Abstract
The biology of migratory plant parasitic nematodes has been less studied than that of the sedentary endoparasites. The damage they cause is less obvious, their presence and number are more difficult to quantify and they are difficult organisms to study. Nevertheless, they are economically serious pests of many crops, from wheat and barley grown in low rainfall areas to horticultural crops (e.g. Lilium longiflorum) and tropical crops such as coffee, banana and sugarcane. The most studied migratory nematodes are the root lesion nematodes, Pratylenchus spp., the burrowing nematode Radopholus similis and the rice root nematode Hirschmanniella oryzae. In the life cycle of migratory nematodes apart from the egg, all stages of juveniles and adults are motile and can enter and leave host roots. They do not induce the formation of a permanent feeding site, but feed from individual host cells. They create pathways for entry of other root pathogens, often resulting in lesions, stunted roots, yellowing of leaves and plants showing symptoms of water stress, leading to yield loss and decreased quality of produce. In terms of genetic plant defences, no major genes for resistance to migratory nematodes have been found, and resistance breeding is usually based on QTL analysis and marker-assisted selection to combine the best minor resistance genes. Feeding damage reduces root function, and root damage and necrotic lesions the nematodes cause can then make them leave the root and seek others to parasitise. Infestation induces classical plant defence responses and changes in host metabolism which reflects the damage they cause, although detailed studies are lacking. New genomic resources are becoming available to study migratory endoparasites, and the knowledge gained can contribute to improved understanding of their interactions with hosts. Notably transcriptomes of Pratylenchus coffeae, Pratylenchus thornei, Pratylenchus zeae, R. similis and H. oryzae and the first genomic sequence, for P. coffeae, are now available. From these data, some candidate effector genes required for parasitism have been identified: many effectors similar to those found in sedentary endoparasites are present, with the exception of those thought to be involved in formation of feeding sites induced by the sedentary parasites. Belowground defence, in the form of enhanced resistance to migratory parasites, may also be achieved by transgenic expression of modified cysteine protease inhibitors (cystatins), anti-root invasion peptides and host-induced gene silencing (RNAi) strategies, demonstrating that migratory nematodes are amenable to control by these technologies. New more environmentally friendly nematicides, combined with better biological control agents, can be applied or used in seed coatings in integrated pest management approaches to defend roots from attack by migratory nematodes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The health status of roots at the soil–root interface is thought to underlie about 80 % of all problems of plant growth: root infestation with plant parasitic nematodes is a major contributor to these problems. The responses of plant roots to nematode attack depend on the invading nematode and its lifestyle. Feeding and lifestyle strategies used by plant parasitic nematodes vary and can be divided into ectoparasitic, in which the nematodes remain outside the plant and penetrate tissues with only a small portion of their body, and endoparasitic in which nematodes enter plant tissues completely or with a large portion of their body—the latter are subdivided into migratory and sedentary groups, depending on whether all life stages remain motile or whether they induce feeding sites and become sedentary (Dropkin 1989). These parasitic habits are summarised in Table 1.
The sedentary endoparasites which attack plant roots are discussed in chapter ‘Belowground Signalling and Defence in Host–Pythium Interactions’: in this chapter the biology and plant defence strategies against migratory parasitic nematodes are discussed. The focus is on migratory endoparasites, in particular Pratylenchus species usually referred to as root lesion nematodes, the burrowing nematode R. similis and Hirschmanniella species, which include the rice nematode H. oryzae. This largely reflects the view that, from an economic point of view, root lesion nematodes are regarded as the third most important group of plant parasitic nematodes after root-knot (Meloidogyne spp.) and cyst nematodes (Heterodera and Globodera), with the burrowing nematode R. similis the fourth most important (Jones et al. 2013).
This ranking for economic importance perhaps partially reflects the fact that infestation by the sedentary endoparasites is much easier to recognise than that for the migratory nematodes, since obvious galls or cysts are not present, and the ranking clearly does not hold for all crops and environments. Migratory nematodes are the most damaging nematodes in cereal crops in many areas of dry land agriculture, such as in the Australian wheat belt (Vanstone et al. 2008) and the Pacific Northwest of the USA (Smiley et al. 2014): the increasing practice of no-till agriculture in such regions to preserve topsoil and moisture tends to increase the occurrence of root lesion nematodes. They are also major pests in tropical regions for crops such as sugarcane grown on fine-textured soils (Blair and Stirling 2007) and horticultural crops including coffee and banana (Castillo and Vovlas 2007). In addition, migratory endoparasites such as Hirschmanniella spp. are significant pests of rice crops in flooded ecosystems (Bauters et al. 2014; Kyndt et al. 2014).
2 The Biology of Migratory Parasitic Nematodes
Three genera of the Pratylenchidae family are documented as significant pests: these include genera belonging to the subfamilies Pratylenchinae, Hirschmanniellinae and Radopholinae (De Ley and Blaxter 2002; Haegeman et al. 2010). Although many of the root lesion nematodes (Pratylenchus species) have been described as economically significant plant pests, of the Radopholinae only R. similis is regarded as a major pest, particularly of banana, citrus and black pepper, and of the Hirschmanniella species (rice root nematode), H. oryzae is the predominant pest (Kyndt et al. 2014).
The number of species of root lesion nematodes (Pratylenchus spp.) described so far is between 70 and 89 (Castillo and Vovlas 2007; Subbotin et al. 2008). They are mostly polyphagous, as evidenced by the ability of species such as P. thornei and P. zeae, isolated, respectively, from the monocots wheat and sugarcane, to be maintained on dicot carrot discs (Tan et al. 2013; Jordaan and De Waele 1988). Pratylenchus spp. are migratory, intracellular root endoparasites, and depending on species, host and temperature, their life cycle lasts between 3 and 9 weeks.
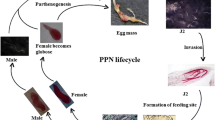
A diagrammatic representation of the life cycle of a root lesion nematode is provided in Fig. 1 (from Jones and Fosu-Nyarko 2014), and the life cycles of R. similis and Hirschmanniella spp. are essentially similar. These migratory nematodes develop within the eggshell to the first stage juvenile (J1), which moults to the second-stage juvenile (J2) and then emerges from the eggshell (Fig. 1). However, the difference between migratory and sedentary nematodes is that all subsequent juvenile and adult stages (J2, J3, J4, adults) of the former are worm-like and mobile, and both juvenile and adult stages can enter and leave host plant roots. Some migratory species also infest tuber tissues, and nematodes such as P. coffeae and the migratory Scutellonema bradys cause major losses when infesting yam tubers in West Africa, in which they continue to multiply in storage. Although these species are migratory endoparasites which usually spend most of their life cycle in host plant roots, they can also be found at the root surface and in nearby soil. Mature females lay eggs both inside infested roots and in nearby soil, and under adverse conditions, these nematodes can survive in soil for several years (Castillo and Vovlas 2007). Reproduction is usually by parthenogenesis, but males occur in some species.
A diagrammatic representation of the life cycle of Pratylenchus (from Jones and Fosu-Nyarko 2014, with permission)
As for other plant parasitic nematodes, root-feeding migratory parasitic nematodes feed by puncturing cells using their hollow mouth stylet. For root lesion nematodes, the J2s tend to feed from the epidermis and root hair cells, but with maturity the nematodes enter roots using their mouth stylet, possibly aided by secretion of plant cell wall-modifying enzymes, and migrate within the root cortex, feeding from the cytoplasm of individual cells, which subsequently die. Dead cells become necrotic, and with additional feeding and tissue damage, typical dark lesions develop in the roots. Development of lesions and further root damage occurs because the nematodes provide entry points for other soil pathogens, such as bacterial (e.g. Pseudomonas spp.) and fungal pathogens (e.g. Fusarium and Verticillium spp.), developing disease complexes which add to the necrosis and root damage (Castillo and Vovlas 2007). The nematodes may leave the roots, particularly from necrotic areas, to feed from new cells or find new host roots. Affected plants are stunted, leaves show early signs of yellowing and roots are short and stubby, with dark lesions. Field infestation is often manifested as patches of poor growth, with more severely affected plants at the centre. Severity is greater under conditions of poor nutrition or water stress.
