Abstract
Tocotrienol is a member of vitamin E family and is well-known for its antioxidant and anti-inflammatory properties. It is also a suppressor of mevalonate pathway responsible for cholesterol and prenylated protein synthesis. This review aimed to discuss the health beneficial effects of tocotrienol, specifically in preventing or treating hyperlipidaemia, diabetes mellitus, osteoporosis and cancer with respect to these properties. Evidence from in vitro, in vivo and human studies has been examined. It is revealed that tocotrienol shows promising effects in preventing or treating the health conditions previously mentioned in in vivo and in vitro models. In some cases, alpha-tocopherol attenuates the biological activity of tocotrienol. Except for its cholesterol-lowering effects, data on the health-promoting effects of tocotrienol in human are limited. As a conclusion, the encouraging results on the health beneficial effects of tocotrienol should motivate researchers to explore its potential use in human.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Tocotrienol
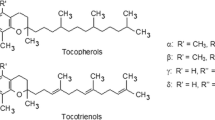
Tocotrienol and tocopherol are collectively known as tocochromanols, or commonly as vitamin E. They share a similar chemical structure, consisting of a chromanol ring and a long carbon tail. The difference between tocotrienol and tocopherol is that the carbon tail of tocotrienol contains three double bonds (an unsaturated farnesyl tail), whereas the carbon tail of tocopherol contains only single bonds (a saturated phytyl tail). The difference in the long carbon tail dictates the difference in biological activity between these two compounds. Similar to tocopherol, tocotrienol can be further divided into 4 isomers (alpha, beta, gamma and delta) based on the position of the methyl side chain on the chromanol ring. Beta- and gamma-tocotrienols are structural isomers and possess the same number of methyl group on the chromanol ring. Alpha-tocotrienol has an additional methyl group whereas delta-tocotrienol has one less methyl group on the chromanol ring compared to beta- and gamma-tocotrienol [6, 14] (Fig. 5.1).
Tocotrienol and tocopherol isomers are found together in a wide variety of plants. Tocotrienol is more commonly found in the fruit and seed. Some of the natural sources that yield the highest amount of tocotrienol include seeds of Bixa orellana (achiote, annatto) (1.53 mg/g dry weight), Zea mays (maize) (1.42 mg/g dry weight), Garcinia mangostana (purple mangosteen) (1.33 mg/g dry weight) and oil of Elaeis guineensis (oil palm) (1.08 mg/g dry weight). Latex of Hevea brasiliensis (rubber tree) (2.59 mg/g dry weight) also contains high amount of tocotrienol, but this is not found in other latex-producing plants (summarized by Müller et al. [98]). Usually alpha-tocopherol is found together with tocotrienol isomers in varying proportions in natural sources. In palm oil, the proportion of alpha-tocopherol is around 30 % of the total vitamin E content [111]. In rice bran, the average percentage of tocotrienol with respect to total vitamin E content is 61 % [156]. Vitamin E derived from the seed of Bixa orellana contains 10 % gamma-tocotrienol and 90 % delta-tocotrienol with no traceable amount of alpha-tocopherol [42]. Several studies on the tocotrienol content of common food have been performed [31, 157]. The tocotrienol intake has been found to be very low (1.9–2.1 mg/person/day) compared to alpha-tocopherol (8–10 mg/day/person) in a Japanese study. This amount is insufficient compared to the doses used in experimental studies to achieve the health-promoting benefits of tocotrienol. Currently, the recommended dietary allowance (RDA) for vitamin E is 15 mg (22.4 IU) for male and female aged 14 years and above [61]. This estimation is based on alpha-tocopherol because it is the only fraction retained in the blood. The RDA for tocotrienol has not been established.
The absorption of tocotrienol is increased with fat-rich food because it is a lipophilic compound [185]. Tocotrienol and tocopherol are absorbed in the lumen of the small intestine, whereby they enter the enterocytes via passive diffusion, and secreted into the lymphatics [43]. They are transported in the form of chylomicron. The release of vitamin E isomers from the liver to the blood stream is regulated by alpha-tocopherol transporter protein. The transfer protein has the highest affinity towards alpha-tocopherol (100 % binding affinity towards RRR-alpha-tocopherol) and lower binding affinity towards other vitamin E isomers (12 % towards alpha-tocotrienol, 38 % towards beta-tocopherol, 9 % towards gamma-tocopherol and 2 % towards delta-tocopherol) [53]. Tocotrienol is distributed by the bloodstream to various tissues. Significant accumulation of tocotrienol can be found in the adipose and skin tissue, as demonstrated by rodent experiments [72, 147]. Similar to tocopherols, tocotrienols are also metabolised and excreted in the form of carboxyethyl-hydroxychromans [81].
Tocotrienol features a variety of biological activities, which enable it to be used as a candidate agent to prevent chronic diseases. The prominent antioxidant and anti-inflammatory properties of tocotrienol will be discussed in the following sections. Tocotrienol is also a known suppressor of the mevalonate pathway involved in cholesterol synthesis, bone metabolism and carcinogenesis. This will be discussed in the respective sections.
5.2 Antioxidant Effects of Tocotrienol
Oxidants such as reactive oxygen species (ROS) and reactive nitrogen species (NOS) are essential for defence against infectious agents, for cell signalling and for redox homeostasis [170]. They are balanced by endogenous and exogenous antioxidants in our body, such as antioxidant enzymes, vitamins, thiols and glutathione. Oxidative stress occurs when the production of oxidants overwhelms the production of antioxidants in our body [152, 153]. Free radical species are capable of damaging macromolecules such as carbohydrate, protein and DNA in the body and generate more free radicals, thereby forming a vicious cycle [48]. Many adverse health conditions, such as neurodegeneration [12], diabetes mellitus [9], cardiovascular disease [82] and osteoporosis [20], have been linked to oxidative stress. This highlights a possible role of tocotrienol, a strong antioxidant, in preventing these oxidative stress-related diseases.
Both tocotrienol and tocopherol exert their free radical scavenging activity by donating phenolic hydrogen at the sixth position of the chromanol ring [113]. Tocotrienol has been shown to be a superior membrane antioxidant compared to its tocopherol counterpart [161]. This is due to the uniform distribution of tocotrienol in the membrane and its stronger ability to disorder membrane lipids, thus enhancing its recycling efficiency [145]. In studies using lipid peroxidation in microsomes extracted from rat liver, Serbinova et al. showed that the antioxidant effect of alpha-tocotrienol was 40–60 times stronger than alpha-tocopherol [145]. They attributed the superior antioxidant effect of tocotrienol to its higher recycling efficacy from chromanoxyl radicals, uniform distribution in the membrane bilayers and stronger disordering of membrane lipids structure, resulting in a higher efficacy in its interaction with free radicals [145]. Palozza et al. showed that tocotrienol isomers inhibited lipid peroxidation, ROS production and heat shock protein expression in rat liver microsomal membrane and in RAT-1 immortalized fibroblasts challenged with free radicals [121]. The effectiveness of delta-tocotrienol was found to be greater than gamma-tocotrienol, and similar between gamma- and alpha-tocotrienol [121]. It was proposed that decreased methylation of the chromanol ring, as in delta-tocotrienol, allowed the molecule to be better incorporated into cell membranes [121]. Kamat et al. showed that palm tocotrienol significantly reduced the lipid and protein peroxidation products in the microsomal extracts of the brain and liver from rats [66, 67]. Among the individual isomers, they identified that gamma-tocotrienol had the highest antioxidant efficacy compared to delta- and alpha-tocotrienol [66, 67].
The antioxidant properties of tocotrienol were also demonstrated in cell culture studies. Tan et al. showed that alpha-tocotrienol prevented the decrease of glutathione and mitochondrial depolarization induced by hydrogen peroxide in hepatocytes [166]. The increase of cellular ROS and malondialdehyde, a lipid peroxidation product, induced by hydrogen peroxide and paracetamol in hepatocytes were also suppressed by alpha-tocotrienol. Nizar et al. showed that gamma-tocotrienol prevented the apoptosis of primary osteoblast induced by hydrogen peroxide [115]. In a subsequent experiment, they showed that this was achieved by the preservation of cellular antioxidant defence system, such as superoxide dismutase, catalase and glutathione peroxidase, and the reduction of lipid peroxidation product in the treated cells [1].
Tocotrienol also exhibited its antioxidant effect in animal models. Nesaretnam et al. demonstrated that long-term feeding of rats with diet containing palm oil rich in tocotrienol significantly reduced the peroxidation potential of hepatic mitochondria and microsomes compared to corn oil control [106]. The initial rate of lipid peroxidation was also found to be slower in the palm oil supplemented group compared to the corn oil control [106]. Lee et al. showed that rats supplemented with tocotrienol-rich fraction at 25 or 50 mg/kg body weight for 28 days and forced to undergo a swimming endurance test showed higher liver superoxide dismutase, catalase and glutathione peroxidase activity but lower liver lipid peroxidation and reduced liver and muscle protein carbonyl levels compared to the vehicle and alpha-tocopherol (25 mg/kg body weight) groups [79]. Supplementation of alpha-tocotrienol (0.04 % weight diet) for one month in rats fed with vitamin E-deficient diet reduced the level of hydroxyoctadecadienoic acid and isoprostane, both markers of lipid peroxidation, in plasma and various organs of the rats [186].
The limited examples presented above showcased the antioxidant potential of tocotrienol ex vivo and in vivo. The role of tocotrienol in suppressing oxidative stress in relation to specific diseases will be discussed separately in the later sections.
5.3 Anti-inflammatory Effects of Tocotrienol
Inflammation has been implicated in the pathogenesis of various diseases, such as cardiovascular disease [80], diabetes mellitus [35], rheumatoid arthritis [30] and osteoporosis [99]. Prostaglandin and leukotriene generated from arachidonic acid are important mediators of the inflammatory process. Prostaglandin E2 (PGE2) synthesized by cyclooxygenase (COX) 1 and 2 are important in activating cytokine formation, apart from eliciting pain and fever. Leukotriene B4 is another important chemotactic agent synthesized by 5-lipoxygenase (5-LOX). Nuclear factor kappa-B, JAK–STAT6/3 (signal transducer and activator of transcription 6/3) and CCAAT-enhancer binding protein β (C/EBPβ) are transcription factors vital in inducing the expression of proinflammatory cytokines (reviewed by Jiang [63]).
Wu et al. showed that palm tocotrienol-rich fraction inhibited the release of nitric oxide and PGE2 in lipopolysaccharide (LPS)-treated human monocytic cells [181]. This was achieved by the suppression of inducible nitric oxide synthase and COX-2 (but not COX-1) expression by tocotrienol-rich fraction [181]. Concurrently, the production of interleukin-4, interleukin-8 and tumor necrosis factor-alpha was suppressed by tocotrienol treatment [181]. Using LPS-treated murine peritoneal macrophages, Ng and Ko demonstrated that tocotrienol-rich fraction inhibited the production of nitric oxide, PGE2 and proinflammatory cytokines, such as tumor necrosis factor-alpha, interferon-gamma, interleukin-1 beta and interleukin-6 in these cells [110]. This contributed to the suppressed protein expression of inducible nitric oxide synthase, COX (but not COX-1) and nuclear factor kappa-B [110]. They further showed that the anti-inflammatory effects of tocotrienol-rich fraction were better than alpha-tocopherol and alpha-tocopheryl acetate [110]. Yam et al. compared the anti-inflammatory effects between alpha-tocopherol, tocotrienol-rich fraction and individual tocotrienol isomers (alpha-, gamma- and delta-tocotrienol) in LPS-treated RAW 264.7 murine macrophages [183]. Yam et al. found that tocotrienol-rich fraction and all three tocotrienol isomers inhibited interleukin-6 and nitric oxide production [183]. Only alpha-tocotrienol could suppress the production of tumor necrosis factor-alpha. Tocotrienol-rich fraction, alpha- and delta-tocotrienol lowered the production of PGE2 [183]. The gene expression of COX-2 was downregulated by tocotrienol-rich fraction and individual isomers but not by alpha-tocopherol [183]. Wang and Jiang showed that gamma-tocotrienol inhibited LPS-induced interleukin-6 production in murine RAW267.4 macrophages by blocking the activation of nuclear factor kappa-B [177]. It also suppressed the LPS induced upregulation of C/EBPβ needed for IL-6 production and the expression of its target gene, granulocyte-colony stimulating factor [177]. These effects could be replicated in bone marrow derived macrophages [177]. Wang et al. further showed that gamma-tocotrienol blocked the activation of nuclear factor kappa-B by upregulating its inhibitor, A20, via modulating the synthesis of sphingolipids involved in inflammatory response [178].
In an vivo study, Qureshi et al. showed that 4-week consumption of diet containing tocotrienol derived from annatto seeds (100 ppm) in aged and young mice inhibited the inflammation induced by LPS alone or in combination with interferon-gamma or interferon-beta [133]. This was evidenced by a reduced nitric oxide and tumour necrosis factor-alpha level in the treated group [133]. This was achieved by suppression of genes related to proinflammatory cytokines, such as interleukin-1 beta, interleukin-1 alpha, interleukin-1 RA, interleukin-6, interleukin-12, COX-2, tumor necrosis factor-alpha etc. [133]. In another experiment, Qureshi et al. compared the anti-inflammatory effects of alpha-, gamma- and delta-tocotrienol in LPS-treated BALB/c mice [129]. A dose-dependent decrease of tumor necrosis factor-alpha synthesis was observed in all tocotrienol-treated groups [129]. The inhibition was strongest in the delta-tocotrienol group [129]. Using peritoneal macrophages derived from these mice, it was shown that delta-tocotrienol could suppress the LPS-induced gene expression of tumor necrosis factor-alpha, interleukin-1 beta, interleukin-6 and inducible nitric oxide synthase [129]. Heng et al. demonstrated that supplementation of tocotrienol mixture 400 mg daily for 16 weeks caused a significant reduction of interleukin-1 and tumor necrosis factor-alpha in a group of subjects (aged 20–60 years) with metabolic syndrome [50]. Supplementation of tocotrienol 15 mg daily for 4 weeks significantly decreased the high-sensitivity C-reactive protein in a group of patients with type-2 diabetes [47].
