Abstract
Aging is multidimensional and the loss of physical activity may be an important reason in a number of age-related diseases. Exercise seems to be a required challenge for the modern urban society in order to maintain a healthy life. An individual’s capacity to utilize oxygen is an indicator of their Reactive Oxygen Species (ROS) generation rate. The common feature of all age-related disorders is the reduced quantity and quality of mitochondria due to the overproduction of ROS. Regular aerobic exercise induces the biogenesis and functionality of mitochondria via various pathways such as peroxisome proliferator-activated receptor-γ coactivator 1 alpha (PGC-1a) during aging. According to recent findings revealing that the sources of aging and exercise-induced ROS are both mitochondrial and extramitochondrial. Although going over of the physiological limits of ROS has detrimental effects, it allows the cells to adapt to exercise-induced stress within individual physiological limits. Thus, regular antioxidant supplementation might be beneficial for the elderly with regards to exercise performance. Albeit redox homeostasis is fundamentally related to aerobic type of exercise, anaerobic exercise has proven to be beneficial during aging. Regular exercise of low intensity and short duration, that targets endurance, strength, balance, and flexibility, would be effective in enhancing quality of life and aging longevity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Life expectancy and advancement in the healthcare system have been improving coordinately for the last 200 years, and the current research has been targeting a further improvement in quality of life particularly in the context of elderly mortality (Madreiter-Sokolowski et al. 2020). Although the advancement in health systems, including vaccination development can lead to a healthier lifestyle, the prevalence of age-related complications remains high. The misdiagnosis of health problems being age-related for the general population by the scientific community is among the factors that hinder the understanding of the aging process and diseases. Recent papers have recommended that external methods such as behavioral changes can influence delayed aging and diseases. Exercise is one of the main behavioral strategies that can delay aging and various age-related chronic diseases including cardiovascular diseases, obesity, diabetes and neurodegenerative diseases such as Alzheimer’s or Parkinson’s diseases (Ezzati et al. 2008; Booth et al. 2012; Jaul and Barron 2017). However, there is no specific exercise-linked mechanism that delays aging and age-related diseases that has been so far established. This demands us to focus on other mechanistic theories of how aging processes are regulated. The most studied theory so far in aging is the oxidative stress theory of aging first postulated by Denham Harman in the 1950s which is otherwise called the free radical theory of aging, an idea that tells that production of oxygen-free radicals during the metabolic process can regulate the aging phenomenon through inducing oxidative damage in the cellular structures. However, recent studies contradict this concept that within a certain level of ROS can regulate various signaling which leads to a delay in the aging processes and diseases. During oxidative metabolism, the superoxide radical anion (O2−) formation is the initial formation that further produces various ROS either by chemical reactions or by cellular enzymes resulting in redox shifts. For the human coping system to deal with oxidative stress, powerful antioxidants including superoxide dismutase (SOD), catalase (CAT), and glutathione system (GSH) can intoxicate ROS or make O2− to become less reactive ROS or inert products. These less reactive ROS such as hydrogen peroxide (H2O2) may function as a signal or mimic some important signaling including age-related signaling. For example, a common oxidative product of methionine named methionine sulfoxide increases during aging, and it can be restored by the enzyme called methionine sulfoxide reductases (MSR) A and B. The overexpression of MSR is linked with an increased stress resistance and extended lifespan, but the expression of these antioxidants during aging may be limited, which demands additional factors that could induce this enzyme or keep the oxidative stress at the physiological level. Physical exercise is one of the factors that can maintain cells under a physiological oxidative stress level.
Advanced technologies have generally increased a physically inactive lifestyle. Exercise is a required challenge for the elderly in order to keep up homeostasis which demands an integrated organ systems response, supporting the form, structure, and functions of the body. Exercise is a keyway to sustain physical activity at an optimum level. The general types of exercise are strengthening, stretching, balance, and aerobic exercise. Being healthy necessitates all of these. However, aerobic exercise has a special emphasis on aging and the metabolism of free radicals. Aerobic exercise, i.e., endurance exercise, is any type of repetitive activity which provides work to the lungs, cardiac, and skeletal muscles. In other words, it requires a higher rate of oxygen consumption and mitochondrial activity, which facilitates higher ROS production, while exercises like strengthening can produce site-specific oxidative stress, which may not reflect on the entire organism resulting in ROS-mediated signaling failure. The failure in the implementation of exercise regimens for the general society of aging people leads to inactivity induced age-related diseases. Mismanaging exercise regimens such as timing, duration, intensity, and individual training status may additionally lead to inducing pathological changes in the cells instead of maintaining ROS homeostasis. This scenario allows any organism to become more prone to age-related disorders and apoptosis instead of delaying aging. Focusing on all these aspects not only provides better management of increasing muscle strengthening and gait training, but also provides protection against aging and age-related diseases.
2 Aging, Exercise, and Free Radicals
Accelerated free radical generation may occur as a response to acute and habitual exercise. Previous studies investigated that physical activity positively affects the quality of life via decreasing age-related physical inactivity, frailty, and supporting mental performance in aged rats but has no effect on extending their life span (Deepa et al. 2017; Cao et al. 2012). Also, it was demonstrated that exercise does have health benefits, due to the resulting optimal physiological rate of ROS formation.
In addition, excessive amounts of antioxidants supplementation have been widely preferred to reduce the detrimental effects of free radicals generated during exercise. However, scientific findings have revealed contrary results. It has been demonstrated that a megadose of vitamin C supplementation in training inhibits exercise adaptation, mitochondrial biogenesis, and the synthesis of antioxidant enzymes (Gomez-Cabrera et al. 2008). Later, Ristow et al. (2009) stated that antioxidant intake during exercise hindered exercise-related physical performance enhancement and overall health improvement.
