Abstract
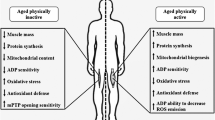
Aging is associated with a chronic state of oxidative stress and inflammation that is mediated by complex and interconnected pathways. Regular exercise counteracts the deleterious effects of aging through its antioxidant and anti-inflammatory actions. The inflammatory actions of exercise are mainly exerted on adipose tissue (by reducing its mass and inflammatory environment), on the immune system (by shifting immune cells towards the less inflammatory phenotype, modulating the cytokines profiles towards more anti-inflammatory and reduced proinflammatory cytokines, and stimulating glucocorticoids), and on skeletal muscle (by stimulating mitochondrial biogenesis, improving muscle mass and strength, reducing pro-inflammatory cytokines and increasing the anabolic myokine IL-15 and protective heat shock proteins). It is likely that regular exercise exerts the most substantial anti-inflammatory effects in patients having high baseline inflammatory biomarkers, particularly when associated with visceral fat loss.
Exercise exerts antioxidant effects by suppressing inflammatory pathways and therefore inhibiting prominent sources of reactive oxygen species and reactive nitrogen species generation. Importantly, exercise also activates redox-sensitive transcription factors, mainly nuclear factor kappa B, activator protein-1 and peroxisome proliferator-activated receptor gamma coactivator 1-alpha, leading to the enhancement of the antioxidant defense mechanisms by modulating the expression and activities of superoxide dismutase, catalase, glutathione peroxidase, and thioredoxin reductase 1. Exercise also upregulates repair proteins such as heat shock proteins, proteasome complex, oxoguanine DNA glycosylase, uracil DNA glycosylase and telomerase. It is important to note that the effects of exercise depends on the type, intensity, frequency and duration, and also on the individual’s age, health status and endurance capacity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Exercise and Ageing
There is much evidence indicating that regular exercise counteracts the negative effects of ageing. For example, regular exercise is associated with reduced risks of all-cause and cardiovascular mortality [1–3], and also with increased longevity [4–7]. Furthermore, exercise reduces the risk of cardiovascular diseases [8], type 2 diabetes [9], metabolic syndrome [10], colon cancer [11], obesity [12], osteoporosis (Kelley 1998; Marques et al. 2012), sarcopenia [13], anxiety (Wipfli et al. 2008), and cognitive impairment [14]. Most importantly, exercise improves the quality of life of elderly people [15].
2 Ageing and Oxidative Stress
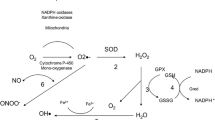
Ageing is associated with oxidative stress that is mainly due to defective (leaky) mitochondria [16], probably resulting from lower cytochrome C oxidase (complex IV) activity (Navarro et al. 2003) and peroxidative damage of mitochondrial membrane lipids [17]. Hence more electrons escape from the mitochondria, generating a long trail of reactive oxygen species (ROS) [18, 19] leading to progressive mitochondrial dysfunction and further exacerbating ROS generation, thus creating a vicious cycle of oxidative damage. Age-associated increases in ROS production occur in skeletal muscles [20] and other organs such as the heart, liver, brain, and kidney [5, 18, 21].
Reduced protein synthesis limits antioxidant defense mechanisms and repair capacity in aged individuals, which further contributes to the state of oxidative stress. The free radicals theory of aging hypothesizes that oxidative stress causes damage to macromolecules, including lipids, proteins and nucleic acids that overwhelms cellular antioxidant defense and repair mechanisms, leading to progressive deleterious changes over time [22, 23].
3 Aging and Inflammation
Age-associated progressive loss of muscle mass and strength, known as sarcopenia [24, 25], increases the incidence of muscle injury [6] and subsequently increases the infiltration of immune cells into the injured muscles. Activated immune cells release ROS, reactive nitrogen species (RNS) and proinflammatory mediators during the respiratory burst [26]. Similarly, the injured muscles generate and release proinflammatory mediators, which bind with membrane receptors and activate specific ROS-generating enzymes such as lipooxygenase, NADPH oxidase, and xanthine oxidase [27–32].
Sarcopenia can also lead to reduced physical activity and increased adiposity. Accumulation of excess adipose tissues induces a state of low-grade but chronic inflammation through the release of a multitude of pro-inflammatory cytokines including tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6) and interleukin-1beta (IL-1β) [33–35]. Indeed, ageing is associated with increased levels of circulating pro-inflammatory cytokines such as TNF-α, IL-6, and interleukin-1 receptor agonist (IL-1ra) and systemic inflammatory biomarkers such as C-reactive protein (CRP) as well as higher count of inflammatory cells (neutrophil and monocytes) [36–38]. Hence, aging is associated with a state of oxidative stress and chronic inflammation. The major events related to age-associated oxidative stress and inflammation is shown in Fig. 1.
4 Oxidative Stress and Inflammation Overlapping Signaling Pathways
Oxidative stress and inflammation share common and overlapping signaling pathways. ROS initiate and augment inflammation, and are also products of inflammation. During the inflammatory response, particularly during the respiratory burst, immune cells generate ROS and RNS via NADPH oxidase and nitric oxide synthase (NOS), and also release proinflammatory cytokines such as TNF-α, IL-1β and IL-6 [26, 39, 40]. Similarly, the injured tissues release proinflammatory cytokines. These cytokines/proinflammatory mediators bind to membrane receptors and activate specific ROS-generating enzymes, such as lipooxygenase, NADPH oxidase, myeloperoxidase and xanthine oxidase [27–32] and specific RNS generating pathways such as NOS, protein kinase B (Akt) and Sph1P (sphingosine-1-phosphate) [41–43].
ROS overproduction activates redox-sensitive transcription factors, namely nuclear factor kappa B (NF-kB) and activator protein-1 (AP-1) via stress kinases such as extracellular signal regulated kinases (ERKs), c-jun N-terminal kinases (JNKs), mitogen activated protein kinase p38 (MAPK p38), protein kinase C (PKC), phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/Akt, Src family kinases (SFKs) leading to increased expression of inflammatory target proteins genes such as matrix metalloproteinase-9 (MMP-9), intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2) and cytosolic phospholipase A2 (cPLA2) (Kim et al. 2008, 2014; Lee et al. 2012) [44–50].
Interestingly, NF-kB has been shown to regulate the transcription of TNF-α gene [51] as well as other proinflammatory mediators such as IL-1, and interleukin 8 (IL-8) [50]. Furthermore, many of NF-kB-induced proteins such as NOS, COX and PGE2 are prominent sources of ROS and RNS [52] forming an auto-activating loop which feeds the vicious cycle of inflammation and oxidative stress. In short, proinflammatory mediators such as TNF-α, IL-1, IL-6 activate redox-sensitive transcription factors such as NF-kB and AP-1 through redox signaling, resulting in the generation of large amounts of these proinflammatory mediators and ROS (Fig. 2). Indeed, aging is associated with adverse health conditions characterized by elevated levels of both oxidative stress and inflammatory markers such as atherosclerosis, metabolic syndrome, sarcopenia, arthritis, and chronic obstructive pulmonary disease [53].
Overlapping signaling pathways of oxidative stress and inflammation in aging. AP-1 activator protein-1, COX-2 cyclooxygenase-2, cPLA2 cytosolic phospholipase A2, ICAM-1 intercellular adhesion molecule-1, IL-1 interleukin-1, IL-8 interleukin-8, iNOS inducible nitric oxide synthase, MMP-9 matrix metalloproteinase-9, NF-kB nuclear factor kappa B, PGE 2 prostaglandin E2, RNS reactive nitrogen species, ROS reactive oxygen species, TNF-α tumor necrosis factor-alpha, VCAM-1 vascular cell adhesion molecule-1
Not surprisingly, ROS can also induce proteins such as heat-shock proteins (HSPs), HSP70 in particular [54] and heme oxygenate 1 oxygenase (HO-1) (Lee et al. 2012) that can protect cells and tissues from the deleterious effects of inflammation. However, in aging the balance of antioxidant/anti-inflammatory to oxidant/inflammatory proteins is tilted towards the latter
5 Exercise: Anti-inflammatory and Antioxidant Effects
Regular exercise reduces the risk of a wide range of oxidative stress and inflammation associated diseases including cardiovascular diseases [8], type 2 diabetes [9], metabolic syndrome [10], cancer [11], obesity [12], and sarcopenia [13].
5.1 Anti-inflammatory Effects of Exercise
Acute bouts of exercise cause transient damage to the contracting skeletal muscles, so triggering an inflammatory response that increases the levels of pro-inflammatory cytokines and acute-phase reactants in the blood [55, 56]. However, regular exercise is associated with reduced levels of systemic inflammatory markers such as CRP, IL-6 and TNF-α that occur independently of weight loss in young and middle aged adults [57–62], as well as in the elderly [57, 63–70]. Also, many interventional studies report that exercise reduces inflammatory markers, particularly CRP, TNF-α, monocyte chemoattractant protein-1 (MCP-1) and (IL-8), soluble TNF-α receptor 2 (sTNFR2) and soluble IL-6 receptor (sIL-6R), and increases the anti-inflammatory mediators interleukin-10 (IL-10), interleukin-4 (IL-4), and transforming growth factor beta 1 (TGFβ1) [71–80]. However, only a few randomized controlled trials were conducted to confirm that [81–84]. These benefits of exercise were also evident in the elderly [81, 85–89]. It is likely that exercise causes the most significant anti-inflammatory effects in patients with high baseline inflammatory biomarkers, particularly when exercise is associated with weight loss.
