Abstract
Microbial fuel cells (MFCs) have significant interest in the research community due to their ability to generate electricity from biodegradable organic matters. Anode materials and their morphological structures play a crucial role in the formation of electroactive biofilms that enable the direct electron transfer. In this work, modified electrodes with nanomaterials, such as multiwalled carbon nanotubes (MWCNTs), reduced graphene oxide (rGO), Al2O3/rGO or MnO2/MWCNTs nanocomposites were synthesized, characterized and utilized to support the growth of electrochemically active biofilms. The MFC's performance is optimized using anode-respiring strains isolated from biofilm-anode surface, while the adjusted operation is conducted with the consortium of (Enterobacter sp.). Besides the formation of matured biofilm on its surface, MnO2/MWCNTs nanocomposite produced the highest electrical potential outputs (710 mV) combined with the highest power density (372 mW/m2). Thus, a correlation between the anode nanostructured materials and the progression of the electrochemically active biofilms formation is presented, allowing new thoughts for enhancing the MFC's performance for potential applications ranging from wastewater treatment to power sources.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microbial fuel cells (MFCs) are promising alternative technology that could reduce energy crisis and environmental pollution [1]. In MFCs, organic substrates or organic waste biomass are biologically oxidized by exoelectrogenic bacteria and transfer electrons extracellularly to a terminal electron acceptor to produce electric current [2]. Despite advances that have been made so far, this technology has not yet been commercially applied, due to the low energy production. One of the main reasons behind the low power density produced by the MFCs is the sluggish of electron transfer from living organisms to the anode surface [3]. To date, several extracellular electron transfer (EET) mechanisms between living microorganisms and electrodes have been proposed [4]: (a) Extracellular direct electron transfer (EDET) through a physical contact between outer-membrane redox proteins, such as cytochromes or bacterial-conductive pili and electrode surface [5,6,7], (b) Mediated electron transfer (MET) which is promoted by implementing synthetic chemical mediators or natural secreted redox molecules to barge electrons from the intracellular redox centres of metabolically active cells to the electrode surface [8, 9]. Nevertheless, the use of artificial redox mediators (electron carriers) has many drawbacks in the progress of MFC applications, e.g. cytotoxicity and electrochemical cross-reactivity or interference [10]. Anode materials, as the host for exoelectrogens, are significantly affecting the direct electron transfer process from living microorganisms [11,12,13,14]. Thus, anodic respiration via the anode-respiring microorganisms along with the direct electron transport process are regulated by the physical and architecture characteristics of anode surface [12, 15, 16]. Hence, new anode materials with specific inspections were developed [17, 18]. Owing to their high surface-to-volume ratio, biocompatibility, electro-catalytic activity, mechanical and electrical properties, nanomaterials showed great impact on the improvement of MFCs efficiency [19,20,21]. In addition, nanomaterials with unique electrochemical properties provide strong charge interactions with organic compounds and the direct electrochemistry process between bacteria and the anode [22]. Although nanomaterials have those unique properties, disadvantages of using nanomaterials in electrochemical systems may be the difficulty in re-usability, as they get polluted or deactivated during the process.
Bio-electrochemical analysis of the biofilm formation at different electrode modifiers was conducted to understand the role of electrode materials on the microbial adherence and the progression of biofilm. Specifically, nanomicrobial electrochemical systems were engineered towards a better understanding of the relationship between anode architectures and rate of biofilm formation along with the mechanisms of electron transfer mechanism [23,24,25]. In this regard, changes in bacterial cell wall dynamic structure, i.e. the phase transition from planktonic cells to the biofilm matrix, were successfully detected using cyclic voltammetric technique [21, 26, 27]. In that report, electrode modifications with conductive nanostructured elements promoted the Pseudomonas aeruginosa biofilm formation and enabled direct extracellular electron transfer by developing bio-conductive cell wall communications through iron-containing materials acting as microbial connectors. It was concluded that the firmly attached microbes to the electrode surface, the higher the bioelectrochemical signals [27,28,29].
