Abstract
Carotenoids are essential components for human nutrition and health, mainly due to their antioxidant and pro-vitamin A activity. Foods with enhanced carotenoid content and composition are essential to ensure carotenoid feasibility in malnourished population of many countries around the world, which is critical to alleviate vitamin A deficiency and other health-related disorders. The pathway of carotenoid biosynthesis is currently well understood, key steps of the pathways in different plant species have been characterized and the corresponding genes identified, as well as other regulatory elements. This enables the manipulation and improvement of carotenoid content and composition in order to control the nutritional value of a number of agronomical important staple crops. Biotechnological and genetic engineering-based strategies to manipulate carotenoid metabolism have been successfully implemented in many crops, with Golden rice as the most relevant example of β-carotene improvement in one of the more widely consumed foods. Conventional breeding strategies have been also adopted in the bio-fortification of carotenoid in staple foods that are highly consumed in developing countries, including maize, cassava and sweet potatoes, to alleviate nutrition-related problems. The objective of the chapter is to summarize major breakthroughs and advances in the enhancement of carotenoid content and composition in agronomical and nutritional important crops, with special emphasis to their potential impact and benefits in human nutrition and health.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
1.1 Importance of Carotenoids for Human Nutrition and Health
Carotenoids are plants pigments that play essential and multiple roles in plants, in addition to provide coloration to flowers, fruits and seeds in many species. They are important during photosynthesis , as part of the light-harvesting complex and in the protection from photo-oxidation. Carotenoids are precursors of some volatiles, play a role in the attraction of pollinators and are also precursors of the plant hormones abscisic acid (ABA) and strigolactones (Krinsky 1989; Havaux 1998; Niyogi 1999; de Saint Germain et al. 2013). Besides these functions in plant physiology and development, carotenoids are key compounds for human nutrition and health. Four carotenoids (α-, β- and γ-carotene, and β-cryptoxanthin) have provitamin A activity, since they are precursors for the synthesis of retinoid , retinol (vitamin A), retinal (main visual pigment), and retinoic acid (Fraser and Bramley 2004; Krinsky and Johnson 2005). β-carotene has the greatest vitamin A activity while β-cryptoxanthin and α-carotene have approximately half the vitamin A activity of β-carotene. Vitamin A deficiency is still a cause of childhood blindness and is associated with reduced immune function and increased risk of mortality from gastrointestinal disease and measles (Maida et al. 2008) but in specific populations of developing countries, dietary carotenoids do not provide yet the required daily vitamin A intake (Olson 1994). On the other hand, zeaxanthin and lutein are also important for maintaining eye health since these two carotenoids are macular pigments that can prevent age-related macular degeneration (Carpentier et al. 2009). Furthermore, carotenoids have antioxidant properties, serving as antioxidant scavengers and inhibitors of pro-inflammatory and pro-thrombotic factors, hence they may provide potential benefits in the prevention of cardiovascular and other chronical diseases (Fassett and Coombes 2012; Mordente et al. 2011). Moreover, higher levels of lycopene in serum are inversely correlated with the incidence of prostate cancer (Etminan et al. 2004). Other studies on the mechanisms of action of this carotenoid revealed that lycopene and its derivatives (metabolites or oxidation products) are antagonists of nuclear receptors such as transacting antioxidant response elements (Zaripheh et al. 2006). Therefore, increasing carotenoid content in crop plants to improve their nutritional value and the benefits for human nutrition, preventing the risk of several food-related diseases, have been a major goal and the objective of many research programs worldwide. Over the last three decades significant progress have been done in the manipulation of carotenoid content and composition in a large number of crops by either genetic engineering or conventional breeding, which have been the subject of exhaustive reviews (Giuliano et al. 2008, 2014; Fraser et al. 2009; Rosati et al. 2009; Farre et al. 2010, 2011; Ye and Bhatia 2012). The aim of this chapter is to summarize major breakthroughs and advances in the enhancement of carotenoid content and composition in agronomical and nutritional important crops , with special emphasis to their potential impact and the benefits for human nutrition and health.
Carotenoids have multiple functions in human nutrition and thus in order to provide food crops with a specific benefit or a particular health-promoting effect, the enhancement of total carotenoid content is not necessarily the best strategy. Rather, strategies should be designed to manipulate specific steps of the carotenoid biosynthetic pathway to enhance when possible the content of the carotenoid of interest, trying to avoid metabolic interferences which may originate negative consequences in the concentration of other metabolically-related carotenoids (Farre et al. 2010). Moreover, carotenoid biosynthetic pathway is complex and each plant species have adopted particular regulatory mechanisms to accumulate a specific carotenoid compliment and that may be also tissue-specific and dependent of the stage of development (Nisar et al. 2015). Then, experimental strategies to enhance carotenoid content and composition in the different crops should be based in a precise understanding of the pathway for each plant species and tissue, identifying key regulatory genes, limiting steps and the mechanism governing the synthesis and degradation of the carotenoid that should be modified. Examples of efficient enhancement of total carotenoids and/or to some particularly useful carotenoids have been obtained in agronomical and nutritional relevant crops, such as rice (Paine et al. 2005), tomato (D’Ambrosio et al. 2014), potato (Diretto et al. 2006), carrot (Jayaraj et al. 2008) or canola (Shewmaker et al. 1999; Ravanello et al. 2003).
1.2 Carotenoid Biosynthesis in Plants and Bacteria
Carotenoids are hydrophobic tetraterpenoids (C40 isoprenoids) that are mostly synthesized in the plastids of photosynthetic organisms such as plants, algae , bacteria and fungi, and also in chromoplasts . In contrast, animals cannot synthesize carotenoids and due to their vital importance for nutrition and health, they must obtain them through the diet. Some animals display visible amounts of carotenoids which are obtained by their feeding, e.g. flamingo feathers, lobster shells or salmon flesh among others (Olson 1994). Exceptionally, the red pea aphid (Acyrthosiphon pisum) and the two spotted spider mite (Tetranychus urticae) have adquired the mechanisms to produce carotenoids by horizontal gene transfer from fungi (Moran and Jarvick 2010; Altinciceck et al. 2012).
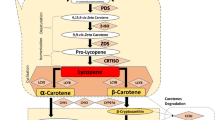
Carotenoid biosynthetic pathway is complex and have been elucidated in many organism (Chaps. 2 and 3). Alternatively, synthesis of carotenoids in bacteria is different and simpler than in plants, and has been used for the generation of transgenic plants (Table 12.1, Chap. 3). Condensation of two molecules of GGPP to form 15-cis-phytoene is catalyzed by the crtB gene, and all desaturations and isomerization are fulfilled by the single crtI gene.
Furthermore, the ketocarotenoid astaxanthin is derived from β-carotene by 3-hydroxylation and 4-ketolation at both ionone groups by the action of β-carotene hydroxylase and β-carotene ketolase, respectively. These reactions are catalyzed by two types of enzymes, a non-hemo hydroxylase (CBHX) and three heme-containing cytochrome 450-hydroxylases (Nisar et al. 2015, Chap. 3).
Although some plant species may perform ketolation of carotenoid, as pepper fruits, ketocarotenoids are mainly found in marine organisms, and also many bacteria contain a ketocarotenoid pathway (Bouvier et al. 1994; reviewed in Zhu et al. 2009). However, many ketocatotenoids have important commercial interest. Astaxanthin and canthaxanthin represent more than 55 % of the global market for carotenoids, since they are extensively used as animal feeding additive to improve coloration. Other evidences also support the importance of ketocarotenoids in human health, since astaxanthin has potential health-promoting effects in the prevention of many diseases, such as cancers, metabolic syndrome, and cardiovascular, gastrointestinal, liver and neurodegenerative diseases (reviewed in Giuliano et al. 2008; Yuan et al. 2011). Therefore, there are several plants that have been genetically engineered to produce ketocarotenoids using the ketocarotenoid pathway genes form bacteria and algae (Gerjets and Sandmann 2006; Morris et al. 2006; Jayaraj et al. 2008; Zhu et al. 2008; Fujisawa et al. 2009; Harada et al. 2014, Chap. 8).
2 Metabolic Engineering to Enhance Carotenoid Content in Crop Plants
Different experimental strategies have been used to generate food crops with enhanced carotenoid content and composition. Conventional plant breeding and genetic modifications (also referred as genetic engineering) are the two basic approaches addressed in most crops. The rapid development of many biotechnological techniques and procedures, as transformation strategies, introgression lines, new mutant collections and genome sequencing, among others, with no doubt have facilitated and assisted both strategies. Conventional breeding or marker-assisted breeding have produced some progress in the selection of carotenoid improved lines, but the process is slow, time-consuming and restricted to a limited number of species. Collection of genetic variants and mutants with altered carotenoid content and composition are especially useful. Vitamin A biofortification in maize, sweet potato and cassava are excellent examples in which conventional and assisted breeding have allowed substantial enhancement of β-carotene to improve the nutritional and health-related benefits of the population of developing countries (Hotz et al. 2012; Ceballos et al. 2013; Pixley et al. 2013). Genetic manipulation of carotenoid content provides many advantages and has been extensively used in many species (Fig. 12.1), even tough has also disadvantages over conventional techniques. Targeting of specific genes in a controlled manner to increase or to suppress their expression is a direct approach allowing modification of the concentration of the specific carotenoid of interest. Moreover, the short time required for transformation and regeneration, and the increasing availability of genes from the same species have leaded to a rapid an efficient selection of crops with enhanced carotenoid composition over conventional techniques. However, public concerns in some countries and that the technical requirements and background necessary for genetic manipulation is not available in many developing countries, have limited their expansion.
Plants with enhanced carotenoid content and composition. Bio-fortified maize from the HarvestPlus program showing (a) cobs with white (null) and pale-yellow (moderately-enriched) grains of segregating populations and, (b) highly β-carotene -enriched deep-orange grains. (c) Internal and external appearance of mature pineapple (wilt-type) sweet orange fruits (left side) and transgenic fruits in which the β-carotene hydroxylase (CsbCHX) gene was silencing by iRNA, showing the ‘Golden Orange’ phenotype (right side). (d) Variation in color intensity of dried root powder from bio-fortified β-carotene-enriched cassava genotypes. (e) Transversal sections of roots from bio-fortified β-carotene-enriched cassava genotypes showing variations in color by differential accumulation of β-carotene. Pictures are courtesy of Dra. Natalia Palacios (CIMMYT/Harvest Plus, Mexico) (a and b); Dra. Elsa Pons and Dr. Leandro Peña (IBMCP, Spain and Fundecitrus, Brasil) (c); and Dr. Hernan Ceballos (CIAT, Colombia) (d and e)
Significant progresses have been done in the generation of genetic modified crops with altered carotenoid content and composition (Fig. 12.1). The rapid development of these technologies and their application in many crops have allowed not only the enhancement of different carotenoids which are deficient in such a crops but also the modulation and extension of the pathway to produce new carotenoids, such as ketocarotenoids (Farre et al. 2010). These strategies have been shown feasible and efficient to enhance carotenoid content in many plant species, from the model plant Arabidopsis or tomato to staple foods, such rice, maize, or sweet potato, that are the basis of the diet of malnourished population in many developing countries. A revised list of important carotenoid-enhanced crops which are especially relevant for their health-promoting properties are summarized in Table 12.1.