3 Exercise-Induced Extramitochondrial ROS
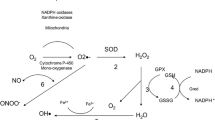
Albeit mitochondria are generally accepted as the source of free radical formation in aging, recently scientists believe that this radical-centered aging theory has lost its importance. Aging is probably a multidimensional cause process. Mitochondria are also considered as the ROS formation center during exercise. Indeed, recent scientific findings suggest that there are other sources of free radical formation in exercise. Even the relationship between free radical metabolism and exercise during aging still requires further studies, while the beneficial effects of exercise on all physiological systems is unquestionable. The most studied tissues related to ROS formation in exercise are the heart, lungs, white blood cells, and skeletal muscles (Gomez-Cabrera et al. 2009; Powers and Jackson 2008). Superoxide and nitric oxide (NO) are the radicals mainly formed in cells and both can potentially react to generate a series of other ROS formations and reactive nitrogen species (RNS). As mentioned above, there may be other sources for ROS formation outside of mitochondria in exercise, such as nicotinamide adenine dinucleotide phosphate (NAD(P)H) oxidase (NOX) from transverse tubules (T-tubules), the sarcolemma, the sarcoplasmic reticulum, and neutrophils secondarily, via myeloperoxidase. As the family members of NOX are differentially expressed among tissues, NOX1 to NOX5 and DUOX1 and DUOX2 make up the NOX family while DUOX1 and DUOX2 are homologs of NOX2, which are composed of the NOX-like region at the C-terminal half, two EF-hands, a membrane-spanning region, and a peroxidase-like domain at the N terminus. Therefore, they are called dual-oxidase, DUOX (Katsuyama et al. 2012). NOX1, NOX3, and NOX5 form a superoxide, while DUOX1, DUOX2, and NOX4 seem to mainly produce H2O2 (Katsuyama 2010). NOX2, NOX4, and their partner proteins (p22phox, p67phox, p47phox, and p40phox) are expressed in skeletal muscle (Cheng et al. 2001) and are found in T-tubules, the sarcolemma, and the sarcoplasmic reticulum while NOX4 also exists in mitochondria (Sakellariou et al. 2013). The flavin adenine dinucleotide (FAD) and NADPH-binding sites and six transmembrane alpha-helices with cytosolic N- and C-termini exist in NOX isoforms (Bedard and Krause 2007) which seem to play significant roles in the skeletal muscle fibers’ adaptation to exercise in response to physiological stimuli (Sakellariou et al. 2013). In contrast, the overactivity of NO synthase (NOS) isoforms in glycolytic muscle causes atrophy in experimental rats (Cunha et al. 2017). In this process, the NF-κB was activated and the p38 phosphorylation was increased while on the other hand, aerobic exercise training diminished p38 phosphorylation and NF-κB activation. The aforementioned aerobic exercise was of a long-term, low-intensity exercise in which the mitochondrial energy system was used more actively. This type of exercise might cause ROS formation and the activation of upper cascade molecules of PGC1a, which might lead to a PGC1a expression. The PGC1a might have activated the expression of antioxidant enzymes with the genes involved in mitochondrial biogenesis in oxidative muscle as the antioxidant enzymes’ activities are very low in glycolytic muscle fibers. The antioxidants expressed in slow-twitch muscles by way of aerobic exercise detoxify over-formed ROS in the cellular environment since a superoxide is very labile and reduced to H2O2. This non-radical, weak oxidant is more stable and can diffuse across cell membranes. H2O2 also generates the hydroxyl radical (OH·) by the Fenton Reaction if there is free iron or transition metals in the cellular environment. Iron is freely present in skeletal muscle at a rate of 10–15% and is mostly found in mitochondria and myoglobin while OH·, which is highly reactive, is known to cause damage to lipids, proteins, and DNA. Nevertheless, H2O2 is considered as second messenger due to its feature of being more stable and more suitable for intercellular transfer.
The necessity of phospholipase A2’s function has been shown in both tiring and repetitive muscle contractions (Gong et al. 2006). Phospholipase A2 enzyme enhances intercellular ROS formation while also causing the activation of NAD(P)H oxidase enzymes and is effective in separating arachidonic acids from membranes. Arachidonic acids are a substrate for ROS-forming enzyme systems like cyclooxygenase and lipoxygenase. There are two types of phospholipase A2 enzyme isoforms in skeletal muscles: calcium-dependent and calcium-independent. Both of these isoforms are active in the formation of ROS in muscle contraction while only calcium-independent isoform supports the formation of cytosolic ROS (Zhao et al. 2002), only calcium-dependent isoform plays a role in generating mitochondrial ROS in skeletal muscle (Zhao et al. 2002). Furthermore, calcium-independent phospholipase A2 is found in the myotubes of adult mice tissues lile, diaphragm, soleus, extensor digitorum longus, gastrocnemius, and heart. The expression and protein levels of phospholipase A2 muscular cells exhibit no difference in levels among rat muscles. Phospholipase A2 was reported to arrange cytosolic oxidant activity and contractile function in murine skeletal muscle cells while the blockage of phospholipase A2 caused reduced cytosolic oxidant activity in myotubes and intact soleus muscle fibers. As a result, a diminished soleus muscle function and depressed force production with a rightward shift in the force–frequency relationship was observed. The scientists also reported all these changes could be repeated by the depletion of superoxide anions (Gong et al. 2006). The findings of this research show that calcium-independent phospholipase A2 regulates the oxidant activity of skeletal muscle by supporting the formation of ROS, and thus, it might affect muscle contraction and fatigue during physical activity.
The xanthine oxidase (XO) is the most ROS productive enzymatic system among the other enzyme systems and exists in most tissues and organs as well as in the vascular endothelium. XO delivers electrons to molecular oxygen and forms O2 ·– by a one-electron reduction or H2O2 by a two-electron reduction. Then, hydroxyl radicals can be generated in the presence of iron or other transition metals. Vida et al. (2011) investigated the activity and expression of the XO in the liver, kidney, and thymus of different age groups of mice, including long-lived ones. A higher activity and expression of XO were determined in all the tissues of old mice. The inhibition of XO by allopurinol revealed that XO may have a special relevance in the formation of H2O2 in older animals. Furthermore, among the other ROS generating enzymes, XO had the highest activity and expression amongst all tissues, therefore XO might play an important role in the etiology of aging. Additionally, when Lee et al. (2009) reviewed ischemia–reperfusion and heart failure in association with XO, they reported the inhibition of XO diminishes the formation of ROS as well as uric acid production in ischemia and reperfusion injury. During exercise, hypoxia occurs at tissue level for short periods of time so, XO might be more effective during these times as XO was informed as an oxidant producer in high-intensity intermittent exercise (Gomez-Cabrera et al. 2006). This type of exercise fundamentally meets its energy requirement from anaerobic glycolysis. Low levels of ATP results in an accumulation of hypoxanthine and xanthine and conversion of xanthine dehydrogenase to XO. This process is a step for O2 generation in the replenishment of oxygen to a relatively hypoxic muscle (Kaminsky and Kosenko 2009). By inhibiting XO by allopurinol, a reduction in muscle oxidative stress after strenuous exercise in both rats and humans was observed (Gomez-Cabrera et al. 2010), though human skeletal muscle has low levels of xanthine dehydrogenase or oxidase (Hirschfield et al. 2000). These outcomes suggest that XO might generate ROS effectively in anaerobic exercise. Moreover, the decrease in lung capacity and the consumption of less oxygen during aging may be one of the reasons why this enzyme is more effective in ROS production in the elderly.