3 Diagnosing Migratory Nematodes
Understanding the effects of migratory nematodes and finding appropriate strategies for their control first require their identification, and conventional taxonomy based on morphometric measurements is a specialist activity. This has been largely superseded by the development of molecular diagnostic tests, based on differences in ribosomal gene DNA, particularly the Internal Transcribed Spacer (ITS) regions (Al-Banna et al. 2004; Subbotin et al. 2008; Holterman et al. 2009; De Luca et al. 2011; Subbotin et al. 2013), further developed as quantitative polymerase chain reaction (PCR) tests (e.g. Sato et al. 2007; Berry et al. 2008; Yan et al. 2012). Correct identification of the species present is important, because plant resistance to one species does not mean it will be resistant to any other species. For example, wheat cultivars with resistance or tolerance to P. thornei are not necessarily resistant or tolerant to P. neglectus and vice versa: resistance and tolerance to each species are genetically independent (Smiley and Nicol 2009). A measure of nematode numbers is also important, because overall crop damage reflects the number of nematodes present, and the number of nematodes per gramme of soil at the start of a growing season can be used to predict potential losses and can determine the best cultivar to grow or treatment to apply. The reason why each plant resistance, tolerance or susceptibility may differ when attacked by different root lesion species may be explained partly by differences in the effectors that different nematodes use to enable successful parasitism, and for root lesion nematodes, this is still a developing research topic (see Sect. 5.2).
4 Virus Transmission by Migratory Ectoparasitic Nematodes
It is now well established that many species of migratory ectoparasitic nematodes from the Dorylaimida (Longidorus, Paralongidorus, Xiphinema) and Triplonchida (Trichodorus, Paratrichodorus), such as the dagger nematodes Xiphinema index and Xiphinema diversicaudatum, can act as vectors to transmit viruses of the viral genera Nepovirus and Tobravirus. They acquire and transmit the viruses by feeding on infected and then uninfected roots, either persistently or non-persistently: viruses they transmit include Tobacco ringspot virus (TRSV) and Tobacco rattle virus (TRV). The nepoviruses Grapevine fanleaf virus (GFLV) and Arabis mosaic virus (ArMV) are transmitted in a non-circulative manner and are economically important viruses of vines: precise interactions are required between the components both of the virus and the nematode stylet for virus transmission (Schellenberger et al. 2011). The main defence against virus diseases transmitted by these migratory nematodes is to avoid the introduction of virus-transmitting nematodes using plant biosecurity strategies, if infested to eradicate the nematodes using chemical nematicides or if available to use nematode resistance germplasm or rootstocks.
5 Natural Mechanisms of Plant Resistance to Nematode Attack
Under natural growing conditions, plants are exposed to a range of biotic and abiotic stresses. Among the biotic stresses are various herbivorous organisms feeding on the aboveground and belowground parts of the plant. Belowground attack involves various microorganisms which include nematodes, a diverse and abundant group of multicellular organisms. Plants normally have structural barriers and physiological processes in place that are able to exclude some microbes, parasites and pests from attack or invasion. Conversely, some parasites and pests have evolved mechanisms which aid successful parasitism or infestation of host plants. A compatible parasite–host interaction is when development and reproduction of the parasite are fully supported: the host plant is then referred to as susceptible to infection or infestation. When the development of a parasite is still supported because the host defences do not confer resistance but the parasite grows reasonably well with little apparent damage to the host plant, then the host is tolerant. However, in an incompatible interaction, in which a plant is considered resistant to infection or infestation, its natural, structural, biochemical or physiological defences can prevent invasion, development and/or reproduction of the invading organism. The strategies used by plants to defend themselves against the arsenal of effectors employed by migratory nematodes are discussed in the next sections.
5.1 Root Structure and Barriers to Nematode Infection
For higher plants the root is the main belowground organ and can be invaded by soil-inhabiting migratory parasitic nematodes (although other belowground organs such as tubers can also be attacked). Plants have many natural physical and chemical barriers which can provide protection against pathogens and pests. During root growth in soil, border cells of the root cap become detached (a process termed rhizodeposition) and can secrete antimicrobial proteins, phytoalexins, arabinogalactan proteins and pectins into the extracellular matrix or rhizosphere (Driouich et al. 2013). Border cells or associated extracellular matrix can both attract and repel pathogenic microorganisms. There is ample evidence that M. incognita second-stage juveniles (J2) are attracted to and accumulate rapidly around a 1- to 2-mm apical region of pea roots ensheathed by border cells, whereas no such reaction occurs at the root tip of snap bean, indicating possible differences in the perception or response of different plant species to similar root parasites (Zhao et al. 2000). A similar study on the mechanism of resistance to R. similis examined the effect that rhizodeposition (root cap cells and exudates) has on infective nematodes: rhizodeposition from both susceptible and resistant cultivars of banana (Musa acuminata) attracted nematodes, but the susceptible cultivar appeared to induce temporary quiescence in R. similis which lasted for 24 h, whereas nematode quiescence lasted for up to 3 days for the resistant cultivar Yangambi km5 (Wuyts et al. 2006a). Although these authors concluded that overall there was no indication that rhizodeposition played a part in preformed resistance of Yangambi km5 against R. similis, the relatively longer period of induced quiescence, and cellular responses of border cells to other factors such as aluminium and fungi, suggests that the tightly regulated production of border cells and associated extracellular matrix may play a role in the protection of root tips from some biotic and abiotic stresses (Hawes et al. 2000).
For migratory nematodes or pathogens that reach epidermal cells of the root of host plants, the next physical barrier to overcome is the cell wall. For both monocots and dicots, the plant cell wall is complex: it is composed of polysaccharides, mainly held together by non-covalent bonds, and cell wall proteins. Cellulose constitutes the most abundant polysaccharide and forms the framework to which matrix components are bound. These cellulose microfibrils are composed of associated linear β-1, 4-glucan chains linked by hydrogen bonds, to form an inelastic and insoluble structure. The cellulose microfibrils are embedded in a matrix of non-cellulosic sugar polymers, which include pectins and hemicelluloses, which is further reinforced by structural proteins such as glycoproteins and aromatic compounds (Carpita and Gibeaut 1993; McCann and Roberts 1994). The matrix of primary cell walls of higher plants consists of pectic substances, and the matrix of secondary cell walls are composed of hemicelluloses. Although the overall structures of cell walls of higher plants are similar in both monocots and dicots, there are substantial differences in polysaccharide composition that vary with cell type, cell function, phase of growth and differentiation. Differences in wall composition may well account for some level of resistance/inhibition to invading nematodes (Carpita and McCann 2000). However, the variation in cell wall composition in many instances seems not to present an insurmountable barrier to migratory endoparasitic nematodes, as reflected by the wide host range of many nematodes, encompassing both monocot and dicot plants. With the exception of some migratory ectoparasites, such as dorylaimids with long stylets, which may only use mechanical penetration of host cells, this suggests that successful invasion of host roots reflects strategies that enable invading nematodes to modify cell walls with a range of differences in composition. The latter seems to be a specialty for plant parasitic nematodes in general and migratory endoparasitic nematodes in particular.
5.2 How Migratory Endoparasitic Nematodes Overcome Plant Defences
Many migratory endoparasites have wide host ranges: for this they must have physical attributes, and physiological and evolutionary strategies, that enable them to avoid detection and successfully parasitise many plants. In a compatible interaction, a nematode can breach the barriers presented by cell walls, feed from host cell cytoplasm and suppress host defences. However, in reality, not all available infective juveniles actually succeed in finding and penetrating roots and develop to adults: this suggests that after the initial invasion, host plants may still employ structural, molecular or physiological defences to limit nematode growth and reproduction.
Secretions of the pharyngeal gland cells are thought to play a number of roles. These include suppression of host defences, enabling migration in plant tissues, promotion of nematode feeding (e.g. anticoagulation for migratory endoparasites, formation of feeding tubes for sedentary endoparasites) and digestion of ingested cytoplasm. (Additional functions are proposed for effectors of endoparasites which are involved in processes of host cell modification in the induction of syncytia or giant cells.) The secreted components which are responsible for these activities are generally described as ‘effectors’. Here we include cell wall-modifying enzymes as effectors, since they are an important component of the gene products required for plant parasitism and are a unique feature of plant parasitic nematodes.
