Abstract
Background and purpose
In patients with prostate cancer (PC) and biochemical relapse after radical prostatectomy, salvage radiotherapy (SRT) could improve PC-specific survival (PCSS) but the timing for initiation is still under discussion. We have demonstrated a low rate of biochemical relapses in a patient series with very low pre-SRT PSA levels after a median follow-up of 42 months. Here, we present an update of that study.
Patients and methods
Overall, 151 patients were analyzed. A biochemical relapse after SRT was diagnosed when the PSA exceeded the post-SRT nadir by 0.2 ng/ml with subsequent increase. Parameters with significant impact on biochemical progression-free survival (BPFS), PCSS, and overall survival (OS) in univariate analysis were included in a multiple Cox regression analysis.
Results
After a median follow-up of 82 months, 18 patients (12 %) had died with 10 (6.6 %) deaths being PC-related. A biochemical progression was diagnosed in 83 patients (55 %). Univariate analysis revealed a significant impact of pre-SRT PSA level, Gleason score, and PSA doubling time (PSADT) on BPFS and for initial tumor stage and Gleason score on OS. Multivariate analysis confirmed the impact of pre-SRT PSA level, Gleason score, and PSADT on BPFS and tumor stage on OS.
Conclusion
In this update, the rate of biochemical relapses increased compared with our previous data. Compared to similar studies, we found a remarkably low rate of PC-related deaths. Our data support early initiation of SRT. However, this treatment strategy, triggered by very low PSA levels, could carry the risk of overtreatment in at least a subset of patients.
Zusammenfassung
Hintergrund und Ziel
Bei Patienten mit Prostatakarzinom und biochemischem Rezidiv nach radikaler Prostatektomie kann eine Salvage-Strahlentherapie das tumorspezifische Überleben verbessern. Der Zeitpunkt des Therapiebeginns wird kontrovers diskutiert. Wir haben in unserer Serie eine geringe Rate biochemischer Rezidive bei Patienten mit sehr niedrigen präradiotherapeutischen PSA-Werten gezeigt. Die vorliegende Arbeit präsentiert die Langzeitdaten dieser monozentrischen Studie.
Patienten und Methoden
Insgesamt wurden 151 Patienten analysiert. Ein biochemischer Progress wurde diagnostiziert, wenn ein steigender PSA-Wert den postradiotherapeutischen Nadir um 0,2 ng/ml überschritten hat. Parameter mit signifikantem Einfluss auf biochemisch-progressionsfreies Überleben, tumorspezifisches Überleben und Gesamtüberleben in der univariaten Analyse wurden in einer multivariaten Cox-Regressionsanalyse analysiert.
Ergebnisse
Nach einer medianen Nachbeobachtungszeit von 82 Monaten waren 18 Patienten (12 %) verstorben, davon 10 (6,6 %) tumorbedingt. Ein biochemischer Progress trat bei 83 Patienten (55 %) auf. Die univariate Analyse zeigte einen signifikanten Einfluss des präradiotherapeutischen PSA-Werts, des Gleason-Scores und der PSA-Verdopplungszeit auf das biochemisch-rezidivfreie Überleben sowie des initialen Tumorstadiums und des Gleason-Scores auf das Gesamtüberleben. Die multivariate Analyse bestätigte den Einfluss des präradiotherapeutischen PSA-Werts, des Gleason-Scores und der PSA-Verdopplungszeit auf das biochemisch rezidivfreie Überleben sowie des initialen Tumorstadiums auf das Gesamtüberleben.
Schlussfolgerung
In unserer aktualisierten Serie ist die Rate biochemischer Rezidive im Vergleich zu der initialen Auswertung erhöht. Im Vergleich zu anderen Studien fanden wir eine bemerkenswert niedrige Rate tumorbezogener Todesfälle. Unsere Daten unterstützen den frühen Beginn der Salvage-Strahlentherapie. Jedoch beinhaltet diese, von sehr geringen PSA-Werten getriggerte Behandlungsstrategie auch das Risiko einer Übertherapie zumindest einiger Patienten.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A large retrospective study documented a positive impact of salvage radiotherapy (SRT) on prostate cancer-specific survival (PCSS) in patients with prostate cancer (PC) and biochemical relapse after radical prostatectomy (RP) [19]. Thus, SRT is considered a first-choice therapeutic option for such patients. Currently, local recurrences after RP are best treated by SRT with 64–66 Gy at a PSA serum level < 0.5 ng/ml [7]. To our knowledge, the median pre-SRT PSA level in our study was lower compared with almost all previously published studies. In our first data analysis [23], the median follow-up time was only 42 months. Therefore, we have now updated our data.
