Abstract
Spinal cord tumor surgery requires the use of intraoperative neurophysiologic monitoring in order to continuously assess the functional integrity of the neural pathways, particularly the corticospinal tracts, during the operation. The utilization of motor evoked potentials is extremely useful in shaping the surgical strategy in order to preserve as much neural function as possible and achieve significant tumor removal at the same time.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Key Learning Points
-
Loss or deterioration of SSEPs during myelotomy is a common finding and NOT associated with motor deficits.
-
Presence of muscle MEPs always correlates to the absence of significant motor deficits.
-
Transient paraparesis is neurophysiologically characterized by loss of uni- or bilateral muscle MEPs and preservation of D-Wave.
Introduction
Intradural spinal tumorsare much less common than primary intracranial tumors, and overall represent 2–4 % of all primary tumors of the central nervous system (CNS). They may be intradural extramedullary tumors, that is, they are located inside the dural sac but outside the spinal cord, and thus exert external compression on the cord. This group comprises meningiomas and nerve sheath tumors (schwannomas, neurofibromas). They are more frequent in adults . The other, more delicate group are intramedullary tumors, also called intrinsic tumors, as they are located within the substance of the spinal cord. They are the predominant spinal tumor type in children. Histologically the most frequent intramedullary tumor is the ependymoma in adults, and the pilocytic astrocytoma in children. These and the great majority of all other tumor types are histologically benign, and graded 1 or 2 by the World Health Organization (WHO) system. Higher grade tumors are rare. Glioblastomas are extremely rare.
The typical presenting signs and symptoms of intradural tumors, and intramedullary tumors in particular, include pain, which is more pronounced in the reclining position. The typical patient has disturbed sleep because of pain in the neck, the shoulders, the arms and legs, uni- or bilaterally, often combined with mild sensory symptoms like numbness and paresthesias. Typically, this type of pain improves or resolves during the day. Neurologic dysfunction may occur in the form of deterioration of fine motor skills for the hands, gait and balance disturbance, or outright paresis. The great majority of patients have slow onset or slow progression of symptoms. Rapidly developing symptoms would indicate the rare case of higher grade tumor.
In recent years, due to the immense progress of clinical oncology, more and more patients with intramedullary metastasis have been presented and the occasional surgery to remove a metastatic lesion has been done.
Spinal cord tumors may also be vascular ; the hemangioblastoma being the most frequent type. This may occur with or without the genetic predisposition of von Hippel-Lindau disease . Cavernomas and arteriovenous malformations occur in the spinal cord, but particularly the latter are exceedingly rare.
The other genetic disorder associated with spinal cord tumors is neurofibromatosis . Type 1 frequently is associated with nerve sheath tumors; type 2 additionally with ependymomas.
A peculiar variant of ependymoma is also well known in the spinal canal: the myxopapillary ependymoma is called such because of its characteristic histologic appearance. It is usually located in or around the conus medullaris and the cauda equina. In fact, it may be located intra- and extramedullary at the same time, which makes resection treacherous. In terms of their internal structure, intramedullary tumors may be solid or cystic with various combinations of the two. The presence of cysts is a predisposition to cause spinal deformity, particularly scoliosis. Often, the presence of cysts facilitates the tumor removal as the cyst opening quickly provides space to directly access the tumor without going along the cord–tumor interface all along.
In terms of treatment , the widespread consensus, based on a lot of experience and some evidence, is that intramedullary tumors should be removed microsurgically. Because most tumors are benign, this mostly results in long survival. The rare malignant tumors have a poor prognosis. Adjuvant treatment, i.e., radiation and chemotherapy, is given only in exceptional circumstances of inoperability or persistent recurrence, or for the rare tumor which is higher grade (WHO 3 or 4).
The oncologic outcome is characterized by long survival. An intramedullary tumor very rarely changes the life expectancy. The neurologic outcome of surgery is characterized by a small motor morbidity. The rate of significant motor deficit may be under 5 %. However, the loss of some sensory function is surely much higher, probably above 50 %. This can result in ataxia, superficial sensory dysfunction or, most severely, loss of joint position sense.
