Abstract
Osteoclasts are cells derived from bone marrow macrophages and are important in regulating bone resorption during bone homeostasis. Understanding what drives osteoclast differentiation and activity is important when studying diseases characterized by heightened bone resorption relative to formation, such as osteoporosis. In the last decade, studies have indicated that reactive oxygen species (ROS), including superoxide and hydrogen peroxide, are crucial components that regulate the differentiation process of osteoclasts. However, there are still many unanswered questions that remain. This review will examine the mechanisms by which ROS can be produced in osteoclasts as well as how it may affect osteoclast differentiation and activity through its actions on osteoclastogenesis signaling pathways. In addition, the contribution of ROS to the aging-associated disease of osteoporosis will be addressed and how targeting ROS may lead to the development of novel therapeutic treatment options.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone is a dynamic structure dependent upon the constant activities of osteoblastic bone formation and osteoclastic bone resorption. These two actions must be in balance or else pathology results [1–3]. There is a growing amount of evidence that oxidative stress induced by reactive oxygen species (ROS), which increase with aging or with the onset of an inflammatory state, can adversely affect bone homeostasis so that a pro-resorptive environment is favored [1, 4–11]. Novel therapeutic treatment development, therefore, requires an understanding of how ROS is involved in modulating bone biology, physiology, and pathology. This review will predominantly focus on the major findings in the field that link ROS involvement in osteoclast biology with a particular emphasis on mechanisms for ROS formation and actions on signaling pathways. Lastly, how targeting RO S may be a method for treatment in diseases of excess bone resorption, such as osteoporosis, will be addressed.
Importance of ROS in osteoclast biology and mechanisms for ROS formation
Although the deleterious effects of ROS in promoting oxidative stress and disease have elicited much attention in recent years, there is a large body of evidence that ROS, specifically H2O2 and in some cases superoxide, are important for a wide variety of different signaling events within the cell including regulation of mitogen-activated protein kinases (MAPKs), intracellular Ca2+ levels, and transcription factors [12]. Although there are other forms of ROS, such as hydroxyl radicals, the hydroxyl ion, and peroxide, the majority of the osteoclast-ROS literature revolves around superoxide and H2O2. Nitrogen radicals have also been implicated in the regulation of osteoclasts and will be discussed in a subsequent section. In addition to signaling, ROS production also serves as a beneficial function in mediating the oxidative burst process by hematopoietic cells in the innate immune system to protect the body from foreign invaders [13]. It comes as no surprise then, that ROS, particularly superoxide and H2O2, are important in another cell of the hematopoietic lineage—the osteoclast.
Early research in the 1990s that laid the foundation for connecting ROS and osteoclast function predominantly involved the use of in-vitro cultures and in-vivo mouse calvarial models whereby oxidants or antioxidant enzymes were added and osteoclast activity was assessed. For example, some of the first evidence for ROS participation in osteoclast biology was described by Dr. Greg Mundy’s group [9] where they found that addition of the superoxide-producing enzyme, xanthine oxidase, in vivo and in vitro resulted in increased osteoclast numbers and activity. This effect was attenuated when the superoxide-degrading enzyme, superoxide dismutase, was added; however, no change was seen upon the addition of catalase, which scavenges H2O2. Therefore, they concluded that the superoxide radical rather than H2O2 or hydroxyl was important in mediating the enhanced bone resorption. Subsequent studies from other groups also found that superoxide production was present within the osteoclast and suggested that it may be derived from nicotinamide adenine dinucleotide phosphate (NADPH) oxidase localized to the bone–osteoclast interface within the osteoclast ruffled border [14–16]. Further evidence for the involvement of NADPH oxidase was found when addition of a specific inhibitor of NADPH oxidase, diphenylene iodonium (DPI), resulted in a reduction in superoxide production and bone resorption [17, 18]. Other studies, however, indicated that rather than superoxide, H2O2 was the primary ROS responsible for promoting osteoclast formation and activity [8, 19–21]. Specifically, Fraser et al. [19] showed that when xanthine and xanthine oxidase were added to cultures, no increase in osteoclast formation or bone resorbing activity was detected unless the cultures were also supplemented with superoxide dismutase to promote superoxide conversion to H2O2. However, in contradiction to the aforementioned studies supporting the direct involvement of ROS in bone resorption, Hall and colleagues suggested that ROS instead had an indirect effect in activating signaling pathways such as nuclear factor κB (NF-κB) that would promote osteoclast formation and activity [22]. This finding by Hall et al. has been supported by more recent studies, which have explored the mechanism behind the role of ROS in the osteoclast. Although it is still uncertain whether the indirect effect is mediated by superoxide or H2O2, much progress has been made in the past decade in deciphering the mechanism for ROS involvement in the osteoclast. Specifically, ROS has been found to influence several different signaling pathways and are produced following receptor activator of NF-κB ligand (RANKL) stimulation of the undifferentiated cell.
RANKL is also known as TRANCE (tumor necrosis factor (TNF)-related activation-induced cytokine), ODF (osteoclast differentiation factor), and OPGL (osteoprotegerin ligand) and is found on the surface of osteoblasts, osteocytes, stromal cells, and T cells [23]. RANKL interaction with the RANK receptor found on the surface of osteoclasts and precursors leads to the induction of signaling pathways that culminate in the expression of genes that promote osteoclastogenesis and osteoclast activity. The RANK receptor on the plasma membrane is a member of the TNF receptor family and, as such, shares many characteristics with others in this family including the production of ROS upon receptor binding. Some of the first evidence for RANKL-mediated ROS production was provided by Ha and colleagues when they showed that, after 5 min of RANKL stimulation, ROS presence could be detected by an increase in CM-DCF (general ROS detector, dichlorodihydrofluorescein) as well as Amplex Red (H2O2 detector) fluorescence, which could be attenuated by the addition of antioxidants [24]. Also, it was found that N-acetylcysteine (NAC) addition led to a decrease in RANKL-mediated signaling pathways and subsequent decreases in the number of osteoclasts formed as well as osteoclast activity. The RANKL-mediated ROS induction was confirmed in a paper published shortly thereafter where RANKL administration led to an increase in dichlorodihydrofluorescein diacetate (DCFH-DA) fluorescence in both bone marrow macrophage (BMM) and RAW 264.7 cells that was both dose and time dependent with maximal fluorescence occurring at 10 min post stimulation before returning to near baseline levels [25]. In addition, as was seen in the previous paper by Ha et al., supplementing the RANKL-stimulated cells with NAC, a general antioxidant, attenuated the DCFH-DA fluorescence as well as total osteoclast numbers. A more recent study confirmed the deleterious effects of antioxidants on osteoclastogenesis in mouse models lacking the FoxO transcription factors, which drive transcription of the antioxidant catalase [26]. Loss of FoxO resulted in a bone loss phenotype characterized by increased osteoclast numbers and H2O2 accumulation. Direct or indirect regulation of transcription factors is a likely mechanism governing levels of ROS and the process of osteoclastogenesis.
