Abstract
The neurodegeneration is one of the features of aging and age-related disorders. Yet, only several antiaging interventions are known to affect the processes of neurodegeneration. Here we show that overexpression of the pro-longevity gene D-GADD45 in Drosophila neurons leads to a postponed manifestation of histological and ultrastructural features of age-dependent neurodegeneration, such as decrease in the packing density of neurons, increasing the degree of neuron cytoplasmic vacuolization, and morphological defects of mitochondrial cristae. Thus, the previously observed (Plyusnina, Biogerontology 12: 211–226, 2011) life extending effect of D-GADD45 overexpression in the nervous system is associated with delayed neurodegeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aging process in different models is associated with progressive degeneration of the nervous system (Miquel et al. 1979; Lee et al. 2000). Also, the neurodegeneration characterizes premature aging syndromes in human, such as Hutchinson-Gilford syndrome and Werner’s syndrome (Coppede and Migliore 2010), as well as causes age-related diseases such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis (Boerrigter et al. 1992; Coppede and Migliore 2010). The aging as well as most of the neurodegenerative diseases and progeroid syndromes is characterized by an accumulation of DNA damages, suggesting that the impaired DNA repair mechanisms and altered DNA-damage responses might be relevant to both aging process and neurodegeneration (Coppede and Migliore 2010; Moskalev et al. 2013).
Recently, we have shown that overexpression of the DNA repair gene Gadd45 (Growth Arrest and DNA Damage-inducible 45) in the nervous system significantly increases Drosophila lifespan without a decrease in fecundity and locomotor activity (Plyusnina et al. 2011). Proteins of the GADD45 family are involved in stress signaling in response to different physiological or environmental stress conditions (Moskalev et al. 2012a, b). Depending on the level of DNA damages, activation of mammalian GADD45s results in cell cycle arrest, DNA repair, cell senescence or apoptosis (Moskalev et al. 2012b). The flies with D-GADD45 overexpression in the nervous system were more stress resistant than ones without overexpression (Moskalev et al. 2012a). Some evidence has been accumulated pointing to an involvement of Gadd45 proteins in age-related pathologies, including neurodegeneration (Moskalev et al. 2012b). For example, Gadd45 is highly expressed in neurons of Alzheimer’s disease patients and protects the neurons from apoptosis, induced by extracellular accumulation of β-amyloid (Torp et al. 1998; Santiard-Baron et al. 1999, 2001). The upregulation of Gadd45 was also observed in the in vitro model (human neuroblastoma cells) of dopamine-induced neurotoxicity (Stokes et al. 2002). An increased expression of Gadd45 in age-related neurodegeneration is likely a protective mechanism, aimed at coping with the neurotoxic stress.
Here we show in Drosophila model that D-GADD45 overexpression in the nervous system alters histological and ultrastructural features of the aging brain and mitigates some signs of neurodegeneration.
Materials and methods
Drosophila melanogaster strains
All stocks were maintained and raised under standard conditions. Expression patterns of the D-GADD45 gene were visualized by using of the D-GADD45-GFP strain, containing a construct with 2 kb of the D-GADD45 promoter region, fused with a GFP coding frame, additionally destabilized by a degradation domain of mouse ornithine decarboxylase (half-life time 8 h) (Moskalev et al. 2012a). We also used the UAS-GFP (#4776, Drosophila Stock Center, Bloomington, USA) strain and drivers D-GADD45-GAL4 (#112149, Drosophila Genetic Resource Center, Kyoto, Japan) and Hsp70-GAL4 (#1799, Drosophila Stock Center, Bloomington, USA).
Neuron specific activation of the D-GADD45 gene was achieved with the UAS-D-GADD45 strain (a kind gift from Dr. Uri Abdu, Ben-Gurion University, Israel) and the Elav-GS-GAL4 strain, carrying the mifepristone-inducible GAL4 driver in the nervous system (kindly granted by Dr. Haig Keshishian, Yale University, USA). Previously it was shown (Plyusnina et al. 2011), the relative D-GADD45 expression values in the nervous tissue of flies with Elav-GS-GAL4/UAS-D-GADD45 genotype not treated with mifepristone exhibited a threefold overexpression in males and twofold overexpression in females.