The role of tocotrienol in suppressing inflammation in individual diseases will be discussed in detail in later sections.
5.4 Tocotrienol as a Hypocholesterolemic Agent
The mevalonate pathway is responsible for the synthesis of cholesterol and other isoprenoids. The determining step in this multi-cascade pathway is the synthesis of 3-hydroxy-3-methyglutaryl-CoA (HMG-CoA) from acetyl-CoA via the enzyme HMG-CoA reductase (HMGR). The expression of HMGR is in turn regulated by sterol regulatory element-binding proteins (SREBPs), whereby the absence of sterol isoprenoids in the cells upregulates its expression (reviewed by Buhaescu and Izzedine [17]). Cholesterol is carried in lipoproteins with specific apolipoproteins which direct the load to specific tissues. Excretion of cholesterol occurs in the form of bile acid. The rate-determining enzyme in this process is cholesterol-7-alpha-hydroxylase [65]. Hypercholesterolemia is an important contributing factor to cardiovascular disease and coronary heart disease [159].
Tocotrienol is known as an inhibitor of the mevalonate pathway. The structure of tocotrienol with its three double bonds is similar to farnesyl, the compound preceding the formation of squalene in cholesterol synthesis [122]. Tocotrienol promotes the formation of farnesol from farnesyl, thus reducing the formation of squalene [122]. Gamma- and delta-tocotrienol have been shown to regulate HMGR in different ways [155]. Delta-tocotrienol stimulates ubiquitination and degradation of HMGR and blocks processing of SREBPs, while gamma-tocotrienol is more selective in enhancing HMGR degradation than blocking SREBP processing [155]. In other studies, gamma-tocotrienol has been shown to stimulate apolipoprotein B degradation by decreasing its translocation into the endoplasmic reticulum lumen [168]. This also causes a reduction in the number of apolipoprotein B in lipoprotein particles [168]. Gamma- and delta-tocotrienol have also been demonstrated to downregulate the expression of genes involved in lipid homeostasis, such as DGAT2. APOB100, SREBP1/2 and HMGR [190]. These tocotrienols also enhance efflux of low density lipoprotein (LDL) cholesterol through increasing LDL receptor expression [190]. Gamma-tocotrienol has been shown to decrease triglyceride (TG) level in liver cells by decreasing the expression of fatty acid synthase, SERBP1 and increasing the expression of carnitine palmitoyl transferase 1A [19] (Fig. 5.2).
The effects of tocotrienol on lipid profile have been tested in several animal models of dyslipidaemia. Different species of animals have been used, including chicken, swine and rodents (rats, hamsters, guinea pigs). Qureshi et al. found that chicken supplemented with delta-tocotrienol (50 ppm for 4 weeks) showed reduced level of total and LDL cholesterol [130]. It also suppressed the lipid elevating effects of dexamethasone and potentiated the TG-lowering effects of riboflavin [130]. Using chickens fed with different dosage (50–2000 ppm) of vitamin E isomers and mixtures, Yu et al. showed that gamma- and delta-tocotrienol reduced the level of total and LDL cholesterol most significantly, followed by tocotrienol-rich fraction and alpha-tocotrienol [188]. Alpha-tocopherol did not exhibit lipid-lowering effects in their experiment [188]. In another experiment, Qureshi and Peterson demonstrated that a combination of tocotrienol-rich fraction (50 ppm) and lovastatin (50 ppm) for 4 weeks suppressed HMGR activity more effectively than lovastatin alone in chickens [125]. The combination was also more effective in reducing serum total and LDL cholesterol, apolipoprotein B, TG, thromboxane B2 and platelet factor 4 than individual treatments [125]. Qureshi et al. also showed that alpha-tocopherol attenuated the inhibition of HMGR by gamma-tocotrienol in chickens [124]. They concluded that the effective preparation of tocotrienol mixture to maximize its hypocholesterolemic effects should contain 15–20 % alpha-tocopherol and 60 % gamma- or delta-tocotrienol [124]. In an experiment using genetically hypercholesterolemic swines, Qureshi et al. showed that 6 weeks treatment of 50 mg tocotrienol-rich fraction derived from rice bran, gamma-tocotrienol, desmethyl (d-P21-T3) or didesmethyl (d-P25-T3) tocotrienol individually could cause significant reduction in serum total and LDL cholesterol, TG, apolipoprotein B, platelet factor 4, thromboxane B2, glucose and glucagon [126]. These tocotrienols also lowered HMGR activity but did not affect cholesterol-7-alpha-hydroxylase activity [126]. After termination of the treatment, the cholesterol-lowering effects of tocotrienols persisted for 10 weeks [126]. In another study using palm tocotrienol-rich fraction (50 mg/g diet for 42 days), genetically hypercholesterolemic swine responded to the treatment by showing a significant decrease in serum total and LDL cholesterol, apolipoprotein B, thromboxane B2 and platelet factor 4 [127]. However, the effects on normal swine were limited to reduction of total cholesterol and apolipoprotein B [127]. The activity of HMGR in adipose tissue was reduced with treatment in both normal and hypercholesterolemic swine [127].
In experiment using rats, hamsters or genuine pigs, tocotrienol was shown to prevent diet or chemical-induced hyperlipidaemia. Burdeos et al. demonstrated that F344 rats on high fat diet receiving 5 or 10 mg rice bran tocotrienol per day for 3 weeks showed a reduction in TG and phospholipid hydroperoxides (oxidative stress marker) in the liver and plasma [18]. This reduction could be due to suppressed production and accumulation of TG by the hepatocytes [18]. However, the cholesterol level did not change in these rats [18]. In a similar experiment by Watkins et al., rats were fed with an atherogenic diet for 6 weeks and the treatment groups received 50 mg gamma-tocotrienol and 500 mg alpha-tocopherol per kg diet [179]. The treatment group showed lower plasma cholesterol, LDL, very–low-density lipoprotein (VLDL), TG, malondialdehyde and fatty acid hydroperoxides [179]. Minhajuddin et al. demonstrated that rats on a 3-week atherogenic diet showed significant reduction in TG, total and LDL cholesterol, malondialdehyde and conjugated dienes after supplementation with tocotrienol-rich fraction (8–48 mg/kg) for 1 week [91]. The activity of HMGR was suppressed with atherogenic diet, probably due to physiological feedback mechanism [91]. Supplementation of tocotrienol-rich fraction led to further reduction in the activity of HMGR [91].
Using a chemically induced hypercholesterolemic model, Iqbal et al. showed that supplementation of rice bran tocotrienol-rich fraction (10 mg/kg/day) significantly suppressed the increase in plasma total and LDL cholesterol in rats administered with 7,12-dimethylbenz[alpha]anthracene [62]. It also suppressed the activity and protein mass of hepatic HMGR [62]. Salman Khan et al. demonstrated that Tocomin, a palm tocotrienol mixture, reduced the level of plasma lipoproteins, cholesterol, apolipoprotein B, small dense LDL and LDL in inflammation-induced hypercholesterolemia in Syrian hamsters [142]. In the same experiment using computational modelling, they showed that the inhibition of HMGR was greater with delta-tocotrienol, followed by gamma-, beta- and alpha-tocotrienol [142]. Raederstorff et al. compared the hypocholesterolemic effects of pure gamma-tocotrienol and tocotrienol mixture in hamsters [134]. They found that both gamma-tocotrienol (at 58 and 263 mg/kg) and tocotrienol mixture (at 263 mg/kg) reduced plasma total and LDL cholesterol but TG and high density lipoprotein (HDL) cholesterol were not affected [134]. Thus, the hypocholesterolemic effect of pure gamma-tocotrienol was greater than the tocotrienol mixture [134]. Khor and Ng showed that presence of alpha-tocopherol might attenuate the hypocholesterolemic effect of tocotrienol [74]. In male hamsters fed with diet containing low-dose alpha-tocopherol (30 ppm), the activity of HMGR was inhibited [74]. However, diet containing high-dose alpha-tocopherol (81 ppm) significantly stimulated the activity of HMGR [74]. On the other hand, in guinea pigs treated with 10 mg tocotrienol (i.p.), showed significant inhibition of HMGR activity but mixture of 5 mg alpha-tocopherol and 10 mg tocotrienol caused less inhibition [74]. This observation in turn validated the experiment of Qureshi et al. using chickens [124]. In a further experiment using guinea pigs injected with palm olein triglycerides containing either tocotrienol or alpha-tocopherol, Khor et al. indicated that tocotrienol (1–5 mg) significantly reduced the activity of liver microsomal HMGR and cholesterol-7-alpha-hydroxylase [73]. The inhibition by alpha-tocopherol was less effective [73]. Furthermore, the inhibitory effect of alpha-tocopherol was lost at 5 mg [73]. At a higher dose (50 mg), alpha-tocopherol significantly elevated the activity of HMGR and reduced the inhibitory effects of tocotrienol [73].
Effects of tocotrienol supplementation on lipid profile in humans were heterogeneous. This could be due to the differences in the composition of the tocotrienol mixture used, population studied, diet of the subjects, etc. In an earlier study using palm vitamin E (containing 18 mg tocopherols and 42 mg tocotrienols), Tan et al. showed that supplementation for 30 days lowered serum total and LDL cholesterol but the TG and HDL cholesterol were not affected [167]. However, this is not a randomized controlled trial and the number of subjects was small. Qureshi et al. showed that palm vitamin E at 200 mg caused a reduction in serum total and LDL cholesterol, apolipoprotein B, thromboxane, platelet factor 4 and glucose in subjects during the four week-treatment [128]. A separate hypocholesterolemic group given 200 mg gamma-tocotrienol showed greater reduction in total cholesterol compared to palm vitamin E group [128]. Daud et al. demonstrated that supplementation of tocotrienol for 16 weeks (180 mg tocotrienols with 40 mg tocopherols) decreased the normalized plasma TG and increased HDL in patients undergoing chronic hemodialysis [36]. These changes might be associated with higher plasma apolipoprotein and lower cholesteryl-ester transfer protein activity [36]. It should be noted that the results of this study was not adjusted for medication used by the subjects. Chin et al. showed that when elderly (>50 years) and young (<50 years) subjects were supplemented with tocotrienol-rich fraction (160 mg/day, containing 74 % tocotrienols and 26 % tocopherols) for 6 months, the elderly subjects responded better by showing an increase in HDL cholesterol compared to placebo group [29]. The products of oxidation, such as protein carbonyl and advance-glycation end products, were also reduced only in the elderly subjects [29]. Baliarsingh et al. supplemented 19 type 2 diabetic subjects with hyperlipidemia with tocotrienol-rich fraction (3 mg/kg body weight) for 60 days and total lipids, total and LDL cholesterol were reduced with treatment [11]. In the study by Qureshi et al., hypercholesterolemic subjects on American Heart Association step 1 diet were supplemented with rice bran tocotrienol-rich fraction at different doses (25–200 mg/kg) [132]. They observed that 100 mg/kg tocotrienol-rich fraction produced maximum decrease in total and LDL cholesterol, apolipoprotein B and TG compared to baseline [132]. In another study, Qureshi demonstrated that hypercholesterolemic subjects on American Heart Society step 1 diet supplemented with rice bran tocotrienol-rich fraction and lovastatin in combination for 25 weeks showed more significant reduction than individual treatments [131]. The combination of alpha-tocopherol and lovastatin did not produce similar changes [131].
Tomeo et al. supplemented subjects presented with hyperlipidemia, cerebrovascular disease and carotid stenosis with 300 mg palm vitamin E (16 mg alpha-tocopherol and 40 mg tocotrienols in 240 mg palm olein) for 18 months [169]. Apparent carotid atherosclerotic regression was found in supplemented subjects while some controls showed progression [169]. Malondialdehyde was decreased in the treatment group but was increased in the placebo group [169]. However, no changes in total and LDL cholesterol, TG and HDL were found in both groups [169]. In men with mildly elevated serum lipid level, supplementation of tocotrienol (4 capsules daily for 6 weeks, each containing 35 mg tocotrienols and 20 mg alpha-tocopherol) produced no significant effects on serum LDL and HDL cholesterol, TG, lipoprotein a, lipid peroxide, platelet aggregation velocity and maximum aggregation, and thromboxane B2 formation compared to men receiving 20 mg alpha-tocopherol only [90]. In the study of O’Byrne et al., subjects followed a low-fat diet for 4 weeks, then were randomized into treatment groups receiving placebo, alpha-, gamma-, delta-tocotrienol acetate supplements (250 mg/d) for 8 weeks while were still maintained on the low-fat diet [120]. It was found that alpha-tocotrienol increased the in vitro LDL oxidation resistance and decreased its rate of oxidation [120]. However, tocotrienol treatments did not affect total and LDL cholesterol, and apolipoprotein B level [120]. In another study, hypercholesterolemic men and women were randomized to receive three different brands of tocotrienol from palm or rice bran, or the placebo. The subjects were put on the American Heart Association Step 1 diet, 21 days before and throughout the trial period [101]. There were no significant changes in lipid profile between placebo and treatment after 28 days [101]. Rasool et al. observed that in healthy male subjects taking placebo or tocotrienol-rich vitamin E at 80, 160 and 320 mg/day for 2 months, arterial compliance, plasma total antioxidant status, serum total and LDL cholesterol were not different among the groups [136].