Nox (NADPH oxidase) isoforms as mediators of ROS production
Although there is ample evidence to suggest that redox signaling through ROS is essential during the osteoclast differentiation process, the source of ROS is less understood. The earliest point in RANKL signaling that has been linked to ROS production is the binding of TNF receptor-associated factor 6 (TRAF6) to the cytoplasmic domain of the RANK receptor. Overexpression of a dominant negative TRAF6 mutant in a cell line resulted in decreased DCF fluorescence after RANKL stimulation [25]. Primary BMM lacking TRAF6 similarly showed a decrease in RANKL-dependent ROS production. Because TRAF6 does not directly produce ROS, another link in this chain had to be identified. In the same paper, Lee and colleagues suggested that the main producer of ROS had to be one of the isoforms of NADPH oxidase. When BMM were treated with the NADPH inhibitor DPI before RANKL addition, the RANKL-mediated rise in ROS was negated. Furthermore, DPI also blocked activation of the MAPK and reduced total numbers of osteoclasts formed during the differentiation process in a dose-dependent manner. In further support of Nox involvement, the involvement of Rac1, a guanosine triphosphate (GTP)ase that is important in activating Nox, was assessed. When a dominant negative form of Rac1 was expressed in RAW264.7 cells, osteoclastogenesis was greatly reduced. Also, although Rac1 activity increased when RANKL was added to the cells, the activity was abolished in cells that also expressed the TRAF6 dominant negative mutant, suggesting that the activation of Rac1 is dependent on TRAF6. In another paper by the same group, expression of the dominant negative form of Rac1 also resulted in decreased activation of downstream signaling pathways such as NF-κB [27]. Furthermore, in primary cells from Rac1-deficient mice, osteoclastogenesis was also found to be impaired as well as ROS production [28].
In order to determine which Nox isoform was mediating this effect, Lee et al. assessed mRNA levels of Nox 1, 2, 3, and 4. In unstimulated BMM, they found that Nox2 was present at the highest levels, whereas Nox1 levels were very low and Nox3 and 4 were undetectable. This was also confirmed by a separate group who assessed Nox miRNA levels during the differentiation process. They found that although Nox2 was present in the unstimulated BMMs, miRNA levels were significantly decreased 72 h after RANKL addition [29, 30]. The opposite result was seen for Nox1 where initial levels were low but were increased by 72 h after RANKL. In contrast to these findings, earlier reports indicate that Nox2 (also called gp91phox) is higher in the osteoclast compared to precursors as determined through reverse transcription polymerase chain reaction (RT-PCR) and immunocytochemistry [17]. Also, although Nox4 was not detected in BMM, mRNA was detected after RANKL addition. This is in agreement with previous studies that have shown that Nox4 is present in osteoclasts and may be important in producing superoxide to facilitate bone resorption [31, 32]. When the different Nox isoforms were silenced to evaluate relative contributions to ROS production, siRNA knockdown of Nox2 did not affect RANKL-mediated ROS production or osteoclast formation. Nox1 silencing, on the other hand, greatly reduced ROS production and osteoclastogenesis, suggesting that Nox1 is the main producer of ROS upon RANKL stimulation [25]. The discrepancy between the findings in the literature is a clear indicator that more research needs to be done in order to define the roles of the different Nox isoforms in the osteoclast.
The importance of Nox1 in producing ROS to initiate osteoclastogenesis has been put to question since BMM from Nox1−/− can produce the same number of osteoclasts as WT [29]. There was also no impact on superoxide production in Nox1−/− BMMs or osteoclasts. Contrary to previous studies, siRNA knockdown of Nox1 had no effect on RANKL-mediated ROS production in their experimental conditions, and a reduction was only seen in conditions where both Nox1 and Nox2 were reduced. The results suggest that the loss of Nox1 may be compensated by other Nox isoforms such as Nox2 during the early stages of differentiation and that loss of either alone is not detrimental to osteoclastogenesis. Furthermore, they conclude that osteoclastogenesis is dependent upon Nox isoform switching. While BMMs contain Nox2, which is a potent producer of superoxide that is used during the innate immune response, osteoclasts depend on different isoforms that are functionally similar but produce less superoxide that is sufficient for its cellular processes. Therefore, RANKL addition leads to a switch to the use of Nox1 in early differentiation in order to promote signaling and then later to Nox4, which could be important in mediating bone resorption.
Another major issue that has come up is that although initial studies indicated that Nox2 was important for bone resorption, osteoclasts from mice lacking Nox2 produce the same amount of superoxide and do not exhibit signs of osteopetrosis, which would be expected if bone resorption by osteoclasts were impaired [31]. In these mice, it appears that Nox4 is making up for the lack of Nox2. In a separate study, overexpression of Nox4 was shown to greatly enhance superoxide production and osteoclast resorption activity [32]. This finding as well as other evidence of Nox4 superoxide production in osteoclasts requires further clarification, since many papers in the literature maintain that Nox4 predominantly produces H2O2 rather than superoxide [33, 34]. However, there are studies to support Nox4 superoxide production [35, 36] in other cells in addition to studies using the osteoclast, suggesting that the ROS produced by Nox4 may be dependent upon the cell and conditions.
