Abstract
Proteins of the GADD45 family play an essential role in the integration of cellular response to a wide variety of stressors and maintenance of homeostasis at the level of a cell, a tissue and an organism. The basic homeostatic processes are implicated in the determination of the progression of aging and development of major age-related disorders. Moreover, GADD45s mediate several well-known aging-associated signaling pathways through the interaction with such proteins as FOXO, p53, ATM, ATR, SIRT1, mTOR and some other. These reasons point out the role of the GADD45 proteins in the aging and life span regulation. Indeed, we have shown that constitutive and conditional (mifepristone-inducible) D-GADD45 overexpression in Drosophila melanogaster nervous system extends median and maximum life span, and increases the resistance to genotoxic, oxidative, thermal stress, and starvation. The life span-extending effect was apparently due to more efficient recognition and repair of DNA damage, because the spontaneous DNA damage in the larva neuroblasts with D-GADD45 overexpression was reduced. However, data obtained for flies with conditional ubiquitous D-GADD45 overexpression demonstrates a negative effect of this intervention on the life span and stress resistance.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Proteins of the GADD45 family (Growth Arrest and DNA Damage -inducible) play an essential role in the integration of cellular response to a wide variety of stressors and maintenance of homeostasis at the level of a cell, a tissue and an organism (Liebermann and Hoffman 1994; Zhang et al. 1999; Fornace et al. 2002; Moskalev et al. 2012b; Salvador et al. 2013) as well as in the determination of aging-related processes and longevity (for review, see Moskalev et al. 2012b).
Majority of gerontogenes (genes whose activity determines organism life span) are members of conserved biological pathways across different groups of species (for review, see Moskalev et al. 2014). Likewise, GADD45 orthologs first appeared in molluscs, and were also found in anemones, polychaete worms, insects, fish, amphibians and mammals . The number of GADD45 proteins in each species varies from one in lower organisms to 5–6 in fish, and decreases to 2–3 in amphibians and mammals (GADD45α, GADD45β and GADD45γ). Additionally, several isoforms were described, which are generated as a result of alternative mRNA splicing (Flicek et al. 2011). This indicates that the GADD45 family is relatively “young” and has undergone duplications and deletions in the course of evolution (Moskalev et al. 2012b). In Drosophila there is a single ortholog of the GADD45 family D-GADD45 (CG11086) (Peretz et al. 2007).
The gerontogenes are classified as life span regulators, mediators, effectors, housekeeping genes, genes involved in mitochondrial function , and genes regulating cellular senescence and apoptosis (for review, see Moskalev et al. 2014). GADD45s can be relevant to the last category of regulatory genes . Protein products of GADD45 genes are small (about 18–20 kDa), acidic (pH 4.0–4.2) proteins with high level of homology (55–57 % of identic aminoacids). Mainly, they have nucleus location and are associated with ribonucleoprotein speckles (Abdollahi et al. 1991; Zhang et al. 1999; Vairapandi et al. 2002; Sytnikova et al. 2011). GADD45 proteins form homo- and hetero-dimers and olygomers (Kovalsky et al. 2001; Schrag et al. 2008). The median half-life of the GADD45 mRNA is unusually short (less than 1 h), suggesting a regulatory rather than metabolic function for GADD45 proteins (Sharova et al. 2009). They don’t exercise enzymatic properties, and function through the interactions with other proteins and RNA (Sytnikova et al. 2011), or by way of modification of DNA/RNA accessibility for enzymes (Carrier et al. 1999; Moskalev et al. 2012b). The expression of the GADD45 proteins is detected in different tissues, including heart , brain, spleen, lungs, liver, skeletal muscles, kidneys, testicles, placenta (Zhang et al. 1999).
Most of the longevity genes described are related to stress response (for review, see Moskalev et al. 2014). GADD45 proteins are highly expressed after exposure to different physical, chemical and biological agents, and physiological factors. During stress response , they control such processes as DNA repair , cell cycle regulation, cellular senescence, apoptosis, inflammatory response , maintaining of the stem cell pool and cell differentiation . However, their inducibility is reduced with age (Edwards et al. 2004) which can be a reason of age-dependent descent of resistance to spontaneous and induced stress influences, and organism survivability . Processes that provide basic homeostasis reactions are implicated in the determination of the progression of aging and age-related diseases (ARDs) (for review, see Moskalev et al. 2012b). Indeed, there are evidences that GADD45 proteins are involved in the development of major ARDs, including cancer , metabolic, cardiovascular and autoimmunity disorders (Budovsky et al. 2009; Wolfson et al. 2009; Moskalev et al. 2012b). Moreover, GADD45 activity is responsible for embryogenesis and ontogenesis , and its imbalance is implicated in the development of such pathologic reactions as preeclampsia (Xiong et al. 2009; Geifman-Holtzman et al. 2013). GADD45s mediate several well-known aging-associated signaling pathways through the interaction with such proteins as FOXO , p53 , ATM, ATR, SIRT1 , mTOR and some other (Furukawa-Hibi et al. 2002; Kobayashi et al. 2005; Bortoff et al. 2010; Moskalev et al. 2012b; Salvador et al. 2013). All these reasons point out the role of the GADD45 proteins in the aging and life span regulation. Recently, we have shown the life span-extending effects and stress resistance stimulation due to neuro-specific D-GADD45 overexpression using the fruit fly Drosophila melanogaster (Plyusnina et al. 2011; Moskalev et al. 2012b; Plyusnina et al. 2012). This data confirm the involvement of GADD45 proteins in longevity determination, and suggest their ectopic expression can appear as beneficial method for the organism life span extension.
GADD45 family is evolutionary conserved in multicellular animals. In this chapter we considered participation of GADD45 genes in different aging-related processes, based on the data obtained in different model objects. At the same time we focused on the results of our investigations, which demonstrate the role of D-GADD45 gene in Drosophila melanogaster longevity .
2 Involvement of GADD45 Proteins in Stress Resistance Regulation
2.1 GADD45 in DNA Repair and Epigenetic Regulation
The expression of GADD45s is one of the critical conditions at the early stages of the DNA damage response with following activation of DNA repair machinery. The GADD45 promoters contain binding motifs for FOXO (AFX/FOXO4, FKHRL1/FOXO3A, and AKT /FOXO1 transcription factors), p53 , p33 (ING1), p73, OCT-1, BRCA1. Their activation under stress conditions leads to GADD45 upregulation with subsequent stimulation of DNA repair, blockage of cell cycle in the G1/S and G2/M checkpoints, and apoptosis (Kastan et al. 1992; Guillouf et al. 1995; Vairapandi et al. 1996; el-Deiry 1998; De Laurenzi and Melino 2000; Jin et al. 2000; MacLachlan et al. 2000; Cheung et al. 2001; Jin et al. 2001; Furukawa-Hibi et al. 2002; Tran et al. 2002; Ju et al. 2014).
The role of GADD45 in DNA repair is supported by the studies on in vitro and in vivo models. GADD45α-null mouse embryo fibroblasts and GADD45α-deficient human colon cancer cells exhibited slow base excision repair and delays removal of AP sites after treatment with methyl methanesulfonate (MMS) (Jung et al. 2007). The GADD45a −/− mice demonstrate genomic instability , reduced nucleotide excision repair, increased level of mutations and high susceptibility to chemical oncogenes (Hollander et al. 1999, 2001). The disrupted expression of GADD45β caused by the hepatitis C viral infection suppresses the DNA excision repair as well (Higgs et al. 2010). GADD45 proteins participate in base and nucleotide excision repair through the links with DNA repair enzymes and regulation of their activity. GADD45α and GADD45β interact with the apurinic/apyrimidinic endonuclease 1 (APE1) (Jung et al. 2007), xeroderma pigmentosum proteins XPC and XPG (Hartman and Ford 2002; Chang et al. 2003; Ma et al. 2009; Le May et al. 2010; Schäfer et al. 2010), and proliferating cell nuclear antigen (PCNA) (Smith et al. 1994; Hall et al. 1995; Vairapandi et al. 2000).
Another way of GADD45 involvement in DNA repair is its involvement in the repair-mediated active DNA demethylation (Barreto et al. 2007; Rai et al. 2008; Cortellino et al. 2011; Schomacher 2013). A possible GADD45-mediated demethylation mechanism involves nucleotide excision repair associated with the endonuclease activity of XPG protein. Specifically, 5-methylcytosine containing nucleotides could be recognized and removed through GADD45–XPG complex, ultimately resulting in the demethylation of CpG dinucleotides (Ma et al. 2009; Schmitz et al. 2009; Le May et al. 2010; Schäfer et al. 2010; Schomacher 2013). Additionally, GADD45 and XPG are involved in base excision repair, which could be another DNA repair mechanism associated with removal of methylated DNA (Jung et al. 2007). Additionally, GADD45 is able to bind histones and modify accessibility of damaged DNA for repair enzymes , and participates in chromatin decondensation (Carrier et al. 1999; Ma et al. 2009; Schomacher 2013). Thus, GADD45 recruits nucleotide and/or base excision repair factors to gene-specific loci and acts as an adapter between repair factors and chromatin, thereby creating a nexus between epigenetics and DNA repair (for review, see Niehrs and Schäfer 2012).
Recently, GADD45α was shown to bind RNA, forming ribonucleoprotein particles. GADD45 was detected inside nuclear speckles which are sites of active transcription, RNA splicing and processing (Sytnikova et al. 2011). Thus, GADD45 could exert its epigenetic effects both through active DNA demethylation, chromatin remodeling and post-transcriptional RNA regulation.