5.5 Tocotrienol as Antidiabetic Agent
Diabetes mellitus represents a group of chronic metabolic conditions characterized by increased blood glucose. Type 1 diabetes results from the destruction of beta-cell in the pancreas due to autoimmunity, thus causing cessation in insulin production. Type 2 diabetes is caused by abnormal resistance of the body to the action of insulin, and the insufficiency of insulin production to overcome this resistance. It is estimated that 5–10 % of the total diabetes cases belong to type 1 and 90–95 % belong to type 2. Diabetes can affect many organ systems and lead to serious complications such as damage to the nervous, renal, cardiovascular system and the eyes [38]. Oxidative stress and inflammation are involved in the development and exacerbation of diabetic complications [39].
The antidiabetic effects of tocotrienol have been explored mainly in animal studies. Diabetes is mostly induced by streptozotocin (STZ), a chemical that can cause cellular ATP reduction, inhibition of insulin production, DNA alkylation and death of pancreatic beta cells [75]. Tocotrienol has been shown to improve the glycemic status and diabetic complications in many instances. Wan Nazaimoon et al. showed that a diet enriched with tocotrienol-rich fraction (1 g/kg diet) significantly reduced the blood glucose level and glycated haemoglobin of STZ-induced diabetic male rats [176]. However, serum-advanced glycation end product and malondialdehyde were not affected [176]. Matough et al. showed that supplementation of tocotrienol-rich fraction at 200 mg/kg body weight for 4 weeks in STZ-induced diabetic rats reduced the plasma glucose level [85]. The supplementation also increased the erythrocytic superoxide dismutase and glutathione levels but decreased the level of reduced glutathione and malondialdehyde [85]. Comet assays revealed reduced DNA damage in the supplemented group [85].
Tocotrienol was shown to prevent diabetic nephropathy in several animal models. Kuhad and Chopra showed that tocotrienol mixture at 25, 50 and 100 mg/kg for 8 weeks dose-dependently reduced diabetic proteinuria, polyuria and increased serum creatinine and blood urea nitrogen levels and their clearance [77]. The improvement in renal profile was accompanied by a dose-dependent decrease in lipid peroxidation product and an increase in non-protein thiol, superoxide dismutase and catalase activity in the kidney [77]. Nitrosative stress in the kidney was also decreased with tocotrienol supplementation as evidenced by a lower nitric oxide level [77]. Tocotrienol was shown to reduce the level of tumor necrosis factor-alpha associated with renal hypertrophy and hyperfunction, transforming growth factor-beta associated with renal fibrosis and the expression of nuclear factor kappa-b p65 subunit indicative of inflammation in the nuclear fraction and apoptosis in the kidney [77]. Siddiqui et al. compared the hypoglycemic effects of tocotrienol-rich fraction derived from palm oil and rice bran oil in two separate experiments [151]. Diabetic male rats were treated with either 200 mg/kg body weight palm oil tocotrienol or rice bran tocotrienol [151]. Both treatments were found to reduce the fasting blood glucose and percentage of glycosylated haemoglobin [151]. Improvements in renal profile, indicated by reductions in serum creatinine and urine protein level, were also seen in both treatment groups [151]. Histopathological examination revealed that both tocotrienol-rich mixtures prevented glomerular hypertrophy, mesangial expansion, tubular atrophy, tubular basement membrane thickening and proteinaceous cast formation in the lumen [151]. The improvement could be attributed to the antioxidative properties of tocotrienol, as evidenced by the decrease in lipid peroxidation products and the increase in superoxide dismutase and catalase activity in the kidney with tocotrienol treatment [151]. Overall, efficacy of tocotrienol from palm was higher compared to rice bran [151]. In the second experiment, Siddiqui et al. fed male Wistar rats with high fat diet for 5 weeks, then switched them to standard diet and induced diabetes in them with STZ at the same time [150]. The hypoglycemic effects between 16-week supplementation with palm tocotrienol at 200 mg/kg body weight and rice bran tocotrienol 400 mg/kg body weight were compared [150]. In agreement with their previous findings, palm and rice bran tocotrienol prevented the rise of blood glucose level as early as week 6 [150]. Percentage of glycosylated hemoglobin was also reduced by both treatments [150]. They also suppressed the increase in TG, total, LDL and VLDL cholesterol and elevated the HDL cholesterol level [150]. Improvement in renal profile, indicated by decreased urinary creatinine and blood urea nitrogen and increased creatinine clearance, was seen with both treatments [150]. These positive changes might be related to improved oxidative status in the kidney (reduced malondialdehyde level, increased superoxide dismutase, catalase, glutathione peroxidase and reductase activities) [150]. In addition, the protein expression of collagen type IV, transforming growth factor-beta and fibronectin related to renal fibrosis were reduced significantly by palm tocotrienol [150]. This was accompanied by an improvement in renal histopathology in both treatment groups [150]. Palm tocotrienol, even though at a lower dosage, exhibited higher efficacy in preventing diabetic nephropathy than rice bran tocotrienol [150].
The presence of cardiovascular risk factors, such as hypertension and hypercholesterolemia, in diabetic patients lead to poorer outcomes compared to those without [38]. In fact, the risk for cardiovascular death is nearly doubled in diabetic patients compared with the non-diabetic [21]. As described in the earlier section, tocotrienol is an effective hypocholesterolemic agent and its effectiveness has been proven in chemical and diet-induced hypercholesterolemic models. Several animal studies also demonstrated that the hypocholesterolemic effect of tocotrienol persisted in diabetic model, accompanied by other cardio and vascular protective effects. Budin et al. fed STZ-induced diabetic rats with palm tocotrienol-rich fraction 200 mg/kg body weight for 8 weeks and observed positive changes in risk factors for cardiovascular disease [16]. Adverse changes in fasting blood glucose, HbA1c and lipid profile were attenuated in the tocotrienol-treated group [16]. Besides, malondialdehyde-4-hydroxynonenal in aorta and plasma, and DNA damage in lymphocytes were reduced and plasma superoxide dismutase activity was increased with tocotrienol treatment [16]. Tocotrienol also reduced the degenerative changes in aortic wall by reducing the proliferation and degeneration of vascular smooth muscle cells and defects in the aortic walls [16]. Using a diet-induced diabetic rat model, Patel et al. showed that co-supplementation of alpha-lipoic acid (1.6 g/kg diet) and palm tocotrienol-rich fraction (0.84 g/kg diet) for 8 weeks could prevent and reverse the increase in plasma glucose, systolic blood pressure, interstitial collagen deposition in the left ventricle, diastolic stiffness and plasma malondialdehyde [123].
Tocotrienol was able to prevent diabetic-associated neuropathy. Kuhad and Chopra et al. showed that tocotrienol mixture at 25, 50 and 100 mg/kg for 10 weeks could prevent the increase in plasma glucose in STZ-induced diabetic rats [76]. Transfer latency and percentage of time spend in the target quadrant in a water maze were decreased in all tocotrienol treated groups compared to the control, indicating better memory and learning performance [76]. This was attributed to decreased oxidative stress, indicated by a reduction in lipid peroxide level and an increase in non-protein thiols, superoxide dismutase and catalase activity, and decreased nitrosative stress indicated by reduced nitric oxide level in the cerebral cortex and hippocampus [76]. Inflammation was also prevented by tocotrienol supplementation, as evidenced by decreased protein expression of interleukin-1b and p65 subunit of nuclear factor kappa-B in the brain [76]. The reduction in both oxidative stress and inflammation led to reduced apoptosis of the neurons [76]. In the subsequent experiment, Kuhad and Chopra supplemented STZ-induced diabetic rats with 25, 50 and 100 mg/kg tocotrienol mixture and assessed the effects on hyperalgesia [78]. Tocotrienol mixture prevented the reduction in nociceptive threshold in the diabetic rats, indicated by increased tolerance for thermal and mechanical stimuli, and tactile allodynia [78]. They also found that lipid peroxide and total nitric oxide levels were reduced; while non-protein thiol levels and activities of superoxide dismutase and catalase were increased in the sciatic nerve with tocotrienol treatment [78]. Inflammation was also reduced with tocotrienol treatment, indicated by a reduction in tumor necrosis factor-alpha, transforming growth factor-1 beta and interleukin-1 beta in the sciatic nerve [78]. Apoptosis indicated by caspase 3 expression was also decreased in the sciatic nerve [78].
There are limited studies attempted to validate the hypoglycemic effects of tocotrienol in humans and the results are heterogenous. In a longitudinal study (median follow-up period 10.2 years) assessing the intake of antioxidants and the risk of type 2 diabetes in 29,133 Finnish male smokers aged 50–69 years, Kataja-Tuomola et al. found that all tocotrienol isomers were not associated with the risk of the disease after multiple adjustment. This could be contributed to the low levels of tocotrienols in the subjects [71]. In the study of Baliarsingh et al., type 2 diabetic subjects supplemented with tocotrienol-rich fraction (3 mg/kg body weight) for 60 days showed no changes in fasting and postprandial plasma sugar, HbA1c and serum creatinine [11]. This could be due to the limited number of subjects in this study (n = 19) and the low dose of tocotrienol. In a study by Haghighat et al., type 2 diabetic subjects taking non-insulin medication were randomized to take 15 mg/day tocotrienol mixture or blank canola oil with meals for 4 weeks [47]. It was observed that urinary microalbumin and serum high-sensitivity C-reactive protein were significantly lowered in the tocotrienol group compared to the placebo, indicating a better renal profile and a reduced risk for cardiovascular disease [47].
5.6 Tocotrienol as Antiosteporotic Agent
Osteoporosis is a metabolic bone disease characterized by low bone mass and deterioration of bone microarchitecture, which subsequently leads to fragility fracture [68]. Despite the high prevalence in women, osteoporosis affects men as well, with female to male ratio of 6:1 [64]. Osteoporosis is a result of the imbalanced bone remodelling process consisting of bone resorption regulated by osteoclast and bone formation regulation by osteoblast. Sex hormone deficiency is the predominant condition underlying the imbalance in bone remodelling, although recent studies have switched the focus to the role of oxidative stress and inflammation [2, 84]. Oxidative stress and inflammation inhibit differentiation of osteoblast (osteoblastogenesis), but promote differentiation of osteoclast (osteoclastogenesis) [2, 10, 171]. Hence, an antioxidant and anti-inflammatory agent like tocotrienol putatively has beneficial effects in preventing bone loss.
The effects of tocotrienol in promoting differentiation of osteoblast and suppressing the differentiation of osteoclast have been demonstrated in cell culture studies. Ha et al. demonstrated that alpha-tocotrienol but not alpha-tocopherol could suppress osteoclastogenesis in an osteoblast-bone marrow co-culture [46]. This was brought about by an inhibition of receptor activator of nuclear factor kappa-b (RANKL) expression in osteoblasts [46]. In osteoclasts, alpha-tocotrienol suppressed the RANKL-mediated differentiation process by inhibiting c-Fos expression via suppression of extracellular signal-regulated kinases and nuclear factor kappa-b activation [46]. Alpha-tocotrienol also prevented the bone resorption activity of mature osteoclasts [46]. Brook et al. compared the suppressive effects of different tocotrienol isomers (alpha, gamma and delta) on human-derived CD14+ cells [15]. It was found that gamma and delta-tocotrienol were effective in suppressing the formation of osteoclast-like cells and the resorbing activity of these cells was significantly inhibited. Gamma-tocotrienol was found to be with less toxic to CD14+ cells, indicating that it is safer compared to delta-tocotrienol [15].
The effectiveness of tocotrienol in preventing bone loss has been demonstrated in several animal models of osteoporosis. The methods of assessing bone health in the animals included densitometry which measures bone mineral density; histomorphometry which measures bone structure, cells and mineralization activity; biomechanical test which measures the stiffness and strength of the bone; bone mineral concentration and serum bone remodelling markers which measure the bone formation and resorption activity (reviewed in [24, 27]).
