Abstract
The pericardium is an important structure of unique interest to the cardiac surgeon who routinely encounters its elegant design. The pericardium itself is a lubricated compartment that protects the heart by maintaining its position in the chest and limiting dilation from volume overload. The pericardium has the unique quality of passive noncompliance. Surgery of the pericardium is mainly required for hemodynamic effects of a pressurized sac around the heart that can occur with the presence of fluid or constriction.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
For Physicians and Health Care Providers
Introduction
The pericardium is an important structure of unique interest to the cardiac surgeon who routinely encounters its elegant design. The pericardium itself is a lubricated compartment that protects the heart by maintaining its position in the chest and limiting dilation from volume overload. It is <2 mm thick and composed of collagen and elastin [1]. The visceral and parietal layers are contiguous with each other at the origins of the great vessels. Entry into the pericardium for surgery reveals not only the heart but also other structures of anatomic significance. Openings are present at the superior vena cava, inferior vena cava, pulmonary artery, and aorta. The phrenic nerves adhere to the pericardium laterally and are critical for preservation of respiratory function [2].
The pericardium has the unique quality of passive noncompliance [2]. Surgery of the pericardium is mainly required for hemodynamic effects of a pressurized sac around the heart that can occur with the presence of fluid or constriction.
Resection of Pericardial Cysts, Tumors, and Congenital Defects
Rare conditions of the pericardium occasionally require surgical intervention. Pericardial cysts are fluid-filled sacs that are often located at the pericardiophrenic angle, more commonly on the right side [3]. Generally, these are of little clinical significance. These cysts may form as a result of inflammation, bacterial infection, trauma, or cardiac surgery. Most cysts are discovered incidentally. Symptoms can occur and may include chest pain, dyspnea, cough, or palpitations [4]. If symptomatic, surgical excision is indicated and may be performed in an open or minimally invasive fashion [5].
Cardiac neoplasms are also rare, found in 1–3 % of patients at autopsy [6]. The pericardium itself may have primary or metastatic tumors that require resection [7]. Mesothelioma is the most common primary malignancy of the pericardium [7]. Symptoms such as chest pain, constrictive pericarditis, or cardiac tamponade have been described [7]. Malignant pericardial effusion is most often caused by metastatic cancer.
Congenital defects of the pericardium occur in 1/10,000 autopsy specimens, with 70 % of these defects occurring on the left side [8, 9]. A partial defect may be symptomatic and can be complicated by herniation and strangulation of the heart through the defect. Total deficit of the pericardium occurs in 1/14,000 births and these patients are usually asymptomatic [9]. They are at risk for traumatic type A aortic dissection [10]. Patch replacement of the pericardium (pericardioplasty) has been described with good success [11, 12].
Mediastinal Exploration
Exploration of the mediastinum and opening of the pericardium are required in a variety of clinical settings. These include trauma, iatrogenic injury, postoperative cardiac surgery, and aortic dissection.
Trauma
Traumatic pericardial effusion after blunt or penetrating injury is a rare but clear indication for drainage and possibly exploration [13–15]. In the current era, diagnosis of a traumatic injury may still be difficult despite rapid ultrasound, echocardiography, and computerized tomography scans. Prompt drainage is important regardless of method and early identification of injury is the key [16]. Traumatic cardiac rupture requiring repair has been found in up to 5.7 % of patients who require drainage [16].
Iatrogenic Injury
Iatrogenic injury to the heart can occur after catheter-based procedures and may require surgical intervention. Percutaneous cardiac procedures are complex and rapidly advancing in the current era. Transseptal puncture to access the left heart takes place during electrophysiology procedures on the left atrium. Percutaneous transaortic valve replacement and valve dilations involve complex vascular access and imaging techniques. Guide wires, sheaths, dilators, balloons, leads, or ablation techniques may result in cardiac injury [17]. The incidence of perforation in atrial fibrillation ablation is reported as 6 % [18]. Pacemakers have an incidence of perforation of 1.7 % [19]. When fluid accumulates in the pericardial space after catheter-based procedures, it is usually a result of cardiac perforation [17]. It is important to remember that in traumatic situation <100 cc of fluid in the pericardial space can cause hemodynamic compromise [20, 21]. Similar to traumatic injury, prompt recognition is the key. These injuries are routinely treated by percutaneous drainage alone. However, depending on the location and size of injury, median sternotomy and exploration may be required [17].