2.2 GADD45 in Cell Cycle Regulation and Cellular Senescence
ATM- and p53-mediated activation of GADD45 is essential for a DNA damage-induced G1 and G2/M cell cycle arrest during stress response (Wang et al. 1999). Thus, human and mouse GADD45-dificient fibroblasts and lymphocytes failed to arrest at G2/M after exposure to stress stimulus (Wang et al. 1999). Microinjection of the GADD45α expression vector into human primary fibroblasts arrests the cells in G2/M phase (Wang et al. 1999). On the same hand, ectopic expression of GADD45α, GADD45β or GADD45γ in cancer cells (M1 human myeloblastic leukemia and H1299 lung carcinoma) leads to accumulation of cells arrested in the G1 phase that later underwent apoptosis (Zhang et al. 2001). Additionally, it was found that GADD45α expression and G1 cell cycle arrest are activated by c-Jun N-terminal kinase (SAPK/JNK) under fucoxanthin treatment of LNCap prostate cancer cells, while the inhibition of SAPK/JNK attenuated the induction of G1 arrest and GADD45α expression (Satomi 2012). To achieve cell cycle arrest, GADD45 proteins interact with the protein kinase cell division cycle 2 (Cdc2), Cyclin B1, PCNA and cyclin-dependent kinase inhibitor p21 (Liebermann and Hoffman 2003). The interaction of GADD45α and GADD45β with the Cdc2/Cyclin B1 kinase complex leads to its dissociation and following G2/M cell cycle arrest as well as the inhibition of Cdc2 kinase activity (Zhan et al. 1999; Zhang et al. 1999; Vairapandi et al. 2002; Hsu et al. 2014). The interaction of all three GADD45 proteins with p21 induces both the G1 and G2/M arrest (Smith et al. 1994; el-Deiry 1998; Xiong et al. 2009; Zhang et al. 2014a).
GADD45 mediates cellular senescence in the case of unrepaired DNA damages, through the cell cycle arrest in the G1 phase with following unresponsiveness to growth factors (for review, see Moskalev et al. 2012b). A significant increase in GADD45α expression was observed upon stress-induced cellular senescence triggered by hydrogen dioxide (Duan et al. 2005). On the other hand, ectopic expression of GADD45γ robustly elicited senescence in hepatocellular carcinoma cells and suppressed tumor growth in vivo (Zhang et al. 2014b). Induction of GADD45 expression with subsequent cellular senescence can be activated by p53-dependent and JAK/STAT3 signaling pathways (Jackson and Pereira-Smith 2006; Zhang et al. 2014b). GADD45-mediated cellular senescence involves an increased expression of p21, mitochondrial dysfunction and generation of reactive oxygen species through the GADD45/p38 MAPK/GRB2/TGFBR2/TGFβ signaling pathway (Passos et al. 2010; Zhang et al. 2014a).
2.3 GADD45 Role in Cell Death and Survival
GADD45 family members play a dual role during mediation of apoptosis associated with two major components—p38/JNK mitogen-activated kinase (MAPK) and NF-κB signaling pathways (Takekawa and Saito 1998; Harkin et al. 1999; Lu et al. 2001; Hildesheim et al. 2002; Yoo et al. 2003; Tront et al. 2006). In fact, the MAPK/GADD45/NF-κB axis responds to a variety of extracellular stimuli, converting them to intracellular responses (Yang et al. 2009; Moskalev et al. 2012b). It is noteworthy that GADD45 proteins and stress kinases form a feedback regulatory loop: the expression of GADD45 is also under the control from p38 and JUNK MAPKs. GADD45γ and GADD45β bind to MEK kinase 4 (MEKK4) and promote phosphorylation and activation of the p38 and JNK MAP kinases by MEKK4 (Takekawa and Saito 1998). However, specific inhibitor of p38 MAPK SB202190 suppresses the expression of all three GADD45 genes (Oh-Hashi et al. 2001). NF-κB and GADD45s form a positive feedback regulatory loop as well (Gupta et al. 2006).
In the case of irreparable damages, GADD45 proteins exert a pro-apoptotic function. For example, the GADD45 proteins mediate the endoplasmic reticulum stress-induced apoptosis in mouse liver cells (Ji et al. 2005). Ectopic expression of GADD45 triggers apoptosis via the TGFβ/MEKK4/p38/JNK pathway in human leukemic cells or in mouse hepatocytes (Selvakumaran et al. 1994; Yoo et al. 2003). At the same time, blocking of early expression of GADD45β suppresses the apoptosis induced by TGFβ in myeloid leukemia cells (Selvakumaran et al. 1994).
However, GADD45α and GADD45β also can fulfil an anti-apoptotic function. For example, their activity increases hematopoietic cell survival under UV-irradiation or treatment with certain chemotherapeutic drugs (Gupta et al. 2005). Bone marrow cells obtained from GADD45α −/− and GADD45β −/− mice show an impaired ability for differentiation and increased sensitivity to the induction of apoptosis after being stimulated by cytokines (Gupta et al. 2006). GADD45α-deficient E1A + Ras cells treated with HDAC inhibitors demonstrated a higher level of pro-apoptotic signals, whereas the anti-apoptotic program is suppressed (Igotti Abramova et al. 2014). Anti-apoptotic function of GADD45 is realized through two mechanisms: activation of the p38/NF-κB anti-apoptotic pathway by GADD45α (Zhang et al. 2005; Gupta et al. 2006) and inhibition of the MKK7/JNK pro-apoptotic pathway by GADD45β (Papa et al. 2004b; Tornatore et al. 2008). Additionally, interactions of GADD45 with PCNA may promote cell survival , apoptosis inhibition together with DNA repair (Vairapandi et al. 2000; Azam et al. 2001).
It should be noted that the MAPK-mediated effect of GADD45 activation on the apoptosis onset is cell type specific. For example, activation of p38 and JNK kinases by GADD45 is associated with apoptosis in endothelial and epithelial cells (Harkin et al. 1999; Hildesheim et al. 2002), whereas it increases survival of hematopoietic cells (Platanias 2003). Additionally, induction of GADD45β by NF-κB downregulates pro-apoptotic JNK signaling in mouse embryonic fibroblasts (De Smaele et al. 2001) and in hepatocytes during liver regenerations after partial hepatectomy (Papa et al. 2008).
2.4 GADD45 in Antioxidant System Regulation and Heat Shock Response
GADD45 proteins can be involved in the prevention of cellular damages and heat shock response induction. Indeed, it was found that D-GADD45 gene was upregulated in fly heads after treatment with free radical inductor paraquat and high temperatures. Furthermore, flies with D-GADD45 overexpression in the nervous system were more resistant to this stressor compared with ones without overexpression (Moskalev et al. 2012a). In mammalian cells GADD45β-mediated activation of the protein kinases MEKK4 and JNK (Takekawa and Saito 1998; Papa et al. 2004a) increases the level of the ASK1 (Ko et al. 2001), which acts in opposition to the SOD1 protein. The GADD45 proteins affect the expression level of the transcription factors PPARγ (Hamza et al. 2009) and RXRA (Wu et al. 2004) as a part of JAK and MEK kinase signaling cascades, and participate in the induction of downstream antioxidant systems (SOD1, thioredoxin and glutaredoxin enzymes). Thus, in the process of oxidative stress response, GADD45 family proteins are involved in the control of the activity and maintenance of the balance between antioxidant enzymes and determine the fate of cells (Moskalev et al. 2012a).
Additionally, the transcription factors PPAR-γ and RXRA increase the expression of the heat shock protein HSP22 (Hamza et al. 2009). HSP22 is responsible for activating another heat shock protein , HSP27 (Sun et al. 2004) and following HSP70 induction (Whitlock et al. 2005). For example, GADD45 proteins can participate in the heat shock response through PPAR-γ and RXRA. Another way of involvement of the GADD45 family members in the heat shock protein activation is mediated by its interaction with the CDK1 protein kinase . All three GADD45 proteins bind and inhibit CDK1, which phosphorylates the transcription factor SP1 (Chuang et al. 2012). The tanscription factor SP1 activates the expression of heat shock proteins HSP27 and HSP60 (Reed et al. 2008; Friedman et al. 2009). Finally, GADD45s activates the transcription factor HSF1 by inhibiting the p38 protein kinase (Moskalev et al. 2012a). HSF1 is a key element for pathways activating heat shock proteins, such as HSP60, HSP90, HSPA4, HSP70 , HSP27 and HSP22 .
2.5 GADD45 in Inflammatory Response and Immunity
GADD45 proteins also contribute to cellular inflammation response and organism survival by modulation of the immune response (for review, see Schmitz 2013). It was shown using the Drosophila model, that the inflammation induced by bacterial infection increases both the level of D-GADD45 mRNA and protein (Peretz et al. 2007; Moskalev et al. 2012a). The GADD45 genes are induced by the pro-inflammatory transcription factor NF-κB (Balliet et al. 2001), cytokines including interleukins TNFα, TNFβ, IL-2, IL-6, IL-8, IL-12, IL-18 (Fan et al. 1999; Zhang et al. 1999; Yang et al. 2001; Salerno et al. 2012) and oncostatin M (Nakayama et al. 1999). The main function of GADD45 in the inflammation response is determined by its interactions with mitogen-activated protein kinase p38, cyclin-dependent kinase p34 (Yang et al. 2000), and PCNA (Smith et al. 1994). For example, after IL-12 and IL-18 treatment GADD45β activated p38 and selectively increased cytokine-induced interferon γ (IFNγ) production (Yang et al. 2001). Additionally, the GADD45 proteins affect the transcription of IFNγ by interacting with PCNA-p300 family (Nakayama et al. 2001). Regulation of this pathway is mediated by interaction of GADD45s with the transcription factors PPARα , C/EBPβ and c-Jun (Moskalev et al. 2012a).
The GADD45β and GADD45γ proteins provide proliferation of T helper 1 (Th1) cells and induce the production of IFNγ in these cells (Yang et al. 2001). The essential role of GADD45 in elevating the level of IFNγ is evidenced by the absence of this process in GADD45γ- (Lu et al. 2001) and GADD45β-deficient mice (Ju et al. 2009). Furthermore, GADD45 proteins play an important role in the process of Th1-mediated anti-tumor immune responses (Ju et al. 2009) and in autoimmunity reaction development (Liu et al. 2005).
It was found that GADD45α and GADD45β are also essential for differentiation of bone marrow cells into macrophage and granulocyte. The GADD45α- and GADD45β-deficient mice were characterized by increased apoptosis during differentiation and reduced clonogenicity (Gupta et al. 2006). Additionally, GADD45s activation of the p38 kinase is implicated in the response of granulocytes to lipopolysaccharide (a component of gramm-negative bacterial cells) mediated chemotaxis, whereas Gadd45α and Gadd45β curtailment of JNK activation was linked to chemotaxis of macrophages in response to this inflammatory stimulus (Salerno et al. 2012). Moreover, Gadd45β regulates the autophagy process, a catabolic pathway that also degrades intracellular pathogens (Schmitz 2013). The Gadd45β-MEKK4 pathway specifically directs p38 to autophagosomes and mediate phosphorylation of the autophagy regulator autophagy-related 5 (ATG5) protein. This process results in an accumulation of autophagosomes through the p38-mediated inhibition of lysosome fusion (Keil et al. 2013).