Neurosurgical resection of spinal cord tumors greatly benefits from the use of intraoperative neurophysiologic monitoring. At this time, it is generally accepted that somatosensory-evoked potential (SSEP), D wave, and motor-evoked potential (MEP) data represent the functional integrity of the respective sensory and motor pathways in the spinal cord. There continues to be a debate about the evidence base as to whether or not the utilization of monitoring influences the overall outcome of spinal cord tumor resection.
The degree of resection, survival, and the neurologic outcome greatly depend on several crucial factors: well-delineated tumors where a “plane of dissection ” can be developed in surgery are more likely to be completely resectable and have an almost zero recurrence rate (ependymomas, hemangioblastomas). Astrocytomas may vary in their configuration and mostly have at least in part of their surface a diffuse interface to the normal cord and thus are much less well resectable. The most important neurologic factor for postoperative neurologic outcome is the preoperative neurologic status: the patient with intact function and only minor symptoms has much less risk of surgery-induced deterioration than the patient with barely preserved function preoperatively. Consequently it is almost never possible to reverse a once-established neurologic deficit, meaning that a patient who comes to surgery already paraplegic usually cannot recover, even with complete tumor resection.
From the perspective of a neurosurgeon experienced in spinal cord surgery, the most important factors for successful resection include the expert application of intraoperative neurophysiologic techniques at all steps of the operation. Certainly, the surgical experience is an essential prerequisite anyway. But the interpretation of continuously acquired monitoring data in an environment of constant and easy communication between surgeon and monitoring team is indispensable.
It was not neurosurgeons but orthopedic surgeons who first implemented intraoperative monitoring with sensory-evoked potentials for the spinal cord in an effort to reduce neurologic morbidity of spinal surgeries [1]. The technology has vastly improved since these early times. In addition to the slow and unreliable [2] spinal monitoring with SSEPs , concepts for direct monitoring of the motor pathways were developed in the 1980s [3–9], implemented for practical use in the 1990s [10–14], and further refined and widely applied in the following decade [15].
Neurophysiology
Motor potentials are evoked with transcranial electrical motor cortex stimulation. The stimulus points are C3, C4, C1, C2, Cz, and a point 6 cm in front of Cz (International 10/20 EEG electrode system). Cork-screw electrodes are optimal for fixation to the scalp, but straight needle electrodes as well as surface electrodes are also in use.
Electrical stimulation with rectangular constant current impulses of 0.5 ms duration and intensities between 15 and 220 mA is used.
D waves [3] are elicited with single stimuli. This is therefore called the “single stimulus technique.” The D waves are recorded as traveling waves directly from the spinal cord with an electrode placed over the spinal cord usually in the spinal epidural space. Baseline recordings are obtained during the surgical opening. The signal usually does not require averaging although recording quality often improves with a few averages. The stimulations are repeatable at a rate of 0.5–2 Hz. This provides practically “real-time” feedback. The relevant D wave parameter is its peak-to-peak amplitude. A decrease of more than 50 % of the baseline amplitude is considered critical and has been found to be associated with a motor deficit [12]. Latency changes of the D wave are mostly not due to surgery but to factors such as temperature [16]. Higher stimulation intensities are followed by shorter D wave latencies [8]. This is likely due to the corticospinal tract fiber activation occurring deeper in the white matter of the brain.
Muscle MEPs are elicited with transcranial electrical stimulation over the same electrodes as for the D wave. A train of five to seven stimuli with 4 ms interstimulus intervals [17, 18] is used. The technique is called “multipulse technique” [13] or “train stimulus technique” [9]. Compound muscle action potentials are recorded with needle electrodes from target muscles in all four extremities (thenar, anterior tibialis, abductor hallucis). Practically all muscles, including those innervated by cranial nerves and even the diaphragm and the external anal sphincter, can be used as recording sites. Muscle MEPs also do not require averaging and can be repeated at a rate of 0.5–2 Hz. With the focal anode as the stimulating electrode, a montage of C1/2 (anode at C1, cathode at C2) or C2/1 is tried first to elicit muscle MEPs in all four extremities. In individual cases, C3/4, C4/3, or Cz/6 are used as alternative stimulation points [19].