Postoperative Tamponade
Cardiac surgeons open the pericardium in order to access the heart. Although drainage tubes are left within the mediastinum after surgery, coagulopathy and postoperative bleeding can occur. This is more common after long cardiopulmonary bypass times and complex procedures. Strict criteria for reoperation for bleeding are maintained in order to prevent complications and organ dysfunction in the postoperative period. In addition, surgeons must carry a high suspicion of tamponade in patients with significant or persistent high-volume bleeding initially who have a sudden drop in output with corresponding hemodynamic changes. These may include: equalization of right and left heart pressures, low cardiac index, low mixed venous saturation, tachycardia, hypotension requiring increased pressors, and elevated central venous pressure. A stat chest x-ray (CXR) or echocardiogram may confirm tamponade but this is ultimately a clinical diagnosis. Patients are promptly returned to the operating room for evacuation of hematoma and exploration through the previous sternotomy incision.
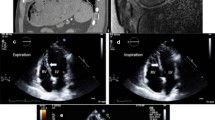
Postpericardiotomy syndrome is another situation in which inflammatory fluid accumulates in the pericardial space after surgery. This syndrome occurs in a delayed fashion after cardiac surgery and has been documented in up to 1.5 % of patients [22] (Fig. 12.1).
Despite over 70 years experience of cardiac surgery, the question of pericardial closure after cardiac procedures has not been definitively answered [23]. Some surgeons close the pericardium, others do not, and many believe that neither is of clinical significance. There has been little scientific study and no clinical randomized trials. What is known is that the pericardium maintains compliance and the integrity of the Starling curve as it limits hypertrophy with exercise. It is structurally protective with mechanical membranous and ligamentous function. Benefits of closing the pericardium include making potential reoperation safer with fewer adhesions, return of the mediastinum back to the original setting, and the possibility of improved hemodynamics [23]. Others are concerned that closure results in increased risk of tamponade, negative hemodynamics, increased use of inotropes, and possible graft compromise [23]. Tension- free substitutes do exist but come with financial cost and possible infectious complications [23].
Aortic Dissection
Pericardial effusion and tamponade may occur in the setting of Type A aortic dissection. In this setting, surgery for the dissection should be performed immediately rather than drainage of the hemopericardium, which may result in further bleeding [4].
Pericardial Window
The etiology of pericardial fluid causing compression varies and includes infections, post-irradiation sequelae, collagen vascular diseases, myocardial infarction, and malignancy [24–30] (Table 12.1). Medical therapy may be started initially but large effusions associated with pericarditis may be unresponsive to non-steroidal anti-inflammatory drugs, corticosteroids, or colchicine [29]. The diagnosis of a large pericardial effusion can be made from CXR, computerized tomography (CT) scan or echocardiography (Fig. 12.2). The definitive treatment for large pericardial effusions or cardiac tamponade is pericardial drainage. If hemodynamic compromise has occurred, medical treatment has failed, or a diagnosis is needed, then intervention is required. The specific presence of purulent pericardial fluid may have a distinct effect separate from tamponade physiology and has a characteristically high mortality without drainage [31].
An open surgical procedure offers several advantages. Firstly, complete drainage can be achieved. There is ample access to pericardial tissue for histopathological and microbiological diagnoses. Loculated or mixed effusions can be evacuated (Fig. 12.3). There is little risk of traumatic injury as there is direct visualization of the pericardial space.
Sub-Xiphoid Pericardial Window
Sub-xiphoid pericardial window, or subxiphoid pericardiostomy, is a common approach to pericardial drainage [25–29, 32]. General anesthesia is preferred for this procedure, but it may be performed with monitored or local anesthesia [33]. Rapid induction can proceed with careful attention to blood pressure throughout the process. The patients are placed supine during the procedure. If the patient is suspected to be in true cardiac tamponade, the patient’s chest is often prepared and draped for surgery while he remains awake. In case of hemodynamic collapse upon induction, rapid evacuation of fluid may be performed. Sub-xiphoid drainage under local anesthesia is also an acceptable choice for patients who are unstable.
