phase ½ study of radiation therapy with concurrent gefitinib for newly diagnosed glioblastoma patients. Int J Radiat Oncol Biol Phys. 2013;85(5):1206–11.
Rich JN, Reardon DA, Peery T, et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol. 2004;22(1):133–42.
Franceschi E, Cavallo G, Lonardi S, et al. Gefitinib in patients with progressive high-grade gliomas: a multicenter phase II study by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Br J Cancer. 2007;96:1047–51.
Reardon DA, Quinn JA, Vredenburgh JJ, et al. Phase I trial of gefitinib plus sirolimus in adults with recurrent malignant glioma. Clin Cancer Res. 2006;12(3 Pt 1):860–8.
Kreisl TN, Lassman AB, Mischel PS, et al. A pilot study of everolimus and gefitinib in the treatment of recurrent glioblastoma (GBM). J Neurooncol. 2009;92(1):99–105.
YungWK, Vredenburgh JJ, Cloughesy TF, et al. Safety and efficacy of erlotinib in first-relapse glioblastoma: a phase II open-label study. Neuro Oncol. 2010;12(10):1061–70.
Raizer JJ, Abrey LE, Lassman AB, et al. A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro Oncol. 2010;12(1):95–103.
Peereboom DM, Shepard DR, Ahluwalia MS, et al. Phase II trial of erlotinib with temozolomide and radiation in patients with newly diagnosed glioblastoma multiforme. J Neurooncol. 2010;98(1):93–9.
Prados MD, Chang SM, Butowski N, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme and gliosarcoma. J Clin Oncol. 2009;27(4):579–84.
Brown PD, Krishnan S, Sarkaria JN, et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J Clin Oncol. 2008;26(34):5603–9.
Krishnan S, Brown PD, Ballman KV, et al. Phase I trial of erlotinib with radiation therapy in patients with glioblastoma multiforme: results of North Central Cancer Treatment Group protocol N0177. Int J Radiat Oncol Biol Phys. 2006;65(4):1192–9.
Prados MD, Lamborn KR, Chang S, et al. Phase 1 study of erlotinib HCl alone and combined with temozolomide in patients with stable or recurrent malignant glioma. Neuro Oncol. 2006;8(1):67–78.
De Groot JF, Gilbert MR, Aldape K, et al. Phase II study of carboplatin and erlotinib (Tarceva, OSI-774) in patients with recurrent glioblastoma. J Neurooncol. 2008;90(1):89–97.
Sathornsumetee S, Dejardins A, Vredenburgh JJ, et al. Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma. Neuro Oncol. 2010;12(12):1300–10.
Nghiemphu PL, Lai A, Green RM, et al. A dose escalation trial for the combination of erlotinib and sirolimus for recurrent malignant gliomas. J Neurooncol. 2012;110(2):245–50.
Reardon DA, Desjardin A, Vredenburgh JJ, et al. Phase 2 trial of erlotinib plus sirolimus in adults with recurrent glioblastoma. J Neurooncol. 2010;96(2):219–30.
Neyns B, Sadones J, Joosens E, et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann Oncol. 2009;20:1596–603.
Hasselbalch B, Lassen U, Hansen S, Holmberg M, Sørensen M, Kosteljanetz M, Broholm H, Stockhausen MT, Poulsen HS. Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: a phase II trial. Neuro Oncol. 2010;12(5):508–16. Doi:10.1093/neuonc/nop063 Epub 2010 Feb 5.
Thiessen B, Stewart C, Tsao M, Kamel-Reid S, Schaiquevich P, Mason W, Easaw J, Belanger K, Forsyth P, McIntosh L, Eisenhauer E. A phase I/II trial of GW572016 (lapatinib) in recurrent glioblastoma multiforme: clinical outcomes, pharmacokinetics and molecular correlation. Cancer Chemother Pharmacol. 2010;65(2):353–61. Doi:10.1007/s00280-009-1041-6.
Reardon DA, Groves MD, Wen PY, Nabors L, Mikkelsen T, Rosenfeld S, Raizer J, Barriuso J, McLendon RE, Suttle AB, Ma B, Curtis CM, Dar MM, de Bono J. A phase I/II trial of pazopanib in combination with lapatinib in adult patients with relapsed malignant glioma. Clin Cancer Res. 2013;19(4):900–8. Doi:10.1158/1078-0432.CCR-12-1707 Epub 2013 Jan 30.
