Abstract
A green and sustainable approach to azo dye degradation by an electrochemically active biofilm (EAB) with Au@TiO2 nanocomposite assistance (average size of Au ~8 nm) has been developed with high efficiency and mineralization of toxic intermediates. The EAB-Au@TiO2 system degraded the dye more rapidly than the EAB without the nanocomposite, which indicated the catalytic role of the Au@TiO2 nanocomposite on the dye degradation. Toxicity measurements showed that the dye wastewater treated by the EAB-Au@TiO2 system was almost non-toxic while the dye wastewater treated by the EAB without the nanocomposite showed a high toxicity compared to the parent dye. Quantized charging and Fermi level equilibration within the Au@TiO2 nanocomposite may be attributed to the excellent catalytic activity of the nanocomposite on the dye degradation. A mechanism of the catalytic activity is also proposed. Redox behavior and quantized charging of the nanocomposite were confirmed by cyclic voltammetry (CV) and differential pulse voltammetry (DPV), respectively. The proposed protocol can be effectively utilized in wastewater treatment applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Azo dyes are commonly used in textile industries. Treatment of dye wastewater prior to discharge is one of the daunting tasks as most of the dyes are recalcitrant molecules and highly toxic in nature (Husain 2010). There are many available methods for the degradation of azo dyes including chemical, physical, and biological methods; however, their performances are far from satisfactory (Robinson et al. 2001). Usually, intermediates formed after the degradation of dyes are highly toxic and sometimes even more toxic than the parent dye. Hence, a further treatment is needed for the degradation of the toxic intermediates. There are many metal nanoparticles such as Au, Ag, and Pt which were employed for the degradation of dyes by using various chemical reducing agents such as NaBH4 (Gupta et al. 2011). The major disadvantage of these methods is the use of toxic chemicals which causes a secondary pollution.
In recent years, coupling of semiconductors with noble metals has been regarded as an interesting approach to enhance the performance of semiconductor-based nanostructures (Choi et al. 2012; Takai and Kamat 2011). For example, marrying TiO2 nanoparticles with Au nanoparticles can enhance the electrochemical performance of the nanocomposite by promoting electron transfer to adsorbed species such as H+ to make hydrogen molecule (Choi et al. 2012). It has been reported that Au@TiO2 nanocomposite can boost photocurrent generation in dye-sensitized solar cells by capturing and discharging electrons (Choi et al. 2012). Deposition of Au nanoparticles on semiconductors enhances charge accumulation property of the nanocomposite and the metal nanoparticles facilitating electron discharge on demand (Choi et al. 2012).
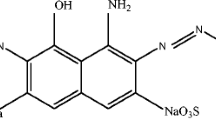
Recently, bioelectrochemical systems (BESs) such as microbial fuel cells (MFCs) (Kalathil et al. 2012a; Kalathil et al. 2011a; Pant et al. 2010) and microbial electrolysis cells (Pant et al. 2010) have received great attraction for simultaneous treatment of dye wastewater and for bioelectricity generation. However, BESs are in early stages and still not practically established (Pant et al. 2010). Electrochemically active biofilms (EABs) developed by electrically active microorganisms have emerged as one of the promising tools for environmental remediation, chemical production, and bioelectricity generation (Borole et al. 2011; Erable et al. 2010). Electrically active microorganisms can be found in various natural sites such as sea water sediments, soils, and various wastewaters such as municipal, dye, and swine wastewaters (Erable et al. 2010). The real attraction of EABs is the bio-anode catalyst for the MFCs (Borole et al. 2011; Erable et al. 2010) where the EAB biologically oxidizes organic substrates to electrons and protons. Murray and co-workers have reported that noble metals especially Au nanoparticles possess the property of storing electrons in a quantized fashion (Chen et al. 1998; Murray 2008). In this study, methyl orange (MO) was degraded with mineralization of toxic intermediates by EABs using Au@TiO2 nanocomposite as catalysts via quantized charging effect. To our best knowledge, it is the first time EAB with a nanocomposite is utilized for the rapid degradation of an azo dye.
Experimental methods
Au@TiO2 nanocomposite synthesis
EABs were developed according to previous reports (Kalathil et al. 2011b; Kalathil et al. 2012b). The Au@TiO2 nanocomposite was synthesized using an EAB (Kalathil et al. 2012b). In brief, an as-prepared EAB was dipped in a solution (200 mL) containing sodium acetate (1 g/L) as electron donor, 4 mM Degussa-TiO2, and 1 mM HAuCl4 (gold precursor) under anaerobic condition by sparging N2 gas for 5 min before the reaction. A color change after 1 h signaled the formation of the Au@TiO2 nanocomposite. The as-synthesized nanocomposite solution was centrifuged and kept for drying in an oven (50 °C) overnight.