However, it is worth noting that some interventional and randomized controlled trials studies did not detect a significant effect of regular exercise on systemic inflammatory biomarkers in adults [59, 90–92], or in aged adults [93–96]. A meta-analysis conducted in 2006 found only five randomized controlled trials that examined the effects of regular aerobic exercise (at least 4 weeks duration) in adults and concluded that aerobic exercise did not reduce CRP levels [97]. It is likely that these discrepancies may be attributed to the smaller sample size used in the clinical trials examined.
On the other hand, the effects of resistance exercise on inflammatory mediators are mostly negative [98–100], although Brooks et al. [101] reported that 16-week resistance training reduced CRP and increased adiponectin levels in older diabetic patients. Clearly, the effects of exercise depends on the type (aerobic/resistance), intensity (mild/moderate/intense/exhaustive), and frequency (sessions per day/week/month) of exercise, and also on the subject’s basic condition and endurance capacity.
5.1.1 Anti-inflammatory Signaling Pathways of Exercise
The signaling pathways underlying the anti-inflammatory effects of exercise are complex and not completely understood but for the sake of convenience, can be divided into three main pathways according to the site of action. The main sites of action are the adipose tissue, the immune system and skeletal muscles.
-
Anti-inflammatory effects of exercise on adipose tissue
Obesity is associated with chronic inflammation. Adipose tissue , particularly visceral fat depots, and macrophages trapped in the adipose tissue release pro-inflammatory cytokines such as IL-6 and TNF-α [33–35, 102]. Exercise increases energy expenditure and burns off some of the body fat, which can result in weight loss, particularly visceral fat loss [12, 103, 104]. Subsequently, the production and release of pro-inflammatory adipokines such as IL-6 and TNF-α are reduced [79, 105–107]. Exercise also induces the release of adiponectin from adipose tissues [108, 109]. Adiponectin exerts anti-apoptotic, anti-inflammatory and anti-oxidative activities [110, 111]. Exercise inhibits the infiltration of M1-type macrophages into adipose tissue and also induces the switch of macrophages from the more inflammatory phenotype M1-type to the less inflammatory phenotype M2-type in obese mice [112].
-
Anti-inflammatory effects of exercise on the immune system
Aerobic exercise downregulates the innate immune response and activates the adaptive immune system with consequent suppression of inflammation. Exercise modulates the immune system by reducing the number of inflammatory CD14+CD16+ monocytes [113], increasing the number of CD4CD25 regulatory T cells [114, 115], increasing the dominance of Type 2 helper T cell over Type 1 helper T cell [116–118], and reducing the expression of toll like receptor-4 (TLR4) on monocyte surfaces [119, 120]. TLR4 signaling participates in several innate immunity and inflammatory processes [121]. Exercise also reduces the production of proinflammatory cytokines such as interferon gamma (INFγ), TNFα, IL-1α, IL-8, MCP-1 and the receptors for TNF-α (sTNFR2) and IL-6 (sIL-6R). In addition, exercise releases anti-inflammatory cytokines such as IL-10, IL-4, TGFβ1 and adiponectin [71–76, 78–81, 85–89, 108, 122].
Exercise is a positive stressor to the body; it stimulates the sympathetic nervous system and the hypothalamic–pituitary–adrenal axis. Therefore, exercise increases serum glucocorticoid levels [123] to cause a subsequent inhibition of the immune system [124].
-
Anti-inflammatory effects of exercise on skeletal muscles
By improving muscle mass and strength, exercise renders skeletal muscles less vulnerable to acute injury and the associated inflammatory responses [125–129]. Also, by stimulating mitochondrial biogenesis [130] and enhancing mitochondrial oxidative capacity [131], exercise mitigates mitochondrial aging and interrupts the vicious cycle of oxidative damage.
Exercise induces the release of several cytokines (myokines) from skeletal muscle, most notably IL-6 [132, 133]. IL-6 triggers the release of several anti-inflammatory cytokines such as IL-1 receptor antagonist (IL-1ra) and IL-10, in addition to cortisol [134, 135]. IL-10 inhibits the synthesis of several pro-inflammatory cytokines such as TNF-α and IL-1β [136]. Exercise also reduces TNF-α and IL-1β production in skeletal muscles [137–141], and upregulates the expression of the anabolic myokine IL-15 [136, 142] and HSPs in skeletal muscles [143–145]. The mechanisms underlying the anti-inflammatory actions of exercise are summarized in Fig. 3 .
Signaling pathways underlying the anti-inflammatory actions of exercise. HSPs heat shock proteins, IL-1α interleukin-1 alpha, IL-1ra interleukin-1 receptor antagonist, IL-1β interleukin-1 beta, IL-6 interleukin-6, IL-8 interleukin-8, IL-10 interleukin-10, IL-15 interleukin-15, INFγ interferon gamma, M1 macrophage phenotype 1, M2 macrophage phenotype 2, ROS reactive oxygen species, sTNFR2 soluble TNF-α receptor 2, sIL-6R soluble IL-6 receptor, TLR4 toll like receptor-4, TGFβ1 transforming growth factor beta 1, TNF-α tumor necrosis factor-alpha, Th1 Type 1 helper T cell, Th2 Type 2 helper T cell
5.2 Anti-oxidant Effects of Exercise
There is little doubt that generation of ROS is increased acutely during exercise. However, the incidence of diseases associated with oxidative stress is reduced by regular exercise. Exercise training attenuates oxidative damage in the brain [5, 146–149], liver [5, 150–152] (Radak et al. 2004), kidney [5], skeletal muscles [153] and heart [5, 154].
Importantly, regular exercise ameliorates age-associated oxidative stress in the heart [154, 155], liver (Radak et al. 2004), and skeletal muscle [156] (Radak et al. 2002). In the study of Navarro et al. [5], exercise reduced age-associated mitochondrial oxidative damage and upregulated mitochondrial NADH-cytochrome-c reductase and cytochrome oxidase activities in brain, heart, liver, and kidney of 52 week old rats. However, exercise caused an increase in oxidative damage in skeletal muscles [157] and hearts [158] of aged rats.
In elderly people, exercise reduced serum levels of myeloperoxidase, a marker of inflammation and oxidative stress [125] and thiobarbituric-reactive acid substances, a marker of lipid peroxidation [159]. However, de Gonzalo-Calvo et al. [160] reported that although regular exercise increased protein carbonyl content and lipid peroxidation levels in the plasma and erythrocytes of long-term trained elderly men, their overall health condition was markedly improved. Another clinical study showed that 8 weeks of walking exercise did not significantly change low density lipoprotein (LDL) oxidation or nitration in the elderly [161].
5.2.1 Anti-oxidant Signaling Pathways of Exercise
By suppressing inflammatory pathways, exercise inhibits prominent sources of ROS and RNS generation and thus exerts beneficial antioxidant effects. Exercise also upregulates the antioxidant defense mechanisms and repair proteins in the body via redox-sensitive transcription factors, mainly NF-kB, AP-1 and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α).
The energy demand of contracting muscles increases during exercise and the body responds by increasing oxygen uptake and delivery to muscles. The increased metabolic rate results in greater ROS production not only in muscles [20, 148, 149], but in other organs as well [21, 162]. Sources other than the mitochondrial electron transport chain enzymes, such as xanthine oxidase [163–165] and NADPH oxidase [20, 166] contribute to ROS generation during exercise.
-
Effects of exercise on NF-kB and AP-1 signaling
Exercise-induced increase in ROS levels preconditions the body against oxidative damage by evoking an adaptive process that is mediated via mitogen-activated protein kinases (MAPK p38, ERK 1 and ERK 2) [163, 167–169], cAMP-response-element binding (CREB) [170, 171], and synapsin [170, 171]. These effects lead to activation of redox-sensitive transcription factors such as NF-kB [163, 172, 173] and AP-1 [169, 173], resulting in increased expression of antioxidant enzymes [174] such as superoxide dismutase [163, 173] and catalase [173], repair proteins such as heat shock proteins HSP25, HSP60, HSP72, HSP70, heat shock cognate 70 HSC70 [157, 173–176], proteasomes complex and NOS [150, 163]. These signaling cascades were demonstrated in skeletal muscles [163, 172], brain [170, 171], leukocytes [177] and hearts [169] of experimental animals as well as in humans [167, 177] and in aged animals [173, 176, 178] and humans [177]. However, other studies reported that exercise-induced activation of NF-kB and AP-1 [173] and upregulation of HSP70 were attenuated in fast skeletal muscles of old rats [179]. Interestingly, ageing also increased ROS production and NF-KB activity in the livers of aged rats; these effects were attenuated by exercise (Radak et al. 2004) [150].