Technique
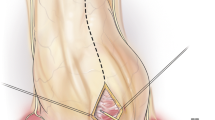
A midline incision is made from the xiphisternal junction to below the tip of the xiphoid. Alternatively a 5 cm transverse incision can be made at the tip of the xiphoid [1]. The upper linea alba is divided in the midline and the xiphoid is incised or completely resected. The tissue plane between the posterior wall of the sternum and the anterior pericardium is developed by blunt finger dissection. The distal sternum is then elevated for visualization of the pericardium. The anterior pericardium is grasped directly and incised to drain the fluid. Culture swabs, fluid analysis, and cytology specimens are collected. Pericardial fluid is analyzed for hematocrit and cell count, amylase, lactate dehydrogenase, protein, glucose, culture, and cytology. The pericardial space is then evaluated by direct vision, digital exam, and echocardiographic visualization if needed. The pericardium may be explored digitally to identify adhesions or tumor deposits. The optional use of intraoperative transesophageal echocardiography may facilitate removal of complex loculated collections and can insure complete drainage. A piece of pericardium is excised, typically 4–5 cm in size (Fig. 12.4). A single chest tube is placed through the pericardiotomy, exiting the body through a separate incision. Both firm large bore and soft flexible drains may be used to insure optimal drainage, particularly in the case of a bloody effusion. The chest tube is left in place for several days after the operation until the drainage is minimal, usually <50 cc/day. This time period is the key to this procedure. The irritative nature of the chest tubes within the space can help form adhesions between the pericardium and epicardium to help prevent recurrence. The chest incision is closed in two layers and covered with sterile dressings.
Outcomes
Multiple retrospective studies over the past 25 years have reviewed outcomes of subxiphoid pericardial window, after its initial success in providing drainage and preventing recurrent effusion. Early studies, however, reported 30-day mortality rates of up to 20 %, with deaths due to associated cancer rather than the procedure itself [34]. Current studies show 30-day mortality of 0.8–4.8 % [27, 35, 36] and a recurrence rate of 2–10 % [35–37].
Risk factors for short-term mortality include the occurrence of postoperative low cardiac output syndrome (PLCOS). This syndrome is early and rapid cardiac failure after relief of tamponade and can occur in up to 4.8 % of patients [36]. It accounted for all the postoperative deaths (0.8 %) in a recent study [27]. The mechanism for this complication is not completely clear but is likely due to chronic external support of the heart by pericardial fluid. When this support is removed, the heart may immediately overdilate, resulting in systolic dysfunction and failure. These patients need close monitoring, as the mortality from this syndrome is very high.
Complications of the procedure include recurrence and transient arrhythmias [36]. Constrictive pericarditis develops in up to 3 % of patients who survive after 1 year, most commonly in those patients with tuberculous pericarditis or non-tuberculous bacterial pericarditis [27]. Direct injury to the heart may occur in 0.8 % and requires median sternotomy for treatment [27]. Wound infections may occur in up to 5 % of patients [27].
Underlying disease, specifically malignancy, is an important risk factor for decreased survival after subxiphoid pericardiostomy [28]. The presence of malignant pericardial effusion leads to limited life expectancy; better survival is found in those patients who have malignant cells but no tumor in the pericardium [38]. The type of malignancy also plays a role: patients with hematologic malignancy were found to have significantly longer survival when compared with patients with other malignancies [36]. Metastatic lung cancer to the pericardium has been shown to have a very poor survival rate, particularly when compared to other cancers [28, 39]. Detectable malignant invasion of the thorax on CT scan and positive echocardiographic findings compatible with tamponade are two independent risk factors for poor outcome [28].
Comparison with Percutaneous Drainage
The optimal management for pericardial effusions with acute pericardial tamponade remains controversial. The two most commonly performed techniques include subxiphoid window and percutaneous catheter drainage (Table 12.2). Percutaneous catheter drainage may be performed with local anesthesia and requires a needle to be placed in the pericardial space, usually under echocardiographic guidance. A guide wire can be inserted and a drainage catheter is passed over the wire [40].