The principle of evoking muscle responses is understood in the context of the D wave concept: each individual electrical stimulus on the motor cortex, either on the exposed cortex or using transcranial stimulation [6], elicits a D wave in the corticospinal tract. A fast train of five stimuli at 250 Hz elicits five consecutive D waves, which then travel down the corticospinal tract 4 ms apart. The spinal alpha-motorneurons receive these five D waves and that increases their membrane potential up to firing threshold [4] even under the conditions of general anesthesia. The parameter monitored is the presence or absence of muscle MEPs in the target muscles within a stimulus intensity range of 15–220 mA. This all-or-none concept has been adopted because of the enormous variability of muscle MEP amplitudes [10, 20, 21] and because a motor deficit occurred only when the muscle responses were lost [7, 10, 14, 21].
With more and more MEP monitoring performed, worldwide safety concerns have required further study into the biologic effects of electric neural stimulation. So far this growing use has not resulted in significant numbers of reported complications resulting from tissue damage [22, 23] or seizures. Nevertheless, this issue continues to be growing in importance [24].
Anesthesia for Neurophysiologic Monitoring
Total intravenous anesthesia with an as constant as possible infusion of propofol (100–150 μg/kg/min) and fentanyl (1 μg/kg/h) is ideal when MEP monitoring is utilized for an operation. Propofol for anesthesia with MEP monitoring has been reported with various stimulation techniques [25–30]. Bolus injections of both intravenous (IC) agents should be avoided because this appears to temporarily disrupt muscle MEP recording. This can be particularly problematic during the critical resection part of any spinal cord tumor surgery.
We found the addition of ketamine, 0.25 mg/kg/min, a particularly useful addition to the anesthetic management [31].
Halogenated anesthetics should not be used [23], even though low doses may be tolerable. They elevate muscle MEP stimulus thresholds and block muscle MEPs in a dose-dependent fashion [32]. Using them adds an uncontrollable variable without improving anesthesia.
Short-acting muscle relaxants are given for intubation only. Neurosurgeons and anesthesiologists may be somewhat uncomfortable with some patient movement during surgery, which results from the effects of transcranial motor cortex stimulation. “Partial” muscle relaxation [21] to improve on this problem continues to be debated. We doubt that it improves anesthesia management, but are convinced that it makes monitoring data less reliable. The proven specificity of muscle MEP data would likely suffer, and some patient movement from stimulation could still not be entirely avoided. Therefore, this would combine poor monitoring with poor relaxation.
Case 1
History, Clinical Assessment, and Imaging
This 55-year-old man had a several years’ history of subtle signs of neurologic dysfunction, such as nighttime back pain, slow deterioration of endurance and stamina in sports, mild urinary dysfunction, and subtle reduction in sexual function. At diagnosis, the symptoms had escalated to a significant degree of gait dysfunctionand a walking distance reduced to about 100 m. Magnetic resonance imaging (Fig. 36.1) revealed a large mass in the lower thoracic spinal cord with extensive upper thoracic cord edema and venous engorgement around the conus. The tumor matrix appeared dark on T2, which indicates an above average degree of fibrous tissue. Urgent resection was recommended due to the presence of significant neurologic dysfunction and recent neurologic deterioration, as the degree of preoperative neurologic dysfunction correlates with the degree of perioperative deterioration [12, 33].
Magnetic resonance images of an extensive intramedullary tumor in lower thoracic spinal cord with complete obstruction of the spinal canal (a), significant enhancement (b), and dark T2-signal indicating fibrous tissue (c). The large size on imaging does not even allow the differential diagnosis of an intramedullary versus an intradural-extramedullary tumor
Surgery and Intraoperative Monitoring
The monitoring routines for surgery of intramedullary spinal cord tumors include MEP and SSEP recordings from the upper and lower extremities. Thenar, tibialis anterior, flexor hallucis brevis bilaterally were used. Cortical SSEP responses were recorded from median and tibial nerve stimulation. In thoracic tumors, the upper extremity recordings serve as controls for the surgically relevant lower extremity recordings. In addition, recordings of bulbocavernosus reflex, pudendal nerve SSEPs and anal MEPs can be attempted.
The patient was positioned prone with the head in a soft face padding mounted on a mirror plate. During the approach phase of the operation, a sudden drop in the cortical amplitude of the right median nerve was noted (Fig. 36.2). An immediate assessment of the situation showed that unchanged recordings from the left side excluded the possibility of a systemic effect such as a change in anesthesia regimen. The surgery was excluded because the relevant spinal level was low thoracic and therefore not corresponding to the median SSEP. Inspection of the electrodes and cables as well as the patient’s positioning showed an insufficient padding and malposition of the right arm. This was corrected and the recordings normalized at the next set of recordings.