Karavasilis V, Kotoula V, Pentheroudakis G, Televantou D, Lambaki S, Chrisafi S, Bobos M, Fountzilas G. A phase I study of temozolomide and lapatinib combination in patients with recurrent high-grade gliomas. J Neurol. 2013;260(6):1469–80. Doi:10.1007/s00415-012-6812-z Epub 2013 Jan 5.
Reardon DA, Conrad CA, Cloughesy T, et al. Phase I study of AEE788, a novel multitarget inhibitor of ErbB and VEGF receptor-family tyrosine kinases, in recurrent glioblastoma patients. Cancer Chemother Pharmacol. 2012;69(6):1507–18.
Minkovsky N, Berezov A. BIBW-2992, a dual receptor tyrosine kinase inhibitor for the treatment of solid tumors. Curr Opin Investig Drugs. 2008 Dec;9(12): 1336–46. PMID 19037840.
ClinicalTrials.gov. BIBW 2992 (Afatinib) With or Without Daily Temozolomide in the Treatment of Patients With Recurrent Malignant Glioma. Identifier: NCT00727506. https://clinicaltrials.gov/ct2/show/NCT00727506?term=Afatinib+glioma&rank=1.
ClinicalTrials.gov. Open Label Trial to Explore Safety of Combining Afatinib (BIBW 2992) and Radiotherapy With or Without Temozolomide in Newly Diagnosed Glioblastoma Multiform. Identifier: NCT00977431. https://clinicaltrials.gov/ct2/show/NCT00977431?term=Afatinib+glioma&rank=3.
ClinicalTrials.gov. PF-00299804 in Adult Patients With Relapsed/Recurrent Glioblastoma. Identifier: NCT01112527 https://clinicaltrials.gov/ct2/show/NCT01112527?term=Dacomitinib+GLIOMA&rank=.
ClinicalTrials.gov. Safety and Efficacy of PF-299804 (Dacomitinib), a Pan-HER Irreversible Inhibitor, in Patients With Recurrent Glioblastoma With EGFR Amplification or Presence of EGFRvIII Mutation. A Phase II CT. Identifier: NCT01520870. https://clinicaltrials.gov/ct2/show/NCT01520870?term=Dacomitinib+GLIOMA&rank=1.
Solomón MT, Selva JC, Figueredo J, Vaquer J, Toledo C, Quintanal N, Salva S, Domíngez R, Alert J, Marinello JJ, Catalá M, Griego MG, Martell JA, Luaces PL, Ballesteros J, de-Castro N, Bach F, Crombet T. Radiotherapy plus nimotuzumab or placebo in the treatment of high grade glioma patients: results from a randomized, double blind trial. BMC Cancer. 2013 Jun 19;13:299. Doi:10.1186/1471-2407-13-299.
Westphal M, Heese O, Steinbach JP, Schnell O, Schackert G, Mehdorn M, Schulz D, Simon M, Schlegel U, Senft C, Geletneky K, Braun C, Hartung JG, Reuter D, Metz MW, Bach F, Pietsch T. A randomised, open label phase III trial with nimotuzumab, an anti-epidermal growth factor receptor monoclonal antibody in the treatment of newly diagnosed adult glioblastoma. Eur J Cancer. 2015;51(4):522–32. Doi:10.1016/j.ejca.2014.12.019 Epub 2015 Jan 20.
Matsui T, Heidaran M, Miki T, Popescu N, La Rochelle W, Kraus M, Pierce J, Aaronson S. Isolation of a novel receptor cDNA establishes the existence of two PDGF receptor genes. Science. 1989;243(4892):800–4. Doi:10.1126/science.2536956.
Stenman G, Rorsman F, Huebner K, Betsholtz C. The human platelet-derived growth factor alpha chain (PDGFA) gene maps to chromosome 7p22. Cytogenet Cell Genet. 1992;60(3–4):206–7. Doi:10.1159/000133337.
Alentorn A, Marie Y, Carpentier C, et al. Prevalence, clinicopathological value, and co-occurrence of PDGFRA abnormalities in diffuse gliomas. Neuro Oncol. 2012;14:1393-140.
Buchdunger E, Cioffi CL, Law N, et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-Kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000;295:139–45.
Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7.
Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80.
Wen PY, Yung WK, Lamborn KR, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99-08. Clin Cancer Res. 2006;12(16):4899–907.
Razis E, Selviaridis P, Labropoulos S, et al. Phase II study of neoadjuvant imatinib in glioblastoma: evaluation of clinical and molecular effects of the treatment. Clin Cancer Res. 2009;15(19):6258–66.