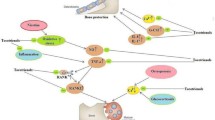
Norazlina supplemented ovariectomized rats with palm vitamin E, which was rich in tocotrienol at either 30 or 60 mg/kg body weight for 8 months and measured the femoral and vertebral bone mineral density [118]. It was found palm vitamin E prevented the decline of bone mineral density in both supplemented groups [118]. Muhammad et al. observed that palm tocotrienol at 60 mg/kg body weight for 8 weeks could improve bone structural indices (increased bone volume and trabecular number, and decreased trabecular separation) in an oestrogen-deficient model of osteoporosis induced by ovariectomy [96]. A decrease in osteoclast number was found in the tocotrienol supplemented group [96]. The effects of alpha-tocopherol at 60 mg/kg body weight were found to be comparable with palm tocotrienol [96]. In a subsequent experiment, Muhammed et al. supplemented the ovariectomized rats with tocotrienol-rich fraction (60 mg/kg for 8 weeks) and found that it improved bone structural indices (increased bone volume and trabecular thickness, and decreased trabecular separation) [97]. Its effects were comparable to calcium (15 mg/kg) and oestrogen treatment (64.5 µg/kg) [97]. Aktifanus et al. demonstrated that tocotrienol-rich fraction at 60 mg/kg body weight for 8 weeks improved bone dynamic indices (decreased single-labelled surface, increased double-labelled surface, mineral apposition rate and bone formation rate) in ovariectomized rats [8]. The effects of tocotrienol were better than oestrogen replacement (64.5 µg/kg body weight) [8]. Using palm tocotrienol at 60 mg/kg body weight for 8 weeks, Soelaiman et al. demonstrated similar findings and its effects were superior to calcium supplementation (1 % calcium in drinking water ad libitum) [154]. Abdul-Majeed et al. supplemented ovariectomized rats with annatto tocotrienol (60 mg/kg body weight for 8 weeks) or in combination with lovastatin (11 mg/kg body weight for 8 weeks) [3, 4]. Annatto tocotrienol alone was found to improve bone structural (increased bone volume, trabecular number and trabecular thickness, decreased trabecular separation) and cellular indices (increased osteoblast number, osteoid surface and osteoid volume, and decreased osteoclast number and eroded surface), bone biomechanical strength (increased load, strain, stress and Young’s modulus) and bone remodelling markers (increased osteocalcin, a bone formation marker and decreased type-1 C-terminal telopeptide crosslink, a bone resorption marker) [3, 4]. The combination of annatto tocotrienol and lovastatin further improved these variables, suggesting an additive or synergistic effect between the two compounds [3, 4]. Deng et al. supplemented ovariectomized mice with 100 mg/kg emulsified gamma-tocotrienol subcutaneously. The supplemented mice showed significant improvements in femoral bone mineral density, bone structural (increased bone volume, trabecular number, trabecular thickness and decreased trabecular separation), dynamic (increased mineral apposition rate and bone formation rate) and cellular indices (increased osteoblast number and decreased osteoclast number) [37]. Elevated gene expression of transcription factors runt-2 and osterix in the bone of supplemented groups suggested increased osteoblast differentiation [37]. This was coupled with increased gene expression of osteoprotegerin and decreased gene expression of RANKL, indicating an inhibition of osteoclast formation [37]. Feeding mevalonate (25 mg/kg body weight) to the supplemented group abolished all the beneficial effects of tocotrienol treatment, suggesting the involvement of mevalonate pathway in the protective action of tocotrienol on bone [37].
Using an orchidectomy-induced testosterone deficient model of osteoporosis, Ima-Nirwana et al. showed that supplementation of palm vitamin E at 30 mg/kg for 8 months prevented the deterioration of femoral and vertebral bone mineral density and calcium level [58]. Other studies also showed that annatto tocotrienol at 60 mg/kg could prevent deterioration of bone cancellous microarchitecture assessed with X-ray microtomography (decreased trabecular separation), bone structural (increased bone volume and trabecular number, decreased trabecular separation), dynamic (decreased single-labelled surface, increased double-labelled surface) and cellular (increased osteoblast number, osteoid surface and osteoid volume, decreased osteoclast number and eroded surface) indices [25, 28]. This was brought about by increased gene expression of alkaline phosphatase, beta-catenin, collagen type 1 alpha 1 and osteopontin, indicating increased osteoblast activity [28]. However, no significant changes in bone resorption genes were observed [28]. Annatto tocotrienol was less efficacious compared to supraphysiological testosterone enanthate injection (7 mg/kg weekly) in the same experiment [28].
Smoking is a non-modifiable risk factor for osteoporosis. In a model of osteoporosis induced by nicotine, Hermizi et al. showed that supplementation of either tocotrienol-rich fraction or gamma-tocotrienol at 60 mg/kg body weight improved bone structural (increased bone volume and trabecular thickness), dynamic (reduced single-labelled surface and increased mineral apposition rate and bone formation rate) and cellular indices (reduced osteoclast and eroded surface) [51]. Abukhadir et al. indicated that these positive changes were brought about by the ability of tocotrienol to reverse nicotine-induced suppression of runt-2, osterix and bone morphogenetic protein-2 [5]. Tocotrienol also inhibited nicotine-induced elevation of interleukin-1 and interleukin-6 [117, 119]. Nazrun et al. demonstrated that palm tocotrienol at 100 mg/kg was able to increase trabecular thickness, bone formation rate, osteoblast number and decrease eroded surface in the osteoporotic animal [7, 105]. This effect was also brought about by the suppression of interleukin-1 and interleukin-6 by tootrienol [7]. The effects of tocotrienol in preventing bone loss due to glucocorticoid treatment were also studied. Ima-Nirwana and Fakhrurazi supplemented palm vitamin E in dexamethasone-treated adrenalectomized male rats and observed no significant changes on bone mineral density and bone calcium level [56]. In a subsequent attempt, supplementation of gamma-tocotrienol at 60 mg/kg body weight for 8 weeks increased the fourth lumbar vertebra bone calcium content in dexamethasone-treated adrenalectomized male rats [59]. Alpha-tocopherol at the same dose did not exert a similar effect [59].
The beneficial effects of tocotrienol were also shown in healthy male rats supplemented with alpha-tocopherol, delta-tocotrienol or gamma-tocotrienol at 60 mg/kg for 8 weeks [89, 149]. Gamma-tocotrienol supplementation improved all bone structural, dynamic and cellular indices and biomechanical strength while alpha-tocopherol and delta-tocotrienol only improved some of the indices, indicating a superior bone-anabolic effect of gamma-tocotrienol over the other vitamin E isomers [89, 149]. Maniam et al. showed that palm tocotrienol at 100 mg/kg for 4 months reduced malondialdehyde and increased glutathione peroxidase activity in the bone of healthy male rats. Alpha-tocopherol at the same dose did not exert similar effects on bone [83].
Despite there are many observational studies on the association between vitamin E and bone health, most studies measured the intake and blood level of alpha-tocopherol. Vitamin E (alpha-tocopherol) was associated with increased bone mineral density or a reduced fracture risk in most studies [26]. However, there has been no report on the effects of tocotrienol on bone health in humans to date. The suggested mechanism of tocotrienol in preventing bone loss is summarized in Fig. 5.3.
5.7 Anticancer Activity
Anticancer activity of vitamin E was discovered and studied extensively since the first experimental evidence of inhibition of tumorigenesis by vitamin E rich palm oil [164]. Anticancer property of tocotrienol was found to be independent of its antioxidant property [32, 162]. Tocotrienol isomer with lower antioxidant properties like delta- and gamma-tocotrienol usually showed greater potency in killing cancerous cells. Based on quantitative structure–activity relationship studies, chromanol structure and phytyl carbon tail were shown to be essential in apoptosis induction [13]. Decreasing methyl groups on the chromanol head increased anti-proliferative potency of tocotrienol (delta-tocotrienol > gamma-tocotrienol > beta-tocotrienol > alpha-tocotrienol) [162]. Shortening the unsaturated phytyl tail of gamma-tocotrienol by one isoprenyl unit also resulted in greater apoptotic activity [13]. In silico analysis suggested that esterification of lysine with gamma-tocotrienol could enhanced its anticancer activity [114]. Tocotrienol metabolites such as carboxyethyl-hydroxychromans were found to be a less effective anti-proliferative agent [34].
Besides that, tumor cell uptake and intracellular accumulation of tocotrienol also determine its anticancer activity. Due to the lipophilic nature of tocotrienols, they are readily transferred between the membranes and accumulated in cells in a time and concentration-dependent manner [187]. Studies have shown that serum physiological tocotrienol level was usually low or absent due to slow absorption, rapid metabolism and lack of a specific carrier protein [164]. Besides that, tocotrienol is highly tissue-dependent and it is only found in a few tissues/organs like the liver and pancreas [52, 55]. Interestingly, in animal models, gamma-tocotrienol and delta-tocotrienol were found to accumulate in tumors [52, 55]. Different tocotrienol isomers possess different cellular accumulation tendency, i.e. delta > gamma > alpha-tocotrienol [52, 87, 88, 116, 141, 144]. This might explain the poor anticancer activity of alpha-tocotrienol.
Tocotrienol and tocotrienol-rich fraction were demonstrated to induce anti-proliferation and apoptosis selectively in several cancerous cell lines originating from the mammary gland [32, 41, 44, 87, 109, 146, 173–175, 189], lung [70, 86, 184], liver [49, 52, 140, 141, 172], prostate [33, 86, 158], melanoma [22, 86], colon [40, 182] and leukemic cells [100]. The inhibitory potency of tocotrienol-rich fraction, tocotrienol isoforms or their succinate synthetic derivatives in different cancerous cells generally follows the order of delta- >gamma- and beta- >alpha-tocotrienol [22, 33, 40, 52, 140, 172]. Studies also demonstrated that tocotrienol possessed little or no adverse effect on normal cell viability or growth in in vitro [49, 70, 87, 88, 141, 158, 184] or animal models [112, 135, 137]. In animal studies, tocotrienols and tocotrienol-rich fraction were also demonstrated to reduce tumor load. Oral administration of 0.05 % tocotrienol-rich vitamin E mixture significantly reduced liver and lung carcinogenesis in C3H/He mice and 4NQO-treated ddY mice model [172]. Gamma-tocotrienol or delta-tocotrienol diet (0.1 %) significantly delayed tumor growth in C3H/HeN mice inoculated subcutaneously with murine hepatoma MH134 cells [52]. There was no apparent adverse effect and insignificant body and tissue weight changes in animals fed with 0.1 % of gamma-tocotrienol or delta-tocotrienol diet [52].
Studies also demonstrated that tocotrienol as a farnesol-mimetic molecule and HMGR inhibitor possessed great potential to produce synergistic anticancer activity with other chemotherapeutic agents [86]. Combination of low concentration of gamma-tocotrienol with several individual statins synergistically inhibited HMGR activity and Rap1A & Rab6 prenylation [174]. Besides that, combination of tocotrienol with flavonoids such as genistein, naringenin, hesperetin, tangeretin, nobiletin, quercetin, apigenin, epigallocatechin gallate or resveratrol demonstrated synergistic activity in inhibiting human breast cancer MCF7 or/and MDA-MB-435 cells proliferation, which was enhanced further with the addition of tamoxifen [45, 54]. The combination of tocotrienol with docetaxel also resulted in higher proportion of apoptotic cells [22]. Combination of tocotrienols with tamoxifen increased the arrest of DNA synthesis and triggered an endoplasmic reticulum (ER)-independent anti-proliferation event in the breast cancer cells [44, 189]. Combination of tocotrienol isomers (delta-tocotrienol and gamma-tocotrienol) was also found to induce cell death synergistically in DU145 and PC3 [33]. In animal studies, gamma-tocotrienol also acted synergistically with lovastatin to reduce tumor load in C57BL/6 mice inoculated with B16 cells [86].
There are limited clinical trials on tocotrienol and cancer conducted to date. Malaysian Palm Oil Board had conducted two clinical trials on the efficacy of tocotrienol in breast cancer patients [107]. The first study was conducted to identify tocopherol and tocotrienol concentrations in malignant and benign adipose tissues of breast cancer patients after a palm oil diet. The results revealed a higher concentration of tocotrienols (alpha-, delta- and gamma-isomers), in the adipose tissues of patients with benign breast lumps compared with that of patients with malignant tumors [107]. The second study was a 5-year double-blind, placebo-controlled pilot trial conducted to compare tamoxifen with or without tocotrienol-rich fraction on early breast cancer [108]. The risk of dying due to breast cancer decreased by 70 % and risk of recurrence decreased by 20 % in patients taking the adjuvant tocotrienol and tamoxifen compared with the patients receiving only tamoxifen [107]. However, there was no significant association between adjuvant tocotrienol therapy with breast cancer survival rate [108].
5.7.1 Tocotrienol-Induced Caspase Activation and Mode of Cell Death
Caspases are constitutively present in cells in an inactive precursor form, the procaspases [164]. In receptor-mediated apoptosis, there is early activation of death receptors (Fas, TNF or TRAIL) which lead to caspase-8 activation. In mitochondria-mediated apoptosis, there are apoptotic signals from damaged DNA or loss of mitochondrial membrane potential, which will lead to activation of caspase-9. Initiator caspases (caspase-8 and -9) activate effector caspases (caspase-3, -6, and -7) which will then execute the cells by cleaving DNA, PARP and other structural proteins [164]. Both death receptor- [33, 140, 141] and mitochondria-mediated [22, 33, 103, 139, 160, 165, 182, 184] pathway of apoptosis induced by tocotrienol-rich fraction, tocotrienol isomers, ether or succinate synthetic derivatives were reported. Tocotrienol-rich fraction and tocotrienol isomer-induced apoptosis are selective and greatly dependent on cell types and treatment conditions. It might involve the upregulation of Bax and tBid protein with the increased of Bax/Bcl-2 ratio [140].
Besides that, gamma-tocotrienol was also found to induce caspase-12 activation in +SA cellular apoptosis [173]. Caspase-12 activation is closely associated with endoplasmic reticulum ER stress-mediated cell death. Further experiments identified an early involvement of PERK/eIF2a/ATF4 phosphorylation and CHOP-dependent TRB3-mediated ER stress response pathway in gamma-tocotrienol-induced +SA cellular apoptosis [173]. Furthermore, gamma-tocotrienol, delta-tocotrienol, gamma-tocotrienol succinate and delta-tocotrienol succinate were also found to trigger caspase-independent cell death in DU145, PC3 and LNCap cells whereby the event of apoptosis could not be abrogated by general caspase inhibitors [33].
5.7.2 Tocotrienol-Induced Upstream Apoptotic Pathway Modulation
The inhibition of HMGR by tocotrienol not only suppresses cholesterol synthesis but also inhibits cell proliferation via prenylation of Ras, RhoA or Rap1A signalling molecules through generation of prenyl intermediates [184]. Farnesyl side chain of tocotrienol is essential for the post-transcriptional suppression of HMGR. Gamma-tocotrienol was shown to downregulate HMGR levels in +SA cells via post-translational protein degradation [173]. However, the gamma-tocotrienol-induced downregulation of HMGR was not able to block Rap1A & Rab6 prenylation and trigger +SA cell death [174]. Delta-tocotrienol-induced growth arrest might be due to HMGR inhibition, causing marked decrease of cyclin D1/cyclin-dependent kinase CDK4 complex but not cyclin E/CDK2 complex, which subsequently reduced the phosphorylation of retinoblastoma RB protein and E2F1 expression [41, 103].