2.1 Fleshy Fruits
2.1.1 Tomato as the Model Fruit-System
Tomato fruits have become the favorite model plant for research in many aspects of fruit development. The large collection of mutant and more recently isogenic or near-isogenic lines, relatively rapid plant cycle, genetic background, well established genetic transformation protocols, well characterized physiological and biochemical changes during fruit ripening , have turned tomato as a favorite model plant for fleshy fruit studies. Tomato fruits display a characteristic ripening behavior in which the transformation from chloroplast to chromoplast and the concomitant accumulation of carotenoids is controlled by the autocatalytic rise in ethylene production (Seymour et al. 2013). Mature tomato fruits accumulate significant amounts of the linear red-carotene lycopene, but only trace amounts of xanthophylls . Regulation of carotenoid biosynthesis and accumulation during tomato fruit ripening is probably one of the most extensively studied (Fraser et al. 1994; Liu et al. 2015). Evidences from these studies indicate that stimulation of PSY gene expression at the onset of ripening is the key regulatory factor challenging the flux of carotenoid trough the pathway, and the down regulation of LCYB and LCYE genes facilitate the characteristic accumulation of lycopene during ripening. The knowledge of regulatory steps of the pathway and the availability of genes have enabled the generation of many transgenic plants with modified fruit carotenoid content, that in addition have revealed the interaction with other metabolic pathway (Fraser et al. 2007).
The first tomato line transformed with the endogenous PSY gene presented detrimental phenotypic effects associated with the presence of the transgenes. Overexpression of the gene restored synthesis of the carotenoid lycopene in the yellow-flesh mutant fruits but produced also pigment accumulation in other cell types, and some lines also exhibited inhibition of carotenoid production by the phenomenon of co-suppression (Fray and Grierson 1993). Moreover, the transformation of tomato plants with the PSY1 gene and the CaMV 35S promoter induced the ectopic accumulation of carotenoids, but generated dwarf plants with reduced chlorophyll contents. Interestingly, the dwarf phenotype was due by an important reduction of gibberellins concentration (Fray et al. 1995). These results indicate a competition of two pathways for the common substrate GGPP, in a way that increasing PSY activity could redirect the channeling of GGPP to the carotenoid pathway at expenses of GA synthesis and the phytyl chain of chlorophylls. Similarly, a feedback repression of the endogenous PSY genes was also observed in transgenic plants expressing the bacterial CrtI genes, encoding the enzyme converting phytoene into lycopene. Fruit of transformed plants did not display an increase in total carotenoids although the content of β-carotene content increased near 3-times (Römer et al. 2000). The use of fruit specific promoters and chloroplast transit peptides greatly overcome the undesirable side effects produced by the ectopic expression of carotenoid biosynthetic genes. Thus, up to fourfold increase in total carotenoid content was obtained in tomato fruits overexpression of crtB gene from Pantoea ananatis (formerly Erwinia uredovora) under the tomato polygalacturonase promoter, and the tomato phytoene synthase-1 transit sequence (Fraser et al. 2002; Moise et al. 2013).
Modification of carotenoid biosynthetic by alteration of upstream metabolic pathways has been also attempted in tomato fruits. Synthesis of IPP was studied by transformation of tomato with the 1-deoxy-d-xylulose-5-phosphate synthase (DXS) and 3-hydroxymethylglutaryl CoA (HMGR-1) genes from the methylerythritol-4-phosphate (MEP) and the mevalonic acid (MVA) pathways, respectively (Rodriguez-Concepción 2010). Fruits from plants containing DXS targeted to the plastid showed a 1.6-fold increase in carotenoid content while those containing an additional HMGR-1 did not show any change in carotenoids (Enfissi et al. 2005). Hence, it appears that the IPP required for carotenoid biosynthesis was solely or at least mainly derived from the MEP pathway. In addition, the manipulation of the last steps of the pathway e.g. silencing by iRNA the SlNCED gene, also turned out to be efficient enhancing carotenoid accumulation, indicating that at least in tomato, such deficiency in the end-product of the pathway doesn’t results in altered carotenoid profiling (Sun et al. 2012).
Since mature tomato fruits accumulate negligible amounts of β-carotene, attempts have been done to increase the concentration of this carotenoid with significant provitamin A activity. Orange-colored fruits with a 2–4 times increase in β-carotene was obtained with a constitutive overexpression of the crtI from Erwinia. Other xanthophylls downstream β-carotene were also enhanced but the content of lycopene was reduced. These metabolic effects appear to be due to the stimulation of the endogenous LCY genes (Römer et al. 2000). The use of other transformation procedures with the Arabidopsis LCYB gene or the CtrY from Erwinia enabled the enhancement of β-carotene without substantial reduction of lycopene (Rosati et al. 2000; Wurbs et al. 2007). Another more complex strategy generated tomato plants overexpressing the LCYB from Arabidopsis and the pepper CHYB genes, and the corresponding fruits contained high levels of β-carotene and the xanthophylls β-cryptoxanthin and zeaxanthin, that were virtually absent in wild type fruits (Dharmapuri et al. 2012). A recent study also reported an elevation of β-carotene in tomato fruits overexpressing the endogenous β-carotene hydroxylase 2 gene (CrtR-b2). However, immature fruits were yellow by the reduced content of chlorophyll and mature fruits accumulated violaxanthin and large amount of esterifies xanthophylls (D’Ambrosio et al. 2011). Lycopene levels in tomato fruit may be also increased by the silencing of both lycopene cyclase and β-carotene hydroxylase genes but carotenoid content in vegetative tissue was unaffected by using fruit-specie promoters (Rosati et al. 2000). Collectively, these evidences indicate the feasibility of manipulating tomato fruit carotenoid content and composition, but a complex balance/equilibrium appears to be operative during fruit development and ripening, and that alteration in enzyme activity in one step or co-suppression of one gene may lead to an unbalanced pathway and to unexpected carotenoid profiling (Fraser et al. 2009; Fantini et al. 2013).
Enhancement of carotenoid content in tomato fruit has been also accomplished by modification of genes involved in other metabolic process accessory to carotenogenesis . Particularly relevant are genes implicated in photomorphogenesis and light perception, which illustrate the complexity of signals involved in the regulation of carotenoid biosynthesis that direct or indirectly may modulate the carotenoid profiling. Indeed, silencing the expression of the morphogenic repressors de-etiolated homolog 1 (DET1) and Le COP1LIKE produce transgenic tomato fruits with enhanced carotenoid and flavonoid contents, indicating common regulatory nodes and the significant improvement of the nutritional value of the fruits (Davuluri et al. 2005). Similarly, overexpression of the blue light receptor cryptochrome 2 (CRY2) resulted in the overproduction of flavonoids and lycopene in fruits (Giliberto et al. 2005). Silencing of other genes involved in light signaling (LeHY5) also reduced lycopene content (Liu et al. 2004). Thus, all these evidences demonstrated the feasibility of signals that may be manipulated in order to enhance carotenoid content and composition and open new avenues and technological strategies to improve the health-related benefits of many other food crops. Other endogenous signals and regulators such as brassinosteroids (BR), has been also showed to influence carotenoid content. Transgenic tomato lines overexpressing the mutant BZR1-1D gene from Arabidopsis resulted in an up-regulation of carotenoid biosynthetic genes and increasing lycopene content (Liu et al. 2013). Fibrillins are proteins involved in the formation of lipoprotein structures , such as plastoglobules and in pepper chromoplasts have been implicated in the over-production of pigments due to a sink effect. When the pepper fibrillin gene was expressed in tomato fruits, fibrils were not observed but a two-fold increase in lycopene, total carotenoids and carotenoid-derived flavor volatiles were observed (Simikin et al. 2007).
Transplastomic plants are genetically modified plants in which the new gene is inserted into the chloroplastic DNA. The major advantage of this technology is that in many plant species plastid DNA is not transmitted through pollen, therefore avoiding gene flow from the genetically modified plant to the neighboring plants. Engineering of the plastids genome for the nutritional enhancement of tomatoes has been developed (Wurbs et al. 2007; Apel and Bock 2009). Plastid expression of a bacterial crtY gene triggered the conversion of lycopene to β-carotene and produced fruits that contained fourfold more provitamin A than the wild type (Wurbs et al. 2007). Similarly, transplastomic tomatoes with an inserted LCYB from daffodil (Narcissus pseudonarcissus) efficiently converted lycopene into provitamin A (β-carotene) and showed a 50 % increase in total carotenoids (Apel and Bock 2009).
The production of ketocarotenoids in tomato fruits has been also achieved by the co-expression of the β-carotene ketolase gene from the algal Chlamydomonas reinhardtii and β-carotene hydroxylase from Haematococcus pluvialis . Transgenic tomato plants up-regulated most carotenogenic genes and increased the carbon flux into carotenoids, resulting in the massive accumulation of free astaxanthin in leaves (3.12 mg/g) and esterified astaxanthin in fruits (16.1 mg/g, Huang et al. 2013), which are carotenoids that are not usually produced in tomato.
2.1.2 Citrus
Citrus fruits are hesperidium berries, characterized by having a leathery peel surrounding the edible and juicy portion of the pulp. Citrus peel and pulp are a complex source of carotenoids, and more than 100 different carotenes, xanthophylls and derivate have been described. Particular accumulation of carotenoids and xanthophylls originate the high color diversity among fruits of the different species and varieties, from the yellow of lemon and grapefruits, to the red pulp of some grapefruits and orange mutants, through the characteristic bright-orange of mandarins and oranges (Gross 1987; Rodrigo et al. 2004; Alos et al. 2006, 2008). Citrus fruits are widely recognized by the high consumption as both juice and fresh fruits, and therefore constitute an important source of carotenoids for the human diet. Carotenoid content is much higher in the peel than in the pulp, being violaxanthin the primary xanthophyll in mature orange fruits, and violaxanthin and β-cryptoxanthin predominate in the pulp of mandarins and hybrids (Rodrigo et al. 2013). The potential provitamin A activity in Citrus fruits is, however, moderated, since β-cryptoxanthin is the only xanthophyll with this activity in the pulp of mandarin fruits but its presence in orange is negligible (Alquezar et al. 2008). Thus, increasing the provitamin A activity in Citrus would be of much interest and has been addressed. The CsPSY gene from CaraCara orange was overexpressed in kumquat, a Citrus relative family developing small fruits (Fortunella hindsii). Fruits of the transgenic plants showed a deeper orange color and contained a significant increase in phytoene, lycopene, β-carotene , and β,β-cryptoxanthin concentrations while lutein and violaxanthin contents remained almost unchanged, indicating an enhancement of the flux to the β,β-branch of the xanthophylls pathway (Zhang et al. 2009).
