Abstract
Microorganisms (bacteria) naturally form biofilms on solid surfaces. Biofilms can be found in a variety of natural sites, such as sea water sediments, soils, and a range of wastewaters, such as municipal, dye, agricultural, and industrial wastewaters. The biofilms are normally dangerous to human health due to their inherited robustness. Electrochemically active biofilms (EABs) generated by electrochemically active microorganisms (EAMs) have potential applications in bioenergy production, green chemical synthesis, bioremediation, bio-corrosion mitigation, and biosensor development. EABs have attracted considerable attention in bioelectrochemical systems, such as microbial fuel cells (MFCs) and microbial electrolysis cells, where they act as living bio-anode or bio-cathode catalysts. EABs are an anode material in MFCs that generate an excess of electrons and protons by biologically oxidizing substrates, such as sodium acetate or organic waste, and the flow of these electrons produces significant amounts of electricity. Recently, it was found that EABs can be used as a biogenic-reducing tool to synthesize metal nanoparticles and metal–metal oxide nanocomposites. The EAB-mediated synthesis of metal nanoparticles and metal–metal oxide nanocomposites is expected to provide a new avenue for the greener synthesis of nanomaterials with high efficiency than other synthetic procedures. It was also found that EABs could be effectively used as a tool to provide electrons and protons by biologically decomposing acetate which is later used in the presence of a suitable catalyst for the bio-hydrogen production. These nanoparticles as well as nanocomposites syntheses and bio-hydrogen production takes place in water at 30 °C and does not involve any energy input which make these approaches highly efficient. These findings show that EAB is a fascinating biogenic tool for MFCs, nanomaterials synthesis, bioremediation, and bio-hydrogen production.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Electrochemically active microorganisms
- Electrochemically active biofilms
- Biogenic tool
- Microbial fuel cells
- Nanomaterials synthesis
- Bio-hydrogen production
4.1 Introduction
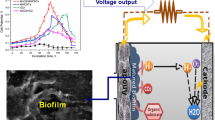
In general, microorganisms naturally form biofilms on solid surfaces for their mutual benefits such as protection from environmental strains caused by contaminants, nutritional depletion, or imbalances. Biofilms can be found in a variety of natural sites, such as sea or river water sediments, soils, and a range of wastewaters, such as domestic, municipal, dye, agricultural, and industrial wastewaters (Borole et al. 2011; Babauta et al. 2012). The biofilms are normally hazardous to human and animal health due to their inherited sturdiness and infectious nature. On the other hand, recent studies suggested that electrochemically active biofilms (EABs) (Fig. 4.1) generated by electrically active microorganisms (EAMs) have properties and potential that can be utilized to catalyze or control the electrochemical reactions in a range of applications, such as bioenergy production, biogenic chemical synthesis, bio-remediation, bio-corrosion mitigation, and bio-sensor development (Borole et al. 2011; Babauta et al. 2012; Erable et al. 2010; Rittmann et al. 2008; Halan et al. 2012; Kalathil et al. 2013a). EABs have attracted considerable attention in bioelectrochemical systems (BESs), such as microbial electrolysis cells (MECs) and microbial fuel cells (MFCs), where they act as living bio-anode or bio-cathode catalysts (Kalathil et al. 2013a). EABs are an anode material in MFCs that generate an excess of electrons and protons by biologically oxidizing substrates, such as sodium acetate or other organic wastes. The flow of these biologically generated electrons produces significant amounts of electricity, whereas the produced protons (H+) moves to the cathodic chamber of MFC where it may be reduced by electrons to H2 gas in the presence of a suitable catalyst such as gold or oxidized to H2O (Dulon et al. 2007; Logan et al. 2005; Khan et al. 2014). The discoveries of EAMs forming biofilms which are able to transfer directly electrons on electrode surfaces have boosted the development of MFCs. The mechanisms of electron transfer have been demonstrated to be either direct, involving membrane-bound cytochromes for instance, or through natural electron mediators that are produced by the microorganisms and remain entrapped in the biofilm (Bond et al. 2002). The involvement of conductive pili in electron transfer has also been demonstrated (Babauta et al. 2012; Erable et al. 2010; Rittmann et al. 2008; Halan et al. 2012). MFCs utilize microbial EABs as catalysts to convert the chemical energy contained in a large variety of organic compounds directly into electricity and various other products such as H2O, H2, etc. MFCs produce a lower power density than fuel cells but the increasing interest in sustainable energy sources is promoting intense research leading to fast improvements.