Reardon DA, Dresemann G, Taillibert S, et al. Multicentre phase II studies evaluating imatinib plus hydroxyurea in patients with progressive glioblastoma. Br J Cancer. 2009;101(12):1995–2004.
Dresemann G, Weller M, Rosenthal MA, et al. Imatinib in combination with hydroxyurea versus hydroxyurea alone as oral therapy in patients with progressive pretreated glioblastoma resistant to standard dose temozolomide. J Neurooncol. 2010;96(3):393–402.
Desjardins A, Quinn JA, Vredenburgh JJ, Sathornsumetee S, Friedman AH, Herndon JE, McLendon RE, Provenzale JM, Rich JN, Sampson JH, Gururangan S, Dowell JM, Salvado A, Friedman HS, Reardon DA. Phase II study of imatinib mesylate and hydroxyurea for recurrent grade III malignant gliomas. J Neurooncol. 2007;83(1):53–60 Epub 2007 Jan 24.
Reardon DA, Egorin MJ, Quinn JA, et al. Phase II study of imatinib mesylate plus hydroxyurea in adults with recurrent glioblastoma multiforme. J Clin Oncol. 2005;23(36):9359–68.
Lassman AB, Pugh SL, Gilbert MR, Aldape KD, Geinoz S, Beumer JH, Christner SM, Komaki R, DeAngelis LM, Gaur R, Youssef E, Wagner H, Won M, Mehta MP. Phase 2 trial of dasatinib in target-selected patients with recurrent glioblastoma (RTOG 0627). Neuro Oncol. 2015;17(7):992–8. Doi:10.1093/neuonc/nov011 Epub 2015 Mar 10.
ClinicalTrials.gov. Dasatinib and Bevacizumab in Treating Patients With Recurrent or Progressive High-Grade Glioma or Glioblastoma Multiforme. Identifier: NCT00892177. https://clinicaltrials.gov/ct2/show/NCT00892177?term=Dasatinib+glioma&rank=5.
ClinicalTrials.gov. Dasatinib or Placebo, Radiation Therapy, and Temozolomide in Treating Patients With Newly Diagnosed Glioblastoma Multiforme Identifier: NCT00869401. https://clinicaltrials.gov/ct2/show/NCT00869401?term=Dasatinib+glioma&rank=11.
Franceschi E, Stupp R, van den Bent MJ, et al. EORTC 26083 phase I/II trial of dasatinib in combination with CCNU in patients with recurrent glioblastoma. Neuro Oncol. 2012;12(12):1503–10.
Reardon DA, Vredenburgh JJ, Desjardins A, Peters KB, Sathornsumetee S, Threatt S, Sampson JH, Herndon JE 2nd, Coan A, McSherry F, Rich JN, McLendon RE, Zhang S. Friedman HS.Phase 1 trial of dasatinib plus erlotinib in adults with recurrent malignant glioma. J Neurooncol. 2012;108(3):499–506. Doi:10.1007/s11060-012-0848-x Epub 2012 Mar 10.
ClinicalTrials.gov. Tandutinib in Treating Patients With Recurrent or Progressive Glioblastoma. Identifier: NCT00379080. https://clinicaltrials.gov/ct2/show/NCT00379080?term=Tandutinib+glioma&rank=31.
ClinicalTrials.gov. Tandutinib Plus Bevacizumab to Treat Recurrent Brain Tumors. Identifier: NCT00667394. https://clinicaltrials.gov/ct2/show/NCT00667394?term=Tandutinib+glioma&rank=1.
Watanabe K, Tachibana O, Sata K, Yonekawa Y, Kleihues P, Ohgaki H. Overexpression of the EGF receptor and p53 mutations are mutually exclusive in the evolution of primary and secondary glioblastomas. Brain Pathol. 1996;6:217–23.
Wong AJ, Ruppert JM, Bigner SH, et al. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci USA. 1992;89:2965–9.
Manning BD, Cantley LC. AKT/PKB Signaling: Navigating Downstream. Cell. 2007;129(7):1261–74. Doi:10.1016/j.cell.2007.06.009.
Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18(16):1926–45. Doi:10.1101/gad.1212704. PMID 15314020.
Akhavan D, Cloughesy TF, Mischel PS. mTOR signaling in glioblastoma: lessons learned from bench to bedside. Neuro Oncol. 2010;12:882–9.