Nox4 is unique in that it is constitutively active and is found on intracellular membranes such as on the endoplasmic reticulum, mitochondria, and nucleus [34, 36]. A recent study has even suggested that Nox4 may be involved in producing ROS during osteoclastogenesis [37]. In this paper, knocking out Nox4 in mice promoted an osteopenic phenotype that was not due to a defect in bone formation as seen from calcein staining. Further analysis revealed that osteoclast numbers were significantly reduced in addition to circulating bone resorption markers. Loss of Nox4 also downregulated both ROS and Ca2+ levels at day 2 of differentiation, suggesting that Nox4 may play a role in producing H2O2 during that time. Although Nox4 levels are relatively low during the initial time of RANKL stimulation, Nox4 deficiency is also associated with decreased c-Jun N-terminal kinases (JNK) phosphorylation after 15 min of RANKL treatment. Unlike other papers discussing Nox isoforms in osteoclasts, this paper also assessed Nox4 levels in bone loss models. Nox4 osteoclast immunostaining was increased in bones from patients with untreated osteoporosis and Nox4 mRNA levels in bone samples from osteoporosis and Paget’s disease patients were also increased. Furthermore, mouse ovariectomy-induced bone loss led to an increase in Nox4 mRNA and protein levels in bones. When a Nox inhibitor was given to these mice, the bone loss was attenuated compared to controls. Together, the data suggest that NADPH oxidases are essential enzymes that produce ROS during osteoclast differentiation and bone resorption, but it is clearly evident due to the conflicting data that more research needs to be done in this area.
Actions of ROS on osteoclastogenesis signaling pathways
Upon binding of RANKL to the RANK receptor, a multitude of signaling pathways are initiated including MAPKs and those regulated to Ca2+ release that conclude in the activation of transcription factors that will promote the expression of genes to begin the differentiation process. The most important of these is nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1), often described as the master regulator in osteoclastogenesis, which promotes transcription of several genes including TRAP, calcitonin receptor (CTR), cathepsin K, and pro-fusion genes [38]. Importantly, a few days after the initial activation of NFATc1, further signaling thought to come from long-lasting Ca2+ oscillations will trigger auto-amplification of NFATc1 that is essential in greatly increasing gene transcription to drive the precursors towards fusion and maturity [39]. In order to effectively determine how ROS may fit into this regulation scheme, it is important to understand how the different signaling pathways work and where the enzymes may be susceptible to redox regulation. Importantly, the ability of ROS, such as H2O2 and in some cases superoxide, to serve as second messengers in signaling processes has been well described, particularly in signaling pathways that are involved in osteoclastogenesis including NFκB, MAPK, and Ca2+-mediated signaling.
NF-κB
NF-κB was the first eukaryotic transcription factor that was shown to respond to ROS and oxidative stress [40]. Although H2O2 is able to activate NF-κB in some cell types such as certain subtypes of T and B cells as well as human breast MCF-7 cells, ROS-induced activation is not universal across all cell types. In BMMs, antioxidant addition leads to a decrease in NF-κB protein expression upon RANKL administration [24]. However, antioxidants did not affect NF-κB expression when TNF-α was added instead of RANKL, suggesting that osteoclastogenesis initiation mediated by that cytokine may not be dependent upon early ROS signaling. Rather than a direct effect of H2O2 on NF-κB, the evidence suggests that ROS is mediating IκBα phosphorylation and degradation. This is supported by evidence that RANKL-mediated phosphorylation of IκBα can be inhibited by NAC [24].
MAPK
The MAPK species JNK, ERK, and p38 have also been shown to be redox regulated in different circumstances via changes in the thiol oxidation state. For example, JNK and p38 can be activated by H2O2 in perfused rat hearts; however, in vascular smooth muscle, ERK was activated by superoxide rather than H2O2 [12]. In the osteoclast, the current literature points towards MAPK activation being mediated predominantly by H2O2 although the effects of superoxide have not been evaluated. As was the case with NF-κB, antioxidant addition to BMMs supplemented with RANKL was able to decrease the phosphorylated forms of the MAPK [24, 25]. Furthermore, in order to assess the direct effects of ROS on MAPK activation, exogenous H2O2 was added alone and was able to activate the MAPK in a dose-dependent manner [25].
Mutual regulation of ROS and Ca2+ release in the cytosol and mitochondria
There are several different ways that ROS can influence the mobilization of Ca2+ stores from intracellular compartments. One example is a ROS-mediated direct modification of inositol 1,4,5-trisphosphate (IP3) receptor thiol groups that promotes Ca2+ release from the endoplasmic reticulum [41]. ROS-mediated Ca2+ regulation has been shown in osteoclast precursors during the differentiation process [39]. Kim and colleagues used a mouse model that lacked peroxiredoxin II (PrxII), which is a peroxide reductase and can scavenge H2O2 into H2O. Global loss of PrxII leads to a decrease in bone density due to enhanced osteoclast function in vivo. Interestingly, BMM from the PrxII−/− mouse exhibited 2.5× higher levels of intracellular ROS and also had spontaneous Ca2+ oscillations that were present even without RANKL stimulation, suggesting that the oscillations were largely dependent upon ROS. They also found that the Ca2+ oscillations required RANKL-mediated Rac1 activation, which peaks after 2 days of stimulation with RANKL. In order to determine if ROS is important in the maintenance of Ca2+ oscillations, they next pretreated BMM with N-acetylcysteine (NAC) before RANKL stimulation or added NAC during the oscillations. They found that pretreatment resulted in a complete ablation of Ca2+ response while concurrent treatment also inhibited the oscillations, suggesting that ROS is necessary to maintain continued Ca2+ signaling. Furthermore, the patterns of ROS and Ca2+ levels during the differentiation process were very similar with both peaking at around day 2 of differentiation, which is when NFATc1 levels are also at a maximum. This data strongly suggests that not only are ROS essential in maintaining Ca2+ signaling throughout differentiation, but also that they may be crucial in inducing NFATc1 amplification in order to promote committed cell fusion and expression of osteoclast genes.
Since the endoplasmic reticulum is in close contact with mitochondria, localized increases in cytosolic Ca2+ concentrations likely have an effect on mitochondrial function, such as increasing mitochondrial ROS production as well as regulating oxidative phosphorylation [42]. If Ca2+ concentrations become excessive, such as in conditions brought about by cellular stress, the increased Ca2+ will adversely alter mitochondrial membrane potential and ATP synthesis. Furthermore, if many mitochondria within the cell are affected, cellular apoptosis or necrosis may be triggered [43]. However, localized release of Ca2+ that functions as a secondary signaling molecule is likely at a concentration that will not overly stress the cell, but is still likely to influence mitochondrial ROS production.