Study of sedentary endoparasites has been underpinned by the availability of genomic and transcriptomic resources for the bacterial feeding model nematode Caenorhabditis elegans and more recently for root-knot and cyst nematodes: similar studies on migratory endoparasites are now emerging. Sequencing of ESTs of R. similis and the application of ‘next-generation’ sequencing technologies to sequence transcriptomes of H. oryzae and mixed stages of P. coffeae, P. thornei and P. zeae and more recently the genome of P. coffeae now provide the opportunity to identify and characterise effectors that make these migratory nematodes successful parasites (Jacob et al. 2008; Haegeman et al. 2010, 2011; Nicol et al. 2012; Bauters et al. 2014; Fosu-Nyarko et al. 2015; Burke et al. 2015; Fosu-Nyarko and Jones 2016). Putative effectors of migratory nematodes can now be predicted using software that identifies sequences for proteins likely to be secreted, combined with in situ hybridisation to identify transcripts expressed in gland cells, and sequence similarities and common structural features with effectors already characterised for sedentary endoparasites. Although the focus of nematode-secreted effectors has been on proteins or peptides secreted from the pharyngeal gland cells, other sources of secretions include the chemosensory amphids, the hypodermis, the cuticle, the excretory system and the rectal glands (Truong et al. 2015). For migratory nematodes, little is known about possible secretions from these sources. The current status of potential effectors of migratory nematodes is provided in Table 2.
Probably the best-characterised group of effectors present in plant parasitic nematodes are the cell wall-modifying enzymes. A cocktail of these enzymes (including a range of pectinases, hemicellulases, cellulases and expansins, Wieczorek 2015) appear to be secreted during nematode–host entry and migration and contribute to modifying the structure of host cell walls. Combined with probing with the sclerotised stylet, these enzymes enable nematodes to penetrate and move either intracellularly or intercellularly through root tissues to select appropriate cells to feed from. In situ hybridisation of transcripts and the presence of granules (implying secretory activity) in the subventral gland cells of sedentary endoparasites during migration suggest that these cells are the source of cell wall-modifying enzymes. However, for Pratylenchus spp., the subventral glands do not contain obvious granules. Nevertheless, identification of similar transcripts of effectors from recent transcriptomes and genome sequencing data of Pratylenchus spp. indicates that they also employ a similar range of cell wall-modifying enzymes to those identified for sedentary endoparasites. Their function is expected to be similar, that is, in hydrolysis of bonds of various polymeric components of primary and secondary cell walls, including pectins, hemicellulose and cellulose (Table 2, Jones and Fosu-Nyarko 2014). Current analysis of available sequences for R. similis (7,726 sequences in NCBI) and published reports suggest that this nematode employs only four of the cell wall-modifying enzymes identified for sedentary types; these are beta 1, 4- endoglucanase, xylanase, pectate lyase and cellulose-binding proteins. More work needs to be done to understand how these wall-modifying enzymes function, particularly the role of each in the host–parasite interaction (Jacob et al. 2008; Maier et al. 2013). The transcriptome analysis of H. oryzae provides evidence for transcripts putatively encoding a similar repertoire of cell wall-modifying enzymes to that of Pratylenchus spp. (Jones and Fosu-Nyarko 2014; Bauters et al. 2014).
In considering the roles of other candidate effectors, the presence of genes encoding proteins secreted by the dorsal glands of plant nematodes further reflects the battle between plants and invading nematodes. In this battle these nematode effectors are responsible for counteracting the effects of plant defences. Such effectors have been characterised better in sedentary nematodes and include proteins suggested to be secreted by nematodes to counter reactive oxygen species (ROS) produced by plants in response to nematode invasion. For example, superoxide dismutase and glutathione peroxidase present at the surface of plant and animal parasitic nematodes have been associated with the role of neutralising oxyradical attack by their host (Waetzig et al. 1999; Robertson et al. 2000; Jones and Fosu-Nyarko 2014). There is also ample evidence that sedentary endoparasites secrete effectors that modulate host cellular functions during establishment and functioning of feeding sites. Some effectors found in root-knot nematodes are involved in the formation of giant cell formation, such as 7E12, CLE peptide and 16D10 CLE-related proteins, whereas others interact with host metabolism to facilitate development of syncytia by cyst nematodes, such as the Hs19C07, Hg30C02 and 10A06 effectors (Huang et al. 2006; Hewezi et al. 2010; Lee et al. 2011; Souza et al. 2011; Hamamouch et al. 2012). Because migratory nematodes do not induce such intricate feeding structures in host tissues, it is not surprising that homologues of the effectors thought to be required for giant cell or syncytium formation have not been identified in migratory nematodes. Nevertheless, in addition to cell wall-modifying enzymes which have now been found in all plant nematodes where there is sufficient molecular data, other common effectors have been identified in secretions and genomes of both sedentary and migratory nematodes. Some are thought to be expressed highly at the parasitic stages (e.g. venom allergen-like proteins, transthyretin-like proteins) or to have roles in other interactions with plant hosts, including targeting and modifying plant signalling pathways (e.g. calreticulin, galectin) (Table 2). Haegeman et al. (2010) suggest a note of caution when extrapolating molecular insights from one group (e.g. Pratylenchus spp.) to another (e.g. Radopholus spp.) because the taxonomic relationship of R. similis and Pratylenchus spp. is not firm. Nevertheless, with increasing genomic information on migratory nematodes, our understanding of the function of demonstrated and candidate effectors from specific nematodes will shed more light on how plants defend themselves against migratory nematodes and how in turn the nematodes overcome plant defences.
5.3 Pathogen- and Damage-Associated Molecular Patterns During Nematode Infection
Apart from physical barriers and other basal mechanisms that contribute to resistance to plant pests and pathogens, several defence responses are triggered following root parasitism, including the innate immunity response. Host plants can detect the presence of pathogens using molecules present on the exterior or secreted by the invaders. These molecular signatures, often referred to as pathogen- or microbe-associated molecular patterns (PAMPs or MAMPs), are detected by cell surface receptors or pattern recognition receptors, PRRs. When PRRs of plants survey the apoplast and detect the presence of PAMPs, a PAMP-triggered immunity (PTI) is induced against the invading pathogen (Zipfel 2009). Characteristics of PAMPs and PTI defence against fungi and bacteria have been well studied, and parallels of the process have been drawn for nematode–host interactions. It has been suggested that derivatives of chitin of plant parasitic nematodes may induce PTI, although the nematode cuticle does not contain chitin (Libault et al. 2007). It is however possible that chitin or some of its derivatives may be present in nematode stylets, and on insertion into the plant cell walls, these molecular signatures could be detected by plants, which could lead to responses such as callose deposition which may reduce further invasion by the pathogen (Golinowski et al. 1997). Another facet of PAMP is effector-triggered immunity (ETI), which is specific to strains of a pathogen which secrete unique effectors. As part of the continuing battle between pathogen and host, there is good evidence that fungal plant pathogens and pests can evolve to counteract PAMP-induced plant defences, by selection of mutations of effectors such that they are no longer recognised by the plant or by secreting proteins which prevent PAMP recognition by plant receptors (De Jonge and Thomma 2009). Candidate ETI suppressors or genes linked to possible ETI to nematodes have been reported for sedentary endoparasitic nematodes (Semblat et al. 2001; Sacco et al. 2009; Rehman et al. 2009). For example, the SPRYSEC 19 effector, secreted by the cyst nematode Globodera rostochiensis, is known to interact with the leucine-rich repeat domains of receptor proteins in tomato and in doing so possibly suppresses receptor activity (Rehman et al. 2009). At present there is no functional evidence that migratory nematodes secrete such an effector, and for Pratylenchus spp., H. oryzae and R. similis for which transcriptomic and/or genomic sequence data are available, no such specific effector that could trigger ETI has yet been identified (Haegeman et al. 2011; Nicol et al. 2012, Fosu-Nyarko et al. 2015).