6-O-carboxypropyl-alpha-tocotrienol (alpha-T3E) was more potent compared to alpha-tocotrienol in inducing apoptosis in A549 cells and H28 cells [70, 184]. 6-O-carboxypropyl-alpha-tocotrienol also harbours the similar farnesyl side chain and might possess HMG-CoA reductase inhibitory activity. 6-O-carboxypropyl-alpha-tocotrienol can inhibit RhoA geranyl-geranylation and Ras molecules farnesylation, subsequently blocking MEK, ERK and p38 phosphorylation and lead to cyclin D and Bcl-xL downregulation during G1 arrest and apoptosis [160, 174, 184]. Besides that, 6-O-carboxypropyl-alpha-tocotrienol also inactivated Src kinase via phosphorylation at Tyr527 site and dephosphorylation at Tyr416 site, which decreased the phosphorylated form of EGFR and its activity [70]. Another study revealed that gamma-tocotrienol inhibited PI3k/PDK-1/Akt pathway via suppression of EGFR (ErbB3)-receptor tyrosine phosphorylation [143]. 6-O-carboxypropyl-alpha-tocotrienol also inhibited Stat-3 activation which might be due to Src kinase inactivation [70]. 6-O-carboxypropyl-alpha-tocotrienol was shown to partially inhibit Src kinase. However, this was inadequate to trigger cell death on A549 cells under hypoxia [69]. Survival and invasion capacity of A549 cells under hypoxia were suppressed by 6-O-carboxypropyl-alpha-tocotrienol via inhibition of the Src/HIF-2alpha/PAI-1 and PI3k/PDK-1/Akt signalling pathways [69]. Gamma-tocotrienol was also demonstrated to inhibit PI3k/PDK-1/Akt pathway and subsequently suppressed the FLIP level [163]. Gamma-tocotrienol also induced +SA cells apoptosis via TRB3-mediated ER stress [173]. TRB3 inhibited Akt signalling pathway and this also contributed to the decrease of Akt activity [173].
Combination of gamma-tocotrienol with several individual statins also synergistically induced +SA cells growth arrest and apoptosis which was associated with inactivation of ERK, p38, JNK MAP kinases and Akt kinases [174, 175]. Downstream Stat-3 inactivation leads to similar downregulation of cyclin D1 and Bcl-xL [69]. Therefore, HMG-CoA reductase might be involved in upstream regulation of RhoA/Ras, Src/HIF-2alpha/PAI-1 and PI3k/Akt pathway.
Previous studies demonstrated that tocotrienol-rich fraction, alpha-, gamma- and delta-tocotrienol inhibited proliferation and induced apoptosis in human breast cancer MCF-7, MDA-MB-435 and MDA-MB-231 cells, regardless of their oestrogen receptor status [32, 41, 44, 109, 189]. Delta-tocotrienol was showed to downregulate cyclin B1 and CDK1 expression in MDA-MB-231 cellular growth arrest [41]. Combination of gamma-tocotrienol with either epigallocatechin gallate or resveratrol suppressed MCF-7 cell proliferation via downregulation of cell cycle regulatory proteins E2F, CDK4 and cyclin D1 [54]. In vitro and in silico studies revealed that gamma- and delta-tocotrienol specifically bound and activated estrogen receptor β signalling molecule [32]. Activated estrogen receptor β served as an upstream signal of apoptosis and suppressed ER alpha-mediated cell survival and proliferation [32]. Estrogen receptor β upregulated MIC-1, cathepsin D and EGR-1 mRNAs expression which triggered subsequent caspase-regulated cell death [32].
Epidermal growth factor (EGF)-dependent mitogenesis is associated with the activation of phospholipid-dependent protein kinase C (PKC) and cyclic AMP (cAMP)-dependent proteins [88]. Tocotrienol-rich fraction, alpha-, gamma-, and delta-tocotrienol induced apoptosis and inhibit the proliferation of EGF-induced preneoplastic mammary epithelial CL-S1 cells, neoplastic mammary epithelial-SA cells and +SA cells [87, 146, 174, 175]. Tocotrienol-rich fraction also reduced EGFR-independent and cAMP-dependent EGF-induced G-protein mitogenic signalling in CL-S1 cells [162]. Combination of tocotrienol-rich fraction with G-protein stimulants (cholera and pertussis toxin) or cAMP agonists (forskolin and 8-Br-cAMP) completely reversed anti-proliferative effects of tocotrienol-rich fraction on CL-S1 cells [162]. Besides that, tocotrienol was shown to inhibit PKC, adenylate cyclase and cyclic AMP-dependent protein activation which might be related to its anti-proliferative effects [23, 88, 94]. Delta- and beta-tocotrienol could inhibit PKC activity leading to transcriptional inhibition of c-myc and hTERT, thus reducing telomerase activity [40]. In addition, gamma-tocotrienol also induced downregulation of Id family proteins and EGFR protein with concomitant activation of JNK MAP kinase pathway [22]. Alpha-tocotrienol but not its acetate analogue was able to inhibit intrinsic cellular 20S proteasome activity which might contribute to THP-1 anti-proliferation activity [100]. Tocotrienol-rich fraction, alpha-, gamma- and delta-tocotrienol might serve as potent DNA synthesis inhibitors with the rank order of gamma-tocotrienol > delta-tocotrienol > alpha-tocotrienol [189].
5.7.3 Tocotrienol-Induced Anti-angiogenesis and Anti-invasion
Anti-angiogenic therapy has recently emerged as a strategy for cancer therapy. As oxidative stress is an important factor in angiogenesis, it is possible that established antioxidants such as tocotrienol may minimize the formation of new blood vessels [60]. An in vitro study revealed that tocotrienol significantly inhibited the proliferation and tube formation of BAEC as well as FGF- or VEGF-induced HUVEC cells in the order of delta- >beta- >gamma- >alpha-tocotrienol [60, 93–95, 103, 180]. Tocotrienol also suppressed VEGF secretion in colon carcinoma cells DLD-1 and HepG2 cells under normoxic and hypoxic conditions in the order of delta- >beta- >gamma- >alpha-tocotrienol [148]. In vivo studies revealed that tocotrienol-rich oil was able to inhibit DLD-1 cells-induced angiogenesis via mouse dorsal air sac (DAS) assay [93, 103]. Tocotrienol-rich fraction and delta-tocotrienol were also demonstrated in vivo to possess anti-angiogenic effects as assessed by chick embryo chorioallantoic membrane (CAM) assay [94, 103, 180]. Tocotrienol-rich fraction supplementation also suppressed serum VEGF level in BALB/c mice inoculated with mouse mammary cancer 4T1 cells which indicated its potential anti-angiogenic activity [180].
Western blotting analysis identified that anti-angiogenic activity of delta-tocotrienol was mediated via apoptosis induction and inactivation of PI3K/PDK/Akt pathway [93]. The inactivation of PI3K/PDK/Akt pathway then suppressed the phosphorylation of ERK1/2, eNOS, and GSK3 alpha/beta and increased the phosphorylated ASK-1 and p38 and p21 which were associated with apoptosis [103]. Besides that, DNA microarray analysis revealed that delta-tocotrienol also downregulated VEGF receptor expression and subsequently blocked intracellular VEGF signalling (phospholipase C-gamma and PKC) in HUVEC cells [94]. Delta-tocotrienol also managed to suppress IL-8 and angiogenic factor secretion and downregulate HIF-1 alpha and IL-8 mRNA in hypoxic conditions [148]. The downregulation of hypoxia-induced HIF-1 alpha expression then inhibited VGEF transcriptional activation [148]. Furthermore, tocotrienol was demonstrated to inhibit human and mice DNA polymerase λ activity in the order of delta- >beta- >gamma- >alpha-tocotrienol [95]. Tocotrienol-induced DNA polymerase λ inhibition required direct binding of tocotrienol to N-terminal region of polymerase [95]. Inhibition of DNA polymerase λ might be closely related to anti-angiogenic activity of tocotrienol.
Tocotrienol-rich fraction and tocotrienols also possessed anti-migration and anti-invasion properties. Delta-tocotrienol inhibited HUVEC and BAEC cells migration as shown in scratch assay test [92, 180]. Delta-tocotrienol also demonstrated profound inhibitory effect on human monocyte U937 cells adhesion to HAECs via blockage of HAECs surface VCAM-1 mRNA and expression [102]. Both alpha-tocotrienol and gamma-tocotrienol suppressed VCAM-1 expression, which inhibited human monocyte THP-1 cells adhesion to endothelial HUVEC cells with the order of alpha-tocotrienol > gamma-tocotrienol [116]. Gamma-tocotrienol also inhibited melanoma cells invasion via restoration of E-cadherin and gamma-catenin expression, together with downregulation of Snail, alpha-SMA, vimentin and twist expression [22].
The anticancer mechanism of tocotrienol is summarized in Fig. 5.4.
5.8 Safety of Tocotrienol
A toxicity study conducted by Ima-Nirwana et al. in the mice revealed that palm tocotrienol at the doses of 500 and 1000 mg/kg body weight (oral) for 14 days (subacute) and 42 days (subchronic) increased the bleeding the clotting time [57]. After conversion based on body surface area [138], this is equivalent to 40.54 and 81.08 mg/kg in humans. Nakamura et al. observed changes in haematology, blood serum enzyme levels and organ histology in rats fed with palm tocotrienol (0.75–3 % weight of diet) for 13 weeks [104]. The no-observed-adverse-effect level derived from this study was 120 mg/kg body weight for male rats and 130 mg/kg body weight for female rats [104]. This is equivalent to 19.46 mg/kg in men and 21.08 mg/kg in women. In view of these studies, it is recommended that patients who are taking anticoagulants such as warfarin, heparin and aspirin to refrain from using tocotrienol because it may increase haemorrhagic risk. Prolonged use of tocotrienol at high dose should also be avoided because it may exert adverse effects on the liver. Taking these into consideration, the use of tocotrienol is relatively safe. The highest dose used in human recorded in this review is 400 mg [47], equivalent to 5.71 mg/kg for a 70 kg adults, which is far below the toxic doses.
5.9 Conclusion
The two groups of vitamin E, the tocotrienols and the tocopherols possess distinct similarities and differences. These differences may be seen even between isomers within the same group. Extensive in vivo and in vitro studies on vitamin E mixtures from various natural sources, as well as the pure isomers, have been done. Most of these studies have shown beneficial effects of the tocotrienol and tocopherol isomers in varying degrees. The source of the naturally occurring vitamin E mixtures are from palm oil, rice bran or annatto oil. However, in the reported literature, the vitamin E mixtures differ in composition, some consisting of mixtures of tocopherols and tocotrienols, while some are mixtures of tocotrienol isomers only. The doses used also differ between studies. Even experiments using individual tocotrienol or tocopherol isomers differ in their effective doses. Thus, it is not easy to summarize the results, but in general the preclinical literature shows that tocotrienols are beneficial in metabolic disease conditions associated with elevated free radicals and inflammatory cytokines, such as dyslipidaemia, diabetes mellitus and osteoporosis. Some clinical trials on tocotrienols and dyslipidaemia have been reported, while none on diabetes mellitus or osteoporosis are available so far. Data from the available clinical trials are inconclusive, and generally the beneficial effects are slight and not as significantly obvious as the effects seen in the in vivo and in vitro studies. This begs the question as to whether dose of the tocotrienols used in the human studies were too low, or the duration of treatment insufficient, or even whether the sampling methods were adequate. Another cause for the discrepancy could be that the distribution and metabolism of the tocotrienols differ between humans and the rodent models used. It will be interesting to see whether the same sort of observations will be seen in clinical trials on the effects of tocotrienols in diabetics or in osteoporotic patients.
Many well-reported studies on the anticancer effects of tocotrienol were found in the literature. Most of these studies are in vitro studies using cancer cell lines. Most of the studies are on breast cancer cells, while some studies are on liver, colon, lung and leukaemic cell lines. The mechanisms have been elucidated as being apoptotic and anti-angiogenetic. The anticancer effects of tocotrienol are thought to be separate from the antioxidant and anti-inflammatory effects. Limited animal studies have been done using genetically modified mice, and only two reported clinical trials on the effects of tocotrienols on breast cancer are found in literature. Again the data from the human studies are scant and inconclusive.
From the above review, tocotrienol presents an exciting possibility as an alternative or adjuvant therapy for diseases associated with increased oxidative stress and inflammation, such as dyslipidaemia and diabetes. Its application in preventing osteoporosis in humans still awaits results from clinical trial. More coordinated, well-designed clinical trials are needed to determine the effects on humans, however, the consistently beneficial effects in in vivo studies indicate that the potential for human therapeutic use is real. As for the anticancer properties, the potential for use presents a real challenge to researchers. The apoptotic and anti-angiogenetic properties seen in the cell culture studies appears very promising and can be the alternative to the stressful chemotherapy, or at least as an adjuvant to reduce the side-effects of chemotherapy.
