Abstract
Carotenoids are the most important biocolor isoprenoids responsible for yellow, orange and red colors found in nature. In plants, they are synthesized in plastids of photosynthetic and sink organs and are essential molecules for photosynthesis, photo-oxidative damage protection and phytohormone synthesis. Carotenoids also play important roles in human health and nutrition acting as vitamin A precursors and antioxidants. Biochemical and biophysical approaches in different plants models have provided significant advances in understanding the structural and functional roles of carotenoids in plants as well as the key points of regulation in their biosynthesis. To date, different plant models have been used to characterize the key genes and their regulation, which has increased the knowledge of the carotenoid metabolic pathway in plants. In this chapter a description of each step in the carotenoid synthesis pathway is presented and discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Carotenoid plant models
- Synthesis regulation
- Carotenoid gene characterization
- Multienzymatic complex
- Key enzymes
1 Introduction
In nature, colors obtained from fruits, flowers, roots, vegetables and microalgae are called biocolors because of their biological origin (Pattnaik et al. 1997). Biocolors are mainly attributed to chemically distinct pigments such as carotenoids, flavonoids, betalains, and chlorophylls , among others, with carotenoids being mainly responsible for yellow, orange and red colors (Grotewold 2006). Carotenoids are synthesized in plastids of all photosynthetic organisms (Cuttriss et al. 2011) as well as in fruits, flowers, seeds and reserve roots (Ruiz-Sola and Rodríguez-Concepción 2012). Some bacteria and fungi are also capable of producing carotenoids in response to growth and environmental conditions (Velayos et al. 2000; Iniesta et al. 2008). Other organisms, such as animals do not synthesize carotenoids and must acquire them through dietary intake as these pigments, especially α-carotene , β-carotene and β-cryptoxanthine, are precursors of vitamine A, retinal and retinoic acid, which play essential roles in nutrition, vision and cellular differentiation, respectively (Krinsky et al. 1994). Some curious exceptions exist in nature such as the synthesis of carotenoids in human protist parasites and aphids (Tonhosolo et al. 2009; Moran and Jarvik 2010), which are explained by the existence of a remnant plastid, known as an apicoplast, and as a result of lateral transfer of carotenogenic genes from a fungus respectively. This makes aphids the only known animal to date capable of synthesizing their own carotenoids.
In animals, the regular ingestion of carotenoids also delays aging due to their antioxidant properties (Bartley and Scolnik 1995; Snodderly 1995; Mayne 1996; Giuliano 2000; Delcourt et al. 2006; Rao and Rao 2007; Zu et al. 2014). Birds, fish and crustaceans utilize carotenoids for pigmentation and nutritional purposes too. For example, the cetocarotenoid astaxanthin is responsible for the orange color of salmon meat and lobster shells (Grotewold 2006, see Chap. 3). Carotenoids also have commercial relevance in the food and cosmetic industries (Klaui 1982; Bjerkeng 2000) as well as in the animal feed industry where they are mainly manufactured as poultry and fish feed additives (Bjerkeng 2000).
From a chemical point of view, carotenoids are the second most abundant naturally occurring pigments on earth, with more than 750 structurally different compounds (Nisar et al. 2015). Also, carotenoids are undoubtedly the most important natural polyunsaturated isoprenoids, widely distributed in the world (Britton et al. 2004). These molecules typically consist of a C-40 hydrocarbon backbone, composed of eight isoprene units joined in a head-to-tail fashion except the central unit, which has a reverse connection (Álvarez et al. 2013, see Chap. 3). This means that carotenoids are polyisoprenoid compounds and can be divided into two main groups:
-
(i)
Carotenes or hydrocarbon carotenoids, which are composed of carbon and hydrogen atoms
-
(ii)
Xanthophylls that are oxygenated hydrocarbon derivatives with cyclic and acyclic structures that contain at least one oxygen function such as hydroxyl, keto, epoxy, methoxy, or carboxylic acid groups (Armstrong 1994).
Several prefixes, preceded by the number of the carbon atom(s) that contain the modification, are used for the systematic nomenclature of modified carotenoids. For example, the apo prefix designates the shortening of the skeleton by the formal removal of fragments from one or both ends of the carotenoid molecule, epoxy is used to indicate oxygen bridges and hydro or dehydro designates the addition or removal of hydrogens, and applies to the elimination of CH3, CH2, or CH groups (Álvarez et al. 2013).
In plants, carotenoids are synthesized in both light- and dark-grown tissues, such as leaves, endosperm, and roots. The carotenoid biosynthetic pathway occurs in plastids were they are also stored and accumulated in envelope/thylakoid membranes and plastoglobuli (Jeffrey et al. 1974; Siefermann-Harms 1978; Austin et al. 2006). It has been suggested that the differential distribution of carotenoids between etioplasts and chloroplasts might also require differential localization of their biosynthetic enzymes (Shumskaya et al. 2012, see Chap. 9).
2 Biological Functions of Carotenoids
Carotenoids play an important role in human health and nutrition. Vertebrates do not synthesize carotenoids and depend on dietary carotenoid sources for making their retinoids , such as retinal (the main visual pigment), retinol (vitamin A) and retinoic acid (a substance controlling morphogenesis) (Fraser and Bramley 2004; Krinsky and Johnson 2005, see Chap. 14). The main precursor of retinoids is β-carotene , also called provitamin A, which contains two unsubstituted beta-ionone rings at the two ends of the molecule (Grune et al. 2010). β-carotene deficiency in human diet causes symptoms ranging from night-blindness to those of xerophthalmia and keratomalacia, leading to total blindness (Berson 1982; Sommer and Vyas 2012). Furthermore, vitamin A deficiency exacerbates afflictions like diarrhea, respiratory diseases and childhood diseases such as measles (Mayo-Wilson et al. 2011). On the other hand, certain carotenoids, such as α- and β-carotene, are good antioxidants and are necessary for human health. Supplementation of the human diet with the carotenoids lycopene or β-carotene has been shown to reduce the risk for the incidence of a number of diseases such as macular degeneration (Delcourt et al. 2006), certain types of cancer (van Poppel and Goldbohm 1995; Zu et al. 2014), neurodegenerative disease (Lu et al. 2010; Obulesu et al. 2011) and coronary heart diseases (Mayne 1996; Karppi et al. 2013).
In plants, carotenoids and their derivatives have an indispensable and prominent role. These molecules endow flowers and fruits with distinct colors and fragrance. Indeed flower color is considered one of the major attractants to pollinators in insect pollinated plants by offering a visual signal (Kevan and Baker 1983). In addition, volatile apocarotenoids mediate plant–animal interactions for pollination or seed dispersal and enhance the flavor characteristics of food crops. Certain carotenoids in the endosperm tissue of some food crops provide nutritional value and have been targets for improvement, especially in cereal crops of the grass family (Kevan and Baker 1983; Harjes et al. 2008; Vallabhaneni and Wurtzel 2009; Yan et al. 2010; Wurtzel et al. 2012, see Chap. 13). Furthermore, carotenoids play essential and multiple roles in photoprotection against photooxidative damage and heat stress to plant cells via energy dissipation and free radical detoxification, which limits damage to membranes and proteins (DellaPenna and Pogson 2006). Therefore, carotenoid antioxidants increase heat and light stress tolerance by protecting membranes from reactive oxygen species and lipid peroxidation (Davison et al. 2002; Havaux et al. 2007; Johnson et al. 2007; Li et al. 2008; Havaux 2014). For example, two molecules of β-carotene in the reaction centre of photosystem II (PSII) participate in singlet oxygen quenching and in the cyclic electron flow along with Cyt b-559 (Miyake et al. 2002; Telfer et al. 2003, see Chap. 4). In addition, plants with reduced zeaxanthin levels exhibit increased sensitivity to light stress, as zeaxanthin prevents the oxidative damage of the thylakoid membranes (Havaux and Niyogi 1999; Verhoeven et al. 2001). Carotenoids are also involved in photosystem assembly where, from the antenna complex, they harvest light in a broader range of wavelengths in the blue region of the visible light spectrum and subsequently transfer the energy to chlorophyll (Grotewold 2006; Dall’Osto et al. 2007; Walter and Strack 2011).
Carotenoids play a key role as precursors to fitohormones such as ABA and strigolactones which influence processes as diverse as morphogenesis, seed dormancy, and environmental adaptation of plants (Auldridge et al. 2006; Paszkowski 2006; Gomez-Roldan et al. 2008; Messing et al. 2010; Walter et al. 2010; Walter and Strack 2011, see Chap. 12). Particularly, ABA is a C15 apocarotenoid derived from the cleavage of xanthophylls (Nambara and Marion-Poll 2005) and it has been linked to several physiological processes in plants, among them the regulation of seed dormancy and germination, and the response to abiotic stress (temperature, light and drought) (Raghavendra et al. 2010). On the other hand, the biosynthetic pathway to strigolactones has recently been shown to involve carotenoid cleavage dioxygenases (CCDs) , CCD7 and CCD8, to convert β-carotene to a lactone, given the name carlactone (Alder et al. 2012, see Chap. 12). In addition to their important role as rhizosphere signaling molecules (Akiyama et al. 2005; Bouwmeester et al. 2007), it has been demonstrated that strigolactones also act as a new hormone class that inhibits shoot branching in plants and hence regulates above-ground plant architecture (Gomez-Roldan et al. 2008; Umehara et al. 2008, see Chap. 12).
3 Vegetal Models as a Tool Box to Study Plant Carotenogenesis
Biochemical and biophysical approaches based on in vitro and in vivo testing have provided significant advances in our understanding of the structural and functional roles of carotenoids in plants. The use of molecular genetics has been critical in the study of carotenoid synthesis and function by using genetically disruption or over expression of key components of the pathway in planta. The identification, characterization and utilization of mutant plant lines, in which the carotenoid composition has been predictably manipulated at the genetic level, has allowed insights into the in vivo role of carotenoids and carotenogenic genes in plants. Today, the ongoing research in this field has allowed the study of carotenoid biosynthesis and function in different carotenoid-enriched plants used as models, including Arabidopsis (Arabidopsis thaliana), pepper (Capsicum annuum), tomato (Solanum lycopersicum), daffodil (Narcissus sp.), citrus (Citrus sp.), tobacco (Nicotiana tabacum), carrots (Daucus carota) among others (Dogbo et al. 1988; Beyer et al. 1989; Cunningham and Gantt 1998; Hirschberg 2001; Fraser and Bramley 2004; Stange et al. 2008; Fuentes et al. 2012; Kachanovsky et al. 2012; Ruiz-Sola and Rodríguez-Concepción 2012). Particularly, extensive study of carotenoid biosynthesis in Zea mays (maize), a major food crop, has benefitted from the diverse germplasm collection, phenotypic mutants, genetic and physical mapping, and quantitative trait loci (QTL) affecting carotenoid biosynthesis (Wurtzel et al. 2012). Other interesting models for understanding carotenogenesis include saffron (Frusciante et al. 2014) and kiwifruit (Ampomah-Dwamena et al. 2009). However, much remains to be understood about the regulation of carotenoid biosynthesis in the context of the manifold roles of carotenoids in plants.
To date, the knowledge generated has helped to define and characterize the key enzymes for carotenoids production in plants (Farré et al. 2011; Quinlan et al. 2012; Ruiz-Sola and Rodríguez-Concepción 2012) and provided insight into the integration of carotenoid synthesis with components of other metabolic pathways.
4 Carotenoid Biosynthetic Pathway: Genes, Proteins and Products
Carotenoids, as well as other isoprenoids, are built from the 5-carbon (5C) compound isopentenyl pyrophosphate (IPP). In plants, there are two sources of IPP: The cytosolic mevalonic acid pathway (MVA, see Chap. 1, Fig. 1.1) and the plastid methylerythritol 4-phosphate (MEP, Chap. 1, Fig. 1.1) pathway (Lichtenthaler et al. 1997; Rodríguez-Concepción and Boronat 2002; Eisenreich et al. 2004). In the cytosolic pathway, IPP is formed from three molecules of acetyl-CoA via 3-hydroxy-3-methyl glutaryl-CoA (HMG-CoA), mevalonic acid (MVA), mevalonic acid 5-phosphate (MVAP) and mevalonic acid 5-diphosphate (MVAPP). Hence, this pathway is called the mevalonic acid pathway (MVA). Before chain elongation begins IPP isomerase (IDI) catalyzes IPP conversion into its allylic isomer, dimethylallyl pyrophosphate (DMAPP), which is the initial, activated substrate for the formation of sesquiterpenes (C15) and triterpenes (C30) such as sterols (Laule et al. 2003; Hsieh and Goodman 2005). DMAPP condenses with a molecule of IPP to give the C10 compound, geranyl pyrophosphate.
In contrast to the MVA pathway, the MEP pathway is localized in plastids and is the major source for plant isoprenoids biosynthesis (Bramley 2002, Lichtenthaler 1999). The IPP and DMAPP used for carotenoid biosynthesis in plants are derived from the MEP pathway (Rodríguez-Concepción and Boronat 2002; Eisenreich et al. 2004). In addition, a diverse range of compounds including monoterpenes, tocopherols, phylloquinone, gibberellins (GA), chlorophylls, and plastoquinone are also derived from IPP and DMAPP (Rodríguez-Concepción 2010).
Along the pathway, carotenoids can be cleaved into apocarotenoids by enzymes of the carotenoid cleavage dioxygenase (CCD) family (Auldridge et al. 2006, see Chap. 12). Apocarotenoids are a class of terpenoid compounds, which include some important volatile compounds such as β-ionone, geranyl acetone, pseudoionone, α-ionone and 3-hydroxy-β-io-none (Simkin et al. 2004). The Arabidopsis CCD family has nine members, including four CCDs (CCD 1, 4, 7 and 8) and five 9-cis-epoxycarotenoid dioxygenases (NCED 2, 3, 5, 6 and 9, see Chap. 11). NCEDs specifically catalyze the first step of the ABA biosynthesis pathway and use 9-cis-viola-xanthin and 9-cis-neoxanthin as substrates (Nambara and Marion-Poll 2005).
4.1 Plastid Localization of the Carotenogenic Pathway
The core of the carotenoid biosynthetic pathway consists of around 10 enzymes. In plants, the enzymatic formation of carotenoids occurs on plastid membranes and is mediated by nuclear-encoded enzymes (DellaPenna and Pogson 2006; Cuttriss et al. 2011). However, the location of the biosynthetic pathway as a complete entity for controlling the unique spatial distribution of carotenoids remains unclear, even though biochemical studies made clear that carotenoid enzyme location is critical for activity. This suggests that carotenoids destiny may be different on the plastid envelope (e.g. conversion to apocarotenoids involved in mediating signaling) as compared to carotenoids on thylakoid membranes, where these pigments function as structural components for photosynthesis or photoprotection. According to Cunningham and Gantt (Cunningham and Gantt 1998) the carotenoid biosynthetic enzymes are likely to be organized in protein complexes in plants. Evidence of in vivo channeling (De la Guardia et al. 1971; Candau et al. 1991), and detection of high molecular weight complexes containing biosynthetic enzymes (Al-Babili et al. 1996; Lopez et al. 2008) support the fact for the limited detection of pathway intermediates (Wurtzel 2004; Quinlan et al. 2012).