Patients and methods
We analyzed patients with increasing PSA levels after RP who received SRT without hormonal treatment. The radiotherapy total dose was 66.6 Gy (range 59.4–68.4 Gy) in 1.8-Gy daily fractions. Three-dimensional (3D) conformal radiotherapy (RT) techniques have been published previously [23]. Acute and long-term side effects were recorded using the definition of the National Cancer Institute Common Terminology Criteria for Adverse Events (CTC-AE) version 3.0. A biochemical relapse after SRT was diagnosed using the definition of Stephenson et al. [14]: a PSA increase of at least 0.2 mg/ml above the post-STR nadir confirmed by one further rise. The date determining the biochemical relapse was the date of the confirmatory second measurement. The PSA doubling time (PSADT) between RP and the start of SRT was calculated by linear regression analysis of the natural log (PSA) of all available PSA values. Negative PSADT values were set to 100 months, as described previously [19]. The follow-up time was calculated from initiation of SRT until the last contact with or death of the patient. Additionally, we calculated a follow-up starting at RP.
Statistical analyses were performed using IBM SPSS version 19 (SPSS Inc., Chicago, Ill., USA). Probabilities for biochemical progression-free survival (bPFS), overall survival (OS), and PCSS were analyzed using the method of Kaplan and Meier. The impact of pretherapeutic parameters in univariate analyses was calculated by the log-rank test. Significant parameters (p ≤ 0.05) were included in a Cox regression model. Continuous variables were dichotomized at their medians. Ordinal variables with more than two categories were dichotomized (tumor stage) or trichotomized (Gleason score) in analogy to previous studies.
Results
Overall, 151 patients were evaluated. Eleven patients without pelvic lymphadenectomy but with negative CT scans for enlarged lymph nodes were classified as cN0 (Table 1). For 53 patients (35 %), the pre-SRT PSA level was below 0.2 ng/ml (Table 2). Caused by an outlying PSA value, PSADT was negative and corrected to 100 months for one patient.
After a median follow-up time of 82 months, the probabilities for bPFS, OS, and PCSS were 40 %, 87 %, and 92 %, respectively (Fig. 1). During follow-up, 18 patients died with 10 deaths being PC-related. Among 133 surviving patients, three experienced a clinical progression. At the last follow-up, a biochemical progression was recorded in 83 patients (55 %). The median time from RP to the last follow-up was 104.5 months (range 17–248).
In univariate analysis, pre-SRT PSA level, Gleason score, and PSADT showed a statistically significant impact on bPFS, and initial tumor stage and Gleason score had a statistically significant impact on OS (Table 3).
Cox regression analysis confirmed the pre-SRT PSA level [p = 0.003, hazard ratio (HR) 2.057, 95 % confidence interval (CI) 1.285–3.294], Gleason score (p = 0.008, HR 1.507, 95 % CI 1.112–2.042), and PSADT (p = 0.035, HR 0.621, 95 % CI 0.399–0.966) as independent variables with statistically significant impact on bPFS and the initial tumor stage (p = 0.029, HR 4.083, 95 % CI 1.154–14.442) on OS. The Gleason score showed no significant impact on OS (p = 0.068, HR 1.831, 95 % CI 0.957–3.504).
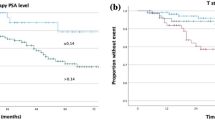
Additionally, the impact of the pre-SRT PSA level on bPFS was assessed in four groups (cut-points at quartiles) with varying pre-SRT PSA ranges (Fig. 2).
No acute grade III or IV genitourinary or gastrointestinal side effects were observed. Six patients presented with urethral strictures requiring interventions and 4 patients showed grade III cystitis, resulting in a total number of 10 patients with grade III genitourinary late toxicities.
Discussion
Three large, prospective, randomized studies showed a benefit of an adjuvant RT (ART) in patients with locally advanced PC [1, 16, 22]. A retrospective analysis and a matched-controlled analysis of a multi-institutional study comparing ART with SRT suggested superiority for ART [4, 18], but these data should be interpreted cautiously as explained in a critical review [8]. Recently, Briganti et al. [3] suggested that timely administration of SRT is comparable to ART in improving bPFS in the majority of patients with pT3 pN0 PC. With the RAVES trial of the Trans-Tasman Radiation Oncology Group (ClinicalTrials.gov; Identifier: NCT00860652) and a trial of the University of Florida (ClinicalTrials.gov; Identifier: NCT00969111), there are at least two ongoing prospective randomized trials investigating the role of SRT versus ART, but reliable results are not yet available.