-
Effects of exercise on PGC-1α signaling
Exercise stimulates mitochondrial biogenesis [130] and ameliorates the age-associated decline in mitochondrial oxidative capacity in skeletal muscles [131], and other organs [180] (Navarro et al. 2003) via PGC-1α signaling [181, 182]. PGC-1α is a redox-sensitive transcription factor that is activated by 5′-AMP-activated protein kinase (AMPK), [168, 183–185] to trigger the transcription of nuclear respiratory factor 1 (NRF-1) and expression of mitochondrial transcription factor A (mtTFA), a key regulator of mitochondrial DNA replication [186]. PGC-1α also increases the expression of antioxidant proteins such as glutathione peroxidase (GPX) and SOD-2 [187]. Safdar et al. [188] reported that exercise reversed most of the multisystem pathology and premature mortality in mice which were genetically modified to accumulate mitochondrial mutations. The effects of exercise on AMPK, and PGC-1α were preserved in the hippocampus of aging rats. However, results from Derbré et al. [181] suggest a blunted effect of exercise response in PGC-1α, NRF-1 in skeletal muscles of aged rats.
-
Effects of exercise on antioxidant enzymes expression and activity
The NF-kB and PGC-1α signaling cascades converge to upregulate antioxidant defense mechanisms in cells to counteract and interrupt the vicious cycle of inflammation and oxidative stress associated with ageing. The most studied antioxidant enzymes systems in laboratory animals and in humans are SOD, catalase, GPX and glutathione reductase.
Regular exercise increases the activities of SOD in the brain [189, 190], erythrocytes [191–193], heart [154, 158, 194–196], tissues from the lung [197], and liver [197]. Exercise increases the protein expression of SOD in blood vessels [195, 198, 199, 200] (Lee et al. 2001), liver [201] and blood [202].
The activity of GPX was increased by exercise in the brain [189, 190], erythrocytes [191, 192, 203], blood (Elosua et al. 2008), liver, heart, lung [18, 197] and skeletal muscles containing a high percentage of type I or type IIa fibers of old rats [156]. Similarly, exercise increases the activities of plasma glutathione reductase (Elosua et al. 2008) and catalase in erythrocytes [193], heart [154, 158, 204] and liver [18]. Some studies reported no changes in the activities of SOD, CAT, or GPX in the brain [205] or skeletal muscle [153].
Several studies investigated the effects of exercise on antioxidant enzymes at old age. For example, exercise enhances the activities of SOD in the heart [154, 158, 195, 197], brain [189], and lung [197] and increases GPX activities in the brain, liver, heart, lung and skeletal muscles of old rats [156, 189, 197, 206] and in erythrocytes of elderly people [203]. Catalase activity is upregulated by exercise in the liver [197] and heart [154, 158] of aged rats. In the study of Navarro et al. [5], exercise reduced the extent of age-associated decline in SOD and catalase activities in brain, heart, liver, and kidney of 52 week old but not older mice (72 week old). Exercise also up-regulated the protein expression of SOD-1 and GPX in the hippocampus of aged rats [147].
Exercise-induced adaptation of antioxidant enzymes is highly isoform, tissue and time course specific. Exercise modulates the three SOD isoforms differently [195, 199, 201, 202, 207, 208] as the promoter region of SOD-2 contains more ROS-sensitive binding sites [209]. Exercise-induced protein expression of SOD is time dependent (Navarro et al. 2003); SOD-1 protein expression was increased in rat skeletal muscles 48 h post exercise, whereas SOD-2 protein content was increased after 10 and 24 h, but not 48 h [208].
-
Effects of exercise on repair mechanisms
Exercise can also stimulate the proteasome complex, which is responsible for the degradation of oxidatively damaged proteins [150, 210, 211] (Radak et al. 2000), and therefore enhances the cellular repair processes. Exercise also modulates the activity of DNA repair enzymes, particularly oxoguanine DNA glycosylase (OGG1) and uracil DNA glycosylase (UDG), and thus reduces the accumulation of nuclear 8-hydroxydeoxyguanosine (8-OHdG) and mutations in skeletal muscles (Radak et al. 2002) [212, 213], but not brains of aged rats [214]. Exercise increased thioredoxin reductase 1 (TrxR1), one of the thioredoxin system enzymes with direct and indirect antioxidant effects, in peripheral blood mononuclear cells in humans [125] (Wadley et al. 2015).
Exercise increases the content of the brain-derived neurotrophic factor (BDNF), a critically important neurotrophic factor that is involved in higher cognitive function [170, 171].
Telomeres are considered ‘the guardians of the genome’. Telomere dysfunction activates p53, leading to suppression of PGC-1α and PGC-1β promoters with consequent metabolic and organ failure [215]. The leukocyte telomere was 200 nucleotides longer in people who exercise regularly, which roughly corresponds to a 10-year increase in longevity [216]. Exercise increases the activity of telomerase, and induces the expression of telomere repeat-binding factor 2 and Ku70 in the thoracic aorta and in leukocytes from mice and humans [217]. The signaling pathways underlying the antioxidant actions of exercise is summarized in Fig. 4 .
Signaling pathways underlying the antioxidant actions of exercise. AMPK AMP-activated protein kinase, AP-1 activator protein-1, CREB cAMP-response-element binding, HSPs heat shock proteins, GPX glutathione peroxidase, MAPKs mitogen activated protein kinases, NF-kB nuclear factor kappa B, OGG1 oxoguanine DNA glycosylase, PGC-1α peroxisome proliferator-activated receptor gamma, coactivator 1-alpha, SOD superoxide dismutase, ROS reactive oxygen species, TrxR1 Thioredoxin reductase 1, UDG uracil DNA glycosylase
References
Nocon M, Hiemann T, Müller-Riemenschneider F, Thalau F, Roll S, Willich SN. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil. 2008;15(3):239–46.
Samitz G, Egger M, Zwahlen M. Domains of physical activity and all-cause mortality: systematic review and dose-response meta-analysis of cohort studies. Int J Epidemiol. 2011;40(5):1382–400.
Taylor RS, Brown A, Ebrahim S, Jolliffe J, Noorani H, Rees K, Skidmore B, Stone JA, Thompson DR, Oldridge N. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116(10):682–92.
Franco OH, de Laet C, Peeters A, Jonker J, Mackenbach J, Nusselder W. Effects of physical activity on life expectancy with cardiovascular disease. Arch Intern Med. 2005;165(20):2355–60.
Navarro A, Gomez C, López-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Regul Integr Comp Physiol. 2004;286(3):R505–11.
Paffenbarger Jr RS, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328(8):538–45.
Sandvik L, Erikssen J, Thaulow E, Erikssen G, Mundal R, Rodahl K. Physical fitness as a predictor of mortality among healthy, middle-aged Norwegian men. N Engl J Med. 1993;328(8):533–7.
Wilund KR. Is the anti-inflammatory effect of regular exercise responsible for reduced cardiovascular disease? Clin Sci (Lond). 2007;112(11):543–55.
LaMonte MJ, Blair SN, Church TS. Physical activity and diabetes prevention. J Appl Physiol. 2005;99(3):1205–13.
Gaesser GA. Exercise for prevention and treatment of cardiovascular disease, type 2 diabetes, and metabolic syndrome. Curr Diab Rep. 2007;7:14–9.
Thune I, Furberg AS. Physical activity and cancer risk: dose-response and cancer, all sites and site-specific. Med Sci Sports Exerc. 2001;33(6 Suppl):S530–50. discussion S609–10.
Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, Janssen I. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133(2):92–103.
Glass D, Roubenoff R. Recent advances in the biology and therapy of muscle wasting. Ann N Y Acad Sci. 2010;1211:25–36.
Tseng CN, Gau BS, Lou MF. The effectiveness of exercise on improving cognitive function in older people: a systematic review. J Nurs Res. 2011;19(2):119–31.
Ozaki A, Uchiyama M, Tagaya H, Ohida T, Ogihara R. The Japanese Centenarian Study: autonomy was associated with health practices as well as physical status. J Am Geriatr Soc. 2007;55(1):95–101.
Navarro A, López-Cepero JM, Sánchez del Pino MJ. Skeletal muscle and aging. Front Biosci. 2001;6:D26–44.
Chen JJ, Yu BP. Alterations in mitochondrial membrane fluidity by lipid peroxidation products. Free Radic Biol Med. 1994;17(5):411–8.
Kim JD, McCarter RJ, Yu BP. Influence of age, exercise, and dietary restriction on oxidative stress in rats. Aging (Milano). 1996;8(2):123–9.
Miquel J, Fleming J. Theoretical and experimental support for an oxygen radical mitochondrial injury hypothesis of cell aging. In: Johnson J, Walford R, Harman D, Miquel J, editors. Biology of aging. New York: Alan R. Liss Inc.; 1986. p. 51–76.