3 Role of GADD45 Proteins in Aging and Life Span Regulation
The GADD45 family members are deeply implicated in the maintaining of cellular, tissue and organism homeostasis which determines the aging rate and longevity . Indeed, some well-known regulators of aging-associated processes and longevity are partner proteins for GADD45s (Budovsky et al. 2009; Wolfson et al. 2009; Moskalev et al. 2012b). The GADD45 proteins contain the FOXO- and p53-binding motifs (Kastan et al. 1992; Guillouf et al. 1995; el-Deiry 1998; Furukawa-Hibi et al. 2002; Tran et al. 2002; Ju et al. 2014), and are activated by the ATM- and ATR-dependent way (Kastan et al. 1992; O’Prey et al. 2003; Jang et al. 2010). In particular, RNA interference of FOXO3a leads to inhibition of GADD45 stress-induced expression (Tran et al. 2002). Another example demonstrated that in human epithelial cells an inhibitor of ATM/ATR prevented induction of GADD45 and growth arrest by flavonoid treatment (O’Prey et al. 2003). The SIRT1 histone deacetylase is another key longevity regulator (Guarente 2011; Satoh et al. 2013; Hubbard and Sinclair 2014), that is involved in the GADD45 functioning regulation (Kobayashi et al. 2005; Scuto et al. 2013). The FOXO-mediated GADD45 induction was markedly impaired in cells, which depleted SIRT1 expression by RNA-interference (Kobayashi et al. 2005). Additionally, there are evidences that GADD45 expression is linked with the target of rapamycin (TOR) signaling. Insulin induces GADD45β transcription by activating the mTOR pathway (Bortoff et al. 2010), well known for its association with aging, longevity, and ARDs (for review, see Blagosklonny 2008; Zoncu et al. 2011). These examples of the relationship between GADD45 family members and longevity-associated genes confirm their immixture in the life span control.
Another way of GADD45 participation in the aging and longevity determination at the tissue level is subjected by the role in stem cell pool maintenance (for review, see Moskalev et al. 2012b). The Gadd45 proteins participate also in the maintenance of the pool of myeloid quiescent stem cells . Gadd45α or Gadd45β deletion was shown to suppress the quiescent stem cell population or lower the survival rate of progenitor cells , leading to the depletion of the stem cell compartment, and to affect the clonogenic potential of these cells (Hoffman and Liebermann 2007). Gadd45γ is involved in stem cell pool maintenance as well. A possible regulatory mechanism of stem cell pool maintenance is mediated by the Nucleus accumbens-1 (NAC-1) protein which is important for self-regeneration and pluripotency of embryonic stem cells, negatively regulates the expression of Gadd45γ-interacting protein 1 (Gadd45γ-ip1), preventing its suppressive activity towards Gadd45γ (Jinawath et al. 2009).
Aging negatively affects the ability of cells to express GADD45 proteins in response to stress conditions. For example, treatment of cardiomyocytes of young mice with free radical inducer paraquat led to the significantly increased expression of GADD45 isoforms, but did not stimulate its expression in the myocardium of old animals (Edwards et al. 2004). Decreased inducibility of GADD45 members may have far-reaching consequences including genome instability, accumulation of DNA damage, and disorders in cellular homeostasis—all of which may eventually contribute to the aging process (for review, see Moskalev et al. 2012b).
The GADD45 family members as well as longevity-associated genes are concerned with ARDs. One of the main implications of the GADD45 proteins in the ARDs is associated with the cancerogenesis determination (for review, see Liebermann et al. 2011; Hoffman and Liebermann 2013). It was shown that the GADD45α-deficient mice were characterized by genomic instability , increased sensitivity to cancerogenes, and high aptitude to ovarian, hepatocellular and vascular tumors (Hollander et al. 1999, 2001; Tront et al. 2006). Mice with the GADD45β gene knockout are more susceptible to ionizing radiation and chemical carcinogens, and display a lower immune response against implanted melanoma cells (Ju et al. 2009). In the in vivo model of Ras-overexpressing mice with different GADD45α expression levels (Ras/GADD45α +/+, Ras/GADD45α+/−, and Ras/GADD45α−/−), it was shown that Ras-driven genesis and growth of breast tumors is a GADD45α-dependent process (Tront et al. 2006). Clinical patients with solid and hematopoietic cancers including breast, lung, nasopharyngeal, brain, liver, prostate cancer, and lymphoma showed disruption in GADD45 expression pattern (Hoggard et al. 1995; Sun et al. 2003; Jiang and Wang 2004; Qiu et al. 2004; Ying et al. 2005; Cretu et al. 2009; Na et al. 2010; Liebermann et al. 2011; Hoffman and Liebermann 2013).
The main cause of GADD45 expression loss in cancers is epigenetic modifications, particularly, DNA methylation (for review, see Moskalev et al. 2012b). For example, the methylation of the GADD45γ promoter was significantly higher in different types of cancers than in normal tissues (Zhang et al. 2010). On the other hand, treatment of cancer cells with DNA methyltransferase inhibitors restored GADD45β expression to its level in the non-tumorous cells (Qiu et al. 2004). One of the pathways that determine GADD45 methylation is NF-κB signaling. This is supported by the effect of NF-κB inhibition in cancer cells which leads to the GADD45α- and γ-dependent induction of apoptosis and reduction in tumor growth (Zerbini et al. 2004). The NF-κB transcription factor induces the expression of proto-oncogene c-Myc, which binds to the GC-rich sites of the GADD45 promoters and significantly reduces the GADD45 inducibility in response to genotoxic stress (Amundson et al. 1998; Zerbini et al. 2004; Zerbini and Libermann 2005). It is known that hipermethylation of gene promoters provides the aging process as well (Muñoz-Najar and Sedivy 2011), thus methylation of the GADD45 promoters can be involved in the age-dependent reduction of its expression and inducibility.
Conversely, ectopic expression of the GADD45 members blocks cell growth by arresting the cells in the G2/M phase (Zhu et al. 2009) and G1/G0 phase (Higgins et al. 2009), and/or induces apoptosis in human tumor cell lines (Zhan et al. 1994; Vairapandi et al. 1996; Zhang et al. 2001; Sun et al. 2003; Jiang and Wang 2004; Ying et al. 2005). For example, GADD45β overexpression in LβT2 mouse gonadotrope cells blocked tumor cell proliferation and increased rates of apoptosis in response to growth factor withdrawal (Michaelis et al. 2011). Anti-cancer activity of the GADD45 proteins is conditioned by its role in apoptosis and cell cycle control as well as in negative regulation of oncogenes, such as p63 and β-catenin (Hildesheim et al. 2004).
However, in some cases, GADD45α may exert a pro-cancer action, depending on the type of the oncogenic stimuli. For example, the Myc-driven breast cancer is promoted by GADD45α activity, which dramatically decreased level of the enzyme MMP10 and led to angiogenesis. In Myc expressing tumors loss of GADD45α was accompanied by apoptosis or cellular senescence (Tront et al. 2010).
Additionally, other ARDs are associated with changes in GADD45 expression. The GADD45 proteins participate in the development of the nervous system during ontogenesis and provide long-term memory formation, as well as their deregulation results in neuronal pathologies including brain cancers, ischemia, insults, seizures, memory decline, autism, Alzheimer’s disease , psychosis (for review, see Sultan and Sweatt 2013). For example, Alzheimer’s disease patients are characterized by highly increased level of GADD45 expression in neurons , that prevents neuronal cells from apoptosis induced by accumulation of β-amyloid (Torp et al. 1998; Santiard-Baron et al. 1999, 2001). The upregulation of GADD45 was also observed in the in vitro model (human neuroblastoma cells) of dopamine-induced neurotoxicity , which is a part of some neurodegenerative disorders (for example, Parkinson’s disease) and normal brain aging (Stokes et al. 2002). The same changes were found in cultures of human endothelial cells derived from atherosclerotic aorta or coronary arteries, as well as in the mouse model of atherosclerosis (Thum and Borlak 2008). Thus, the GADD45 proteins apparently are induced during neurodegenerative processes and atherosclerosis providing a vicarious protective mechanism.
Chronic inflammation is largely attributed to an age-related increase in pro-inflammatory cytokines TNFα, IL-1β, IL-6 and NF-κB (Finch 1990; Chung et al. 2009; Coppé et al. 2010), that induce GADD45 proteins. For example, the induction of GADD45 was observed in the course of liver inflammation (Gant et al. 2003). Additionally, the GADD45 proteins can be involved in the process of the epithelial to mesenchymal transition (EMT). The EMT is a crucial process in the development of different tissues in the embryo and its reactivation in the adult is a part of inflammatory responses useful for the healing damaged tissue. However, abnormality of its control leads to tumorogenesis and organ fibrosis development (López-Novoa and Nieto 2009). GADD45s closely cooperate with key EMT regulators NF-κB , β-catenin, and matrix metalloproteases (Moskalev et al. 2012b).
Aging-dependent induction of oxidative stress and inflammation processes contributes to a process known as immunosenescence . Immunosenescence manifests in a decreased immune responsiveness to foreign and self-antigens, leading to an increased susceptibility to infection, cancer and autoimmune diseases. A decreased ability to maintain tolerance against self-antigens may result in autoimmune disorders (for review, see Moskalev et al. 2012b). Mice with deficiency in GADD45β and GADD45γ spontaneously develop signs of autoimmune lymphoproliferative syndrome and systemic lupus erythematosus. The reduced inducibility of GADD45s in immune cells is one of the possible factors that increase frequency of autoimmune conditions in aging (Liu et al. 2005).