Therefore, in this work, the impact of nanostructured anode materials on the biofilm formation and the direct electron transfer is investigated and exploited for bioelectricity generation and wastewater treatment using air–cathode single chamber–membrane–less microbial fuel cells. The amended nanostructured anodes presented significant enhancements in the acceleration of electrochemically active biofilms and increased the rate of direct electron transfer that led to increasing the power density as well as the current outputs.
Materials and methods
Synthesis and characterization of nanostructured anodes
Preparation of carbon paste electrodes (CPEs) modified with nanomaterials was performed by sonicating 0.95 g of synthetic graphite powder with 0.05 g of each of nanomodifiers, e.g. manganese dioxide (MnO2), multi-walled carbon nanotubes (MWCNTs), MnO2/MWCNTs, reduced graphene oxide (rGO) or Al2O3/rGO nanomaterials, for 30 min before adding 400 μL of paraffin oil to achieve a homogenous mixture. The prepared pastes were mechanically packed into electrode assemblies and well compressed using a stainless rod to provide modified electrodes with a surface area of 5 mm [28]. For surface regeneration, the working electrode surface was polished and smoothed on weighing paper to obtain a uniform surface before running the electrochemical experiments. Electrocatalytic activity of the prepared electrodes was characterized using 200 μM potassium ferricyanide (FCN) as a redox probe in 0.1 M KCl. The cyclic voltammetric experiments were conducted using the computer controlled potentiostat/galvanostat (Gamry, ZR-G750 system).
Electrochemical impedance spectroscopy (EIS) was performed in ferricyanide (1 mM) as the redox probe to characterize the charge transfer resistances of the modified electrode surfaces. The EIS was conducted over a frequency range of 10 kHz–0.1 Hz with a sinusoidal perturbation amplitude of 5 mV.
XRD analysis
The composite structure was analyzed by powder X-ray diffraction (XRD) analyzer (Philips X-ray diffractometer, PW 1390), with Cu–Kα radiation (1.54 Å) operating in the Bragg–Brentano reflection geometry. The analysis was performed indoor at the National Research Centre (NRC, Cairo, Egypt).
MFC fabrication and assembly
A sterile homemade cylindrical-shaped single chamber-membrane-less microbial fuel cell was fabricated for testing the effect of anode materials. The MFC was constructed from transparent polyacrylic materials and the distance between anode and cathode is about 7 cm and the projected surface area of anode or cathode is about 4 cm2 [29, 30]. For the anode functionalization, silver gauzes were individually coated with each of the prepared nanomaterials (MnO2/MWCNTs nanocomposite, rGO, Al2O3/rGO or graphite alone as control). In order to maintain consistency of experimental procedures, the same type and size of silver mesh was used as a cathode material for all tests. After assembling the MFC, the anode/cathode chips were positioned at both sides before they were connected with copper wire and external variable resistors. The designed MFCs were operated at 25 ± 1 °C in a temperature-controlled microbiology lab. The nanostructured anodes were inoculated with pre-acclimated bacterial suspensions, while the generated electrical voltage was recorded.
Biofilm formation and MFC operation
To monitor the capacity of each electrode material for enhancing the adherence and growth of electrochemically active biofilms, in the MFC, 200 mL of bacterial cell suspension (a mixed culture, with the cell density of OD600 ≈ 0.4) was incubated in brain heart infusion broth medium (with the initial COD concentration of 25 mg/mL). Close circuit continuous experiments were carried out for 15 days, while the output voltage over the incubation time was recorded using (The LabJack U6/U6-Pro). At a fixed external resistance (150 kΩ), the MFC cycles were conducted with fed-batch mode, while the power and current densities were computed.
Polarization curves
Duplicate MFCs were operated simultaneously with different external resistances (0.265, 0.500, 1.20, 9.10, 20.0, 52.0, 100.0, 200.0 and 550.0 kΩ). Accordingly, the output voltage was collected during the running time which is two weeks. Subsequently, the potential and power density curves were plotted vs. the anodic current density to generate the polarization curves.