A sudden drop in cortical signal amplitude from the right median nerve SEP prompted analysis and insufficient padding and suboptimal arm position was found and corrected. This resulted in rapid recovery of the response. The contralateral side showed no change at the same time, indicating that it could not be caused by systemic factors
During resection of the spinal cord tumor, significant difficulty was encountered as the tumor was found to be very firm and thus could not be grabbed or easily decompressed. Internal decompression was achieved using a microsurgical laser [34]. This brought the size of the mass sufficiently down so that the remaining tumor mass could be dissected out of the edematous spinal cord in toto. There was no further significant change in either motor- or sensory-evoked potential recordings.
The patient awoke from anesthesia without significant motor deficits. Postoperative MRI showed complete tumor removal.
Case Summary Interpretation and Discussion
A 55-year-old man with progressive paraparesis underwent resection of an intramedullary tumor eventually diagnosed as a solitary fibrous tumor (Fig. 36.1) [35]. During surgery, a unilateral sudden decrease of median nerve SSEP was noted (Fig. 36.2) and found to be caused by less-than-optimal padding of the right forearm. Improved positioning rapidly improved the response. This was a mechanical problem unrelated to the operation per se, due to patient positioning which was identified and corrected immediately.
This case demonstrates that routinely recording evoked potentials from upper and lower extremities is useful even when the surgery takes place caudal to the level of the cervical spinal cord. Upper extremity recordings may serve as controls for difficult lower extremity recordings and as controls to systemic effects of core temperature change and the effects of anesthetic agents. Considering the possible influences of these factors, it became evident that the observed change must have been caused by a nonsurgical (far cranial to the surgery) influence and a nontechnical (no artifacts, correct electrode positions), nonsystemic (stable recordings contralaterally and on the lower extremities) problem. Positioning-related compressive neuropathy is not an infrequent occurrence in complex surgical positioning [36]. Early detection is possible with an evoked potential change such as the one shown here [37]. The case also provides evidence that even SEPs requiring averaging can provide fast information upon potential and correctable problems.
Case 2
History, Clinical Assessment, and Imaging
A 4-year-old boy with nighttime neck pain and progressive clumsiness of the right hand was diagnosed with a cervical intramedullary spinal cord tumor. On MRI, the lesion showed a marked heterogeneity and significant internal compression of the spinal cord by both solid tumor masses and cystic formations (Fig. 36.3). Tumor resection was recommended to obtain a histologic diagnosis and as optimal primary tumor treatment.
Surgery and Intraoperative Monitoring
Routine monitoring for cervical intramedullary spinal cord tumors includes MEP and SSEP recordings from the upper and lower extremities. Thenar, tibialis anterior, flexor hallucis brevis are used. In this context, cortical SSEP responses are recorded from ulnar and tibial nerves, respectively. If possible, an epidural electrode is inserted to record D waves.
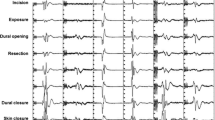
The patient was positioned prone with the head fixed in neutral position in a four-pronged Sugita headholder. A laminotomy C3 to T3 was made for surgical exposure. After a dorsal myelotomy , the extensive tumor tissue was removed in piecemeal fashion using the Cavitron ultrasonic aspirator (CUSA), regular suction, and the microsurgical laser. MEP and SEP signals for the entire right side were permanently lost during resection on the right side (Fig. 36.4). Waiting, irrigation, induced hypertension, and additional steroid administration did not achieve recovery of the recordings. The resection remained subtotal. No attempt was made to remove tumor residuals adherent to the still-functioning left side of the cord. The patient awoke with a severe right-sided hemiparesis. Over the long run he recovered motor function of the left side, learned how to walk and run, but remained with a severely impaired right hand and an abnormal gait.