Engineering of β-carotene content in orange fruits has been successfully accomplished in a recent study. The rational of that work was to silence the endogenous β-carotene hydroxylase gene (Csβ-CHX) that is involved in the conversion of β-carotene into xanthophylls, under the expression of the regulatory gene FLOWERING LOCUS T from sweet orange that accelerates flowering and reduces the fruiting time. As expected, β-carotene content was enhanced by more than 36-times and the content of downstream xanthophylls were reduced, producing fruits of yellow color both in the peel and pulp which were then termed as “Golden Orange” (Fig. 12.1c). The enhanced nutritional capacity of the juice of the β-carotene-enriched oranges was assessed in the model nematode Caenorhabditis elegans. The antioxidant effect of the golden-orange juice against an in vivo oxidative stress was a 20 % higher than that of the conventional orange juice, providing a direct evidence of the enhanced health-related benefits of biotechnological modified orange fruits (Pons et al. 2014).
2.2 Cereals
Cereals are considered an important source of nutrients, as carbohydrates, proteins or vitamins, and major contributors to the diary food consumption of the population of many under-developed and developing countries. The content of carotenoids in cereal grains is relatively low compared to the majority of fruits and vegetables and, therefore, their fortification may have an enormous impact on the nutrition and health benefits of the consumers (Mellado-Ortega and Hornero-Mendez 2015). Enhancement of carotenoid content and composition in cereal crops, such as rice, wheat, sorghum and maize have received much attention in past decades, and international effort have been developed (Harvestplus programs; http://www.harvestplus.org) to provided enriched-carotenoid cereal products to malnourished populations. Following is a summary of the main contributions to the enhancement of carotenoid content in selected cereal crops.
2.2.1 Rice
Rice is probably the staple food more widely distributed and consumed, and constitutes the basis of the diet of large populations in many countries. Unfortunately, rice is rich in carbohydrates but the endosperm doesn’t contain carotenoids and the deficiency in vitamin A may results in millions of people suffering from blindness and several other diseases in developing countries. In the early 1990s and trying to alleviate vitamin A deficiencies, research projects initiated in Europe leaded to one of the major biotechnological breakthroughs in food breeding. Rice lines of the japonica and indica background with enhanced β-carotene content were obtained, and international effort has been done to overcome restrictions from public and private patents, allowing application of conventional breeding to introduce the enhanced β-carotene phenotype in local varieties (http://www.goldenrice.org/).
Transformation of rice with the daffodil (Narcissus pseudonarcissus) phytoene synthase gene (PSY) was the first attempt to increase carotenoid content but only produced accumulation of phytoene in rice endosperm but no other downstream carotenoids (Burkhardt et al. 1997). The first generation of β-carotene-enhanced rice endosperm was obtained by the expression of a mini-pathway, containing the PSY and LCYB genes from daffodil and the bacterial desaturase/isomerase crtI (Pantoea ananatis) under the control of different promotors. Transformed grains contained up to 1.6 μg/g dry weight of β-carotene, and also lutein, zeaxanthin and α-carotene (Ye et al. 2000), and because the yellow color of the endosperm it was termed as “Golden Rice ”. This increment in β-carotene in rice endosperm was still insufficient for the recommended daily vitamin A intake from a usual rice meal and a second generation of Golden Rice was generated. The rational for the new strategy was incorporating a more active PSY gene from maize which increased up to 23-times the concentration of β-carotene (37 μg/g dry weight) in the endosperm of the so-called Golden Rice2 (Paine et al. 2005) and fulfill the daily requirement of vitamin A. Thereafter, stability of the transgenes and their introgression into locally adapted and consumed rice cultivars have been developed (Datta et al. 2006, 2007) and it is expected that new β-carotene-enriched rice cultivars would be available in a near future (http://www.goldenrice.org/).
2.2.2 Maize
Maize is an important staple crop with a world production over 1000 million Tm and an harvested area of 184 million Ha in 2013 (http://faostat.fao.org). In the population of many underdeveloped countries where maize is the most important food crop, the incidence of child and maternal vitamin A deficiency is very high (West 2002). Although traditional corn varieties have provitamin A, the concentration in seeds is too low to cover vitamin A deficiencies. Therefore, the nutritional enhancement of provitamin A in maize is of paramount relevance for their impact in the amelioration of the diet and the nutritional status of malnourished populations.
The first biotechnological attempt to obtain vitamin A-enriched maize grains was based on the successful experiments of the Golden Rice 2 project. Transgenic plants of Hi-II corn expressed the bacterial crtB and crtI genes in an endosperm-specific manner, using a modified and highly active β-zein promoter fused to transit peptide of the small subunit of the pea Rubisco (rbcS). Interestingly, the seeds of the transgenic Hi-II corn contained up to 34-fold increase in total carotenoids with a significant accumulation of β-carotene which seemed to be due to the upregulation of the endogenous LCYB (Aluru et al. 2008). Then, a highly ambitious strategy was designed to introduce a ‘mini-pathway’ of five transgenes into the elite variety M37W, in order to increase simultaneously the concentration of three vitamins; A, C and B9 (folate). The successful approach produced new corn transgenic lines accumulating 60 μg/g DW β-carotene, 23 μg/g lycopene, and 36 μg/g zeaxanthin in the endosperm, which represent concentration substantially higher than those found in previous studies (Naqvi et al. 2009). Moreover, when PSY (Zea mays), crtI (Pantoea ananatis) and LCYB (Gentiana lutea) were introgressed into yellow corn varieties, zeaxanthin production was enhanced and reached 56 μg/g dry weight, suggesting the existence of metabolic synergy between endogenous and heterologous pathways (Naqvi et al. 2011). Furthermore, the insertion of five carotenogenic genes (PSY, from Zea mays; crtI from Pantoea ananatis, CHBX and crtW from Paracoccus spp and LCYB from Gentiana lutea) under the control of different endosperm-specific promoters into a white maize variety, generated plants with significantly higher levels of β-carotene and other carotenoids, including complex mixtures of hydroxycarotenoids and ketocarotenoids (Zhu et al. 2008). These examples illustrate the potential of biotechnological transformation strategies to enhance not only carotenoid content and composition, but also other nutrients to deliver new superior maize cultivars. There are, however, vast germplasm collections of maize cultivars available worldwide, and significant progress have been done by conventional and marker-assistant breeding in the search of favorable alleles and to fortify carotenoids in new maize lines (Pixley et al. 2013; Mellado-Ortega and Hornero-Mendez 2015) that are discussed below.
2.2.3 Wheat
Wheat is also a staple food and its grain provides a fifth of the calories and the protein for the world’s population (Shiferaw et al. 2013). Although wheat breeding research has been intensively developed, numerous features of wheat grains remain to be ameliorated as for example seed nutritional content. Lutein is the main carotenoid found in the wheat endosperm and is, in most cases, accompanied by lower amounts of zeaxanthin, β-cryptoxanthin and β-carotene that may be also heavily esterified (Kaneko et al. 1995; Hentschel et al. 2002; Rodríguez-Suárez et al. 2014; Mellado-Ortega and Hornero-Mendez 2015). Hence, the provitamin A content in wheat seeds is quite low and consequently a number of strategies through conventional breeding and transgenic techniques have been carried out for the last decades. In the hexaploid wheat variety EM12 cotransformed with the PSY (Zea mays) and crtI (Pantoea ananatis) genes under the control of an endosperm-specific promoter (1Dx5) or the constitutive CaMV 35S, total carotenoid content increased around 11-fold (4.96 μg/g dry weight). Moreover, the yellow color of the transgenic wheat lines was not due to the upregulation of the endogenous wheat carotenoid biosynthetic pathway (Schaub et al. 2005; Cong et al. 2009). Another approach to increase the carotenoid content of wheat seeds by biotechnological applications was the insertion of crtB and crtI genes from Pantoea ananatis, which produced a similar enhancement of total carotenoids content (4.76 μg/g dry weights). Importantly, β-carotene was the predominant carotenoid, accounting for 67 % of the total. On the other hand, transformation with either crtB or crtI alone was not sufficient to enhance the accumulation of carotenoids in the seeds, indicating a reduced activity of the endogenous pathway and that introgression of at least these two enzymes is necessary to obtain carotenoid-enriched seeds by biotechnological modification (Wang et al. 2014). A comprehensive review summarizes current genetic and genomic resources and achievements in wheat in relation to carotenoid metabolism (Wurtzel et al. 2012). Other attempts to get superior wheat lines with high carotenoid concentration in the endosperm have been carried out by conventional breeding and are discussed below in the chapter (see Sect. 12.3).
2.2.4 Sorghum
Sorghum and millet are two key components of the Africa Sub-Saharan diets. Consumption of sorghum in African countries is very relevant among cereals, with an average of 20 million Tm per year, which is one third of the total world production. Sorghum seeds are rich in carbohydrates but poor in vitamins and other essential nutrients. Hence, biofortified sorghum lines are being developed in order to reduce the malnutrition associated to the seed-based diets. The primary target of the Biofortified Sorghum project is to provide seeds with increased bioavailable vitamin A, lysine, and iron and zinc (Lipkie et al. 2013; http://www.biosorghum.com). For an efficient malnutrition control not only a sufficient amount of provitamin A in the food should be provided but also appropriate bioaccessibility is required for the assimilation of the compounds. Biotechnological modified sorghum with enhanced levels of total carotenoids and β-carotene has been obtained and the bioaccessibility of carotenoids was assessed, as well that of conventionally breed lines, using porridge preparations. Bioaccessible β-carotene in transgenic sorghum (Homo188-A) was near 5-times higher than that non-transformed. Bioavailability and bioconversion of provitamin A in these sorghum grains should be confirmed in vivo, but results provide direct evidences supporting the beneficial nutritional effects of the β-carotene-enriched sorghum lines to enhance total and bioaccessible provitamin A carotenoid levels (Lipkie et al. 2013).
2.3 Roots
2.3.1 Carrot
Carrot (Daucus carota) storage roots accumulate massive amounts of carotenoid pigments, including β-carotene and, to a lower extent, α-carotene. Carrot germplasm display a variety of color (purple, red, yellow, white) envisaging a complex regulation of carotenoid biosynthesis and accumulation. Moreover, carotenoid accumulation and chromoplast formation in carrot is accomplished under dark, contrary to the situation in aerial-growing tissues (Stange et al. 2008; Fuentes et al. 2012; Rodriguez-Concepcion and Stange 2013, Chap. 7). Overexpression of the Arabidopsis thaliana PSY gene in carrot resulted in roots of intense color, enhanced total carotenoids and the formation of crystals. The content of β-carotene was substantially increased but also other upstream carotenoids, such as phytoene, phytofluene and ζ-carotene, suggesting that carotene desaturation may be a rate-limiting step (Maass et al. 2009). Moreno et al. (2013) described that the overexpression of Daucus carota LCYB (DcLLCYB1) correlated with the increase in carotenoid levels in roots and leaves. Moreover, transgenic carrot with higher or reduced levels of DcLCYB1 displayed increased or decreased, respectively, content of total carotenoids and β-carotene in leaves and in storage roots. Alteration of the transgene DcLcyb1 was accompanied by a modulation in the expression of endogenous carotenogenic genes, indicating a tight regulation of carotenoid biosynthesis in carrot root in which the cyclization of lycopene may be a essential step (Moreno et al. 2013). On the other hand, transgenic carrot plants have been experimentally tested for ketocaronoid production and accumulation. The ketocarotenoid pathway was introduced by the insertion of the β-carotene ketolase gene (Haematococcus pluvialis ) under three different promoters. All the transformed lines presented high root expression, and the expression of the endogenous β-carotene hydroxylase genes was also enhanced in transgenic leaves and roots. Interestingly, up to 70 % of the total carotenoids were converted to novel ketocarotenoids (2400 mg/g root dry weight). The most predominant carotenoids were astaxanthin , adonirubin, and canthaxanthin, and therefore demonstrated that high potential of carrots as biopharming for the production of ketocarotenoid (Jayaraj et al. 2008).