For conditional overexpression of the D-GADD45 gene in the Drosophila nervous system, UAS-D-GADD45 females were crossed with Elav-GS-GAL4 males, and the offspring were kept on a medium with conditional inducer of GAL4 expression RU486/mifepristone (Sigma, USA). It is known that mifepristone on its own does not affect the Drosophila melanogaster lifespan (Ford et al. 2007); therefore the changes in the studied parameters of experimental animals were caused by D-GADD45 overexpression and/or genetic background. Flies, obtained by crossing of UAS-D-GADD45 females with Elav-GS-GAL4 males and kept on a medium without mifepristone, as well as flies of the parental UAS-D-GADD45 genotype were used as a control when studying the effects of conditional D-GADD45 overexpression. For mifepristone treatment, stock solution of mifepristone with 25 mg/ml concentration in 100 % ethanol was used. It was mixed with yeast paste (60.4 % water, 39 % active yeast, 0.5 % acetic acid, 0.1 % ethanol) in a proportion of 0.1 ml initial mifepristone solution per 100 ml yeast paste. 0.3 ml of prepared yeast paste was added to the surface of the medium (Poirier et al. 2008). For experiments without mifepristone, the medium surface was covered with yeast paste that was prepared in the same way, but with an equivalent amount of ethyl alcohol, added instead of the mifepristone solution. Since Ford et al. (2007) showed that mifepristone in it self does not affect males life span we inspect if there are any effect in females (see Supplementary Material) and showed the that females life span is indeed not affected by this substance as well.
Normal D-GADD45 expression pattern
To study the natural expression of the D-GADD45 gene at the larval stage (third instar), organs was dissected in Hanks solution, fixed 20 min in 3.7 % formaldehyde, prepared on 1 × PBS, washed in 1 × PBS. GFP fluorescence of the reporter were analyzed by Leica DM4000 (Leica Microsystems, Germany) microscope (FITC filter). Adult brain were dissected in Hanks solution as was described by Gu and O’Dowd (2007). Reporter fluorescence was analyzed similarly.
Electron microscopy
To perform ultrastructural study, flies were anaesthetized, placed in 0.1 M phosphate buffer (pH 7.4) and subjected for the brains isolation. Brains were fixed in 2.5 % glutaraldehyde solution, prepared on 0.1 M phosphate buffer during 2 h, washed 3 times in 0.1 M phosphate buffer. Further, the brains were postfixed in 1 % OsO4 solution, prepared on phosphate buffer during 1 h. The material was dehydrated in ethanol of rising concentrations (50, 60, 70 % on ice during 10 min; 80, 90, 96 %––during 10 min at the room temperature and 100 %––two times for 15 min). After that, the material was contrasted in uranyl acetate and phospho-wolframic acid (5 mg/ml), dissolved in 100 % ethanol for 30 min at the room temperature. Epoxy resin treatment was done in two steps. First, the preparations was placed into the solution of equal volumes of 100 % ethanol and epon mix (Epon 812, DDSA, MNA и DMP-30) for 1 h at 37 °C, and then to epon mix without ethanol for 2 h at 37 °C. Polymerization step was done in capsules with epon mix for 48 h at 65 °C.
Results
D-GADD45 expression pattern of in vivo reporters
To understand how D-GADD45 overexpression, induced by the Elav-GS-GAL4 driver, affects adult brain structure during aging, it is necessary not only to study the brain structure at the different ages under overexpression condition, but also to inspect the spatial and time evolution of D-GADD45 expression patterns under normal conditions. To reach the aim, we used the D-GADD45-GFP strain, containing GFP-reporter of the D-GADD45 gene (Moskalev et al. 2012b) and also the flies with D-GADD45-GAL4/UAS-GFP genotype, were the UAS-GFP construct is controlled by the D-GADD45-GAL4 driver.