Mass spectrometry studies of the chromoplast proteome found individual carotenoid enzymes mostly in plastoglobuli (Ytterberg et al. 2006) or in membrane fractions (Wang et al. 2013). Plastoglobulies attached to thylakoid membrane of chloroplasts contain PSY (with the exception of the “yellow endosperm” allele for maize PSY1), which would suggest that PSY is physically separated from the rest of the enzymes of the pathway (Shumskaya et al. 2012). The envelope localization of carotenoid enzymes can be explained by the need to supply carotenoids for abscisic acid (ABA) synthesis, which occurs outside of the plastid (Cutler et al. 2010). In particular, in recent proteomic studies on Arabidopsis thaliana chloroplasts (Joyard et al. 2009; Ferro et al. 2010), many, but not all carotenoid biosynthetic enzymes are found in envelope membranes, while only a few carotenoid enzymes are identified in thylakoids (Ytterberg et al. 2006; Joyard et al. 2009; Ruiz-Sola and Rodríguez-Concepción 2012). These enzymes include xanthophyll cycle enzymes and phytoene desaturase (PDS). In pepper (Capsicum annuum) fruit chromoplasts, most carotenoid enzymes are localized to plastoglobuli. In maize (Zea mays), through proteomic studies, PDS and ZDS were the only carotenoid enzymes detected in membrane fractions of bundle sheath and mesophyll cells, respectively (Friso et al. 2010). Although, carotenoids are found in both cell types, no other carotenoid biosynthetic enzymes were detected. In maize PSY, isozymes differ in chloroplast suborganellar localization and can be found either in plastoglobuli or in the stroma and thylakoid membranes (Shumskaya et al. 2012). Even though all of the pathway enzymes are known today and most OMIC approaches have reported the location of individual enzymes, the three-dimensional structure of the “pathway” and a putative metabolomic organization is not yet understood (Shumskaya and Wurtzel 2013).
4.2 MEP Pathway to GGPP
The plastid-localized MEP pathway combines glyceraldehyde-3-phosphate and pyruvate to form deoxy-D-xylulose 5-phosphate (DXP), a reaction catalysed by DXP synthase (DXS) (Fig. 2.1). MEP is subsequently formed via an intramolecular rearrangement and reduction of DXP by the enzyme DXP reductoisomerase (DXR). Both DXS and DXR are important in carotenoid flux regulation. In Arabidopsis, both enzymes are encoded by single genes and appear to be rate-determining enzymes. Indeed, the Arabidopsis Cla1 mutant, in which the DXS gene of the MEP pathway is disrupted, present photo bleaching due to the absence of protective carotenoids (Araki et al. 2000; Estévez et al. 2000). In contrast, over expression of DXS and DXR in Arabidopsis seedlings increases carotenoid production (Estévez et al. 2001; Carretero-Paulet et al. 2006) and the expression of PSY resulted in increased carotenoid accumulation and a concomitant accumulation of the DXS enzyme (Rodríguez-Villalón et al. 2009). Moreover, Recent work has also suggested the post-transcriptional regulation of DXS activity mediated by the involvement of J-protein (J20) and heat shock protein 70 (Hsp70) chaperones. Indeed, mutants defective in J20 activity exhibit reduced DXS enzyme activity and accumulate DXS protein only in an inactive form (Pulido et al. 2013). It is suggested that plastidial J20 protein appears to assist Hsp70 chaperone in the proper folding and assembly of DXS and participate in the regulation of MEP-derived isoprenoid biosynthesis (Pulido et al. 2013). In poplar, a feedback inhibition of DXS by DMAPP reveals an important regulatory mechanism of the MEP pathway and thus carotenoid biosynthesis (Ghirardo et al. 2014). The enzymes of the MEP and carotenoid pathways are preferred targets for herbicides (Withers and Keasling 2007) because these pathways are not present in animals and the blockage of any of the steps preceding the formation of lycopene inhibits carotenoid synthesis. The herbicide clomazone inhibits DXS (Müller et al. 2000, Fig. 2.1) by an indirect mechanism once it is absorbed into the plant. Clomazone is oxidized by cytochrome P450 monooxygenases (P450s) to form the putative active herbicidal form (Müller et al. 2000), ketoclomazone. This requirement for metabolic activation was shown in plants treated with P450s inhibitors such as phorate, where the herbicidal effect of clomazone was prevented (Ferhatoglu et al. 2005; Ferhatoglu and Barrett 2006). Although the antibiotic fosmidomycin is not used as an herbicide, this compound inhibits DXR (Singh et al. 2007) (Fig. 2.1), the second step of the MEP pathway (Rohmer 1998; Rohmer et al. 2004). The potential benefits of developing new herbicides which interfere with pathways leading to carotenoids, such as the MEP and isoprenoid pathways, has been addressed recently (Hale et al. 2012; Corniani et al. 2014) taking into account the emergence of resistance to herbicides as an increasing problem facing agriculture (Service 2007, 2013). A number of steps are then required in the condensation between IPP and its allylic isomer DMAPP (Fig. 2.1), to form the C10 compound, geranyl pyrophosphate (GPP). The further addition of two IPP units results in the formation of C20 geranylgeranyl pyrophosphate (GGPP) the precursor of carotenoid biosynthesis (Bramley 2002; Cunningham 2002; Fraser and Bramley 2004). The sequential addition of three IPP molecules to a DMAPP molecule is catalyzed by GGPP synthase (GGPPS).
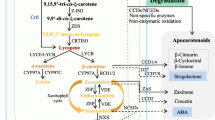
Isoprenoid and Carotenoid pathway in plants. Schematic representation of the plastidial MEP (2-C-methyl-D-erythritol-4-P) pathway: DXS: deoxyxylulose 5-phosphate synthase, DXR: deoxyxylulose 5-phosphate reductoisomerase, DXP: Deoxy-D-xylulose-5-P, CMS: 2C-methyl-D-erythritol 4-phosphate cytidyltransferase, CMK: 4-(cytidine 5-diphospho)-2-C-methyl-D-erythritol kinase, MCS: 2C-methyl-D-erythritol 2,4-cyclodiphosphate synthase, HDS: 4-hydroxy-3-methylbut-2-enyl diphosphate synthase; HDR: 4-hydroxy-3-methylbut-2-enyl diphosphate reductase, IPP (isopentenyl pyrophosphate), GGPPS: geranylgeranyl pyrophosphate synthase, DMAPP: dimethylallyl pyrophosphate, IPI: isopentenyl pyrophosphate isomerase, GGPP: geranylgeranyl pyrophosphate. The relevant genes for carotenoid synthesis are: PSY: phytoene synthase, PDS: phytoene desaturase, Z-ISO: ζ-carotene isomerase, ZDS: ζ-carotene desaturase, CRTISO: carotene isomerase, LCYB: lycopene β-cyclase, LCYE: lycopene ε-cyclase, BCH: carotenoid β hydroxylase, CHYE: carotenoid ε-hydroxylase (CYP97C1, CYP97A3), ZEP: zeaxanthin epoxidase, VDE: violaxanthin deepoxidase, NSY: neoxanthin synthase, NCED: 9-cis-epoxycarotenoid dioxygenase. Reported mutants in isoprenoid and carotenogenic genes are written in blue. Chemical inhibitors of some enzymes are included with a red T symbol. Light, referred as a yellow ray can replace Z-ISO and CRTISO activity in photosynthetic organs
4.3 GGPP to Phytoene: Phytoene Synthase
Phytoene synthase (PSY) is the first dedicated enzyme of the carotenoid biosynthesis pathway and catalyzes the condensation of two GGPP molecules into phytoene as a 15-cis isomer by introducing a central 15-Z double bond during hydrogen elimination (Cunningham and Gantt 1998). Due to the fact that PSY is the enzyme that catalyzes the first committed step in the plant carotenoid pathway, it has been postulated to be rate-limiting, regulating the reaction flux. In fact, dosage effect of the maize Y1 allele upon seed carotenoid content was noted as early as the 1940s (Randolph and Hand 1940), where three copies of the dominant Y1 allele in the triploid endosperm conditioned the most yellow seeds (endosperm) in contrast to homozygous white y1 seeds. Cloning and subsequent sequence analyses identified the Y1 gene as the gene coding for PSY1 (Buckner et al. 1996). Considering PSY as rate-limiting, it has been a preferred target for genetic manipulation. Fray et al. (1995) were the first to report ectopic quantitative and qualitative changes in carotenoids through fruit-specific expression of a phytoene synthase cDNA under a CaMV 35S promoter in tomato. Interestingly, the overexpression lines present a dwarf phenotype with a 30-fold reduction in gibberellin A1 (GA1) and chlorophyll levels. The dwarf character was inherited with an inverse relationship between plant height and expression of PSY, which redirects GGPP to phytoene and diverts this intermediate away from the gibberellin (GA) and phytol biosynthetic pathways. On the other hand, the overexpression of an exogenous daffodil PSY in Japonica rice model variety Taipei 309 leads to phytoene accumulation in endosperm, which was the first instance of carotenoid engineering in rice (Burkhardt et al. 1997). The regulation and rate-limiting role of PSY was also evident in Brassica napus expressing an additional PSY gene from the bacterium Erwinia uredovora, crtB (Shewmaker et al. 1999). The transgenic plants resulted in a 50-fold increase in total carotenoid content, predominantly α-carotene , β-carotene and phytoene. A similar study was reported in transgenic tomato carrying the additional E. uredovora crtB gene under the control of fruit-specific polygalacturonase promoter (Fraser et al. 2002). The PSY activity in these transgenic plants was increased five to ten-fold. This was accompanied by a two- to four-fold increase in the total carotenoid content of primary transformants whereas phytoene was increased 2.4 fold. In another study, E. uredovora crtB was overexpressed in potato (Ducreux et al. 2005, see Chap. 13). In addition, constitutive PSY overexpression has enhanced total carotenoid contents and substantially increased synthesis of β-carotene in a variety of many other vegetal species such as linseed flax, carrots , maize, and cassava roots (Fujisawa et al. 2008; Maass et al. 2009; Naqvi et al. 2009; Welsch et al. 2010, see Chap. 13). It is important to take into account that most of those results obtained after over expression of the PSY gene in carotenoid-producing fruits may result in a shift in the regulatory control point within the pathway. Furthermore, the bigger increase in the carotenoid content in those cases of using a plant PSY instead of the bacterial crtB could be due to the less effective protein-protein interaction in the multienzymatic complexes produced with CRTB compared with those formed with plant PSYs.
Recently, a phytoene synthase-RNAi construct was delivered into the Oncidium hybrid orchid as the first report using RNAi approaches to break down carotenogenesis in this plant (Liu et al. 2014). Transgenic lines displayed a semi-dwarf phenotype and brilliant green leaves as a consequence of changes in the carotenoid, gibberellin, abscisic acid and chlorophyll biosynthetic pathway (Liu et al. 2014).
The localization of PSY within the chloroplast is another highlight finding reported recently (Shumskaya et al. 2012; Shumskaya and Wurtzel 2013). While the majority of phytoene synthases, such as maize (PSY2 and PSY3), rice and Arabidopsis PSYs, are found to localize to plastoglobuli, maize PSY1 isozymes are localized to distinct chloroplast suborganellar sites (e.g. globular or fibrillar) based on their allelic variation, suggesting that PSY1 sequence variation can affect suborganellar localization of carotenoid storage and bioavailability.
4.4 Lycopene Synthesis: Teamwork of Desaturases and Isomerases
In plants and cyanobacteria, phytoene undergoes four successive dehydrogenations and isomerizations to produce lycopene. The first two desaturation steps are catalyzed by phytoene desaturase (PDS), while the latter two reactions are catalyzed by (zeta) ζ-carotene desaturase (ZDS). These desaturation reactions introduce a series of carbon–carbon double bonds that constitute the chromophore in carotenoid pigments, and they transform the colorless phytoene into the red-colored lycopene. These reactions lead to a poly-cis pathway in contrast to the all-trans pathway carried out by CRTI in bacteria and fungi (Sandmann 1994; Armstrong 1997, see Chap. 1). Therefore, in concert to these steps, ζ-carotene isomerase (Z-ISO) and carotenoid isomerase (CRTISO) catalyze cis–trans reactions to isomerize the four cis-bonds introduced by the desaturases (Fig. 2.1). Z-ISO activity occurs upstream of CRTISO and catalyzes the cis to trans conversion of the product of PDS to form the substrate of ZDS (Li et al. 2007) whereas CRTISO is involved in the conversion of poly-cis lycopene to trans-lycopene (Figs. 2.1 and 1.3. Isaacson et al. 2002; Park et al. 2002). Reactions performed by Z-ISO and PDS precede ZDS which requires substrates with desaturated 11 and 11′ bonds (Albrecht et al. 1996) and 15-15′ in trans (Breitenbach and Sandmann 2005). The isomerization reaction carried out by CRTISO is the last reaction since it requires substrates to have adjacent 7- and 9-cis bonds (Isaacson et al. 2004). It is not understood why bacteria and fungi accomplish these reactions with a single enzyme, CRTI. The most likely explanation refers to possible roles of the cis-intermediates in regulatory signaling pathways (Kachanovsky et al. 2012, see Chap. 1)
4.4.1 Desaturases
Two sequential desaturations of phytoene, catalyzed by PDS, result in the formation of phytofluene, followed by the formation of ζ-carotene . Additional desaturations, catalyzed by Z-ISO and ZDS, give rise to 7,9,9′-cis-neurosporene. The deduced peptide sequences of PDS and ZDS show a high degree of similarity between different plant species. Nevertheless, PDS shows 33% to 35% identity to ZDS, they can be grouped together in a phylogenetic tree, indicating a common ancestor for both proteins (Beyer et al. 1989; Albrecht et al. 1995; Hirschberg et al. 1997). The desaturases require FAD and the association with a membrane to be active (Nievelstein et al. 1995; Bonk et al. 1997). The FAD appears to feed an electron transport chain involving quinones and the plastid terminal oxidase (PTOX). Particularly, quinones act as electron acceptors for PDS and ZDS desaturation reactions, as demonstrated in daffodil and Arabidopsis (Beyer 1989; Mayer et al. 1990; Norris et al. 1995), in which the impaired plastoquinone biosynthesis in pds1 and pds2 mutants resulted in albino phenotypes and phytoene accumulation (Norris et al. 1995; Norris et al. 1998). The cloning of the IMMUTANS gene encoding PTOX also revealed a link between phytoene desaturation and redox poise of the chloroplast (Al-Babili et al. 1996; Josse et al. 2000; Carol and Kuntz 2001). The disruption of the IMMUTANS gene prevents oxidation during chlorophyll biosynthesis and results in the accumulation of phytoene (Carol et al. 1999; Wu et al. 1999; Aluru et al. 2001).