Multiple studies have directly compared these two techniques (Table 12.3). Allen et al. performed a direct comparison of the two procedures in 1999 [35]. The mortality, complication, and recurrence rates were significantly higher for percutaneous drainage (4.3, 17.3, and 33.3 %, respectively) than for subxiphoid drainage (0, 1.1, and 1.1 % respectively) [35]. This same study combined published collected data from 1977 to 1999 and found 560 patients undergoing subxiphoid pericardial window for pericardial tamponade. These patients had a mortality rate of 0.6 %, a complication rate of 1.5 %, and a recurrence rate of 3.2 % [35]. Percutaneous catheter drainage (331 patients) demonstrated increased mortality, morbidity and effusion recurrence of 4.3, 10.6, and 13.9 %, respectively [35]. Subxiphoid pericardiostomy is a safe and durable technique for chronic effusion despite the less invasive nature of the percutaneous drain.
Others studies concur that recurrent effusion is more frequent in the percutaneous versus the open group, (28.9 % vs. 2.8 %), [41] and (15.6 and 4.7 %) [37]. Mortality rates that are higher in the percutaneous group are difficult to extract from data as variable comorbid conditions and hemodynamic states leading to percutaneous drainage are confounding factors. It has been shown that extended catheter drainage may help decrease this rate of recurrence in percutaneous techniques, likely secondary to the inflammatory/adhesion forming qualities of the drains themselves [42].
Ultimately, an individualized, patient-centered approach is necessary in making decisions about percutaneous versus open procedures. Patients with positive cytology on previous pericardiocentesis or a limited lifespan where recurrence is unlikely may benefit from percutaneous drainage. Effusions that are loculated, posterior, or of mixed density, may be better served with an open subxiphoid window. The presence of malignancy, direct invasion of the pericardium, poor long term prognosis, and clinical conditions all play a role in this decision process.
Thoracoscopic Pericardial Window
An alternative technique to subxiphoid window is video-assisted thoracoscopic surgery (VATS) drainage of pericardial fluid into the pleural space. Anesthesia preparation is more complicated and time-intensive. It requires bronchoscopic-directed placement of a double-lumen endotracheal tube to achieve single lung ventilation. Lateral decubitus positioning is also required. Thoracoscopy is performed through a 10-mm camera port placed in the seventh intercostal space in the mid-axillary line. The pericardial resection and any additionally procedures are completed through one or two working incisions. A section of pericardium approximately 4–5 cm in diameter is resected anterior to the phrenic nerve, which creates a window into the pleural space [43] (Fig. 12.5). A flexible chest tube may be placed into the pericardial space along with a pleural tube. Alternatively, a single chest tube may be placed into the pleural space for drainage of both cavities.
This procedure has specific advantages and disadvantages. One benefit of the thoracoscopic approach is that it allows simultaneous access to the pleural and pericardial spaces, which is helpful in the setting of large pleural effusion or concomitant pleural disease. Thoracoscopy affords better visualization of the pleural cavity and pericardium, which allows for more direct sampling of suspicious sites [43, 44]. The entry into another body cavity is a potential disadvantage depending on the co-morbidities of the patient. Generally, sub-xiphoid windows are preferred in a setting in which the patient is unstable to avoid the prolonged anesthetic and preparation time that is associated with VATS.
In a study of 71 patients directly comparing sub-xiphoid and VATS, O’Brien et al. found significant differences in complication rates [44]. The sub-xiphoid group had a 2 % morbidity rate while the VATS group had a 27 % morbidity rate [44]. Complications included pneumothoraces, an on-going air leak requiring discharge with a Heimlich valve, and readmission for self-limited drainage from the chest tube site. The 30-day mortality rate was 13 and 0 % for subxiphoid and VATS, respectively, but all mortalities were nonspecific to the procedure and were attributed to advancing malignancy or worsening of underlying medical illness in the absence of recurrent effusion [44]. Patients with greater comorbidities were selected for sub-xiphoid drainage. Recurrence was similar in both groups (10 % of the sub-xiphoid group and 8 % of the VATS). Malignant effusions were found to have a greater risk of recurrence [44].
A recent study found that limited survival is not a contraindication for VATS pericardial window as selected patients could achieve improvement through palliation [45]. Prognostic factors of poor survival included pericardial cytology with metastatic involvement of the pericardium, similar to other studies [44, 28]. Others have found that there are few differences in recurrences or complications, but that operative time is longer for VATS [46].
In summary, the sub-xiphoid approach is simpler, faster, and slightly less morbid. This is the preferred approach if the patient’s life expectancy is likely to be limited due to major comorbidities or extensive metastatic disease. In contrast, those patients with benign disease, malignancy that has not metastasized extensively or is responsive to chemotherapy, and those who require concomitant intrapleural procedures, would benefit from VATS [44] (Table 12.4).