The expression patterns of the constructs in different larval organs are shown at Fig. 1. It is clear that the pattern of D-GADD45-GFP in the brain is wider than in the case of D-GADD45-GAL4/UAS-GFP (Fig. 1A, E). Similar observations can be done for wing (Fig. 1B, F) and leg (Fig. 1C, G) imaginal discs. Evidently, while in the case of D-GADD45-GFP the fluorescent signal present in all the gut cells, in the D-GADD45-GAL4/UAS-GFP case only a small fraction of the gut cells are expressing the reporter (Fig. 1D, H). Those data show that the D-GADD45 larval expression patterns in different organs are essentially affected by regulatory elements situated outside of the cloned 2 kb promoter zone, or by the chromatin state near the gene.
Next, we studied the D-GADD45-GFP expression in the female adult brain. Figure 2 shows that one day old females only a weak expression (compared to the larval brain) in small regions can be detected. At the later developmental stages the expression completely vanished.
We wondered whether inherent stability of GFP, once it is synthesized, may affect our results and measured the half-life of GFP in the adult brain of Hsp70-GAL4/UAS-GFP flies. Fig. 3 shows some level of GFP expression in the optic centers. Since 2 h after heat shock, the pattern is the same-not enough time for GFP synthesis. After 6 h, the intensive reporter expression can be seen. Expression occurs to be weak since 3 days after heat shock, and after 5 days is completely disappearing. So, the half time of GFP in the adult brain appears to be 3 days.
Expression dynamic of Hsp70-GAL4/UAS-GFP in the adult female brain after heat shock induction. A the GFP reporter fluorescence without heat shock treatment. Expression in optic centers can be seen. B expression since 2 h after heat shock-not enough time for GFP protein synthesis, no difference from a control. C since 6 h after heat shock, the strong expression was detected. D after 3 days the reporter expression becomes weaker. E since 5 days after heat shock, the expression becomes weaker than in not treated control. 3–4 brains per a time point. Bar = 100 μm
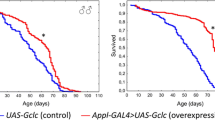
We showed that the expression patterns in the cases of D-GADD45-GFP and D-GADD45-GAL4 differ, and studied the level of D-GADD45-GAL4/UAS-GFP expression in the adult brain during aging. Figure 4 demonstrates that the expression lasts about 37 days. Taking in account that the expression of once synthesized GFP protein in the adult brain lasts 5 days, we conclude that normally D-GADD45 is expressed in the female adult brain during a month and than vanished.
D-GADD45 overexpression in the adult brain (histology and ultrastructure)
The Elav-GS-GAL4/UAS-D-GADD45 females, living on the mifepristone containing medium (experiment) and without it (control-1), were subjected to histological analysis at the 5, 45 and 55 days after eclosion. 5 days UAS-D-GADD45 females, living on the mifepristone free medium, were also studied (control-2). For the further analysis, we took the sections, containing neuropile and nearby neurons (Fig. 5). The comparison of the regions in the case of 5 days individuals from control-1 and control-2 do not show any significant morphological difference. However, at the ages of 45 and 55 days considerable difference was found between the experiment and the control.
Brain histology of the Elav-GS-GAL4/UAS-D-GADD45 females at the different ages in the case of ectopic expression of D-GADD45 and without it. A—5 days without mifepristone (control-1), B—45 days without mifepristone, C—45 days with mifepristone, D—55 days without mifepristone, E—55 days with mifepristone. Bar = 4 μm
Figure 5 illustrates histological analysis of the female brain. It could be seen that in the case of 5 days females neurons adjacent to neuropil are densely packed. In 45 days females, living without mifepristone, the neuron package are less dense. In the case of D-GADD45 overexpression the package is more dence than in the case without it. Similar observation can be done in the case of 55 days females. Thus, the D-GADD45 overexpression diminishes age related neurodegeneration.