High GADD45 expression may sustain the age-related immune dysfunctions, particularly, rheumatoid arthritis (for review, see Lindstrom and Robinson 2010). It is known that infiltrated Th1 cells in the synovial fluid of patients with rheumatoid arthritis are resistant to apoptosis. This resistance is accompanied by the high levels of GADD45β resulting from stimulation by pro-inflammatory cytokines TNFα and IL-12 (Du et al. 2008). The activated Th1 cells avoid utilization, which leads to chronic inflammation and tissue destruction. The important role of GADD45β in this process also follows from the finding that silencing of GADD45β by RNA interference abolished the anti-apoptotic effect of rheumatoid arthritis synovial fluid (for review, see Moskalev et al. 2012b). Another disorder associated with elevated expression of GADD45α protein is preeclampsia. Inflammatory and immune activation in preeclampsia may function in a feedback loop to maintain elevated expression of GADD45α protein (Geifman-Holtzman et al. 2013). GADD45α activates Mkk3-p38 and/or JNK signaling that leads to immunological and inflammatory changes as well as to triggering the production of circulating factors such as sFlt-1 (Xiong et al. 2009; Geifman-Holtzman et al. 2013; Xiong et al. 2013).
In hepatocytes both injury and growth stimulation remarkably increase the expression of the GADD45β protein. In liver cancer, promoter methylation frequently silences GADD45β, demonstrating a suppressive proapoptotic function. This contrasts with normal hepatocytes, where GADD45β facilitates cell survival , growth, and proliferation. GADD45β protects the liver through two ways: binding MKK7 to block damaging signal transduction or binding CAR to coactivate anabolic transcription (Tian et al. 2011; Tian and Locker 2013). Furthermore, the GADD45γ protein deregulation may be a reason of liver hypertrophy and liver tumor as well through the interaction with cyclins and cyclin-dependent kinase inhibitors (Ozawa et al. 2011).
Thus, GADD45 proteins are involved in major aging-associated conditions including oxidative stress , chronic inflammation, immunosenescence and fibroproliferative repair that contribute to the development of ARDs and aging progression (for review, see Moskalev et al. 2012b).
Aging-related changes in organism fertility can be caused by GADD45 expression alterations as well. In a model mice with deficiency of GADD45 isoforms, it was shown that GADD45s determine male fertility, testis development, and primary sex determination (Johnen et al. 2013).
In a view of its functions, it seems reasonable that GADD45 overexpression might promote longevity , in particular, by increasing the efficiency of DNA repair (for review, see Moskalev et al. 2012b). Recently, we have confirmed this hypothesis in the Drosophila melanogaster model and have shown a life span-extending effects of D-GADD45 overactivation in the nervous system (Plyusnina et al. 2011; Moskalev et al. 2012a; Plyusnina et al. 2012).
4 Life Span and Stress Resistance in Fruit Flies with D-Gadd45 Overexpression
Research of the life span and stress resistance in model animals such as fruit fly Drosophila melanogaster with overexpressed investigated genes is a promising method to reveal their life span-extending properties. Therethrough, we investigated the effect of conditional and constitutive overexpression of the GADD45 gene both in the nervous system and the whole body on the life span and some age-dependent physiological parameters. To realize this aim the UAS/GAL4 system was used.
It was obtained that despite the fact that the overall spontaneous activity of the D-GADD45 gene increases with age, the level of expression of this gene in the nervous system is practically eliminated (Moskalev et al. 2012a). The dramatic decrease of its activity within the nervous system with age might be one of the reasons for the decrease in the organism’s stress resistance . Moreover, flies with constitutive D-GADD45 overexpression in the nervous system showed a reduction in mRNA levels of D-GADD45 with age as well (Moskalev et al. 2012a) that may indicate an epigenetic cause of the low level of activity of this gene in old flies.
Peretz et al. (2007) have shown that D-GADD45 overexpression in Drosophila melanogaster has diverse phenotypic manifestations depending on the target tissue. Ubiquitous overexpression of this gene in Drosophila flies from the first stages of life cycle is lethal. Our investigations revealed that flies with conditional (mifepristone-inducible) ubiquitous D-GADD45 overexpression in the imaginal developmental stage are viable, but characterized by 22–46 % decreased life span (Fig. 2.1) (our unpublished data) and low resistance to the acute γ-irradiation and oxidative stress induced by paraquat treatment. The reason of this effect may be an insufficient epigenetic regulation of D-GADD45 activity. For example, in human fibroblasts increased activity of DNA repair genes slows the replicative senescence only under simultaneous overexpression of histone deacetylase SIRT6 (Mao et al. 2012). The GADD45 expression is depends on the sirtuins activity as well (Kobayashi et al. 2005; Scuto et al. 2013). Another reason could be associated with a high energy rate of repair processes (Halmosi et al. 2001). Ubiquitous D-GADD45 overexpression can lead to excessive energy expenditure, which violates other processes.
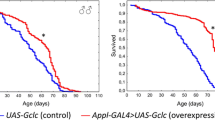
Opposite to ubiquitous overexpression, tissue-specific D-GADD45 overexpression in the nervous system leads to life span prolongation. Our data indicate that constitutive D-GADD45 overexpression in the Drosophila nervous system leads to median life span extension (by 22–77 %) in comparison with flies without overexpression (Fig. 2.2a). Furthermore, the maximum life span was also increased, which is an evidence of slowing down aging (Plyusnina et al. 2011). To avoid effect of heterosis we also studied the life span effects of the conditional (mifepristone-inducible) D-GADD45 overexpression in the nervous system. We found that the median life span of individuals with conditional overexpression of the D-GADD45 gene in the nervous system was higher in comparison with animals with the same genotype kept on a medium without mifepristone (by 3–102 %) (Fig. 2.2b) (Plyusnina et al. 2011). It must be noted that the life span-extending effect of D-GADD45 overexpression in Drosophila nervous system was not accompanied by decreases in fertility and locomotor activity parameters (Plyusnina et al. 2011). We proposed that D-GADD45 overexpression causes more effective functioning of stress response mechanisms. Indeed, the DNA comet assay showed that neuroblasts of third-instar larvae with D-GADD45 overexpression had the decreased DNA damage level (by 21–27 %). Therefore, overexpression of the D-GADD45 gene in Drosophila nervous system results in more efficient recognition and elimination of spontaneous DNA damage caused by physiological processes and environmental factors (Plyusnina et al. 2011).
Survival curves for Drosophila melanogaster flies with and without D-GADD45 overexpression in the nervous system: a Survival curves for Drosophila melanogaster males (♂♂) and females (♀♀) with the parental UAS-D-GADD45 and GAL4-1407 genotypes and constitutive D-GADD45 overexpression in the nervous system (combined results of three replications), b Survival curves for Drosophila melanogaster males (♂♂) and females (♀♀) with the UAS-D-GADD45/ELAV genotype not treated with mifepristone and treated with mifepristone (conditional D-GADD45 overexpression in the nervous system) (combined results of two replications), * p < 0.05, *** p < 0.001 (Kolmogorov–Smirnov test) (Plyusnina et al. 2011)
In further experiment we found additional evidences of increased resistance of Drosophila melanogaster individuals with constitutive and conditional D-GADD45 overexpression in the nervous system. In most cases, these flies are characterized by increased survival under conditions of genotoxic stress (chronic and acute γ-irradiation), oxidative stress (paraquat), hyperthermia and starvation (Figs. 2.3, 2.4 and 2.5) (Moskalev et al. 2012a). Additionally, the involvement of the D-GADD45 gene in the formation of biological responses to γ-irradiation was shown in the experiment on the fruit flies with homozygous and heterozygous D-GADD45 mutation. Our results revealed the effects of hormesis and radioadaptive response for the wild-type flies irradiated by the low 40 cGy dose. Over against, the D-GADD45 mutations led to elimination of these reactions (Moskalev et al. 2012a).
Survival curves of Drosophila males (♂♂) and females (♀♀) with the parental UAS-D-GADD45 and GAL4-1407 genotypes and constitutive D-GADD45 overexpression in the nervous system under different irradiation conditions: a chronic 40 cGy γ-irradiation, b acute 30 Gy γ-irradiation, *** p < 0.001 (Kolmogorov–Smirnov test) (Moskalev et al. 2012a)
Survival curves of Drosophila males (♂♂) and females (♀♀) with the parental UAS-D-GADD45 and GAL4-1407 genotypes and constitutive D-GADD45 overexpression in the nervous system under different stress conditions: a paraquat (20 mM), b hyperthermia (35 °C), c starvation , * p < 0.05, ** p < 0.01, *** p < 0.001 (Kolmogorov–Smirnov test) (Moskalev et al. 2012a)
Survival curves for Drosophila melanogaster males (♂♂) and females (♀♀) with the UAS-D-GADD45/ELAV genotype not treated with mifepristone and treated with mifepristone (conditional D-GADD45 overexpression in the nervous system) under different stress conditions: a paraquat (20 mM), b hyperthermia (35 °C), c starvation , * p < 0.05, ** p < 0.01, *** p < 0.001 (Kolmogorov–Smirnov test) (Moskalev et al. 2012a)
Thus, ubiquitous D-GADD45 overexpression leads to decrease of life span and stress resistance. At the same time, neuron-specific overexpression of the D-GADD45 gene demonstrates a high life span-extending potential of controlled manipulation with this gene.
5 Concluding Remarks
Proteins of the GADD45 family are essential for stress resistance, and display antiaging and prolongevity activities (Fig. 2.6). Particularly, GADD45s provide a maintenance of basic homeostatic reactions and regulate a balance between cell (DNA) repair, eliminating (apoptosis) or preventing the expansion of potentially dangerous cells (cell cycle arrest, cellular senescence), maintaining of the stem cell pool and cellular differentiation. These processes provide survival of cells from different tissues and contribute to tissue regeneration. In turn, a decreased inducibility of the GADD45 family members may have far reaching consequences including genome instability, accumulation of DNA damage, and disorders in cellular homeostasis. All these negative processes may eventually lead to the age-dependent decline of organism system and organ functioning, aging progression, and promotion of carcinogenesis and development of other ARDs. It must be noted, that the GADD45 protein members are deeply involved in major longevity-associated signaling pathways, which confirm their role in aging and longevity determination.