Phytoene desaturase is also a rate-limiting enzyme in carotenoid synthesis (Chamovitz et al. 1993). Maize mutants viviparous5 (vp5), vp2 and white3 (w3) are associated with the function of PDS by the accumulation of phytoene both in leaf and seed (Treharne et al. 1966; Neill et al. 1986; Hable et al. 1998) The vp5 locus was established as the structural gene for PDS. A soybean cDNA encoding PDS was cloned using a heterologous probe derived from the Synechococcus PDS gene (Bartley et al. 1991). The tomato PDS cDNA was cloned by a similar approach and its identity was confirmed by expression in E. coli, which resulted in the formation of ζ-carotene (Pecker et al. 1992). The pepper PDS coding sequence, isolated using antibodies raised against the purified protein, showed high homology to soybean and tomato PDS genes and active PDS recombinant enzyme was obtained in E. coli (Hugueney et al. 1992). Transgenic tobacco plants overexpressing PDS had no phenotypic or metabolic effects (Busch et al. 2002; D’Haeze 2002). However, PDS down-regulation led to a dramatic accumulation of phytoene (Busch et al. 2002; D’Haeze 2002), a lethal phenotype in homozygous seedlings (Busch et al. 2002) and albinism (Travella et al. 2006; Kirigia et al. 2014). Transgenic tobacco plants showing different degrees of albinism caused by PDS silencing presented a decrease in total carotenoid, chlorophyll and PSII efficiency (Wang et al. 2009).
The protective function of carotenoids depends on the complete conjugated double-bond system. As a consequence, any step in carotene biosynthesis up to lycopene formation is a potential target for a herbicidal inhibitor (Böger and Sandmann 1998). PDS is the molecular target site for several herbicides such as fluridone, norflurazon, flurtamone and picolinafen (Dayan and Duke 2003). These herbicides compete for the binding site of plastoquinone (Breitenbach et al. 2001) as an essential cofactor for this enzyme (Norris et al. 1995). ZDS is essential for cell growth, stress responses and carotenoid biosynthesis , providing a precursor for cyclization reactions (Bartley et al. 1999). Coding sequences for ZDS have been isolated and characterized in maize (Matthews et al. 2003), pepper (Breitenbach et al. 1999), common fig (Araya-Garay et al. 2012) and goji berry plants (Li et al. 2015). Maize ZDS did not function efficiently in a heterologous complementation system where PDS did (Matthews et al. 2003). Suggested reasons for the low activity of carotenoid desaturases include: End product inhibition of ZDS (Sandmann and Böger 1989; Breitenbach et al. 1999), failure to reconstitute the interactions of an assembly complex responsible for substrate channeling (Breitenbach et al. 1999), the absence of a carotene isomerase (Bartley et al. 1999), among others.
Mutation of the ZDS gene from Arabidopsis resulted in impaired carotenoid biosynthesis and subsequent spontaneous cell death from the increased content of superoxide (Dong et al. 2007).
Moreover, it has been shown that loss of function of the ζ -carotene desaturase encoded by ZDS/CLB5/SPC1/PDE181 arrests chloroplast biogenesis at a very early stage of development and Arabidopsis plants also exhibit dramatic alterations in leaf morphology and in the expression of a variety of chloroplast proteins and genes required for leaf development. These phenotypes were not observed in mutants deficient in the closely related desaturase PDS (Avendaño-Vázquez et al. 2014). The vp9 maize ZDS mutant has reduced or suppressed ABA and carotenoid accumulation in both endosperm and vegetative tissues, even when it accumulates ζ-carotene (Matthews et al. 2003), while the non dormant-1 (nd-1) mutant of sunflower (Helianthus annuus L.) was characterized by an albino and viviparous phenotype (Conti et al. 2004).
Compared to inhibitors of phytoene desaturation, fewer compounds interfere with the ZDS reaction. The following compounds have been reported to accumulate ζ-carotene in whole plants: Amitrole, Dichlormate (Burns et al. 1971), Methoxyphenone (NK049) (Fujii et al. 1977), KM 143-958 (Chollet et al. 1990), WL 110547 (Sandmann et al. 1996; Kerr and Whitaker 1987), LS 80707 (Sandmann et al. 1985), J852 (Chollet et al. 1990).
The modes of action of J852 and LS 80707 (including related compounds) in carotene biosynthesis are well defined. Inhibitors treatment causes ζ-carotene accumulation and the concurrent bleaching of plants (Sandmann and Böger 1989; Chollet et al. 1990). Pepper leaves treated with the herbicide J852 showed an accumulation of phytoene and ζ-carotene. Unexpectedly, none of the genes coding for PSY, PDS, ZDS or the Terminal Oxidase associated with phytoene desaturation were induced upon herbicide treatment in pepper leaves or seedlings (Simkin et al. 2000).
4.4.2 Isomerases
Plants do not only require desaturases to convert 15-cis-phytoene into all-trans lycopene. In fact, while PDS is active it prefers 15-cis substrates (Breitenbach and Sandmann 2005). ZDS is only active against substrates with a 15-trans conformation, (Breitenbach and Sandmann 2005) indicating the requirement of two types of isomerase enzymes. Particularly 15-cis phytoene is transformed by PDS into 15,9′-di-cis-phytofluene, and eventually 9,15,9′-tri-cis-ζ-carotene, which is isomerized at the 15-cis-double bond to form the substrate of ZDS, 9,9′-di-cis-ζ-carotene (Bartley et al. 1999; Matthews et al. 2003; Breitenbach and Sandmann 2005). The isomerization step of the 15-cis bond of ζ-carotene to trans, can be mediated either by light or by the 15-cis-ζ-carotene isomerase (Z-ISO) occurring in the chloroplasts of photosynthetic tissues or in other plastid types, respectively (Breitenbach and Sandmann 2005; Li et al. 2007, Fig. 2.1). However, the loss-of-function phenotypes detected in mutants of maize and Arabidopsis strongly suggest that Z-ISO activity is required even in the presence of light. Plants with insufficient Z-ISO also grow poorly under the stress of fluctuating temperature. Z-ISO is predicted to be an integral membrane protein with several membrane-spanning domains, but there is no available information about its sub organellar localization, showing whether the protein is found in the envelope or the thylakoid membranes (Janick-Buckner et al. 2001, Ruiz-Sola and Rodríguez-Concepción 2012; Beltrán et al 2015). Because climatic variations alter the need for photosynthetic and non-photosynthetic carotenoids, Z-ISO facilitates plant adaptation to environmental stress, a major factor affecting crop yield. It was also described that Z-ISO exists in a high-molecular-weight protein complex of about 480 kDa (Shumskaya and Wurtzel 2013), similarly to other carotenoid enzymes (Zybailov et al. 2008; Ishikawa et al. 2009). Z-ISO homologues can be found in plants and cyanobacteria. A mutant in the Z-ISO gene of maize, referred to as pale yellow 9 (y9), in addition to the isolation of Arabidopsis zic mutant, showed 9,15,9′-tri-cis-ζ-carotene accumulation in the dark, leading to the identification of 15-cis-ζ-carotene isomerase (Z-ISO). Expression of the Z-ISO transcript is highly correlated with the expression of other carotenogenic genes. Single copy genes appear to encode Z-ISO in most plants (reviewed in Ruiz-Sola and Rodríguez-Concepción 2012), but no evidence of alternate transcripts was found in plants such as maize or rice, except for Arabidopsis (Chen et al. 2010). The single Arabidopsis gene encoding Z-ISO (At1g10830) produces two alternate transcripts, Z-ISO1.1, which is highly expressed and encodes a functional enzyme, and a shorter Z-ISO1.2, which encodes a truncated protein that lacks the predicted C-terminal transmembrane domain and shows no Z-ISO activity. Therefore, the effect of the deletion suggests that the C-terminal TM domain is important for the function of Z-ISO, either for activity or proper folding (Chen et al. 2010). Screening for impaired carotenoid formation in dark germinated seeds of maize (Zea mays) and Arabidopsis mutants were performed to identify the gene by functional complementation assays in E. coli, expressing bacterial GGPPS and PSY. In maize Z-ISO is an enzyme related to nitrite and nitric oxide reductase U (NnrU), which is normally present in denitrifying bacteria. Considering that the bacterial denitrification pathway produces nitrogen oxides as alternate electron acceptors for anaerobic growth, it has been suggested that plant carotenogenesis evolved by recruitment of genes from noncarotenogenic bacteria (Li et al. 2007; Chen et al. 2010).
Based on the central location of the double bond in the substrate of Z-ISO, it is predicted that this enzyme recognizes the carotenoid molecule differently in comparison to the mechanism proposed for CRTI, PDS, ZDS or CRTISO, which act on one half of the molecule. The lack of sequence homology of Z-ISO to any known carotenogenic enzyme also suggests a different mechanism of reaction. Recently, it was shown that Z-ISO is a bona fide enzyme, which independently catalyzes the cis-trans isomerization of the 15–15′ carbon-carbon double bond in 9,15,9′-cis-ζ-carotene through a unique mechanism requiring a redox-regulated heme β cofactor that undergoes redox-regulated ligand switching between the heme iron and alternate Z-ISO amino acid residues (Beltrán et al. 2015). Reduction of the heme iron switches coordination of the heme to bis-histidine and exposes the active site for substrate binding. These results let to the proposal that Z-ISO as a metalloprotein, representing a new prototype for heme β proteins that potentially uses a new chemical mechanism.
Following the production of 9,9′-di-cis-ζ-carotene by Z-ISO, the enzyme ZDS carries out the stereospecific abstraction of protons from 7 and 7′, which results in the formation of the 7 and 7′ cis bonds in 7,9,9′-tri-cis-neurosporene (Breitenbach and Sandmann 2005; Sandmann 2009) followed by the synthesis of 7,9,9′,7′-tetra-cis-lycopene (also called pro-lycopene) with very low efficiency (Bartley et al. 1999). Because 7,9,7′,9′-tetra-cis-lycopene is not a substrate for β or ε-cyclases (Schnurr et al. 1996; Yu et al. 2011), the formation of the poly-cis intermediates requires the recruitment of an additional isomerase to generate the all-trans-lycopene, a suitable substrate for β and ε-cyclases (Breitenbach and Sandmann 2005). In chloroplasts , the isomerization of pro-lycopene to all-trans-lycopene can occur non-enzymatically in the presence of light whereas the activity of CRTISO (Fig. 2.1) fulfills a critical role in converting the cis-double bonds introduced by PDS and ZDS to all-trans in tissues receiving no light exposure and in non-photosynthetic tissues (Isaacson et al. 2002; Park et al. 2002). Therefore, CRTISO-deficient plants can still synthesize carotenoids but at slower rate and only in specific tissues. The isomerase activity of CRTISO requires the presence of membranes and the flavin adenine dinucleotide (FAD) binding motif, which bonds to the reduced form of the cofactor (FADred) to catalyze a reaction without net redox changes (Isaacson et al 2004; Yu et al. 2011). The regional specificity and the kinetics of the isomerization reaction were recently determined (Yu et al. 2011).
CRTISO is thought to be evolved from the bacterial-type desaturase (CRTI) considering the high sequence homology between both proteins (Isaacson et al. 2002; Park et al. 2002; Isaacson et al. 2004; Chen et al. 2010). Nevertheless, in terms of mechanisms of reaction related to the reduced flavin requeriment for its activity, it is especially interesting that CRTISO seems to be more related to CRTY (bacterial lycopene cyclase) than to its possible ancestor CRTI (Mialoundama et al. 2010; Yu et al. 2010; Yu et al. 2011).
The Arabidopsis, tomato, and melon mutants of CRTISO (ccr2, tangerine, and yolf, respectively) accumulate cis-carotenes in etioplasts of seedlings or chromoplasts of fruits (Isaacson et al. 2002; Park et al. 2002; Galpaz et al. 2013). However, it is interesting to note that the CRTISO activity can partially be substituted by exposure to light in green tissues via photoisomerization (Isaacson et al. 2002; Park et al. 2002). Photoisomerization of the cis bonds facilitates carotene synthesis in the chloroplasts of the ccr2 mutant, but delays greening and chlorophyll accumulation during photomorphogenesis. Similarly, loss of CRTISO causes partial inhibition of lutein biosynthesis in light-grown tissues and varying degrees of chlorosis in newly developed leaves of tomato and rice (Isaacson et al. 2002; Masamoto et al. 2004; Fang et al. 2008; Wei et al. 2010; Chai et al. 2011). In contrast to green tissues, CRTISO activity cannot be substituted by light in non-photosynthetic tissues. The etiolated tissues of ccr2, tangerine, and yolf fruits exhibit an orange color due to the accumulation of cis-lycopene (Isaacson et al. 2002; Park et al. 2002; Galpaz et al. 2013). Such accumulation is associated with a metabolite-dependent feedback regulation of early carotenoid synthesis genes. The feedback regulation of early tomato carotenoid genes (PSY1, PDS, and ZDS) observed in tangerine elucidates the recently discovered epistasis effect of tangerine over the r mutation in PSY, which partially restores PSY1 expression by cis-carotene accumulation in tangerine (Kachanovsky et al. 2012).
Even though most carotenoids found in nature are primarily in the more stable all-trans configurations (Britton 1995), a small proportion of cis isomers are encountered. Interestingly, they have different biological potency than their trans counterparts (e.g. lower pro vitamin A activity); they are constituents of the light harvesting complex (LHC) such as 9-cis-neoxanthin (Liu et al. 2004) and supply substrates for the biogenesis of plant hormone, ABA such as 9-cis-epoxyxanthophylls (Schwartz et al. 1997).
4.5 Lycopene to Cyclic Carotenes: Cyclases
Lycopene is the starting compound of various end group modifications that produces a large variety of carotenoids with different physical properties. In higher plants two enzymes, lycopene β-cyclase (LYCB) (Pecker et al. 1996) and lycopene ε-cyclase (LYCE) (Cunningham and Gantt 2001, Fig. 2.1) compete for lycopene as substrate, leading to carotenoids with either β or ε-ionone rings. Therefore, cyclization of lycopene represents the first branching point in the carotenogenic pathway (Fig. 2.1).
The amino acid sequence of lycopene cyclases from plants and cyanobacteria are polypeptides with around 400 amino acids and have a molecular mass of 43 kDa (Schnurr et al. 1996). The enzymes from plants also have an additional N-terminal transit sequence of 100 amino acids (Krubasik and Sandmann 2000).