Although several retrospective studies evaluating SRT have been published within the past two decades, only the study of Trock et al. [19] demonstrated a PCSS benefit. In this study, a group of 160 patients treated with SRT alone and a group of 78 patients, who received SRT and hormonal treatment, were compared with a cohort of 397 patients, who did not receive any salvage therapy. After a median follow-up of 5 years following SRT, 18 PC-related deaths (11.3 %) were observed in the SRT-only group. Our data showed a lower rate of PC-related deaths, while the time from RP to last follow-up in both studies was almost similar (108 vs. 104.5 months). One reason for this difference might be the fact that we commenced SRT at lower pre-SRT PSA levels (medians 0.34 vs. 0.7 ng/ml). The study of Trock et al. [19] revealed that SRT lost its impact on PCSS, when the time interval between biochemical relapse after RP and start of SRT exceeds 2 years.
Recently, Ohri et al. [11] published a systematic review and regression meta-analysis with radiobiological modeling. The authors identified 25 published series reporting treatment outcomes following SRT, including a total of 3,828 patients. The median follow-up ranged from 38 to 127 months (median: 50); The 5-year bPFS ranged from 25 to 70 %. On multivariate analysis, bPFS increased with SRT dose by 2.5 % per Gy and decreased with pre-SRT PSA by 18.3 % per ng/ml (p < 0.001). Radiobiological models demonstrate the interaction between pre-SRT PSA, SRT dose, and bPFS. For example, an increase in pre-SRT PSA from 0.4 to 1.0 ng/ml increases the SRT dose required to achieve a 50 % bPFS rate from 60 to 70 Gy [11].
Following RP, a confirmed PSA value of > 0.2 ng/ml (i.e., two consecutive increases) represents recurrent cancer [14]. Currently, local recurrences after RP are best treated by SRT at lower levels of PSA: a PSA serum level of ≤ 0.5 ng/ml. This recommendation was incorporated into the German, the European, and the American guidelines [7, 17, 21].
Recently, King presented a systematic review of 41 published SRT-studies. The PSA level before SRT (and RT dose) had a significant and independent association with bPFS with an average 2.6 % loss of relapse-free survival for each incremental 0.1 ng/ml PSA at the start of SRT [9]. These data support an early initiation of SRT. However, it remains unclear whether this observation is in part due to a shift of treatment initiation to an earlier time point of the natural course of disease recurrence.
Although usually regarded as a risk factor, the short PSADT in our patient series might have contributed to a low rate of PC-related deaths. Interestingly, the patients who showed benefits from SRT in the study of Trock were mostly those with a PSADT of less than 6 months. More than half of our patient cohort had a shorter PSADT, which possibly indicates a higher sensitivity to SRT.
Our multivariate analysis for OS revealed the initial tumor stage as the only variable with statistically significant impact. None of the three patients with a pT4 tumor stage died during a follow-up time of 53–103 months. Hence, our data do not support a restriction for SRT to patients with low tumor stages.
In previous SRT studies with data on the number of PC-related deaths (besides the study of Trock), the percentages ranged from 2.8 to 4.7% [5, 10, 12, 15, 20] (Table 4). Considering the longer follow-up time in our patient series, the number of PC-specific deaths in our study was low by comparison.
Apart from controversies concerning the optimal time point to commence SRT, there are also discussions regarding the optimal RT dose [2, 13]. In our study, the majority of patients received a dose of 66.6 Gy to the prostate bed. Recently, Goenka et al. [6] have shown in a retrospective analysis of 285 patients that high-dose intensity-modulated RT (IMRT) up to 72 Gy can be delivered safely with an associated reduction in late grade ≥ 2 gastrointestinal toxicity compared with three-dimensional conformal radiation therapy. The optimal dose for SRT is currently under investigation in prospective randomized trials of the Swiss Group for Clinical Cancer Research (ClinicalTrials.gov; identifier number: NCT01272050) and of the University of Miami Sylvester Comprehensive Cancer Center (ClinicalTrials.gov; identifier number: NCT01411345).
Conclusion
In agreement with several previous studies, the pre-SRT PSA level in our series consistently showed its statistical impact on bPFS after SRT alongside with the Gleason score and PSADT. Whether this is a true or only a virtual effect remains unclear at least to some extent. However, SRT could increase PCSS, and severe acute and late toxicities of this therapy are rare. Our data support an immediate initiation of SRT upon detection of a biochemical relapse of PC, even at very low PSA values. In our opinion, this strategy should currently always be considered in patients with detectable PSA after PR that is confirmed and rising. However, results from ongoing prospective trials are needed to substantiate this treatment approach.