Bejma J, Ji LL. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol. 1999;87(1):465–70.
Bejma J, Ramires P, Ji LL. Free radical generation and oxidative stress with ageing and exercise: differential effects in the myocardium and liver. Acta Physiol Scand. 2000;169(4):343–51.
Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300.
Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R18–36.
Rogers MA, Evans WJ. Changes in skeletal muscle with aging: effects of exercise training. In: Holloszy JO, editor. Exercise and sport sciences reviews, vol. 21. Baltimore: Williams & Wilkins; 1993. p. 65–102.
Roubenoff R. Physical activity, inflammation, and muscle loss. Nutr Rev. 2007;65:S208–12.
Cannon JG, Blumberg JB. Acute phase immune responses in exercise. In: Sen CK, Packer L, Hanninen O, editors. Exercise and oxygen toxicity. New York: Elsevier Science; 1994. p. 447–79.
Flohé L, Brigelius-Flohé R, Saliou C, Traber MG, Packer L. Redox regulation of NF-kappa B activation. Free Radic Biol Med. 1997;22(6):1115–26.
Gao X, Belmadani S, Picchi A, Xu X, Potter BJ, Tewari-Singh N, Capobianco S, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation. 2007;115(2):245–54.
Li JM, Fan LM, Christie MR, Shah AM. Acute tumor necrosis factor alpha signaling via NADPH oxidase in microvascular endothelial cells: role of p47phox phosphorylation and binding to TRAF4. Mol Cell Biol. 2005;25:2320–30.
Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res. 2006;99(1):69–77.
Reeves EP, Nagl M, Godovac-Zimmermann J, Segal AW. Reassessment of the microbicidal activity of reactive oxygen species and hypochlorous acid with reference to the phagocytic vacuole of the neutrophil granulocyte. J Med Microbiol. 2003;52:643–51.
Thabut G, El-Benna J, Samb A, Corda S, Megret J, Leseche G, Vicaut E, Aubier M, Boczkowski J. Tumor necrosis factor-α increases airway smooth muscle oxidants production through a NADPH oxidase-like system to enhance myosin light chain phosphorylation and contractility. J Biol Chem. 2002;277:22814–21.
Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–50.
Mohamed-Ali V, Pinkney JH, Coppack SW. Adipose tissue as an endocrine and paracrine organ. Int J Obes Relat Metab Disord. 1998;22:1145–58.
Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92(3):347–55.
Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhøj P, Pedersen BK. A high plasma concentration of TNF-alpha is associated with dementia in centenarians. J Gerontol A Biol Sci Med Sci. 1999;54:M357–64.
Bruunsgaard H. The clinical impact of systemic low-level inflammation in elderly populations. With special reference to cardiovascular disease, dementia and mortality. Dan Med Bull. 2006;53:285–309. PubMed: 17092450.
Vasto S, Candore G, Balistreri CR, Caruso M, Colonna-Romano G, Grimaldi MP, Listi F, Nuzzo D, Lio D, Caruso C. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128:83–91.
Pizza FX, Hernandez IJ, Tidball JG. Nitric oxide synthase inhibition reduces muscle inflammation and necrosis in modified muscle use. J Leukoc Biol. 1998;64(4):427–33.
Pyne DB. Regulation of neutrophil function during exercise. Sports Med. 1994;17:245–58.
De Palma C, Meacci E, Perrotta C, Bruni P, Clementi E. Endothelial nitric oxide synthase activation by tumor necrosis factor alpha through neutral sphingomyelinase 2, sphingosine kinase 1, and sphingosine 1 phosphate receptors: a novel pathway relevant to the pathophysiology of endothelium. Arterioscler Thromb Vasc Biol. 2006;26(1):99–105.
Zemse SM, Chiao CW, Hilgers RH, Webb RC. Interleukin-10 inhibits the in vivo and in vitro adverse effects of TNF-alpha on the endothelium of murine aorta. Am J Physiol Heart Circ Physiol. 2010;299(4):H1160–7.
Zhong L, You J, Sun Q. The role of NF-kB in the TNF-α-induced endothelial cell apoptosis. Zhonghua Yixue Zazhi. 1999;79:863–6.
Lee IT, Yang CM. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem Pharmacol. 2012;84(5):581–90.
Lee CW, Lin CC, Lee IT, Lee HC, Yang CM. Activation and induction of cytosolic phospholipase A2 by TNF-α mediated through Nox2, MAPKs, NF-kB, and p300 in human tracheal smooth muscle cells. J Cell Physiol. 2011;226:2103–14.
Lee S, Park Y, Dellsperger KC, Zhang C. Exercise training improves endothelial function via adiponectin-dependent and independent pathways in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2011;301(2):H306–14.
Lin CP, Huang PH, Tsai HS, Wu TC, Leu HB, Liu PL, Chen YH. Monascus purpureus-fermented rice inhibits tumor necrosis factor-α-induced upregulation of matrix metalloproteinase 2 and 9 in human aortic smooth muscle cells. J Pharm Pharmacol. 2011;63(12):1587–94.
Luo SF, Chang CC, Lee IT, Lee CW, Lin WN, Lin CC, Yang CM. Activation of ROS/NF-kappaB and Ca2+/CaM kinase II are necessary for VCAM-1 induction in IL-1beta-treated human tracheal smooth muscle cells. Toxicol Appl Pharmacol. 2009;237(1):8–21.
Phalitakul S, Okada M, Hara Y, Yamawaki H. Vaspin prevents TNF-a-induced intracellular adhesion molecule-1 via inhibiting reactive oxygen species-dependent NF-kB and PKCu activation in cultured rat vascular smooth muscle cells. Pharmacol Res. 2011;64:493–500.
Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J. 2000;16:534–54.
Liu H, Sidiropoulos P, Song G, Pagliari LJ, Birrer MJ, Stein B, Anrather J, Pope RM. TNF-alpha gene expression in macrophages: regulation by NF-kappa B is independent of c-Jun or C/EBP beta. J Immunol. 2000;164(8):4277–85.
Wolin MS. Reactive oxygen species and vascular signal transduction mechanisms. Microcirculation. 1996;3(1):1–17.
Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8(1):18–30.
Jacquier-Sarlin MR, Fuller K, Dinh-Xuan AT, Richard MJ, Polla BS. Protective effects of hsp70 in inflammation. Experientia. 1994;50(11–12):1031–8.
Moldoveanu AI, Shephard RJ, Shek PN. The cytokine response to physical activity and training. Sports Med. 2001;31:115–44.
Zaldivar F, Wang-Rodriguez J, Nemet D, Schwindt C, Galassetti P, Mills PJ, Wilson LD, Cooper DM. Constitutive pro- and anti-inflammatory cytokine and growth factor response to exercise in leukocytes. J Appl Physiol. 2006;100(4):1124–33.
Abramson JL, Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch Intern Med. 2002;162:1286–92.
Albert MA, Glynn RJ, Ridker PM. Effect of physical activity on serum C reactive protein. Am J Cardiol. 2004;93:221–5.
Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clin Chim Acta. 2010;411(11–12):785–93.
Church TS, Barlow CE, Earnest CP, Kampert JB, Priest EL, Blair SN. Associations between cardiorespiratory fitness and C-reactive protein in men. Arterioscler Thromb Vasc Biol. 2002;22:1869–76.
King DE, Carek P, Mainous III AG, Pearson WS. Inflammatory markers and exercise: differences related to exercise type. Med Sci Sports Exerc. 2003;35:575–81.
Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Rimm EB. Leisure-time physical activity and reduced plasma levels of obesity-related inflammatory markers. Obes Res. 2003;11:1055–64.
Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, Pahor M, Taaffe DR, Brach J, Rubin S, Harris TB. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52(7):1098–104.
Elosua R, Bartali B, Ordovas JM, Corsi AM, Lauretani F, Ferrucci L. Association between physical activity, physical performance, and inflammatory biomarkers in an elderly population: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2005;60:760–7.
Geffken D, Cushman M, Burke G, Polak J, Sakkinen P, Tracy R. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153:242–50.
Jankord R, Jemiolo B. Influence of physical activity on serum IL-6 and IL-10 levels in healthy older men. Med Sci Sports Exerc. 2004;36:960–4.
Reuben DB, Judd-Hamilton L, Harris TB, Seeman TE. The associations between physical activity and inflammatory markers in high-functioning older persons: MacArthur Studies of Successful Aging. J Am Geriatr Soc. 2003;51:1125–30.
Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2000;55(12):M709–15. PubMed PMID: 11129392.
Wannamethee SG, Lowe GD, Whincup PH, Rumley A, Walker M, Lennon L. Physical activity and hemostatic and inflammatory variables in elderly men. Circulation. 2002;105:1785–90.
Yu Z, Ye X, Wang J, Qi Q, Franco OH, Rennie KL, Pan A, Li H, Liu Y, Hu FB, Lin X. Associations of physical activity with inflammatory factors, adipocytokines, and metabolic syndrome in middle-aged and older Chinese people. Circulation. 2009;119(23):2969–77.