In addition, the N terminus of plant LCYB and CCS contains dinucleotide-binding motifs characteristic of an N-terminal Rossmann fold of FAD-dependent oxidoreductases (Rossmann et al. 1974; Cunningham et al. 1994; Hugueney et al. 1995). Indeed, it has been reported that CCS-bound FAD is required for enzyme activity in the presence of NADPH, which functions as a reductant of FAD (Cunningham et al. 1994). Erwinia uredovora LCYB, which cyclizes lycopene into β-carotene , strictly requires NADPH but proceeds without any net redox change (Schnurr et al. 1996; Hornero-Méndez and Britton 2002).
LCYB and LCYE share significant similarities in their amino acid sequences, suggesting that they have originated from a common ancestor through gene duplication (Sandmann 2002). There are also two other related carotenoid cyclase enzymes: the capsanthin-capsorubin synthase (CCS) of pepper (Cunningham et al. 1996) and the neoxanthin synthase (NSY) of tomato (Bouvier et al. 2000) and potato (Al-Babili et al. 2000). CCS catalyzes the formation of the unusual five-carbon k ring (Bouvier et al. 1994), converting antheraxanthin or violaxanthin into the k-cyclic carotenoids capsanthin or capsorubin, respectively, by a similar mechanism to lycopene cyclization (Bouvier et al. 1997). Interestingly, CCS exhibits LCYB activity when lycopene is provided as a substrate (Hugueney et al. 1995).
The LCYB enzyme catalyzes the cyclization of one end of the linear lycopene molecule to produce γ-carotene. Subsequently, the enzyme forms a second β-ring at the other extreme to yield β-carotene (Fig. 2.1). LCYE introduces a single ε-ring into lycopene to produce δ-carotene and a subsequent cyclization of the ψ- acyclic end of δ-carotene by LCYB generates α-carotene. Carotenoids containing two ε-rings are rarely found in plants and algae (Goodwin 1980; Cunningham et al. 1996) with the notable exception of Lactuca sativa (lettuce), in which a unique LCYE can cyclize both ψ-ends of lycopene to generate ε,ε-carotene and the dihydroxy derivative lactucaxanthin (Siefermann-Harms et al. 1981; Phillip and Young 1995; Cunningham and Gantt 2001). Several specific domains and amino acid residues have been identified which determine whether LCYE introduces one or two ε-ionone rings (Cunningham and Gantt 2001).
Lycopene cyclases, LCYB and LCYE, are involved in determining carotenoid content and composition in different plants. Some plants fine-tune carotenoid content using tissue-specific isoforms of lycopene cyclases such as those chromoplast-specific LCYB genes expressed in the fruits and flowers of tomato, papaya and saffron that correlate with the accumulation of β-carotene and/or downstream xanthophylls (Ronen et al. 2000; Ahrazem et al. 2010; Devitt et al. 2010). Two color mutations in tomato, Beta and old-gold (og) affect the fruit and flower-specific LCYB which is encoded by the B gene (Ronen et al. 2000). Beta is a partially dominant, single-locus mutation that causes an orange color in the fully ripened fruit because of the accumulation of β-carotene at the expense of lycopene due to an important increase in the transcription of the B gene. By contrast, the old-gold (og) mutant carries a null allele in the locus B resulting in an elevated concentration of lycopene and a reduction of β-carotene (Ronen et al. 2000).
A mutation in the papaya LCYB2 dramatically reduces its expression and is responsible for the difference between red- and yellow-fleshed fruits as consequence of lycopene accumulation (Blas et al. 2010; Devitt et al. 2010).
Transcript analysis of homo- and heterozygous Arabidopsis lut2 mutants demonstrates that lut2 is semidominant and their biochemical phenotype is consistent with a disruption of epsilon ring cyclization (Pogson et al. 1996). In particular, lut2 mutation has significantly higher levels of the xanthophyll cycle pigments (violaxanthin, antheraxanthin, and zeaxanthin) as well as β-carotene. In tomato, expression of the gene for lycopene epsilon-cyclase is down-regulated during ripening and is elevated in the mutant Delta where a single dominant gene, Del, changes the fruit colour to orange as a result of accumulation of delta-carotene at the expense of lycopene (Ronen et al. 1999). Metabolic engineering to manipulate the lycopene cyclase expression and enzyme activity can bias the flux toward one or the other branch of the carotenogenic pathway (Yu and Beyer 2012; Giorio et al. 2013). Enhanced levels of β-carotene were observed in LCYE tuber-specific silencing of potato (Diretto et al. 2006). Similarly, LCYE downregulation in canola results in the enhanced accumulation of β-carotene, zeaxanthin, violaxanthin, and lutein (Yu et al. 2008). Suppression of LCYE in sweet potato and tobacco showed increased synthesis of a β-branch–specific pathway and enhanced tolerance to abiotic stress (Kim et al. 2013; Shi et al. 2014). Maize endosperm tissues lacking LCYB activity accumulate ε,ε-carotene , δ-carotene, and ε-carotene produced by a dual mono- or bicyclic LCYE enzyme activity (Bai et al. 2009).
The expression levels of LCYB and LCYE in tomato, rice, and Arabidopsis affect the relative cyclase activities as well as the synthesis of cyclic carotenoids playing a critical role in determining the β-carotene/α-carotene ratio (Yu and Beyer 2012; Giorio et al. 2013).
Inhibition of these enzymes produces growth abnormalities as well as herbicidal effects. The only specific non-competitive inhibitors of lycopene cyclases are N,N-diethyl-N-[2-(4-chlorophenylthio)ethyl]amine (CPTA) and N,N-diethyl-N-[2-(4-methylphenoxy)ethyl]amine (MPTA) (Yokoyama et al. 1977; Spurgeon, 1983; Schnurr et al. 1998). CPTA exhibited preferential inhibition of the LCYB over LCYE, resulting in an accumulation of the monocyclic carotenoid precursor δ-carotene as well as lycopene (at the expense of β-carotene and xanthophylls) (La Rocca et al. 2007). MPTA belongs to a group of substituted triethylamines that have a number of effects including inhibition of lycopene cyclases (Phillip and Young 2006; Liu and Puckhaber 2011). Another inhibitor of carotenoid biosynthesis on the step of cyclization of lycopene is the bleaching herbicide amitrole (AM) (Agnolucci et al. 1996; La Rocca et al. 1998). It has been reported that amitrole (AM) treatment decreased salt tolerance of Salicornia Europaea by inhibiting lycopene cyclization (Chen et al. 2011). However amitrole also has other effects in plants such as inhibition of geranylgeraniol hydrogenation to phytol in chlorophyll synthesis of wheat seedlings that have been transferred from darkness to light (Rüdiger and Benz 1979).
The bleaching herbicidal compound N,N-diethyl-N-(2-undecynyl) amine (NDUA) was also identified as an inhibitor of lycopene cyclase in Lepidium sativum plants. Plants under NDUA treatment showed both lycopene accumulation and chlorotic effects similar to CPTA and MPTA treatments (Fedtke et al. 2001).
4.6 Cyclic Carotenes to Xanthophylls Cycle: Dynamic Role of Hydroxylases, Epoxidases and More
From a chemical point of view, xanthophylls are oxygenated hydrocarbon derivatives of carotenoids that contain at least one oxygen function, such as carboxylic acid, epoxy, hydroxyl, keto or methoxy groups. Xanthophylls are among the main carotenoid pigments in the photosystems of plants. The oxygen-containing xanthophylls are produced from either α- or β-carotene and require ring-specific hydroxylation reactions. β–Hydroxylase catalyzes two hydroxylation reactions, converting β-carotene to zeaxanthin via β-cryptoxanthin whereas α-carotene is twice hydroxylated by two different enzymatic reactions catalyzed by the ε- and β–hydroxylases. In Arabidopsis thaliana, these reactions are catalyzed by a set of four enzymes (Kim et al. 2009). Two non-heme di-iron enzymes (β-carotene hydroxylase-1 (BCH1) and -2 (BCH2)) are primarily responsible for β-ring hydroxylation of β-carotene and produce zeaxanthin, while two heme-containing cytochrome P450 enzymes (CYP97A3 and CYP97C1) preferentially hydroxylate the ε- and β-ionone rings of α-carotene, yielding lutein (Fig. 2.1). In carrot , unusually high levels of α- and β-carotene are accumulated in leaves due to the presence of a defective carotene hydroxylase CYP97A3 (Arango et al. 2014).
Zeaxanthin is further epoxidized by the enzyme zeaxanthin epoxidase (ZEP), leading to antheraxanthin, violaxanthin and neoxanthin, a reversible reaction carried out by violaxanthin de-epoxidase (VDE), representing the xanthophyll cycle (Niyogi et al. 1998). Six types of xanthophyll cycles have been described. Four of them are based on β-xanthophylls and two on α-xanthophylls (Garcia-Plazaola et al. 2007). The common factor in all xanthophyll cycles is the light-dependent transformation of epoxidized xanthophylls to de-epoxidized ones, which facilitates the dissipation of excitation energy, and their reversion to epoxidized xanthophylls in low light (Muller et al. 2001; Latowski et al. 2004). Two of the xanthophyll cycles have been described for land plants : The violaxanthin cycle, in which violaxanthin is reversibly converted to zeaxanthin via antheraxanthin (Sapozhnikov et al. 1957; Yamamoto et al. 1962; Jahns et al. 2009) and the lutein epoxide cycle, in which lutein epoxide is reversibly converted into lutein (Bungard et al. 1999; Garcia-Plazaola et al. 2007). Both cycles are involved in the light-regulated switching of PSII from a light-harvesting state (with epoxidized xanthophylls, violaxanthin and lutein epoxide present in low light or darkness) to an energy dissipating state (with de-epoxidized xanthophylls, antheraxanthin, zeaxanthin and lutein, present in high light). Therefore, these cycles facilitate the short- and long-term acclimation of plants to varying light conditions. Although the violaxanthin cycle is present in all land plants, the lutein epoxide cycle is restricted to some species only (Esteban et al. 2009). The most commonly occurring type of xanthophyll cycles in plants and the most intensively studied is the violaxanthin cycle, also called the xanthophyll cycle, where the main product of strong light-stimulated de-epoxidation is zeaxanthin. The violaxanthin or xanthophyll cycle, first described in lettuce by Yamamoto et al. (1962), consists of the light-driven de-epoxidation of the diepoxide violaxanthin through the intermediate monoepoxide antheraxanthin into zeaxanthin, and the dark epoxidation of zeaxanthin via antheraxanthin into violaxanthin. The cycle is catalyzed by VDE and ZEP that act in two successive steps. These enzymes are differentially located in chloroplast thylakoids; VDE is located in the thylakoid lumen and ZEP is on the stromal side (Hager 1980; Pfundel et al. 1994). Low pH and ascorbate are required by VDE that converts violaxanthin into zeaxanthin via the intermediate antheraxanthin (Pfundel et al. 1994; Bratt et al. 1995; Kramer et al. 1999). Under low light and relatively alkaline conditions (optimum pH of 7.5), ZEP hydroxylates β-rings of zeaxanthin in two consecutive steps to yield antheraxanthin and then violaxanthin, thus forming an integrated cycle. This cycle is present in all higher plants studied to date, as well as in ferns, mosses, lichens, and some algae (Phaeophyta, Chlorophyta and Rhodophyta).
Finally violaxanthin is converted to neoxanthin by neoxanthin synthase (NSY), which also represents the final step in the core carotenoid biosynthetic pathway.
The lutein epoxide cycle was reported in green tomato fruits (Rabinowitch et al. 1975) and was later found in the photosynthetic stems of the parasitic plant Cuscuta reflexa Roxb. (Bungard et al. 1999). It involves the de-epoxidation of lutein epoxide (monoepoxide) to lutein and the epoxidation of lutein to lutein epoxide.
For the lutein epoxide cycle, two different types have been described:
-
(i)
A complete lutein epoxide cycle, in which lutein Lut is reconverted to lutein epoxide overnight
-
(ii)
A truncated lutein epoxide cycle, in which lutein Lut reconversion is not observed overnight (Garcia-Plazaola et al. 2007).
Essential photoprotective functions have been assigned to lutein and the xanthophyll cycle pigments antheraxanthin and zeaxanthin, particularly related to the heat dissipation of excess light energy (Non-Photochemical Quenching).
The xanthophyll precursor pool plays an important role in the biosynthesis of the phytohormone abscisic acid (ABA) (Fig. 2.1, Li and Walton 1990; Seo and Koshiba 2002; Wasilewska et al. 2008). De novo synthesis of ABA requires ZEP-catalyzed epoxidation of zeaxanthin to violaxanthin. Subsequently, the violaxanthin-derivatives neoxanthin and xanthoxin are converted into ABA through a series of isomerization and dehydrogenation reactions (Milborrow 2001). In the ABA-deficient mutant aba1 (an allele of npq2), ZEP is not functional and causes accumulation of zeaxanthin in parallel with the decrease in the epoxy-xanthophylls antheraxanthin, violaxanthin and neoxanthin (Duckham et al. 1991; Marin et al. 1996). In the xanthophyll cycle, VDE requires ascorbate as reductant to convert violaxanthin to zeaxanthin (Bratt et al. 1995). As a result, reduced levels of ascorbate in the Arabidopsis vtc1 (vitamin C1) mutant stimulate ABA production (Pastori et al. 2003). In contrast, enhanced VDE activity can reduce ascorbate levels and antagonize ABA synthesis (Pastori et al. 2003). Therefore, the regulation of the xanthophyll cycle allows ABA levels to be modified.
4.7 Neoxanthin Synthase
The final step of the β,β-branch in the classic carotenoid pathway is the conversion of yellow-colored violaxanthin into a xanthophyll carrying an allenic double bond, named neoxanthin which represents the classical final step in plant xanthophyll formation (Li and Walton 1990; Parry and Horgan 1991). Neoxanthin together with violaxanthin can further be used for the production of the apocarotenoid hormone, ABA. Interestingly, in tomato violaxanthin is a sufficient precursor for ABA production in vivo (Neuman et al. 2014).