Investigations carried out in Drosophila melanogaster model disclosed the life span-extending activity of the D-GADD45 gene due to its neuron-specific overactivation (Plyusnina et al. 2011, 2012) but not ubiquitous overexpression. It was shown that both constitutive and conditional D-GADD45 overactivation in Drosophila nervous system extends median and maximum life span without negative changes in fertility and locomotor activity. This effect is apparently conditioned by elevated efficiency of recognition and elimination of spontaneous DNA damages. Additionally, neuron-specific D-GADD45 overexpression can stimulate the resistance to different stress agents including genotoxic (γ-radiation), oxidative (paraquat) and thermal stressor as well as starvation.
Obtained results suggest that controlled manipulations of GADD45s and its interacting partners may also bring benefits to humans. Indeed, the increased GADD45 expression can be induced by several anti-tumor, anti-oxidant, anti-inflammatory pharmacological agents with potential life span-extending action, such as troglitazone (Yin et al. 2004), arsenic trioxide (Li et al. 2003), cucurbitacin E (Hsu et al. 2014), xanthatin (Takeda et al. 2011, 2013), quercetin (Yoshida et al. 2005), fucoxanthin (Kumar et al. 2013), epicatechin (Saha et al. 2010), ibuprofen (Bonelli et al. 2011). Thus, future studying the GADD45 family may provide prosperous therapeutic targets for promoting longevity and combating ARDs, as well as for stimulation of organism stress-resistance and enhancement of survivability.
References
Abdollahi A, Lord KA, Hoffman-Liebermann B, Liebermann DA (1991) Sequence and expression of a cDNA encoding MyD118: a novel myeloid differentiation primary response gene induced by multiple cytokines. Oncogene 6(1):165–167
Amundson SA, Zhan Q, Penn LZ, Fornace AJ Jr (1998) Myc suppresses induction of the growth arrest genes gadd34, gadd45, and gadd153 by DNA-damaging agents. Oncogene 17(17):2149–2154
Azam N, Vairapandi M, Zhang W, Hoffman B, Liebermann DA (2001) Interaction of CR6 (GADD45γ) with proliferating cell nuclear antigen impedes negative growth control. J Biol Chem 276(4):2766–2774
Balliet AG, Hatton KS, Hoffman B, Liebermann DA (2001) Comparative analysis of the genetic structure and chromosomal location of the murine MyD118 (Gadd45beta) gene. DNA Cell Biol 20(4):239–247
Barreto G, Schäfer A, Marhold J, Stach D, Swaminathan SK, Handa V, Döderlein G, Maltry N, Wu W, Lyko F, Niehrs C (2007) Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature 445(7128):671–675
Blagosklonny MV (2008) Prevention of cancer by inhibiting ageing. Cancer Biol Ther 7(10):1520–1524
Bonelli P, Tuccillo FM, Calemma R, Pezzetti F, Borrelli A, Martinelli R, De Rosa A, Esposito D, Palaia R, Castello G (2011) Changes in the gene expression profile of gastric cancer cells in response to ibuprofen: a gene pathway analysis. Pharmacogenomics J 11(6):412–428
Bortoff KD, Keeton AB, Franklin JL, Messina JL (2010) Anti-inflammatory action of insulin via induction of Gadd45-β transcription by the mTOR signaling pathway. Hepat Med 2001(2):79–85
Budovsky A, Tacutu R, Yanai H, Abramovich A, Wolfson M, Fraifeld V (2009) Common gene signature of cancer and longevity. Mech Ageing Dev 130(1–2):33–39
Carrier F, Georgel PT, Pourquier P, Blake M, Kontny HU, Antinore MJ, Gariboldi M, Myers TG, Weinstein JN, Pommier Y, Fornace AJJ (1999) Gadd45, a p53-responsive stress protein, modifies DNA accessibility on damaged chromatin. Mol Cell Biol 19(3):1673–1685
Chang HC, Tsai J, Guo YL, Huang YH, Tsai HN, Tsai PC, Huang W (2003) Differential UVC-induced gadd45 gene expression in xeroderma pigmentosum cells. Biochem Biophys Res Commun 305(4):1109–1115
Cheung KJJr, Mitchell D, Lin P, Li G (2001) The tumor suppressor candidate p33ING1 mediates repair of UV-damaged DNA. Cancer Res 61(13):4974–4977
Chuang JY, Wang SA, Yang WB, Yang HC, Hung CY, Su TP, Chang WC, Hung JJ (2012) Sp1 phosphorylation by cyclin-dependent kinase 1/cyclin B1 represses its DNA-binding activity during mitosis in cancer cells. Oncogene 31(47):4946–4959
Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C (2009) Molecular inflammation: underpinnings of ageing and age-related diseases. Ageing Res Rev 8(1):18–30
Coppé JP, Desprez PY, Krtolica A, Campisi J (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5:99–118
Cortellino S, Xu J, Sannai M et al (2011) Thymine DNA glycosylase is essential for active DNA demethylation by linked deamination-base excision repair. Cell 146(1):67–79
Cretu A, Sha X, Tront J, Hoffman B, Liebermann DA (2009) Stress sensor Gadd45 genes as therapeutic targets in cancer. Cancer Ther 7(A):268–276
De Laurenzi V, Melino G (2000) Evolution of functions within the p53/p63/p73 family. Ann N Y Acad Sci 926:90–100
De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G (2001) Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature 414(6861):308–313
Du F, Wang L, Zhang Y, Jiang W, Sheng H, Cao Q, Wu J, Shen B, Shen T, Zhang JZ, Bao C, Li D, Li N (2008) Role of GADD45 beta in the regulation of synovial fluid T cell apoptosis in rheumatoid arthritis. Clin Immunol 128(2):238–247
Duan J, Zhang Z, Tong T (2005) Irreversible cellular senescence induced by prolonged exposure to H2O2 involves DNA-damage-and-repair genes and telomere shortening. Int J Biochem Cell Biol 37(7):1407–1420
Edwards MG, Sarkar D, Klopp R, Morrow JD, Weindruch R, Prolla TA (2004) Impairment of the transcriptional responses to oxidative stress in the heart of aged C57BL/6 mice. Ann N Y Acad Sci 1019:85–95
el-Deiry WS (1998) Regulation of p53 downstream genes. Semin Cancer Biol 8(5):345–357
Fan W, Richter G, Cereseto A, Beadling C, Smith KA (1999) Cytokine response gene 6 induces p21 and regulates both cell growth and arrest. Oncogene 18(47):6573–6582
Finch CE (1990) Longevity, senescence, and the genome. University of Chicago Press, Chicago
Flicek P, Amode MR, Barrell D et al (2011) Ensembl 2011. Nucleic Acids Res 39(Database issue):D800–D806
Fornace AJ Jr, Amundson SA, Do KT, Meltzer P, Trent J, Bittner M (2002) Stress-gene induction by low-dose gamma irradiation. Mil Med 167(2 Suppl):13–15
Friedman MJ, Li S, Li XJ (2009) Activation of gene transcription by heat shock protein 27 may contribute to its neuronal protection. J Biol Chem 284(41):27944–27951
Furukawa-Hibi Y, Yoshida-Araki K, Ohta T, Ikeda K, Motoyama N (2002) FOXO forkhead transcription factors induce G(2)-M checkpoint in response to oxidative stress. J Biol Chem 277(30):26729–26732
Gant TW, Baus PR, Clothier B, Riley J, Davies R, Judah DJ, Edwards RE, George E, Greaves P, Smith AG (2003) Gene expression profiles associated with inflammation, fibrosis, and cholestasis in mouse liver after griseofulvin. EHP Toxicogenomics 111(1T):37–43
Geifman-Holtzman O, Xiong Y, Holtzman EJ (2013) Gadd45 stress sensors in preeclampsia. Adv Exp Med Biol 793:121–129
Guarente L (2011) Sirtuins, ageing, and metabolism. Cold Spring Harb Symp Quant Biol 76:81–90
Guillouf C, Grana X, Selvakumaran M, De Luca A, Giordano A, Hoffman B, Liebermann DA (1995) Dissection of the genetic programs of p53-mediated G1 growth arrest and apoptosis: blocking p53-induced apoptosis unmasks G1 arrest. Blood 85(10):2691–2698
Gupta M, Gupta SK, Balliet AG, Hollander MC, Fornace AJ, Hoffman B, Liebermann DA (2005) Hematopoietic cells from Gadd45a- and Gadd45b-deficient mice are sensitized to genotoxic-stress-induced apoptosis. Oncogene 24(48):7170–7179
Gupta M, Gupta SK, Hoffman B, Liebermann DA (2006) Gadd45a and Gadd45b protect hematopoietic cells from UV-induced apoptosis via distinct signaling pathways, including p38 activation and JNK inhibition. J Biol Chem 281(26):17552–17558
Hall PA, Kearsey JM, Coates PJ, Norman DG, Warbrick E, Cox LS (1995) Characterisation of the interaction between PCNA and Gadd45. Oncogene 10(12):2427–2433
Halmosi R, Berente Z, Osz E, Toth K, Literati-Nagy P, Sumegi B (2001) Effect of poly(ADP-ribose) polymerase inhibitors on the ischemia-reperfusion-induced oxidative cell damage and mitochondrial metabolism in Langendorff heart perfusion system. Mol Pharmacol 59(6):1497–1505
Hamza MS, Pott S, Vega VB, Thomsen JS, Kandhadayar GS, Ng PW, Chiu KP, Pettersson S, Wei CL, Ruan Y, Liu ET (2009) De-novo identification of PPARgamma/RXR binding sites and direct targets during adipogenesis. PLoS ONE 4(3):e4907
Harkin DP, Bean JM, Miklos D, Song YH, Truong VB, Englert C, Christians FC, Ellisen LW, Maheswaran S, Oliner JD, Haber DA (1999) Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell 97(5):575–586