4 Exercise-Induced NO
Nitric oxide, which is a highly diffusible gas, is synthesized via NO synthase’s enzymes in a number of cell types. There are three NOS isoforms: neuronal NOS (nNOS/NOS1); endothelial NOS (eNOS/NOS3); and inducible NOS (iNOS/NOS2). L-Arginine is converted into NO and citrulline via these NO synthases. NO reacts with oxygen to form nitric dioxide and reacts with superoxide to generate peroxynitrite which is a strong oxidizing agent. Albeit, NO is synthesized in various cells, it has an important role in the cardiovascular system, nervous system, and skeletal muscle contraction. NO diminishes leukocytes adhesion, aids vasodilation, and lessens thrombosis and apoptosis. Arterial hypertension, atherosclerosis, heart failure, and neurodegenerative diseases have endothelial dysfunction, which are related to a reduction in NO bioavailability in aging while eNOS expression and activity decrease in aged vascular system. Indeed, aged endothelial dysfunction is related to a higher expression of proinflammatory enzymes, i.e., cyclooxygenase-2 (COX-2) and iNOS. Augmented NO synthesis is based on iNOS, which reacts with ROS to produce RNS and deforms post-translational modification of proteins. All these result in a higher vasoconstriction tonus in the arteries of the elderly (Novella et al. 2013).
All three NOS enzymes are found in skeletal muscle. The eNOS and nNOS are Ca2+-dependent isoforms, while iNOS is Ca2+-independent. These three enzymes are normally responsible for NO synthesis at a lower physiological level. NO, like ROS, shows its toxic effects depending on its levels. When NO is expressed in high levels in the cellular environment, it shows its toxic effects. The nNOS isoform is expressed at a higher ratio in fast-twitch muscle fibers when compared with slow-twitch fibers (Reid 1998). The iNOS expression rate increases with respect to aging. In other words, iNOS’s role in muscle contractile function decreases while increasing its inflammatory role (Song et al. 2009). It is informed that NO synthesis increases during muscle contraction, while muscle fibers form lower physiological levels of NO during inactivity (Pye et al. 2007). Moreover, NOS inhibitors lead to an increase of muscle force production, though NO providers cause a depression in muscle force generation during submaximal tetanic contractions (Andrade et al. 1998). Song et al. (2009) investigated the effects of endurance exercise on NOS enzymes in the soleus and white gastrocnemius muscles in aged rats. In aged, sedentary rats, the protein level and enzyme activity of iNOS was found to be high in both fast- and slow-twitch muscles while the protein level of nNOS was found to be lower in each muscle, the protein level of eNOS was only found to be lower in the white gastrocnemius. In addition, after endurance exercise, an increase in the protein level of nNOS was detected in both muscles, while an increase in the protein level in the fast-twitch muscle was detected only in the eNOS enzyme. When the eNOS and nNOS enzyme activities were evaluated together after exercise, only in the white gastrocnemius muscle was an increase found. No significant change in the protein level and enzyme activity of iNOS were found in both muscles from endurance exercise (Song et al. 2009). This study was supported by another research. Chung et al. (2001) reported that a caloric restriction diminished age-related augmentations in the proinflammatory mediator cytokines, iNOS, and NF-kB in the kidney, heart, and brain. In summary, the findings show that there is an increased iNOS expression in the brain, skeletal muscle, and endothelial cells, though there is a decreased expression of nNOS and eNOS in these tissues. Unlike nNOS and eNOS, that are responsible for lower levels of NO synthesis, iNOS is responsible for a higher ratio of NO synthesis. In the study of Song et al. (2009), endurance exercise increased the expression of the nNOS and eNOS ratios in the fast-twitch muscle, while there was no change in the expression level of these enzymes in the slow-twitch muscle. These findings show that different mechanisms may be more effective in the slow-twitch muscle during aging.
5 Aging, Exercise, and Nrf2 Signaling
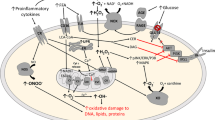
Redox shift, inflammation, and mitochondrial dysfunction alter the way of signaling pathways from the way they should behave in aging cells which finally contribute to degrading the molecular process and manifest age-related pathologies. Studies have reported that ROS can mediate to crosstalk these signaling pathways (Silva-Palacios et al. 2018; Yanar et al. 2020). However, how this is orchestrated during aging is not well established particularly at the transcriptional level of the antioxidants’ expressions. The transcription factor nuclear erythroid-2-p45-related factor-2 (Nrf-2) is not only the master regulator of antioxidants, but it can also contribute to regulate several cytoprotective genes (approximately 250) including detoxifying enzymes and drug transporters (Silva-Palacios et al. 2018). Generally, Nrf-2 is coupled with the cysteine-rich Keap-1 protein which is associated with the Cullin-3 (Cul-3) and Ring-box protein. This scenario leads to Nrf2 ubiquitination and proteasomal degradation. ROS exposure induces the cysteine oxidation within the Keap-1 (Cys 151, Cys273, Cys288), which results in the release of Nrf2 and the further re-establishment of redox status. The activation of Nrf2 is not only dependent on ROS exposure, but also depends on aging. Nrf2 expression declines with aging and this decrease may be related to the decrease of Nrf-2 target genes such as quinone 1 (NQO1) and heme-oxygenase 1 (HO-1) (Done et al. 2016). Furthermore, an external stimulus can activate Nrf2 and extend its half-life. Exercise is a known external stimulus that induces various stresses on the skeletal system such as oxidative, mechanical, and thermal stresses. Therefore, it is clear that exercise can induce a redox balance; however, how the exercise protocol can activate Nrf2-ARE pathway and what is the duration or intensity necessary to activate the Nrf2-ARE pathway remains unknown. Yet, studies have shown that Nrf2 activation depends on exercise duration (>60 min of exercise) (Li et al. 2015; Wang et al. 2016). However, the duration could be varied even more during the condition of aging as elderly individuals may respond poorly to this redox balance. Consequently, Nrf2 accumulation and its stability becomes lower with aging, suggesting that there is an important role to Nrf2 and biological aging (Yates et al. 2007). It was observed that acute exercise impaired the Nrf2 nuclear accumulation and its downstream antioxidant genes in the aging animals, but moderate 6-week exercise restores the Nrf2 accumulation and antioxidant genes (Gounder et al. 2012). However, studies have shown that exercise only influences the gene expression rate during aging, and does not impact the level of protein response such as SOD1 and HMOX to exercise (Done et al. 2016). This may be due to having a defect in the sampling collection which will delay in the detection of protein after the exercise stimulus, suggesting that the change in the findings of the protein synthesis need several hours or even days or weeks after exercise. Nonetheless, exercise’s influence on mRNA expression within hours of exercise is assumed to be as a response to aging (Done et al. 2016). Therefore, focusing on exercise duration and the number of stimuli that influence Nrf2 expression and by following its target genes in the subjects will pave the way to developing exercise as non-pharmacological approach to preventing aging and diseases (Fig. 6.1). The Nrf-2 and further antioxidant activation via the redox balance during exercise is demonstrated in Fig. 6.1.