Even though, neoxanthin is synthesized from violaxanthin in plants (Li and Walton 1990; Parry and Horgan 1991; Rock and Zeevaart 1991; Galpaz et al. 2008; Qin et al. 2008), the identity of the gene coding for neoxanthin synthase (NSY) and the mechanism of formation of this xanthophyll, are still not known. Two previous reports have suggested that NSY is encoded by a gene of the lycopene cyclase gene family (Al-Babili et al. 2000; Bouvier et al. 2000). Indeed NSY enzyme was practically identical to the chromoplast-targeted lycopene β-cyclase from tomato (LCYB2/CYC-B isoform) (Ronen et al. 2000). However, the ortholog of this gene in pepper codes for capsanthin–capsorubin synthase, (CCS) an enzyme involved in the production of ketocarotenoids in pepper fruit chromoplasts (Bouvier et al. 1994). Genetic and molecular analyses of two alleles of nxd1, a recessive neoxanthin-deficient mutation in tomato, identified a gene of unknown function, which is necessary for neoxanthin synthesis in tomato (Neuman et al. 2014). Two unrelated mutations in the gene NXD1 were identified in tomato and a loss-of-function mutation in the orthologous NXD1 gene of Arabidopsis showed an identical phenotype of lack of neoxanthin in leaves, but the study was unable to demonstrate any neoxanthin synthesis activity by NXD1 in a functional expression assay in E. coli (Neuman et al. 2014). Although, no NSY homologous gene has been identified in the genome of Arabidopsis, the ABA4 (At1g67080) gene of Arabidopsis (North et al. 2007) led to increased accumulation of trans-neoxanthin when overexpressed, whereas the loss-of-function in aba4 mutant was defective in this xanthophyll, indicating that the ABA4 protein has a direct role in neoxanthin synthesis. However, in vitro activity of the cloned gene has never been demonstrated.
Considering the results obtained at present, the identification of a functional NSY has several hindrances in view of that this reaction is not readily accessible by classical methods of protein purification, since it has not been possible until now to perform the reaction in vitro starting from exogenous synthetic violaxanthin. The high degree of lipophilicity of the substrate and product, suggest that the enzymatic activity could be dependent on membrane structures. Also the incapability of transformed E. coli to synthesize epoxidated xanthophylls in vivo and the very high spectral similarity of violaxanthin and neoxanthin (Marin et al. 1996) rule out color complementation methods in Escherichia coli even though this strategy have proven to be successful with other carotenoid biosynthetic enzymes. Therefore, the identification of a mutant defective in this biosynthetic step is expected to give better insights on the identification and characterization of NSY.
5 Concluding Remarks
The updated information provided in this chapter aims to confirm that until now the plant carotenoid pathway and the characterization of each enzymatic step is ongoing. Several efforts have been done to understand the metabolic flux in different plant models. Although most knowledge has been obtained from Arabidopsis thaliana and mutants obtained thereof, we may conclude that each plant is a whole and unique specie for carotenoid synthesis regulation .
At present most of the genes and enzymes have been identified and characterized, which has been very useful and amenable to continuously improve the carotenogenic metabolism of many different vegetal species by genetic engineering.
This obviously provides opportunities for the improvement of the nutritional value of various food plants and it also opens up the possibility for the metabolic engineering of compounds further downstream in the carotenoid pathway (Chap. 13), for which naturally accumulating mutants or genotypes are not currently available. Those of more than 750 structurally different yellow, orange, and red colored molecules found in both eukaryotes and prokaryotes that were classified in simplified terms in this chapter comprised an estimated global market totaled $1.5 billion in 2014. This market is expected to reach nearly $1.8 billion in 2019, with a compound annual growth rate (CAGR) of 3.9% (According to “The Global Market for Carotenoids” from PR Newswire Association LLC). This projected market size reflects the multiple functions and uses of carotenoids as cells protectors against photooxidative damage involving important applications in disease control, environment, food and nutrition, and as potent antimicrobial agents.
References
Agnolucci L, Dalla Vecchia F, Barbato R, Tassani V, Casadoro G, Rascio N (1996) Amitrole effects on chloroplasts from barley plants grown at different temperatures. J Plant Physiol 147:493–502
Ahrazem O, Rubio-Moraga A, Lopez RC, Gomez-Gomez L (2010) The expression of a chromoplast-specific lycopene beta cyclase gene is involved in the high production of saffron’s apocarotenoid precursors. J Exp Bot 61:105–119
Akiyama K, K-i M, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435:824–827
Al-Babili S, Hugueney P, Schledz M, Welsch R, Frohnmeyer H, Laule O, Beyer P (2000) Identification of a novel gene coding for neoxanthin synthase from Solanum tuberosum. FEBS Lett 485:168–172
Al-Babili S, von Lintig J, Haubruck H, Beyer P (1996) A novel, soluble form of phytoene desaturase from Narcissus pseudonarcissus chromoplasts is Hsp70-complexed and competent for flavinylation, membrane association and enzymatic activation. Plant J 9:601–612
Albrecht M, Klein A, Hugueney P, Sandmann G (1995) Molecular cloning and functional expression in E. coli of a novel plant enzyme mediating ξ-carotene desaturation. FEBS Lett 372(2–3):199–202
Albrecht M, Linden H, Sandmann G (1996) Biochemical characterization of purified zeta-carotene desaturase from Anabaena PCC 7120 after expression in Escherichia coli. Eur J Biochem 236:115–120
Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P, Ghisla S, Bouwmeester H, Beyer P, Al-Babili S (2012) The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 335:1348–1351
Aluru MR, Bae H, Wu D, Rodermel SR (2001) The Arabidopsis immutans mutation affects plastid differentiation and the morphogenesis of white and green sectors in variegated plants. Plant Physiol 127:67–77
Álvarez R, Vaz B, Gronemeyer H, de Lera ÁR (2013) Functions, therapeutic applications, and synthesis of retinoids and carotenoids. Chem Rev 114:1–125
Ampomah-Dwamena C, McGhie T, Wibisono R, Montefiori M, Hellens RP, Allan AC (2009) The kiwifruit lycopene betacyclase plays a significant role in carotenoid accumulation in fruit. J Exp Bot 60(13):3765–3779
Araki N, Kusumi K, Masamoto K, Niwa Y, Iba K (2000) Temperature -sensitive Arabidopsis mutant defective in 1-deoxy-dxylulose 5-phosphate synthase within the plastid non-mevalonate pathway of isoprenoid biosynthesis. Physiol Plant 108:19–24
Arango J, Jourdan M, Geoffriau E, Beyer P, Welsch R (2014) Carotene hydroxylase activity determines the levels of both alpha-carotene and total carotenoids in orange carrots. Plant Cell 26:2223–2233
Araya-Garay JM, Feijoo-Siota L, Veiga-Crespo P, Sánches-Pérez A, González V (2012) Cloning and functional expression of Z-carotene desaturase, a novel carotenoid biosynthesis gene from Ficus Carica. J Bioprocess Biotech 2:125
Armstrong GA (1994) Eubacteria show their true colors: genetics of carotenoid pigment biosynthesis from microbes to plants. J Bacteriol 176:4795–4802
Armstrong GA (1997) Genetics of eubacterial carotenoid biosynthesis: a colorful tale. Annu Rev Microbiol 51:629–659
Auldridge ME, McCarty DR, Klee HJ (2006) Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr Opin Plant Biol 9:315–321
Austin JR, Frost E, Vidi P-A, Kessler F, Staehelin LA (2006) Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes. Plant Cell 18:1693–1703
Avendaño-Vázquez A-O, Cordoba E, Llamas E, San Román C, Nisar N, De la Torre S, Ramos-Vega M, Gutiérrez-Nava ML, Cazzonelli CI, Pogson BJ, León P (2014) An uncharacterized apocarotenoid-derived signalgenerated in ζ-carotene desaturase mutants regulates leaf development and the expression of chloroplast and nuclear genes in arabidopsis. Plant Cell 26:2524–2537
Bai L, Kim EH, DellaPenna D, Brutnell TP (2009) Novel lycopene epsilon cyclase activities in maize revealed through perturbation of carotenoid biosynthesis. Plant J 59:588–599
Bartley GE, Scolnik PA (1995) Plant carotenoids: pigments for photoprotection, visual attraction, and human health. Plant Cell 7:1027–1038
Bartley GE, Scolnik PA, Beyer P (1999) Two Arabidopsis thaliana carotene desaturases, phytoene desaturase and ζ‐carotene desaturase, expressed in Escherichia coli, catalyze a poly‐cis pathway to yield pro‐lycopene. Eur J Biochem 259:396–403
Bartley GE, Viitanen PV, Pecker I, Chamovitz D, Hirschberg J, Scolnik PA (1991) Molecular cloning and expression in photosynthetic bacteria of a soybean cDNA coding for phytoene desaturase, an enzyme of the carotenoid biosynthesis pathway. Proc Natl Acad Sci USA 88:6532–6536
Beltrán J, Kloss B, Hosler JP, Geng J, Liu A, Modi A, Dawson JH, Sono M, Shumskaya M, Ampomah-Dwamena C, Love JD, Wurtzel ET (2015) Control of carotenoid biosynthesis through a heme-based cis-trans isomerase. Nat Chem Biol 11:598–605
Berson EL (1982) Nutrition and retinal degenerations: vitamin A, taurine, ornithine, and phytanic acid. RETINA 2:236
Beyer P (1989) Physiology, biochemistry, and genetics of nongreen plastids. In: Carotene biosynthesis in daffodil chromoplasts: on the membrane-integral desaturation and cyclization reactions. American Society of Plant Physiologists, Rockville, pp 157–170
Beyer P, Mayer M, Kleinig H (1989) Molecular oxygen and the state of geometric isomerism of intermediates are essential in the carotene desaturation and cyclization reactions in daffodil chromoplasts. Eur J Biochem 184(1):141–150
Bjerkeng B (2000) Carotenoid pigmentation in salmonid fishes- recent progress. In: Cruz-Suarez LE, Ricque-Marie D, Tapia-Salazar M, Olvera-Novoa MA, Civera-Cereceda R (eds) Avances en Nutrición Acuícola, Memorias del V Simposium Internacional de Nutrición Acuícola, Ciudad de Mérida, México, pp 71–89
Blas AL, Ming R, Liu Z, Veatch OJ, Paull RE, Moore PH, Yu Q (2010) Cloning of the papaya chromoplast-specific lycopene beta-cyclase, CpCYC-b, controlling fruit flesh color reveals conserved microsynteny and a recombination hot spot. Plant Physiol 152:2013–2022
Böger P, Sandmann G (1998) Carotenoid biosynthesis inhibitor herbicides – mode of action and resistance mechanisms. Pestic Outlook 9:29–35
Bonk M, Hoffmann B, von Lintig J, Schledz M, Al-Babili S, Hobeika E, Kleinig H, Beyer P (1997) Chloroplast import of four carotenoid biosynthetic enzymes in vitro reveals differential fates prior to membrane binding and oligomeric assembly. Eur J Biochem 247:942–950
Bouvier F, D’Harlingue A, Backhaus RA, Kumagai MH, Camara B (2000) Identification of neoxanthin synthase as a carotenoid cyclase paralog. Eur J Biochem/FEBS 267:6346–6352
Bouvier F, D’Harlingue A, Camara B (1997) Molecular analysis of carotenoid cyclase inhibition. Arch Biochem Biophys 346:53–64
Bouvier F, Hugueney P, D’Harlingue A, Kuntz M, Camara B (1994) Xanthophyll biosynthesis in chromoplasts: isolation and molecular cloning of an enzyme catalyzing the conversion of 5,6-epoxycarotenoid into ketocarotenoid. Plant J 6:45–54
Bouwmeester HJ, Roux C, Lopez-Raez JA, Bécard G (2007) Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci 12:224–230
Bramley PM (2002) Regulation of carotenoid formation during tomato fruit ripening and development. J Exp Bot 53:2107–2113
Bratt CE, Arvidsson PO, Carlsson M, Akerlund HE (1995) Regulation of violaxanthin de-epoxidase activity by pH and ascorbate concentration. Photosynth Res 45:169–175
Breitenbach J, Kuntz M, Takaichi S, Sandmann G (1999) Catalytic properties of an expressed and purified higher plant type zeta-carotene desaturase from Capsicum annuum. Eur J Biochem 265:376–383
Breitenbach J, Sandmann G (2005) zeta-Carotene cis isomers as products and substrates in the plant poly-cis carotenoid biosynthetic pathway to lycopene. Planta 220:785–793
Breitenbach J, Zhu C, Sandmann G (2001) Bleaching herbicide norflurazon inhibits phytoene desaturase by competition with the cofactors. J Agric Food Chem 49:5270–5272
Britton G (1995) Structure and properties of carotenoids in relation to function. FASEB J 9(15):1551–1558
Britton G, Liaaen-Jensen S, Pfander H (2004) Carotenoids. In: Handbook. Springer, New York, p 647
Buckner B, Miguel PS, Janick-Buckner D, Bennetzen JL (1996) The y1 gene of maize codes for phytoene synthase. Genetics 143:479–488
Bungard RA, Ruban AV, Hibberd JM, Press MC, Horton P, Scholes JD (1999) Unusual carotenoid composition and a new type of xanthophyll cycle in plants. Proc Natl Acad Sci USA 96:1135–1139
Burkhardt PK, Beyer P, Wünn J, Klöti A, Armstrong GA, Schledz M, von Lintig J, Potrykus I (1997) Transgenic rice (Oryza sativa) endosperm expressing daffodil (Narcissus pseudonarcissus) phytoene synthase accumulates phytoene, a key intermediate of provitamin A biosynthesis. Plant J 11:1071–1078
Burns ER, Buchanan GA, Carter MC (1971) Inhibition of carotenoid synthesis as a mechanism of action of amitrole, dichlormate, and pyriclor. Plant Physiol 47:144–148