Conraads VM, Beckers P, Bosmans J, De Clerck LS, Stevens WJ, Vrints CJ, et al. Combined endurance/resistance training reduces plasma TNF-alpha receptor levels in patients with chronic heart failure and coronary artery disease. Eur Heart J. 2002;23:1854–60.
Kohut ML, McCann DA, Russell DW, Konopka DN, Cunnick JE, Franke WD, Castillo MC, Reighard AE, Vanderah E. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav Immun. 2006;20(3):201–9. Epub 2006 Feb 28.
Larsen AI, Aukrust P, Aarsland T, Dickstein K. Effect of aerobic exercise training on plasma levels of tumor necrosis factor alpha in patients with heart failure. Am J Cardiol. 2001;88:805–8.
LeMaitre JP, Harris S, Fox KA, Denvir M. Change in circulating cytokines after 2 forms of exercise training in chronic stable heart failure. Am Heart J. 2004;147:100–5.
Mattusch F, Dufaux B, Heine O, Mertens I, Rost R. Reduction of the plasma concentration of C-reactive protein following nine months of endurance training. Int J Sports Med. 2000;21:21–4.
Smith JK, Dykes R, Douglas JE, Krishnaswamy G, Berk S. Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA. 1999;281:1722–7.
Stefanov T, Vekova A, Bonova I, Tzvetkov S, Kurktschiev D, Blüher M, Temelkova-Kurktschiev T. Effects of supervised vs non-supervised combined aerobic and resistance exercise programme on cardiometabolic risk factors. Cent Eur J Public Health. 2013;21(1):8–16.
Straczkowski M, Kowalska I, Dzienis-Straczkowska S, Stepién A, Skibińska E, Szelachowska M, Kinalska I. Changes in tumor necrosis factor-alpha system and insulin sensitivity during an exercise training program in obese women with normal and impaired glucose tolerance. Eur J Endocrinol. 2001;145(3):273–80.
Troseid M, Lappegard KT, Claudi T, Damas JK, Morkrid L, Brendberg R, Mollnes TE. Exercise reduces plasma levels of the chemokines MCP-1 and IL-8 in subjects with the metabolic syndrome. Eur Heart J. 2004;25:349–55.
Tsukui S, Kanda T, Nara M, Nishino M, Kondo T, Kobayashi I. Moderate intensity regular exercise decreases serum tumor necrosis factor-alpha and HbA1c levels in healthy women. Int J Obes Relat Metab Disord. 2000;24:1207–11.
Campbell PT, Campbell KL, Wener MH, et al. A yearlong exercise intervention decreases CRP among obese postmenopausal women. Med Sci Sports Exerc. 2009;41:1533–9.
Kadoglou NP, Iliadis F, Angelopoulou N, Perrea D, Ampatzidis G, Liapis CD, Alevizos M. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Cardiovasc Prev Rehabil. 2007;14:837–43.
Tisi PV, Hulse M, Chulakadabba A, Gosling P, Shearman CP. Exercise training for intermittent claudication: does it adversely affect biochemical markers of the exercise-induced inflammatory response? Eur J Vasc Endovasc Surg. 1997;14:344–50.
Walther C, Möbius-Winkler S, Linke A, Bruegel M, Thiery J, Schuler G, Halbrecht R. Regular exercise training compared with percutaneous intervention leads to a reduction of inflammatory markers and cardiovascular events in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2008;15(1):107–12.
Greiwe JS, Cheng B, Rubin DC, Yarasheski KE, Semenkovich CF. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J. 2001;15:475–82.
Milani RV, Lavie CJ, Mehra MR. Reduction in C-reactive protein through cardiac rehabilitation and exercise training. J Am Coll Cardiol. 2004;43:1056–61.
Nicklas BJ, Hsu FC, Brinkley TJ, Church T, Goodpaster BH, Kritchevsky SB, Pahor M. Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J Am Geriatr Soc. 2008;56(11):2045–52.
Wegge JK, Roberts CK, Ngo TH, Barnard RJ. Effect of diet and exercise intervention on inflammatory and adhesion molecules in postmenopausal women on hormone replacement therapy and at risk for coronary artery disease. Metabolism. 2004;53:377–81.
You T, Berman DM, Ryan AS, Nicklas BJ. Effects of hypocaloric diet and exercise training on inflammation and adipocyte lipolysis in obese postmenopausal women. J Clin Endocrinol Metab. 2004;89:1739–46.
Gielen S, Adams V, Möbius-Winkler S, Linke A, Erbs S, Yu J, Kempf W, Schubert A, Schuler G, Hambrecht R. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol. 2003;42(5):861–8.
Marcell TJ, McAuley KA, Traustadottir T, Reaven PD. Exercise training is not associated with improved levels of C-reactive protein or adiponectin. Metabolism. 2005;54:533–41.
Rauramaa R, Halonen P, Väisänen SB, Lakka TA, Schmidt-Trucksäss A, Berg A, Penttilä IM, Rankinen T, Bouchard C. Effects of aerobic physical exercise on inflammation and atherosclerosis in men: the DNASCO Study: a six-year randomized, controlled trial. Ann Intern Med. 2004;140(12):1007–14.
Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Martin BS, Mackey JR. Effect of exercise training on C-reactive protein in postmenopausal breast cancer survivors: a randomized controlled trial. Brain Behav Immun. 2005;19(5):381–8.
Hammett CJ, Oxenham HC, Baldi JC, Doughty RN, Ameratunga R, French JK, White HD, Stewart RA. Effect of six months’ exercise training on C-reactive protein levels in healthy elderly subjects. J Am Coll Cardiol. 2004;44(12):2411–3.
Kemmler W, Von SS, Engelke K, Kalender WA. Exercise decreases the risk of metabolic syndrome in elderly females. Med Sci Sports Exerc. 2009;41:297–305.
Nicklas BJ, Ambrosius W, Messier SP, Miller GD, Penninx BW, Loeser RF, Palla S, Bleecker E, Pahor M. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr. 2004;79(4):544–51.
Kelley GA, Kelley KS. Effects of aerobic exercise on C-reactive protein, body composition, and maximum oxygen consumption in adults: a meta-analysis of randomized controlled trials. Metabolism. 2006;55:1500–7.
Brochu M, Malita MF, Messier V, Doucet E, Strychar I, Lavoie JM, Prud’homme D, Rabasa-Lhoret R. Resistance training does not contribute to improving the metabolic profile after a 6-month weight loss program in overweight and obese postmenopausal women. J Clin Endocrinol Metab. 2009;94(9):3226–33.
Levinger I, Goodman C, Peake J, Garnham A, Hare DL, Jerums G, Selig S. Inflammation, hepatic enzymes and resistance training in individuals with metabolic risk factors. Diabet Med. 2009;26(3):220–7.
Rall LC, Roubenoff R, Cannon JG, Abad LW, Dinarello CA, Meydani SN. Effects of progressive resistance training on immune response in aging and chronic inflammation. Med Sci Sports Exerc. 1996;28:1356–65.
Brooks N, Layne JE, Gordon PL, Roubenoff R, Nelson ME, Castaneda-Sceppa C. Strength training improves muscle quality and insulin sensitivity in Hispanic older adults with type 2 diabetes. Int J Med Sci. 2007;4:19–27. PubMed: 17211497.
Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumié A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clément K. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54(8):2277–86.
Giannopoulou I, Ploutz-Snyder LL, Carhart R, Weinstock RS, Fernhall B, Goulopoulou S, Kanaley JA. Exercise is required for visceral fat loss in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab. 2005;90(3):1511–8.
Ross R, Bradshaw AJ. The future of obesity reduction: beyond weight loss. Nat Rev Endocrinol. 2009;5:319–25.
Esposito EK, Giugliano G, Scuderi N, Giugliano D. Role of adipokines in the obesity inflammation relationship: the effect of fat removal. Plast Reconstr Surg. 2006;118:1048–57.
Forsythe LK, Wallace JM, Livingstone MB. Obesity and inflammation: the effects of weight loss. Nutr Res Rev. 2008;21:117–33.
You T, Nicklas BJ. Chronic inflammation: role of adipose tissue and modulation by weight loss. Curr Diabetes Rev. 2006;2:29–37.
de Lemos ET, Reis F, Baptista S, Pinto R, Sepodes B, Vala H, Rocha-Pereira P, Silva AS, Teixeira F. Exercise training is associated with improved levels of C-reactive protein and adiponectin in ZDF (type 2) diabetic rats. Med Sci Monit. 2007;13(8):BR168–74.
Mujumdar PP, Duerksen PJ, Firek AF, Hessingere DA. Long-term, progressive, aerobic training increases adiponectin in middle-aged, overweight, untrained males and females. Scand J Clin Lab Invest. 2011;71:101–7.
Andrade-Oliveira V, Câmara NO, Moraes-Vieira PM. Adipokines as drug targets in diabetes and underlying disturbances. J Diabetes Res. 2015;2015:681612. Epub 2015 Apr 8. Review.