References
Abd Manan N, Mohamed N, Shuid AN (2012) Effects of low-dose versus high-dose gamma-tocotrienol on the bone cells exposed to the hydrogen peroxide-induced oxidative stress and apoptosis. Evid Based Complement Altern Med 2012:680834
Abdelmagid SM, Barbe MF, Safadi FF (2015) Role of inflammation in the aging bones. Life Sci 123:25–34
Abdul-Majeed S, Mohamed N, Soelaiman I-N (2012) Effects of tocotrienol and lovastatin combination on osteoblast and osteoclast activity in estrogen-deficient osteoporosis. Evid Based Complement Altern Med 2012:960742
Abdul-Majeed S, Mohamed N, Soelaiman IN (2015) The use of delta-tocotrienol and lovastatin for anti-osteoporotic therapy. Life Sci 125:42–48
Abukhadir SSA, Mohamed N, Makpol S, Muhammad N (2012) Effects of palm vitamin e on bone-formation-related gene expression in nicotine-treated rats. Evid Based Complement Altern Med 2012:656025
Aggarwal BB, Sundaram C, Prasad S, Kannappan R (2010) Tocotrienols, the vitamin E of the 21(St) Century: it’s potential against cancer and other chronic diseases. Biochem Pharmacol 80(11):1613–1631
Ahmad NS, Khalid BAK, Luke DA, Ima Nirwana S (2005) Tocotrienol offers better protection than tocopherol from free radical-induced damage of rat bone. Clin Exp Pharmacol Physiol 32(9):761–770
Aktifanus AT, Shuid AN, Rashid NH et al (2012) Comparison of the effects of tocotrienol and estrogen on the bone markers and dynamic changes in postmenopausal osteoporosis rat model. Asian J Anim Vet Adv 7(3):225–234
Atli T, Keven K, Avci A et al (2004) oxidative stress and antioxidant status in elderly diabetes mellitus and glucose intolerance patients. Arch Gerontol Geriatr 39(3):269–275
Baek KH, Oh KW, Lee WY et al (2010) Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif Tissue Int 87(3):226–235
Baliarsingh S, Beg ZH, Ahmad J (2005) The therapeutic impacts of tocotrienols in type 2 diabetic patients with hyperlipidemia. Atherosclerosis 182(2):367–374
Barnham KJ, Masters CL, Bush AI (2004) Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 3(3):205–214
Birringer M, EyTina JH, Salvatore BA, Neuzil J (2003) Vitamin E analogues as inducers of apoptosis: structure-function relation. Br J Cancer 88(12):1948–1955
Brigelius-Flohe R, Kelly FJ, Salonen JT, Neuzil J, Zingg JM, Azzi A (2002) The European perspective on vitamin E: current knowledge and future research. Am J Clin Nutr 76(4):703–716
Brooks R, Kalia P, Ireland DC, Beeton C, Rushton N (2011) Direct inhibition of osteoclast formation and activity by the vitamin E isomer gamma-tocotrienol. Int J Vitam Nutr Res 81(6):358–367
Budin SB, Othman F, Louis SR, Bakar MA, Das S, Mohamed J (2009) The effects of palm oil tocotrienol-rich fraction supplementation on biochemical parameters, oxidative stress and the vascular wall of streptozotocin-induced diabetic rats. Clinics (Sao Paulo) 64(3):235–244
Buhaescu I, Izzedine H (2007) Mevalonate pathway: a review of clinical and therapeutical implications. Clin Biochem 40(9–10):575–584
Burdeos GC, Nakagawa K, Kimura F, Miyazawa T (2012) Tocotrienol attenuates triglyceride accumulation in Hepg2 cells and F344 rats. Lipids 47(5):471–481
Burdeos GC, Nakagawa K, Watanabe A, Kimura F, Miyazawa T (2013) Gamma-tocotrienol attenuates triglyceride through effect on lipogenic gene expressions in mouse hepatocellular carcinoma hepa 1-6. J Nutr Sci Vitaminol (Tokyo) 59(2):148–151
Callaway DA, Jiang JX (2015) Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J Bone Miner Metab 33(4):359–370
Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM (2012) Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care 35(9):1835–1844
Chang PN, Yap WN, Lee DT, Ling MT, Wong YC, Yap YL (2009) Evidence of Γ-tocotrienol as an apoptosis-inducing, invasion-suppressing, and chemotherapy drug-sensitizing agent in human melanoma cells. Nutr Cancer 61(3):357–366
Chatelain E, Boscoboinik DO, Bartoli G-M et al (1993) Inhibition of smooth muscle cell proliferation and protein kinase C activity by tocopherols and tocotrienols. Biochim Biophys Acta (BBA) Mol Cell Res 1176(1–2):83–89
Chin K-Y, Ima-Nirwana S (2012) Vitamin E as an antiosteoporotic agent via receptor activator of nuclear factor kappa-B ligand signaling disruption: current evidence and other potential research areas. Evid Based Complement Altern Med 2012:747020
Chin KY, Abdul-Majeed S, Fozi NF, Ima-Nirwana S (2014) Annatto tocotrienol improves indices of bone static histomorphometry in osteoporosis due to testosterone deficiency in rats. Nutrients 6(11):4974–4983
Chin KY, Ima-Nirwana S (2014) The effects of alpha-tocopherol on bone: a double-edged sword? Nutrients 6(4):1424–1441
Chin KY, Ima-Nirwana S (2015) the biological effects of tocotrienol on bone: a review on evidence from rodent models. Drug Des Devel Ther 9:2049–2061
Chin KY, Ima Nirwana S (2014) The effects of annatto-derived tocotrienol supplementation in osteoporosis induced by testosterone deficiency in rats. Clin Interv Aging 9:1247–1259
Chin SF, Ibahim J, Makpol S et al (2011) Tocotrienol rich fraction supplementation improved lipid profile and oxidative status in healthy older adults: a randomized controlled study. Nutr Metab (Lond) 8(1):42
Choy EHS, Panayi GS (2001) Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 344(12):907–916
Chun J, Lee J, Ye L, Exler J, Eitenmiller RR (2006) Tocopherol and tocotrienol contents of raw and processed fruits and vegetables in the United States Diet. J Food Compos Anal 19(2–3):196–204
Comitato R, Nesaretnam K, Leoni G et al (2009) A novel mechanism of natural vitamin E tocotrienol activity: involvement of Erβ signal transduction. Am J Physiol Endocrinol Metab 297:E427–E437
Constantinou C, Hyatt JA, Vraka PS et al (2009) Induction of caspase-independent programmed cell death by vitamin E natural homologs and synthetic derivatives. Nutr Cancer 61(6):864–874
Conte C, Floridi A, Aisa C, Piroddi M, Floridi A, Galli F (2004) Γ-tocotrienol metabolism and antiproliferative effect in prostate cancer cells. Ann N Y Acad Sci 1031:391–394
Dandona P, Aljada A, Bandyopadhyay A (2004) Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 25(1):4–7
Daud ZA, Tubie B, Sheyman M et al (2013) Vitamin E tocotrienol supplementation improves lipid profiles in chronic hemodialysis patients. Vasc Health Risk Manag 9:747–761
Deng L, Ding Y, Peng Y et al (2014) Γ-tocotrienol protects against ovariectomy-induced bone loss via mevalonate pathway as Hmg-Coa reductase inhibitor. Bone 67:200–207
Deshpande AD, Harris-Hayes M, Schootman M (2008) Epidemiology of diabetes and diabetes-related complications. Phys Ther 88(11):1254–1264
Di Marco E, Jha JC, Sharma A, Wilkinson-Berka JL, Jandeleit-Dahm KA, de Haan JB (2015) Are reactive oxygen species still the basis for diabetic complications? Clin Sci (Lond) 129(2):199–216
Eitsuka T, Nakagawa K, Miyazawa T (2006) Down-regulation of telomerase activity in Dld-1 human colorectal adenocarcinoma cells by tocotrienol. Biochem Biophys Res Commun 348(1):170–175
Elangovan S, Hsieh TC, Wu JM (2008) Growth inhibition of human Mda-Mb-231 breast cancer cells by Δ-tocotrienol is associated with loss of cyclin D1/Cdk4 expression and accompanying changes in the state of phosphorylation of the retinoblastoma tumor suppressor gene product. Anticancer Res 28:2641–2648
Frega N, Mozzon M, Bocci F (1998) Identification and estimation of tocotrienols in the annatto lipid fraction by gas chromatography-mass spectrometry. J Am Oil Chem Soc 75(12):1723–1727
Gee PT (2011) Unleashing the untold and misunderstood observations on vitamin E. Genes Nutr 6(1):5–16
Guthrie N, Gapor A, Chambers AF, Carroll KK (1997) Inhibition of proliferation of estrogen receptor-negative Mda-Mb-435 and -Positive Mcf-7 human breast cancer cells by palm oil tocotrienols and tamoxifen, alone and in combination. J Nutr 127:544S–548S
Guthrie N, Gapor A, Chambers AF, Carroll KK (1997) Palm oil tocotrienols and plant flavonoids act synergistically with each other and with tamoxifen in inhibiting proliferation and growth of estrogen receptor-negative Mda-Mb-435 and -positive Mcf-7 human breast cancer cells in culture. Asia Pac J Clin Nutrition 6(1):41–45
Ha H, Lee JH, Kim HN, Lee ZH (2011) Alpha-tocotrienol inhibits osteoclastic bone resorption by suppressing rankl expression and signaling and bone resorbing activity. Biochem Biophys Res Commun 406(4):546–551
Haghighat N, Vafa M, Eghtesadi S, Heidari I, Hosseini A, Rostami A (2014) The effects of tocotrienols added to canola oil on microalbuminuria, inflammation, and nitrosative stress in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Int J Prev Med 5(5):617–623
Halliwell B (1994) Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet 344(8924):721–724
Har CH, Keong CK (2005) Effects of tocotrienols on cell viability and apoptosis in normal murine liver cells (Bnl Cl.2) and liver cancer cells (Bnl 1me A.7r.1), in Vitro. Asia Pac J Clin Nutr 14(4):374–380
Heng KS, Hejar AR, Johnson Stanslas J, Ooi CF, Loh SF (2015) Potential of mixed tocotrienol supplementation to reduce cholesterol and cytokines level in adults with metabolic syndrome. Malays J Nutr 21(2):231–243
Hermizi H, Faizah O, Ima-Nirwana S, Ahmad Nazrun S, Norazlina M (2009) Beneficial effects of tocotrienol and tocopherol on bone histomorphometric parameters in Sprague-Dawley male rats after nicotine cessation. Calcif Tissue Int 84(1):65–74
Hiura Y, Tachibana H, Arakawa R et al (2009) Specific accumulation of Γ- and Δ-tocotrienols in tumor and their antitumor effect in vivo. J Nutr Biochem 20(8):607–613
Hosomi A, Arita M, Sato Y et al (1997) Affinity for alpha-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett 409(1):105–108
Hsieh TC, Wu JM (1992) Suppression of cell proliferation and gene expression by combinatorial synergy of Egcg, resveratrol and Γ-tocotrienol in estrogen receptor-positive Mcf-7 breast cancer cells. Int J Oncol 33(4):851–859
Husain K, Francois RA, Hutchinson SZ et al (2009) Vitamin E Δ-tocotrienol levels in tumor and pancreatic tissue of mice after oral administration. Pharmacology 83(3):157–163
Ima-Nirwana S, Fakhrurazi H (2002) Palm vitamin E protects bone against dexamethasone-induced osteoporosis in male rats. Med J Malaysia 57(2):133–141
Ima-Nirwana S, Nurshazwani Y, Nazrun AS, Norliza M, Norazlina M (2011) Subacute and subchronic toxicity studies of palm vitamin E in mice. J Pharmacol Toxicol 6:166–173
Ima Nirwana S, Kiftiah A, Zainal A, Norazlina M, Abd. Gapor M, Khalid BAK (2000) Palm vitamin E prevents osteoporosis in orchidectomised growing male rats. Nat Prod Sci 6(4):155–160
Ima Nirwana S, Suhaniza S (2004) Effects of tocopherols and tocotrienols on body composition and bone calcium content in adrenalectomized rats replaced with dexamethasone. J Med Food 7(1):45–51
Inokuchi H, Hirokane H, Tsuzuki T, Nakagawa K, Igarashi M, Miyazawa T (2003) Anti-angiogenic activity of tocotrienol. Biosci Biotechnol Biochem 67(7):1623–1627
Institute of Medicine (2000) Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. The National Academies Press, Washington
Iqbal J, Minhajuddin M, Beg ZH (2003) Suppression of 7,12-dimethylbenz[alpha]anthracene-induced carcinogenesis and hypercholesterolaemia in rats by tocotrienol-rich fraction isolated from rice bran oil. Eur J Cancer Prev 12(6):447–453
Jiang Q (2014) Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med 72:76–90
Johnell O, Kanis J (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17(12):1726–1733
Jones MP, Pandak WM, Heuman DM, Chiang JY, Hylemon PB, Vlahcevic ZR (1993) Cholesterol 7 alpha-hydroxylase: evidence for transcriptional regulation by cholesterol or metabolic products of cholesterol in the rat. J Lipid Res 34(6):885–892