Plants also respond to cell damage and stresses that cause mechanical injury to aboveground and belowground parts. This response is mostly against damage-associated molecular pattern (DAMP) molecules released following cellular injury or damage caused by pathogens such as bacteria and fungi (Lotze et al. 2007). Responses to DAMPs are usually systemic and can include the release of redox-sensitive proteins as well as trigger induction of hormone signalling pathways. Movement of migratory nematodes through host roots and the mechanical probing of host cells with the stylet during feeding are likely to cause injury that may elicit such responses from host plants. Generally, plant hormone signalling pathways such as salicylic acid (SA), jasmonic acid (JA) and ethylene (ET) pathways are activated upon infection by many pathogens. While biotrophic pathogens would normally induce the SA pathway, wounding or infection by necrotrophic pathogens often activates the JA and ET pathways (Pieterse and van Loon 1999). It has been suggested that ETI initially activates all three signalling pathways and the plant mobilises resources to support the most effective pathway in combating a particular pathogen (Katagiri and Tsuda 2010). On infection of rice with the migratory nematode H. oryzae, JA and ET pathways are activated, while the SA pathway is suppressed, but one week after infection, JA and ET signalling is repressed. Foliar application of JA and ethephon, an exogenous ET, induces systemic defence response in roots against the sedentary endoparasite Meloidogyne graminicola, whereas for the migratory endoparasitic H. oryzae in rice, all three SA, JA and ET hormonal pathways appear to be essential for defence (Nahar et al. 2011, 2012).
5.4 Biochemical Responses in Host Plants Following Migratory Nematode Infection
In response to mechanical damage caused by nematodes, plants produce a range of compounds including ROS. These compounds are toxic to nematodes, but both animal and plant parasitic nematodes are well equipped to metabolise ROS, for example, via the secretion of proteins with antioxidant properties such as peroxiredoxins (Robertson et al. 2000). Production of ROS is associated with a suite of plant defence responses which include activation of signalling pathways and processes which can result in cell wall deposition, synthesis of terpenes, phenolic compounds and nitrogen- and sulphur-containing compounds (Mazid et al. 2011). These responses can be generic and are normally induced locally to eliminate or counteract the invading pathogen but can also be systemic in nature (Bezemer et al. 2004; van Dam 2009). For example, infection of black mustard (Brassica nigra) by P. penetrans results in increased synthesis of phenolic compounds and glucosinolates in roots, and this innate defence response was also effective in reducing the growth rate of larvae and number of pupae produced by the shoot feeding crucifer insect Pieris rapae (L.) (van Dam et al. 2005). The accumulation of isoflavonoid conjugates in roots of alfalfa (Medicago sativa) following infection by the stem nematode Ditylenchus dipsaci is a classical example of how some plant defence responses are generic and presumptive in nature (Edwards et al. 1995). Transcriptional changes in genes involved in metabolic pathways such as the phenylalanine metabolism, carotenoid biosynthesis and phenylpropanoid biosynthesis following infection by Pratylenchus spp. have been associated with induction of plant defence mechanisms (Baldridge et al. 1998; Zhu et al. 2014).
6 Breeding for Resistance to Migratory Nematodes
Some natural genes which confer host resistance to plant parasitic nematodes have been identified in cultivated and wild relatives of crop plants. For sedentary endoparasites, several dominant or semi-dominant resistance genes have been identified, mapped to chromosomal locations or linkage groups, characterised at the molecular level and implemented in a range of economically important crops (Fuller et al. 2008). There has been much less study of genes that confer resistance to migratory nematodes compared to sedentary types, and major dominant genes conferring resistance to migratory species have not yet been found. Not surprisingly, research on mechanisms of host resistance to migratory species has been undertaken mainly in countries and on crops where they cause most damage. For example, for Pratylenchus spp., the most detailed work to identify and combine sources of natural resistance to these species has been done with cereals and in most detail on bread wheat (Triticum aestivum) and barley (Hordeum vulgare) in Australia and the Pacific Northwest of the USA, where infection levels of root lesion nematodes and losses in wheat growing areas are significant (Vanstone et al. 2008; Smiley and Machado 2009; Jones and Fosu-Nyarko 2014).
Eight Pratylenchus species are known to attack wheat. In the southern and western wheat belts of Australia, P. neglectus, P. thornei, Pratylenchus quasiterioides (former species teres), P. penetrans, P. zeae, P. brachyurus and P. scribneri are present, with P. neglectus the most important (Vanstone et al. 2008), whereas in the northern wheat belt, P. thornei and P. penetrans cause the most damage (Smiley and Nicol 2009). Genotypes of wheat with different levels of resistance (and tolerance) to specific Pratylenchus species have been identified in many breeding programmes using tools for marker-assisted breeding (Table 3). This usually involves large-scale screening of germplasm from wild ancestors or progenitors of crop plant cultivars and mapping of quantitative trait loci (Table 3). A recent marker-assisted selection study for resistance in barley has also identified five QTLs contributing to resistance to P. neglectus in barley germplasm (Table 3). However, no major gene conferring resistance to root lesion nematodes has been found, and the mechanisms that underlie resistance to Pratylenchus spp. in wheat and barley are not known. Although the identification of QTLs for resistance to migratory endoparasites is an important advance, there is a need for further detailed study to identify new, more effective and durable sources of natural resistance to these nematodes.
7 Resistance to Migratory Nematodes in Tropical Crops
Banana and plantain (Musa spp.) constitute the eighth most important staple food crop worldwide. The most damaging migratory nematodes of these crops are the endoparasites R. similis, Pratylenchus goodeyi, P. coffeae and the spiral nematode Helicotylenchus multicinctus, together with Meloidogyne spp., with combinations of these nematode pests varying with locality (Karakaş 2007; Tripathi et al. 2015). The search for resistance genes against these species, especially against R. similis, has largely focussed on Musa spp. Using traditional nematode screening methods either by inoculating samples in vitro or in glasshouses or using existing infection at field conditions, many recent Musa cultivars have been scored for resistance to nematodes, mainly to R. similis but to a lesser extent to Pratylenchus spp. and H. multicinctus (Elsen et al. 2002; Moens et al. 2005). Among the most well-known nematode-resistant Musa spp. are a triploid AAA cultivar, Yangambi km5, with high resistance to both R. similis and P. goodeyi, and the AA diploid Pisang Jari Buaya, resistant to R. similis (Pinochet and Rowe 1979; Wehunt et al. 1978; Sarah et al. 1993; Price 1994; Fogain and Gowen 1998). Accessions from gene pools of these resistant cultivars have been used as sources of resistance in Musa breeding programmes with some success (Pinochet and Rowe 1979; Viaene et al. 2003). In one of the few reports on genetic resistance screening, using 81 banana diploid hybrids, it appeared that resistance to R. similis is controlled by two dominant genes, both with additive and interactive effects (Dochez et al. 2009).
Otherwise, investigations on mechanisms of resistance of Musa spp. to R. similis and Pratylenchus spp. have largely focussed on characteristics of root structures and the biochemical responses of resistant and susceptible cultivars on infection. The presence of more preformed phenolic cells in roots of the resistant cultivar Yangambi km5 suggests that the formation and this type of cell play a role in its defence (Fogain and Gowen 1998). However, resistant cultivar Pisang Jari Buaya may have a different resistance mechanism, because it has fewer preformed phenolic cells in roots, but appears to have more cells with lignified walls than cultivars susceptible to R. similis (Fogain and Gowen 1998). A possible role of cell wall lignification may also be evident for other resistant and partially resistant Musa cultivars, and this suggests that infection by migratory endoparasites may induce lignification and suberisation of endodermal cells, so limiting invasion of the vascular bundle (Collingborn et al. 2000; Valette et al. 1998). Differential accumulation of the secondary metabolites phenylalanine ammonia-lyase, peroxidase and polyphenol oxidase in roots of resistant and susceptible cultivars of banana infected with R. similis has been associated with levels of resistance to the nematode pest (Wuyts et al. 2006b).
8 Cultural, Biological and Chemical Control of Migratory Nematodes
8.1 Rotations with Non-host Crops
Apart from natural resistance genes or transgenic approaches, the three main approaches used to control plant parasitic nematodes are cultural, biological and chemical. Cultural control relates to developing crop rotation systems which include one or more crop plants which are non-hosts for a particular nematode. The nematode population should then be reduced substantially during the non-host period of the rotation, with the aim of reducing the threshold levels of the damaging nematode to levels below those that result in crop losses. Rotation is more effective if more than one non-host crop species is available in the rotation, and the effectiveness depends on the nematode species and also whether it has an ability to survive for long periods in the absence of a good host. For migratory nematodes with a wide host range, this strategy may not always work well.