Recently, it was found that EABs can be directly used as a biogenic tool (Fig. 4.2) to synthesize metal nanoparticles and metal–metal oxide nanocomposites (Kalathil et al. 2011, 2012; Khan et al. 2012, 2013a, b; Ansari et al. 2013a). The EAB-mediated synthesis of metal nanoparticles and metal–metal oxide nanocomposites is expected to provide a new way for the greener synthesis of nanomaterials with comparatively high efficiency than the other synthetic procedures. It was also established that EABs could be effectively used as a tool to provide electrons and protons by biologically decomposing acetate which is later used in the presence of a suitable catalyst for the bio-hydrogen production (Khan et al. 2013c, 2014; Kalathil et al. 2013b). Further, it was also found that EABs could be exploited to narrow the band gap of metal oxides such as TiO2, ZnO, SnO2, and CeO2 (Kalathil et al. 2013a, b, c; Ansari et al. 2013b, 2014). These EAB-mediated nanoparticles as well as nanocomposites syntheses, bio-hydrogen production, and metal oxide modification processes do not involve any external energy input (energy supply) which makes these methodologies highly efficient and useful. These findings show that EAB is a fascinating biogenic tool for MFCs, nanomaterials syntheses, bioremediation, and bio-hydrogen production.
4.2 Applications of EABs as a Bioenergy Source
The chemical energy stored in the bonds of organic compounds (such as acetate) is utilized using EAMs which oxidizes organic compounds into harmless by-products such as protons, electrons, and CO2 (Kim et al. 2012; Logan and Rabaey 2012; Pant et al. 2012; Han et al. 2013; Rozendal et al. 2009). Recently, it was established by many researchers that these electrons and protons can be used for various purposes in MFCs such as electricity generation, CO reduction, etc. (Kim et al. 2012; Logan and Rabaey 2012; Pant et al. 2012). Very recently, it was also reported that nanomaterials such as metal nanoparticles and metal-metal oxide nanocomposites can be in-situ synthesized successfully using EABs (Kalathil et al. 2011, 2012; Khan et al. 2012, 2013a, b; Ansari et al. 2013a, 2014). Following are the few examples which show that how the energy stored in the organic compounds is biologically exploited for various green synthesis, environmental remediation, bioelectricity, and bio-hydrogen production. In general, when one mole of acetate is biologically decomposed by EABs, it gives two moles of HCO3 −, nine moles of H+, and eight moles of electrons as shown by following reaction (Logan and Rabaey 2012; Rozendal et al. 2009; Khan et al. 2014).
4.2.1 Bioelectricity Production
EABs are used as living bioanode catalysts in MFCs to generate electricity (Kim et al. 2012; Logan and Rabaey 2012; Pant et al. 2012; Han et al. 2013). The EAB oxidizes organic substrates, such as acetate to electrons, protons, and CO2 without combustion. The electrons produced are transferred through an external circuit, whereas the protons migrate to the cathode via a cation exchange membrane to cathode and react with oxygen to produce water (Fig. 4.3). The most striking feature of this technology is that a simultaneous wastewater treatments, nanomaterials synthesis, bio-hydrogen production, and bioelectricity generation can be achieved without the need of energy input (Han et al. 2013). Though the produced electricity is not too high, but no energy input, nanomaterials synthesis, bio-hydrogen production, and wastewater treatment, makes this approach efficient (Kalathil et al. 2013b; Han et al. 2013).
4.2.2 Synthesis of Metal Nanoparticles
Metal nanoparticles such as gold nanoparticles (AuNPs) (Khan et al. 2013c; Kalathil et al. 2013b), silver nanoparticles (AgNPs) (Kalathil et al. 2011), and cysteine-capped silver nanoparticles (cys-AgNPs) (Khan et al. 2012) were reported to be synthesized by EABs as a reducing tool in the presence of sodium acetate as an electron donor (Kalathil et al. 2013b; Logan et al. 2005). Here, sodium acetate acts as carbon source and biologically oxidizes to electrons, protons, and CO2. Respective precursors were used to synthesize the different metal nanoparticles in the presence of sodium acetate as a carbon source which provides plenty of electrons for the reduction of metal ions into zero-valent metal nanoparticles. Figure 4.4a shows the synthesis of AuNPs using EAB formed on stainless steel as a support. Similar approach was used to synthesize AgNPs (Kalathil et al. 2011). Presence of stainless steel as a support for EAB enhances the availability of electrons by Cl- penetration into it (Khan et al. 2013 c; Han et al. 2013). Figure 4.4b shows the synthesis of cys-AgNPs using EABs and sodium acetate as an electron source (Khan et al. 2012). The synthesized nanoparticles were used for different applications, for example, bio-hydrogen production (Khan et al. 2013d) and anti-microbial activity (Khan et al. 2012).