Pitz MW, Eisenhauer EA, MacNeil MV, Thiessen B, Easaw JC, Macdonald DR, Eisenstat DD, Kakumanu AS, Salim M, Chalchal H, Squire J, Tsao MS, Kamel-Reid S, Banerji S, Tu D, Powers J, Hausman DF, Mason WP. Phase II study of PX-866 in recurrent glioblastoma. Neuro Oncol. 2015 Sep;17(9):1270–4. Doi:10.1093/neuonc/nou365 Epub 2015 Jan 20.
ClinicalTrials.gov. A Phase I Dose Escalation Study of BKM120 With Radiation Therapy and Temozolomide in Patients With Newly Diagnosed Glioblastoma. Identifier: NCT01473901. https://clinicaltrials.gov/ct2/show/NCT01473901?term=NCT01473901&rank=1.
ClinicalTrials.gov. Phase II Study of BKM120 for Subjects With Recurrent Glioblastoma. Identifier: NCT01339052. https://clinicaltrials.gov/ct2/show/NCT01339052?term=NCT01339052&rank=1.
ClinicalTrials.gov. Combination of BKM120 and Bevacizumab in Refractory Solid Tumors and Relapsed/Refractory Glioblastoma Multiforme. Identifier: NCT01349660. https://clinicaltrials.gov/ct2/show/NCT01349660?term=pi3k+glioma&rank=6.
ClinicalTrials.gov. Exploratory Study of XL765 (SAR245409) or XL147 (SAR245408) in Subjects With Recurrent Glioblastoma Who Are Candidates for Surgical Resection. Identifier: NCT01240460. https://clinicaltrials.gov/ct2/show/NCT01240460?term=pi3k+glioma&rank=5.
ClinicalTrials.gov. A Study of XL765 (SAR245409) in combination with temozolomide with and without radiation in adults with malignant gliomas. Identifier: NCT00704080. https://clinicaltrials.gov/ct2/show/NCT00704080?term=pi3k+glioma&rank=2.
ClinicalTrials.gov. Evaluation of the treatment effectiveness of glioblastoma/ gliosarcoma through the suppression of the PI3K/Akt pathway in compared with MK-3475. Identifier: NCT02430363. https://clinicaltrials.gov/ct2/show/NCT02430363?term=pi3k+glioma&rank=4.
Butowski N, Chang SM, Lamborn KR, Polley MY, Pieper R, Costello JF, Vandenberg S, Parvataneni R, Nicole A, Sneed PK, Clarke J, Hsieh E, Costa BM, Reis RM, Hristova-Kazmierski M, Nicol SJ, Thornton DE, Prados MD. Phase II and pharmacogenomics study of enzastaurin plus temozolomide during and following radiation therapy in patients with newly diagnosed glioblastoma multiforme and gliosarcoma. Neuro Oncol. 2011;13(12):1331–8. Doi:10.1093/neuonc/nor130 Epub 2011 Sep 6.
Wick W, Steinbach JP, Platten M, Hartmann C, Wenz F, von Deimling A, Shei P, Moreau-Donnet V, Stoffregen C, Combs SE. Enzastaurin before and concomitant with radiation therapy, followed by enzastaurin maintenance therapy, in patients with newly diagnosed glioblastoma without MGMT promoter hypermethylation. Neuro Oncol. 2013;15(10):1405–12. Doi:10.1093/neuonc/not100 Epub 2013 Aug 1.
Wick W, Puduvalli VK, Chamberlain MC, van den Bent MJ, Carpentier AF, Cher LM, Mason W, Weller M, Hong S, Musib L, Liepa AM, Thornton DE, Fine HA. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28(7):1168–74. Doi:10.1200/JCO.2009.23.2595 Epub 2010 Feb 1.
Kreisl TN, Kotliarova S, Butman JA, Albert PS, Kim L, Musib L, Thornton D, Fine HA. A phase I/II trial of enzastaurin in patients with recurrent high-grade gliomas. Neuro Oncol. 2010;12(2):181–9. Doi:10.1093/neuonc/nop042 Epub 2010 Jan 22.
ClinicalTrials.gov. Clinical and Molecular-Metabolic Phase II trial of perifosine for recurrent/progressive malignant gliomas. Identifier: NCT00590954. https://clinicaltrials.gov/ct2/show/NCT00590954?term=akt+glioma&rank=5.
ClinicalTrials.gov. Perifosine and Torisel (Temsirolimus) for recurrent/progressive malignant gliomas. Identifier: NCT02238496. https://clinicaltrials.gov/ct2/show/NCT02238496?term=akt+glioma&rank=4.