In order to study alternative crops suitable for rotations with wheat in the Pacific Northwest of the USA, Smiley et al. (2014) surveyed 30 crop species and cultivars to look for cultivars with reduced reproductive efficiency or as potential non-hosts of P. neglectus and P. thornei. Poor hosts of both species were identified in chickpea, pea, safflower and sunflower cultivars and some grasses, but more crop cultivars were found to be good hosts for both species: the latter included cultivars of oat, chickpea and lentil. Ten brassica species (canola, mustard, camelina), sudan grass and a sudan grass/sorghum hybrid were good hosts only of P. neglectus, and other cultivars of lentil and pea were good hosts for P. thornei. The defence mechanisms of these non-host plants to migratory nematodes have not been investigated: such information would contribute to development of resistance to economically important hosts of these damaging nematode pests. Similar studies have been undertaken in Australia, which showed, for example, that densities of P. neglectus, but not of P. thornei, were likely to be increased after canola (Taylor et al. 2000; Hollaway et al. 2000), although in Australian environments the choices available for alternative cash crops to wheat or barley are relatively limited. The use of non-host crops in rotations to reduce populations of migratory nematodes is a simple approach but needs further study. Smiley et al. (2014) commented that it is likely that reduced efficiency of wheat production is associated with rotations that include multiple crops that are each good hosts of Pratylenchus spp., such as now appears to be very likely for some wheat–food legume or wheat–brassica rotations.
8.2 Biological and Chemical Control of Migratory Nematodes
A range of nematophagous bacteria and fungi can be found in nematode-suppressive soils, but in the past the success of biological control agents, such as natural predators or pathogens, used to reduce nematode numbers, was limited (Kerry 1997). Biological control was more inconsistent, less effective and slower acting than control normally achieved with chemicals. The use of nematicidal chemicals for nematode control is not always cost effective or environmentally acceptable, especially for broadscale agriculture or for small-scale farms in developing countries. In addition, the phasing out of long-standing chemical nematicides, such as Temik (aldicarb), Mocap (ethoprophos) and Nemacur (fenamiphos), has spurred research to develop more effective and environmentally benign methods of chemical and biological control of plant nematodes. Research by various commercial organisations has led to the development of new seed coating technologies and biocontrol agents which are now commercially available and are much more effective than previous generations of biological control agents. For example, Bayer CropScience now markets VOTiVO, based on Bacillus firmus root colonising bacteria which colonises root surfaces and reduces nematode access to root-feeding sites, and Velum (fluopyram), a new class of chemical nematicide which inhibits mitochondrial respiration in nematodes; Syngenta markets AVICTA (abamectin), which has broader anthelmintic and insecticidal properties; and a contact nematicide Nimitz (fluensulfone) has been passed for nematode control for vegetable crops. Other biological control agents such as the entomopathogenic fungus Paecilomyces and the parasitic bacterium Pasteuria penetrans are also available commercially (the latter was initially developed to control sting nematodes in turfgrass by Pasteuria Bioscience, which was acquired by Syngenta in 2012). Such biological control agents can be included in an integrated pest management approach and are stable enough to be applied as a seed coating, so reducing the chemical load on the field: most are toxic to migratory nematodes. Early protection and establishment of crop seedlings provides a much greater opportunity for a crop to reach its full yield potential.
9 Transgenic Approaches to Migratory Nematode Resistance
Much research has been undertaken to develop transgenic (biotechnological) strategies for nematode control. These include interfering with nematode location of roots, reducing entry into and migration in roots, preventing formation or disturbing the functions of feeding cells of endoparasites and delivery of compounds via plants that interfere with different aspects of nematode life cycles (Fosu-Nyarko and Jones 2015). The focus of the vast majority of such studies has been on sedentary endoparasites.
The earliest transgenic strategies for nematode control were based on plant cystatins, inhibitors of nematode cysteine proteases which interfere with nematode digestion (Urwin et al. 1997; Vain et al. 1998; Samac and Smigocki 2003). The range of available cystatins has been expanded, with reports of effective resistance against the migratory endoparasite Ditylenchus destructor (Gao et al. 2011). The focus of these and subsequent experimental work was on cyst and root-knot nematodes.
To find and enter host roots, invading nematodes must respond to root stimuli and physical and chemical gradients in the rhizosphere: these are mediated by chemosensory and mechanosensory neurons. Interference with nematode chemoreceptors can reduce the ability of nematodes to find host roots, and this strategy has been followed by development of peptides that inhibit acetylcholinesterase, which appear to be taken up by chemoreceptor sensillae via retrograde transport along their neurons to cholinergic synapses (Lilley et al. 2011a). Transgenic plants that secreted this peptide from roots driven by a constitutive promoter (CaMV35S) reduced establishment of Globodera pallida (Lilley et al. 2004; Liu et al. 2005): the delivery was refined using expression of the peptide driven by a root cap promoter (MDK4-20) (Lilley et al. 2011b).
The two experimental approaches outlined above have been progressed to confined field tests for transgenic plantain (Musa spp.) in Uganda, Africa, to control key migratory nematode pests, which include R. similis, H. multilinctus, P. coffeae, P. goodeyi and also endoparasitic root-knot nematodes (Tripathi et al. 2015). In this work, an antifeedant cysteine proteinase inhibitor from maize and an anti-root invasion synthetic peptide were expressed either jointly or separately in banana and subjected to nematode challenge. The results focussing on R. similis and H. multicinctus showed that the best peptide-expressing transgenic line showed improved agronomic performance relative to non-transgenic controls and provided about 99 % nematode resistance at harvest and that the anti-root invasion peptide appeared to be more effective than the cystatin: in plants expressing both genes, the cystatin appeared to contribute little additional resistance (Tripathi et al. 2015). This work demonstrated that expression of cystatins and/or an anti-root invasion peptide can confer resistance to migratory endoparasites as well as sedentary endoparasites and provide a potential new mode of control of nematodes for banana and other tropical crops (e.g. yam, cassava) which are staple foods of small-scale farmers in Central and West Africa.
As further evidence that root lesion nematode infestation can be reduced by a cystatin, expression of a modified rice cystatin (Oc-IDD86) in the flower crop Lilium longiflorum also conferred enhanced resistance to Pratylenchus penetrans, reducing nematode numbers by about 75 %, resulting in enhanced growth performance (Vieira et al. 2014).
An alternative approach to that described above is generally described as ‘host-induced gene silencing’ (HIGS) and involves using transgenic plants to deliver a gene silencing (RNAi) signal in the form of dsRNA to silence a vital gene in the nematode when it ingests cell contents (e.g. Lilley et al. 2012; Jones and Fosu-Nyarko 2014). Research in this area on migratory endoparasitic nematodes lagged behind that on sedentary endoparasitic nematodes, partly because of a lack of genomic resources, combined with the fact that migratory nematodes are more difficult to work with than most sedentary endoparasites. However, increasing genomic and transcriptomic data is now becoming available for migratory endoparasitic nematodes, providing a new resource to identify target genes for their control. As discussed above, ‘next-generation sequencing’ has been used to generate transcriptome data on P. coffeae, P. thornei, P. zeae, H. oryzae and R. similis (Haegeman et al. 2011; Nicol et al. 2012; Fosu-Nyarko et al. 2015; Bauters et al. 2014), and genomic data for P. coffeae is now also available (Burke et al. 2015). These data now enable identification of new gene targets for RNAi-based control of migratory nematodes (Fosu-Nyarko and Jones 2015).
The most common approach to determining what target genes to use for nematode control involves (1) a bioinformatics phase to identify potential target genes, often based on comparative data from the effects of gene knockout in C. elegans, or identified effectors required for successful plant parasitism; (2) their cloning and generation of dsRNA to their sequences; (3) in vitro feeding of motile stages with dsRNA, often in the presence of a neurostimulant to make the nematodes take up the external solution, and assessment of the effects of gene knockdown in the nematodes; (4) based on results from in vitro feeding, production of transgenic plants expressing dsRNA to the nematode target gene; and (5) challenge of the transgenic plants with nematodes in glasshouse experiments to quantify the effects on nematode reproduction.