Morphological characterization of biofilm-based anodes
The functionalized nanostructured anodes were incubated with the cell suspensions of microbial communities in a closed chamber for two weeks. Afterwards, the electrodes were removed from the microbial cultures, washed carefully with PBS and dried (for 2 h at 60 ± 1 °C) at ambient conditions. Scanning electron microscope (SEM), (JEOL, JXA-840A) at an accelerating applied potential of 15 keV was used for characterizing the morphology of formed biofilms at each anode material. Each electrode surface was imaged by the SEM after being sputtered with a thin film of gold. As a control, all anode surfaces were imaged using SEM before getting in the microbial cultures.
Effect of nanomaterial concentration on MFC's performance
To evaluate the effects of concentration of MnO2/MWCNTs composite, anodes surfaces were modified with different concentrations of the composite (0%, 5%, 25%, 50% and 75%) and deployed in single chamber MFCs which inoculated with the mixed microbial culture. Computed power densities were considered as the key parameter that assessed the performance of each MFC with respect to each concentration. Polarization curves were made after 2 weeks to determine the maximum power density achieved by each system.
Testing the MFCs performance using a single bacterial strain or bacterial consortium
Simultaneously, the performance of the designed MFC using MnO2/MWCNTs as the anode was incubated with different bacterial cultures either using a single isolated bacterial strain (Enterobacter cloacae), bacterial consortium or raw anaerobic mixed raw culture. The biodegradation rate of the organic load was quantified during the sequential MFC operations. For all running MFCs, chemical oxygen demand (COD) was kinetically determined according to the reference method.
Result and discussion
Electrocatalytic properties of nanostructured electrodes
In microbial electrochemical systems, electron transfer and electricity generation are linked directly to the formation of biofilms. In addition to providing quantitative information about the influence of new materials on the catalytic activity, the cyclic voltammetry (CV) is considered as a reliable characterization technique for studying the electron transfer between living-attached microorganisms to the conductive electrode surfaces. Basically, CV comprises sweeping an electrode potential as a function of time and measuring the resulting faradic current that rounds through the circuit. The CV measurement in this work was conducted with a typical 3-electrode electrochemical setup, with Ag/AgCl (in 3M KCl) being reference electrode, Pt wire as the counter electrode and nanomaterial-based electrode as the working electrode.
Thus, in the current work, the CV was used to characterize the electrochemical properties of new modified electrodes with 5%, w/w of each of the following: MnO2 nanorods, MWCNTs, MnO2/MWCNTs, rGO and Al2O3/rGO nanocomposite. As a result, when ferricyanide was used as redox probes, there was a discernible difference between the unmodified and all the modified surfaces, particularly the MnO2/MWCNT-based electrode displayed the highest oxidation/reduction peaks (Fig. 1a). The obtained synergetic electrochemical activity is attributed to the fast electron transfer which is collected by the modified surface of the composite that is made of a combination of highly catalytic materials (MnO2 conjugated with MWCNTs).
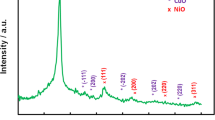
The EIS analysis was carried out in ferricyanide for all modified electrodes and the nyquist plot indicated the role of the electrode modification in the enhancement of the charge transfer (Rct), whereas the Rct of the modified surfaces were significantly lowered when the response was compared with the EIS pattern of the bare electrode (Fig. 1b). On the other hand, X-ray diffraction analysis (XRD) was carried out to determine the phase structure of the prepared composite. In the diffractograms, sharp and intense diffraction peaks were observed at 2θ = 28.45°, 37.25°, 44.45°, 56.58° and 64.84°, respectively. The diffraction patterns displayed the crystalline nature of the composite (Fig. 1c).