Hartman AR, Ford JM (2002) BRCA1 induces DNA damage recognition factors and enhances nucleotide excision repair. Nat Genet 32(1):180–184
Higgins S, Wong SH, Richner M, Rowe CL, Newgreen DF, Werther GA, Russo VC (2009) Fibroblast growth factor 2 reactivates G1 checkpoint in SK-N-MC cells via regulation of p21, inhibitor of differentiation genes (Id1-3), and epithelium-mesenchyme transition-like events. Endocrinology 150(9):4044–4055
Higgs MR, Lerat H, Pawlotsky JM (2010) Downregulation of Gadd45beta expression by hepatitis C virus leads to defective cell cycle arrest. Cancer Res 70(12):4901–4911
Hildesheim J, Belova GI, Tyner SD, Zhou X, Vardanian L, Fornace AJJ (2004) Gadd45a regulates matrix metalloproteinases by suppressing DeltaNp63alpha and beta-catenin via p38 MAP kinase and APC complex activation. Oncogene 23(10):1829–1837
Hildesheim J, Bulavin DV, Anver MR, Alvord WG, Hollander MC, Vardanian L, Fornace AJJ (2002) Gadd45a protects against UV irradiation-induced skin tumors, and promotes apoptosis and stress signaling via MAPK and p53. Cancer Res 62(24):7305–7315
Hoffman B, Liebermann DA (2007) Role of gadd45 in myeloid cells in response to hematopoietic stress. Blood Cells Mol Dis 39(3):344–347. doi:S1079-9796(07)00127-1
Hoffman B, Liebermann DA (2013) Gadd45 in modulation of solid tumors and leukemia. Adv Exp Med Biol 793:21–33
Hoggard N, Hey Y, Brintnell B, James L, Jones D, Mitchell E, Weissenbach J, Varley JM (1995) Identification and cloning in yeast artificial chromosomes of a region of elevated loss of heterozygosity on chromosome 1p31.1 in human breast cancer. Genomics 30(2):233–243
Hollander MC, Sheikh MS, Bulavin DV, Lundgren K, Augeri-Henmueller L, Shehee R, Molinaro TA, Kim KE, Tolosa E, Ashwell JD, Rosenberg MP, Zhan Q, Fernandez-Salguero PM, Morgan WF, Deng CX, Fornace AJ Jr (1999) Genomic instability in Gadd45a-deficient mice. Nat Genet 23(2):176–184
Hollander MC, Kovalsky O, Salvador JM, Kim KE, Patterson AD, Haines DC, Fornace AJ Jr (2001) Dimethylbenzanthracene carcinogenesis in Gadd45α-null mice is associated with decreased DNA repair and increased mutation frequency. Cancer Res 61(6):2487–2491
Hsu YC, Huang TY, Chen MJ (2014) Therapeutic ROS targeting of GADD45γ in the induction of G2/M arrest in primary human colorectal cancer cell lines by cucurbitacin E. Cell Death Dis 5:e1198
Hubbard BP, Sinclair DA (2014) Small molecule SIRT1 activators for the treatment of ageing and age-related diseases. Trends Pharmacol Sci 35(3):146–154
Igotti Abramova MV, Pojidaeva AK, Filippova EA, Gnedina OO, Svetlikova SB, Pospelov VA (2014) HDAC inhibitors induce apoptosis but not cellular senescence in Gadd45α-deficient E1A+ Ras cells. Int J Biochem Cell Biol 51:102–110
Jackson JG, Pereira-Smith OM (2006) p53 is preferentially recruited to the promoters of growth arrest genes p21 and GADD45 during replicative senescence of normal human fibroblasts. Cancer Res 66(17):8356–8360
Jang ER, Choi JD, Park MA, Jeong G, Cho H, Lee JS (2010) ATM modulates transcription in response to histone deacetylase inhibition as part of its DNA damage response. Exp Mol Med 42(3):195–204
Ji C, Mehrian-Shai R, Chan C, Hsu YH, Kaplowitz N (2005) Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol Clin Exp Res 29(8):1496–1503
Jiang F, Wang Z (2004) Gadd45gamma is androgen-responsive and growth-inhibitory in prostate cancer cells. Mol Cell Endocrinol 213(2):121–129. doi:10.1016/j.mce.2003.10.050
Jin S, Zhao H, Fan F, Blanck P, Fan W, Colchagie AB, Fornace AJ Jr, Zhan Q (2000) BRCA1 activation of the GADD45 promoter. Oncogene 19(35):4050–4057
Jin S, Fan F, Fan W, Zhao H, Tong T, Blanck P, Alomo I, Rajasekaran B, Zhan Q (2001) Transcription factors Oct-1 and NF-YA regulate the p53-independent induction of the GADD45 following DNA damage. Oncogene 20(21):2683–2690
Jinawath N, Vasoontara C, Yap KL, Thiaville MM, Nakayama K, Wang TL, Shih IM (2009) NAC-1, a potential stem cell pluripotency factor, contributes to paclitaxel resistance in ovarian cancer through inactivating Gadd45 pathway. Oncogene 28(18):1941–1948
Johnen H, González-Silva L, Carramolino L, Flores JM, Torres M, Salvador JM (2013) Gadd45g is essential for primary sex determination, male fertility and testis development. PLoS ONE 8(3):e58751
Ju S, Zhu Y, Liu L, Dai S, Li C, Chen E, He Y, Zhang X, Lu B (2009) Gadd45b and Gadd45g are important for anti-tumor immune responses. Eur J Immunol 39(11):3010–3018
Ju Y, Xu T, Zhang H, Yu A (2014) FOXO1-dependent DNA damage repair is regulated by JNK in lung cancer cells. Int J Oncol 44(4):1284–1292
Jung HJ, Kim EH, Mun JY, Park S, Smith ML, Han SS, Seo YR (2007) Base excision DNA repair defect in Gadd45a-deficient cells. Oncogene 26(54):7517–7525
Kastan MB, Zhan Q, el-Deiry WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Vogelstein B, Fornace AJ Jr (1992) A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 71(4):587–597
Keil E, Höcker R, Schuster M, Essmann F, Ueffing N, Hoffman B, Liebermann DA, Pfeffer K, Schulze-Osthoff K, Schmitz I (2013) Phosphorylation of Atg5 by the Gadd45β-MEKK4-p38 pathway inhibits autophagy. Cell Death Differ 20(2):321–332
Ko YG, Kang YS, Park H, Seol W, Kim J, Kim T, Park HS, Choi EJ, Kim S (2001) Apoptosis signal-regulating kinase 1 controls the proapoptotic function of death-associated protein (Daxx) in the cytoplasm. J Biol Chem 276(42):39103–39106
Kobayashi Y, Furukawa-Hibi Y, Chen C, Horio Y, Isobe K, Ikeda K, Motoyama N (2005) SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int J Mol Med 16(2):237–243
Kovalsky O, Lung FD, Roller PP, Fornace AJ Jr (2001) Oligomerization of human Gadd45a protein. J Biol Chem 276(42):39330–39339
Kumar SR, Hosokawa M, Miyashita K (2013) Fucoxanthin: a marine carotenoid exerting anti-cancer effects by affecting multiple mechanisms. Mar Drugs 11(12):5130–5147
Le May N, Egly JM, Coin F (2010) True lies: the double life of the nucleotide excision repair factors in transcription and DNA repair. J Nucleic Acids 2010:616342
Li X, Ding X, Adrian TE (2003) Arsenic trioxide induces apoptosis in pancreatic cancer cells via changes in cell cycle, caspase activation, and GADD expression. Pancreas 27(2):174–179
Liebermann DA, Hoffman B (1994) Differentiation primary response genes and proto-oncogenes as positive and negative regulators of terminal hematopoietic cell differentiation. Stem Cells 12(4):352–369
Liebermann DA, Hoffman B (2003) Myeloid differentiation (MyD) primary response genes in hematopoiesis. Blood Cells Mol Dis 31(2):213–228
Liebermann DA, Tront JS, Sha X, Mukherjee K, Mohamed-Hadley A, Hoffman B (2011) Gadd45 stress sensors in malignancy and leukemia. Crit Rev Oncog 16(1–2):129–140
Lindstrom TM, Robinson WH (2010) Rheumatoid arthritis: a role for immunosenescence? J Am Geriatr Soc 58(8):1565–1575
Liu L, Tran E, Zhao Y, Huang Y, Flavell R, Lu B (2005) Gadd45 beta and Gadd45 gamma are critical for regulating autoimmunity. J Exp Med 202(10):1341–1347
López-Novoa JM, Nieto MA (2009) Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med 1(6–7):303–314
Lu B, Yu H, Chow C, Li B, Zheng W, Davis RJ, Flavell RA (2001) GADD45gamma mediates the activation of the p38 and JNK MAP kinase pathways and cytokine production in effector TH1 cells. Immunity 14(5):583–590
Ma DK, Guo JU, Ming GL, Song H (2009) DNA excision repair proteins and Gadd45 as molecular players for active DNA demethylation. Cell Cycle 8(10):1526–1531
MacLachlan TK, Somasundaram K, Sgagias M, Shifman Y, Muschel RJ, Cowan KH, El-Deiry WS (2000) BRCA1 effects on the cell cycle and the DNA damage response are linked to altered gene expression. J Biol Chem 275(4):2777–2785
Mao Z, Tian X, Van Meter M, Ke Z, Gorbunova V, Seluanov A (2012) Sirtuin 6 (SIRT6) rescues the decline of homologous recombination repair during replicative senescence. Proc Natl Acad Sci U S A 109(29):11800–11805
Michaelis KA, Knox AJ, Xu M, Kiseljak-Vassiliades K, Edwards MG, Geraci M, Kleinschmidt-DeMasters BK, Lillehei KO, Wierman ME (2011) Identification of growth arrest and DNA-damage-inducible gene beta (GADD45beta) as a novel tumor suppressor in pituitary gonadotrope tumors. Endocrinology 152(10):3603–3613
Moskalev A, Plyusnina E, Shaposhnikov M, Shilova L, Kazachenok A, Zhavoronkov A (2012a) The role of D-GADD45 in oxidative, thermal and genotoxic stress resistance. Cell Cycle 11(22):4222–4241