Optimisation of in vitro feeding conditions and treatment with dsRNA of target genes show that P. coffeae, P. thornei and P. zeae are all amenable to a level of control using RNAi (Haegeman et al. 2011; Tan et al. 2013), and this also holds for transgenic plant resistance (Tan 2015). Thus, there is good reason to expect that all the migratory endoparasitic nematodes are equally amenable to control by the RNAi-based HIGS strategy. Such plant-mediated gene silencing traits in nematodes may be transmitted to the next generation and reduce pathogenicity of nematode offspring on non-RNAi plants, which suggests that there can be epigenetic inheritance of the silencing effect (Elling 2015). The level of resistance obtained by HIGS, if expressed as the percentage reduction in the number of nematodes present compared with susceptible controls, is never 100 %, but a percentage reduction in nematode numbers of up to 90 % or more can be obtained, and this will greatly reduce nematode populations over time. There are many reasons why 100 % resistance by this measure is not achieved (Fosu-Nyarko and Jones 2015), but stacking two (or more) different modes of resistance, such as an RNAi trait and an antifeedant peptide or cystatin, might provide the most effective and durable form of transgenic resistance, preferably in a crop cultivar genotype which expresses the best levels of conventional resistance.
10 Conclusions
The losses caused to crops by infestation with migratory nematodes are difficult to quantify accurately, but in many cases they are equal to or more important than losses caused by sedentary endoparasites. The biology of migratory nematodes is becoming better understood, especially with the availability of new genomic resources. In terms of conventional plant breeding, host plant defences can be improved by marker-assisted selection, which is valuable in combining the best QTLs contributing to resistance against major species. There is also clear evidence that migratory nematodes are amenable to various forms of transgenic control, and new integrated approaches to chemical and biological control are also showing success in protecting crop plant roots against migratory nematodes. In many ways understanding of migratory parasitic nematodes and their interactions with host roots is now emerging from biological darkness into the light.
References
Al-Banna L, Ploeg AT, Williamson VM, Kaloshian I (2004) Discrimination of six Pratylenchus species using PCR and species-specific primers. J Nematol 36:142–146
Baldridge GD, O’Neill NR, Samac DA (1998) Alfalfa (Medicago sativa L.) resistance to the root-lesion nematode, Pratylenchus penetrans: defense-response gene mRNA and isoflavonoid phytoalexin levels in roots. Plant Mol Biol 38:999–1010
Bauters L, Haegeman A, Kyndt T, Gheysen G (2014) Analysis of the transcriptome of Hirschmanniella oryzae to explore potential survival strategies and host-nematode interactions. Mol Plant Pathol 15:352–363
Berry AD, Fargette M, Spaull VW, Morand S, Cadet P (2008) Detection and quantification of root-knot nematode (Meloidogyne javanica), lesion nematode (Pratylenchus zeae) and dagger nematode (Xiphinema elongatum) parasites of sugarcane using real-time PCR. Mol Cell Probes 22:168–176
Bezemer TM, Wagenaar IR, van Dam NM, van der Putten WH, Wackers FL (2004) Above- and below-ground terpenoid aldehyde induction in cotton, Gossypium herbaceum, following root and leaf injury. J Chem Ecol 30:53–67
Blair BL, Stirling GR (2007) The role of plant-parasitic nematodes in reducing yield of sugarcane in fine textured soils in Queensland, Australia. Aust J Exp Agric 2007:620–634
Burke, M, Scholl EH, Bird DMcK, Schaff JE, Coleman, S, Crowell R, Diener S, Gordon O, Graham S, Wang X, Windham E, Wright GM and Opperman CH (2015) The plant parasite Pratylenchus coffeae carries a minimal nematode genome. Nematology (in press). doi: 10.1163/15685411-00002901
Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3(1):1–30
Carpita N, McCann M (2000) The cell wall. In: Buchanan B et al (eds) Biochemistry and molecular biology of plants. ASPP, Rockville, pp 52–108
Castillo P, Vovlas N (2007) Pratylenchus (Nematoda: Pratylenchidae): diagnosis, biology, pathogenicity and management, nematology monographs and perspectives. Brill Leiden, Boston
Collingborn FM, Gowen SR, Mueller-Harvey I (2000) Investigations into the biochemical basis for nematode resistance in roots of three musa cultivars in response to Radopholus similis infection. J Agric Food Chem 48:5297–5301
De Jonge R, Thomma BPHJ (2009) Fungal LysM effectors: extinguishers of host immunity? Trends Microbiol 17:151–157
De Ley P, Blaxter M (2002) Systemic position and phylogeny. In: Lee DL (ed) The biology of nematodes. Taylor and Francis, New York, pp 1–30
De Luca F, Reyes A, Troccoli A, Castillo P (2011) Molecular variability and phylogenetic relationships among different species and populations of Pratylenchus (Nematoda: Pratylenchidae) as inferred from the analysis of the ITS rDNA. Eur J Plant Pathol 130:415–426
Dochez C, Tenkouano A, Ortiz R, Whyte J, De Waele D (2009) Host plant resistance to Radopholus similis in a diploid banana hybrid population. Nematology 11:329–335
Driouich A, Follet-Gueye ML, Vicré-Gibouin M, Hawes M (2013) Root border cells and secretions as critical elements in plant host defense. Curr Opin Plant Biol 16(4):489–495
Dropkin VH (1989) Introduction to nematology, 2nd edn. Wiley, New York, p 304
Edwards R, Mizen T, Cook R (1995) Isoflavonoid conjugate accumulation in the roots of lucerne (Medicago sativa) seedlings following infection by the stem nematode (Ditylenchus dipsaci). Nematologica 41:51–66
Elling AA (2015) RNA interference as a nematode control strategy. In: Proceedings of the 54th annual meeting of the society of nematologists, East Lansing, p 47
Elsen A, Stoffelen R, Thi Tuyet N, Baimey H, Dupré de Boulois H, De Waele D (2002) In vitro screening for resistance to Radopholus similis in Musa spp. Plant Sci 163:407–416
Fogain R, Gowen SR (1998) Investigations on possible mechanisms of resistance to nematodes in Musa. Euphytica 92(3):375–381
Fosu-Nyarko J, Jones MGK (2015) Application of biotechnology for nematode control in crop plants. Adv Bot Res 73:339–376
Fosu-Nyarko J, Tan JCH, Gill R, Agrez VG, Rao U, Jones MGK (2015) De novo analysis of the transcriptome of Pratylenchus zeae to identify transcripts for proteins required for structural integrity, sensation, locomotion and parasitism. Mol Plant Pathol. doi: 10.1111/mpp.12301
Fosu-Nyarko J, Jones MGK (2016) Advances in understanding the molecular mechanisms of root lesion nematode host interactions. Annu Rev Phytopathol 54. doi:10.1146/annurev-phyto-080615-100257
Fuller VL, Lilley CJ, Urwin PE (2008) Nematode resistance. New Phytol 180:27–44
Galal A, Sharma S, Abou-Elwafa SF, Sharma S, Kopisch-Obuch F, Laubach E, Perovic D, Ordon F, Jung C (2014) Comparative QTL analysis of root lesion nematode resistance in barley. Theor Appl Genet 127:1399–1407
Gao S, Yu B, Yuan L, Li Y, Zhai H, He A, Liu WCL (2011) Production of transgenic sweet potato plants resistant to stem nematodes using oryzacystatin-I gene. Sci Hortic 128:408–414
Golinowski W, Sobczak M, Kurek W, Grymaszewska G (1997) The structure of syncytia. In: Fenoll C, Grundler FMW, Ohl S (eds) Cellular and molecular aspects of plant–nematode interactions. Kluwer Academic, Dordrecht, pp 80–97