Busch M, Seuter A, Hain R (2002) Functional analysis of the early steps of carotenoid biosynthesis in tobacco. Plant Physiol 128:439–453
Candau R, Bejarano ER, Cerdá-Olmedo E (1991) In vivo channeling of substrates in an enzyme aggregate for beta-carotene biosynthesis. Proc Natl Acad Sci USA 88:936–4940
Carretero-Paulet L, Cairo A, Botella-Pavia P, Besumbes O, Campos N, Boronat A, Rodriguez-Concepcion M (2006) Enhanced flux through the methylerythritol 4-phosphate pathway in Arabidopsis plants overexpressing deoxyxylulose 5-phosphate reductoisomerase. Plant Mol Biol 62:683–695
Carol P, Kuntz M (2001) A plastid terminal oxidase comes to light: implications for carotenoid biosynthesis and chlororespiration. Trends Plant Sci 6:31–36
Carol P, Stevenson D, Bisanz C, Breitenbach J, Sandmann G, Mache R, Coupland G, Kuntz M (1999) Mutations in the Arabidopsis gene IMMUTANS cause a variegated phenotype by inactivating a chloroplast terminal oxidase associated with phytoene desaturation. Plant Cell 11:57–68
Chai C, Fang J, Liu Y, Tong H, Gong Y, Wang Y, Liu M, Wang Y, Qian Q, Cheng Z et al (2011) ZEBRA2, encoding a carotenoid isomerase, is involved in photoprotection in rice. Plant Mol Biol 75:211–221
Conti A, Pancaldi S, Fambrini M, Michelotti V, Bonora A, Salvini M, Pugliesi C (2004) A deficiency at the gene coding for zeta-carotene desaturase characterizes the sunflower non dormant-1 mutant. Plant Cell Physiol 45:445–455
Corniani N, Velini ED, Silva FML, Nanayakkara NPD, Witschel M, Dayan FE (2014) Novel bioassay for the discovery of inhibitors of the 2-C-methyl-D-erythritol 4-phosphate (MEP) and terpenoid pathways leading to carotenoid biosynthesis. PLoS One 9:e103704
Cunningham FX Jr, Pogson B, Sun Z, McDonald KA, DellaPenna D, Gantt E (1996) Functional analysis of the beta and epsilon lycopene cyclase enzymes of Arabidopsis reveals a mechanism for control of cyclic carotenoid formation. Plant Cell 8:1613–1626
Cunningham FX, Gantt E (1998) Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 49:557–583
Cunningham FX Jr, Gantt E (2001) One ring or two? Determination of ring number in carotenoids by lycopene epsilon-cyclases. Proc Natl Acad Sci USA 98:2905–2910
Cunningham FX Jr, Sun Z, Chamovitz D, Hirschberg J, Gantt E (1994) Molecular structure and enzymatic function of lycopene cyclase from the cyanobacterium Synechococcus sp strain PCC7942. Plant Cell 6:1107–1121
Cunningham FX Jr (2002) Regulation of carotenoid synthesis and accumulation in plants. Pure Appl Chem 74:1409–1417
Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61:651–679
Cuttriss AJ, Cazzonelli CI, Wurtzel ET, Pogson BJ (2011) Carotenoids. Adv Bot Res 58:1–36
Chamovitz D, Sandmann G, Hirschberg J (1993) Molecular and biochemical characterization of herbicide-resistant mutants of cyanobacteria reveals that phytoene desaturation is a rate-limiting step in carotenoid biosynthesis. J Biol Chem 268:17348–17353
Chen X, Han H, Jiang P, Nie L, Bao H, Fan P, Lv S, Feng J, Li Y (2011) Transformation of beta-lycopene cyclase genes from Salicornia europaea and Arabidopsis conferred salt tolerance in Arabidopsis and tobacco. Plant Cell Physiol 52:909–921
Chen Y, Li F, Wurtzel ET (2010) Isolation and characterization of the Z-ISO gene encoding a missing component of carotenoid biosynthesis in plants. Plant Physiol 153:66–79
Chollet R, Sandmann G, Diethelm R, Felix H, Milzner K, Böger P (1990) zeta-Carotene accumulation and bleaching by new pyrimidine compounds. Pest Sci 30:326–329
D’Haeze W (2002) Inhibiting carotenoids. Genome Biol 3:reports0031
Dall’Osto L, Fiore A, Cazzaniga S, Giuliano G, Bassi R (2007) Different roles of alpha- and beta-branch xanthophylls in photosystem assembly and photoprotection. J Biol Chem 282:35056–35068
Davison PA, Hunter CN, Horton P (2002) Overexpression of β-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 418:203–206
Dayan FE, Duke SO (2003) Herbicides, carotenoid biosynthesis inhibitors. In: Encyclopedia of agrochemicals. Wiley, Hoboken, pp 744–749
De la Guardia MD, Aragón CM, Murillo FJ, Cerdá-Olmedo E (1971) A carotenogenic enzyme aggregate in Phycomyces: evidence from quantitive complementation. Proc Natl Acad Sci USA 68:2012–2015
Delcourt C, Carrière I, Delage M, Barberger-Gateau P, Schalch W, Group PS (2006) Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: the POLA Study. Invest Ophthalmol Vis Sci 47:2329–2335
DellaPenna D, Pogson BJ (2006) Vitamin synthesis in plants: tocopherols and carotenoids. Annu Rev Plant Biol 57:711–738
Devitt LC, Fanning K, Dietzgen RG, Holton TA (2010) Isolation and functional characterization of a lycopene beta-cyclase gene that controls fruit colour of papaya (Carica papaya L.). J Exp Bot 61:33–39
Diretto G, Tavazza R, Welsch R, Pizzichini D, Mourgues F, Papacchioli V, Beyer P, Giuliano G (2006) Metabolic engineering of potato tuber carotenoids through tuber-specific silencing of lycopene epsilon cyclase. BMC Plant Biol 6:13
Dogbo O, Laferriére A, D’Harlingue A, Camara B (1988) Carotenoid biosynthesis: Isolation and characterization of a bifunctional enzyme catalyzing the synthesis of phytoene. Proc Natl Acad Sci USA 85:7054–7058
Dong H, Deng Y, Mu J, Lu Q, Wang Y, Xu Y, Chu C, Chong K, Lu C, Zuo J (2007) The Arabidopsis Spontaneous Cell Death1 gene, encoding a |[zeta]|-carotene desaturase essential for carotenoid biosynthesis, is involved in chloroplast development, photoprotection and retrograde signalling. Cell Res 17:458–470
Duckham S, Linforth R, Taylor I (1991) Abscisic-acid-deficient mutants at the aba gene locus of Arabidopsis thaliana are impaired in the epoxidation of zeaxanthin. Plant Cell Environ 14:601–606
Ducreux LJM, Morris WL, Hedley PE, Shepherd T, Davies HV, Millam S, Taylor MA (2005) Metabolic engineering of high carotenoid potato tubers containing enhanced levels of beta-carotene and lutein. J Exp Bot 56:81–89
Eisenreich W, Bacher A, Arigoni D, Rohdich F (2004) Biosynthesis of isoprenoids via the non-mevalonate pathway. Cell Mol Life Sci 61:1401–1426
Esteban R, Olano JM, Castresana J, Fernandez-Marin B, Hernandez A, Becerril JM, Garcia-Plazaola JI (2009) Distribution and evolutionary trends of photoprotective isoprenoids (xanthophylls and tocopherols) within the plant kingdom. Physiol Plant 135:379–389
Estévez JM, Cantero A, Romero C, Kawaide H, Jiménez LF, Kuzuyama T, Seto H, Kamiya Y, León P (2000) Analysis of the expression of CLA1, a gene that encodes the 1-deoxyxylulose 5-phosphate synthase of the 2-C-methyl-D- erythritol-4-phosphate pathway in Arabidopsis. Plant Physiol 124:95–104
Estévez JM, Cantero A, Reindl A, Reichler S, León P (2001) 1-Deoxyxylulose 5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. J Biol Chem 276:22901–22909
Fang J, Chai C, Qian Q, Li C, Tang J, Sun L, Huang Z, Guo X, Sun C, Liu M, Zhang Y, Lu Q, Wang Y, Lu C, Han B, Chen F, Cheng Z, Chu C (2008) Mutations of genes in synthesis of the carotenoid precursors of ABA lead to pre-harvest sprouting and photo-oxidation in rice. Plant J 54:177–189
Farré G, Bai C, Twyman RM, Capell T, Christou P, Zhu C (2011) Nutritious crops producing multiple carotenoids – a metabolic balancing act. Trends Plant Sci 16:532–540
Fedtke C, Depka B, Schallner O, Tietjen K, Trebst A, Wollweber D, Wroblowsky HJ (2001) Mode of action of new diethylamines in lycopene cyclase inhibition and in photosystem II turnover. Pest Manag Sci 57:278–282
Ferhatoglu Y, Avdiushko S, Barrett M (2005) The basis for the safening of clomazone by phorate insecticide in cotton and inhibitors of cytochrome P450s. Pestic Biochem Physiol 81:59–70
Ferhatoglu Y, Barrett M (2006) Studies of clomazone mode of action. Pestic Biochem Physiol 85:7–14
Ferro M, Brugière S, Salvi D, Seigneurin-Berny D, Court M, Moyet L, Ramus C, Miras S, Mellal M, Le Gall S, Kieffer-Jaquinod S, Bruley C, Garin J, Joyard J, Masselon C, Rolland N (2010) AT_CHLORO, a comprehensive chloroplast proteome database with subplastidial localization and curated information on envelope proteins. Mol Cell Proteomics 9:1063–1084
Fraser PD, Bramley PM (2004) The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res 43:228–265
Fraser PD, Römer S, Shipton CA, Mills PB, Kiano JW, Misawa N, Drake RG, Schuch W, Bramley PM (2002) Evaluation of transgenic tomato plants expressing an additional phytoene synthase in a fruit-specific manner. Proc Natl Acad Sci USA 99:1092–1097
Fray RG, Wallace A, Fraser PD, Valero D, Hedden P, Bramley PM, Grierson D (1995) Constitutive expression of a fruit phytoene synthase gene in transgenic tomatoes causes dwarfism by redirecting metabolites from the gibberellin pathway. Plant J 8:693–701
Friso G, Majeran W, Huang M, Sun Q, van Wijk KJ (2010) Reconstruction of metabolic pathways, protein expression, and homeostasis machineries across maize bundle sheath and mesophyll chloroplasts: large-scale quantitative proteomics using the first maize genome assembly. Plant Physiol 152:1219–1250
Frusciante S, Diretto G, Bruno M, Ferrante P, Pietrella M, Prado-Cabrero A, Rubio-Moraga A, Beyer P, Gomez-Gomez L, Al-Babili S, Giuliano G (2014) Novel carotenoid cleavage dioxygenase catalyzes the first dedicated step in saffron crocin biosynthesis. Proc Natl Acad Sci USA 111(33):12246–12251
Fuentes P, Pizarro L, Moreno JC, Handford M, Rodríguez-Concepción M, Stange C (2012) Light-dependent changes in plastid differentiation influence carotenoid gene expression and accumulation in carrot roots. Plant Mol Biol 79:47–59
Fujii Y, Kurokawa T, Inoue Y, Yamaguchi I, Misato T (1977) Inhibition of carotenoid biosynthesis as a possible mode of herbicidal action of 3, 3′-dimethyl-4-methoxybenzophenone (NK-049). J Pest Sci 2:431–437
Fujisawa M, Watanabe M, Choi S-K, Teramoto M, Ohyama K, Misawa N (2008) Enrichment of carotenoids in flaxseed (Linum usitatissimum) by metabolic engineering with introduction of bacterial phytoene synthase gene crtB. J Biosci Bioeng 105:636–641
Galpaz N, Wang Q, Menda N, Zamir D, Hirschberg J (2008) Abscisic acid deficiency in the tomato mutant high-pigment 3 leading to increased plastid number and higher fruit lycopene content. Plant J 53:717–730
Galpaz N, Burger Y, Lavee T, Tzuri G, Sherman A, Melamed T, Eshed R, Meir A, Portnoy V et al (2013) Genetic and chemical characterization of an EMS induced mutation in Cucumis melo CRTISO gene. Arch Biochem Biophys 539(2):117–125
Garcia-Plazaola J, Matsubara S, Osmond C (2007) The lutein epoxide cycle in higher plants: its relationships to other xanthophyll cycles and possible functions. Funct Plant Biol 34:759–773
Ghirardo A, Wright LP, Bi Z, Rosenkranz M, Pulido P, Rodríguez-Concepción M, Niinemets Ü, Brüggemann N, Gershenzon J, Schnitzler JP (2014) Metabolic flux analysis of plastidic isoprenoid biosynthesis in poplar leaves emitting and nonemitting isoprene. Plant Physiol 165:37–51
Giorio G, Yildirim A, Stigliani AL, D’Ambrosio C (2013) Elevation of lutein content in tomato: a biochemical tug-of-war between lycopene cyclases. Metab Eng 20:167–176
Giuliano G, Aquilani R, Dharmapuri S (2000) Metabolic engineering of plant carotenoids. Trends Plant Sci 5(10):406–409
Goodwin TW (1980) The biochemistry of the carotenoids, vol 1, 2nd edn. Chapman and Hall, London
Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot J-P, Letisse F, Matusova R, Danoun S, Portais J-C, Bouwmeester H, Bécard G, Beveridge CA, Rameau C, Rochange SF (2008) Strigolactone inhibition of shoot branching. Nature 455:189–194
Grotewold E (2006) The genetics and biochemistry of floral pigments. Annu Rev Plant Biol 57:761–780
Grune T, Lietz G, Palou A, Ross AC, Stahl W, Tang G, Thurnham D, S-a Y, Biesalski HK (2010) Beta-carotene is an important vitamin A source for humans. J Nutr 140:2268S–2285S
Hable WE, Oishi KK, Schumaker KS (1998) Viviparous-5 encodes phytoene desaturase, an enzyme essential for abscisic acid (ABA) accumulation and seed development in maize. Mol Gen Genet 257:167–176
Hager A (1980) The reversible light-induced conversions of xanthophylls in the chloroplast. In: Czygan FC (ed) Pigments in plants. Fischer, Stuttgart, pp 57–79
Hale I, O’Neill PM, Berry NG, Odom A, Sharma R (2012) The MEP pathway and the development of inhibitors as potential anti-infective agents. Med Chem Commun 3:418–433
Harjes CE, Rocheford TR, Bai L, Brutnell TP, Kandianis CB, Sowinski SG, Stapleton AE, Vallabhaneni R, Williams M, Wurtzel ET, Yan J, Buckler ES (2008) Natural genetic variation in lycopene epsilon cyclase tapped for maize biofortification. Science 319:330–333
Havaux M (2014) Carotenoid oxidation products as stress signals in plants. Plant J 79:597–606
Havaux M, Dall’Osto L, Bassi R (2007) Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol 145:1506–1520
Havaux M, Niyogi KK (1999) The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci USA 96:8762–8767
Hirschberg J (2001) Carotenoid biosynthesis in flowering plants. Curr Opin Plant Biol 4(3):210–218
Hirschberg J, Cohen M, Harker M, Lotan T, Mann V, Pecker I (1997) Molecular genetics of the carotenoid biosynthesis pathway in plants and algae. Pure Appl Chem 69:2151–2158
Hornero-Méndez D, Britton G (2002) Involvement of NADPH in the cyclization reaction of carotenoid biosynthesis. FEBS Lett 515:133–136