Testing the nano-anode's performance
Different anode surfaces were tested for enhancing the microbial fuel cell's performance that were powered in parallel by anaerobic mixed cultures (bacterial cell density is 0.4 OD600nm) under closed circuit operation. As shown in Fig. 2, the potential–time curves displayed a huge difference between the modified and unmodified anodes, since the nano-modified surfaces produced significantly higher voltage than the unmodified. The ascending order of the MFC-output voltages revealed that the maximum values were obtained by the MnO2/MWCNTs (690 mV), followed by MWCNTs (500 mV), rGO (494 mV), Al2O3/rGO nanocomposite (294 mV), eventually the unmodified anode (85 mV). On other hand, polarization curves and power outputs were calculated at given external resistances (0.265, 0.500, 1.20, 9.10, 20.0, 52.0, 100.0, 200.0 and 550.0 kΩ) (Fig. 3). Effectively, anode surface modification using the MnO2/MWCNTs improved the power generation for about 112-fold (56.6 mW/m3) higher than the unmodified anode surface (0.5 mW/m3). These results confirmed the obtained synergetic electrocatalytic activity of the integration of MnO2 with the multiwalled carbon nanotubes. By introducing a nanocomposite anode material, efficient electrocatalysis was obtained and a significant enhancement in the microbial fuel cell performance was achieved. The anode modification supported the formation of electrochemically active biofilms, as a result, a direct extracellular electron transfer (DEET) between the bacteria and the MnO2/MWCNTs-architecture is accomplished.
Unexpectedly, the Al2O3/rGO nanocomposite produced weak electrical potential (294 mV) combined with lower power density (16 mW/m2). This weak electrochemical signal could be attributed to the gained conductance and capacitance properties of Al2O3 particles that are linked to the partial reduced graphene oxide which still carries a part of the graphene oxide nature [31]. Thus, the modified anode with the Al2O3/rGO nanocomposite is storing the electrons rather than delivering them to the electrode surfaces as the final electron acceptor.
Morphological characterization of biofilm assisted by nano-anodes
In order to investigate the biofilm growth on the nano-anode surface, each modified electrode was morphologically analyzed by the scanning electron microscopy after 15-days of incubation with a bacterial consortium under anaerobic conditions. The SEM images of all nano-anodes (Fig. 4) were captured before and after the incubation with the bacterial cultures to acquire a better understanding on the correlation between the bacterial coverage area (on each surface) and the power density outputs. Accordingly, formation of compacted and condensed-layer of colonized cells was obtained when the MnO2/MWCNTs is used as the anode surface, followed by the Al2O3/rGO nanocomposite, as shown in Fig. 4c, e, respectively. On the other hand, weaker colonization of scattered cells was observed on the surface of the unmodified or the modified with the rGO, as shown in Fig. 4a, d, respectively. Therefore, we can conclude that, the higher the electrochemical activity of anode material, the faster the formation of biofilm. This is reflecting a clear relationship between the catalytic activities of anode materials with the direct electron transfer through the physically contacted microbial cells with the nano-anode surfaces.
Impact of MnO2/MWCNTs concentration
Influence of the MnO2/MWCNTs concentrations (0%, 5%, 25%, 50%, 75%) on the MFC's performance was kinetically evaluated by correlating the increase in the incubation time with the resulted power density and the generated voltage of the closed circuit at the fixed external resistance (150 KΩ). Apparently, the MnO2/MWCNTs (50%) produced the highest electrochemical performance (710 mV) combined with the highest power density (372 mW/m2), as can be seen in Fig. 5a, b. It worth mentioning here that the power density with the MnO2/MWCNTs (50% w/w) is about three folds higher than the previous reports, whereas 120 mW/m2 and 109 mW/m2 were obtained by Fu et al. and Kalathil et al. [32, 33].