MEP recordings from the right and left hypothenar (bottom traces) and the right tibialis anterior and abductor hallucis muscles, respectively (top traces). The lower extremity recordings disappear simultaneously at a well-defined point. The right hypothenar recordings disappeared gradually and remained stable on the left side
Case Summary Interpretation and Discussion
A 4-year-old boy underwent surgical resection of a cervical intramedullary spinal cord pilocytic astrocytoma . At a critical stage of the resection, a significant change of MEPs of both upper and lower extremities occurred on the right side. MEP and SSEP signals for the entire right side were permanently lost. The resection remained incomplete and the patient had a severe right-sided hemiparesis. The leg recovered partially, the upper extremity remained significantly impaired.
This case demonstrates both the immediate feedback provided by the loss of MEPs on one side and the significant neurologic consequences of a complete loss of both upper and lower extremity recordings. Usually the leg recovers faster and easier than the hand, and unilateral loss of lower extremity MEPs usually recovers completely [14]. However, the complete loss of both extremities on one side appears to have graver consequences. This young patient recovered gross motor function of the arm but not the motor skills adequate for the dominant hand. He developed left-handedness and his rehabilitation was aimed at achieving this goal. His walking improved rapidly to independence but his gait remained massively abnormal.
The preoperative anatomy allowed a preassessment that the right side was under significant risk and that the left side must be preserved at all cost. This concept unfolded during the operation in that indeed the right side suffered, but the left could be preserved and thus useful functioning and social independence was saved. Continuation of the surgery to achieve an anatomically complete resection even of the tumor components involving the left side of the cord would likely have resulted in a complete loss of motor function bilaterally.
Case 3
History, Clinical Assessment, and Imaging
A 54-year-old woman with a year-long progressive history of nighttime back pain presented with a sensory level below Th7 and MR imaging revealed an intradural-extramedullary tumor at the level of Th9 (Fig. 36.5).
Surgery and Intraoperative Monitoring
The monitoring routines for surgery of intradural-extramedullary tumors and intramedullary spinal cord tumors are identical with MEP and SEP recordings from the upper and lower extremities. Thenar, tibialis anterior, flexor hallucis brevis bilaterally were used.
The patient was positioned prone. During the laminectomy, baseline recordings were obtained. Upon opening of the dura, significant hemorrhage occurred from around the tumor and epidurally. Adequate exposure and hemostasis required some time and some blood loss. Left tibialis anterior MEPs disappeared at that time. Blood loss was rapidly compensated with fluid replacement. There was no significant hypotension during that time. MEPs reappeared after about 40 min (Fig. 36.6).
Summary, Interpretation, and Discussion
A 54-year-old woman underwent laminectomy and resection of an intradural meningioma at T9. This case differs little from intramedullary tumor resections and is therefore presented in this context. After dural opening, a significant hemorrhage occurred. Rapid fluid replacement was considered important and started immediately. Thus, significant hypotension was avoided. Nevertheless, during this time a temporary loss of muscle MEPs from the left tibialis anterior occurred. Both systemic and local factors could have contributed to these findings: local pressure on the tumor and subsequently to the spinal cord during hemostasis and initial tumor resection could have caused temporary MEP loss . In addition, the blood loss compensated through fluid replacement could have contributed to the MEP loss despite stable blood pressure in the situation of both local and systemic vulnerability of the corticospinal system originally due to tumor compression. We consider the proactive maintenance of stable circulatory parameters by the anesthesiologist essential to avoid lasting neurologic dysfunction. It would be an error to wait with fluid replacement until the blood pressure indeed decreases. Correction of the volume deficit at such a late time would be trailing events, carrying more risk for ischemic damage.
Discussion
The use of MEP data, both D wave and muscle MEPs, has found significant acceptance and implementation in the growing community of intraoperative monitoring specialists worldwide. Intramedullary and extramedullary tumors (like the one in Case 3) are basically monitored using the same techniques, practical setups, and interpretation criteria: preservation of muscle MEPs is always associated with preserved motor function, loss of muscle MEPs is highly likely to indicate temporary loss of motor function as long as the D wave is preserved. Loss of both muscle MEPs and D wave is indicating irreversible loss of motor function. There is a scenario of present muscle MEPs but absent or unrecordable D wave. This is interpreted as a desynchronization of the D wave and is sometimes observed in patients with intradural-extramedullary tumors as well as in patients who underwent prior radiation therapy.