4.2.3 Synthesis of Metal-Metal Oxide Nanocomposites
Another very interesting use of EABs was to synthesize different types of nanocomposites. New reports show the use of EABs as a biogenic tool to synthesize metal–metal oxides nanocomposites such as Au@TiO2, Ag@TiO2, and Ag@ZnO nanocomposites in the presence of sodium acetate as a carbon source (Kalathil et al. 2012; Khan et al. 2013a; Ansari et al. 2013a). Figure 4.5 shows a common proposed mechanism for the synthesis of nanocomposites. Here too, the electrons produced by the EABs were used for the reduction of the metal ions at the surface of metal oxides. This leads to the formation and anchoring of metal nanoparticles at the surface of metal oxides. The reported methods are green as the entire synthesis takes place in water at 30 °C. The advantage of this protocol is that it does not involve any energy input and the products obtained are quite free from any impurities or by-products. The synthesized nanocomposites were used for various applications such as sensing (Khan et al. 2013b), dye degradation (Kalathil et al. 2012; Khan et al. 2013a; Ansari et al. 2013a), etc.
4.2.4 Modifications of Metal Oxides
Recently another use of EABs was discovered which is highly motivated, i.e., band gap engineering of metal oxides such as TiO2, ZnO, SnO2, and CeO2. The approach is quite simple, efficient, and produces the defected metal oxides having reduced band gap in comparison to pure metal oxides (Kalathil et al. 2013a; Ansari et al. 2013b, 2014). Figure 4.6 shows the proposed mechanism to narrow down the band gap of the different metal oxides using EAB as a band gap engineer. The EAB produced electrons and protons interacted with the metal oxides and produced some defects such as oxygen vacancies, low valent ion formation, etc. (Kalathil et al. 2013a; Ansari et al. 2013b, 2014). The defected metal oxides were used as visible light active photocatalyst materials for environmental remediation. The band gap-narrowed metal oxides were used for several exciting studies and applications such as visible light-induced photocurrent and dyes degradation of different classes induced by visible light (Kalathil et al. 2013a; Ansari et al. 2013b, 2014).
4.2.5 Bio-hydrogen Production
The use of EABs seems to be fictions; however, it is a fact and also reported for bio-hydrogen production in presence of gold nanoparticles as catalyst and sodium acetate as a carbon source which provides electrons as well as protons. Figure 4.7 shows the proposed mechanism for the bio-hydrogen production. The biologically produced electrons and protons combine at the surface of AuNPs following the Volmer-Heyrovsky mechanism (Kalathil et al. 2013c; Brust and Gordillo 2012). The observed bio-hydrogen production rate was ~105 ± 2 mL/L/day (Khan et al. 2013d). The bio-hydrogen production in MFC was also reported and found ~1.5 mL/h (Kalathil et al. 2013c).
4.2.6 Environmental Remediation
Recently, it was also reported that EABs could be directly used for the environmental remediation such as dye (methylene blue) degradation in the presence of suitable catalyst such as Au@TiO2 (Kalathil et al. 2013d). Here too, the degradation process does not need any energy which makes it efficient.
In summary, EABs are biogenic tool that is used for various applications such as nanomaterials synthesis, band gap engineering, bio-hydrogen production, and environment remediation. The beauty of EABs is that its use does not need any energy input and the products obtained are free from impurities. The energy stored in the organic molecules are released with the help of EABs and used up for various applications. These approaches show that EABs acts as a fascinating biogenic tool which is easy to prepare and use.
References
Ansari SA, Khan MM, Ansari MO, Lee J, Cho MH (2013a) Biogenic synthesis, photocatalytic, and photoelectrochemical performance of Ag-ZnO nanocomposite. J Phys Chem C 117: 27023–27030
Ansari SA, Khan MM, Kalathil S, Nisar A, Lee J, Cho MH (2013b) Oxygen vacancy induced band gap narrowing of ZnO nanostructures by an electrochemically active biofilm. Nanoscale 5:9238–9246
Ansari SA, Khan MM, Ansari MO, Lee J, Cho MH (2014) Highly photoactive SnO2 nanostructures engineered by electrochemically active biofilm. New J Chem 38:2462–2469
Babauta J, Renslow R, Lewandowski Z, Beyenal H (2012) Electrochemically active biofilms: facts and fiction. A review. Biofouling 28:789–812
Bond DR, Holmes DE, Tender LM, Lovely DR (2002) Electrode-reducing microorganisms that harvest energy from marine sediments. Science 295:483–485