2.3.2 Cassava
Cassava, in the Sub-Saharan Africa, is the second most important food crop after maize in terms of total energy consumption and the first in land area planted (www.faostat.fao.org). It is worth noting that cassava is more drought tolerant than maize and their roots can be banked in the soil for up to 3 years with little loss due to herbivory (Ihemere and Sayre 2008). Thus, cassava provides increased food security in comparison to other major crops in Africa (Sayre et al. 2011a, b).
The BioCassava Plus program (BC+) was one of the projects funded by Bill and Melinda Gatess Foundation to alleviate malnutrition in sub-Saharan African populations. In general, the aims of the project were to improve several limiting nutrients for biofortification in cassava including protein, β-carotene iron and zinc as well as value-added traits (low cyanogens, virus resistance, and increased shelf life) (Njoku et al. 2011). Biofortification through conventional breeding in cassava have generated several lines with increasing β-carotene and intensive studies have demonstrated that the provitamin A activity is substantially higher to fulfill daily requirements and is retained after processing (Montagnac et al. 2009; Ceballos et al. 2012, 2013). Representative pictures of other carotenoid-enriched cassava roots obtained by conventional breeding are shown in Fig. 12.1d, e, and discussed below.
Biotechnological transformation of cassava roots to enrich β-carotene accumulation has been also addressed. Failla et al. (2012) reported the generation of transgenic lines overexpressing two transgenes, the crtB from Pantoea, and the Arabidopsis DXS under the control of a potato root-specific promoter. Carotenoid content was between 15–34-fold greater in the new lines that in wild-type roots, and β-carotene was the primary carotenoid, accounting for about 92 % of the total. These results illustrate the potential for biotechnological enrichment of β-carotene and indicate that cassava roots have the metabolic capability to drive the carotenoid flux to the formation of β-carotene if enough precursors are provided. This assumption is consistent with previous findings demonstrating that a single nucleotide polymorphism in one of the PSY genes is able to enhanced catalytic activity, leading to carotenoid accumulation and yellow-colored cassava root (Welsch et al. 2010). Then, similarly to rice PSY appears to be the rate-limiting for carotenoid biosynthesis in cassava roots.
In addition to the concentration of β-carotene in a food matrix , as cassava roots, the type of processing may affect its stability and the absorption by intestinal cells. Analysis of the β-carotene bioavailability in cassava roots using a in vitro digestion system and absorption in Caco-2 cells demonstrated that the amount of β-carotene transformed is proportional to the concentration in the roots and then the quantity provide by an enriched-cassava is higher that the corresponding wild-type (Thakkar et al. 2007, 2009). Although all the methods of root processing decreased to different extend the content and stability of β-carotene, the in vitro digestion system indicated that the β-carotene-enriched cassava still provide greater amounts of bioaccessible provitamin A to the consumers than conventional roots (Failla et al. 2012).
2.4 Tubers
2.4.1 Potato
Potato is the most widely consumed vegetable in the world and then a primary food source for population of many countries. Potato tubers are rich in carbohydrates, micronutrients and vitamin C, but unfortunately very poor in carotenoids and provitamin A. Despite potato shows a great diversity in germplasm, β-carotene is not available in commercial and wild species of potato although many tubers varieties may contain also anthocyanins (Morris et al. 2004; Brown et al. 2005). Because of the relevance of potato for a large world population, intense effort has been done in last decade first to decipher regulation of carotenoid biosynthesis (Taylor and Ramsay 2005; Zhou et al. 2011) and second to enrich the tuber in nutritionally important carotenoids, primary β-carotene to reduce vitamin A deficiency.
Because the negligible amount of early carotenes in potato tubers, a biotechnological strategy to increase carotenoid content was to enhance the flux of precursors by the introgression of early biosynthetic genes. Overexpression of the bacterial DXS increased phytoene and total carotenoid content, but also shifted the flux of the pathway to the accumulation of the cytokinine trans-zeatine riboside and produced an early sprouting (Morris et al. 2006). An important increment (sixfold) in β-carotene was obtained by the overexpression of the bacterial CrtB under the control of the tissue-specific patatin promotor (Ducreux et al. 2005). Similar approaches overexpressing the β-carotene ketolase (CrtO) (Gerjets and Sandmann 2006) from several organisms, as Synech ocistis, or green algae (Morris et al. 2006) or a combination of the crtW and crtZ forma bacteria and the Or from cauliflower (Campbell et al. 2015) substantially enhanced the concentration of astaxanthin and also total ketocarotenoids, up to 12 % of total carotenoids.
The spontaneous cauliflower orange mutant Or, allowed the identification of a gene, named as Or, that when mutated produces accumulation of β-carotene in chromoplasts. The function of the gene is not yet defined by interacts with plastid-associated proteins that modified the capability to sequesters carotenoids in specialized structures originating accumulation of β-carotene head crystals (Li et al. 2001; Lu et al. 2006). Overexpression of the Or gene in potato produced the same phenotype as in cauliflower and tubers accumulated more than tenfold the normal levels of β-carotene. Moreover, Or-overexpressing potatoes also accumulated phytoene and phytofluene, and the amyloplast also accumulated β-carotene crystals that were stable under cold (Lopez et al. 2008).
A major enhancement of carotenoid content in potato was accomplished by Diretto et al. (2007a) overexpressing a mini-pathway from Erwinia composed by the CrtB, phytoene synthase CrtI and lycopene βcyclase CrtY. Constitutive expression of the genes interfered with the endogenous expression of the genes in leaves and produced unusual accumulation of carotenoids. By contrast, coordinated expression of the three genes under a tuber-specific promoter originated a massive accumulation of total carotenoids (20-fold increment) and reached levels of β-carotene as high as 47 μ g/g dry weight. These tubers displayed a characteristic deep-yellow color (Golden phenotype) and accumulated also xanthophylls.
Other rational to enhance carotenoid content in potato was the silencing of genes of both branch of lycopene cyclization. Decreasing the expression of LCYE increased the levels of upstream carotenoids but it interfered with the endogenous expression of carotenoid biosynthetic genes (Diretto et al. 2006). A more severe phenotype was obtained by silencing LCYE, which eliminates the competence from the α-carotene pathway, and also CHY in a tuber-specify manner, generating tubers with a 38-fold increment in β-carotene and 4.5-fold in total carotenoids (Diretto et al. 2007b). However, and as expected, a partial reduction in downstream xanthophylls was also detected, probably by modification of the endogenous pathway. A similar strategy was adopted by Van Eck et al. (2007) in other potato cultivars in which silencing only the CHBY gene generated potatoes enriched in β-carotene. Finally, silencing a downstream gene in the pathway, as ZEP, increased zeaxanthin content without affecting β-carotene and violaxanthin, but unexpectedly there was also an increment in the concentration of tocopherol (Römer et al. 2002). All these results illustrate the feasibility to modify carotenoid content in potato tuber, and particularly, β-carotene, by different biotechnological strategies and it is expected that may be the basis for further improvement of the nutritional value of this crop.
2.4.2 Sweet Potato
Sweet potato (Ipomoea batatas L.) is also a nutritionally important staple food for the population of many locations of Asia and Africa countries. Sweet potato exhibit a large diversity in flesh coloration by the presence of different proportion of the two pigments carotenoids or anthocyanins, and large germplasm collections can be found in many sweet potato-producing countries. This tuber is a good example of intensive international effort in biofortification by conventional genetic breeding a many new orange-fleshed varieties have been obtained (Tunmegamire et al. 2014). However, molecular studies of the regulation of carotenoid biosynthesis and its manipulation are scarce. Park et al. (2015) overexpressed the homologues of the Or gene in sweet potato and found an elevation of the carotenoid that are present in non-transformed control, α- and β-carotene , lutein, zeaxanthin and β-cryptoxanthin. Moreover, the enriched-carotenoid sweet potato was more resistant to salt and oxidative stress, indicating an enhanced antioxidant capacity . In other recent study, genetic transformation of sweet potato with transcription factors of anthocyanin biosynthesis resulted in tuber with an altered carotenoid biosynthesis (Goo et al. 2015). Highly anthocyanin-pigmented tubers contained less carotenoid and vice versa, indicating that both biosynthetic pathways may be connected. If this relationship is by a directly metabolic connection or by an indirect mechanism, as the antioxidant status remains to be elucidated.
2.5 Other Crops
2.5.1 Canola
Genetic engineering of canola for the production of carotenoids in seeds was one of the pioneering works addressing the biotechnological enrichment of carotenoid content in foods (Chap. 13). Earlier studies expressing the Crtb gene under a seed-specific promoter were very efficient increasing carotenoid content in canola seeds. Total carotenoids were enhanced by more than 50-times compared with un-transformed plants, increasing primary β-carotene and α-carotene in a ratio 2:1 (Shewmaker et al. 1999). Thereafter, new strategies were developed to obtain new canola transgenic lines with improved carotenoid content and composition. Hence, the expression of additional bacterial genes for the enzymes geranylgeranyl diphosphate synthase (crtE), phytoene desaturase (crtI), crtY and the plant LCYB from Brassica napus were engineered in transgenic canola seed in combination with phytoene synthase (crtB). From the different gene combination assayed, the insertion of crtB, crtI and crtY from Pantoea ananatis was the only one increasing significantly the ratio β- to α-carotene, from 2:1 to 3:1 (Ravanello et al. 2003). Silencing of the LCYE gene using RNAi in canola, revealed that this step may be rate-limiting in carotenoid biosynthesis , because total carotenoid content as well as β-carotene, zeaxanthin, violaxanthin and unpredictably lutein concentration increased in the transgenic seeds (Yu et al. 2013).