The mitochondria contain a uniporter and ryanodine receptors that are able to take up intracellular Ca2+ released from the endoplasmic reticulum. Although the uniporter has such low affinity that it was initially thought to not play a role in Ca2+ homeostasis at physiological levels, intracellular Ca2+ probes have proven otherwise [42]. The increase in intermitochondrial Ca2+ is then able to escalate the rate of oxidative phosphorylation by activating isocitrate dehydrogenase, α-ketoglutarate dehydrogenase, and pyruvate dehydrogenase [44], which will also increase the amount of superoxide produced. With respect to the osteoclast, it has been shown that mitochondrial ROS production is necessary during osteoclastogenesis since addition of an antioxidant specifically targeted to scavenge mitochondrial ROS leads to decreased osteoclast formation [45]. This paper also showed that conditions of hypoxia led to increased mitochondrial ROS production, which promoted higher calcinuerin and NF-κB activity as well as increased numbers of osteoclasts. A recent publication provided additional evidence for the importance of mitochondrial ROS in osteoclastogenesis by using a mouse model where human catalase was expressed in the mitochondria only in cells with the LysM promoter [26]. They found that mitochondrial catalase expression led to an increase in bone mass due to a reduction in the number and activity of osteoclasts. Furthermore, the antioxidant expression was sufficient to prevent ovariectomy-induced bone loss, suggesting that a primary mechanism mediating bone loss following hormonal withdrawal involves increased H2O2 within the mitochondria. However, the origin and regulating mechanisms for mitochondrial H2O2 production remain to be uncovered.
Iron regulation by Steap4 and TfR1
Other interesting pathways that are activated by ROS pertain to mitochondrial biogenesis and iron regulation. Zhou et al. [46] showed that Steap4, which is an endosomal ferrireductase that reduces Fe3+ to Fe2+, is upregulated during osteoclast differentiation. Loss of Steap4 led to attenuated osteoclast differentiation as well as decreased intracellular and mitochondrial ROS, and iron content in osteoclast precursors. Subsequently, the decreased iron content suppressed mitochondrial biogenesis as seen from decreased mitochondrial markers. In a previous paper by Ishii et al. [47], iron concentration was shown to also be regulated by transferrin receptor 1 (TfR1), which is upstream of Steap4 and binds extracellular Fe3+. TfR1 loss also resulted in a reduction of osteoclastogenesis, mitochondrial biogenesis, and mitochondrial ROS levels. With regards to signaling pathways, Steap4 knockdown inhibited cAMP response element-binding protein (CREB) activation in the osteoclast precursors, but there were relatively minor effects on activated MAPKs, Akt, and IĸB. The authors suggest that this discrepancy could be because the subcellular localization of ROS production is important in activating different pathways (i.e., plasma membrane generation for Nox signaling versus mitochondrial for CREB signaling). Furthermore, there may be a temporal component as well where mitochondrial ROS generation is more important in activating signaling pathways later in the differentiation process such as the Ca2+/calmodulin pathway that facilitate NFATc1 auto-amplification.
Nrf2/Keap1
Nrf2, which is a transcriptional regulator of several cytoprotective enzymes including heme oxygenase-1, NAD-(P)H:quinone reductase, γ-glutamylcysteine synthetase, and glucose-6-phosphate dehydrogenase becomes downregulated during osteoclastogenesis in order to decrease the transcription of the aforementioned enzymes so that ROS levels can be maintained [48, 49]. This is accomplished through the upregulation of Keap1, which prevents Nrf2 translocation to the nucleus. Furthermore, loss of Nrf2 is associated with increased ROS levels and total osteoclast numbers whereas Nrf2 overexpression had the opposite effect. As the papers elucidating the effects of Nrf2 and Keap1 on osteoclastogenesis are very recent, their relative contributions to the process will likely be investigated further in the future.
Reactive nitrogen species (RNS)
Although this review has focused predominantly on ROS such as superoxide and H2O2 as mediators of osteoclastogenesis regulation, we would be remiss if we did not include a brief discussion of the potential role for RNS in this process. Several groups have shown evidence that it may be a central regulator of bone resorption with implications for diseases involving increased inflammation such as arthritis and osteoporosis [50]. In particular, nitric oxide (NO), which is produced by NO synthase in response to inflammatory cytokines, inhibits osteoclast differentiation and activity when present at high levels through calcium-mediated changes in cell shape and motility [50–54]. However, when present at low levels, NO instead potentiates bone resorption, which was seen when the NO synthase inhibitor, L-NG-monomethyl arginine (LMMA) was added in vitro [52, 54]. Therefore, it has been postulated that low constitutive levels of NO are important in order to promote osteoclast differentiation and function, particularly through actin remodeling [55]. Furthermore, NO can react with ROS in order to generate the more toxic radicals hydroxyl and peroxynitrite, which are the most reactive radical species and are known to readily react with cellular macromolecules and induce tissue damage [52]. These radicals may also play a role in osteoclastogenesis, particularly in contributing to disease states; however, more work remains to be done.
Therapeutic approaches targeting osteoclastic ROS
Although the deleterious effects of ROS in damaging macromolecules and causing cellular stress are evident, identifying how it is involved in signaling will have tremendous implications as the research moves forward. For example, many bone pathologies such as osteoporosis, inflammatory bone diseases, and certain cancers have been linked to states of higher ROS. With regard to post-menopausal osteoporosis, one paper calculated the total antioxidant and oxidant, and used these values to obtain an oxidative stress index (OSI) and found that the OSI had a significant negative correlation with bone mineral density (BMD) of the femoral neck and lumbar vertebrae in postmenopausal osteoporosis patients compared to healthy controls [4]. Furthermore, the total oxidant status and oxidative stress index were significantly higher while the antioxidant status was lower in women with post-menopausal osteoporosis. Also, Baek and colleagues [56] compared the serum 8-hydroxy-20-deoxyguanosine (8-OH-dG) levels, a marker for oxidative damage to DNA, with BMD measurements in a group of healthy post-menopausal women. They found that the 8-OH-dG levels were negatively correlated with BMD levels in the lumbar spine, total hip, femoral neck, and trochanter. In addition, they also found a positive correlation with DNA damage and type I collagen C-telopeptide (ICTP) levels, suggesting that oxidative stress in the bone is associated with increased bone resorption in post-menopausal women. Antioxidant defenses in postmenopausal osteoporotic women as well as in mouse postmenopausal models are significantly decreased, leading to increased levels of ROS and bone loss [5–7]. Furthermore, treatment with pegylated catalase in one study was able to attenuate the ovariectomy-induced bone loss [57]. In another study involving osteoporotic males, a negative correlation was seen for superoxide dismutase activity and lumbar BMD; however, although the authors concluded that there was an increase in free radical levels, no link between free radicals and bone turnover was found [10]. Because of the link between oxidative stress and bone pathology, understanding how ROS interacts with osteoclasts as well as other cells in the bone including osteocytes and osteoblasts could lead to the development of novel therapeutic treatments to target ROS and halt excessive bone resorption.