Hsieh M-H, Goodman HM (2005) The Arabidopsis IspH homolog is involved in the plastid nonmevalonate pathway of isoprenoid biosynthesis. Plant Physiol 138:641–653
Hugueney P, Badillo A, Chen HC, Klein A, Hirschberg J, Camara B, Kuntz M (1995) Metabolism of cyclic carotenoids: a model for the alteration of this biosynthetic pathway in Capsicum annuum chromoplasts. Plant J 8:417–424
Hugueney P, Römer S, Kuntz M, Camara B (1992) Characterization and molecular cloning of a flavoprotein catalyzing the synthesis of phytofluene and ζ‐carotene in Capsicum chromoplasts. Eur J Biochem 209:399–407
Iniesta AA, Cervantes M, Murillo FJ (2008) Conversion of the lycopene monocyclase of Myxococcus xanthus into a bicyclase. Appl Microbiol Biotechnol 79:793–802
Isaacson T, Ohad I, Beyer P, Hirschberg J (2004) Analysis in vitro of the enzyme CRTISO establishes a poly-cis-carotenoid biosynthesis pathway in plants. Plant Physiol 136:4246–4255
Isaacson T, Ronen G, Zamir D, Hirschberg J (2002) Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of beta-carotene and xanthophylls in plants. Plant Cell 14:333–342
Ishikawa M, Fujiwara M, Sonoike K, Sato N (2009) Orthogenomics of photosynthetic organisms: bioinformatic and experimental analysis of chloroplast proteins of endosymbiont origin in Arabidopsis and their counterparts in Synechocystis. Plant Cell Physiol 50:773–788
Jahns P, Latowski D, Strzalka K (2009) Mechanism and regulation of the violaxanthin cycle: the role of antenna proteins and membrane lipids. Biochim Biophys Acta 1787:3–14
Janick-Buckner D, O’Neal JM, Joyce EK, Buckner B (2001) Genetic and biochemical analysis of the y9 gene of maize, a carotenoid biosynthetic gene. Maydica 46:41–46
Jeffrey SW, Douce R, Benson AA (1974) Carotenoid transformations in the chloroplast envelope. Proc Natl Acad Sci USA 71:807–810
Johnson MP, Havaux M, Triantaphylidès C, Ksas B, Pascal AA, Robert B, Davison PA, Ruban AV, Horton P (2007) Elevated zeaxanthin bound to oligomeric LHCII enhances the resistance of Arabidopsis to photooxidative stress by a lipid-protective, antioxidant mechanism. J Biol Chem 282:22605–22618
Josse EM, Simkin AJ, Gaffé J, Labouré AM, Kuntz M, Carol P (2000) A plastid terminal oxidase associated with carotenoid desaturation during chromoplast differentiation. Plant Physiol 123:1427–1436
Joyard J, Ferro M, Masselon C, Seigneurin-Berny D, Salvi D, Garin J, Rolland N (2009) Chloroplast Proteomics and the Compartmentation of Plastidial Isoprenoid Biosynthetic Pathways. Mol Plant 2:1154–1180
Kachanovsky DE, Filler S, Isaacson T, Hirschberg J (2012) Epistasis in tomato color mutations involves regulation of phytoene synthase 1 expression by cis-carotenoids. Proc Natl Acad Sci USA 109:19021–19026
Karppi J, Laukkanen JA, Mäkikallio TH, Ronkainen K, Kurl S (2013) Serum β-carotene and the risk of sudden cardiac death in men: A population-based follow-up study. Atherosclerosis 226:172–177
Kevan PG, Baker HG (1983) Insects as flower visitors and pollinators. Ann Rev Entomol 28:407–453
Kerr MW, Whitaker DF (1987) The mode of action of the herbicide W L110547. Br Crop Prot Conf Weeds 3:1005–1013
Kim J, Smith JJ, Tian L, DellaPenna D (2009) The evolution and function of carotenoid hydroxylases in Arabidopsis. Plant Cell Physiol 50:463–479
Kim SH, Kim YH, Ahn YO, Ahn MJ, Jeong JC, Lee HS, Kwak SS (2013) Downregulation of the lycopene epsilon-cyclase gene increases carotenoid synthesis via the beta-branch-specific pathway and enhances salt-stress tolerance in sweetpotato transgenic calli. Physiol Plant 147:432–442
Kirigia D, Runo S, Alakonya A (2014) A virus-induced gene silencing (VIGS) system for functional genomics in the parasitic plant Striga hermonthica. Plant Methods 2005 1:4, vol 10. BioMed Central, p 16
Klaui H (1982) Industrial and commercial uses of carotenoids. In: Carotenoid chemistry and biochemistry. Pergamon Press, Oxford, pp 308–317
Kramer D, Sacksteder C, Cruz J (1999) How acidic is the lumen? Photosynth Res 60:151–163
Krinsky N, Wang XD, Tang T, Russell R (1994) Cleavage of β-carotene to retinoid. In: Livrea MA, Vidali G (eds) Retinoids: from basic science to clinical applications. Birkhauser Press, Birkhaüser, Basel, pp 21–28
Krinsky NI, Johnson EJ (2005) Carotenoid actions and their relation to health and disease. Mol Aspects Med 26:459–516
Krubasik P, Sandmann G (2000) Molecular evolution of lycopene cyclases involved in the formation of carotenoids with ionone end groups. Biochem Soc Trans 28:806–810
La Rocca N, Bonora A, Dalla Vecchia F, Barbato R, Rascio N (1998) Effects of amitrole and norflurazon on carotenogenesis in barley plants grown at different temperatures. In: Garab G (ed) Photosynthesis: mechanisms and effects, vol V. Kluwer, Dordrecht, pp 3459–3462
La Rocca N, Rascio N, Oster U, Rudiger W (2007) Inhibition of lycopene cyclase results in accumulation of chlorophyll precursors. Planta 225:1019–1029
Latowski D, Grzyb J, Strzalka K (2004) The xanthophyll cycle – molecular mechanism and physiological significance. Acta Physiol Plant 26:197–212
Laule O, Fürholz A, Chang H-S, Zhu T, Wang X, Heifetz PB, Gruissem W, Lange M (2003) Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 100:6866–6871
Li F, Murillo C, Wurtzel ET (2007) Maize Y9 encodes a product essential for 15-cis-zeta-carotene isomerization. Plant Physiol 144:1181–1189
Li F, Vallabhaneni R, Yu J, Rocheford T, Wurtzel ET (2008) The maize phytoene synthase gene family: overlapping roles for carotenogenesis in endosperm, photomorphogenesis, and thermal stress tolerance. Plant Physiol 147:1334–1346
Li Y, Walton D (1990) Violaxanthin is an abscisic acid precursor in water-stressed dark-grown bean leaves. Plant Physiol 92:551–559
Li Z, Wu G, Ji J, Wang G, Tian X, Gao H (2015) Cloning and expression of a ζ-carotene desaturase gene from Lycium chinense. J Genet 94:287–294
Lichtenthaler HK (1999) The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 50:47–65
Lichtenthaler HK, Rohmer M, Schwender J (1997) Two independent biochemical pathways for isopentenyl diphosphate and isoprenoid biosynthesis in higher plants. Physiol Plant 101:643–652
Liu Y, Roof S, Ye Z, Barry C, van Tuinen A, Vrebalov J, Bowler C, Giovannoni J (2004) Manipulation of light signal transduction as a means of modifying fruit nutritional quality in tomato. Proc Natl Acad Sci USA 101:9897–9902
Liu J, Puckhaber LS (2011) The Induction of lycopene in germinating cottonseed with 2-(4-Methylphenoxy) triethylamine (MPTA). Am J Agric Biol Sci 6:1–6
Liu J-X, Chiou C-Y, Shen C-H, Chen P-J, Liu Y-C, Jian C-D, Shen X-L, Shen F-Q, Yeh K-W (2014) RNA interference-based gene silencing of phytoene synthase impairs growth, carotenoids, and plastid phenotype in Oncidium hybrid orchid. Springerplus 3:478
Lopez AB, Yang Y, Thannhauser TW, Li L (2008) Phytoene desaturase is present in a large protein complex in the plastid membrane. Physiol Plant 133:190–198
Lu Y-P, Liu S-Y, Sun H, Wu X-M, Li J-J, Zhu L (2010) Neuroprotective effect of astaxanthin on H(2)O(2)-induced neurotoxicity in vitro and on focal cerebral ischemia in vivo. Brain Res 1360:40–48
Maass D, Arango J, Wüst F, Beyer P, Welsch R (2009) Carotenoid crystal formation in Arabidopsis and carrot roots caused by increased phytoene synthase protein levels. PLoS One 4:e6373
Marin E, Nussaume L, Quesada A, Gonneau M, Sotta B, Hugueney P, Frey A, Marion-Poll A (1996) Molecular identification of zeaxanthin epoxidase of Nicotiana plumbaginifolia, a gene involved in abscisic acid biosynthesis and corresponding to the ABA locus of Arabidopsis thaliana. EMBO J 15:2331–2342
Masamoto K, Hisatomi S, Sakurai I, Gombos Z, Wada H (2004) Requirement of carotene isomerization for the assembly of photosystem II in Synechocystis sp. PCC 6803. Plant Cell Physiol 45:1325–1329
Matthews PD, Luo R, Wurtzel ET (2003) Maize phytoene desaturase and zeta-carotene desaturase catalyse a poly-Z desaturation pathway: implications for genetic engineering of carotenoid content among cereal crops. J Exp Bot 54:2215–2230
Mayer MP, Beyer P, Kleinig H (1990) Quinone compounds are able to replace molecular oxygen as terminal electron acceptor in phytoene desaturation in chromoplasts of Narcissus pseudonarcissus L. Eur J Biochem 191:359–363
Mayne ST (1996) Beta-carotene, carotenoids, and disease prevention in humans. FASEB J 10:690–701
Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA (2011) Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ 343:d5094
Messing SAJ, Gabelli SB, Echeverria I, Vogel JT, Guan JC, Tan BC, Klee HJ, McCarty DR, Amzel LM (2010) Structural insights into maize viviparous14, a key enzyme in the biosynthesis of the phytohormone abscisic acid. Plant Cell 22:2970–2980
Mialoundama AS, Heintz D, Jadid N, Nkeng P, Rahier A, Deli J, Camara B, Bouvier F (2010) Characterization of plant carotenoid cyclases as members of the flavoprotein family functioning with no net redox change. Plant Physiol 153:970–979
Milborrow BV (2001) The pathway of biosynthesis of abscisic acid in vascular plants: a review of the present state of knowledge of ABA biosynthesis. J Exp Bot 52:1145–1164
Miyake C, Yonekura K, Kobayashi Y, Yokota A (2002) Cyclic electron flow within PSII functions in intact chloroplasts from spinach leaves. Plant Cell Physiol 43:951–957
Moran NA, Jarvik T (2010) Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science 328:624–627
Müller C, Schwender J, Zeidler J, Lichtenthaler HK (2000) Properties and inhibition of the first two enzymes of the non-mevalonate pathway of isoprenoid bioynthesis. Biochem Soc Trans 28:792–793
Muller P, Li XP, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Nambara E, Marion-Poll A (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56:165–185
Naqvi S, Zhu C, Farré G, Ramessar K, Bassie L, Breitenbach J, Perez Conesa D, Ros G, Sandmann G, Capell T, Christou P (2009) Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc Natl Acad Sci USA 106:7762–7767
Neill SJ, Horgan R, Parry AD (1986) The carotenoid and abscisic acid content of viviparous kernels and seedlings ofZea mays L. Planta 169:87–96
Neuman H, Galpaz N, Cunningham FX Jr, Zamir D, Hirschberg J (2014) The tomato mutation nxd1 reveals a gene necessary for neoxanthin biosynthesis and demonstrates that violaxanthin is a sufficient precursor for abscisic acid biosynthesis. Plant J 78:80–93
Nievelstein V, Vandekerchove J, Tadros MH, Lintig JV, Nitschke W, Beyer P (1995) Carotene desaturation is linked to a respiratory redox pathway in Narcissus pseudonarcissus chromoplast membranes. Involvement of a 23-kDa oxygen-evolving-complex-like protein. Eur J Biochem 233:864–872
Nisar N, Li L, Lu S, Khin NC, Pogson BJ (2015) Carotenoid metabolism in plants. Mol Plant 8:68–82
Niyogi KK, Grossman AR, Björkman O (1998) Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10:1121–1134
Norris SR, Barrette TR, DellaPenna D (1995) Genetic dissection of carotenoid synthesis in arabidopsis defines plastoquinone as an essential component of phytoene desaturation. Plant Cell 7:2139–2149
Norris SR, Shen X, DellaPenna D (1998) Complementation of the Arabidopsis pds1 mutation with the gene encoding p-hydroxyphenylpyruvate dioxygenase. Plant Physiol 117:1317–1323
North HM, De Almeida A, Boutin JP, Frey A, To A, Botran L, Sotta B, Marion-Poll A (2007) The Arabidopsis ABA-deficient mutant aba4 demonstrates that the major route for stress-induced ABA accumulation is via neoxanthin isomers. Plant J 50:810–824
Obulesu M, Dowlathabad MR, Bramhachari PV (2011) Carotenoids and Alzheimer’s disease: an insight into therapeutic role of retinoids in animal models. Neurochem Int 59:535–541
Park H, Kreunen SS, Cuttriss AJ, DellaPenna D, Pogson BJ (2002) Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis. Plant Cell 14:321–332
Parry AD, Horgan R (1991) Carotenoids and abscisic-acid (ABA) biosynthesis in higher-plants. Physiol Plant 82:320–326
Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH (2003) Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 15:939–951
Paszkowski U (2006) Mutualism and parasitism: the yin and yang of plant symbioses. Curr Opin Plant Biol 9:364–370
Pattnaik P, Roy U, Jain P (1997) Biocolours : new generation additives for food. Indian Food Ind 16:21–25
Pecker I, Chamovitz D, Linden H, Sandmann G, Hirschberg J (1992) A single polypeptide catalyzing the conversion of phytoene to zeta-carotene is transcriptionally regulated during tomato fruit ripening. Proc Natl Acad Sci USA 89:4962–4966
Pecker I, Gabbay R, Cunningham FX Jr, Hirschberg J (1996) Cloning and characterization of the cDNA for lycopene b-cyclase from tomato reveals decrease in its expression during fruit ripening. Plant Mol Biol 30:807–819
Pfundel EE, Renganathan M, Gilmore AM, Yamamoto HY, Dilley RA (1994) Intrathylakoid pH in isolated pea chloroplasts as probed by violaxanthin deepoxidation. Plant Physiol 106:1647–1658
Phillip D, Young AJ (1995) Occurrence of the carotenoid lactucaxanthin in higher plant LHC II. Photosyn Res 43:273–282
Phillip DM, Young AJ (2006) Preferential inhibition of the lycopene epsilon-cyclase by the substituted triethylamine compound MPTA in higher plants. J Plant Physiol 163:383–391