We also undertook ultrastructural examination of age-related cellular changes in those cases. Figure 6 illustrates cytoplasm vacuolization in the case of 55 days Elav-GS-GAL4/UAS-D-GADD45 females in comparison with 5 days control-1. In the case of mifepristone addition vacuolization significantly less manifested. As it is illustrated at Fig. 7, mitochondrial cristae destruction can be detected for 45 day females without D-GADD45 overexpression, compared to the control-1 [Control-1––5 flies (395 sections), 45 days without MF—5 flies (117 sections), 45 days MF—5 flies 115 sections, 55 days without MF—5 flies (181 sections), 55 days MF—5 flies (277 sections)]. 45 days females recover cristae structure in the case of D-GADD45 overexpression. We also observed that in some cases 5 days females as well as 45 day females with overexpression contain multilayered glial wrappings around neurons (Fig. 8).
Discussion
The nervous system is characterized by a high metabolic activity, as a result of which a lot of damage is accumulated in neurons during aging (Cortopassi et al. 1992; Hamilton et al. 2001). Aging of the brain causes progression of neurological disorders which accelerate and aggravate the aging process (Lee et al. 2000). Recent researches have shown that signals, coming from the nervous system by the neuroendocrine regulation, affect the stress resistance and lifespan of the organism as a whole. For example, IGF-I and growth hormone, produced by neurosecretory cells in the brain, can regulate the lifespan in mammals (Kappeler et al. 2008; Holzenberger 2009). The gene therapy for the inhibition of NF-κB signaling in the hypothalamus or in the brain of mice delays the aging process and increases the lifespan by 20 % (Zhang et al. 2013). On the other hand, the lifespan of Caenorhabditis elegans with the mutation of the age-1 gene can be completely restored to wild genotype level upon expression of AGE-1 in just the neurons, whereas expression in other tissues results in no such effect (Morley and Morimoto 2004). Despite the fact that the nervous system is one of main targets of aging the histological and ultrastructural features of the antiaging interventions is poorly understood currently.
Recently we have shown that D-GADD45 overexpression in the imago brain extends the Drosophila life span (Plyusnina et al. 2011). Here we study the gene expression patterns in the normal conditions by two different genetic constructs. D-GADD45-GFP contains 5′ fragment of the gene regulatory zone (2 kb); the distance for 3′ end of neighboring Ady43A gene was 3.3 kb, therefore significant part of 5′ regulatory zone was under study. This regulatory fragment was fused with coding the GFP region that was also destabilized by degradation domain of mouse ornithine decarboxylase (half-time 8 h). In another approach we used the D-GADD45-GAL4 driver whose activity was visualized with the UAS-GFP reporter. The results illustrated at Fig. 1 show the D-GADD45 patterns, revealed by those two approaches differ. In all the cases D-GADD45-GFP was wider than in the case of D-GADD45-GAL4/UAS-GFP. This is very clear in the case of gut fragment, where all the cells are expressing reporter in the case of D-GADD45-GFP, while only a few cells showed fluorescence in the case of D-GADD45-GAL4/UAS-GFP. This may be a consequence of the use of incomplete regulatory zone in the D-GADD45-GFP construct or the essential role of neighboring chromatin in the regulation of normal GADD45 activity that present in the case D-GADD45-GAL4 but absent in the D-GADD45-GFP case.
Our data also showed that the D-GADD45-GFP expression vanished at the first days after eclosion. Reporter expression in the case of D-GADD45-GAL4/UAS-GFP lasts more than 30 days of the adult life (Fig. 4). To check if the difference is due to the presence of degradation domain of reporter, we studied the GFP half-time of life in the Hsp70-GAL4/UAS-GFP adult female brain after a single heat shock treatment. As it can be seen at Fig. 3, the protein degrades in 3 days. Thus, we concluded that the difference in the half time of GFP life in the cases D-GADD45-GFP and D-GADD45-GAL4/UAS-GFP could be attributed to the degradation domain. Thus, our results showed that in a study of age related gene activity it is preferable to use the D-GADD45-GAL4 insertional drivers in comparison with the regulatory zone constructs, inserted into non-homological chromatin region. In the case of D-GADD45-GAL4, more fine details of temporal and spatial aspects of the pattern could be revealed.