References
Bolla M, van PH, Tombal B et al (2012) Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet 380:2018–2027
Bottke D, Bartkowiak D, Schrader M et al (2012) Radiotherapy after radical prostatectomy: immediate or early delayed? Strahlenther Onkol 188:1096–1101
Briganti A, Wiegel T, Joniau S (2012) Early salvage radiation therapy does not compromise cancer control in patients with pT3N0 prostate cancer after radical prostatectomy: results of a match-controlled multi-institutional analysis. Eur Urol 62:472–487
Budiharto T, Perneel C, Haustermans K et al (2010) A multi-institutional analysis comparing adjuvant and salvage radiation therapy for high-risk prostate cancer patients with undetectable PSA after prostatectomy. Radiother Oncol 97:474–479
Cremers RG, van Lin EN, Gerrits WL (2010) Efficacy and tolerance of salvage radiotherapy after radical prostatectomy, with emphasis on high-risk patients suited for adjuvant radiotherapy. Radiother Oncol 97:467–473
Goenka A, Magsanoc JM, Pei X (2011) Improved toxicity profile following high-dose postprostatectomy salvage radiation therapy with intensity-modulated radiation therapy. Eur Urol 60:1142–1148
Heidenreich A, Bastian PJ, Bellmunt J (2014) EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 65:467–479
King CR (2012) Adjuvant versus salvage radiotherapy after prostatectomy: the apple versus the orange. Int J Radiat Oncol Biol Phys 82:1045–1046
King CR (2012) The timing of salvage radiotherapy after radical prostatectomy: a systematic review. Int J Radiat Oncol Biol Phys 84:104–111
Neuhof D, Hentschel T, Bischof M (2007) Long-term results and predictive factors of three-dimensional conformal salvage radiotherapy for biochemical relapse after prostatectomy. Int J Radiat Oncol Biol Phys 67:1411–1417
Ohri N, Dicker AP, Trabulski EJ et al (2012) Can early implementation of salvage radiotherapy for prostate cancer improve the therapeutic ratio? A systematic review and regression meta-analysis with radiobiological modelling. Eur J Cancer 48:837–844
Pisansky TM, Kozelsky TF, Myers RP (2000) Radiotherapy for isolated serum prostate specific antigen elevation after prostatectomy for prostate cancer. J Urol 163:845–850
Siegmann A, Bottke D, Faehndrich J et al (2011) Dose escalation for patients with decreasing PSA during radiotherapy for elevated PSA after radical prostatectomy improves biochemical progression-free survival: results of a retrospective study. Strahlenther Onkol 187:467–472
Stephenson AJ, Scardino PT, Kattan MW et al (2007) Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol 25:2035–2041
Stephenson AJ, Shariat SF, Zelefsky MJ (2004) Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA 291:1325–1332
Thompson IM, Jr., Tangen CM, Paradelo J et al (2006) Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA 296:2329–2335
Thompson IM, Valicenti RK, Albertsen P et al (2013) Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO guideline. J Urol 190:441–449
Trabulsi EJ, Valicenti RK, Hanlon AL et al (2008) A multi-institutional matched-control analysis of adjuvant and salvage postoperative radiation therapy for pT3-4N0 prostate cancer. Urology 72:1298–1302
Trock BJ, Han M, Freedland SJ et al (2008) Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 299:2760–2769
Ward JF, Zincke H, Bergstralh EJ (2004) Prostate specific antigen doubling time subsequent to radical prostatectomy as a prognosticator of outcome following salvage radiotherapy. J Urol 172:2244–2248
Wenz F, Martin T, Bohmer D et al (2010) The German S3 guideline prostate cancer: aspects for the radiation oncologist. Strahlenther Onkol 186:531–534
Wiegel T, Bottke D, Steiner U et al (2009) Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol 27:2924–2930
Wiegel T, Lohm G, Bottke D et al (2009) Achieving an undetectable PSA after radiotherapy for biochemical progression after radical prostatectomy is an independent predictor of biochemical outcome–results of a retrospective study. Int J Radiat Oncol Biol Phys 73:1009–1016
Compliance with ethical guidelines
Conflict of interest
G. Lohm, J. Lütcke, B. Jamil, S. Höcht, K. Neumann, W. Hinkelbein, T. Wiegel, and D. Bottke state that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lohm, G., Lütcke, J., Jamil, B. et al. Salvage radiotherapy in patients with prostate cancer and biochemical relapse after radical prostatectomy. Strahlenther Onkol 190, 727–731 (2014). https://doi.org/10.1007/s00066-014-0612-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-014-0612-6
Keywords
- Prostate cancer
- Salvage radiotherapy
- Biochemical relapse
- Radical prostatectomy
- Prostate-specific antigen