Canola plants have been also successfully used to understand the role of novel regulators of carotenoid biosynthesis initially identified in the model plant Arabidopsis thaliana. Up to 41-fold increase in β-carotene content was detected in seeds of canola plants transformed with the chloroplast signal recognition particle 54 kDa subunit gene (cpSRP54, Arabidopsis) under the napin promoter (Yu et al. 2012). Moreover, constitutive expression of AtmiR156b in Brassica napus resulted in enhanced levels of lutein and β-carotene in the seed (Wei et al. 2010). These results illustrated that additional genes and regulatory factors of unknown function may be relevant in the regulation of carotenoid biosynthesis and they modification would result in altered pigment content. Manipulation of canola to produce ketocarotenoids has been also achieved by the insertion of seven key genes involved in ketocarotenoid formation (Chap. 13), namely: IDI (Paracoccus sp.), crtE, crtB, crtI and crtY (Pantoea ananatis) and crtZ and crtW (Brevundimonas sp.). The total carotenoid content increased 19-30-fold and, in addition, the total amount of ketocarotenoids ranged from 60–190 μg/g fresh weight (Fujisawa et al. 2009).
2.5.2 Soybean
Soybean contain an unusually complete aminoacid composition, and a diversity of minerals, vitamins, isoflavones and a highly polyunsaturated fatty acid content that makes soy- derived products convenient for a healthy diet (Erdman and Fordyce 1989). Because of the importance of soybean in the total world production, researches and public companies pay attention to the generation of nutritional-enhanced soybean early than in other crops. Despite insect and herbicide resistant soybean were created several decades ago (Hinchee et al. 1988), carotenoid-enhanced soybean phenotypes were more recently generated. Insertion of two carotenoid biosynthetic genes, PSY from Capsicum and crtI from Pantoea in Korean soybean, increased total carotenoid contents up to 62-fold, being 77 % β-carotene (Kim et al. 2012). Remarkably, the seed-specific overexpression of the Pantoea ananatis crtB gene targeted to plastids resulted in the accumulation of high levels of β-carotene (over 1500-fold compared with un-transformed lines). In addition, the transgenic seeds also displayed two collateral traits: elevated de-saturated oil and increased protein content which are important to enhance the nutritional value of the crop (Schmidt et al. 2014).
2.5.3 Lettuce
Lettuce is a leafy vegetable highly consumed in the world, but unfortunately it has a low nutritional value (Mou 2009). Therefore, even small increases in the concentrations of dietary constituents in lettuce could have widespread effects. Metabolic engineering has been applied to generate lettuce plants enriched in vitamin E and folate (Lee et al. 2007; Nunes et al. 2009; Yabuta et al. 2013). In addition, the red pigment astaxanthin which has diverse clinical benefits against age-related diseases, and muscle or eye fatigue (Guerin et al. 2003; Kidd 2011), was also synthesized in transformed lettuce plants. The plastid genome of lettuce has been site-specifically modified with the insertion of three transgenes from marine bacterium, crtZ, crtW (Brevundimonas sp.) and ipi (Paracoccus). Astaxanthin is naturally produced by a unicellular green alga Haematococcus pluvialis . This carotenoid extracted from the green algae comprised a fatty acid diester (28 %), a monoester (70 %), and the free form (2 %) (Okada et al. 2009). Astaxanthin has been produced in the edible organs of several crops such as maize (Zhu et al. 2008), potato (Morris et al. 2006; Gerjets and Sandmann 2006), tomato (Huang et al. 2013), carrot (Jayaraj et al. 2008), and rapeseed (Fujisawa et al. 2009). In general, the free form of astaxanthin may accumulate in all the crops with the exception of tomato fruits (Huang et al. 2013). Transformed lettuce leaves produced astaxanthin fatty acid (myristate or palmitate) diester (49.2 % of total carotenoids), astaxanthin monoester (18.2 %), and the free form of astaxanthin (10.0 %) and other ketocarotenoids (17.5 %). These results indicated that artificial ketocarotenoids account for up to 95 % of total carotenoids (230 µg/g fresh weight) while the native carotenoids lactucaxanthin and lutein represented only the 3.8 and 1.3 %, respectively (Harada et al. 2014).
3 Bio-fortification and Quantitative Trait Loci Related to High Carotenoid Contents in Plants
As discussed in previous sections, bio-fortification is a conventional breeding strategy to enhance the content in micronutrients, minerals, pigments or any other element in a staple food to improve its nutritional value and its health-beneficial effect for malnourish population. This genetic improvement of a food crops is particularly suitable for rural-based population and in the case of carotenoid has been very successful and has allowed the alleviation of vitamin A deficiency that is one of the major nutrition-related problems in many underdeveloped and developing countries (Bouis et al 2011; Dwivedi et al 2012). Major achievement has been done in selected crops by international networking strategies and collaborative projects, in which the HarvestPlus (http://www.harvestplus.org) and other institutions have been essential and played determinant roles. Staple food with enhanced β-carotene content, such as maize (Pixley et al. 2013), cassava (Ceballos et al. 2012) or sweet potato (Tunmegamire et al. 2014) have been obtained and locally adapted and spread in Asian and Africa countries.
Other genetic analysis, as quantitative trait loci (QTL) has been applied to the study of the DNA regions of a plant genome related to carotenoid accumulation. QTL studies have improved the knowledge on DNA fragments involved in the variation of carotenoid content and have provided information on the genetic architecture of this trait in several plant species. Moreover, QTLs have led to the identification of candidate genes that could participate in the regulation of carotenoid concentration. A recombinant inbred (RI) population with 233 RI maize lines derived from a cross between By804 and B73 was used to detect QTL revealing that much of the phenotypic variation in carotenoids contents may be explained by two loci (y1 and y9). A gene targeted marker (Y1ssr) in the candidate gene phytoene synthase 1 (PSY1) tightly linked to a major QTL explained up to 27 % of phenotypic variation for carotenoids content (Chander et al. 2007). Other experiment showed that a 378-bp InDel upstream of the transcription start site and a SNP in the fifth exon resulting in a Thr to Asn substitution may be functional sites associated with total carotenoid levels in maize grain (Fu et al. 2013). PSY has also been studied in wheat and the allelic variation at PSY1-A1 was associated with the yellow pigment trait (YP). Hence, the PSY1-A1o allele was associated with elevated pigment in a validation population comprising 93 diverse cultivars and breeding lines (Singh et al. 2009).
Major QTLs for carrot color are positionally associated with carotenoid biosynthetic genes and interact epistatically in a domesticated x wild carrot cross. Based on the progeny of a wild white carrot (QAL), which doesn’t accumulate pigments crossed with domesticated orange carrot (B493), one of the richest sources of carotenoid pigments-mainly provitamin A β- and α- carotene s, two major interacting loci, Y and Y(2), associated with the carotenoid biosynthetic genes zeaxanthin epoxidase and carotene hydroxylase, and carotenoid dioxygenase gene family members, were found to control much variation for carotenoid accumulation in carrot roots (Just et al. 2009).
Two QTLs for increased fruit lycopene content, inherited from a high-lycopene S. pimpinellifolium accession, were detected in tomato chromosomes 7 and 12 using a S. lycopersicum × S. pimpinellifolium RIL population, and were identified as potential targets for marker-assisted selection and positional cloning. Statistical analyses revealed that while lyc7.1 did not significantly increase lycopene content in the heterozygous condition, individuals harboring lyc12.1 (localized to tomato chromosome 12) in the heterozygous condition contained 70.3 % higher lycopene than the recurrent parent. The derived sub-NILs could be used for transferring lyc12.1 to other tomato varieties (Kinkade and Foolad 2013). A major QTL for pigment content in pepper fruit, pc8.1, is associated with variation in plastid compartment size. Quantitative variation in pigment content was studied in a cross between a dark-green Capsicum annuum pepper and a light-green C. chinense pepper. QTL pc8.1, affected carotenoid content in the ripe fruit and found that the QTL exerts its effect via increasing chloroplast compartment size in the dark-green genotypes (Brand et al. 2012).
Other genetic strategies based on genome-wide association (GWAS) are now being used to identify allelic variation for genes controlling carotenoid content and composition. This technique requires wide allelic diversity and a high-density genotyping, that are now only available for selected plant species, as maize (Owens et al. 2014; Suwarno et al. 2015) but it may provide the basis to address similar objectives in other agronomical important plants in the future.
4 Concluding Remarks
Biotechnological manipulation of carotenoid content and composition to enhance the nutritional value and health related benefits of many foods have been successfully addressed in past decades. Strategies have been based on the silencing of particular genes to increase the concentration of upstream carotenoids of the pathway or to overexpress key biosynthetic genes to challenge the pathway to increase metabolic precursors. Evidences accumulate indicate that each plant species and tissue may have particular regulatory and rate-limiting steps but, in general, three points appears to be key in the pathway: first, early step regulating the flux of entrance (phytoene synthase); second, the branching point diverging from lycopene, particularly lycopene β-cyclase; and third, β-carotene hydroxylase, that depletes the level of carotenes with provitamin A activity. Moreover, it becomes evident that other accessory or side-associated steps of the pathway may be also important regulating carotenoid accumulation and are potential targets to enhance their content. Application of genetic engineering strategies has also strong limitations, by scientific and technical reasons in many plants species and by public concerns in many countries. Conventional breeding has becoming successful enriching carotenoid content in important staple foods, and β-carotene-biofortified maize, cassava and sweet potato varieties have been obtained and are spread in countries with malnourished population affected of vitamin A deficiency. The rapid development of new “omic” technologies, transformation of recalcitrant plants and a better understanding of carotenoid biosynthesis and catabolism in different plants species will open new avenues to enrich foods with carotenoid not only to alleviate deficiencies but also to provide a better antioxidant balance and other health-related benefits.