On the other hand, increasing the composite concentration above 50% led to a decrease in the current density because of the excessive distribution of MnO2 particles on the anode surfaces (semi-conductivity of a metal oxide feature is the majority). Thus, hindering the electron transfer between the biofilm and the anode due to the increase in the resistance of the electrode matrix. Therefore, 50% of MnO2/MWCNTs has been selected in this study as the ideal concentration and it has been assigned for the preparation of nano-anodes for further investigations.
Powering the MFCs a single bacterial strain or bacterial consortium
In our previous report, microbiological identification was conducted with the surface of modified working electrodes that were designed to support the formation of electrochemically active biofilms [14, 26]. Thus, the anticipated living bacterial cells involved in the anode-biofilm formation were isolated from the nano-anode surface, and then identified using 16S rRNA gene sequencing. From the analysis of the complete genome sequence, Enterobacter cloacae subsp. (ATCC 13,047 strain) is recognized as the targeted electrochemically active strain. Accordingly, this strain was selected with the anodes made of 50% of MnO2/MWCNTs for testing the effects of microbial diversity on the output voltage and power density. In this regard, three MFCs were operated in parallel using a single strain of E. cloacae, consortium of E. cloacea sp. or using a raw wastewater inoculum. As a result, the highest output voltage (662 mV) is produced by the Eterobacter consortium, followed by the E. cloacae (540 mV) as single strain culture, while the least output voltage (485 mV) was obtained from the raw wastewater inoculum culture (Fig. 6a). By the same pattern, the polarization curve shown in Fig. 6b confirmed the same order of the above-mentioned three culture conditions, whereas the raw culture was producing the lowest power density (65 mW/m2).
a Variation in voltage generation over the incubation time in single chamber MFCs inoculated with E. cloacae single strain, E. cloacea sp. consortium or raw wastewater inoculum. MFCs were running in parallel under closed circuits. b Polarization curves and power output of the MFCs with E. cloacae single strain, E. cloacea sp. consortium and raw wastewater inoculum. c COD removal (biodegradability) over the MFCs operation time inoculated with E. cloacae single strain, E. cloacea sp. consortium and raw wastewater inoculum
Electrochemically active group of Enterobacter spp. that has been colonized on the nano-anode to form the electroactive biofilm is the responsible for generating the high bioelectrochemical signals. However, interferences from electrochemically in-active organisms (nonelectrogenic organisms) are existing in the raw culture. This was confirmed by the COD measurements (Fig. 6c) which showed that the raw culture produced the maximum biodegradation rate after 11 days of operation accompanied by the lowest power density production. The MFC inoculated with E. cloacae culture produced a power density (80 mW/m2) almost equal to that obtained by previous report [34]. These results are in agreement with the previous report which showed that Enterobacter consortium culture has generated higher current outputs than the pure culture (single stain-based MFC) [35].
Conclusion
In the current study, we introduce distinguished anode compositions for supporting electro-active biofilm formation and direct electron transfer. Apparently, the anode material requirements of conductivity, stability and biocompatibility were satisfied by decorating the anode surface with MnO2/MWCNTs. As an accomplishment, a great potential in the increase of current density and power output of the system was successfully achieved. Thus, nanocomposite-based electrodes are introduced as promising anodes materials to accelerate the biofilm formation and to facilitate direct electron transfer. Based on that the commercial application and large-scale production of MFC could be reached through the optimization of electrode materials which will offer a technology for cost-effective bioelectricity generation along with wastewater treatment.