Characterizations of nanocomposite
Formation of Au@TiO2 nanocomposite was monitored by UV–Vis absorption spectroscopy (UV–VIS-NIR, Cary 5000). The crystal structure of the nanocomposite was determined by X-ray diffractometry (PANalytical, X’Pert-PRO MPD). The morphology of the nanocomposite was determined by a high resolution transmission electron microscope (HRTEM; Tecnai G2F20, FEI, USA) operating at an accelerating voltage of 200 kV.
Dye degradation
The dye degradation was carried out by dipping an as-prepared EAB into a MO solution (20 mg/L) using the as-synthesized Au@TiO2 nanocomposite (20 mg/L) as the catalyst containing sodium acetate (1 g/L) as electron donor under magnetic stirring. Also, the MO degradation was carried out by dipping an EAB without using the nanocomposite to investigate the role of the Au@TiO2 nanocomposite on the dye degradation process. The reactions were carried out in dark to avoid photo effect on the dye degradation. The MO degradations were performed at pH 4 (30 °C) as electron transfer reaction, for the dye degradation is highly favorable at lower pH (Liu et al. 2009), and also methanogenic bacteria can be suppressed at lower pH, which inhibit electrically active bacteria (Borole et al. 2011).
Analyses
The dye degradation was monitored using a UV–Vis spectroscopy (UV–VIS-NIR, Cary 5000). Differential pulse voltammetry (DPV) analysis was carried out using a potentiostat (VersaSTAT 3, Princeton Research, USA) using standard three-electrode system. An Ag/AgCl (saturated with KCl) was used as the reference electrode, a Pt foil as the counter electrode, and plain carbon paper as the working electrode with as-synthesized Au@TiO2 nanocomposite colloidal solution as the electrolyte. Cyclic voltammetry (CV, multiple cycles) analysis was also performed by the potentiostat using standard three electrode system. An Ag/AgCl (saturated with KCl) was used as the reference electrode, a Pt foil as the counter electrode, and plain carbon paper as the working electrode with as-synthesized Au@TiO2 nanocomposite colloidal solution as the electrolyte. The scan rate used was 50 mV/s. Toxicity analysis was performed by following the resazurin reduction method (Kalathil et al. 2012a; Kalathil et al. 2011a).
Results and discussion
The metal nanocomposites show characteristic colors due to excitation of surface plasmon vibrations. UV–Vis analysis showed a peak at around 550 nm (Fig. S1) which indicated the formation of the Au@TiO2 nanocomposite (Dawson and Kamat 2001; Kalathil et al. 2012b). XRD measurement showed that sharp peaks correspond to both TiO2 and Au (Fig. S2) indicating high crystallinity of the as-synthesized nanocomposite (Kalathil et al. 2012b). Transmission electron microscopic (TEM) image shows that the product is almost exclusively composed of discrete Au@TiO2 nanoparticles (Fig. S3a). The HRTEM micrograph (Fig. S3b) indicates that average particle diameter of Au nanoparticles is around 8 nm. Selected area electron diffraction (SAED) pattern shows well-resolved (101) lattice fringes (d101 = 0.35 nm) and diffraction cycles indicative of a highly crystalline TiO2 anatase framework (Fig. S3c).
MO reductions by the EAB in the presence of Au@TiO2 and by the EAB without the nanocomposite were monitored using UV–Vis spectroscopy. The UV–Vis spectra (Fig. 1) showed that the MO reduction was completed within 2 h by the EAB-Au@TiO2 nanocomposite system while only around 40 % degradation was observed for the EAB without the nanocomposite, which indicates the role of Au@TiO2 on the degradation. To our pleasant surprise, the toxic intermediates (aromatic amines) formed by the MO degradation were also degraded after 2 h, which was confirmed by the disappearance of the peak around 270 nm by the EAB-Au@TiO2 system (Fig. 1d). However, the MO degradation under strict anaerobic condition by sparging N2 gas continuously during degradation reaction into the EAB-Au@TiO2 system increased the intensity (Fig. 1g) of the toxicity peak (more explanation in mechanism section, Fig. 2). The toxic intermediate peaks at 270 nm were enhanced after the MO degradation by the EAB only (without nanocomposite), which is anticipated in the anaerobic conditions without the catalyst (Fig. 1e–f).