The patient care essentially requires extensive integration of neurosurgery, neurophysiology, and neuroanesthesia. As the three case vignettes presented in this contribution show, an array of interpretation from all three specialties contribute to the integrated picture. The neuroanesthesiologist should not only be a passive “provider of anesthesia” for the operation but an active, and as Case 3 shows, even proactive partner in the entire effort to manage, control, influence and eventually maintain neurologic homeostasis and neurologic integrity of essential neural structures.
Questions
-
1.
During a spinal cord tumor surgery in a 2-year-old child in prone position with the head turned rather sharply to the side and positioned in a ring, the muscle MEPs of the right upper and lower extremities first require higher stimulation intensity and soon disappear soon after baselines were obtained and BEFORE the surgeon even got to laminotomy.
This is most likely due to:
-
A.
Hypothermia resulting from poor covering during anesthesia preparation and monitoring setup.
-
B.
Hypotension due to blood loss.
-
C.
Compression of the spinal cord at the level of the cervicothoracic junction.
-
D.
Poor positioning and position-induced transient spinal cord compression.
-
A.
-
2.
Upon resection of a T2 to T11 intramedullary ganglioglioma in a 14-year-old girl, a loss of muscle MEPs from the right tibialis anterior muscles occurs. The ipsilateral abductor hallucis has not been recordable even at baseline at maximum intensity settings. The D wave is down in amplitude about 30 % from baseline. Thenar MEPs are bilaterally intact and unchanged.
What should you do next?
-
A.
Advise the surgeon of the change and recommend temporarily halting resection and start irrigation.
-
B.
Alert the anesthesiologist to a possible perfusion deficit.
-
C.
Ask the anesthesiologist if the anesthesia level may be too light.
-
D.
Further increase the stimulus intensity and the number of pulses per train and repeat recordings.
-
A.
-
3.
A right-handed adult patient has an intramedullary astrocytoma with asymmetric configuration from C2 to C7. His symptoms are primarily nighttime pain in the ulnar left forearm. The surgical plan is to enter the cord on the left (i.e., symptomatic and nondominant) side.
During this operation it is essential to preserve:
-
A.
SSEPs of the right leg, muscle MEPs of the left arm and the D wave.
-
B.
The D wave.
-
C.
SSEPs of the right arm and leg, D wave, bilateral upper extremity MEPs, and right lower extremity MEPs.
-
D.
Muscle MEPs of both tibialis anterior and abductor hallucis muscles.
-
A.
-
4.
A myxopapillary ependymoma of the conus and cauda equina extending from T11 to L5 is resected in a 5-year-old boy. What is the role of D-Wave recording in this surgery?
-
A.
The D wave must be recorded with priority over muscle MEPs and bulbocavernosus-reflex.
-
B.
The D wave cannot be recorded.
-
C.
The D wave must be recorded using a collision technique.
-
D.
The D wave amplitude must remain above 50 % of baseline to ensure preserved sphincter function.
-
A.
Answers
-
1.
D
-
2.
A
-
3.
B
-
4.
B
References
Nash CL, Lorig RA, Schatzinger L, Brown RH. Spinal cord monitoring during operative treatment of the spine. Clin Orthop Rel Res. 1977;126:100–5.
Lesser RP, Raudzens P, Lüders H, Nuwer MR, Goldie WD, Morris III HH, et al. Postoperative neurological deficits may occur despite unchanged intraoperative somatosensory evoked potentials. Ann Neurol. 1986;19:22–5.
Patton HD, Amassian VE. Single-and multiple unit analysis of cortical stage of pyramidal tract activation. J Neurophysiol. 1954;17:345–63.
Philips CG, Porter R. The pyramidal projection to motoneurones of some muscle groups of the baboon’s forelimb. In: Eccles JC, Schadé JP, editors. Progress in brain research, vol. 12. Amsterdam: Elsevier; 1964. p. 222–43.
Merton PA, Morton HB. Stimulation of the cerebral cortex in the intact human subject. Nature. 1980;285:227.
Katayama Y, Tsubokawa T, Maemjima S, Hirayama T, Yamamoto T. Corticospinal direct response in humans: identification of the motor cortex during intracranial surgery under general anesthesia. J Neurol Neurosurg Psychiatr. 1988;51:50–9.
Zentner J. Noninvasive motor evoked potential monitoring during neurosurgical operations in the spinal cord. Neurosurgery. 1989;24(5):709–12.