References
Fraiwan A, Adusumilli SP, Han D, Steckl AJ, Call DF, Westgate CR, Choi S (2014) Microbial power-generating capabilities on micro-/nano-structured anodes in micro-sized microbial fuel cells. Fuel Cells 14:801–809
Guang L, Koomson DA, Jingyu H, Ewusi-Mensah D, Miwornunyuie N (2020) Performance of exoelectrogenic bacteria used in microbial desalination cell technology. Int J Environ Res Public Health 17:1121–1132
Zhang P, Liu J, Qu Y, Zhang J, Zhong Y, Feng Y (2017) Enhanced performance of microbial fuel cell with a bacteria/multi-walled carbon nanotube hybrid biofilm. J Power Sources 361:318–325
Kumar GG, Sarathi VGS, Nahm KS (2013) Recent advances and challenges in the anode architecture and their modifications for the applications of microbial fuel cells. Biosens Bioelectron 43:461–475
Reardon PN, Mueller KT (2013) Structure of the type IVa major pilin from the electrically conductive bacterial nanowires of Geobacter sulfurreducens. J Biol Chem 288:29260–29266
Fapetu S, Keshavarz T, Clements M, Kyazze G (2016) Contribution of direct electron transfer mechanisms to overall electron transfer in microbial fuel cells utilising Shewanella oneidensis as biocatalyst. Biotechnol Lett 38:1465–1473
Hu Y, Yang Y, Katz E, Song H (2015) Programming the quorum sensing-based AND gate in Shewanella oneidensis for logic gated-microbial fuel cells. Chem Commun (Camb) 51:4184–4187
Selim HMM, Kamal AM, Ali DMM, Hassan RYA (2017) Bioelectrochemical systems for measuring microbial cellular functions. Electroanalysis 29:1498–1505
Hassan RYA, Wollenberger U (2016) Mediated bioelectrochemical system for biosensing the cell viability of Staphylococcus aureus. Anal Bioanal Chem 408:579–587
Hassan RYA, Mekawy MM, Ramnani P, Mulchandani A (2017) Monitoring of microbial cell viability using nanostructured electrodes modified with graphene/alumina nanocomposite. Biosens Bioelectron 91:857–862
Ding WJ, Yu LL, Chen J, Cheng SA (2017) Effects of anode materials on electricity generation and organic wastewater treatment of 6 L microbial fuel cells. Huan Jing Ke Xue 38:1911–1917
Sonawane JM, Yadav A, Ghosh PC, Adeloju SB (2017) Recent advances in the development and utilization of modern anode materials for high performance microbial fuel cells. Biosens Bioelectron 90:558–576
Liu Y, Harnisch F, Fricke K, Schroder U, Climent V, Feliu JM (2010) The study of electrochemically active microbial biofilms on different carbon-based anode materials in microbial fuel cells. Biosens Bioelectron 25:2167–2171
Mahmoud RH, Samhan FA, Ali GH, Ibrahim MK, Hassan RYA (2018) Assisting the biofilm formation of exoelectrogens using nanostructured microbial fuel cells. J Electroanal Chem 824:128–135
Mustakeem M (2015) Electrode materials for microbial fuel cells: nanomaterial approach. Mater Renew Sustain Energy 4:22
Kipf E, Koch J, Geiger B, Erben J, Richter K, Gescher J, Zengerle R, Kerzenmacher S (2013) Systematic screening of carbon-based anode materials for microbial fuel cells with Shewanella oneidensis MR-1. Bioresour Technol 146:386–392
Flores-Sifuentes J, Sánchez-Cardona KV, Acosta-Arreazola F, Sánchez-Domínguez M, Garza-Tovar LL, Sepúlveda-Guzmán S, Garza-Montes-de-Oca NF, Garcia-Gomez NA, Sánchez EM (2018) Preparation of CuO/CNF composite and its performance as anode in a microbial fuel cell with Shewanella oneidensis in a half cell configuration. J Mater Sci Mater Electron 29:15784–15794
Zhou M, Chi M, Luo J, He H, Jin T (2011) An overview of electrode materials in microbial fuel cells. J Power Sources 196:4427–4435