The comparison of temporal D-GADD45-GAL4 activity changes with already studied D-GADD45 viability curves (Plyusnina et al. 2011) shows that the gene expression vanishing correlates with a time of transition of one aging to another. It is already accepted mind that Gompertz curve correctly describes the Drosophila viability with age (Gavrilov and Gavrilova 1991). Gompertz curve has two parameters: R—a “background” component of viability (death intensity at zero time) and α—an exponential parameter of death rate. At the initial time Gompertz curve is close to the linear dependence with the R slope, at the later time the curve decrease exponentially. Our data constitute to the view that the transition from the first regime to the second occurs at the time when the D-GADD45 expression in the female brain vanished. In our opinion, the experiments similar to ours can determine if there is a time-schedule of genes expression vanishing in the brain or it is a stochastic process. Another important question—what genes are vanishing expression at the time when “linear” death is changed by “exponential” one?
Earlier study showed that the ectopic expression of D-GADD45 in the imago brain increase Drosophila life span (Plyusnina et al. 2011). At the same time, the spontaneous level of DNA damage in neuroblasts of the third instar larva with D-GADD45 overexpression was reduced (Plyusnina et al. 2011). Here we study how the ectopic expression affects brain ultrastructure. Histological study showed the effect on neurons package. The 45 days Elav-GS-GAL4/UAS-D-GADD45 individuals, living on mifepristone medium, demonstrate partial rescue of the package. Ultrastructural analysis showed a similar picture—55 days females have strong cytoplasm vacuolation. Less vacuolation was observed when D-GADD45 was expressed ectopically. Mitochondrial ultrastructure changed with the age. In our study this was reflected as the cristae morphology change. D-GADD45 ectopic expression partially restores this phenotype.
It was shown, that expression of a human Hsp70 transgene in flies suppresses neurodegeneration in the Drosophila Machado-Joseph neurodegenerative disease model (Warrick et al. 1999). Those results are consistent with ours and demonstrate a possibility to prevent age related neurodegeneration by induction of the genes responsible for cellular stress response.
We observed multilayered glial wrappings covering neurons and their axons in a control and Elav-GS-GAL4/UAS-D-GADD45 individuals, living on mifepristone, while in the case of Elav-GS-GAL4/UAS-D-GADD45 individuals without mifepritone (i.e. when the aging was rapid) those structures were not found. The frequency glial wrappings identification in the control and Elav-GS-GAL4/UAS-D-GADD45 with MF is about 0.5 per a section. Assuming the frequency of those structures is the same for Elav-GS-GAL4/UAS-D-GADD45 without MF, the probability not found any structure among 117 sections is (0.5)117—very low value. We conclude the frequency of those structures in the case of Elav-GS-GAL4/UAS-D-GADD45 without MF is very low.
Earlier Kretzschmar et al. (1997) showed that rapidly aging sws (Swiss Cheese) mutants have multilayered glial wrappings covering neurons and their axons. From one side those glial structures save neurons, however wrappings overdevelopment disrupts inter-neuron connections. We speculate that the aging in different systems may be realized by different ways in different systems.
Thus, overexpression of the pro-longevity gene D-GADD45 in Drosophila neurons affects age-dependent neurodegeneration. In old flies with ectopic D-GADD45 expression we observed partial rescue of neuronal packing density. Ultrastructural study showed that age-dependent neuron cytoplasmic vacuolation is also less manifested. Similar effect was found for morphological defects of mitochondrial cristae. Thus, D-GADD45 overexpression causes delay of the age related neurodegeneration.