Hui X, Lam KS, Vanhoutte PM, Xu A. Adiponectin and cardiovascular health: an update. Br J Pharmacol. 2012;165(3):574–90.
Kawanishi N, Yano H, Yokogawa Y, Suzuki K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc Immunol Rev. 2010;16:105–18.
Timmerman KL, Flynn MG, Coen PM, Markofski MM, Pence BD. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J Leukoc Biol. 2008;84:1271–8.
Wang J, Song H, Tang X, Yang Y, Vieira VJ, Niu Y, Ma Y. Effect of exercise training intensity on murine T-regulatory cells and vaccination response. Scand J Med Sci Sports. 2012;22(5):643–52.
Yeh SH, Chuang H, Lin LW, Hsiao CY, Eng HL. Regular tai chi chuan exercise enhances functional mobility and CD4CD25 regulatory T cells. Br J Sports Med. 2006;40(3):239–43.
Lancaster GI, Halson SL, Khan Q, Drysdale P, Wallace F, Jeukendrup AE, Drayson MT, Gleeson M. Effects of acute exhaustive exercise and chronic exercise training on type 1 and type 2 T lymphocytes. Exerc Immunol Rev. 2004;10:91–106.
Steensberg A, Toft AD, Bruunsgaard H, Sandmand M, Halkjaer-Kristensen J, Pedersen BK. Strenuous exercise decreases the percentage of type 1 T cells in the circulation. J Appl Physiol. 2001;91:1708–12.
Yeh SH, Chuang H, Lin LW, Hsiao CY, Wang PW, Liu RT, Yang KD. Regular Tai Chi Chuan exercise improves T cell helper function of patients with type 2 diabetes mellitus with an increase in T-bet transcription factor and IL-12 production. Br J Sports Med. 2009;43(11):845–50.
McFarlin BK, Flynn MG, Campbell WW, Craig BA, Robinson JP, Stewart LK, Timmerman KL, Coen PM. Physical activity status, but not age, influences inflammatory biomarkers and toll-like receptor 4. J Gerontol A Biol Sci Med Sci. 2006;61(4):388–93.
Stewart LK, Flynn MG, Campbell WW, Craig BA, Robinson JP, McFarlin BK, Timmerman KL, Coen PM, Felker J, Talbert E. Influence of exercise training and age on CD14+ cell-surface expression of toll-like receptor 2 and 4. Brain Behav Immun. 2005;19(5):389–97.
Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14.
Oberbach A, Tönjes A, Klöting N, Fasshauer M, Kratzsch J, Busse MW, Paschke R, Stumvoll M, Blüher M. Effect of a 4 week physical training program on plasma concentrations of inflammatory markers in patients with abnormal glucose tolerance. Eur J Endocrinol. 2006;154(4):577–85.
Goto S, Radák Z, Nyakas C, Chung HY, Naito H, Takahashi R, Nakamoto H, Abea R. Regular exercise: an effective means to reduce oxidative stress in old rats. Ann N Y Acad Sci. 2004;1019:471–4.
Cupps TR, Fauci AS. Corticosteroid-mediated immunoregulation in man. Immunol Rev. 1982;65:133–55.
Beltran Valls MR, Dimauro I, Brunelli A, Tranchita E, Ciminelli E, Caserotti P, Duranti G, Sabatini S, Parisi P, Parisi A, Caporossi D. Explosive type of moderate-resistance training induces functional, cardiovascular, and molecular adaptations in the elderly. Age (Dordr). 2014;36(2):759–72.
Fitts RH, Troup JP, Witzmann FA, Holloszy JO. The effect of ageing and exercise on skeletal muscle function. Mech Ageing Dev. 1984;27(2):161–72.
Narici MV, Reeves ND, Morse CI, Maganaris CN. Muscular adaptations to resistance exercise in the elderly. J Musculoskelet Neuronal Interact. 2004;4(2):161–4.
Peterson MD, Rhea MR, Sen A, Gordon PM. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev. 2010;9(3):226–37.
Peterson MD, Sen A, Gordon PM. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc. 2011;43:249–58.
Holloszy JO. Biochemical adaptations in muscle: effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–82.
Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52(8):1888–96.
Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J. 2002;16:1335–47.
Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund Pedersen B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000;529(Pt 1):237–42.
Bruunsgaard H. Physical activity and modulation of systemic low-level inflammation. J Leukoc Biol. 2005;78:819–35.
Steensberg A, Fischer CP, Keller C, Møller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285(2):E433–7.
Nielsen AR, Mounier R, Plomgaard P, Mortensen OH, Penkowa M, Speerschneider T, Pilegaard H, Pedersen BK. Expression of interleukin-15 in human skeletal muscle effect of exercise and muscle fibre type composition. J Physiol. 2007;584:305–12.
Pedersen BK, Fischer CP. Physiological roles of muscle-derived interleukin-6 in response to exercise. Curr Opin Clin Nutr Metab Care. 2007;10(3):265–71.
Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, Febbraio M, Saltin B. Searching for the exercise factor: is IL-6 a candidate? J Muscle Res Cell Motil. 2003;24(2–3):113–9.
Pedersen M, Bruunsgaard H, Weis N, Hendel HW, Andreassen BU, Eldrup E, Dela F, Pedersen BK. Circulating levels of TNF-alpha and IL-6-relation to truncal fat mass and muscle mass in healthy elderly individuals and in patients with type-2 diabetes. Mech Ageing Dev. 2003;124(4):495–502.
Schindler R, Mancilla J, Endres S, Ghorbani R, Clark SC, Dinarello CA. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990;75(1):40–7.
Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003;17:884–6.
Quinn LS, Anderson BG, Drivdahl RH, Alvarez B, Argilés JM. Overexpression of interleukin-15 induces skeletal muscle hypertrophy in vitro: implications for treatment of muscle wasting disorders. Exp Cell Res. 2002;280:55–63.
Febbraio MA, Ott P, Nielsen HB, et al. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol. 2002;544(pt 3):957–62.
Thompson HS, Clarkson PM, Scordilis SP. The repeated bout effect and heat shock proteins: intramuscular HSP27 and HSP70 expression following two bouts of eccentric exercise in humans. Acta Physiol Scand. 2002;174:47–56.
Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones. 2001;6:386–93.
Engesser-Cesar C, Anderson AJ, Basso DM, Edgerton VR, Cotman CW. Voluntary wheel running improves recovery from a moderate spinal cord injury. J Neurotrauma. 2005;22(1):157–71.
Marosi K, Bori Z, Hart N, Sárga L, Koltai E, Radák Z, Nyakas C. Long-term exercise treatment reduces oxidative stress in the hippocampus of aging rats. Neuroscience. 2012;226:21–8.
Radak Z, Sasvari M, Nyakas C, Kaneko T, Tahara S, Ohno H, Goto S. Single bout of exercise eliminates the immobilization-induced oxidative stress in rat brain. Neurochem Int. 2001;39(1):33–8.
Radak Z, Taylor AW, Ohno H, Goto S. Adaptation to exercise-induced oxidative stress: from muscle to brain. Exerc Immunol Rev. 2001;7:90–107.
Goto S, Naito H, Kaneko T, Chung HY, Radák Z. Hormetic effects of regular exercise in aging: correlation with oxidative stress. Appl Physiol Nutr Metab. 2007;32(5):948–53.
Nakamoto H, Kaneko T, Tahara S, Hayashi E, Naito H, Radak Z, Goto S. Regular exercise reduces 8-oxodG in the nuclear and mitochondrial DNA and modulates the DNA repair activity in the liver of old rats. Exp Gerontol. 2007;42(4):287–95.
Viboolvorakul S, Niimi H, Wongeak-in N, Eksakulkla S, Patumraj S. Increased capillary vascularity in the femur of aged rats by exercise training. Microvasc Res. 2009;78(3):459–63.
Leeuwenburgh C, Fiebig R, Chandwaney R, Ji LL. Aging and exercise training in skeletal muscle: responses of glutathione and antioxidant enzyme systems. Am J Physiol. 1994;267(2 Pt 2):R439–45.
Starnes JW, Taylor RP, Park Y. Exercise improves postischemic function in aging hearts. Am J Physiol Heart Circ Physiol. 2003;285(1):H347–51.
Quindry J, French J, Hamilton K, Lee Y, Mehta JL, Powers S. Exercise training provides cardioprotection against ischemia-reperfusion induced apoptosis in young and old animals. Exp Gerontol. 2005;40(5):416–25.
Hammeren J, Powers S, Lawler J, Criswell D, Martin D, Lowenthal D, Pollock M. Exercise training-induced alterations in skeletal muscle oxidative and antioxidant enzyme activity in senescent rats. Int J Sports Med. 1992;13(5):412–6.
Thomas MM, Vigna C, Betik AC, Tupling AR, Hepple RT. Initiating treadmill training in late middle age offers modest adaptations in Ca2+ handling but enhances oxidative damage in senescent rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2010;298(5):R1269–78.