References
Alos E, Cercos M, Rodrigo MJ, Zacarias L, Talon M (2006) Regulation of color break in citrus fruits. Changes in pigment profiling and gene expression induced by gibberellins and nitrate, two ripening retardants. J Agric Food Chem 54:4888–4895
Alos E, Roca M, Iglesias DJ, Mínguez-Mosquera MI, Damasceno CM, Thannhauser TW, Rose JK, Talon M, Cercos M (2008) An evaluation of the basis and consequences of a stay-green mutation in the navel negra citrus mutant using transcriptomic and proteomic profiling and metabolite analysis. Plant Physiol 47:1300–1315
Alquezar B, Rodrigo MJ, Zacarías L (2008) Carotenoid biosynthesis and their regulation in citrus fruits. Tree Forest Sci Biotech 2:23–35
Altinciceck B, Kovacs JL, Gerardo NM (2012) Horizontally transferred fungal carotenoid genes in two-spotted spider mite Tetranychus urticae. Biol Lett 8:253–257
Aluru M, Xu Y, Guo R, Wang Z, Li S, White W, Wang K, Rodermel S (2008) Generation of transgenic maize with enhanced provitamin A content. J Exp Bot 59:3551–3562
Apel W, Bock R (2009) Enhancement of carotenoid biosynthesis in transplastomic tomatoes by induced lycopene-to-provitamin A conversion. Plant Physiol 151:59–66
Bouis HE, Hotz C, McClafferty B, Meenakshi JV, Pfeiffer H (2011) Biofotification: a new tol to reduce micronutrient malnutrition. Food Nutr Bull 32:S31–S41
Bouvier F, Hugueney P, D’Harlingue A, Kuntz M, Camara B (1994) Xanthophyll biosynthesis in chromoplasts: isolation and molecular cloning of an enzyme catalyzing the conversion of 5,6-epoxycarotenoid into ketocarotenoid. Plant J 6:45–54
Brand A, Borovsky Y, Meir S, Rogachev I, Aharoni A, Paran I (2012) A major QTL for pigment content in pepper fruit is associated with variation in plastid compartment size. Planta 235:579–588
Brown CR, Culley D, Yang C, Durst R, Wrolstad R (2005) Variation of anthocyanin and carotenoid contents and associated antioxidant values in potato breeding lines. J Amer Soc Hort Sci 130:174–180
Burkhardt PK, Beyer P, Wünn J, Klöti A, Armstrong GA, Schledz M, von Lintig J, Potrykus I (1997) Transgenic rice (Oryza sativa) endosperm expressing daffodil (Narcissus pseudonarcissus) phytoene synthase accumulates phytoene, a key intermediate of provitamin A biosynthesis. Plant J 11:1071–1078
Campbell R, Morris WL, Mortimer CL, Misawa N, Ducreux LJM, Morris JA, Hedley PE, Fraser PD, Taylor MA (2015) Optimizing ketocarotenoid production in potato tubers: effect of genetic background, transgene combinations and environment. Plant Sc 234:27–37
Carpentier S, Knaus M, Suh M (2009) Associations between lutein, zeaxanthin, and age-related macular degeneration: an overview. Crit Rev Food Sci 49:313–326
Ceballos H, Hershey C, Becerra-Lopez-Lavalle LA (2012) New approaches to cassava breeding. Plant Breed Rev 36:427–504
Ceballos H, Morante N, Sanchez T, Ortiz D, Aragon I, Chavez AL, Pizarro M, Calle F, Dufour D (2013) Rapid cycling recurrent selection for increased carotenoids content in cassava roots. Crop Sci 53:2342–2351
Chander S, Guo YQ, Yang XH, Zhang J, Lu XQ, Yan JB, Song TM, Rocheford TR, Li JS (2007) Using molecular markers to identify two major loci controlling carotenoid contents in maize grain. Theor Appl Genet 116:223–323
Cong L, Wang C, Chen L, Liu H, Yang G, He G (2009) Expression of phytoene synthase1 and carotene desaturase crtI genes result in an increase in the total carotenoids content in transgenic elite wheat (Triticum aestivum L.). J Agric Food Chem 57:8652–8660
D’Ambrosio C, Giorio G, MarinoI MA, Petrozza A, Salfi L, Stigliani AL, Cellini F (2004) Virtually complete conversion of lycopene into β-carotene in fruits of tomato plants transformed with the tomato lycopene β-cyclase (tlcy-b) cDNA. Plant Sci 166:207–214
D’Ambrosio C, Stigliani AL, Giorio G (2011) Overexpression of CrtR-b2 (carotene beta hydroxylase 2) from S. lycopersicum L. differentially affects xanthophyll synthesis and accumulation in transgenic tomato plants. Transgenic Res 20:47–60
Datta K, Rai M, Parkhi V et al (2006) Improved “Golden” rice and post-transgeneration enhancement of metabolic target products of carotenoids (b-carotene) in transgenic elite cultivars (IR64 and BR29). Curr Sci 91:935–939
Datta SK, Datta K, Parkhi V et al (2007) Golden rice: introgression, breeding, and field evaluation. Euphytica 154:271–278
Davuluri GR, van Tuinen A, Fraser PD, Manfredonia A, Newman R, Burgess D, Brummell DA, King SR, Palys J, Uhlig J, Bramley PM, Pennings HM, Bowler C (2005) Fruit-specific RNAi-mediated suppression of DET1 enhances carotenoid and flavonoid content in tomatoes. Nat Biotechnol 23:890–895
de Saint GA, Bonhomme S, Boyer FD, Rameau C (2013) Novel insights into strigolactone distribution and signalling. Curr Opin Plant Biol 16:583–589
Dharmapuri S, Rosati C, Pallara P, Aquilani R, Bouvier F, Camara B, Giuliano G (2012) Metabolic engineering of xanthophyll content in tomato fruits. FEBS Lett 519:30–34
Diretto G, Tavazza R, Welsch R, Pizzichini D, Mourgues F, Papacchioli V, Beyer P, Giuliano G (2006) Metabolic engineering of potato tuber carotenoids through tuber-specific silencing of lycopene epsilon cyclase. BMC Plant Biol 6:13
Diretto G, Al-Babili S, Tavazza R, Papacchioli V, Beyer P, Giuliano G (2007a) Metabolic engineering of potato carotenoid content through tuber-specific overexpression of a bacterial mini-pathway. PLoS One 2, e350
Diretto G, Welsch R, Tavazza R, Mourgues F, Pizzichini D, Beyer P, Giuliano G (2007b) Silencing of beta-carotene hydroxylase increases total carotenoid and beta-carotene levels in potato tubers. BMC Plant Biol 7:11
Ducreux LJ, Morris WL, Hedley PE, Shepherd T, Davies HV, Millam S, Taylor MA (2005) Metabolic engineering of high carotenoid potato tubers containing enhanced levels of beta-carotene and lutein. J Exp Bot 409:81–89
Dwivedi SL, Sahrawat KL, Rai KN, Blair MW, Andersson MS, Pfeiffer W (2012) Nutritionally enhanced staple food crops. Plant Breeding Rev 36:169–291
Enfissi EM, Fraser PD, Lois LM, Boronat A, Schuch W, Bramley PM (2005) Metabolic engineering of the mevalonate and non-mevalonate isopentenyl diphosphate-forming pathways for the production of health-promoting isoprenoids in tomato. Plant Biotechnol J 3:17–27
Erdman JW Jr, Fordyce EJ (1989) Soy products and the human diet. Am J Clin Nutr 49:725–737
Etminan M, Takkouche B, Caamaño-Isorna F (2004) The role of tomato products and lycopene in the prevention of prostate cancer: a meta-analysis of observational studies. Cancer Epidemiol Biomarkers Prev 13:340–345
Failla ML, Chitchumroonchokchai C, Siritunga D, De Moura FF, Fregene M, Manary MJ, Sayre RT (2012) Retention during processing and bioaccessibility of β-carotene in high β-carotene transgenic cassava root. J Agric Food Chem 60:3861–3866
Fantini E, Falcone G, Frusciante S, Giliberto L, Giuliano G (2013) Dissection of tomato lycopene biosynthesis through virus-induced gene silencing. Plant Physiol 163:986–998
Faostat (2008) Statistics on Agricultural Production and Trade. Food and Agricultural Organization of the United Nations. www.faostat.com
Farre G, Sanahuja G, Naqvi S, Bai C, Capell T, Zhu C, Christou P (2010) Travel advice on the road to carotenoids in plants. Plant Sci 179:28–48
Farre G, Bai C, Twyman RM, Capell T, Christou P, Zhu C (2011) Nutritious crops producing multiple carotenoids-a metabolic balancing act. Trend Plant Sci 16:532–540
Fassett RG, Coombes JS (2012) Astaxanthin in cardiovascular health and disease. Molecules 17:2030–2048
Fraser PD, Bramley PM (2004) The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res 43:228–265
Fraser PD, Truesdale MR, Bird CR, Schuch W, Bramley PM (1994) Carotenoid biosynthesis during tomato fruit development (evidence for tissue-specific gene expression). Plant Physiol 105:405–413
Fraser PD, Römer S, Shipton CA, Mills PB, Kiano JW, Misawa N, Drake RG, Schuch W, Bramley PM (2002) Evaluation of transgenic tomato plants expressing an additional phytoene synthase in a fruit-specific manner. Proc Natl Acad Sci U S A 99:1092–1097
Fraser PD, Enfissi EM, Halket JM, Truesdale MR, Yu D, Gerrish C, Bramley PM (2007) Manipulation of phytoene levels in tomato fruit: effects on isoprenoids, plastids, and intermediary metabolism. Plant Cell 19:3194–31211
Fraser PD, Enfissi EM, Bramley PM (2009) Genetic engineering of carotenoid formation in tomato fruit and the potential application of systems and synthetic biology approaches. Arch Biochem Biophys 483:196–204
Fray RG, Grierson D (1993) Identification and genetic analysis of normal and mutant phytoene synthase genes of tomato by sequencing, complementation and co-suppression. Plant Mol Biol 22:589–602
Fray RG, Wallace A, Fraser PD, Valero D, Hedden P, Bramley PM, Grierson D (1995) Constitutive expression of a fruit phytoene synthase gene in transgenic tomatoes causes dwarfism by redirecting metabolites from the gibberellin pathway. Plant J 8:693–701
Fu Z, Chai Y, Zhou Y, Yang X, Warburton ML, Xu S, Cai Y, Zhang D, Li J, Yan J (2013) Natural variation in the sequence of PSY1 and frequency of favorable polymorphisms among tropical and temperate maize germplasm. Theor Appl Genet 126:923–935
Fuentes F, Pizarro L, Moreno JC, Handford M, Rodriguez-Concepcion M, Stange C (2012) Light-dependent changes in plastid differentiation influence carotenoid gene expression and accumulation in carrot roots. Plant Mol Biol 79:47–59
Fujisawa M, Takita E, Harada H, Sakurai N, Suzuki H, Ohyama K, Shibata D, Misawa N (2009) Pathway engineering of Brassica napus seeds using multiple key enzyme genes involved in ketocarotenoid formation. J Exp Bot 60:1319–1332
Gerjets T, Sandmann G (2006) Ketocarotenoid formation in transgenic potato. J Exp Bot 57:3639–3645
Giliberto L, Perrotta G, Pallara P, Weller JL, Fraser PD, Bramley PM, Fiore A, Tavazza R, Giuliano G (2005) Manipulation of the blue light photoreceptor cryptochrome 2 in tomato affects vegetative development, flowering time, and fruit antioxidant content. Plant Physiol 137:199–208