UV-Vis spectra of MO degradation by the EAB-Au@TiO2 nanocomposite and EAB only. a represents initial MO spectrum while b, c, and d represent MO degradation after 30 min, 1 h, and 2 h, respectively by the EAB-Au@TiO2 nanocomposite system. e and f show the MO degradation after 2 and 3 h by the EAB only (without nanocomposite). g represents the MO degradation after 2 h by the EAB-Au@TiO2 system under strict anaerobic condition by sparging N2 gas continuously during the degradation reaction. The arrow indicates the increase of intermediates peaks by the EAB only, and * represents the reduction of intermediates peaks by the EAB-Au@TiO2 nanocomposite system
Mechanism involved in the MO degradation by the EAB-Au@TiO2 nanocomposite was depicted in Fig. 2. We used Au@TiO2 nanocomposite instead of pure Au nanoparticles for the dye degradation as the Fermi level energy difference between the TiO2 conduction band and the pure Au nanoparticles allows a much larger potential difference to be accommodated than is possible at the pure Au–H2O interface (Oldfield et al. 2000). The TiO2 can be an appropriate support to anchor the Au nanoparticles, and the anchoring of Au nanoparticles on the TiO2 surfaces should avoid agglomeration and coalescence of Au nanoparticles during catalysis (Primo et al. 2011). The EAB biologically oxidized the acetate to electrons and protons. The electrons produced by the EAB were injected into the conduction band (CB) of TiO2 (Fig. 2), and the electron injection by the EAB to the CB of TiO2 has already been documented (Kalathil et al. 2012b). Chemical injection of electrons to Au@SnO2 nanocomposite by NaBH4 has been already reported (Oldfield et al. 2000). Here, the EAB mimics NaBH4 by giving electrons to the nanocomposite (Fig. 2), which offers a green and sustainable approach for the dye degradation. Spectroscopic studies prove that the electron transfer from a semiconductor to a metal nanoparticle is an ultrafast process (Harris and Kamat 2010). Since the Fermi level of Au (EF = + 0.45 V versus NHE) is more positive than the CB of TiO2 (ECB = −0.5 V versus NHE), the stored electrons in the CB of TiO2 are easily transferred to Au nanoparticles until the Fermi level of the two systems attain equilibration (Choi et al. 2012; Kamat 2007). The electron accumulation increases the Fermi level of Au to more negative potentials, and hence the resultant Fermi level of the nanocomposite shifts closer to the CB of the TiO2 (arrow in Fig. 2), which will impart excellent reductive power to the nanocomposite (Choi et al. 2012; Kamat 2007).
A convenient tool to investigate the charge storage within metal or metal nanocomposites is DPV analysis (Murray 2008). The DPV analysis showed a well-defined quantized charging peak at positive potential similar to many previous reports (Chen et al. 1998; Murray 2008) indicating excellent charge storage property of the nanocomposite (Fig. 3). CV analysis showed a well-pronounced reduction peak which further confirmed the excellent reduction capacity of the nanocomposite (Fig. 4). It can be seen that the second CV cycle is well-pronounced than the first CV cycle as the second cycle should be less influenced by ohmic drop compared to the first cycle (Lovric and Scholz 2003). The stored electrons within the nanocomposite were transferred to the azo bonds (–N = N–) of the MO which cleaved the bonds by producing intermediate aromatic compounds (Fig. 2). These toxic intermediates may be further mineralized by the EAB-Au@TiO2 system which was confirmed by the UV analysis (Fig. 1d). Even though the system was kept as anaerobic, we cannot neglect the possibility of some dissolved oxygen (DO) in the dye degradation system. The DO can accept the electrons stored in the nanocomposite to form superoxide (.O2) radicals (Fig. 2) which can destroy the toxic intermediates (Hoffmann et al. 1995). As observed in Fig. 1g, when the EAB-Au@TiO2 system was kept strictly anaerobic by sparging N2 gas continuously during the reaction time, peak corresponding to toxic intermediates increased. It should be noted that it is not possible to keep a strict anaerobic condition by sparging N2 gas continuously in practical use as it consumes lots of energy, and it is also important to consider that biological systems in practical uses are often temporarily exposed to oxygen. Fortunately, EABs formed with mixed culture bacteria can usually survive at low DO concentration, and the presence of low DO can enhance substrate breakdown and enhances its utilization (Borole et al. 2011). This indicates the possible role of DO on the mineralization of toxic intermediates by forming .O2 radicals, and the .O2 radicals can be generated only in the presence of a catalyst (Tsunoyama et al. 2009).