ClinicalTrials.gov. Evaluation of the treatment effectiveness of glioblastoma/gliosarcoma through the suppression of the PI3K/Akt pathway in compared with MK-3475. Identifier: NCT02430363. https://clinicaltrials.gov/ct2/show/NCT00694837?term=akt+glioma&rank=9.
ClinicalTrials.gov. Study with Nelfinavir and combined radiochemotherapy for glioblastoma identifier: NCT00694837. https://clinicaltrials.gov/ct2/show/NCT00694837?term=akt+glioma&rank=9.
Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–88.
Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–11.
Cloughesy T, Raizer J, Drappatz J, et al. A phase II trial of everolimus in patients with recurrent glioblastoma multiforme. Neuro Oncol. 2011;13(suppl 3):42–3.
Ma DJ, Galanis E, Anderson SK, Schiff D, Kaufmann TJ, Peller PJ, Giannini C, Brown PD, Uhm JH, McGraw S, Jaeckle KA, Flynn PJ, Ligon KL, Buckner JC, Sarkaria JN. A phase II trial of everolimus, temozolomide, and radiotherapy in patients with newly diagnosed glioblastoma: NCCTG N057K. Neuro Oncol. 2015;17(9):1261–9. Doi:10.1093/neuonc/nou328 Epub 2014 Dec 18.
Hainsworth JD, Shih KC, Shepard GC, Tillinghast GW, Brinker BT, Spigel DR. Phase II study of concurrent radiation therapy, temozolomide, and bevacizumab followed by bevacizumab/everolimus as first-line treatment for patients with glioblastoma. Clin Adv Hematol Oncol. 2012;10(4):240–6.
Chang SM, Wen P, Cloughesy T, Greenberg H, Schiff D, Conrad C, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23:357–61.
Galanis E, Buckner JC, Maurer MJ, Kreisberg JI, Ballman K, Boni J, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23:5294–304.
Sarkaria JN1, Galanis E, Wu W, Dietz AB, Kaufmann TJ, Gustafson MP, Brown PD, Uhm JH, Rao RD, Doyle L, Giannini C, Jaeckle KA, Buckner JC. Combination of temsirolimus (CCI-779) with chemoradiation in newly diagnosed glioblastoma multiforme (GBM) (NCCTG trial N027D) is associated with increased infectious risks. Clin Cancer Res. 2010 Nov 15;16(22):5573–80. Doi:10.1158/1078-0432.CCR-10-1453 Epub 2010 Oct 4.
Lassen U, Sorensen M, Gaziel TB, Hasselbalch B, Poulsen HS. Phase II study of bevacizumab and temsirolimus combination therapy for recurrent glioblastoma multiforme. Anticancer Res. 2013;33(4):1657–60.
ClinicalTrials.gov. AZD8055 for adults with recurrent gliomas. Identifier: NCT01316809. https://clinicaltrials.gov/ct2/show/NCT01316809?term=AZD8055&rank=2.
Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95(1):29–39.
Schmidt EE, Ichimura K, Goike HM, Moshref A, Liu L, Collins VP. Mutational profile of the PTEN gene in primary human astrocytic tumors and cultivated xenografts. J Neuropathol Exp Neurol. 1999;58(11):1170–83.
Vivanco I, Rohle D, Versele M, et al. The phosphatase and tensin homolog regulates epidermal growth factor receptor (EGFR) inhibitor response by targeting EGFR for degradation. Proc Natl Acad Sci USA. 2010;107:6459–64.
Bos J. ras oncogenes in human cancer: a review. Cancer Res. 1989;49(17):4682–9.
Downward J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer. 2003 Jan;3(1):11–22.
Hurley JB, Simon MI, Teplow DB, Robishaw JD, Gilman AG. Homologies between signal transducing G proteins and ras gene products. Science. 1984;226(4676):860–2.
Dasgupta B, Li W, Perry A, Gutmann DH. Glioma formation in neurofibromatosis 1 reflects preferential activation of K-RAS in astrocytes. Cancer Res. 2005;65:236–45.
Feldkamp MM, Lau N, Guha A. Growth inhibition of astrocytoma cells by farnesyl transferase inhibitors is mediated by a combination of anti-proliferative, pro-apoptotic and antiangiogenic effects. Oncogene. 1999;18:7514–26.