Giuliano G (2014) Plant carotenoids: genomics meets multi-gene engineering. Curr Opin Plant Biol 19:111–117
Giuliano G, Tavazza R, Diretto G, Beyer P, Taylor MA (2008) Metabolic engineering of carotenoid biosynthesis in plants. Trend Biotech 26:139–145
Goo YM, Han EH, Jeong JC, Kwak SS, Yu J, Kim YH, Ahn MJ, Lee SW (2015) Over expression of the sweet potato IbOr gene results in the increased accumulation of carotenoid and confers tolerance to environmental stresses in transgenic potato. C R Biol 338:12–20
Gross J (1987) Pigments in fruits. Academic, London
Guerin M, Huntley ME, Olaizola M (2003) Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol 21:210–216
Harada H, Maoka T, Osawa A, Hattan J, Kanamoto H, Shindo K, Otomatsu T, Misawa N (2014) Construction of transplastomic lettuce (Lactuca sativa) dominantly producing astaxanthin fatty acid esters and detailed chemical analysis of generated carotenoids. Transgenic Res 23:303–315
Havaux M (1998) Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci 3:147–151
Hentschel V, Katja K, Hollmann J, Lindhauer MG, Böhm V, Bitsch R (2002) Spectrophotometric determination of yellow pigment content and evaluation of carotenoids by high-performance liquid chromatography in durum wheat grain. J Agric Food Chem 50:6663–6668
Hinchee MAW, Connor-Wood DV, Newwll CA, Mcdonnell RE, Sato SJ, Gasser CS, Fiscchhoff DA, Re DB, Fraley RT, Horsch RB (1988) Production of transgenic soybean plant using Agrobacterium-mediated DNA transfer. Nat Biotech 6:915–922
Hotz C, Loechl C, Lubowa A, Tumwine JK, Ndeezi G, Masawi AN, Baingana R, Carriquiry A, de Brauw A, Meenakshi JV, Gilligan DO (2012) Introduction of β-carotene-rich orange sweet potato in rural Uganda results in increased vitamin A intakes among children and women and improved vitamin A status among children. J Nutr 142:1871–1880
Huang JC, Zhong YJ, Liu J, Sandmann G, Chen F (2013) Metabolic engineering of tomato for high-yield production of astaxanthin. Metab Eng 17:59–67
Ihemere U, Sayre RT (2008) Transgenic cassava. In: Kole C, Hall TC (eds) Compendium of transgenic crop plants, vol 7, transgenic sugar, tuber and fiber crops. Blackwell, London
Jayaraj J, Devlin R, Punja Z (2008) Metabolic engineering of novel ketocarotenoid production in carrot plants. Transgenic Res 17:489–501
Just BJ, Santos CA, Yandell BS, Simon PW (2009) Major QTL for carrot color are positionally associated with carotenoid biosynthetic genes and interact epistatically in a domesticated wild carrot cross. Theor Appl Genet 119:1155–1169
Kaneko S, Nagamine T, Yamada T (1995) Esterification of endosperm lutein with fatty acids during the storage of wheat seeds. Biosc Biotech Biochem 59:1–4
Kidd PM (2011) Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern Med Rev 16:355–364
Kim MJ, Kim JK, Kim HJ, Pak JH, Lee JH, Kim DH, Choi HK, Jung HW, Lee JD, Chung YS, Ha SH (2012) Genetic modification of the soybean to enhance the β-carotene content through seed-specific expression. PLoS One 7, e48287
Kinkade MP, Foolad MR (2013) Validation and fine mapping of lyc12.1, a QTL for increased tomato fruit lycopene content. Theor Appl Genet 126:2163–2175
Krinsky NI (1989) Antioxidant function of carotenoids. Free Rad Bio Med 7:617–635
Krinsky NI, Johnson EJ (2005) Carotenoid actions and their relation to health and disease. Mol Aspects Med 26:459–516
Lee K, Lee SM, Park SR, Jung J, Moon JK, Cheong JJ, Kim M (2007) Overexpression of Arabidopsis homogentisate phytyltransferase or tocopherol cyclase elevates vitamin E content by increasing gamma-tocopherol level in lettuce (Lactuca sativa L.). Mol Cells 24:301–306
Li L, Paolillo DJ, Parthasarathy MV, Dimuzio EM, Garvin DJ (2001) A novel gene mutation that confers abnormal patterns of beta-carotene accumulation in cauliflower (Brassica oleracea var. botrytis). Plant J 26:59–67
Li L, Yang Y, Xu Q, Owsiany K, Welsch R, Chitchumroonchokchai C, Lu S, Van EJ, Deng XX, Failla M, Thannhauser TW (2012) The Or gene enhances carotenoid accumulation and stability during post-harvest storage of potato tubers. Mol Plant 5:339–352
Lipkie TE, De Moura FF, Zhao ZY, Albertsen MC, Che P, Glassman K, Ferruzzi MG (2013) Bioaccessibility of carotenoids from transgenic provitamin A biofortified sorghum. J Agric Food Chem 61:5764–5771
Liu Y, Roof S, Ye Z, Barry C, van Tuinen A, Vrebalov J, Bowler C, Giovannoni J (2004) Manipulation of light signal transduction as a means of modifying fruit nutritional quality in tomato. Proc Natl Acad Sci U S A 101:9897–9902
Liu L, Jia C, Zhang M, Chen D, Chen S, Guo R, Guo D, Wang Q (2013) Ectopic expression of a BZR1-1D transcription factor in brassinosteroid signaling enhances carotenoid accumulation and fruit quality attributes in tomato. Plant Biotech J 12:105–115
Liu J-X, Chiou C-Y, Shen C-H, Chen P-J, Liu Y-C, Jian C-D, Shen X-L, Shen F-Q, Yeh K-W (2014) RNA interference-based gene silencing of phytoene synthase impairs growth, carotenoids, and plastid phenotype in Oncidium hybrid orchid, vol 3, Springerplus. Springer, p 478
Liu L, Shao Z, Zhang M, Wang Q (2015) Regulation of carotenoid metabolism in tomato. Mol Plant 8:28–39
Lopez AB, Van Eck J, Conlin BJ, Paolillo DJ, O’Neill J, Li L (2008) Effect of the cauliflower Or transgene on carotenoid accumulation and chromoplast formation in transgenic potato tubers. J Exp Bot 59:213–223
Lu S, Van Eck J, Zhou X, Lopez AB, O’Halloran DM, Cosman KM, Conlin BJ, Paolillo DJ, Garvin DF, Vrebalov J, Kochian LV, Küpper H, Earle ED, Cao J, Li L (2006) The cauliflower Or gene encodes a DnaJ cysteine-rich domain-containing protein that mediates high levels of beta-carotene accumulation. Plant Cell 18:3594–3605
Maass D, Arango J, Wüst F, Beyer P, Welsch R (2009) Carotenoid crystal formation in Arabidopsis and carrot roots caused by increased phytoene synthase protein levels. PLoS One 4, e6373
Maida JM, Mathers K, Alley C (2008) Pediatric ophthalmology in the developing world. Curr Opin Ophthalmol 19:403–408
Mellado-Ortega E, Hornero-Mendez D (2015) Carotenoids in cereals: an ancient resource with present and future applications. Phytochem Rev 14:873–890.
Moise AR, Al-Babili S, Wurtzel ET (2013) Mechanistic aspects of carotenoid biosynthesis. Chem Rev 114:164–193
Montagnac JA, Davis CR, Tanumihardjo SA (2009) Nutritional value of cassava for use as a staple food and recent advances for improvement. Compr Rev Food Sci Food Saf 18:181–194
Moran NA, Jarvick T (2010) Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science 328:624–627
Mordente A, Guantario B, Meucci E, Silvestrini A, Lombardi E, Martorana GE, Giardina B, Böhm V (2011) Lycopene and cardiovascular diseases: an update. Curr Med Chem 18:1146–1163
Moreno JC, Pizarro L, Fuentes P, Handford M, Cifuentes V, Stange C (2013) Levels of lycopene β-cyclase 1 modulate carotenoid gene expression and accumulation in Daucus carota. PLoS One 8, e58144
Morris ML, Ducreux L, Griffiths DW, Stewart D, Davies HV, Taylor MA (2004) Carotenogenesis during tuber development and storage in potato. J Exp Bot 55:975–982
Morris WL, Ducreux LJ, Hedden P, Millam S, Taylor MA (2006) Overexpression of a bacterial 1-deoxy-D-xylulose 5-phosphate synthase gene in potato tubers perturbs the isoprenoid metabolic network: implications for the control of the tuber life cycle. J Exp Bot 57:3007–3018
Mou B (2009) Nutrient content of lettuce and its improvement. Curr Nutr Food Sci 5:242–248
Naqvi S, Zhu C, Farre G, Ramessar K, Bassie L, Breitenbach J, Perez Conesa D, Ros G, Sandmann G, Capell T, Christou P (2009) Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc Natl Acad Sci U S A 106:7762–7767
Naqvi S, Zhu C, Farre G, Sandmann G, Capell T, Christou P (2011) Synergistic metabolism in hybrid corn indicates bottlenecks in the carotenoid pathway and leads to the accumulation of extraordinary levels of the nutritionally important carotenoid zeaxanthin. Plant Biotechnol J 9:384–393
Nisar N, Li L, Lu S, Khin NC, Pogson BJ (2015) Carotenoid metabolism in plants. Molec Plant 8:68–82
Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Mol Biol 50:333–359
Njoku DM, Vernon G, Egesi CN, Asante I, Offe FK, Okogbenin E, Kulakow P, Eke-Okoro ON, Ceballos H (2011) Breeding for enhanced β-carotene content in cassava: constraints and accomplishments. J Crop Improv 25:560–571
Nunes AC, Kalkmann DC, Aragão FJ (2009) Folate biofortification of lettuce by expression of a codon optimized chicken GTP cyclohydrolase I gene. Transgenic Res 18:661–667
Okada Y, Ishikura M, Maoka T (2009) Bioavailability of astaxanthin in Haematococcus algal extract: the effects of timing of diet and smoking habits. Biosci Biotechnol Biochem 73:1928–1932
Olson JA (1994) Needs and sources of carotenoids and vitamin A. Nutr Rev 52:S67–S73
Owens BF, Lipka AE, Magallanes-Lundback M, Tiede T, Diepenbrock CH, Kandianis CB, Kim E, Cepela J, Mateos-Hernandez M, Buell CR, Buckler ES, DellaPenna D, Gore MA, Rocheford T (2014) A foundation for provitamin A biofortification of maize: genome-wide association and genomic prediction models of carotenoid levels. Genetics 198:1699–1716
Paine JA, Shipton CA, Chaggar S, Howells RM, Kennedy MJ, Vernon G, Wright SY, Hinchliffe E, Adams JL, Silverstone AL, Drake R (2005) Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat Biotech 23:482–487
Park SC, Kim YH, Kim SH, Jeong YJ, Kim CY, Lee JS, Bae JY, Ahn MJ, Jeong JC, Lee HS, Kwak SS (2015) Overexpression of the IbMYB1 gene in an orange-fleshed sweet potato cultivar produces a dual-pigmented transgenic sweet potato with improved antioxidant activity. Physiol Plant 153:525–537
Pixley K, Palacios-Rojas N, Babu R, Mutale R, Surie R, Simpungwe E (2013) Biofortification of maize with provitamin A carotenoids. In: Tanumihardjo SA (ed) Carotenoids and human health. Springer Science, New York, pp 271–292