Burke D, Hicks RG, Stephen JPH. Corticospinal volleys evoked by anodal and cathodal stimulation of the human motor cortex. J Physiol. 1990;425:283–99.
Taniguchi M, Schramm J, Cedzich C. Recording of myogenic motor evoked potentials under general anesthesia. In: Schramm J, Møller ÅR, editors. Intraoperative neurophysiologic monitoring in neurosurgery. Berlin: Springer; 1991. p. 72–87.
Jones SJ, Harrison R, Koh KF, Mendoza N, Crockard HA. Motor evoked potential monitoring during spinal surgery: responses of distal limb muscles to transcranial cortical stimulation with pulse trains. Electroencephalogr Clin Neurophysiol. 1996;100:375–83.
Pechstein U, Cedzich C, Nadstawek J, Schramm J. Transcranial high-frequency repetitive electrical stimulation for recording myogenic motor evoked potentials with the patient under general anesthesia. Neurosurgery. 1996;39(2):335–44.
Morota N, Deletis V, Constantini S, Kofler M, Cohen H, Epstein FJ. The role of motor evoked potentials during surgery for intramedullary spinal cord tumors. Neurosurgery. 1997;41(6):1327–36.
Calancie B, Harris W, Broton JG, Alexeeva N, Green BA. “Threshold-level” multipulse transcranial electrical stimulation of motor cortex for intraoperative monitoring of spinal motor tracts: description of method and comparison to somatosensory evoked potential monitoring. J Neurosurg. 1998;88(1):457–70.
Kothbauer KF, Deletis V, Epstein FJ. Motor evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in a series of 100 consecutive procedures. Neurosurg Focus. 1998;4(5). Article 1. http://www.aans.org/journals/online_j/may98/94-95-91.
Sala F, Palandri G, Basso E, Lanteri P, Deletis V, Faccioli F, Bricolo A, et al. Motor evoked potential monitoring improves outcome during surgery for intramedullary spinal cord tumor: a historical control study in 50 patients. Neurosurgery. 2006;58(6):1129–43.
Deletis V. Intraoperative monitoring of the functional integrity of the motor pathways. In: Devinsky O, Beric A, Dogali M, editors. Electrical and magnetic stimulation of the brain and spinal cord. New York: Raven; 1993. p. 201–14.
Deletis V, Rodi Z, Amassian VE. Neurophysiological mechanisms underlying motor evoked potentials in anesthetized humans. Part 2. Relationship between epidurally and muscle recorded MEPs in man. Clin Neurophysiol. 2001;112:445–52.
Deletis V, Isgum V, Amassian VE. Neurophysiological mechanisms underlying motor evoked potentials in anesthetized humans. Part 1. Recovery time of corticospinal tract direct waves elicited by pairs of transcranial electrical stimuli. Clin Neurophysiol. 2001;112:438–44.
Szelenyi A, Kothbauer KF, Deletis V. Transcranial electric stimulation for intraoperative motor evoked potential monitoring: stimulation parameters and electrode montages. Clin Neurophysiol. 2007;118:1586–95.
Woodforth IJ, Hicks RG, Crawford MR, Stephen JP, Burke DJ. Variability of motor-evoked potentials recorded during nitrous oxide anesthesia from the tibialis anterior muscle after transcranial electrical stimulation. Anesth Analg. 1996;82:744–9.
Lang EW, Beutler AS, Chesnut FM, Patel PM, Kennelly NA, Kalkman CJ, et al. Myogenic motor-evoked potential monitoring using partial neuromuscular blockade in surgery of the spine. Spine. 1996;21(14):1676–86.
Agnew WF, McCreery DB. Considerations for safety in the use of extracranial stimulation for motor evoked potentials. Neurosurgery. 1987;20(1):143–7.
Taniguchi M, Cedzich C, Schramm J. Modification of cortical stimulation for motor evoked potentials under general anesthesia: technical description. Neurosurgery. 1993;32(2):219–26.
MacDonald DB. Safety of intraoperative transcranial electrical stimulation motor evoked potential monitoring. J Clin Neurophysiol. 2002;19(5):416–29.
Sloan TB. Intraoperative neurophysiology and anesthesia management. In: Deletis V, Shils J, editors. Neurophysiology in neurosurgery. Vol 1. Amsterdam: Academic, Elsevier; 2002. p. 451–74.