There is some evidence in the literature that different compounds with antioxidant activity may be beneficial to bone health. In one case, dried plum polyphenols, containing antioxidant and anti-inflammatory properties, given to male and female osteoporosis rat models resulted in attenuated bone loss compared to controls [58, 59]. Part of the rescued bone phenotype was attributed to antioxidant effects on the osteoblast since ROS, which increases in animal models after operations to induce sex hormone deficiency, has been shown to upregulate expression of RANKL [60]. Additional studies revealed that dried plum polyphenols also cause decreased osteoclast differentiation, NFATc1 expression, and bone resorption [61]. Simvastatin, a 3-hydroxy-3 methylglutaryl coenzyme A (HMG Co-A) reductase inhibitor, used to treat high cholesterol, has also been shown to function as an antioxidant that can affect bone formation and resorption. Specifically, in the osteoclast, simvastatin administration led to a reduction in ROS levels and subsequent downregulation of osteoclastogenesis signaling pathways and osteoclast formation [62]. Many other compounds in the literature are suggested to improve conditions of excessive bone resorption through antioxidant scavenging and are listed Table 1. Although there are many other compounds in the current literature that are able to suppress osteoclastogenesis, not all examine whether the results could be due to an antioxidant property of the compound. Because of the importance of ROS in osteoclastogenesis, all studies that test how different compounds affect this process should also include ROS measurements when they evaluate possible mechanisms. Importantly, although the aforementioned research appears promising with respect to using antioxidants to target ROS and improve bone quality, it is essential to remember that ROS is present throughout all cells of the body. Therefore, any intervention against ROS will have other effects that are not limited to the bone. Because of the non-specificity of targeting ROS, it is appropriate to question whether antioxidants are the most efficient therapeutic tools to combating bone frailty. As the research in this area moves forward, it is imperative that these issues are addressed, especially in relation to the identification of bone-specific molecules and pathways. However, as many compounds listed in Table 1 with antioxidant properties have shown to improve bone quality in animal models and humans, the potential benefits of targeting ROS cannot be ignored.
Concluding remarks
Combined, the evidence strongly supports that ROS is an essential component in osteoclast biology for the regulation of osteoclast differentiation and resorption. Figure 1 proposes a possible model for the role of ROS during the different stages of osteoclast differentiation by combining the different concepts discussed in this review. While this is a hypothesized model and not a complete picture of how ROS is involved in osteoclast biology, it should bring attention to the many questions that remain to be answered. For example, the mechanism behind the initial production of ROS is unclear due to the mixed results from Nox 1 and 2 knockout models and it has not yet been established whether Nox4 may also be a contributor. In addition it is unknown what particular ROS−H2O2 or superoxide, mediates the different effects on the signaling pathways. Likely, both are involved, but their relative contributions need to be dissected. A very intriguing question concerns the role of mitochondrial ROS production during osteoclastogenesis. Although the literature suggests that mitochondrial ROS may be more important during the late stages of osteoclast differentiation, it has not been conclusively determined. Furthermore, there is also a possibility that mitochondrial ROS either from the electron transport chain or even from Nox4 could be involved in activating signaling pathways during early differentiation. Lastly, the role of ROS in the bone resorption process is unclear. Although it has been suggested that ROS only plays an indirect role in activating pro-resorption pathways, some early papers did show evidence that superoxide or H2O2 was present within the osteoclast ruffled border. As researchers uncover more information about how ROS is involved in regulating osteoclast biology, this will lead the way to understanding mechanisms that promote excessive bone resorption prevalent in various bone pathologies. Already, promising results have been reported involving the use of compounds that scavenge ROS in the treatment of diseases such as osteoporosis. More novel mechanistic and drug discoveries are surely on the horizon, which makes this an exciting time to be involved in osteoclast and bone research.
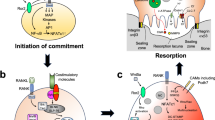
Proposed model for early and late osteoclast differentiation events mediated by ROS. Top Early differentiation. RANKL interaction with the RANK receptor on BMM initiates TRAF6 localization to the cytoplasmic tail of RANK and the initiation of many signaling pathways. Rac1 activation induces superoxide production by Nox, which can then be reduced to H2O2 by SOD1. It is unclear which Nox isoform is responsible although it is likely that it is Nox1, which can be substituted for Nox2 in mice deficient in Nox1. Although Nox4 levels in BMM are low, it may also contribute to ROS production. Nox4 is depicted in the mitochondria (Mito) in this figure, but its subcellular location has not been verified. In addition to ROS production by Nox1 or Nox2, it is possible that mitochondrial ROS also adds to total cellular ROS levels. Red dashed boxes depicted linked to ROS via dashed arrows indicate where ROS regulation may occur as described previously in this review. Green lettering depicts transcription factors. Together, the ROS-mediated signaling pathways induce initial NFATc1 activation, which transcribes osteoclast-specific genes at low levels. Bottom Late differentiation. At this point, the pathways that were initially activated in early differentiation are still present. Importantly, ROS production peaks at this time as well as cellular Ca2+ levels from the endoplasmic reticulum (ER). Current evidence suggests that the heightened ROS production is likely to be mitochondrial in origin. Mitochondrial biogenesis is increased due to Ca2+-mediated activation of PGC-1β, which is aided by increased iron uptake by TfR1 and Steap4. The increased ROS contributes to the long-lasting Ca2+ oscillations and downstream signaling pathways, culminating in NFATc1 auto-amplification and increased transcription of pro-fusion and osteoclast-specific genes. As in the top figure, red dashed boxes connected to ROS via dashed arrows indicate likely areas of ROS interaction