Schematic illustration represents that an exercise-induced redox shift activates the Nrf-2 and a further antioxidant response, which results in a regulation of the molecular processes and delays aging and prevents diseases. Nrf2: nuclear erythroid-2-p45-related factor-2; ARE: antioxidant response element; Keap-1: Kelch-like ECH-associated protein 1
6 Exercise-Induced Mitochondrial ROS
The mitochondria control cell cycles, various aging processes, redox homeostasis, and apoptosis. Exercise is one of the non-pharmacological interventions for alleviating age-related impaired redox status. Oxidative metabolism in the mitochondria is the major source of ROS along with producing ATP through oxidative phosphorylation. Approximately 0.2–2% of molecular oxygen undergoes a one-electron reduction into superoxide radicals in the respiratory complexes I and III. This is further converted into singlet oxygen or H2O2.
An increase in mitochondrial ROS is linked with oxidative damage and a loss of mitochondrial membrane potential which further releases pro-apoptotic factors and induces apoptosis. ROS is also linked with telomere shortening and DNA damage. Biological aging is characterized by the increase in mitochondrial ROS, mutation in the mitochondrial genome, morphological abnormalities in the mitochondria, and ATP synthesis impairment. As mentioned above, exercise can be the rejuvenating method that can alter ROS-mediated intracellular signaling, decrease the energy ratio (NAD/NADH and AMP/ATP), and increase the release of several circulatory factors which all coordinately activate mitochondrial biogenesis pathways such as PGC-1a, SIRT1, and AMPK and antioxidant defense genes including Nrf2-Keap-1. Further, exercise strengthens the immune system and contributes to the removal of damage which ultimately increases the functional capacity of the organs. Aerobic exercise is the gold-standard method to increase mtDNA replication, protein expression, enzymatic antioxidants, and ATP synthesis. For example, Viña et al. (2009) reported that aerobic training improves mitochondrial biogenesis (PGC-1a and Nrf1) and increases the respiratory complex capacity during aging. One of the important things to consider is that the reduction in mitochondrial content is associated with aging or the increased inactivity that comes with aging. Furthermore, whether exercise has an important role in maintaining mitochondrial content and function or whether exercise can aid in the recovery of mitochondrial content during aging is not known. Indeed, scientific findings show that aerobic or anaerobic exercise training is effective in preserving both mitochondrial content and function and has positive effects on the quality and quantity of mitochondria in all age groups. Indeed, prolonged moderate-intensity aerobic exercise at 50–70% of VO2max for 12 weeks showed an increased mitochondrial quality, NADH oxidase, and succinate oxidase enzyme activity levels in the elderly (Menshikova et al. 2005). In a similar manner, regular anaerobic exercise, i.e., strength training, enhanced mitochondrial quality with potentially increased efficiency in mitochondrial complex I and complex II with respect to maximum electron transfer (Porter et al. 2014). Furthermore, progressive strength exercises at 50–75% of one-repetition maximum decrease mtDNA deletions in older adults (Tarnopolsky 2009). Tarnopolosky also reported that strength training partially activated satellite cells, which fused with the myofiber and brought in undamaged wildtype mtDNA. These studies illustrate that both aerobic and anaerobic exercise models have positive effects on mitochondrial content and function. Both types of exercise in these studies are of moderate intensity: Aerobic exercise is at 50–70% of VO2max while anaerobic exercise is at 50–75% of one-repetition maximum. It is expected that ROS formation rates in these exercise types might have been at a lower level. Radak et al. (2005) proposed to apply the hormesis theory to ROS, which appears to plateau during exercise. ROS at lower levels has a modulation effect on the cellular environment like as in signaling, receptor stimulation, and enzymatic stimulation, though an over-generated ratio of ROS damages macromolecules and may result in apoptosis or necrosis.
7 Aging, Exercise, and PGC1a Signaling
PGC-1a can be regulated by multiple signaling pathways during endurance exercise. Silent mating type information regulation 2 homolog 1 (SIRT1), AMP-activated protein kinase (AMPK), calcineurin A, Ca2+/calmodulin-dependent protein kinases, p38 MAPK, NO, and ROS can activate PGC-1a (Erlich et al. 2016; Philp and Schenk 2013; Canto et al. 2009; Serrano et al. 2001; Puigserver et al. 2001). The activation of PGC-1a during exercise is also partially aided by β-adrenergic signaling (Miura et al. 2007), while the cAMP and cAMP response element-binding protein (CREB) seems to activate a PGC-1a expression (Herzig et al. 2001). The activation of AMPK is associated with diminished levels of ATP during exercise in muscles. AMPK either directly phosphorylates PGC-1α or activates it via promoting Sirt1 (Canto et al. 2009). Muscle contractions activate the Ca2/calmodulin-dependent serine/threonine protein phosphatase calcineurin A and Ca2/calmodulin-dependent protein kinases (Serrano et al. 2001). p38 MAPK phosphorylates and activates PGC-1a (Puigserver et al. 2001), which seems to be in the center of exercise-induced PGC1a regulation (Fig. 6.2). The regulation of PGC1a during endurance exercise is illustrated in Fig. 6.2.