Kamat JP, Devasagayam TP (1995) Tocotrienols from palm oil as potent inhibitors of lipid peroxidation and protein oxidation in rat brain mitochondria. Neurosci Lett 195(3):179–182
Kamat JP, Sarma HD, Devasagayam TP, Nesaretnam K, Basiron Y (1997) Tocotrienols from palm oil as effective inhibitors of protein oxidation and lipid peroxidation in rat liver microsomes. Mol Cell Biochem 170(1–2):131–137
Kanis JA, McCloskey EV, Harvey NC, Johansson H, Leslie WD (2015) Intervention thresholds and the diagnosis of osteoporosis. J Bone Miner Res 30(10):1747–1753
Kashiwagi K, Harada K, Yano Y et al (2008) A redox-silent analogue of tocotrienol inhibits hypoxic adaptation of lung cancer cells. Biochem Biophys Res Commun 365(4):875–881
Kashiwagi K, Virgona N, Harada K et al (2009) A redox-silent analogue of tocotrienol acts as a potential cytotoxic agent against human mesothelioma cells. Life Sci 84(19–20):650–656
Kataja-Tuomola MK, Kontto JP, Mannisto S, Albanes D, Virtamo J (2011) Intake of antioxidants and risk of type 2 diabetes in a cohort of male smokers. Eur J Clin Nutr 65(5):590–597
Kawakami Y, Tsuzuki T, Nakagawa K, Miyazawa T (2007) Distribution of tocotrienols in rats fed a rice bran tocotrienol concentrate. Biosci Biotechnol Biochem 71(2):464–471
Khor H, Ng T, Rajendran R (2002) Dose-dependent cholesterolemic activity of tocotrienols. Malays J Nutr 8(2):157–166
Khor HT, Ng TT (2000) Effects of administration of alpha-tocopherol and tocotrienols on serum lipids and liver Hmg Coa reductase activity. Int J Food Sci Nutr 51(Suppl):S3–S11
King AJ (2012) The use of animal models in diabetes research. Br J Pharmacol 166(3):877–894
Kuhad A, Bishnoi M, Tiwari V, Chopra K (2009) Suppression of Nf-Kappabeta signaling pathway by tocotrienol can prevent diabetes associated cognitive deficits. Pharmacol Biochem Behav 92(2):251–259
Kuhad A, Chopra K (2009) Attenuation of diabetic nephropathy by tocotrienol: involvement of Nfkb signaling pathway. Life Sci 84(9–10):296–301
Kuhad A, Chopra K (2009) Tocotrienol attenuates oxidative-nitrosative stress and inflammatory cascade in experimental model of diabetic neuropathy. Neuropharmacology 57(4):456–462
Lee SP, Mar GY, Ng LT (2009) Effects of tocotrienol-rich fraction on exercise endurance capacity and oxidative stress in forced swimming rats. Eur J Appl Physiol 107(5):587–595
Libby P (2006) Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr 83(2):456S–460S
Lodge JK, Ridlington J, Leonard S, Vaule H, Traber MG (2001) Alpha- and gamma-tocotrienols are metabolized to carboxyethyl-hydroxychroman derivatives and excreted in human urine. Lipids 36(1):43–48
Madamanchi NR, Vendrov A, Runge MS (2005) Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 25(1):29–38
Maniam S, Mohamed N, Shuid AN, Soelaiman IN (2008) Palm tocotrienol exerted better antioxidant activities in bone than α-tocopherol. Basic Clin Pharmacol Toxicol 103(1):55–60
Manolagas SC (2010) From Estrogen-Centric to Aging and Oxidative Stress: A Revised Perspective of the Pathogenesis of Osteoporosis. Endocr Rev 31(3):266–300
Matough FA, Budin SB, Hamid ZA, Abdul-Rahman M, Al-Wahaibi N, Mohammed J (2014) Tocotrienol-rich fraction from palm oil prevents oxidative damage in diabetic rats. Sultan Qaboos Univ Med J 14(1):e95–e103
McAnally JA, Gupta J, Sodhani S, Bravo L, Mo H (2007) Tocotrienols potentiate lovastatin-mediated growth suppression in vitro and in vivo. Exp Biol Med (Maywood) 232:523–531
McIntyre BS, Briski KP, Gapor A, Sylvester PW (2008) Antiproliferative and apoptotic effects of tocopherols and tocotrienols on preneoplastic and neoplastic mouse mammary epithelial cells. Proc Soc Exp Biol Med 224(4):292–301
McIntyre BS, Briski KP, Tirmenstein MA, Fariss MW, Gapor A, Sylvester PW (2000) Antiproliferative and apoptotic effects of tocopherols and tocotrienols on normal mouse mammary epithelial cells. Lipids 35(2):171–180
Mehat M, Shuid A, Mohamed N, Muhammad N, Soelaiman I (2010) Beneficial effects of vitamin E isomer supplementation on static and dynamic bone histomorphometry parameters in normal male rats. J Bone Miner Metab 28(5):503–509
Mensink RP, van Houwelingen AC, Kromhout D, Hornstra G (1999) A Vitamin E concentrate rich in tocotrienols had no effect on serum lipids, lipoproteins, or platelet function in men with mildly elevated serum lipid concentrations. Am J Clin Nutr 69(2):213–219
Minhajuddin M, Beg ZH, Iqbal J (2005) Hypolipidemic and antioxidant properties of tocotrienol rich fraction isolated from rice bran oil in experimentally induced hyperlipidemic rats. Food Chem Toxicol 43(5):747–753
Miyazawa T, Inokuchi H, Hirokane H, Tsuzuki T, Nakagawa K, Igarashi M (2004) Anti-angiogenic potential of tocotrienol in vitro. Biochemistry (Moscow) 69(1):67–69
Miyazawa T, Shibata A, Nakagawa K, Tsuzuki T (2008) Anti-angiogenic function of tocotrienol. Asia Pac J Clin Nutr 17(S1):253–256
Miyazawa T, Tsuzuki T, Nakagawa K, Igarashi M (2004) Antiangiogenic potency of vitamin E. Ann N Y Acad Sci 1031:401–404
Mizushina Y, Nakagawa K, Shibata A et al (2006) Inhibitory effect of tocotrienol on eukaryotic DNA polymerase Λ and angiogenesis. Biochem Biophys Res Commun 339(3):949–955
Muhammad N, Luke DA, Shuid AN, Mohamed N, Soelaiman IN (2012) Two different isomers of vitamin E prevent bone loss in postmenopausal osteoporosis rat model. Evid Based Complement Altern Med 2012:161527
Muhammad N, Razali S, Shuid AN, Mohamed N, Soelaiman IN (2013) Membandingkan Kesan Antara Fraksi-Kaya Tokotrienol, Kalsium Dan Estrogen Terhadap Metabolisme Tulang Tikus Terovariektomi. Sains Malays 42(11):1591–1597
Müller M, Cela J, Asensi-Fabado MA, Munné-Bosch S (2012) Tocotrienols in plants: occurrence, biosynthesis, and function. In: Tan B, Watson RR, Preedy VR (eds) Tocotrienols: vitamin E beyond tocopherols. CRC Press, Florida, pp 1–16
Mundy GR (2007) Osteoporosis and inflammation. Nutr Rev 65(suppl 3):S147–S151
Munteanu A, Ricciarelli R, Massone S, Zingg JM (2007) Modulation of proteasome activity by vitamin E in Thp-1 monocytes. IUBMB Life 59(12):771–780
Mustad VA, Smith CA, Ruey PP, Edens NK, DeMichele SJ (2002) Supplementation with 3 compositionally different tocotrienol supplements does not improve cardiovascular disease risk factors in men and women with hypercholesterolemia. Am J Clin Nutr 76(6):1237–1243
Naito Y, Shimozawa M, Kuroda M et al (2005) Tocotrienols reduce 25-hydroxycholesterol-induced monocyte-endothelial cell interaction by inhibiting the surface expression of adhesion molecules. Atherosclerosis 180(1):19–25
Nakagawa K, Shibata A, Yamashita S et al (2007) In vivo angiogenesis is suppressed by unsaturated vitamin E, tocotrienol. J Nutr 137:1938–1943
Nakamura H, Furukawa F, Nishikawa A et al (2001) Oral toxicity of a tocotrienol preparation in rats. Food Chem Toxicol 39(8):799–805
Nazrun AS, Luke DA, Khalid BAK, Ima Nirwana S (2005) Vitamin E protects from free-radical damage on femur of rats treated with ferric nitrilotriacetate. Curr Topics Pharmacol 9(2):107–115
Nesaretnam K, Devasagayam TP, Singh BB, Basiron Y (1993) Influence of palm oil or its tocotrienol-rich fraction on the lipid peroxidation potential of rat liver mitochondria and microsomes. Biochem Mol Biol Int 30(1):159–167
Nesaretnam K, Meganathan P, Veerasenan SD, Selvaduray KR (2012) Tocotrienols and breast cancer: the evidence to date. Genes Nutr 7(1):3–9
Nesaretnam K, Selvaduray KR, Abdul Razak G, Veerasenan SD, Gomez PA (2010) Effectiveness of tocotrienol-rich fraction combined with tamoxifen in the management of women with early breast cancer: a pilot clinical trial. Breast Cancer Res 12(5):R81
Nesaretnam K, Stephen R, Dils R, Darbre P (1998) Tocotrienols inhibit the growth of human breast cancer cells irrespective of estrogen receptor status. Lipids 33(5):461–469
Ng LT, Ko HJ (2012) Comparative effects of tocotrienol-rich fraction, alpha-tocopherol and alpha-tocopheryl acetate on inflammatory mediators and nuclear factor kappa B expression in mouse peritoneal macrophages. Food Chem 134(2):920–925
Ng MH, Choo YM, Ma AN, Chuah CH, Hashim MA (2004) Separation of vitamin E (tocopherol, tocotrienol, and tocomonoenol) in palm oil. Lipids 39(10):1031–1035
Ngah WZW, Jarien Z, San MM et al (1991) Effect of tocotrienols on hepatocarcinogenesis induced by 2-acetylaminofluorene in rats. Am J Clin Nutr 53:1076S–1081S
Niki E (2013) Antioxidant action of tocotrienols. In: Tan B, Watson RR, Preedy VR (eds) Tocotrienols: vitamin E beyond tocopherols. CRC Press, Boca Raton, pp 233–240
Nikolic K, Agababa D (2009) Design and Qsar study of analogs of Γ-tocotrienol with enhanced antiproliferative activity against human breast cancer cells. J Mol Graph Model 27(7):777–783
Nizar AM, Nazrun AS, Norazlina M, Norliza M, Ima Nirwana S (2011) Low dose of tocotrienols protects osteoblasts against oxidative stress. Clin Ter 162(6):533–538
Noguchi N, Hanyu R, Nonaka A, Okimoto Y, Kodama T (2003) Inhibition of Thp-1 cell adhesion to endothelial cells by Α-tocopherol and Α-tocotrienol is dependent on intracellular concentration of the antioxidants. Free Radic Biol Med 34(12):1614–1620
Norazlina M, Hermizi H, Faizah O, Ima-Nirwana S (2010) Vitamin E reversed nicotine-induced toxic effects on bone biochemical markers in male rats. Arch Med Sci 6(4):505–512
Norazlina M, Ima Nirwana S, Abd. Gapor M, Khalid B (2000) Palm vitamin E is comparable to alpha-tocotrienol in maintaining bone mineral density in ovariectomised female rats. Exp Clin Endocrinol Diabetes 108:305–310
Norazlina M, Lee P, Lukman H, Nazrun A, Ima-Nirwana S (2007) Effects of vitamin E supplementation on bone metabolism in nicotine-treated rats. Singapore Med J 48(3):195–199
O’Byrne D, Grundy S, Packer L et al (2000) Studies of Ldl oxidation following alpha-, gamma-, or delta-tocotrienyl acetate supplementation of hypercholesterolemic humans. Free Radic Biol Med 29(9):834–845
Palozza P, Verdecchia S, Avanzi L et al (2006) Comparative antioxidant activity of tocotrienols and the novel chromanyl-polyisoprenyl molecule feaox-6 in isolated membranes and intact cells. Mol Cell Biochem 287(1–2):21–32
Parker RA, Pearce BC, Clark RW, Gordon DA, Wright JJ (1993) Tocotrienols regulate cholesterol production in mammalian cells by post-transcriptional suppression of 3-hydroxy-3-methylglutaryl-coenzyme a reductase. J Biol Chem 268(15):11230–11238
Patel J, Matnor NA, Iyer A, Brown L (2011) A regenerative antioxidant protocol of vitamin E and alpha-lipoic acid ameliorates cardiovascular and metabolic changes in fructose-fed rats. Evid Based Complement Altern Med 2011:120801
Qureshi AA, Pearce BC, Nor RM, Gapor A, Peterson DM, Elson CE (1996) Dietary alpha-tocopherol attenuates the impact of gamma-tocotrienol on hepatic 3-hydroxy-3-methylglutaryl coenzyme a reductase activity in chickens. J Nutr 126(2):389–394
Qureshi AA, Peterson DM (2001) The combined effects of novel tocotrienols and lovastatin on lipid metabolism in chickens. Atherosclerosis 156(1):39–47
Qureshi AA, Peterson DM, Hasler-Rapacz JO, Rapacz J (2001) Novel tocotrienols of rice bran suppress cholesterogenesis in hereditary hypercholesterolemic swine. J Nutr 131(2):223–230
Qureshi AA, Qureshi N, Hasler-Rapacz JO et al (1991) Dietary tocotrienols reduce concentrations of plasma cholesterol, apolipoprotein B, thromboxane B2, and platelet factor 4 in pigs with inherited hyperlipidemias. Am J Clin Nutr 53(4 Suppl):1042s–1046s
Qureshi AA, Qureshi N, Wright JJ et al (1991) Lowering of serum cholesterol in hypercholesterolemic humans by tocotrienols (Palmvitee). Am J Clin Nutr 53(4 Suppl):1021s–1026s
Qureshi AA, Reis JC, Papasian CJ, Morrison DC, Qureshi N (2010) Tocotrienols inhibit lipopolysaccharide-induced pro-inflammatory cytokines in macrophages of female mice. Lipids Health Dis 9:143