Haegeman A, Elsen A, de Waele D, Gheysen G (2010) Emerging molecular knowledge on Radopholus similis, an important nematode pest of banana. Mol Plant Pathol 11:315–323
Haegeman A, Joseph S, Gheysen G (2011) Analysis of the transcriptome of the root lesion nematode Pratylenchus coffeae generated by 454 sequencing technology. Mol Biochem Parasitol 178:7–14
Hamamouch N, Li C, Hewezi T, Baum TJ, Mitchum MG, Hussey RS, Vodkin LO, Davis EL (2012) The interaction of the novel 30C02 cyst nematode effector protein with a plant β-1,3-endoglucanase may suppress host defence to promote parasitism. J Exp Bot 62:3683–3695
Hawes MC, Gunawardena U, Miyasaka S, Zhao X (2000) The role of root border cells in plant defense. Trends Plant Sci 5(3):128–133
Hewezi T, Howe PJ, Maier TR, Hussey RS, Mitchum MG, Davis EL, Baum TJ (2010) Arabidopsis spermidine synthase is targeted by an effector protein of the cyst nematode Heterodera schachtii. Plant Physiol 152:968–984
Hollaway GJ, Taylor SP, Eastwood RF, Hunt CH (2000) Effect of field crops on density of Pratylenchus in southeastern Australia: Part 2. P. thornei. J Nematol 32:600–608
Holterman M, Karssen G, van den Elsen S, van Megen H, Bakker J, Helder J (2009) Small subunit rDNA-based phylogeny of the Tylenchida sheds light on relationships among some high-impact plant-parasitic nematodes and the evolution of plant feeding. Phytopathology 99:227–235
Huang GZ, Dong RH, Allen R, Davis EL, Baum TJ, Hussey RS (2006) A root-knot nematode secretory peptide functions as a ligand for a plant transcription factor. Mol Plant Microbe Interact 19:463–470
Jacob J, Mitreva M, Vanholme B, Gheysen G (2008) Exploring the transcriptome of the burrowing nematode Radopholus similis. Mol Genet Genomics 280:1–17
Jones MGK, Fosu-Nyarko J (2014) Molecular biology of root lesion nematodes (Pratylenchus spp.) and their interaction with host plants. Ann Appl Biol 164:163–181
Jones JT, Haegeman A, Danchin EGJ, Gaur HS, Helder J, Jones MGK, Kikuchi T, Manzanilla-Lopez R, Palomares-Rius JE, Wesemael WML, Perry RN (2013) Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol 14:946–961
Jordaan EM, De Waele D (1988) Host status of five weed species and their effects on Pratylenchus zeae infestation of maize. J Nematol 20(4):620
Karakaş M (2007) Life cycle and mating behavior of Helicotylenchus multicinctus (Nematoda: Hoplolaimidae) on excised Musa cavendishii roots. Biologia 62(3):320–322
Katagiri F, Tsuda K (2010) Understanding the plant immune system. Mol Plant Microbe Interact 23:1531–1536
Kerry B (1997) Biological control of nematodes: prospects and opportunities. In: Maqbool MA, Kerry B (eds) Plant nematode problems and their control in the near east region (FAO Plant Production and Protection Paper—144). ISBN: 92-5-103798-1
Kyndt T, Fernandez D, Gheysen G (2014) Plant-parasitic nematode infections in rice: molecular and cellular insights. Annu Rev Phytopathol 52:135–153
Lee C, Chronis D, Kenning C, Peret B, Hewezi T, Davis EL, Baum TJ, Hussey RS, Bennett M, Mitchum MG (2011) The novel cyst nematode effector protein 19C07 interacts with the Arabidopsis auxin influx transporter LAX3 to control feeding site development. Plant Physiol 155:866–880
Libault M, Wan J, Czechowski T, Udvardi M, Stacey G (2007) Identification of 118 Arabidopsis transcription factor and 30 ubiquitin-ligase genes responding to chitin, a plant-defense elicitor. Mol Plant Microbe Interact 20:900–911
Lilley CJ, Urwin PE, Johnston KA, Atkinson HJ (2004) Preferential expression of a plant cystatin at nematode feeding sites confers resistance to Meloidogyne and Globodera sp. Plant Biotech J 2:3–12
Lilley CJ, Davies LJ, Urwin PE (2011a) RNA interference in plant parasitic nematodes: a summary of the current status. Parasitology 139:630–640
Lilley CJ, Wang D, Atkinson HJ, Urwin PE (2011b) Effective delivery of a nematode-repellent peptide using a root-cap-specific promoter. Plant Biotechnol J 9:151–161
Lilley CJ, Davies LJ, Urwin PE (2012) RNA interference in plant parasitic nematodes: a summary of the current status. Parasitology 139:630–640
Liu B, Hibbard JK, Urwin PE, Atkinson HJ (2005) The production of synthetic chemodisruptive peptides in planta disrupts the establishment of cyst nematodes. Plant Biotechnol J 3:487–496
Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, De Vera ME, Liang X, Tor M, Billiar T (2007) The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Annu Rev Immunol 220:60–81
Maier TR, Hewezi T, Peng J, Baum TJ (2013) Isolation of whole esophageal gland cells from plant-parasitic nematodes for transcriptome analyses and effector identification. Mol Plant Microbe Interact 26:31–35
Mazid M, Khan TA, Firoz M (2011) Role of nitric oxide in regulation of H2O2 mediating tolerance of plants to abiotic stress: a synergistic signalling approach. J Stress Physiol Biochem 7:34–74
McCann MC, Roberts K (1994) Changes in cell wall architecture during cell elongation. J Exp Bot 45(Special Issue):1683–1691
Moens T, Araya M, Swennerf R, De Waele D (2005) Screening of Musa cultivars for resistance to Helicotylenchus multicinctus, Meloidogyne incognita, Pratylenchus coffeae and Radopholus similis. Aust Plant Pathol 34(3):299–309
Nahar K, Kyndt T, De Vleesschauwer D, Hofte M, Gheysen G (2011) The jasmonate pathway is a key player in systemically induced defense against root knot nematodes in rice. Plant Physiol 157:305–316
Nahar K, Kyndt T, Nzogela YB, Gheysen G (2012) Abscisic acid interacts antagonistically with classical defense pathways in rice–migratory nematode interaction. New Phytol 196:901–913
Nicol P, Gill R, Fosu-Nyarko J, Jones MGK (2012) de novo analysis and functional classification of the transcriptome of the root lesion nematode, Pratylenchus thornei, after 454 GS FLX sequencing. Int J Parasitol 42:225–237
Pieterse CM, van Loon LC (1999) Salicylic acid-independent plant defence pathways. Trends Plant Sci 4:52–58
Pinochet J, Rowe P (1979) Progress in breeding for resistance to Radopholus similis on bananas. Nematropica 9:76–78
Price NS (1994) Alternate cropping in the management of Radopholus similis and Cosmopolites sordidus two important pests of banana and plantain. Int J Pest Manage 40:237–244
Rehman S, Postma W, Tytgat T, Prins P, Qin L, Overmars H, Vossen J, Spiridon LN, Petrescu AJ, Goverse A, Bakker J, Smant G (2009) A secreted SPRY domain-containing protein (SPRYSEC) from the plant-parasitic nematode Globodera rostochiensis interacts with a CC-NB-LRR protein from a susceptible tomato. Mol Plant Microbe Interact 22:330–340
Robertson L, Robertson WM, Sobczak M, Bakker J, Tetaud E, Arinagayayam MR, Ferguson MAJ, Fairlamb AH, Jones JT (2000) Cloning, expression and functional characterisation of a thioredoxin peroxidise from the potato cyst nematode Globodera rostochiensis. Mol Biochem Parasitol 111:41–49
Sacco MA, Koropacka K, Grenier E, Jaubert MJ, Blanchard A, Goverse A, Smant G, Moffett P (2009) The cyst nematode SPRYSEC protein RBP-1 elicits Gpa-2 and RanGAP2-dependent plant cell death. PLoS Pathog 5:e1000564
Samac DA, Smigocki AC (2003) Expression of oryzacystatin I and II in alfalfa increases resistance to the root-lesion nematode. Phytopathology 93:799–804
Sarah JL, Sabatini C, Boisseau M (1993) Differences in pathogenicity to banana (Musa Sp., Cv. Poyo) among isolates of Radopholus similis from different production areas of the world. Nemtropica 23:75–79
Sato E, Min YY, Shirakashi T, Wada S, Toyota K (2007) Detection of the root-lesion nematode, Pratylenchus penetrans (Cobb), in a nematode community using real time PCR. Jpn J Nematol 37:87–92