Schematic illustration represents endurance exercise-induced signaling pathways, ROS and RNS activation of the PGC1a, and further gene expressions, which results in regulating mitochondrial biogenesis. ROS: reactive oxygen species; NO: nitric oxide; PGC1a: peroxisome proliferator-activated receptor-γ coactivator 1 alpha; AMPK: AMP-activated protein kinase; SIRT1: silent mating type information regulation 2 homolog 1; Nrf2: nuclear erythroid-2-p45-related factor-2; Tfam: mitochondrial transcription factor A; p38 MAPK: p38 mitogen-activated protein kinases; eNOS: endothelial nitric oxide synthase; nNOS: neural nitric oxide synthase; ATP: adenosine triphosphate; AMP: adenosine monophosphate; NAD: Nicotinamide adenine dinucleotide; NADH: Nicotinamide adenine dinucleotide phosphate
Muscle contraction causes NO generation via both eNOS and nNOS. Both have been reported to be activated by Ca2/calmodulin and AMPK (Pattwell et al. 2004; Tatsumi et al. 2009; Close et al. 2005). However, PGC-1a activation through NO seems to be blunted during exercise. Exercise induces activity in all three NOS enzymes isoforms (endothelial, neural, and inducible isoforms) and the generation of NO. Boushel et al. (2012) reported that NO competes with oxygen in order to bind to a 3-heme-site of cytochrome oxidase at a lower physiological level and reversibly decreases the oxygen consumption of mitochondria. However, NO forms peroxynitrite at a higher level, which irreversibly inhibits complex I and II of the electron transport chain. On the other hand, the upregulation of the slow-twitch myosin heavy chain (MHC I) during overload exercise necessitates NO (Sellman et al. 2006) by the activation of Akt and glycogen synthase kinase-3 (GSK-3) and CnA/NFAT-dependent signaling (Drenning et al. 2008).
Ca2/calmodulin and AMPK activate eNOS and nNOS in skeletal muscle (Tatsumi et al. 2009). In addition, it is reported that NO interacts with AMPK which cooperatively regulate PGC-1α in skeletal muscle cells (McConell et al. 2010). Endurance exercise induces a PGC-1a expression which cannot be hindered by either the deletion of eNOS (or nNOS) genes in mice or the inactivation of NOS enzymes in rats (Wadley and McConell 2007). These outcomes suggest that NO plays to some extent a regulatory role in PGC-1a, but there are some alternative ways to exercise-induce the upregulation of PGC-1a (Fig. 6.2).
8 Exercise and Antioxidant Defense
Free radicals are products of normal cellular function and the natural physiological process. They have both beneficial and toxic effects depending on their cellular levels in the metabolism. When free radicals are over-generated, i.e., when they exceed the antioxidant capacity, their harmful effects cannot be avoided. Antioxidants are basically classified into two groups as endogenous and exogenous. Exogenous antioxidants are nutritionally taken by one’s diet and/or supplements. Endogenous antioxidants are synthesized by the body, and this group of antioxidants is further divided into two subgroups, enzymatic and nonenzymatic. The former group consists of superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase (GR), and catalase (CAT). Nonenzymatic antioxidant group includes glutathione (GSH), protein thiol groups, thioredoxin (TRX), ubiquinone/CoQ10, uric acid, lipoic acid, bilirubin (Kayali et al. 2007, 2009). Some of the free radicals which are formed in the oxidative metabolism may escape from the control of the endogenous antioxidant system. Thus, it may cause oxidative damage to the surrounding mitotic and post-mitotic tissues, and ultimately initiate the molecular aging process (Cakatay et al. 2003). Thavanati et al. (2008) reported that muscular glutathione S-transferase (GST), SOD, and catalase activities were significantly reduced in elderly people. All of this information suggests that elderly individuals are more susceptible to oxidative damage due to their diminished muscular antioxidant defense capacity.
The effects of exercise on antioxidant mechanisms in the elderly are still obscure. Lambertucci et al. (2007a, b) reported that the activities of the CAT, GPX, and Cu,Zn- and Mn-superoxide dismutase (Cu,Zn-SOD) were not changed in the soleus of aged rats, whereas the activities of the Mn-SOD and XO were found to be decreased. Furthermore, the same groups of authors reported that the expression levels of CAT, GPX, and Cu,Zn-SOD were significantly elevated in the soleus of aged rats. There may be many reasons why the outcomes of Lambertucci et al.’s study were unexpected. Some of the possible reasons can be listed as the duration, intensity, and type of exercise, as well as differences in tissue and ages.
As mentioned earlier, exercise-induced ROS formation enhances the expression of PGC1a, which leads to the upregulation of endogenous ROS-scavenger enzymes (St-Pierre et al. 2003, 2006). Moreover, exercise-induced ROS also activates the nuclear factor kappa-B (NF-kB), which has a role in the regulation of antioxidant enzyme coding gene clusters (Lingappan 2018). Furthermore, NF-kB is believed to be an important regulator of muscular adaptation to exercise stress (Cuevas et al. 2005). NF-kB is also reported to modulate redox homeostasis via the upregulation of the expression of antioxidant enzymes (Jarosz et al. 2017). Indeed, elevated muscular antioxidant enzyme activity illustrates a higher tolerance to exercise-induced ROS formation (Pittaluga et al. 2006). An increased muscular antioxidant enzyme activity was reported in aged rodents which undertook a habitual endurance and strength exercise regime (Lambertucci et al. 2007a, b).
Previous studies indicate that the benefits of antioxidant supplements in exercise is controversial as the administration of antioxidants negatively affect the adaptation process of skeletal muscle to endurance exercise (Morrison et al. 2015; Paulsen et al. 2014). On the other hand, antioxidant supplementation may interfere with the muscular redox-signaling pathways during endurance exercise (Gomez-Cabrera et al. 2015).
The administration of antioxidants may be beneficial for elderly individuals during exercise. It was reported that the administration of resveratrol ameliorates the aging-related insufficiency in physical performance in elderly individuals, who have regular exercise habits (Murase et al. 2009). Moreover, the intake of vitamin C enhances the physical performance capacity in the elderly (Saito et al. 2012). However, Nalbant et al. (2009) reported that the supplementation of vitamin E over a 6-month period has no extra beneficial effects on physical performance as well as aerobic exercise. In brief, both of the exercises’ effects on the endogenous antioxidant system and the benefits of exogenous antioxidant supplementation during exercise by the elderly are thought-provoking. Hopefully, future studies will enlighten all these unresolved matters.