Pericardiectomy
Indications
Constrictive pericarditis is a rare but severely disabling condition of the pericardium leading to impaired filling of the ventricles and reduced ventricular function [4]. The majority of cases of constrictive pericarditis are idiopathic [4, 47]. Patients present with symptoms such as dyspnea, orthopnea, jugular venous distention, or ascites. CXR or CT scan may confirm a thickened or calcified pericardium, a classic diagnostic feature of constrictive pericarditis [4]. However constriction may present in up to 18 % of patients with normal pericardial thickness [48]. True constrictive physiology is best defined at cardiac catheterization with findings as described in previous chapters. The hallmarks of constrictive physiology are equalization of diastolic pressures in the ventricles and a dip plateau pattern (square root sign) of the ventricular filling pressure curves [1]. Pericardiectomy for constrictive pericarditis corrects hemodynamic abnormalities and can produce dramatic clinical improvement [47].
Pericardiectomy is indicated once the diagnosis of constrictive pericarditis has been established. Constrictive pericarditis is irreversible and surgical resection is the only effective treatment. Surgeons are sometimes consulted to evaluate patients for pericardiectomy who have frequent and highly symptomatic recurrences that are refractory to medical therapy [4]. Recurring pericarditis management relies on exercise restriction and is treated medically with NSAIDS, colchicine, and/or corticosteroids [4]. Patients who are deemed candidates for surgery should be on a steroid-free regimen for several weeks prior to surgery. Patients with little physiologic effects and significant comorbidities should be delayed until more significant symptoms occur. This is especially true in the case of radiation-induced pericarditis, in which the myocardial tissue is affected [1, 49].
Technique
Pericardiectomy is performed under general anesthesia. Anesthetic considerations are similar to other routine cardiac procedures except for the use of short acting muscle relaxants. It is helpful to have minimal paralysis during dissection near the phrenic nerve. TEE is used routinely to evaluate changes in cardiac size and function and, specifically, to assess the tricuspid valve which may require repair for severe regurgitation and chronic right heart failure [50].
Cardiopulmonary bypass is usually on standby and surgeons will reserve it for extremely difficult dissections, reoperations, or concomitant required cardiac surgery. Pericardiectomy is most commonly approached through a median sternotomy, which provides excellent exposure. Left thoracotomy or bilateral anterior thoracotomy approaches have also been used. Finding the proper plane can be very difficult; the plane between the parietal and visceral layers is avascular. Serious bleeding and/or injury can result if the epicardium is penetrated. Sometimes the parietal layer may be very densely adhered to the epicardium with heavily calcified spicules. These areas can be rongeured, but total removal is often hazardous. The dissection plane in a post-radiated heart provides further challenges to complete decortication [50, 51].
A key step in this procedure is that resection should begin with the left ventricle first. Specific right ventricular dilatation and failure can result when the right ventricle is freed from its pericardial restraints before the left ventricle is freed. This would allow for increased filling of the right ventricle in the setting of persistently increased right ventricular afterload. Pulmonary edema and right ventricular failure due to outflow obstruction can occur if the right ventricle is released first [1]. Ultimately the goal is to do as complete a resection as safely possible, with decortications of both ventricles, both atria, and both cava (Fig. 12.6). Care should be taken to visualize and preserve both phrenic nerves. Hemostasis should be achieved and chest tubes left in place. Utilizing a pulmonary artery catheter, the adequacy of the pericardial resection can be evaluated by measuring mean arterial pressures and RV end-diastolic pressures before and after completion of the operation. Perioperative low output cardiac failure can usually be managed with inotropic medications and occasionally the use of an intra-aortic balloon pump if needed.
Manipulation of the heart can lead to hemodynamic instability in these patients. The need for cardiopulmonary bypass (CPB) must be considered during this procedure and initiated if necessary. This is straightforward in the setting of median sternotomy in which the aorta and right atrial appendage are available for cannulation. The femoral artery and vein are also sites of potential cannulation for bypass. It must be remembered that cardiopulmonary bypass empties the heart and may make dissection of the pericardium more difficult. However, it prevents hemodynamic shifts with lifting and dissection that can cause poor organ perfusion and postoperative dysfunction. CPB requires full anticoagulation with heparin and can increase bleeding, coagulopathy, and a generalized systemic inflammatory response.