Schellenberger P, Sauter C, Lorber B, Bron P, Trapani S, Bergdoll M, Marmonier A, Schmitt-Keichinger C, Lemaire O, Demangeat G, Ritzenthaler C (2011) Structural insights into viral determinants of nematode mediated grapevine fanleaf virus transmission. PLoS Pathog 7:e1002034. doi:10.1371/journal.ppat.1002034
Semblat J-P, Rosso M-N, Husser RS, Abad P, Castagnone-Sereno P (2001) Molecular cloning of a cDNA encoding an amphid secreted putative avirulence protein from the root knot nematode Meloidogyne incognita. Mol Plant Microbe Interact 14:72–79
Sharma S, Sharma S, Kopisch-Obuch FJ, Keil T, Laubach E, Stein N, Graner A, Jung C (2011) QTL analysis of root-lesion nematode resistance in barley: 1. Pratylenchus neglectus. Theor Appl Genet 122:1321–1330
Smiley RW, Machado S (2009) Pratylenchus neglectus reduces yield of winter wheat in dryland cropping systems. Plant Dis 93:263–271
Smiley RW, Nicol JM (2009) Nematodes which challenge global wheat production. In: Carver BF (ed) Wheat science and trade. Wiley, Ames, pp 171–187
Smiley RW, Yan GP, Gourlie JA (2014) Selected Pacific Northwest crops as hosts of Pratylenchus neglectus and P. thornei. Plant Dis 98:1341–1348
Souza Ddos S, de Souza JD, Grossi-de- Sá M, Rocha TL, Fragoso RR, Barbosa AE, de Oliveira GR, Nakasu EY, de Sousa BA, Pires NF et al (2011) Ectopic expression of a Meloidogyne incognita dorsal gland protein in tobacco accelerates the formation of the nematode feeding site. Plant Sci 180:276–282
Subbotin SA, Ragsdale EJ, Mullens T, Roberts PA, Mundo-Ocampo M, Baldwin JG (2008) A phylogenetic framework for root lesion nematodes of the genus Pratylenchus (Nematoda): evidence from 18S and D2-D3 expansion segments of 28S ribosomal RNA genes and morphological characters. Mol Phylogenet Evol 48:491–505
Subbotin SA, Waeyenberge L, Moens M (2013) Molecular taxonomy and phylogeny. In: Perry RN, Moens M (eds) Plant nematology, 2nd edn. CABI, Wallingford, pp 40–728
Tan J, Jones MGK, Fosu-Nyarko J (2013) Gene silencing in root lesion nematodes (Pratylenchus spp.) significantly reduces reproduction in a plant host. Exp Parasitol 133:166–178
Tan JCH (2015) Characterising putative parasitism genes for root lesion nematodes and for use in RNAi studies. PhD thesis, Murdoch University, Perth, Western Australia
Taylor SP, Holloway G, Hunt C (2000) Effect of field crops on population densities of Pratylenchus neglectus and P. thornei in Southeastern Australia; Part 1: P. neglectus. J Nematol 32:591–599
Thompson JP, Brennan PS, Clewett TG, Sheedy JG, Seymour NP (1999) Progress in breeding wheat for tolerance and resistance to root-lesion nematode (Pratylenchus thornei). Australas Plant Pathol 28:45–52
Toktay H, McIntyre CL, Nicol JM, Ozkan H, Elekcioglu HI (2006) Identification of common root-lesion nematode (Pratylenchus thornei Sher et Allen) loci in bread wheat. Genome 49:1319–1323
Tripathi L, Babirye A, Roderick H, Tripathi JN, Changa C, Urwin PE, Tushemereirwe WK, Coyne D, Atkinson HJ (2015) Field resistance of transgenic plantain to nematodes has potential for future African food security. Sci Rep 5:1–10
Truong NM, Nguyen CN, Abad P, Quentin M, Favery B (2015) Function of root-knot nematode effectors and their targets in plant parasitism. Adv Bot Res 73:293–324
Urwin PE, Lilley CJ, McPherson MJ, Atkinson HJ (1997) Resistance to both cyst and root-knot nematodes conferred by transgenic Arabidopsis expressing a modified plant cystatin. Plant J 12:455–461
Vain P, Worland B, Clarke MC, Richard G, Beavis M, Liu H, Kohli A, Leech M, Snape J, Christou P, Atkinson H (1998) Expression of an engineered cysteine proteinase inhibitor (Oryzacystatin-I_D86) for nematode resistance in transgenic rice plants. Theor Appl Genet 96:266–271
Valette C, Andary C, Geiger JP, Sarah JL, Nicole M (1998) Histochemical and cytochemical investigations of phenols in roots of banana infected by the burrowing nematode Radopholus similis. Phytopathology 88(11):1141–1148
van Dam NM (2009) Belowground herbivory and plant defenses. Annu Rev Ecol Evol Syst 40:373–392
van Dam NM, Raaijmakers CE, van der Putten WH (2005) Root herbivory reduces growth and survival of the shoot feeding specialists Pieris rapae on Brassica nigra. Entomol Exp Appl 115:161–170
Vanstone VA, Hollaway GJ, Stirling GR (2008) Managing nematode pests in the southern and western regions of the Australian cereal industry: continuing progress in a challenging environment. Aust Plant Pathol 37:220–234
Viaene N, Duran LF, Mauricio Rivera J, Duenas J, Rowe P, De Waele D (2003) Responses of banana and plantain cultivars, lines and hybrids to the burrowing nematode Radopholus similis. Nematology 5:85–95
Vieira P, Wantoch S, Lilley CH, Chitwood DJ, Atkinson HJ, Kamo K (2014) Expression of a cystatin transgene can confer resistance to root lesion nematodes in Lilium longiflorum cv. ‘Nellie White’. Transgenic Res. doi:10.1007/s11248-014-9848-2
Waetzig GH, Sobczak M, Grundler FMW (1999) Localization of hydrogen peroxide during the defence response of Arabidopsis thaliana against the plant-parasitic nematode Heterodera glycines. Nematology 1:681–686
Wehunt EJ, Hutchison DJ, Edwards DI (1978) Reaction of banana cultivars to the burrowing nematode (Radopholus similis). J Nematol 10(4):368
Wieczorek K (2015) Cell wall alterations in nematode-infected roots. Adv Bot Res 73:61–90
Williams K, Taylor S, Bogacki P, Pallotta M, Bariana H, Wallwork H (2002) Mapping of the root lesion nematode (Pratylenchus neglectus) resistance gene Rlnn1 in wheat. Theor Appl Genet 104:874–879
Wuyts N, de Waele D, Swennen R (2006a) Activity of phenylalanine ammonia-lyase, peroxidase and polyphenol oxidase in roots of banana (Musa acuminata AAA, cvs Grande Naine and Yangambi km5) before and after infection with Radopholus similis. Nematology 8:201–209
Wuyts N, Maung ZTZ, Swennen R, De Waele D (2006b) Banana rhizodeposition: characterization of root border cell production and effects on chemotaxis and motility of the parasitic nematode Radopholus similis. Plant and Soil 283:217–228
Yan G, Smiley RW, Okubara PA (2012) Detection and quantification of Pratylenchus thornei in DNA extracted from soil using real-time PCR. Phytopathology 102:14–22
Zhao X, Schmitt M, Hawes MC (2000) Species-dependent effects of border cell and root tip exudates on nematode behavior. Phytopathology 90(11):1239–1245
Zhu S, Tang S, Tang Q, Liu T (2014) Genome-wide transcriptional changes of ramie (Boehmeria nivea L. Gaud) in response to root-lesion nematode infection. Gene 552:67–74
Zipfel C (2009) Early molecular events in PAMP-triggered immunity. Curr Opin Plant Biol 12:414–420
Zwart RS, Thompson JP, Godwin ID (2005) Identification of quantitative trait loci for resistance to two species of root-lesion nematode (Pratylenchus thornei and P. neglectus) in wheat. Aust J Agric Res 56:345–352
Acknowledgments
We thank Murdoch University (MJ, J F-N) and the Australian Government Endeavour Scholarship scheme (SI) for financial support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Jones, M.G.K., Iqbal, S., Fosu-Nyarko, J. (2016). Belowground Defence Strategies Against Migratory Nematodes. In: Vos, C., Kazan, K. (eds) Belowground Defence Strategies in Plants. Signaling and Communication in Plants. Springer, Cham. https://doi.org/10.1007/978-3-319-42319-7_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-42319-7_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-42317-3
Online ISBN: 978-3-319-42319-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)