9 Conclusion
Aging is linked with several degenerative processes which are related with restricted mobility and a compromised quality of life. At the same, finding a target for pharmacotherapy is not fully achievable since aging corresponds to multifactorial targets including cellular and molecular mechanisms. Redox homeostasis management could be the core of either initiating aging and diseases or regulating age-related signaling. A slight increase in the level of ROS could facilitate the adaptation of cells to a better homeostatic status through activating various redox sensitive signaling mechanisms, by having an effective antioxidant defense system in the cell since the redox balance is significantly altered in the elderly as aging cells are prone to an impaired redox homeostasis and aberrant signaling. Exercise is one of the non-pharmacological approaches that induces an antioxidant enzyme expression and scavenger activity. It was proven that exercise could produce an over formation of ROS. The mismanagement of exercise protocols is linked with an unregulated ROS production and cellular damage. With advancements in techniques developed for the assessment of ROS, exercise-induced ROS sources (including its further pleiotropic effect on cellular signaling), metabolic functions, transcriptional factors, we now know that exercise-induced ROS aids in the cellular adaptations to exercise.
However, ROS has complex interactions with metabolome, which may be the core of several degenerative diseases and disorders. Also, the overall effects of exercise on ROS-aging cell interaction still remain a mystery. For example, we still do not know at what exercise-induced production rate of ROS would be beneficial to the overall redox status and how it could be maintained in aging cells. Exercise professionals need to design exercise regimens carefully with respect to the regulation of the redox status which occurs during aging as the regulation of exercise needs added attention for establishing an optimum redox status.
References
Andrade FH, Reid MB, Allen DG et al (1998) Effect of nitric oxide on single skeletal muscle fibres from the mouse. J Physiol 509(2):577–586. https://doi.org/10.1111/j.1469-7793.1998.577bn.x
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87(1):245–313. https://doi.org/10.1152/physrev.00044.2005
Booth FW, Roberts CK, Laye MJ (2012) Lack of exercise is a major cause of chronic diseases. Compr Physiol 2:1143–1211. https://doi.org/10.1002/cphy.c110025
Boushel R, Fuentes T, Hellsten Y et al (2012) Opposing effects of nitric oxide and prostaglandin inhibition on muscle mitochondrial Vo(2) during exercise. Am J Physiol Regul Integr Comp Physiol 303:R94–R100. https://doi.org/10.1152/ajpregu.00044.201
Cakatay U, Telci A, Kayali R et al (2003) Relation of aging with oxidative protein damage parameters in the rat skeletal muscle. Clin Biochem 36(1):51–55
Canto C, Gerhart-Hines Z, Feige JN et al (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458(7241):1056–1060. https://doi.org/10.1038/nature07813
Cao X, Zhao ZW, Zhou HY et al (2012) Effects of exercise intensity on copy number and mutations of mitochondrial DNA in gastrocnemus muscles in mice. Mol Med Rep 6:426–428. https://doi.org/10.3892/mmr.2012.913
Cheng G, Cao Z, Xu X et al (2001) Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5 269(1–2):131–140. https://doi.org/10.1016/s0378-1119(01)00449-8
Chung HY, Kim HJ, Kim JW et al (2001) The inflammation hypothesis of aging: molecular modulation by calorie restriction. Ann N Y Acad Sci 928:327–335
Close GL, Ashton T, McArdle A et al (2005) Microdialysis studies of extracellular reactive oxygen species in skeletal muscle: factors influencing the reduction of cytochrome c and hydroxylation of salicylate. Free Radic Biol Med 39:1460–1467. https://doi.org/10.1016/j.freeradbiomed.2005.07.009
Cuevas MJ, Almar M, Garcia-Glez JC et al (2005) Changes in oxidative stress markers and NF-κB activation induced by sprint exercise. Free Radic Res 39:431–439. https://doi.org/10.1080/10715760500072149
Cunha TF, Bechara LRG, Bacurau AVN et al (2017) Exercise training decreases NADPH oxidase activity and restores skeletal muscle mass in heart failure rats. J Appl Physiol 122(4):817–827. https://doi.org/10.1152/japplphysiol.00182.2016
Drenning JA, Lira VA, Simmons CG et al (2008) Nitric oxide facilitates NFAT-dependent transcription in mouse myotubes. Am J Physiol Cell Physiol 294:C1088–C1095. https://doi.org/10.1152/ajpcell.00523.2007
Done AJ, Gage MJ, Nieto NC, Traustadóttir T (2016) Exercise-induced Nrf2-signaling is impaired in aging. Free Radic Biol Med 96:130–138
Deepa SS, Bhaskaran S, Espinoza S et al (2017) A new mouse model of frailty: the Cu/Zn superoxide dismutase knockout mouse. Geroscience 39(2):187–198. https://doi.org/10.1007/s11357-017-9975-9
Erlich AT, Tryon LD, Crilly MJ et al (2016) Function of specialized regulatory proteins and signaling pathways in exercise-induced muscle mitochondrial biogenesis. Integr Med Res 5:187–197. https://doi.org/10.1016/j.imr.2016.05.003
Ezzati M, Friedman AB, Kulkarni SC, Murray CJ (2008) The reversal of fortunes: trends in county mortality and cross-county mortality disparities in the United States. PLoS Med 5(4):e66
Gomez-Cabrera MC, Martinez A, Santangelo G et al (2006) Oxidative stress in marathon runners: interest of antioxidant supplementation. Br J Nutr 96(1):S31e3. https://doi.org/10.1079/bjn20061696
Gomez-Cabrera MC, Salvador-Pascual A, Cabo H et al (2015) Redox modulation of mitochondriogenesis in exercise. Does antioxidant supplementation blunt the benefits of exercise training? Free Radic Biol Med 86:37–46. https://doi.org/10.1016/j.freeradbiomed.2015.04.006
Gomez-Cabrera MC, Domenech E, Romagnoli M et al (2008) Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr 87(1):142–149. https://doi.org/10.1093/ajcn/87.1.142