Pogson B, McDonald KA, Truong M, Britton G, DellaPenna D (1996) Arabidopsis carotenoid mutants demonstrate that lutein is not essential for photosynthesis in higher plants. Plant Cell 8:1627–1639
Pulido P, Toledo-Ortiz G, Phillips MA, Wright LP, Rodriguez-Concepcion M (2013) Arabidopsis J-protein J20 delivers the first enzyme of the plastidial isoprenoid pathway to protein quality control. Plant Cell Preview 1–12
Qin X, Yang SH, Kepsel AC, Schwartz SH, Zeevaart JA (2008) Evidence for abscisic acid biosynthesis in Cuscuta reflexa, a parasitic plant lacking neoxanthin. Plant Physiol 147:816–822
Quinlan RF, Shumskaya M, Bradbury LMT, Beltrán J, Ma C, Kennelly EJ, Wurtzel ET (2012) Synergistic interactions between carotene ring hydroxylases drive lutein formation in plant carotenoid biosynthesis. Plant Physiol 160:204–214
Rabinowitch HD, Budowski P, Kedar N (1975) Carotenoids and epoxide cycles in mature-green tomatoes. Planta 122:91–97
Raghavendra AS, Gonugunta VK, Christmann A, Grill E (2010) ABA perception and signalling. Trends Plant Sci 15:395–401
Randolph LF, Hand DB (1940) Relation between carotenoid content and number of genes per cell in diploid and tetraploid corn. J Agric Res 60:51–64
Rao AV, Rao LG (2007) Carotenoids and human health. Pharmacol Res 55:207–216
Rock CD, Zeevaart JA (1991) The aba mutant of Arabidopsis thaliana is impaired in epoxy-carotenoid biosynthesis. Proc Natl Acad Sci USA 88:7496–7499
Rodríguez-Concepción M (2010) Supply of precursors for carotenoid biosynthesis in plants. Arch Biochem Biophys 504:118–122
Rodríguez-Concepción M, Boronat A (2002) Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol 130:1079–1089
Rodríguez-Villalón A, Gas E, Rodriguez-Concepcion M (2009) Colors in the dark: a model for the regulation of carotenoid biosynthesis in etioplasts. Plant Signal Behav 4:965–967
Rohmer M (1998) Isoprenoid biosynthesis via the mevalonate-independent route, a novel target for antibacterial drugs? Prog Drug Res 50:135–154
Rohmer M, Grosdemange-Billiard C, Seemann M, Tritsch D (2004) Isoprenoid biosynthesis as a novel target for antibacterial and antiparasitic drugs. Curr Opin Investig Drugs 5:154–162
Ronen G, Carmel-Goren L, Zamir D, Hirschberg J (2000) An alternative pathway to beta -carotene formation in plant chromoplasts discovered by map-based cloning of beta and old-gold color mutations in tomato. Proc Natl Acad Sci USA 97:11102–11107
Ronen G, Cohen M, Zamir D, Hirschberg J (1999) Regulation of carotenoid biosynthesis during tomato fruit development: expression of the gene for lycopene epsilon-cyclase is down-regulated during ripening and is elevated in the mutant Delta. Plant J 17:341–351
Rossmann MG, Moras D, Olsen KW (1974) Chemical and biological evolution of nucleotide-binding protein. Nature 250:194–199
Rüdiger W, Benz J (1979) Influence of aminotriazol on the biosynthesis of chlorophyll and phytol. Z Naturforsch 34c:1055–1057
Ruiz-Sola MÁ, Rodríguez-Concepción M (2012) Carotenoid biosynthesis in arabidopsis: a colorful pathway. In: http://dx.doi.org/10.1199/tab.0158, vol 10. American Society of Plant Biologists, p e0158
Sandmann G (1994) Carotenoid biosynthesis in microorganisms and plants. Eur J Biochem 223:7–24
Sandmann G (2002) Molecular evolution of carotenoid biosynthesis from bacteria to plants. Physiol Plant 116:431–440
Sandmann G (2009) Evolution of carotene desaturation: the complication of a simple pathway. Arch Biochem Biophys 483:169–174
Sandmann G, Böger P (1989) Inhibition of carotenoid biosynthesis by herbicides. In: Target sites of herbicide action. CRC Press, Boca Raton, pp 25–44
Sandmann G, Bramley PM, Böger P (1985) New herbicidal inhibitors of carotene biosynthesis. J Pest Sci 10:19–24
Sandmann G, Schneider C, Böger P (1996) A new non-radioactive assay of phytoene desaturase to evaluate bleaching herbicides. Z Naturforsch C J Biosci 51:534–538
Sapozhnikov DI, Krasovskaya TA, Maevskaya AN (1957) Change in the interrelationship of the basic carotenoids of the plastids of green leaves under the action of light. Dokl Akad Nauk USSR 113:465–467
Schnurr G, Misawa N, Sandmann G (1996) Expression, purification and properties of lycopene cyclase from Erwinia uredovora. Biochem J 315(Pt 3):869–874
Schnurr G, Boger P, Sandmann G (1998) Interaction of 2-(4-methylphenoxy)triethylamine and related compounds with its herbicide target in the carotenoid biosynthetic pathway. J Pest Sci 23(2):113–116
Schwartz SH, Leon-Kloosterziel KM, Koornneef M, Zeevaart JAD (1997) Biochemical characterization of the aba2 and aba3 mutants in Arabidopsis thaliana. Plant Physiol 114:161–166
Seo M, Koshiba T (2002) Complex regulation of ABA biosynthesis in plants. Trends Plant Sci 7:41–48
Service RF (2007) A growing threat down on the farm. Science 316:1114–1117
Service RF (2013) Agriculture. What happens when weed killers stop killing? Science 341:1329–1329
Shewmaker C, Sheehy J, Daley M, Colburn S, Ke D (1999) Seed-specific overexpression of phytoene synthase: increase in carotenoids and other metabolic effects. Plant J 20:401–412X
Shi Y, Wang R, Luo Z, Jin L, Liu P, Chen Q, Li Z, Li F, Wei C, Wu M, Wei P, Xie H, Qu L, Lin F, Yang J (2014) Molecular cloning and functional characterization of the lycopene epsilon-cyclase gene via virus-induced gene silencing and its expression pattern in Nicotiana tabacum. Int J Mol Sci 15:14766–14785
Shumskaya M, Bradbury LMT, Monaco RR, Wurtzel ET (2012) Plastid localization of the key carotenoid enzyme phytoene synthase is altered by isozyme, allelic variation, and activity. Plant Cell 24:3725–3741
Shumskaya M, Wurtzel ET (2013) The carotenoid biosynthetic pathway: thinking in all dimensions. Plant Sci 208:58–63
Siefermann-Harms D (1978) Light-induced changes of the carotenoid levels in chloroplast envelopes. Plant Physiol 61:530–533
Siefermann-Harms D, Hertzberg S, Borch G, Liaaen-Jensen S (1981) Lactucaxanthin, an ɛ, ɛ-carotene-3,3′-diol from Lactuca sativa. Phytochemistry 20:85–88
Simkin AJ, Breitenbach J, Kuntz M, Sandmann G (2000) In vitro and in situ inhibition of carotenoid biosynthesis in Capsicum annuum by bleaching herbicides. J Agric Food Chem 48:4676–4680
Simkin AJ, Underwood BA, Auldridge M, Loucas HM, Shibuya K, Schmelz E, Clark DG, Klee HJ (2004) Circadian regulation of the PhCCD1 carotenoid cleavage dioxygenase controls emission of beta-ionone, a fragrance volatile of petunia flowers. Plant Physiol 136:3504–3514
Singh N, Cheve G, Avery MA, McCurdy CR (2007) Targeting the methyl erythritol phosphate (MEP) pathway for novel antimalarial, antibacterial and herbicidal drug discovery: inhibition of 1-deoxy-D-xylulose-5-phosphate reductoisomerase (DXR) enzyme. Curr Pharm Des 13:1161–1177
Snodderly D (1995) Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr 62:1448S–1461S
Sommer A, Vyas KS (2012) A global clinical view on vitamin A and carotenoids. Am J Clin Nutr 96:1204S–1206S
Spurgeon SL (1983) Biosynthesis of carotenoids. In: Porter JW, Spurgeon SL (eds) Biosynthesis of isoprenoid compounds. Wiley, New York, pp 1–122
Stange C, Fuentes P, Handford M, Pizarro L (2008) Daucus carota as a novel model to evaluate the effect of light on carotenogenic gene expression. Biol Res 41:289–301
Telfer A, Frolov D, Barber J, Robert B, Pascal A (2003) Oxidation of the two β-carotene molecules in the photosystem II reaction center. Biochemistry 42:1008–1015
Tonhosolo R, D’Alexandri FL, de Rosso VV, Gazarini ML, Matsumura MY, Peres VJ, Merino EF, Carlton JM, Wunderlich G, Mercadante AZ, Kimura EA, Katzin AM (2009) Carotenoid biosynthesis in intraerythrocytic stages of Plasmodium falciparum. J Biol Chem 284:9974–9985
Travella S, Klimm TE, Keller B (2006) RNA interference-based gene silencing as an efficient tool for functional genomics in hexaploid bread wheat. Plant Physiol 142:6–20
Treharne KJ, Mercer EI, Goodwin TW (1966) Carotenoid biosynthesis in some maize mutants. Phytochemistry 5:581–587
Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, Kyozuka J, Yamaguchi S (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455:195–200
Vallabhaneni R, Wurtzel ET (2009) Timing and biosynthetic potential for carotenoid accumulation in genetically diverse germplasm of maize. Plant Physiol 150:562–572
van Poppel G, Goldbohm RA (1995) Epidemiologic evidence for beta-carotene and cancer prevention. Am J Clin Nutr 62:1393S–1402S
Velayos A, Eslava AP, Iturriaga EA (2000) A bifunctional enzyme with lycopene cyclase and phytoene synthase activities is encoded by the carRP gene of Mucor circinelloides. Eur J Biochem 267:5509–5519
Verhoeven AS, Bugos RC, Yamamoto HY (2001) Transgenic tobacco with suppressed zeaxanthin formation is susceptible to stress-induced photoinhibition. Photosyn Res 67:27–39
Walter MH, Floss DS, Strack D (2010) Apocarotenoids: hormones, mycorrhizal metabolites and aroma volatiles. Planta 232:1–17
Walter MH, Strack D (2011) Carotenoids and their cleavage products: biosynthesis and functions. Nat Prod Rep 28:663–692
Wang M, Wang G, Ji J, Wang J (2009) The effect of pds gene silencing on chloroplast pigment composition, thylakoid membrane structure and photosynthesis efficiency in tobacco plants. Plant Sci 177:222–226
Wang Y-Q, Yang Y, Fei Z, Yuan H, Fish T, Thannhauser TW, Mazourek M, Kochian LV, Wang X, Li L (2013) Proteomic analysis of chromoplasts from six crop species reveals insights into chromoplast function and development. J Exp Bot 64:949–961
Wasilewska A, Vlad F, Sirichandra C, Redko Y, Jammes F, Valon C, Frei dit Frey N, Leung J (2008) An update on abscisic acid signaling in plants and more. Mol Plant 1:198–217
Welsch R, Arango J, Bär C, Salazar B, Al-Babili S, Beltrán J, Chavarriaga P, Ceballos H, Tohme J, Beyer P (2010) Provitamin A accumulation in cassava (Manihot esculenta) roots driven by a single nucleotide polymorphism in a phytoene synthase gene. Plant Cell 22:3348–3356
Wei J, Xu M, Zhang D, Mi H (2010) The role of carotenoid isomerase in maintenance of photosynthetic oxygen evolution in rice plant. Acta Biochim Biophys Sin 42:457–463
Withers ST, Keasling JD (2007) Biosynthesis and engineering of isoprenoid small molecules. Appl Microbiol Biotechnol 73:980–990
Wu D, Wright DA, Wetzel C, Voytas DF, Rodermel S (1999) The IMMUTANS variegation locus of Arabidopsis defines a mitochondrial alternative oxidase homolog that functions during early chloroplast biogenesis. Plant Cell 11:43–55
Wurtzel ET (2004) Genomics, genetics, and biochemistry of maize carotenoid biosynthesis. Recent Adv Phytochem 38:85–110
Wurtzel ET, Cuttriss A, Vallabhaneni R (2012) Maize provitamin a carotenoids, current resources, and future metabolic engineering challenges. Front Plant Sci 3:29
Yamamoto HY, Nakayama TOM, Chichester CO (1962) Studies on the light and dark interconversions of leaf xanthophylls. Arch Biochem Biophys 97:168–173
Yan J, Kandianis CB, Harjes CE, Bai L, Kim E-H, Yang X, Skinner DJ, Fu Z, Mitchell S, Li Q, Fernandez MGS, Zaharieva M, Babu R, Fu Y, Palacios N, Li J, DellaPenna D, Brutnell T, Buckler ES, Warburton ML, Rocheford T (2010) Rare genetic variation at Zea mays crtRB1 increases beta-carotene in maize grain. Nat Genet 42:322–327
Yokoyama HHW, Poling SM, Hayman EP, DeBenedict CS (1977) Bioregulators and citrus color. Proc Int Soc Citricul 3:717–722
Ytterberg AJ, Peltier J-B, van Wijk KJ (2006) Protein profiling of plastoglobules in chloroplasts and chromoplasts. A surprising site for differential accumulation of metabolic enzymes. Plant Physiol 140:984–997
Yu B, Lydiate DJ, Young LW, Schafer UA, Hannoufa A (2008) Enhancing the carotenoid content of Brassica napus seeds by downregulating lycopene epsilon cyclase. Transgenic Res 17:573–585
Yu Q, Schaub P, Ghisla S, Al-Babili S, Krieger-Liszkay A, Beyer P (2010) The lycopene cyclase CrtY from Pantoea ananatis (formerly Erwinia uredovora) catalyzes an FADred-dependent non-redox reaction. J Biol Chem 285(16):12109–12120
Yu Q, Ghisla S, Hirschberg J, Mann V, Beyer P (2011) Plant Carotene Cis-Trans Isomerase CRTISO: A new member of the Fadred-dependent Flavoproteins catalyzing non-redox reactions. J Biol Chem 286(10):8666–8676
Yu Q, Beyer P (2012) Reaction specificities of the epsilon-ionone-forming lycopene cyclase from rice (Oryza sativa) elucidated in vitro. FEBS Lett 586:3415–3420
Zu K, Mucci L, Rosner BA, Clinton SK, Loda M, Stampfer MJ, Giovannucci E (2014) Dietary lycopene, angiogenesis, and prostate cancer: a prospective study in the prostate-specific antigen era. J Natl Cancer Inst 106:djt430
Zybailov B, Rutschow H, Friso G, Rudella A, Emanuelsson O, Sun Q, van Wijk KJ (2008) Sorting signals N-terminal modifications and abundance of the chloroplast proteome. PLoS One 3:e1994
Acknowledgements
CONICYT Fondecyt Grant 1130245.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Rosas-Saavedra, C., Stange, C. (2016). Biosynthesis of Carotenoids in Plants: Enzymes and Color. In: Stange, C. (eds) Carotenoids in Nature. Subcellular Biochemistry, vol 79. Springer, Cham. https://doi.org/10.1007/978-3-319-39126-7_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-39126-7_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-39124-3
Online ISBN: 978-3-319-39126-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)