Toxicity analysis (resazurin reduction method) was performed to investigate the toxicity of parent dye and intermediate products. The original MO showed 16 ± 3 % inhibition on the resazurin reduction, which can be considered as toxic. The products formed by the degradation of MO by the EAB-Au@TiO2 nanocomposite system after 2 h showed 6 ± 2 % inhibition which can be considered as almost non-toxic concordant with disappearance of intermediates peak in the UV spectrum (Fig. 1d). On the other hand, the products formed after the MO degradation by the EAB (without nanocomposite) after 2 h showed 28 ± 5 % inhibition which indicates the relatively higher toxicity of the intermediates compared with the parent dye which also satisfies the UV analysis (Fig. 1e).
The proposed EAB-Au@TiO2 dye degradation route is far superior to other existing physical, chemical, and biological methods for the efficient degradation of dyes (Table 1). The dye degradation process was rapid compared to other biological methods using fungi and bacteria which usually consume 24–96 h for the degradation of dyes (Chang et al. 2004; Lin et al. 2010; Saratale et al. 2011; Wang et al. 2009) while our approach degraded the dye within 2 h. Physical methods based on coagulation–flocculation suffer from lower color removal efficiency, use of copious amount of chemicals, and large amount of sludge production (Vandevivere et al. 1998). Adsorption method has been limited by problems associated with regeneration or disposal, high cost, and low effectiveness for the dye degradation (Anjaneyulu et al. 2005). Filtration methods using membranes have been severely hindered by high costs and potential membrane fouling (Robinson et al. 2001). Chemical oxidation methods such as ozonation limit practical applications due to their shorter life time and high cost of ozone (Anjaneyulu et al. 2005). Even though electrochemical oxidation is highly effective for the dye degradation, high cost of electricity limits its application (Morawski et al. 2000). Photo-degradation of dyes by UV light is potentially dangerous and consumes lots of energy (Primo et al. 2011). On the other hand, our proposed degradation protocol doesn’t use any toxic chemicals or reagents which make the protocol eco-friendly. Also, there is no need of energy input as the EAB acts as an electron generator which makes the protocol highly sustainable for practical applications. The toxic intermediates formed during the dye degradation were also removed by the EAB-Au@TiO2 system, and it avoids a secondary treatment of the treated dye wastewater.
Conclusions
A rapid and efficient degradation of MO with mineralization of toxic intermediates was achieved by EAB-Au@TiO2 nanocomposite system. The quantized charging property of the nanocomposite may be responsible for the high catalytic activity of the nanocomposite. Also, Fermi level equilibration in the Au@TiO2 nanocomposite plays a crucial role in improving its catalytic performance. The extension of this study on other semiconductor–metal nanostructures for environmental remediation is on the anvil in our lab and will be reported elsewhere.
References
Anjaneyulu Y, Sreedhara Chary N, Raj SSD (2005) Decolorization of industrial effluents-available methods and emerging technologies—A review. Rev Environ Sci Biotechnol 4:245
Borole AP, Reguera G, Ringeisen B, Wang Z-W, Feng Y, Kim BH (2011) Electroactive biofilms: current status and future research needs. Energy Environ Sci 4:4813–4834
Chang JS, Chen BY, Lin YS (2004) Stimulation of bacterial decolorization of an azo dye by extracellular metabolites from Escherichia coli strain NO3. Bioresource Technol 91:243
Chen S, Ingram RS, Hostetler MJ, Pietron JJ, Murray RW, Schaaff TG, Khoury JT, Alvarez MM, Whetten RL (1998) Gold nanoelectrodes of varied size: transition to molecule-like charging. Science 280:2098–2101
Choi H, Chen WT, Kamat PV (2012) Know thy nano neighbor. Plasmonic versus electron charging effects of metal nanoparticles in dye-sensitized solar cells. ACS Nano 6:4418–4427
Dawson A, Kamat PV (2001) Semiconductor-metal nanocomposites. Photoinduced fusion and photocatalysis of gold capped TiO2 (TiO2/gold) nanoparticles. J Phys Chem B 105:960–966
Erable B, Duteanu NM, Ghangrekar MM, Dumas C, Scott K (2010) Applications of electro- active biofilms. Biofouling 26:57–71