Gomez-Cabrera MC, Vina J, Ji LL (2009) Interplay of oxidants and antioxidants during exercise: implications for muscle health. Phys Sportsmed 37:116e23. https://doi.org/10.3810/psm.2009.12.1749
Gomez-Cabrera MC, Close GL, Kayani A (2010) Effect of xanthine oxidase-generated extracellular superoxide on skeletal muscle force generation. Am J Physiol Regul Integr Comp Physiol 298:R2e8. https://doi.org/10.1152/ajpregu.00142.2009
Gong MC, Arbogast S, Guo Z et al (2006) Calcium-independent phospholipase A2 modulates cytosolic oxidant activity and contractile function in murine skeletal muscle cells. J Appl Physiol 100(2):399-405 (1985).https://doi.org/10.1152/japplphysiol.00873.2005
Gounder SS, Kannan S, Devadoss D et al (2012) Impaired transcriptional activity of Nrf2 in age-related myocardial oxidative stress is reversible by moderate exercise training. PLoS One 7(9):e45697
Herzig S, Long F, Jhala US et al (2001) CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413:179–183. https://doi.org/10.1038/35093131
Hirschfield W, Moody MR, O’Brien WE et al (2000) Nitric oxide release and contractile properties of skeletal muscles from mice deficient in type III NOS. Am J Physiol Regul Integr Comp Physiol 278:R95–R100. https://doi.org/10.1152/ajpregu.2000.278.1.R95
Jarosz M, Olbert M, Wyszogrodzka G (2017) Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 25(1):11–24
Jaul E, Barron J (2017) Age-related diseases and clinical and public health implications for the 85 Years old and over population. Front Public Health 5:335
Kaminsky Y, Kosenko E (2009) Brain purine metabolism and xanthine dehydrogenase / oxidase conversion in hyperammonemia are under control of NMDA receptors and nitric oxide. Brain Res 1294:193–201. https://doi.org/10.1016/j.brainres.2009.07.082
Katsuyama M (2010) NOX/NADPH oxidase, the superoxidegenerating enzyme: its transcriptional regulation and physiological roles. J Pharmacol Sci 114(2):134–146. https://doi.org/10.1254/jphs.10r01cr
Katsuyama M, Matsuno K, Yabe-Nishimura C et al (2012) Physiological roles of NOX/NADPH oxidase, the superoxide-generating enzyme. J Clin Biochem Nutr 50(1):9–22. https://doi.org/10.3164/jcbn.11-06SR
Kayali R, Cakatay U, Uzun H et al (2007) Gender difference as regards myocardial protein oxidation in aged rats: male rats have increased oxidative protein damage. Biogerontology 8(6):653–661
Kayali R, Aydin S, Cakatay U (2009) Effect of gender on main clinical chemistry parameters in aged rats. Curr Aging Sci 2(1):67–71
Lambertucci RH, Levada-Pires AC, Rossoni LV (2007a) Effects of aerobic exercise training on antioxidant enzyme activities and mRNA levels in soleus muscle from young and aged rats. Mech Ageing Dev 128(3):267–275
Lambertucci RH, Levada-Pires AC, Rossoni LV et al (2007b) Effects of aerobic exercise training on antioxidant enzyme activities and mRNA levels in soleus muscle from young and aged rats. Mech Ageing Dev 128:267–275. https://doi.org/10.1016/j.mad.2006.12.006
Lee BE, Toledo AH, Anaya-Prado R et al (2009) Allopurinol, xanthine oxidase, and cardiac ischemia. J Investig Med 57(8):902–909. https://doi.org/10.2310/JIM.0b013e3181bca50c
Li T, He S, Liu S et al (2015) Effects of different exercise durations on Keap1-Nrf2-ARE pathway activation in mouse skeletal muscle. Free Radic Res 49(10):1269–1274
Lingappan K (2018) NF-κB in Oxidative Stress. Curr Opin Toxicol 7:81–86. https://doi.org/10.1016/j.cotox.2017.11.002
Madreiter-Sokolowski CT, Thomas C, Ristow M (2020) Interrelation between ROS and Ca2+ in aging and age-related diseases. Redox Biol 36:101678
McConell GK, Ng GP, Phillips M et al (2010) Central role of nitric oxide synthase in AICAR and caffeine-induced mitochondrial biogenesis in L6 myocytes. J Appl Physiol 108:589–595. https://doi.org/10.1152/japplphysiol.00377.2009
Menshikova EV, Ritov VB, Fairfull L et al (2006) Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol Ser A Biol Sci Med Sci 61:534–540. https://doi.org/10.1093/gerona/61.6.534
Miura S, Kawanaka K, Kai Y et al (2007) An increase in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) mRNA in response to exercise is mediated by beta-adrenergic receptor activation. Endocrinol 148:3441–3448. https://doi.org/10.1210/en.2006-1646
Murase T, Haramizu S, Ota N et al (2009) Suppression of the aging-associated decline in physical performance by a combination of resveratrol intake and habitual exercise in senescence-accelerated mice. Biogerontology 10:423e34. https://doi.org/10.1007/s10522-008-9177-z
Morrison D, Hughes J, Della Gatta PA et al (2015) Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic Biol Med 89:852–862. https://doi.org/10.1016/j.freeradbiomed.2015.10.412
Nalbant O, Toktas‚ N, Toraman NF et al (2009) Vitamin E and aerobic exercise: effects on physical performance in older adults. Aging Clin Exp Res 21:111e21. https://doi.org/10.1007/BF03325218
Novella S, Dantas AP, Segarra G et al (2013) Aging enhances contraction to thromboxane A(2) in aorta from female senescence accelerated mice. Age (Dordrecht) 35(1):117–128. https://doi.org/10.1007/s11357-011-9337-y
Pattwell DM, McArdle A, Morgan JE et al (2004) Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Radic Biol Med 37:1064–1072. https://doi.org/10.1016/j.freeradbiomed.2004.06.026
Paulsen G, Cumming KT, Holden G et al (2014) Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: a double-blind, randomized, controlled trial. J Physiol 592:1887–1901. https://doi.org/10.1113/jphysiol.2013.267419
Philp A, Schenk S (2013) Unraveling the complexities of SIRT1-mediated mitochondrial regulation in skeletal muscle. Exerc Sport Sci Rev 41:174–181. https://doi.org/10.1097/JES.0b013e3182956803
Pittaluga M, Parisi P, Sabatini S et al (2006) Cellular and biochemical parameters of exercise-induced oxidative stress: relationship with training levels. Free Radic Res 40(6):607–614. https://doi.org/10.1080/10715760600623015