This surgery is often technically demanding and tedious. There is potential for myocardial injury, phrenic nerve injury, and coronary artery injury. With longstanding disease there can be remodeling of myocardial anatomy. Changes in filling can also contribute to failure. These patients require intensive care monitoring postoperatively with constant hemodynamic assessment and early cardiac support if needed. This may include vasopressors and inotropic medications.
Outcomes
In the current era, pericardiectomy for constrictive pericarditis has a mortality rate of 6–14 % [48, 50–57]. The complete normalization of cardiac hemodynamics can be expected in about 60 % of patients [58, 59]. In a recent study, George et al. found that a 5–7.6 % morality with 1, 5, and 10-year survival of 82, 64, and 49 %, respectively [54] (Table 12.5).
Several factors have been found to be risk factors for poor outcome, including high NYHA class, female sex, and the underlying etiology of the effusion [53]. Inflammatory and idiopathic etiologies have the best outcome, while post-radiation patients fare the worst [53]. The need for CPB is also associated with increased mortality [54]. Poor prognosis has been associated with increased age, decreased left ventricular systolic dysfunction, elevated pulmonary artery pressure, and increased creatinine [52]. Other studies have confirmed that age [50], preop NYHA class [58, 60–62], and hepatic [62] and renal dysfunction [61] are important. Recently, diabetes mellitus and high early diastolic inflow velocity have been shown to predict high mortality [63]. In 2013, Gopaldas et al. published a nationwide outcomes study of over 13,000 pericardiectomy patients [55]. He found that after risk adjustment, age, female gender, comorbidity index, and primary diagnosis were significant predictors of in-hospital mortality and complications [55].
Complications of pericardiectomy include direct myocardial injury during dissection which can lead to cardiac failure and bleeding. Failure to achieve complete resection may result in suboptimal hemodynamic changes. Low cardiac output syndrome (LCOS) is the most significant complication. It is likely due to myocardial atrophy as the heart is chronically externally supported; once released the heart is subject to over dilation and failure [64]. However, this is not the only issue as it does not explain why patients with constrictive idiopathic disease have much better outcomes after surgery than other groups. LCOS occurs in 14–28 % of patients after pericardiectomy [64]. It is rapid, and can lead to systemic heart failure and death [64]. The treatment is supportive care [64].
Although complete pericardiectomy is technically challenging and can cause significant hemodynamic compromise, it has been found that smaller operations are more poorly tolerated and that a less aggressive pericardiectomy is a risk factor of overall survival [65]. Furthermore, reoperative pericardiectomy has a significant and nearly prohibitive early mortality. Cho et al. showed in 41 patients who presented for reoperative pericardiectomy had a 30 day morality of 12 % with a 5 year survival of only 4 % [66]. Risk factors for these patients included high NYHA class 3 or 4 and less than 1 year between operations [66]. It is clear that a simple anterior pericardiectomy is not sufficient release to normalize cardiac function. Those who survive total pericardiectomy do better in the long term. Patients with underlying restrictive cardiomyopathy and pulmonary hypertension can have a more complicated course.
The etiology of the constriction relates directly to survival (Fig. 12.7). Patients with constrictive pericarditis due to radiation injury have markedly reduced late survival [51]. Many post radiation patients have myocardial fibrosis, restrictive cardiomyopathy, coronary artery disease, and valvular heart disease [58]. If pericarditis is secondary to radiation, it is important to consider that the underlying cardiomyopathy is still present even after release of the heart. Postoperative recovery for patients with previous radiation is complicated by poor lung function and chest wall fibrosis. Long-term survival of these patients demonstrates 40 % 5- year survival and 11 % 10- year survival [54].
Kaplan-Meier curves showing a significant difference (log-rank test, p = 0.0075) in overall survival of patients after pericardiectomy, based on the presumed cause of constrictive pericarditis (Permission obtained from Elsevier Ltd. [52])
Overall survival is also related to the duration of symptoms. If the indication for surgery was established early, long-term survival after pericardiectomy may correspond to that of the general population [50].