References
Wauquier F, Leotoing L, Coxam V, Guicheux J, Wittrant Y (2009) Oxidative stress in bone remodelling and disease. Trends Mol Med 15:468–477
Vaananen HK, Zhao H, Mulari M, Halleen JM (2000) The cell biology of osteoclast function. J Cell Sci 113:377–381
Boyle W, Simonet W, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342
Altindag O, Erel O, Soran N, Celik H, Selek S (2008) Total oxidative/anti-oxidative status and relation to bone mineral density in osteoporosis. Rheumatol Int 28:317–321
Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O’Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC (2007) Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 282:27285–27297
Muthusami S, Ramachandran H, Muthusamy B, Vasudevan G, Prabhu V, Subramaniam V, Jagadeesan A, Narasimhan S (2005) Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin Chim Acta 360:81–86
Ozgocmen S, Kaya H, Fadillioglu E, Aydogan R, Yilmaz Z (2007) Role of antioxidant systems, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Mol Cell Biochem 295:45–52
Bax BE, Alam ASMT, Banerji B, Bax CMR, Bevis PJR, Stevens CR, Moonga BS, Blake DR, Zaidi M (1992) Stimulation of osteoclastic bone-resorption by hydrogen-peroxide. Biochem Biophys Res Commun 183:1153–1158
Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR (1990) Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest 85:632–639
Yalin S, Bagis S, Polat G, Dogruer N, Aksit SC, Hatungil R, Erdogan C (2005) Is there a role of free oxygen radicals in primary male osteoporosis ? Clin Exp Rheumatol 23:689–692
Harman D (1956) Aging—a theory based on free-radical and radiation-chemistry. J Gerontol 11:298–300
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Sareila O, Kelkka T, Pizzolla A, Hultqvist M, Holmdahl R (2011) NOX2 complex-derived ROS as immune regulators. Antioxid Redox Signal 15:2197–2208
Key LL, Ries WL, Taylor RG, Hays BD, Pitzer BL (1990) Oxygen derived free-radicals in osteoclasts—the specificity and location of the nitroblue tetrazolium Reaction. Bone 11:115–119
Key LL, Wolf WC, Gundberg CM, Ries WL (1994) Superoxide and bone-resorption. Bone 15:431–436
Steinbeck MJ, Appel WH, Verhoeven AJ, Karnovsky MJ (1994) Nadph-oxidase expression and in-situ production of superoxide by osteoclasts actively resorbing bone. J Cell Biol 126:765–772
Yang S, Ries WL, Key LL (1998) Nicotinamide adenine dinucleotide phosphate oxidase in the formation of superoxide in osteoclasts. Calcif Tissue Int 63:346–350
Darden AG, Ries WL, Wolf WC, Rodriguiz RM, Key LL (1996) Osteoclastic superoxide production and bone resorption: stimulation and inhibition by modulators of NADPH oxidase. J Bone Miner Res 11:671–675
Fraser JH, Helfrich MH, Wallace HM, Ralston SH (1996) Hydrogen peroxide, but not superoxide, stimulates bone resorption in mouse calvariae. Bone 19:223–226
Suda N, Morita I, Kuroda T, Murota SI (1993) Participation of oxidative stress in the process of osteoclast differentiation. Biochim Biophys Acta 1157:318–323
Kim H, Kim IY, Lee SY, Jeong D (2006) Bimodal actions of reactive oxygen species in the differentiation and bone-resorbing functions of osteoclasts. FEBS Lett 580:5661–5665
Hall TJ, Schaeublin M, Jeker H, Fuller K, Chambers TJ (1995) The role of reactive oxygen intermediates in osteoclastic bone-resorption. Biochem Biophys Res Commun 207:280–287
Wong BR, Josien R, Choi Y (1999) TRANCE is a TNF family member that regulates dendritic cell and osteoclast function. J Leukoc Biol 65:715–724
Ha H, Kwak HB, Lee SW, Jin HM, Kim HM, Kim HH, Lee ZH (2004) Reactive oxygen species mediate RANK signaling in osteoclasts. Exp Cell Res 301:119–127
Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, Kim N, Lee SY (2005) A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 106:852–859
Bartell S, Kim H, Ambrogini E, Han L, Iyer S, Serra Ucer S, Rabinovitch P, Jilka R, Weinstein R, Zhao H, O’Brien C, Manolagas S, Almeida M (2014) FoxO proteins restrain osteoclastogenesis and bone resorption by attenuating H2O2 accumulation. Nat Commun 5:3773
Lee NK, Choi HK, Kim DK, Lee SY (2006) Rac1 GTPase regulates osteoclast differentiation through TRANCE-induced NF-kappa B activation. Mol Cell Biochem 281:55–61
Wang YQ, Lebowitz D, Sun CX, Thang H, Grynpas MD, Glogauer M (2008) Identifying the relative contributions of Rac1 and Rac2 to osteoclastogenesis. J Bone Miner Res 23:260–270
Sasaki H, Yamamoto H, Tominaga K, Masuda K, Kawai T, Teshima-Kondo S, Rokutan K (2009) NADPH oxidase-derived reactive oxygen species are essential for differentiation of a mouse macrophage cell line (RAW264.7) into osteoclasts. J Med Invest 56:33–41
Sasaki H, Yamamoto H, Tominaga K, Masuda K, Kawai T, Teshima-Kondo S, Matsuno K, Yabe-Nishimura C, Rokutan K (2009) Receptor activator of nuclear factor-kappa B ligand-induced mouse osteoclast differentiation is associated with switching between NADPH oxidase homologues. Free Radic Biol Med 47:189–199
Yang S, Madyastha P, Bingel S, Ries W, Key L (2001) A new superoxide-generating oxidase in murine osteoclasts. J Biol Chem 276:5452–5458
Yang S, Zhang YZ, Ries W, Key L (2004) Expression of Nox4 in osteoclasts. J Cell Biochem 92:238–248
Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG (2006) Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18:69–82
Takac I, Schroder K, Zhang LL, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP (2011) The e-loop is involved in hydrogen peroxide formation by the nadph oxidase Nox4. J Biol Chem 286:13304–13313
Case AJ, Li SM, Basu U, Tian J, Zimmerman MC (2013) Mitochondrial-localized NADPH oxidase 4 is a source of superoxide in angiotensin II-stimulated neurons. Am J Physiol Heart Circ Physiol 305:H19–H28
Block K, Gorin Y, Abboud HE (2009) Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci USA 106:14385–14390
Goettsch C, Babelova A, Trummer O, Erben RG, Rauner M, Rammelt S, Weissmann N, Weinberger V, Benkhoff S, Kampschulte M, Obermayer-Pietsch B, Hofbauer LC, Brandes RR, Schroder K (2013) NADPH oxidase 4 limits bone mass by promoting osteoclastogenesis. J Clin Investig 123:4731–4738
Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E, Takayanagi H (2005) Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med 202:1261–1269
Kim MS, Yang YM, Son A, Tian YS, Lee SI, Kang SW, Muallem S, Shin DM (2010) RANKL-mediated reactive oxygen species pathway that induces long lasting Ca2+ oscillations essential for osteoclastogenesis. J Biol Chem 285:6913–6921
Schreck R, Rieber P, Baeuerle PA (1991) Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa-B transcription factor and HIV-1. EMBO J 10:2247–2258
Decuypere JP, Monaco G, Missiaen L, De Smedt H, Parys JB, Bultynck G (2011) IP(3) receptors, mitochondria, and Ca signaling: implications for aging. J Aging Res 2011:920178
Rizzuto R, Brini M, Murgia M, Pozzan T (1993) Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science 262:744–747
Figueira TR, Barros MH, Camargo AA, Castilho RF, Ferreira JCB, Kowaltowski AJ, Sluse FE, Souza-Pinto NC, Vercesi AE (2013) Mitochondria as a source of reactive oxygen and nitrogen species: from molecular mechanisms to human health. Antioxid Redox Signal 18:2029–2074
McCormack JG, Halestrap AP, Denton RM (1990) Role of calcium-ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev 70:391–425
Srinivasan S, Koenigstein A, Joseph J, Sun L, Kalyanaraman B, Zaidi M, Avadhani NG (2010) Role of mitochondrial reactive oxygen species in osteoclast differentiation. Skelet Biol Med 1192:245–252
Zhou J, Ye S, Fujiwara T, Manolagas SC, Zhao H (2013) Steap4 plays a critical role in osteoclastogenesis in vitro by regulating cellular iron/reactive oxygen species (ROS) levels and cAMP response element-binding protein (CREB) activation. J Biol Chem 288:30064–30074
Ishii KA, Fumoto T, Iwai K, Takeshita S, Ito M, Shimohata N, Aburatani H, Taketani S, Lelliott CJ, Vidal-Puig A, Ikeda K (2009) Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med 15:259–266
Kanzaki H, Shinohara F, Kajiya M, Kodama T (2013) The Keap1/Nrf2 protein axis plays a role in osteoclast differentiation by regulating intracellular reactive oxygen species signaling. J Biol Chem 288:23009–23020
Hyeon S, Lee H, Yang Y, Jeong W (2013) Nrf2 deficiency induces oxidative stress and promotes RANKL-induced osteoclast differentiation. Free Radic Biol Med 65:789–799
Brandi ML, Hukkanen M, Umeda T, Moradi-Bidhendi N, Bianchi S, Gross SS, Polak JM, MacIntyre I (1995) Bidirectional regulation of osteoclast function by nitric oxide synthase isoforms. Proc Natl Acad Sci USA 92:2954–2958
Mancini L, Moradi-Bidhendi N, Brandi ML, MacIntyre I (1998) Nitric oxide superoxide and peroxynitrite modulate osteoclast activity. Biochem Biophys Res Commun 243:785–790
Evans DM, Ralston SH (1996) Nitric oxide and bone. J Bone Miner Res 11:300–305
Lowik CW, Nibbering PH, van de Ruit M, Papapoulos SE (1994) Inducible production of nitric oxide in osteoblast-like cells and in fetal mouse bone explants is associated with suppression of osteoclastic bone resorption. J Clin Invest 93:1465–1472
Ralston SH, Ho LP, Helfrich MH, Grabowski PS, Johnston PW, Benjamin N (1995) Nitric oxide: a cytokine-induced regulator of bone resorption. J Bone Miner Res 10:1040–1049
Nilforoushan D, Gramoun A, Glogauer M, Manolson MF (2009) Nitric oxide enhances osteoclastogenesis possibly by mediating cell fusion. Nitric Oxide 21:27–36
Baek KH, Oh KW, Lee WY, Lee SS, Kim MK, Kwon HS, Rhee EJ, Han JH, Song KH, Cha BY, Lee KW, Kang MI (2010) Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif Tissue Int 87:226–235
Lean JM, Jagger CJ, Kirstein B, Fuller K, Chambers TJ (2005) Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation. Endocrinology 146:728–735
Deyhim F, Stoecker BJ, Brusewitz GH, Devareddy L, Arjmandi BH (2005) Dried plum reverses bone loss in an osteopenic rat model of osteoporosis. Menopause 12:755–762
Franklin M, Bu SY, Lerner MR, Lancaster EA, Bellmer D, Marlow D, Lightfoot SA, Arjmandi BH, Brackett DJ, Lucas EA, Smith BJ (2006) Dried plum prevents bone loss in a male osteoporosis model via IGF-I and the RANK pathway. Bone 39:1331–1342
Bai XC, Lu D, Liu AL, Zhang ZM, Li XM, Zou ZP, Zeng WS, Cheng BL, Luo SQ (2005) Reactive oxygen species stimulates receptor activator of NF-kappa B ligand expression in osteoblast. J Biol Chem 280:17497–17506
Bu SY, Lerner M, Stoecker BJ, Boldrin E, Brackett DJ, Lucas EA, Smith BJ (2008) Dried plum polyphenols inhibit osteoclastogenesis by downregulating NFATc1 and inflammatory mediators. Calcif Tissue Int 82:475–488
Moon HJ, Kim SE, Yun YP, Hwang YS, Bang JB, Park JH, Kwon IK (2011) Simvastatin inhibits osteoclast differentiation by scavenging reactive oxygen species. Exp Mol Med 43:605–612
Kim HJ, Chang EJ, Kim HM, Lee SB, Kim HD, Su KG, Kim HH (2006) Antioxidant alpha-lipoic acid inhibits osteoclast differentiation by reducing nuclear factor-kappaB DNA binding and prevents in vivo bone resorption induced by receptor activator of nuclear factor-kappaB ligand and tumor necrosis factor-alpha. Free Radic Biol Med 40:1483–1493