Porter C, Reidy P, Bhattarai N, Rasmussen B et al (2014) Resistance exercise training alters mitochondrial function in human skeletal muscle. Med Sci Sports Exerc 47:1922–1931. https://doi.org/10.1249/MSS.0000000000000605
Powers SK, Jackson MJ (2008) Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88:1243e76. https://doi.org/10.1152/physrev.00031.2007
Puigserver P, Rhee J, Lin J et al (2001) Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell 8:971–982. https://doi.org/10.1016/s1097-2765(01)00390-2
Pye D, Palomero J, Kabayo T et al (2007) Real-time measurement of nitric oxide in single mature mouse skeletal muscle fibres during contractions. J Physiol 581:309–318. https://doi.org/10.1113/jphysiol.2006.125930
Radak Z, Chung HY, Goto S (2005) Exercise and hormesis: oxidative stress-related adaptation for successful aging. Biogerontology 6:71–75. https://doi.org/10.1007/s10522-004-7386-7
Reid MB (1998) Role of nitric oxide in skeletal muscle: synthesis, distribution and functional importance. Acta Physiol Scand 162:401–409. https://doi.org/10.1046/j.1365-201X.1998.0303f.x
Ristow M, Zarse K, Oberbach A et al (2009) Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA 106(21):8665–8670. https://doi.org/10.1073/pnas.0903485106
Saito K, Yokoyama T, Yoshida H et al (2012) A significant relationship between plasma vitamin C concentration and physical performance among Japanese elderly women. J Gerontol A Biol Sci Med Sci 67:295–301. https://doi.org/10.1093/gerona/glr174
Sakellariou GK, Vasilaki A, Palomero J et al (2013) Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid Redox Signal 18(6):603–621. https://doi.org/10.1089/ars.2012.4623
Sellman JE, DeRuisseau KC, Betters JL et al (2006) In vivo inhibition of nitric oxide synthase impairs upregulation of contractile protein mRNA in overloaded plantaris muscle. J Appl Physiol 100:258–265. https://doi.org/10.1152/japplphysiol.00936.2005
Serrano AL, Murgia M, Pallafacchina G (2001) Calcineurin controls nerve activity-dependent specification of slow skeletal muscle fibers but not muscle growth. Proc Natl Acad Sci 98:13108–13113. https://doi.org/10.1073/pnas.231148598
Silva-Palacios A, Ostolga-Chavarría M, Zazueta C, Königsberg M (2018) Nrf2: molecular and epigenetic regulation during aging. Aging Res Rev 47:31–40
St-Pierre J, Lin J, Krauss S et al (2003) Bioenergetic analysis of peroxisome proliferator-activated receptor coactivators 1 and 1 (PGC-1 and PGC-1) in muscle cells. J Biol Chem 278(29):26597–26603. https://doi.org/10.1074/jbc.M301850200
St-Pierre J, Drori S, Uldry M et al (2006) Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127(2):397–408. https://doi.org/10.1016/j.cell.2006.09.024
Song W, Kwak HB, Kim JH et al (2009) Exercise training modulates the nitric oxide synthase profile in skeletal muscle from old rats. J Gerontol A Biol Sci Med Sci 64A(5):540–549. https://doi.org/10.1093/gerona/glp021
Tarnopolsky MA (2009) Mitochondrial DNA shifting in older adults following resistance exercise training. Appl Physiol Nutr Metab 34:348–354. https://doi.org/10.1139/H09-022
Tatsumi R, Wuollet AL, Tabata K et al (2009) A role for calcium-calmodulin in regulating nitric oxide production during skeletal muscle satellite cell activation. Am J Physiol Cell Physiol 296:C922–C929. https://doi.org/10.1152/ajpcell.00471.2008
Thavanati PKR, Kanala KR, de Dios AE et al (2008) Age-related correlation between antioxidant enzymes and DNA damage with smoking and body mass index. J Gerontol A Biol Sci Med Sci 63(4):360–364. https://doi.org/10.1093/gerona/63.4.360
Vida C, Rodríguez-Terés S, Heras V et al (2011) The aged-related increase in xanthine oxidase expression and activity in several tissues from mice is not shown in long-lived animals. Biogerontology 12:551–564. https://doi.org/10.1007/s10522-011-9351-6
Viña J, Gomez-Cabrera MC, Borras T et al (2009) Mitochondrial biogenesis in exercise and in ageing☆. Adv Drug Deliv Rev 61(14):1369–1374. https://doi.org/10.1016/j.addr.2009.06.006
Wadley GD, McConell GK (2007) Effect of nitric oxide synthase inhibition on mitochondrial biogenesis in rat skeletal muscle. J Appl Physiol 102:314–320. https://doi.org/10.1152/japplphysiol.00549.2006
Wang P, Li CG, Qi Z et al (2016) Acute exercise stress promotes Ref1/Nrf2 signalling and increases mitochondrial antioxidant activity in skeletal muscle. Exp Physiol 101(3):410–420
Yanar K, Atayik MC, Simsek B et al (2020) Novel biomarkers for the evaluation of aging-induced proteinopathies. Biogerontology 21(5):531–548. https://doi.org/10.1007/s10522-020-09878-8
Yates MS, Tauchi M, Katsuoka F et al (2007) Pharmacodynamic characterization of hemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol Cancer Ther 6(1):154–162
Zhao X, Bey EA, Wientjes FB et al (2002) Cytosolic phospholipase A2 (cPLA2) regulation of human monocyte NADPH oxidase activity. cPLA2 affects translocation but not phosphorylation of p67(phox) and p47(phox). J Biol Chem 277:25385–25392. https://doi.org/10.1074/jbc.M203630200
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Conflict of Interest:
All authors declare they have no conflict of interest.
Ethical Approval:
This article does not contain any studies performed by any of the authors with human participants or animals.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ağaşcioğlu, E.A., Thirupathi, A. (2022). Aging and Exercise-Induced Reactive Oxygen Species. In: Çakatay, U. (eds) Redox Signaling and Biomarkers in Ageing. Healthy Ageing and Longevity, vol 15. Springer, Cham. https://doi.org/10.1007/978-3-030-84965-8_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-84965-8_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-84964-1
Online ISBN: 978-3-030-84965-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)