Gupta N, Singh HP, Sharma RK (2011) Metal nanoparticles with high catalytic activity in degradation of methyl orange: an electron relay effect. J Molecular Catalysis A: Chemical 335:248–252
Harris C, Kamat PV (2010) Photocatalytic events of CdSe quantum dots in confined media. Electrodic behavior of coupled platinum nanoparticles. ACS Nano 4:7321–7330
Hoffmann MR, Martin ST, Choi W, Bahnemann DW (1995) Environmental applications of semiconductor Photoctalaysis. Chem Rev 95:69–96
Husain Q (2010) Peroxidase mediated decolorization and remediation of wastewater containing industrial dyes: a review. Rev Environ Sci Biotechnol 9:117–140
Kalathil S, Lee J, Cho MH (2011a) Granular activated carbon based microbial fuel cell for simultaneous decolorization of real dye wastewater and electricity generation. New Biotechnol 29:32–37
Kalathil S, Lee J, Cho MH (2011b) Electrochemically active biofilm-mediated synthesis of silver nanoparticles in water. Green Chem 13:1482–1485
Kalathil S, Lee J, Cho MH (2012a) Efficient decolorization of real dye wastewater and bioelectricity generation using a novel single chamber biocathode-microbial fuel cell. Bioresource Technol 119:22–27
Kalathil S, Khan MM, Banerjee AN, Lee J, Cho MH (2012b) A simple biogenic route to rapid synthesis of Au@TiO2 nanocomposites by electrochemically active biofilms. J Nanopart Res 14:1051
Kamat PV (2007) Meeting the clean energy demand: nanostructure architectures for solar energy conversion. J Phys Chem C 111:2834–2860
Lin J, Zhang X, Li Z, Lei L (2010) Biodegradation of reactive blue 13 in a two stage anaerobic/aerobic fluidized beds system with a Pseudomonas sp Isolate. Bioresource Technol 101:34
Liu L, F-b Li, C-h Feng, X-z Li (2009) Microbial fuel cell with an azo-dye feeding cathode. Appl Microbiol Biotechnol 85:175–183
Lovrić M, Scholz F (2003) Modeling cyclic voltammograms of simultaneous electron and ion transfer at a conic film three-phase electrode. J Electroanalytical Chem 540:89–96
Morawski BS, Quan S, Arnold FH (2000) Functional expression and stabilization of horseradish peroxidase by directed evolution in Saccharomyces cerevisiae. Biotechnol Bioeng 76:99
Murray RW (2008) Nanoelectrochemistry: metal nanoparticles, nanoelectrodes and nanopores. Chem Rev 108:2688–2720
Oldfield G, Ung T, Mulvaney P (2000) Au@SnO2 core-shell nanocapacitors. Adv Mater 12:1519–1522
Pant D, Bogaert GV, Diels L, Vanbroekhoven K (2010) A review of substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresource Technol 101:1533–1543
Primo A, Corma A, García H (2011) Titania supported gold nanoparticles as photocatalyst. Phys Chem Chem Phys 13:886–910
Robinson T, McMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textiles effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol 77:247–255
Saratale RG, Saratale GD, Chang JS, Govindwar SP (2011) Bacterial decolorization and degradation of azo dyes: a review. J Taiwan Institute of Chemical Engineers 42:138–157
Takai A, Kamat PV (2011) Capture, store, and discharge. Shuttling photogenerated electrons across TiO2-silver interface. ACS Nano 5:7369–7376
Tsunoyama H, Ichikuni N, Sakurai H, Tsukuda T (2009) Effect of electronic structures of Au clusters stabilized by poly(N-vinyl-2-pyrrolidone) on aerobic oxidation catalysis. J Am Chem Soc 131:7086–7093
Vandevivere PC, Bianchi R, Verstraete W (1998) Treatment and reuse of wastewater from the textile wet-processing industry: review of emerging technologies. J Chem Technol Biotechnol 72:289
Wang H, Zheng SW, Su JQ, Tian Y, Xiong XJ, Zheng TL (2009) Biological decolorization of the reactive dyes reactive black 5 by a novel isolated bacterial strain Enterobacter sp. EC3. J Hazard Mater 171:654
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (Grant No: 2012R1A1A4A01005951). Shafeer Kalathil was supported by the Human Resources Development Program of Korea Institute of Energy Technology Evaluation and Planning (KETEP) Grant (No:20104010100580) funded by the Korean Ministry of Knowledge Economy.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kalathil, S., Lee, J. & Cho, M.H. Catalytic role of Au@TiO2 nanocomposite on enhanced degradation of an azo-dye by electrochemically active biofilms: a quantized charging effect. J Nanopart Res 15, 1392 (2013). https://doi.org/10.1007/s11051-012-1392-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-1392-5