Koh JM, Lee YS, Byun CH, Chang EJ, Kim H, Kim YH, Kim HH, Kim GS (2005) Alpha-lipoic acid suppresses osteoclastogenesis despite increasing the receptor activator of nuclear factor kappaB ligand/osteoprotegerin ratio in human bone marrow stromal cells. J Endocrinol 185:401–413
Polat B, Halici Z, Cadirci E, Albayrak A, Karakus E, Bayir Y, Bilen H, Sahin A, Yuksel TN (2013) The effect of alpha-lipoic acid in ovariectomy and inflammation-mediated osteoporosis on the skeletal status of rat bone. Eur J Pharmacol 718:469–474
Lever JH (2002) Paget’s disease of bone in Lancashire and arsenic pesticide in cotton mill wastewater: a speculative hypothesis. Bone 31:434–436
Szymczyk KH, Kerr BAE, Freeman TA, Adams CS, Steinbeck MJ (2006) Involvement of hydrogen peroxide in the differentiation and apoptosis of preosteoclastic cells exposed to arsenite. Biochem Pharmacol 72:761–769
Xiao XH, Liao EY, Zhou HD, Dai RC, Yuan LQ, Wu XP (2005) Ascorbic acid inhibits osteoclastogenesis of RAW264.7 cells induced by receptor activated nuclear factor kappaB ligand (RANKL) in vitro. J Endocrinol Invest 28:253–260
Le Nihouannen D, Barralet JE, Fong JE, Komarova SV (2010) Ascorbic acid accelerates osteoclast formation and death. Bone 46:1336–1343
Sanbe T, Tomofuji T, Ekuni D, Azuma T, Irie K, Tamaki N, Yamamoto T, Morita M (2009) Vitamin C intake inhibits serum lipid peroxidation and osteoclast differentiation on alveolar bone in rats fed on a high-cholesterol diet. Arch Oral Biol 54:235–240
Kim MH, Ryu SY, Bae MA, Choi JS, Min YK, Kim SH (2008) Baicalein inhibits osteoclast differentiation and induces mature osteoclast apoptosis. Food Chem Toxicol 46:3375–3382
Moon HJ, Ko WK, Han SW, Kim DS, Hwang YS, Park HK, Kwon IK (2012) Antioxidants, like coenzyme Q10, selenite, and curcumin, inhibited osteoclast differentiation by suppressing reactive oxygen species generation. Biochem Biophys Res Commun 418:247–253
Léotoing L, Wauquier F, Guicheux J, Miot-Noirault E, Wittrant Y, Coxam V (2013) The polyphenol fisetin protects bone by repressing NF-kappa B and MKP-1-dependent signaling pathways in osteoclasts. Plos One 8:e68388
Sakai E, Shimada-Sugawara M, Yamaguchi Y, Sakamoto H, Fumimoto R, Fukuma Y, Nishishita K, Okamoto K, Tsukuba T (2013) Fisetin inhibits osteoclastogenesis through prevention of RANKL-induced ROS production by Nrf2-mediated up-regulation of phase II antioxidant enzymes. J Pharmacol Sci 121:288–298
Guo JD, Li L, Shi YM, Wang HD, Hou SX (2013) Hydrogen water consumption prevents osteopenia in ovariectomized rats. Br J Pharmacol 168:1412–1420
Li DZ, Zhang QX, Dong XX, Li HD, Ma X (2013) Treatment with hydrogen molecules prevents RANKL-induced osteoclast differentiation associated with inhibition of ROS formation and inactivation of MAPK, AKT and NF-kappa B pathways in murine RAW264.7 cells. J Bone Miner Metab 32:494–504
Kondo H, Togari A (2011) Continuous treatment with a low-dose beta-agonist reduces bone mass by increasing bone resorption without suppressing bone formation. Calcif Tissue Int 88:23–32
Kondo H, Takeuchi S, Togari A (2013) beta-Adrenergic signaling stimulates osteoclastogenesis via reactive oxygen species. Am J Physiol Endocrinol Metab 304:E507–E515
Rao LG, Krishnadev N, Banasikowska K, Rao AV (2003) Lycopene I–effect on osteoclasts: lycopene inhibits basal and parathyroid hormone-stimulated osteoclast formation and mineral resorption mediated by reactive oxygen species in rat bone marrow cultures. J Med Food 6:69–78
Arshad A, Sengupta S, Sharma S, Ghosh R, Sawlani V, Singh MM (2004) In vitro anti-resorptive activity and prevention of ovariectomy-induced osteoporosis in female Sprague-Dawley rats by ormeloxifene, a selective estrogen receptor modulator. J Steroid Biochem Mol Biol 91:67–78
Kharkwal G, Chandra V, Fatima I, Dwivedi A (2012) Ormeloxifene inhibits osteoclast differentiation in parallel to downregulating RANKL-induced ROS generation and suppressing the activation of ERK and JNK in murine RAW264.7 cells. J Mol Endocrinol 48:261–270
Nomura M, Yoshimura Y, Kikuiri T, Hasegawa T, Taniguchi Y, Deyama Y, Koshiro K, Sano H, Suzuki K, Inoue N (2011) Platinum nanoparticles suppress osteoclastogenesis through scavenging of reactive oxygen species produced in RAW264.7 cells. J Pharmacol Sci 117:243–252
Oka Y, Iwai S, Amano H, Irie Y, Yatomi K, Ryu K, Yamada S, Inagaki K, Oguchi K (2012) Tea polyphenols inhibit rat osteoclast formation and differentiation. J Pharmacol Sci 118:55–64
He X, Andersson G, Lindgren U, Li Y (2010) Resveratrol prevents RANKL-induced osteoclast differentiation of murine osteoclast progenitor RAW 264.7 cells through inhibition of ROS production. Biochem Biophys Res Commun 401:356–362
Kyung TW, Lee JE, Shin HH, Choi HS (2008) Rutin inhibits osteoclast formation by decreasing reactive oxygen species and TNF-alpha by inhibiting activation of NF-kappa B. Exp Mol Med 40:52–58
Horcajada-Molteni MN, Crespy V, Coxam V, Davicco MJ, Remesy C, Barlet JP (2000) Rutin inhibits ovariectomy-induced osteopenia in rats. J Bone Miner Res 15:2251–2258
Kim MH, Ryu SY, Choi JS, Min YK, Kim SH (2009) Saurolactam inhibits osteoclast differentiation and stimulates apoptosis of mature osteoclasts. J Cell Physiol 221:618–628
Han KY, Yang D, Chang EJ, Lee Y, Huang H, Sung SH, Lee ZH, Kim YC, Kim HH (2007) Inhibition of osteoclast differentiation and bone resorption by sauchinone. Biochem Pharmacol 74:911–923
Ahn KS, Sethi G, Chaturvedi MM, Aggarwal BB (2008) Simvastatin, 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, suppresses osteoclastogenesis induced by receptor activator of nuclear factor-kappa B ligand through modulation of NF-kappa B pathway. Int J Cancer 123:1733–1740
Hie M, Tsukamoto I (2011) Administration of zinc inhibits osteoclastogenesis through the suppression of RANK expression in bone. Eur J Pharmacol 668:140–146
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Callaway, D.A., Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J Bone Miner Metab 33, 359–370 (2015). https://doi.org/10.1007/s00774-015-0656-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-015-0656-4