References
Boerrigter ME, Wei JY, Vijg J (1992) DNA repair and Alzheimer’s disease. J Gerontol 47(6):B177–B184
Coppede F, Migliore L (2010) DNA repair in premature aging disorders and neurodegeneration. Curr Aging Sci 3(1):3–19
Cortopassi GA, Shibata D, Soong NW et al (1992) A pattern of accumulation of a somatic deletion of mitochondrial DNA in aging human tissues. Proc Natl Acad Sci USA 89(16):7370–7374
Ford D, Hoe N, Landis GN et al (2007) Alteration of Drosophila life span using conditional, tissue-specific expression of transgenes triggered by doxycyline or RU486/Mifepristone. Exp Gerontol 42(6):483–497
Gavrilov LA, Gavrilova NS (1991) The biology of life span: a quantitative approach. Harwood Academic Publishers, Chur––New York
Gu H, O’Dowd DK (2007) Whole cell recordings from brain of adult Drosophila. J Vis Exp 6:248
Hamilton ML, Van Remmen H, Drake JA et al (2001) Does oxidative damage to DNA increase with age? Proc Natl Acad Sci USA 98(18):10469–10474
Holzenberger M (2009) IGF-1 receptors in the brain control longevity in mice. Med Sci (Paris) 25(4):371–376
Kappeler L, De Magalhaes Filho C, Dupont J et al (2008) Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. PLoS Biol 6(10):e254
Kretzschmar D, Hasan G, Sharma S et al (1997) The swiss cheese mutant causes glial hyperwrapping and brain degeneration in Drosophila. J Neurosci 17(19):7425–7432
Lee CK, Weindruch R, Prolla TA (2000) Gene-expression profile of the ageing brain in mice. Nat Genet 25(3):294–297
Miquel J, Economos AC, Bensch KG et al (1979) Review of cell aging in Drosophila and mouse. Age 2(3):78–88
Morley JF, Morimoto RI (2004) Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell 15(2):657–664
Moskalev A, Plyusnina E, Shaposhnikov M et al (2012a) The role of D-GADD45 in oxidative, thermal and genotoxic stress resistance. Cell Cycle 11(22):4222–4241
Moskalev AA, Smit-McBride Z, Shaposhnikov MV et al (2012b) Gadd45 proteins: relevance to aging, longevity and age-related pathologies. Ageing Res Rev 11(1):51–66
Moskalev AA, Shaposhnikov MV, Plyusnina EN et al (2013) The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res Rev 12(2):661–684
Plyusnina EN, Shaposhnikov MV, Moskalev AA (2011) Increase of Drosophila melanogaster lifespan due to D-GADD45 overexpression in the nervous system. Biogerontology 12(3):211–226
Poirier L, Shane A, Zheng J et al (2008) Characterization of the Drosophila gene-switch system in aging studies: a cautionary tale. Aging Cell 7(5):758–770
Santiard-Baron D, Gosset P, Nicole A et al (1999) Identification of β-amyloid-responsive genes by RNA differential display: early induction of a DNA damage-inducible gene, gadd45. Exp Neurol 158(1):206–213
Santiard-Baron D, Lacoste A, Ellouk-Achard S et al (2001) The amyloid peptide induces early genotoxic damage in human preneuron NT2. Mutat Res 479(1–2):113–120
Stokes AH, Freeman WM, Mitchell SG et al (2002) Induction of GADD45 and GADD153 in neuroblastoma cells by dopamine-induced toxicity. Neurotoxicology 23(6):675–684
Torp R, Su JH, Deng G et al (1998) GADD45 is induced in Alzheimer’s disease, and protects against apoptosis in vitro. Neurobiol Dis 5(4):245–252
Warrick JM, Chan HY, Gray-Board GL et al (1999) Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70. Nat Genet 23(4):425–428
Zhang G, Li J, Purkayastha S et al (2013) Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 497(7448):211–216
Acknowledgments
The study was supported by the projects of UrB RAS No. 12-C-4-1019 and SB RAS No. 81, 82, Grant of RFBR N 14-04-01596.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bgatova, N., Dubatolova, T., Omelyanchuk, L. et al. Gadd45 expression correlates with age dependent neurodegeneration in Drosophila melanogaster . Biogerontology 16, 53–61 (2015). https://doi.org/10.1007/s10522-014-9533-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-014-9533-0