Jellinek D, Jewkes D, Symon L. Noninvasive intraoperative monitoring of motor evoked potentials under propofol anesthesia: effect of spinal surgery on the amplitude and latency of motor evoked potentials. Neurosurgery. 1991;29:551–7.
Kalkman CJ, Drummond JC, Ribberink AA, Patel PM, Sano T, Bickford RG. Effects of propofol, etomidate, midazolam and fentanyl on motor evoked responses to transcranial electrical or magnetic stimulation in humans. Anesthesiology. 1992;76:502–9.
Schmid UD, Boll J, Liechti S, Schmid J, Hess CW. Influence of some anesthetic agents on muscle responses to transcranial magnetic cortex stimulation: a pilot study in man. Neurosurgery. 1992;30(1):85–92.
Taniguchi M, Nadstawek J, Langenbach U, Bremer F, Schramm J. Effects of four intravenous anesthetic agents on motor evoked potentials elicited by magnetic transcranial stimulation. Neurosurgery. 1993;33(3):407–15.
Fennelly ME, Taylor BA, Hetreed M. Anaesthesia and the motor evoked potential. In: Jones SJ, Boyd S, Hetreed M, Smith NJ, editors. Handbook of spinal cord monitoring 1992, vol. 1. Dordrecht: Kluwer Academic; 1993. p. 272–6.
Kothbauer K, Schmid UD, Liechti S, Rösler KM. The effect of ketamine anesthetic induction on muscle responses to transcranial magnetic cortex stimulation studied in man. Neurosci Lett. 1993;154:105–8.
Ubags LH, Kalkman CJ, Been HD. Influence of isoflurane on myogenic motor evoked potentials to single and multiple transcranial stimuli during nitrous oxide/opioid anesthesia. Neurosurgery. 1998;43(1):90–4.
Woodworth GF, Chaichana KL, McGirt MJ, Sciubba DM, Jallo GI, Gokaslan Z, et al. Predictors of ambulatory function after surgical resection of intramedullary spinal cord tumors. Neurosurgery. 2007;61(1):99–105; discussion 105–6.
Jallo GI, Kothbauer KF, Epstein FJ. Contact laser microsurgery. Child’s Nerv Syst. 2002;18:333–6.
Jallo GI, Roonprapunt C, Kothbauer K, Freed D, Allen J, Epstein F. Spinal solitary fibrous tumors: a series of four patients: case report. Neurosurgery. 2005;57(1), E195.
Warner MA, Warner ME, Martin JT. Ulnar neuropathy. Incidence, outcome, and risk factors in sedated or anesthetized patients. Anesthesiology. 1994;81(6):1332–40.
Labrom RD, Hoskins M, Reilly CW, Tredwell SJ, Wong PK. Clinical usefulness of somatosensory evoked potentials for detection of brachial plexopathy secondary to malpositioning in scoliosis surgery. Spine (Phila Pa 1976). 2005;30(18):2089–93.
Suggested Reading
Brotchi J. Intrinsic spinal cord tumor resection. Neurosurgery. 2002;50:1059–63.
Jallo G, Kothbauer K, Epstein FJ. Intrinsic spinal cord tumor resection: operative nuances. Neurosurgery. 2001;49:1124–8.
Kothbauer KF. Intraoperative neurophysiologic monitoring for intramedullary spinal-cord tumor surgery. Neurophysiol Clin. 2007;37:407–14.
Sala F, Kothbauer K. Intraoperative neurophysiological monitoring during surgery for intramedullary spinal cord tumors. In: Nuwer M, editor. Intraoperative monitoring of neural function. Amsterdam: Elsevier; 2008. p. 632–50.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Poblete, B., Kothbauer, K.F. (2017). Intramedullary Spinal Cord Surgery. In: Koht, A., Sloan, T., Toleikis, J. (eds) Monitoring the Nervous System for Anesthesiologists and Other Health Care Professionals. Springer, Cham. https://doi.org/10.1007/978-3-319-46542-5_36
Download citation
DOI: https://doi.org/10.1007/978-3-319-46542-5_36
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-46540-1
Online ISBN: 978-3-319-46542-5
eBook Packages: MedicineMedicine (R0)