Pushpalatha K, Nishanth K, Sathyavelu RK. Myocardial antioxidant status and oxidative stress after combined action of exercise training and ethanol in two different age groups of male albino rats. Acta Biol Hung. 2007;58(2):173–85.
Vincent HK, Bourguignon C, Vincent KR. Resistance training lowers exercise-induced oxidative stress and homocysteine levels in overweight and obese older adults. Obesity. 2006;14(11):1921–30.
de Gonzalo-Calvo D, Fernández-García B, de Luxán-Delgado B, Rodríguez-González S, García-Macia M, Suárez FM, Solano JJ, Rodríguez-Colunga MJ, Coto-Montes A. Chronic training increases blood oxidative damage but promotes health in elderly men. Age (Dordr). 2013;35(2):407–17.
Aldred S, Rohalu M. A moderate intensity exercise program did not increase the oxidative stress in older adults. Arch Gerontol Geriatr. 2011;53(3):350–3.
Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–205.
Gomez-Cabrera MC, Borras C, Pallardo FV, Sastre J, Ji LL, Vina J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol. 2005;567:113–20.
Hellsten Y, Frandsen U, Orthenblad N, Sjødin B, Richter EA. Xanthine oxidase in human skeletal muscle following eccentric exercise: a role in inflammation. J Physiol. 1997;498(Pt 1):239–48.
Radak Z, Asano K, Inoue M, Kizaki T, Oh-Ishi S, Suzuki K, Taniguchi N, Ohno H. Superoxide dismutase derivative reduces oxidative damage in skeletal muscle of rats during exhaustive exercise. J Appl Physiol. 1995;79(1):129–35.
Ji LL, Leeuwenburgh C, Leichtweis S, Gore M, Fiebig R, Hollander J, Bejma J. Oxidative stress and aging. Role of exercise and its influences on antioxidant systems. Ann N Y Acad Sci. 1998;854:102–17.
Aronson D, Violan MA, Dufresne SD, Zangen D, Fielding RA, Goodyear LJ. Exercise stimulates the mitogen-activated protein kinase pathway in human skeletal muscle. J Clin Invest. 1997;99(6):1251–7.
Gibala MJ, McGee SL, Garnham AP, Howlett KF, Snow RJ, Hargreaves M. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1alpha in human skeletal muscle. J Appl Physiol. 2009;106(3):929–34.
Iemitsu M, Maeda S, Jesmin S, Otsuki T, Kasuya Y, Miyauchi T. Activation pattern of MAPK signaling in the hearts of trained and untrained rats following a single bout of exercise. J Appl Physiol. 2006;101:151–63.
Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20(10):2580–90.
Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122(3):647–57.
Ji LL, Gomez-Cabrera MC, Steinhafel N, Vina J. Acute exercise activates nuclear factor (NF)-kappaB signaling pathway in rat skeletal muscle. FASEB J. 2004;18(13):1499–506.
Vasilaki A, McArdle F, Iwanejko LM, McArdle A. Adaptive responses of mouse skeletal muscle to contractile activity: the effect of age. Mech Ageing Dev. 2006;127(11):830–9. Epub 2006 Sep 22.
McArdle A, Jackson MJ. Exercise, oxidative stress and ageing. J Anat. 2000;197(Pt 4):539–41.
Huffman DM, Moellering DR, Grizzle WE, Stockard CR, Johnson MS, Nagy TR. Effect of exercise and calorie restriction on biomarkers of aging in mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1618–27.
Naito H, Powers SK, Demirel HA, Aoki J. Exercise training increases heat shock protein in skeletal muscles of old rats. Med Sci Sports Exerc. 2001;33(5):729–34.
Simar D, Malatesta D, Koechlin C, Cristol JP, Vendrell JP, Caillaud C. Effect of age on Hsp72 expression in leukocytes of healthy active people. Exp Gerontol. 2004;39(10):1467–74.
McArdle A, Vasilaki A, Jackson M. Exercise and skeletal muscle ageing: cellular and molecular mechanisms. Ageing Res Rev. 2002;1(1):79–93.
Vasilaki A, Jackson MJ, McArdle A. Attenuated HSP70 response in skeletal muscle of aged rats following contractile activity. Muscle Nerve. 2002;25(6):902–5.
Boveris A, Navarro A. Systemic and mitochondrial adaptive responses to moderate exercise in rodents. Free Radic Biol Med. 2008;44:224–9.
Derbré F, Gomez-Cabrera MC, Nascimento AL, Sanchis-Gomar F, Martinez-Bello VE, Tresguerres JA, Fuentes T, Gratas-Delamarche A, Monsalve M, Viña J. Age associated low mitochondrial biogenesis may be explained by lack of response of PGC-1α to exercise training. Age (Dordr). 2012;34(3):669–79.
Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104(29):12017–22.
Lee-Young RS, Griffee SR, Lynes SE, Bracy DP, Ayala JE, McGuinness OP, Wasserman DH. Skeletal muscle AMP-activated protein kinase is essential for the metabolic response to exercise in vivo. J Biol Chem. 2009;284(36):23925–34.
O’Neill HM, Maarbjerg SJ, Crane JD, Jeppesen J, Jørgensen SB, Schertzer JD, Shyroka O, Kiens B, van Denderen BJ, Tarnopolsky MA, Kemp BE, Richter EA, Steinberg GR. AMP-activated protein kinase (AMPK) beta1beta2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise. Proc Natl Acad Sci U S A. 2011;108(38):16092–7.
Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward III JL, Goodyear LJ, Tong Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging. 2009;1(9):771–83.
Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res. 2008;79(2):208–17.
St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408.
Safdar A, Bourgeois JM, Ogborn DI, Little JP, Hettinga BP, Akhtar M, Thompson JE, Melov S, Mocellin NJ, Kujoth GC, Prolla TA, Tarnopolsky MA. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci U S A. 2011;108:4135–40.
Devi SA, Kiran TR. Regional responses in antioxidant system to exercise training and dietary vitamin E in aging rat brain. Neurobiol Aging. 2004;25:501–8.
Somani SM, Husain K. Exercise training alters kinetics of antioxidant enzymes in rat tissues. Biochem Mol Biol Int. 1996;38:587–95.
Marzatico F, Pansarasa O, Bertorelli L, Somenzini L, Della Valle G. Blood free radical antioxidant enzymes and lipid peroxides following long-distance and lactacidemic performances in highly trained aerobic and sprint athletes. J Sports Med Phys Fitness. 1997;37(4):235–9.
Miyazaki H, Oh-ishi S, Ookawara T, Kizaki T, Toshinai K, Ha S, Haga S, Ji LL, Ohno H. Strenuous endurance training in humans reduces oxidative stress following exhausting exercise. Eur J Appl Physiol. 2001;84(1–2):1–6.
Siems WG, Brenke R, Sommerburg O, Grune T. Improved antioxidative protection in winter swimmers. QJM. 1999;92(4):193–8.
Hamilton KL, Powers SK, Sugiura T, Kim S, Lennon S, Tumer N, Mehta JL. Short-term exercise training can improve myocardial tolerance to I/R without elevation in heat shock proteins. Am J Physiol Heart Circ Physiol. 2001;281(3):H1346–52.
Lawler JM, Kwak HB, Kim JH, Suk MH. Exercise training inducibility of MnSOD protein expression and activity is retained while reducing prooxidant signaling in the heart of senescent rats. Am J Physiol Regul Integr Comp Physiol. 2009;296(5):R1496–502.
Yamashita N, Hoshida S, Otsu K, Asahi M, Kuzuya T, Hori M. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J Exp Med. 1999;189(11):1699–706.
Gündüz F, Sentürk UK, Kuru O, Aktekin B, Aktekin MR. The effect of one year’s swimming exercise on oxidant stress and antioxidant capacity in aged rats. Physiol Res. 2004;53(2):171–6.
de Moraes C, Davel AP, Rossoni LV, Antunes E, Zanesco A. Exercise training improves relaxation response and SOD-1 expression in aortic and mesenteric rings from high caloric diet-fed rats. BMC Physiol. 2008;8:12.
Rush JW, Turk JR, Laughlin MH. Exercise training regulates SOD-1 and oxidative stress in porcine aortic endothelium. Am J Physiol Heart Circ Physiol. 2003;284:H1378–87.
Moien-Afshari F, Ghosh S, Elmi S, Khazaei M, Rahman MM, Sallam N, Laher I. Exercise restores coronary vascular function independent of myogenic tone or hyperglycemic status in db/db mice. Am J Physiol Heart Circ Physiol. 2008;295(4):H1470–80.
Chang SP, Chen YH, Chang WC, Liu IM, Cheng JT. Increase of anti-oxidation by exercise in the liver of obese Zucker rats. Clin Exp Pharmacol Physiol. 2004;31:506–11.
Ookawara T, Haga S, Ha S, Oh-Ishi S, Toshinai K, Kizaki T, Ji LL, Suzuki K, Ohno H. Effects of endurance training on three superoxide dismutase isoenzymes in human plasma. Free Radic Res. 2003;37(7):713–9.