Qureshi AA, Reis JC, Qureshi N, Papasian CJ, Morrison DC, Schaefer DM (2011) Delta-tocotrienol and quercetin reduce serum levels of nitric oxide and lipid parameters in female chickens. Lipids Health Dis 10:39
Qureshi AA, Sami SA, Salser WA, Khan FA (2001) Synergistic effect of tocotrienol-rich fraction (Trf(25)) of rice bran and lovastatin on lipid parameters in hypercholesterolemic humans. J Nutr Biochem 12(6):318–329
Qureshi AA, Sami SA, Salser WA, Khan FA (2002) Dose-dependent suppression of serum cholesterol by tocotrienol-rich fraction (Trf25) of rice bran in hypercholesterolemic humans. Atherosclerosis 161(1):199–207
Qureshi AA, Tan X, Reis JC et al (2011) Inhibition of nitric oxide in Lps-stimulated macrophages of young and senescent mice by delta-tocotrienol and quercetin. Lipids Health Dis 10:239
Raederstorff D, Elste V, Aebischer C, Weber P (2002) Effect of either gamma-tocotrienol or a tocotrienol mixture on the plasma lipid profile in hamsters. Ann Nutr Metab 46(1):17–23
Rahmat A, Ngah WZW, Top AGM, Khalid BAK (1993) Long term tocotrienol supplementation and glutathione-dependent enzymes during hepatocarcinogenesis in the rat. Asia Pac J Clin Nutr 2:129–134
Rasool AH, Yuen KH, Yusoff K, Wong AR, Rahman AR (2006) Dose dependent elevation of plasma tocotrienol levels and its effect on arterial compliance, plasma total antioxidant status, and lipid profile in healthy humans supplemented with tocotrienol rich vitamin E. J Nutr Sci Vitaminol (Tokyo) 52(6):473–478
Rasool AHG, Yuen KH, Yusoff K, Wong AR, Rahman ARA (2006) Dose dependent elevation of plasma tocotrienol levels and its effect on arterial compliance, plasma total antioxidant status, and lipid profile in healthy humans supplemented with tocotrienol rich vitamin E. J Nutr Sci Vitaminol 52:473–478
Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 22(3):659–661
Rickmann M, Vaquero EC, Malagelada JR, Molero X (2007) Tocotrienols induce apoptosis and autophagy in rat pancreatic stellate cells through the mitochondrial death pathway. Gastroenterology 132(7):2518–2532
Sakai M, Okabe M, Tachibana H, Yamada K (2006) Apoptosis induction by Γ-tocotrienol in human hepatoma Hep3b cells. J Nutr Biochem 17(10):672–676
Sakai M, Okabe M, Yamasaki A, Tachibana H, Yamada K (2004) Induction of apoptosis by tocotrienol in rat hepatoma Drlh-84 cells. Anticancer Res 24:1683–1688
Salman Khan M, Akhtar S, Al-Sagair OA, Arif JM (2011) Protective effect of dietary tocotrienols against infection and inflammation-induced hyperlipidemia: an in vivo and in silico study. Phytother Res PTR 25(11):1586–1595
Samant GV, Sylvester PW (2006) Γ-tocotrienol inhibits Erbb3-dependent Pi3k-Akt mitogenic signalling in neoplastic mammary epithelial cells. Cell Prolif 39:563–574
Sen CK, Khanna S, Roy S, Packer L (2000) Molecular basis of vitamin E action: tocotrienol potently inhibits glutamate-induced Pp60c-Src kinase activation and death of Ht4 neuronal cells. J Biol Chem 275(17):13049–13055
Serbinova E, Kagan V, Han D, Packer L (1991) Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic Biol Med 10(5):263–275
Shah SJ, Sylvester PW (2005) Tocotrienol-induced cytotoxicity is unrelated to mitochondrial stress apoptotic signaling in neoplastic mammary epithelial cells. Biochem Cell Biol 83(1):86–95
Shibata A, Nakagawa K, Shirakawa H et al (2012) Physiological effects and tissue distribution from large doses of tocotrienol in rats. Biosci Biotechnol Biochem 76(9):1805–1808
Shibata A, Nakagawa K, Sookwong P et al (2008) Tocotrienol inhibits secretion of angiogenic factors from human colorectal adenocarcinoma cells by suppressing hypoxia-inducible factor-1α. J Nutr 138(11):2136–2142
Shuid A, Mehat Z, Mohamed N, Muhammad N, Soelaiman I (2010) Vitamin E exhibits bone anabolic actions in normal male rats. J Bone Miner Metab 28(2):149–156
Siddiqui S, Ahsan H, Khan MR, Siddiqui WA (2013) Protective effects of tocotrienols against lipid-induced nephropathy in experimental type-2 diabetic rats by modulation in Tgf-beta expression. Toxicol Appl Pharmacol 273(2):314–324
Siddiqui S, Rashid Khan M, Siddiqui WA (2010) Comparative hypoglycemic and nephroprotective effects of tocotrienol rich fraction (Trf) from palm oil and rice bran oil against hyperglycemia induced nephropathy in type 1 diabetic rats. Chem Biol Interact 188(3):651–658
Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol 82(2):291–295
Sies H (2000) What is oxidative stress? In: Keaney J, Jr. (ed) Oxidative stress and vascular disease. Developments in cardiovascular medicine, vol 224. Springer, New York , pp 1–8
Soelaiman IN, Ming W, Abu Bakar R et al (2012) Palm tocotrienol supplementation enhanced bone formation in oestrogen-deficient rats. Int J Endocrinol 2012:532862
Song BL, DeBose-Boyd RA (2006) Insig-dependent ubiquitination and degradation of 3-hydroxy-3-methylglutaryl coenzyme a reductase stimulated by delta- and gamma-tocotrienols. J Biol Chem 281(35):25054–25061
Sookwong P, Nakagawa K, Murata K, Kojima Y, Miyazawa T (2007) Quantitation of tocotrienol and tocopherol in various rice brans. J Agric Food Chem 55(2):461–466
Sookwong P, Nakagawa K, Yamaguchi Y et al (2010) Tocotrienol distribution in foods: estimation of daily tocotrienol intake of Japanese population. J Agric Food Chem 58(6):3350–3355
Srivastava JK, Gupta S (2006) Tocotrienol-rich fraction of palm oil induces cell cycle arrest and apoptosis selectively in human prostate cancer cells. Biochem Biophys Res Commun 346(2):447–453
Stamler J, Daviglus ML, Garside DB, Dyer AR, Greenland P, Neaton JD (2000) Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA 284(3):311–318
Sun W, Xu W, Liu H et al (2009) Γ-tocotrienol induces mitochondria-mediated apoptosis in human gastric adenocarcinoma Sgc-7901 cells. J Nutr Biochem 20(4):276–284
Suzuki YJ, Tsuchiya M, Wassall SR et al (1993) Structural and dynamic membrane properties of alpha-tocopherol and alpha-tocotrienol: implication to the molecular mechanism of their antioxidant potency. Biochemistry 32(40):10692–10699
Sylvester PW, Nachnani A, Shah S, Briski KP (2002) Role of Gtp-binding proteins in reversing the antiproliferative effects of tocotrienols in preneoplastic mammary epithelial cells. Asia Pac J Clin Nutr 11(Suppl):S452–S459
Sylvester PW, Shah S (2005) Intracellular mechanisms mediating tocotrienol-induced apoptosis in neoplastic mammary epithelial cells. Asia Pac J Clin Nutr 14(4):366–373
Sylvester PW, Shah SJ (2005) Mechanisms mediating the antiproliferative and apoptotic effects of vitamin E in mammary cancer cells. Front Biosci 10(1–3):699
Takahashi K, Loo G (2004) Disruption of mitochondria during tocotrienol-induced apoptosis in Mda-Mb-231 human breast cancer cells. Biochem Pharmacol 67(2):315–324
Tan CY, Saw TY, Fong CW, Ho HK (2015) Comparative hepatoprotective effects of tocotrienol analogs against drug-induced liver injury. Redox Biol 4:308–320
Tan DT, Khor HT, Low WH, Ali A, Gapor A (1991) Effect of a palm-oil-vitamin E concentrate on the serum and lipoprotein lipids in humans. Am J Clin Nutr 53(4 Suppl):1027s–1030s
Theriault A, Wang Q, Gapor A, Adeli K (1999) Effects of gamma-tocotrienol on apob synthesis, degradation, and secretion in Hepg2 cells. Arterioscler Thromb Vasc Biol 19(3):704–712
Tomeo AC, Geller M, Watkins TR, Gapor A, Bierenbaum ML (1995) Antioxidant effects of tocotrienols in patients with hyperlipidemia and carotid stenosis. Lipids 30(12):1179–1183
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39(1):44–84
Vester H, Holzer N, Neumaier M, Lilianna S, Nussler AK, Seeliger C (2014) Green tea extract (Gte) improves differentiation in human osteoblasts during oxidative stress. J Inflamm (Lond) 11:15
Wada S, Satomi Y, Murakoshi M, Noguchi N, Yoshikawa T, Nishino H (2005) Tumor suppressive effects of tocotrienol in vivo and in vitro. Cancer Lett 229(2):181–191
Wali VB, Bachawal SV, Sylvester PW (2009) Endoplasmic reticulum stress mediates Γ-tocotrienol-induced apoptosis in mammary tumor cells. Apoptosis 14(11):1366–1377
Wali VB, Bachawal SV, Sylvester PW (2009) Suppression in mevalonate synthesis mediates antitumor effects of combined statin and Γ-tocotrienol treatment. Lipids 44(10):925–934
Wali VB, Sylvester PW (2007) Synergistic antiproliferative effects of Γ-tocotrienol and statin treatment on mammary tumor cells. Lipids 42(12):1113–1123
Wan Nazaimoon WM, Khalid BA (2002) Tocotrienols-rich diet decreases advanced glycosylation end-products in non-diabetic rats and improves glycemic control in streptozotocin-induced diabetic rats. Malays J Pathol 24(2):77–82
Wang Y, Jiang Q (2013) Gamma-tocotrienol inhibits lipopolysaccharide-induced interlukin-6 and granulocyte colony-stimulating factor by suppressing C/Ebpbeta and Nf-Kappab in macrophages. J Nutr Biochem 24(6):1146–1152
Wang Y, Park NY, Jang Y, Ma A, Jiang Q (2015) Vitamin E gamma-tocotrienol inhibits cytokine-stimulated Nf-kappab activation by induction of anti-inflammatory A20 via stress adaptive response due to modulation of sphingolipids. J Immunol 195(1):126–133
Watkins T, Lenz P, Gapor A, Struck M, Tomeo A, Bierenbaum M (1993) Gamma-tocotrienol as a hypocholesterolemic and antioxidant agent in rats fed atherogenic diets. Lipids 28(12):1113–1118
Weng-Yew W, Selvaduray KR, Ming CH, Nesaretnam K (2009) Suppression of tumor growth by palm tocotrienols via the attenuation of angiogenesis. Nutr Cancer 61(3):367–373
Wu SJ, Liu PL, Ng LT (2008) Tocotrienol-rich fraction of palm oil exhibits anti-inflammatory property by suppressing the expression of inflammatory mediators in human monocytic cells. Mol Nutr Food Res 52(8):921–929
Xu WL, Liu JR, Liu HK et al (2009) Inhibition of proliferation and induction of apoptosis by Γ-tocotrienol in human colon carcinoma Ht-29 cells. Nutrition 25(5):555–566
Yam ML, Abdul Hafid SR, Cheng HM, Nesaretnam K (2009) Tocotrienols suppress proinflammatory markers and cyclooxygenase-2 expression in Raw264.7 macrophages. Lipids 44(9):787–789
Yano Y, Satoh H, Fukumoto K et al (2005) Induction of cytotoxicity in human lung adenocarcinoma cells by 6-O-carboxypropyl-Α-tocotrienol, a redox-silent derivative of Α-tocotrienol. Int J Cancer 115(5):839–846
Yap SP, Yuen KH, Wong JW (2001) Pharmacokinetics and bioavailability of alpha-, gamma- and delta-tocotrienols under different food status. J Pharm Pharmacol 53(1):67–71
Yoshida Y, Hayakawa M, Habuchi Y, Itoh N, Niki E (2007) Evaluation of lipophilic antioxidant efficacy in vivo by the biomarkers hydroxyoctadecadienoic acid and isoprostane. Lipids 42(5):463–472
Yoshida Y, Niki E, Noguchi N (2003) Comparative study on the action of tocopherols and tocotrienols as antioxidant: chemical and physical effects. Chem Phys Lipids 123(1):63–75
Yu SG, Thomas AM, Gapor A, Tan B, Qureshi N, Qureshi AA (2006) Dose-response impact of various tocotrienols on serum lipid parameters in 5-week-old female chickens. Lipids 41(5):453–461
Yu W, Simmons-Menchaca M, Gapor A, Sanders BG, Kline K (1999) Induction of apoptosis in human breast cancer cells by tocopherols and tocotrienols. Nutr Cancer 33(1):26–32
Zaiden N, Yap WN, Ong S et al (2010) Gamma delta tocotrienols reduce hepatic triglyceride synthesis and Vldl secretion. J Atheroscler Thromb 17(10):1019–1032
Acknowledgements
This work is supported by Universiti Kebangsaan Malaysia via grants GGPM-2015-036.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Chin, KY., Pang, KL., Soelaiman, IN. (2016). Tocotrienol and Its Role in Chronic Diseases. In: Gupta, S., Prasad, S., Aggarwal, B. (eds) Anti-inflammatory Nutraceuticals and Chronic Diseases. Advances in Experimental Medicine and Biology, vol 928. Springer, Cham. https://doi.org/10.1007/978-3-319-41334-1_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-41334-1_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-41332-7
Online ISBN: 978-3-319-41334-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)