Borole AP, Reguera G, Ringeisen B, Wang Z, Feng Y, Kim BH (2011) Electroactive biofilms: current status and future research needs. Energy Environ Sci 4:4813–4834
Brust M, Gordillo GJ (2012) Electrocatalytic hydrogen redox chemistry on gold nanoparticles. J Am Chem Soc 134:3318–3321
Dulon S, Parot S, Delia ML, Bergel A (2007) Electroactive biofilms: new means for electrochemistry. J Appl Electrochem 37:173–179
Erable B, Duteanu NM, Ghangrekar MM, Dumas C, Scott K (2010) Application of electro-active biofilms. Biofouling 26:57–71
Halan B, Buehler K, Schmid A (2012) Biofilms as living catalysts in continuous chemical syntheses. Trends Biotechnol 30:453–465
Han TH, Khan MM, Kalathil S, Lee J, Cho MH (2013) Simultaneous enhancement of methylene blue degradation and power generation in a microbial fuel cell by gold nanoparticles. ACS Ind Eng Chem Res 52:8174–8181
Kalathil S, Lee J, Cho MH (2011) Electrochemically active biofilm-mediated synthesis of silver nanoparticles in water. Green Chem 13:1482–1485
Kalathil S, Khan MM, Banerjee AN, Lee J, Cho MH (2012) A simple biogenic route to rapid synthesis of Au@TiO2 nanocomposites by electrochemically active biofilms. J Nanopart Res 14:1051–1060
Kalathil S, Khan MM, Ansari SA, Lee J, Cho MH (2013a) Band gap narrowing of titanium dioxide (TiO2) nanocrystals by electrochemically active biofilms and their visible light activity. Nanoscale 5:6323–6326
Kalathil S, Khan MM, Lee J, Cho MH (2013b) Production of bioelectricity, bio-hydrogen, high value chemicals and bioinspired nanomaterials by electrochemically active biofilms. Biotech Adv 31:915–924
Kalathil S, Lee J, Cho MH (2013c) Gold nanoparticles produced in situ mediate bioelectricity and hydrogen production in a microbial fuel cell by quantized capacitance charging. ChemSusChem 6:246–250
Kalathil S, Lee J, Cho MH (2013d) Catalytic role of Au@TiO2 nanocomposite on enhanced degradation of an azo-dye by electrochemically active biofilms: a quantized charging effect. J Nanopart Res 15:1392–1398
Khan MM, Kalathil S, Lee J, Cho MH (2012) Synthesis of Cysteine Capped Silver Nanoparticles by Electrochemically Active Biofilm and their Antibacterial Activities. Bull Kor Chem Soc 33:2592–2596
Khan MM, Ansari SA, Lee J, Cho MH (2013a) Highly visible light active Ag@TiO2 nanocomposites synthesized by electrochemically active biofilm: a novel biogenic approach. Nanoscale 5:4427–4435
Khan MM, Ansari SA, Lee J, Cho MH (2013b) Novel Ag@TiO2 nanocomposite synthesized by electrochemically active biofilm for nonenzymatic hydrogen peroxide sensor. Mater Sci Eng C 33:4692–4699
Khan MM, Kalathil S, Han TH, Lee J, Cho MH (2013c) Positively charged gold nanoparticles synthesized by electrochemically active biofilm—a biogenic pproach. J Nanosci Nanotechnol 13:6079–6085
Khan MM, Lee J, Cho MH (2013d) Electrochemically active biofilm mediated bio-hydrogen production catalyzed by positively charged gold nanoparticles. Int J Hydr Energy 38:5243–5250
Khan MM, Ansari SA, Lee JH, Lee J, Cho MH (2014) Mixed culture electrochemically active biofilms and their microscopic and spectroelectrochemical studies. ACS Sustain Chem Eng 2:423–432
Kim D, An J, Kim B, Jang JK, Kim BH, Chang IS (2012) Scaling-up microbial fuel cells: configuration and potential drop phenomenon at series connection of unit cells in shared anolyte. ChemSusChem 5:1086–1091
Logan BE, Rabaey K (2012) Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 337:686–690
Logan BE, Murano C, Scott K, Gray ND, Head IM (2005) Electricity generation from cysteine in a microbial fuel cell. Water Res 39:942–952
Pant D, Singh A, Bogaert GV, Olsen SI, Nigam PS, Diels L (2012) Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Adv 2:1248–1263
Rittmann BE, Krajmalnik-Brown R, Halden RU (2008) Pre-genomic, genomic and post-genomic study of microbial communities involved in bioenergy. Nat Rev Microbiol 6:604–612
Rozendal RA, Leonea E, Keller J, Rabaey K (2009) Efficient hydrogen peroxide generation from organic matter in a bioelectrochemical system. Electrochem Commun 11:1752–1755
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Khan, M.M. (2014). Bioenergy Derived from Electrochemically Active Biofilms. In: Hakeem, K., Jawaid, M., Rashid, U. (eds) Biomass and Bioenergy. Springer, Cham. https://doi.org/10.1007/978-3-319-07578-5_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-07578-5_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-07577-8
Online ISBN: 978-3-319-07578-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)