Rousseau AS, Margaritis I, Arnaud J, Faure H, Roussel AM. Physical activity alters antioxidant status in exercising elderly subjects. J Nutr Biochem. 2006;17(7):463–70.
Lennon SL, Quindry JC, Hamilton KL, French JP, Hughes J, Mehta JL, Powers SK. Elevated MnSOD is not required for exercise-induced cardioprotection against myocardial stunning. Am J Physiol Heart Circ Physiol. 2004;287(2):H975–80.
Radak Z, Asano K, Inoue M, Kizaki T, Ohishi S, Suzuki K, Taniguchi N, Ohno H. Acute bout of exercise does not alter the antioxidant enzyme status and lipid peroxidation in rat hippocampus and cerebellum. Pathophysiology. 1995;2:243–5.
Ji LL, Wu E, Thomas DP. Effect of exercise training on antioxidant and metabolic functions in senescent rat skeletal muscle. Gerontology. 1991;37(6):317–25.
Higuchi M, Cartier LJ, Chen M, Holloszy JO. Superoxide dismutase and catalase in skeletal muscle: adaptive response to exercise. J Gerontol. 1985;40:281–6.
Hollander J, Fiebig R, Gore M, Ookawara T, Ohno H, Ji LL. Superoxide dismutase gene expression is activated by a single bout of exercise in rat skeletal muscle. Pflugers Arch. 2001;442:426–34.
Ho YS, Howard AJ, Crapo JD. Molecular structure of a functional rat gene for manganese-containing superoxide dismutase. Am J Respir Cell Mol Biol. 1991;4:278–86.
Grune T, Merker K, Jubg T, Sitte N, Davies KJ. Protein oxidation and degradation during postmitotic senescence. Free Radic Biol Med. 2005;39:1208–15.
Sitte N, Merker K, Grune T. Proteasome-dependent degradation of oxidized proteins in MRC-5 fibroblasts. FEBS Lett. 1998;440:399–402.
Radak Z, Kumagai S, Nakamoto H, Goto S. 8-Oxoguanosine and uracil repair of nuclear and mitochondrial DNA in red and white skeletal muscle of exercise-trained old rats. J Appl Physiol. 2007;102(4):1696–701.
Radak Z, Apor P, Pucsok J, Berkes I, Ogonovszky H, Pavlik G, Nakamoto H, Goto S. Marathon running alters the DNA base excision repair in human skeletal muscle. Life Sci. 2003;72:1627–33.
Radak Z, Toldy A, Szabo Z, Siamilis S, Nyakas C, Silye G, Jakus J, Goto S. The effects of training and detraining on memory, neurotrophins and oxidative stress markers in rat brain. Neurochem Int. 2006;49(4):387–92.
Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, Maser RS, Tonon G, Foerster F, Xiong R, Wang YA, Shukla SA, Jaskelioff M, Martin ES, Heffernan TP, Protopopov A, Ivanova E, Mahoney JE, Kost-Alimova M, Perry SR, Bronson R, Liao R, Mulligan R, Shirihai OS, Chin L, DePinho RA. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–65.
Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, Kimura M, Lu X, Spector TD, Aviv A. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 2008;168(2):154–8.
Werner C, Furster T, Widmann T, Poss J, Roggia C, Hanhoun M, Scharhag J, Buchner N, Meyer T, Kindermann W, Haendeler J, Bohm M, Laufs U. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation. 2009;120:2438–47.
Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–6. PubMed: 11303144.
Cannon JG, St Pierre BA. Cytokines in exertion-induced skeletal muscle injury. Mol Cell Biochem. 1998;179(1–2):159–67.
Das UN. Anti-inflammatory nature of exercise. Nutrition. 2004;20:323–6.
Elosua R, Molina L, Fito M, Arquer A, Sanchez-Quesada JL, Covas MI, Ordoñez-Llanos J, Marrugat J. Response of oxidative stress biomarkers to a 16-week aerobic physical activity program, and to acute physical activity, in healthy young men and women. Atherosclerosis. 2003;167(2):327–34. Erratum in: Atherosclerosis. 2008 Apr;197(2):967.
Fleg JL, Schulman SP, O’Connor FC, Gerstenblith G, Becker LC, Fortney S, Goldberg AP, Lakatta EG. Cardiovascular responses to exhaustive upright cycle exercise in highly trained older men. J Appl Physiol. 1994;77(3):1500–6.
Flynn M, McFarlin BK, Markofski MA. The anti-inflammatory actions of exercise training. Am J Lifestyle Med. 2007;1:220–35.
Gohil K, Rothfuss L, Lang J, Packer L. Effect of exercise training on tissue vitamin E and ubiquinone content. J Appl Physiol. 1987;63(4):1638–41.
Hack V, Strobel G, Rau JP, Weicker H. The effect of maximal exercise on the activity of neutrophil granulocytes in highly trained athletes in a moderate training period. Eur J Appl Physiol Occup Physiol. 1992;65(6):520–4.
Handel ML, McMorrow LB, Gravallese EM. Nuclear factor-kappa B in rheumatoid synovium. Localization of p50 and p65. Arthritis Rheum. 1995;38:1762–70.
Huang G, Gibson CA, Tran ZV, Osness WH. Controlled endurance exercise training and VO2max changes in older adults: a meta-analysis. Prev Cardiol. 2005;8:217–25.
Ji LL. Exercise at old age: does it increase or alleviate oxidative stress? Ann N Y Acad Sci. 2001;928:236–47.
Keller C, Keller P, Giralt M, Hidalgo J, Pedersen BK. Exercise normalises overexpression of TNF-α in knockout mice. Biochem Biophys Res Commun. 2004;321:179–82.
Kim SR, Bae YH, Bae SK, Choi KS, Yoon KH, Koo TH, Jang HO, Yun I, Kim KW, Kwon YG, Yoo MA, Bae MK. Visfatin enhances ICAM-1 and VCAM-1 expression through ROS-dependent NF-kB activation in endothelial cells. Biochim Biophys Acta. 1783;2008:886–95.
Kim SY, Moon KA, Jo HY, Jeong S, Seon SH, Jung E, Cho YS, Chun E, Lee KY. Anti-inflammatory effects of apocynin, an inhibitor of NADPH oxidase, in airway inflammation. Immunol Cell Biol. 2012;90(4):441–8.
Kohrt WM, Malley MT, Coggan AR, Spina RJ, Ogawa T, Ehsani AA, Bourey RE, Martin 3rd WH, Holloszy JO. Effects of gender, age, and fitness level on response of VO2max to training in 60-71 yr olds. J Appl Physiol. 1991;71(5):2004–11.
Konopka AR, Sreekumaran Nair K. Mitochondrial and skeletal muscle health with advancing age. Mol Cell Endocrinol. 2013;379(1–2):19–29.
Lambert CP, Wright NR, Finck BN, Villareal DT. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J Appl Physiol. 2008;105(2):473–8.
Lee IT, Luo SF, Lee CW, Wang SW, Lin CC, Chang CC, Chen YL, Chau LY, Yang CM. Overexpression of HO-1 protects against TNF-alpha-mediated airway inflammation by down-regulation of TNFR1-dependent oxidative stress. Am J Pathol. 2009;175(2):519–32.
Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289(5488):2350–4.
Pedersen BK, Ostrowski K, Rohde T, Bruunsgaard H. The cytokine response to strenuous exercise. Can J Physiol Pharmacol. 1998;76(5):505–11.
Petrone WF, English DK, Wong K, McCord JM. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980;77(2):1159–63.
Radák Z, Chung HY, Naito H, Takahashi R, Jung KJ, Kim HJ, Goto S. Age-associated increase in oxidative stress and nuclear factor kappaB activation are attenuated in rat liver by regular exercise. FASEB J. 2004;18(6):749–50.
Radák Z, Naito H, Kaneko T, Tahara S, Nakamoto H, Takahashi R, Cardozo-Pelaez F, Goto S. Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Arch. 2002;445(2):273–8.
Radák Z, Sasvári M, Nyakas C, Pucsok J, Nakamoto H, Goto S. Exercise preconditioning against hydrogen peroxide-induced oxidative damage in proteins of rat myocardium. Arch Biochem Biophys. 2000;376(2):248–51.
Starnes JW, Cantu G, Farrar RP, Kehrer JP. Skeletal muscle lipid peroxidation in exercised and food-restricted rats during aging. J Appl Physiol. 1989;67(1):69–75.
Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74:139–62.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Sallam, N., Laher, I. (2016). Protective Effect of Exercise on Age-Related Oxidant and Inflammatory Events. In: Bondy, S., Campbell, A. (eds) Inflammation, Aging, and Oxidative Stress. Oxidative Stress in Applied Basic Research and Clinical Practice. Springer, Cham. https://doi.org/10.1007/978-3-319-33486-8_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-33486-8_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33484-4
Online ISBN: 978-3-319-33486-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)