Moskalev A, Smit-McBride Z, Shaposhnikov M, Plyusnina E, Zhavoronkov A, Budovsky A, Tacutu R, Fraifeld VE (2012b) Gadd45 proteins: relevance to ageing, longevity and age-related pathologies. Ageing Res Rev 11(1):51–66
Moskalev A, Aliper A, Smit-McBride Z, Buzdin A, Zhavoronkov A (2014) Genetics and epigenetics of ageing and longevity. Cell Cycle 13(7):1063–1077. doi:10.4161/cc.28433
Muñoz-Najar U, Sedivy JM (2011) Epigenetic control of ageing. Antioxid Redox Signal 14(2):241–259
Na YK, Lee SM, Hong HS, Kim JB, Park JY, Kim DS (2010) Hypermethylation of growth arrest DNA-damage-inducible gene 45 in non-small cell lung cancer and its relationship with clinicopathologic features. Mol Cells 30(1):89–92
Nakayama K, Hara T, Hibi M, Hirano T, Miyajima A (1999) A novel oncostatin M-inducible gene OIG37 forms a gene family with MyD118 and GADD45 and negatively regulates cell growth. J Biol Chem 274(35):24766–24772
Nakayama A, Kawasaki H, Jin C, Munekata E, Taira K, Yokoyama KK (2001) Transcriptional regulation of interferon gamma gene by p300 co-activator. Nucleic Acids Res 1:89–90
Niehrs C, Schäfer A (2012) Active DNA demethylation by Gadd45 and DNA repair. Trends Cell Biol 22(4):220–227
O’Prey J, Brown J, Fleming J, Harrison PR (2003) Effects of dietary flavonoids on major signal transduction pathways in human epithelial cells. Biochem Pharmacol 66(11):2075–2088
Oh-Hashi K, Maruyama W, Isobe K (2001) Peroxynitrite induces GADD34, 45, and 153 VIA p38 MAPK in human neuroblastoma SH-SY5Y cells. Free Radic Biol Med 30(2):213–221
Ozawa S, Gamou T, Habano W, Inoue K, Yoshida M, Nishikawa A, Nemoto K, Degawa M (2011) Altered expression of GADD45 genes during the development of chemical-mediated liver hypertrophy and liver tumor promotion in rats. J Toxicol Sci 36(5):613–623
Papa S, Zazzeroni F, Bubici C, Jayawardena S, Alvarez K, Matsuda S, Nguyen DU, Pham CG, Nelsbach AH, Melis T, De Smaele E, Tang WJ, D’Adamio L, Franzoso G (2004a) Gadd45 beta mediates the NF-kappa B suppression of JNK signalling by targeting MKK7/JNKK2. Nat Cell Biol 6(2):146–153
Papa S, Zazzeroni F, Pham CG, Bubici C, Franzoso G (2004b) Linking JNK signaling to NF-kappaB: a key to survival. J Cell Sci 117(Pt 22):5197–5208
Papa S, Zazzeroni F, Fu YX, Bubici C, Alvarez K, Dean K, Christiansen PA, Anders RA, Franzoso G (2008) Gadd45beta promotes hepatocyte survival during liver regeneration in mice by modulating JNK signaling. J Clin Invest 118(5):1911–1923
Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ, Miwa S, Olijslagers S, Hallinan J, Wipat A, Saretzki G, Rudolph KL, Kirkwood TB, von Zglinicki T (2010) Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol 6:347
Peretz G, Bakhrat A, Abdu U (2007) Expression of the Drosophila melanogaster GADD45 homolog (CG11086) affects egg asymmetric development that is mediated by the c-Jun N-terminal kinase pathway. Genetics 177(3):1691–1702
Platanias LC (2003) Map kinase signaling pathways and hematologic malignancies. Blood 101(12):4667–4679
Plyusnina EN, Shaposhnikov MV, Moskalev AA (2011) Increase of Drosophila melanogaster lifespan due to D-GADD45 overexpression in the nervous system. Biogerontology 12(3):211–226
Plyusnina EN, Shaposhnikov MV, Moskalev AA (2012) Geroprotective effects of activation of D-GADD45 DNA reparation gene in Drosophila melanogaster nervous system. Bull Exp Biol Med 152(3):340–343
Qiu W, Zhou B, Zou H, Liu X, Chu PG, Lopez R, Shih J, Chung C, Yen Y (2004) Hypermethylation of growth arrest DNA damage-inducible gene 45 beta promoter in human hepatocellular carcinoma. Am J Pathol 165(5):1689–1699
Rai K, Huggins IJ, James SR, Karpf AR, Jones DA, Cairns BR (2008) DNA demethylation in zebrafish involves the coupling of a deaminase, a glycosylase, and gadd45. Cell 135(7):1201–1212
Reed BD, Charos AE, Szekely AM, Weissman SM, Snyder M (2008) Genome-wide occupancy of SREBP1 and its partners NFY and SP1 reveals novel functional roles and combinatorial regulation of distinct classes of genes. PLoS Genet 4(7):e1000133
Saha A, Kuzuhara T, Echigo N, Suganuma M, Fujiki H (2010) New role of (-)-epicatechin in enhancing the induction of growth inhibition and apoptosis in human lung cancer cells by curcumin. Cancer Prev Res (Phila) 3(8):953–962
Salerno DM, Tront JS, Hoffman B, Liebermann DA (2012) Gadd45a and Gadd45b modulate innate immune functions of granulocytes and macrophages by differential regulation of p38 and JNK signaling. J Cell Physiol 227(11):3613–3620
Salvador JM, Brown-Clay JD, Fornace AJJ (2013) Gadd45 in stress signaling, cell cycle control, and apoptosis. Adv Exp Med Biol 793:1–19
Santiard-Baron D, Gosset P, Nicole A, Sinet PM, Christen Y, Ceballos-Picot I (1999) Identification of beta-amyloid-responsive genes by RNA differential display: early induction of a DNA damage-inducible gene, gadd45. Exp Neurol 158(1):206–213
Santiard-Baron D, Lacoste A, Ellouk-Achard S, Soulie C, Nicole A, Sarasin A, Ceballos-Picot I (2001) The amyloid peptide induces early genotoxic damage in human preneuron NT2. Mutat Res 479(1–2):113–120
Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, Yamada KA, Imai S (2013) Sirt1 extends life span and delays ageing in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab 18(3):416–430
Satomi Y (2012) Fucoxanthin induces GADD45A expression and G1 arrest with SAPK/JNK activation in LNCap human prostate cancer cells. Anticancer Res 32(3):807–813
Schäfer A, Schomacher L, Barreto G, Döderlein G, Niehrs C (2010) Gemcitabine functions epigenetically by inhibiting repair mediated DNA demethylation. PLoS ONE 5(11):e14060
Schmitz KM, Schmitt N, Hoffmann-Rohrer U, Schäfer A, Grummt I, Mayer C (2009) TAF12 recruits Gadd45a and the nucleotide excision repair complex to the promoter of rRNA genes leading to active DNA demethylation. Mol Cell 33(3):344–353
Schmitz I (2013) Gadd45 proteins in immunity. Adv Exp Med Biol 793:51–68. doi:10.1007/978-1-4614-8289-5_4
Schomacher L (2013) Mammalian DNA demethylation: multiple faces and upstream regulation. Epigenetics 8(7):679–684
Schrag JD, Jiralerspong S, Banville M, Jaramillo ML, O’Connor-McCourt MD (2008) The crystal structure and dimerization interface of GADD45gamma. Proc Natl Acad Sci U S A 105(18):6566–6571
Scuto A, Kirschbaum M, Buettner R, Kujawski M, Cermak JM, Atadja P, Jove R (2013) SIRT1 activation enhances HDAC inhibition-mediated upregulation of GADD45G by repressing the binding of NF-κB/STAT3 complex to its promoter in malignant lymphoid cells. Cell Death Dis 4:e635
Selvakumaran M, Lin HK, Sjin RT, Reed JC, Liebermann DA, Hoffman B (1994) The novel primary response gene MyD118 and the proto-oncogenes myb, myc, and bcl-2 modulate transforming growth factor beta 1-induced apoptosis of myeloid leukemia cells. Mol Cell Biol 14(4):2352–2360
Sharova LV, Sharov AA, Nedorezov T, Piao Y, Shaik N, Ko MS (2009) Database for mRNA half-life of 19 977 genes obtained by DNA microarray analysis of pluripotent and differentiating mouse embryonic stem cells. DNA Res 16(1):45–58
Smith ML, Chen IT, Zhan Q, Bae I, Chen CY, Gilmer TM, Kastan MB, O’Connor PM, Fornace AJ Jr (1994) Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science 266(5189):1376–1380
Stokes AH, Freeman WM, Mitchell SG, Burnette TA, Hellmann GM, Vrana KE (2002) Induction of GADD45 and GADD153 in neuroblastoma cells by dopamine-induced toxicity. Neurotoxicology 23(6):675–684
Sultan FA, Sweatt JD (2013) The role of the Gadd45 family in the nervous system: a focus on neurodevelopment, neuronal injury, and cognitive neuroepigenetics. Adv Exp Med Biol 793:81–119
Sun L, Gong R, Wan B, Huang X, Wu C, Zhang X, Zhao S, Yu L (2003) GADD45gamma, down-regulated in 65 % hepatocellular carcinoma (HCC) from 23 chinese patients, inhibits cell growth and induces cell cycle G2/M arrest for hepatoma Hep-G2 cell lines. Mol Biol Rep 30(4):249–253
Sun X, Fontaine JM, Rest JS, Shelden EA, Welsh MJ, Benndorf R (2004) Interaction of human HSP22 (HSPB8) with other small heat shock proteins. J Biol Chem 279(4):2394–2402
Sytnikova YA, Kubarenko AV, Schäfer A, Weber AN, Niehrs C (2011) Gadd45a is an RNA binding protein and is localized in nuclear speckles. PLoS ONE 6(1):e14500
Takeda S, Matsuo K, Yaji K, Okajima-Miyazaki S, Harada M, Miyoshi H, Okamoto Y, Amamoto T, Shindo M, Omiecinski CJ, Aramaki H (2011) (–)-Xanthatin selectively induces GADD45γ and stimulates caspase-independent cell death in human breast cancer MDA-MB-231 cells. Chem Res Toxicol 24(6):855–865
Takeda S, Nishimura H, Koyachi K, Matsumoto K, Yoshida K, Okamoto Y, Amamoto T, Shindo M, Aramaki H (2013) (–)-Xanthatin induces the prolonged expression of c-Fos through an N-acetyl-L-cysteine (NAC)-sensitive mechanism in human breast cancer MDA-MB-231 cells. J Toxicol Sci 38(4):547–557