Pons E, Alquézar B, Rodríguez A, Martorell P, Genovés S, Ramón D, Rodrigo MJ, Zacarías L, Peña L (2014) Metabolic engineering of β-carotene in orange fruit increases its in vivo antioxidant properties. Plant Biotechnol J 12:17–27
Ravanello MP, Ke D, Alvarez J, Huang B, Shewmaker CK (2003) Coordinate expression of multiple bacterial carotenoid genes in canola leading to altered carotenoid production. Metab Eng 5:255–263
Rodrigo MJ, Marcos JF, Zacarías L (2004) Biochemical and molecular analysis of carotenoid biosynthesis in flavedo of orange (Citrus sinensis L.) during fruit development and maturation. J Agric Food Chem 52:6724–6731
Rodrigo MJ, Alquezar B, Alos E, Lado J, Zacarias L (2013) Biochemical bases and molecular regulation of pigmentation in the peel of Citrus fruit. Sci Hort 163:42–62
Rodríguez-Concepción M (2010) Supply of precursors for carotenoid biosynthesis in plants. Arch Biochem Biophys 504:118–122
Rodriguez-Concepcion M, Stange C (2013) Biosynthesis of carotenoids in carrot: an underground story comes to light. Arch Biochem Biophys 539:110–116
Rodríguez-Suárez C, Mellado-Ortega E, Hornero-Méndez D, Atienza SG (2014) Increase in transcript accumulation of Psy1 and e-Lcy genes in grain development is associated with differences in seed carotenoid content between durum wheat and tritordeum. Plant Molec Biol 84:659–673
Römer S, Fraser PD, Kiano JW, Shipton CA, Misawa N, Schuch W, Bramley PM (2000) Elevation of the provitamin A content of transgenic tomato plants. Nat Biotech 18:666–669
Römer S, Lübeck J, Kauder F, Steiger S, Adomat C, Sandmann G (2002) Genetic engineering of a zeaxanthin-rich potato by antisense inactivation and co-suppression of carotenoid epoxidation. Metab Eng 4:263–272
Rosati C, Aquilani R, Dharmapuri S, Pallara P, Marusic C, Tavazza R, Bouvier F, Camara B, Giuliano G (2000) Metabolic engineering of beta-carotene and lycopene content in tomato fruit. Plant J 24:413–419
Rosati C, Diretto G, Giuliano G (2009) Biosynthesis and engineering of carotenoids and apocarotenoids in plants: state of the art and future prospects. Biotechnol Genetic Eng Rev 26:139–162
Sayre R, Beeching JR, Cahoon EB, Egesi C, Fauquet C, Fellman J, Fregene M, Gruissem W, Mallowa S, Manary M, Maziya-Dixon B, Mbanaso A, Schachtman DP, Siritunga D, Taylor N, Vanderschuren H, Zhang P (2011a) BioCassava plus program: biofortification of cassava for sub- Saharan Africa. Annu Rev Plant Biol 62:251–272
Sayre R, Beeching JR, Cahoon EB, Egesi C, Fauquet C, Fellman J, Fregene M, Gruissem W, Mallowa S, Manary M, Maziya-Dixon B, Mbanaso A, Schachtman DP, Sisitunga D, Taylor N, Vanderschuren H, Zhang P (2011b) The BioCassava plus program: biofortification of cassava for sub-Saharan Africa. Annu Rev Plant Biol 62:251–272
Schaub P, Al-Babili S, Drake R, Beyer P (2005) Why is golden rice golden (yellow) instead of red? Plant Physiol 138:441–450
Schmidt MA, Parrott WA, Hildebrand DF, Berg RH, Cooksey A, Pendarvis K, He Y, McCarthy F, Herman EM (2014) Transgenic soya bean seeds accumulating β-carotene exhibit the collateral enhancements of oleate and protein content traits. Plant Biotechnol J 13:590–600
Seymour GB, Ostergaard L, Chapman NH, Knapp S, Martin C (2013) Fruit development and ripening. Annu Rev Plant Biol 64:219–241
Shewmaker CK, Sheehy JA, Daley M, Colburn S, Ke DY (1999) Seed-specific overexpression of phytoene synthase: increase in carotenoids and other metabolic effects. Plant J 20:401–412
Shiferaw B, Smale M, Braun HJ, Duveiller E, Reynolds M, Muricho G (2013) Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Sec 5:291–317
Simikin AJ, Gaffé J, Alcaraz JP, Carde JP, Bramley PM, Fraser PD, Kuntz M (2007) Fibrillin influence on plastid ultrastructure and pigment content in tomato fruit. Phytochem 68:1545–1556
Singh A, Reimer S, Pozniak CJ, Clarke FR, Clarke JM, Knox RE, Singh AK (2009) Allelic variation at Psy1-A1 and association with yellow pigment in durum wheat grain. Theor Appl Genet 118:1539–1548
Stange C, Fuentes P, Handford M, Pizarro L (2008) Daucus carota as a novel model to evaluate the effect of light on carotenogenic gene expression. Biol Res 41:289–301
Sun L, Yuan B, Zhang M, Wang L, Cui M, Wang Q, Leng P (2012) Fruit-specific RNAi-mediated suppression of SlNCED1 increases both lycopene and β-carotene contents in tomato fruit. J Exp Bot 63:3097–3108
Suwarno WB, Pixley KV, Palacios-Rojas N, Kaeppler SM, Babu R (2015) Genome-wide association analysis reveals new targets for carotenoid biofortification in maize. Theor Appl Genet 128:851–864
Taylor M, Ramsay G (2005) Carotenoid biosynthesis in plant storage organs: recent advances and prospects for improving plant food quality. Physiol Plant 124:143–151
Thakkar SK, Maziya-Dixon B, Dixon AGO, Failla ML (2007) β-carotene micellarization during in vitro digestion and uptake by Caco-2 cells is directly proportional to β-carotene content in different genotypes of cassava. J Nutr 137:2229–2233
Thakkar SK, Huo T, Maziya-Dixon B, Failla ML (2009) Impact of style of processing on retention and bioaccessibility of β-carotene in cassava (Manihot esculanta, Crantz). J Agric Food Chem 57:344–1348
Tunmegamire S, Mwarya R, Andrade ML, Low JW, Ssemakula GN, Laurie AM, Chipungu FP, Ndirigue J, Agili S, Karanja L, Chiona M, Njoku JC, Mtunda K, Ricardo J, Adofo K, Carey E, Cgruneberg WJ (2014). Orange-fleshed sweet potato for Africa. Catalog 2014, 2nd edn. International Potato Center (CIP), Lima Peru, 74 p
Van Eck J, Conlin B, Garvin DF, Mason H, Navarre DA, Brown CR (2007) Enhancing beta-carotene content in potato by RNAi-mediated silencing of the beta-carotene hydroxylase gene. Amer J Potato Res 84:331–342
Wang C, Zeng J, Li Y, Hu W, Chen L, Miao Y, Deng P, Yuan C, Ma C, Chen X, Zang M, Wang Q, Li K, Chang J, Wang Y, Yang G, He G (2014) Enrichment of provitamin A content in wheat (Triticum aestivum L.) by introduction of the bacterial carotenoid biosynthetic genes CrtB and CrtI. J Exp Bot 65:2545–2556
Wei S, Yu B, Gruber MY, Khachatourians GG, Hegedus DD, Hannoufa A (2010) Enhanced seed carotenoid levels and branching in transgenic Brassica napus expressing the Arabidopsis miR156b gene. J Agric Food Chem 58:9572–9578
Welsch R, Arango J, Bar C, Salazar B, Al-Babili S, Beltran J, Chavarriaga P, Ceballos H, Tohme J, Beyer P (2010) Provitamin A accumulation in cassava (Manihot esculenta) roots driven by a single nucleotide polymorphism in a phytoene synthase gene. Plant Cell 22:3348–3356
West KP (2002) Extent of vitamin A deficiency among preschool children and women of reproductive age. J Nutr 132:2857S–2866S
Wurbs D, Ruf S, Bock R (2007) Contained metabolic engineering in tomatoes by expression of carotenoid biosynthesis genes from the plastid genome. Plant J 49:276–288
Wurtzel ET, Cuttriss A, Vallabhaneni R (2012) Maize provitamin A carotenoids, current resources, and future metabolic engineering challenges. Frontiers Plant Sci 29:1–12
Yabuta Y, Tanaka H, Yoshimura S, Suzuki A, Tamoi M, Maruta T, Shigeoka S (2013) Improvement of vitamin E quality and quantity in tobacco and lettuce by chloroplast genetic engineering. Transgenic Res 22:391–402
Ye VM, Bhatia SK (2012) Metabolic engineering strategies for the production of beneficial carotenoids in plants. Food Sci Biotechnol 21:1511–1517
Ye X, Al-Babili S, Klöti A, Zhang J, Lucca P, Beyer P, Potrykus I (2000) Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287:303–305
Yu B, Gruber MY, Khachatourians GG, Zhou R, Epp DJ, Hegedus DD, Parkin IA, Welsch R, Hannoufa A (2012) Arabidopsis cpSRP54 regulates carotenoid accumulation in Arabidopsis and Brassica napus. J Exp Bot 63:5189–5202
Yu B, Lydiate DJ, Young LW, Schäfer UA, Hannoufa A (2013) Enhancing the carotenoid content of Brassica napus seeds by downregulating lycopene epsilon cyclase. Transgenic Res 17:573–585
Yuan JP, Peng J, Yin K, Wang JH (2011) Potential health-promoting effects of astaxanthin: a high-value carotenoid mostly from microalgae. Mol Nutr Food Res 55:150–165
Zaripheh S, Nara TY, Nakamura MT, Erdman JW Jr (2006) Dietary lycopene downregulates carotenoid 15, 15′-monooxygenase and PPAR-gamma in selected rat tissues. J Nutr 136:932–938
Zhang J, Tao N, Xu Q, Zhou W, Cao H, Xu J, Deng X (2009) Functional characterization of Citrus PSY gene in Hongkong kumquat (Fortunella hindsii Swingle). Plant Cell Rep 28:1737–1746
Zhou X, Mcquinn R, Fei Z, Wolters AMA, Van Eck J, Brown C, Giovannoni J, Li L (2011) Regulatory control of high levels of carotenoid accumulation in potato tubers. Plant Cell Environ 34:1020–1030
Zhu C, Naqvi S, Breitenbach J, Sandmann G, Christou P, Capell T (2008) Combinatorial genetic transformation generates a library of metabolic phenotypes for the carotenoid pathway in maize. Proc Natl Acad Sci U S A 105:18232–18237
Zhu C, Naqvi S, Capell T, Christou P (2009) Metabolic engineering of ketocarotenoid biosynthesis in higher plants. Arch Biochem Biophys 483:182–190
Acknowledgements
Research in our laboratory has been supported by grants from the Spanish Ministerio de Ciencia e Innovación, and Ministerio de Economia y Competitividad, and also from the Generalitat Valenciana (Prometeo 2014/0027). LZ and MJR are members of the IBERCAROT network funded by CYTED (ref. 112RT0445). EA was the recipient of a JAE-postdoctoral contract (CSIC-FSE).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Alós, E., Rodrigo, M.J., Zacarias, L. (2016). Manipulation of Carotenoid Content in Plants to Improve Human Health. In: Stange, C. (eds) Carotenoids in Nature. Subcellular Biochemistry, vol 79. Springer, Cham. https://doi.org/10.1007/978-3-319-39126-7_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-39126-7_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-39124-3
Online ISBN: 978-3-319-39126-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)