Di Lorenzo M, Scott K, Curtis TP, Head IM (2010) Effect of increasing anode surface area on the performance of a single chamber microbial fuel cell. Chem Eng J 156:40–48
Gajda I, Greenman J, Ieropoulos IA (2018) Recent advancements in real-world microbial fuel cell applications. Curr Opin Electrochem 11:78–83
Mahmoud RH, Abdo SM, Samhan FA, Ibrahim MK, Ali GH, Hassan RY (2020) Biosensing of algal-photosynthetic productivity using nanostructured bioelectrochemical systems. J Chem Technol Biotechnol 95:1028–1037
Liu Y, Zhang X, Zhang Q, Li C (2020) Microbial fuel cells: nanomaterials based on anode and their application. Energy Technol 8:2000206
Elumalai P, AlSalhi MS, Mehariya S, Karthikeyan OP, Devanesan S, Parthipan P, Rajasekar A (2020) Bacterial community analysis of biofilm on API 5LX carbon steel in an oil reservoir environment. Bioprocess Biosyst Eng 1–14
Armato C, Ahmed D, Agostino V, Traversi D, Degan R, Tommasi T, Margaria V, Sacco A, Gilli G, Quaglio M, Saracco G, Schilirò T (2019) Anodic microbial community analysis of microbial fuel cells based on enriched inoculum from freshwater sediment. Bioprocess Biosyst Eng 42:697–709
Mahmoud RH, Samhan FA, Ibrahim MK, Ali GH, Hassan RYA (2020) Boosting the cathode function toward the oxygen reduction reaction in microbial fuel cell using nanostructured surface modification. Electrochem Sci Adv. https://doi.org/10.1002/elsa.202000002
Sedki M, Hassan RYA, Andreescu S, El-Sherbiny IM (2019) Online-monitoring of biofilm formation using nanostructured electrode surfaces. Mater Sci Eng C 100:178–185
Kang J, Kim T, Tak Y, Lee J-H, Yoon J (2012) Cyclic voltammetry for monitoring bacterial attachment and biofilm formation. J Ind Eng Chem 18:800–807
Mekawy MM, Hassan RYA, Ramnani P, Yu X, Mulchandani A (2018) Electrochemical detection of dihydronicotinamide adenine dinucleotide using Al2O3-GO nanocomposite modified electrode. Arabian J Chem 11:942–949
Khater DZ, El-Khatib KM, Hassan RYA (2018) Effect of vitamins and cell constructions on the activity of microbial fuel cell battery. J Genet Eng Biotechnol 16:369–373
Khater DZ, El-Khatib KM, Hazaa MM, Hassan RYA (2015) Development of bioelectrochemical system for monitoring the biodegradation performance of activated sludge. Appl Biochem Biotechnol 175:3519–3530
Purkait T, Singh G, Kumar D, Singh M, Dey RS (2018) High-performance flexible supercapacitors based on electrochemically tailored three-dimensional reduced graphene oxide networks. Sci Rep 8:640
Fu Y, Yu J, Zhang Y, Meng Y (2014) Graphite coated with manganese oxide/multiwall carbon nanotubes composites as anodes in marine benthic microbial fuel cells. Appl Surf Sci 317:84–89
Kalathil S, Van Nguyen H, Shim JJ, Khan MM, Lee J, Cho MH (2013) Enhanced performance of a microbial fuel cell using CNT/MnO2 nanocomposite as a bioanode material. J Nanosci Nanotechnol 13:7712–7716
Feng C, Li J, Qin D, Chen L, Zhao F, Chen S, Hu H, Yu CP (2014) Characterization of exoelectrogenic bacteria enterobacter strains isolated from a microbial fuel cell exposed to copper shock load. PLoS ONE 9:e113379
Park DH, Zeikus JG (2002) Impact of electrode composition on electricity generation in a single-compartment fuel cell using Shewanella putrefaciens. Appl Microbiol Biotechnol 59:58–61
Acknowledgements
This work has been supported by an internal Grant funded by the NRC (Project code:11050110).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mahmoud, R.H., Samhan, F.A., Ibrahim, M.K. et al. Formation of electroactive biofilms derived by nanostructured anodes surfaces. Bioprocess Biosyst Eng 44, 759–768 (2021). https://doi.org/10.1007/s00449-020-02485-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02485-4