Takekawa M, Saito H (1998) A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell 95(4):521–530
Thum T, Borlak J (2008) LOX-1 receptor blockade abrogates oxLDL-induced oxidative DNA damage and prevents activation of the transcriptional repressor Oct-1 in human coronary arterial endothelium. J Biol Chem 283(28):19456–19464. doi:M708309200
Tian J, Locker J (2013) Gadd45 in the liver: signal transduction and transcriptional mechanisms. Adv Exp Med Biol 793:69–80
Tian J, Huang H, Hoffman B, Liebermann DA, Ledda-Columbano GM, Columbano A, Locker J (2011) Gadd45β is an inducible coactivator of transcription that facilitates rapid liver growth in mice. J Clin Invest 121(11):4491–4502
Tornatore L, Marasco D, Dathan N, Vitale RM, Benedetti E, Papa S, Franzoso G, Ruvo M, Monti SM (2008) Gadd45beta forms a homodimeric complex that binds tightly to MKK7. J Mol Biol 378(1):97–111
Torp R, Su JH, Deng G, Cotman CW (1998) GADD45 is induced in Alzheimer’s disease, and protects against apoptosis in vitro. Neurobiol Dis 5(4):245–252
Tran H, Brunet A, Grenier JM, Datta SR, Fornace AJ Jr, DiStefano PS, Chiang LW, Greenberg ME (2002) DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science 296(5567):530–534
Tront JS, Hoffman B, Liebermann DA (2006) Gadd45a suppresses Ras-driven mammary tumorigenesis by activation of c-Jun NH2-terminal kinase and p38 stress signaling resulting in apoptosis and senescence. Cancer Res 66(17):8448–8454
Tront JS, Huang Y, Fornace AJJ, Hoffman B, Liebermann DA (2010) Gadd45a functions as a promoter or suppressor of breast cancer dependent on the oncogenic stress. Cancer Res 70(23):9671–9681
Vairapandi M, Balliet AG, Fornace AJ Jr, Hoffman B, Liebermann DA (1996) The differentiation primary response gene MyD118, related to GADD45, encodes for a nuclear protein which interacts with PCNA and p21WAF1/CIP1. Oncogene 12(12):2579–2594
Vairapandi M, Azam N, Balliet AG, Hoffman B, Liebermann DA (2000) Characterization of MyD118, Gadd45, and proliferating cell nuclear antigen (PCNA) interacting domains. PCNA impedes MyD118 AND Gadd45-mediated negative growth control. J Biol Chem 275(22):16810–16819
Vairapandi M, Balliet AG, Hoffman B, Liebermann DA (2002) GADD45b and GADD45g are cdc2/cyclinB1 kinase inhibitors with a role in S and G2/M cell cycle checkpoints induced by genotoxic stress. J Cell Physiol 192(3):327–338
Wang XW, Zhan Q, Coursen JD, Khan MA, Kontny HU, Yu L, Hollander MC, O’Connor PM, Fornace AJ Jr, Harris CC (1999) GADD45 induction of a G2/M cell cycle checkpoint. Proc Natl Acad Sci USA 96(7):3706–3711
Whitlock NA, Lindsey K, Agarwal N, Crosson CE, Ma JX (2005) Heat shock protein 27 delays Ca2+-induced cell death in a caspase-dependent and -independent manner in rat retinal ganglion cells. Invest Ophthalmol Vis Sci 46(3):1085–1091
Wolfson M, Budovsky A, Tacutu R, Fraifeld V (2009) The signaling hubs at the crossroad of longevity and age-related disease networks. Int J Biochem Cell Biol 41(3):516–520
Wu Y, Zhang X, Bardag-Gorce F, Robel RC, Aguilo J, Chen L, Zeng Y, Hwang K, French SW, Lu SC, Wan YJ (2004) Retinoid X receptor alpha regulates glutathione homeostasis and xenobiotic detoxification processes in mouse liver. Mol Pharmacol 65(3):550–557
Xiong Y, Liebermann DA, Tront JS, Holtzman EJ, Huang Y, Hoffman B, Geifman-Holtzman O (2009) Gadd45a stress signaling regulates sFlt-1 expression in preeclampsia. J Cell Physiol 220(3):632–639
Xiong Y, Liebermann DA, Holtzman EJ, Jeronis S, Hoffman B, Geifman-Holtzman O (2013) Preeclampsia-associated stresses activate Gadd45a signaling and sFlt-1 in placental explants. J Cell Physiol 228(2):362–370
Yang Q, Manicone A, Coursen JD, Linke SP, Nagashima M, Forgues M, Wang XW (2000) Identification of a functional domain in a GADD45-mediated G2/M checkpoint. J Biol Chem 275(47):36892–36898
Yang J, Zhu H, Murphy TL, Ouyang W, Murphy KM (2001) IL-18-stimulated GADD45 beta required in cytokine-induced, but not TCR-induced IFN-gamma production. Nat Immunol 2(2):157–164
Yang Z, Song L, Huang C (2009) Gadd45 proteins as critical signal transducers linking NF-kappaB to MAPK cascades. Curr Cancer Drug Targets 9(8):915–930
Yin F, Bruemmer D, Blaschke F, Hsueh WA, Law RE, Herle AJ (2004) Signaling pathways involved in induction of GADD45 gene expression and apoptosis by troglitazone in human MCF-7 breast carcinoma cells. Oncogene 23(26):4614–4623
Ying J, Srivastava G, Hsieh WS, Gao Z, Murray P, Liao SK, Ambinder R, Tao Q (2005) The stress-responsive gene GADD45G is a functional tumor suppressor, with its response to environmental stresses frequently disrupted epigenetically in multiple tumors. Clin Cancer Res 11(18):6442–6449
Yoo J, Ghiassi M, Jirmanova L, Balliet AG, Hoffman B, Fornace AJJ, Liebermann DA, Bottinger EP, Roberts AB (2003) Transforming growth factor-beta-induced apoptosis is mediated by Smad-dependent expression of GADD45b through p38 activation. J Biol Chem 278(44):43001–43007
Yoshida T, Maeda A, Horinaka M, Shiraishi T, Nakata S, Wakada M, Yogosawa S, Sakai T (2005) Quercetin induces gadd45 expression through a p53-independent pathway. Oncol Rep 14(5):1299–1303
Zerbini LF, Wang Y, Czibere A, Correa RG, Cho JY, Ijiri K, Wei W, Joseph M, Gu X, Grall F, Goldring MB, Zhou JR, Libermann TA (2004) NF-kappa B-mediated repression of growth arrest- and DNA-damage-inducible proteins 45alpha and gamma is essential for cancer cell survival. Proc Natl Acad Sci U S A 101(37):13618–13623
Zerbini LF, Libermann TA (2005) Life and death in cancer. GADD45 alpha and gamma are critical regulators of NF-kappaB mediated escape from programmed cell death. Cell Cycle 4(1):18–20
Zhan Q, Lord KA, Alamo I Jr, Hollander MC, Carrier F, Ron D, Kohn KW, Hoffman B, Liebermann DA, Fornace AJ Jr (1994) The gadd and MyD genes define a novel set of mammalian genes encoding acidic proteins that synergistically suppress cell growth. Mol Cell Biol 14(4):2361–2371
Zhan Q, Kontny U, Iglesias M, Alamo I Jr, Yu K, Hollander MC, Woodworth CD, Fornace AJ Jr (1999) Inhibitory effect of Bcl-2 on p53-mediated transactivation following genotoxic stress. Oncogene 18(2):297–304
Zhang W, Bae I, Krishnaraju K, Azam N, Fan W, Smith K, Hoffman B, Liebermann DA (1999) CR6: A third member in the MyD118 and Gadd45 gene family which functions in negative growth control. Oncogene 18(35):4899–4907
Zhang W, Hoffman B, Liebermann DA (2001) Ectopic expression of MyD118/Gadd45/CR6 (Gadd45beta/alpha/gamma) sensitizes neoplastic cells to genotoxic stress-induced apoptosis. Int J Oncol 18(4):749–757
Zhang N, Ahsan MH, Zhu L, Sambucetti LC, Purchio AF, West DB (2005) NF-kappaB and not the MAPK signaling pathway regulates GADD45beta expression during acute inflammation. J Biol Chem 280(22):21400–21408
Zhang W, Li T, Shao Y, Zhang C, Wu Q, Yang H, Zhang J, Guan M, Yu B, Wan J (2010) Semi-quantitative detection of GADD45-gamma methylation levels in gastric, colorectal and pancreatic cancers using methylation-sensitive high-resolution melting analysis. J Cancer Res Clin Oncol 136(8):1267–1273
Zhang L, Yang Z, Liu Y (2014a) GADD45 proteins: roles in cellular senescence and tumor development. Exp Biol Med (Maywood) 239(7):773–778
Zhang L, Yang Z, Ma A, Qu Y, Xia S, Xu D, Ge C, Qiu B, Xia Q, Li J, Liu Y (2014b) Growth arrest and DNA damage 45G down-regulation contributes to Janus kinase/signal transducer and activator of transcription 3 activation and cellular senescence evasion in hepatocellular carcinoma. Hepatology 59(1):178–189
Zhu N, Shao Y, Xu L, Yu L, Sun L (2009) Gadd45-alpha and Gadd45-gamma utilize p38 and JNK signaling pathways to induce cell cycle G2/M arrest in Hep-G2 hepatoma cells. Mol Biol Rep 36(8):2075–2085
Zoncu R, Efeyan A, Sabatini DM (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12(1):21–35
Acknowledgments
This work was supported by Russian Science Foundation grant N 14-50-00060.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Moskalev, A.A., Proshkina, E.N., Shaposhnikov, M.V. (2015). Gadd45 Proteins in Aging and Longevity of Mammals and Drosophila . In: Vaiserman, A., Moskalev, A., Pasyukova, E. (eds) Life Extension. Healthy Ageing and Longevity, vol 3. Springer, Cham. https://doi.org/10.1007/978-3-319-18326-8_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-18326-8_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-18325-1
Online ISBN: 978-3-319-18326-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)