Nghiemphu PL1, Wen PY, Lamborn KR, Drappatz J, Robins HI, Fink K, Malkin MG, Lieberman FS, DeAngelis LM, Torres-Trejo A, Chang SM, Abrey L, Fine HA, Demopoulos A, Lassman AB, Kesari S, Mehta MP, Prados MD, Cloughesy TF, North American Brain Tumor Consortium. A phase I trial of tipifarnib with radiation therapy, with and without temozolomide, for patients with newly diagnosed glioblastoma. Int J Radiat Oncol Biol Phys. 2011 Dec 1;81(5):1422–7. Doi:10.1016/j.ijrobp.2010.07.1997 Epub 2010 Oct 8.
Cloughesy TF, Wen PY, Robins HI, Chang SM, Groves MD, Fink KL, et al. Phase II trial of tipifarnib in patients with recurrent malignant glioma either receiving or not receiving enzyme-inducing antiepileptic drugs: a North American Brain Tumor Consortium Study. J Clin Oncol. 2006;24:3651–6.
Glass TL, Liu TJ, Yung WK. Inhibition of cell growth in human glioblastoma cell lines by farnesyltransferase inhibitor SCH66336. Neuro Oncol. 2000;2(3):151–8.
Chaponis D, Barnes JW, Dellagatta JL, Kesari S, Fast E, Sauvageot C, Panagrahy D, Greene ER, Ramakrishna N, Wen PY, Kung AL, Stiles C, Kieran MW. Lonafarnib (SCH66336) improves the activity of temozolomide and radiation for orthotopic malignant gliomas. J Neurooncol. 2011;104(1):179–89. Doi:10.1007/s11060-010-0502-4 Epub 2011 Jan 19.
Desjardins A, Reardon DA, Peters KB, Threatt S, Coan AD, Herndon JE 2nd, Friedman AH, Friedman HS, Vredenburgh JJ. A phase I trial of the farnesyl transferase inhibitor, SCH 66336, with temozolomide for patients with malignant glioma. J Neurooncol. 2011;105(3):601–6. Doi:10.1007/s11060-011-0627-0 Epub 2011 Jul 7.
Yust-Katz S, Liu D, Yuan Y, Liu V, Kang S, Groves M, et al. Phase 1/1b study of lonafarnib and temozolomide in patients with recurrent or temozolomide refractory glioblastoma. Cancer. 2013;119:2747–53.
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110.
Sandmann T, Bourgon R, Garcia J, Li C, Cloughesy T, Chinot OL, Wick W, Nishikawa R, Mason W, Henriksson R, Saran F, Lai A, Moore N, Kharbanda S, Peale F, Hegde P, Abrey LE, Phillips HS, Bais C. Patients With Proneural Glioblastoma May Derive Overall Survival Benefit From the Addition of Bevacizumab to First-Line Radiotherapy and Temozolomide: Retrospective Analysis of the AVAglio Trial. J Clin Oncol. 2015;33(25):2735–44. Doi:10.1200/JCO.2015.61.5005 Epub 2015 Jun 29.
Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT, Martuza RL, Louis DN, Rozenblatt-Rosen O, Suvà ML, Regev A, Bernstein BE. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–401. Doi:10.1126/science.1254257 Epub 2014 Jun 12.
Hegi ME, Diserens AC, Bady P, Kamoshima Y, Kouwenhoven MC, Delorenzi M, et al. Pathway analysis of glioblastoma tissue after preoperative treatment with the EGFR tyrosine kinase inhibitor gefitinib—a phase II trial. Mol Cancer Ther. 2011;10:1102–12.
Chakravarti A, Loeffler JS, Dyson NJ. Insulin-like growth factor receptor I mediates resistance to anti-epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer Res. 2002;62(1):200–7.
van den Bent MJ, Gao Y, Kerkhof M, Kros JM, Gorlia T, van Zwieten K, Prince J, van Duinen S, Sillevis Smitt PA, Taphoorn M, French PJ. Changes in the EGFR amplification and EGFRvIII expression between paired primary and recurrent glioblastomas. Neuro Oncol. 2015 Jul;17(7):935–41. Doi:10.1093/neuonc/nov013 Epub 2015 Feb 16.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Mandel, J., Kesari, S. (2017). Targeting Aberrant Signaling Pathways. In: Moliterno Gunel, J., Piepmeier, J., Baehring, J. (eds) Malignant Brain Tumors . Springer, Cham. https://doi.org/10.1007/978-3-319-49864-5_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-49864-5_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-49863-8
Online ISBN: 978-3-319-49864-5
eBook Packages: MedicineMedicine (R0)