Surgical Management of Pericardial Disease-For Patients and their Families
The pericardium is a double-layered membrane that covers the heart. Excess fluid in the space between the pericardium and the heart is referred to as a pericardial effusion. This can result from a variety of illnesses including bacterial infections, cancer, a reaction to radiation, or a heart attack. The initial management of pericardial effusions is usually with medicines such as NSAIDs or steroids; however, if the effusion is not controlled with medicine or a diagnosis is needed, surgery is required.
Too much fluid in the cavity can also lead to compression of the heart called ‘pericardial tamponade’. This is a potentially dangerous condition as it prevents the heart from beating normally and providing enough blood flow to the body.
The definitive treatment for large pericardial effusions or pericardial tamponade is drainage with a surgical procedure. These procedures open up the pericardium and allow complete drainage of the fluid, thereby relieving the pressure on the heart. The surgical procedures allow for sampling of the pericardial tissue for biopsy and less risk of injury to the heart when compared with placing a needle or thin catheter into the pericardial space. Pericardial fluid is sent to the laboratory for analysis of its contents and to try and determine what caused the fluid to accumulate.
Sub-Xiphoid Pericardial Window
General anesthesia is usually preferred for this procedure. However, a sub-xiphoid pericardial window can be performed with local anesthesia and adequate sedation if the patient is unstable.
The patient is placed lying on his back during the procedure. The procedure is as follows: a small incision is either made vertically over the xiphoid (a bone that hangs off the bottom of the ribcage in the center of the chest) or horizontally right below where the xiphoid ends. The surgeon will then use his fingers to gently dissect down to the heart. The pericardium is cut and the fluid is drained and sent for culture and analysis. The pericardium is then explored for adhesions or tumor deposits before cutting out a small piece, creating a “window”. A chest tube is placed through the window and through a separate skin incision, exiting the body. The chest tube is left in place for several days after the operation until the drainage is minimal, at which time it is removed.
Possible complications from this procedure include developing air between the lung and chest cavity (pneumothorax), irregular heart rhythms (arrhythmia), or damage to the heart muscle. Recurrence of the effusion is also possible, although the rates of this are very low with this technique.
Another commonly performed technique for pericardial fluid drainage is percutaneous catheter drainage. Percutaneous catheter drainage is performed under local anesthesia and is achieved with either blind placement of a needle into the pericardial space using anatomical landmarks or with an ultrasound. A wire is passed through the needle and a drainage catheter is passed over the wire. The wire and needle are removed and the catheter is secured. There are more complications with this procedure when compared with the sub-xiphoid pericardial window such as damage to the heart and a higher chance of recurrence of the effusion. However, very unstable patients may have more benefit from this procedure.
Thoracoscopic Pericardial Window
Another procedure that is used for drainage of pericardial effusions is thoracoscopic drainage of pericardial fluid into the spaces surrounding the lungs (pleural space) and out through a chest tube. General anesthesia must be used in these cases and the time of the procedure is longer because of positioning and other simultaneous lung procedures. Thoracoscopy, or visualizing the lung and heart through a small camera, is achieved with two to three small incisions allowing a camera and small instruments into the chest cavity. A piece of pericardium approximately 4 cm in diameter is cut away and a chest tube is placed in the pleural space. Post-operative management is similar to that of the sub-xiphoid technique.
Advantages of this procedure include simultaneous access to both the pleural and pericardial spaces, better visualization of the pericardium and more direct sampling of anything that looks suspicious (for example, cancer). However, entry into two cavities is also potentially a disadvantage, especially in patients who have many medical problems or those who are unstable. Again, sub-xiphoid pericardial window is the preferred procedure in the setting of an unstable patient.
Total Pericardiectomy
Constrictive pericarditis is a rare but severely disabling disease of the pericardium. The pericardium is a membrane that forms the cavity in which the heart lives. Normally this cavity is filled with a small amount of fluid and the pericardium is usually soft and pliable allowing the heart to expand when filling with blood and contract when ejecting the blood out to the rest of the body. In constrictive pericarditis, there has been some insult to the heart leading to a stiffening of the pericardium and this does not allow the heart to move properly. Most importantly, it leads to an impaired filling of the ventricles and a reduced ventricular function. The majority of cases of constrictive pericarditis are of unknown cause (idiopathic). When a cause can be identified, it is most commonly seen in patients who have had open-heart surgery. Patients may have symptoms such as shortness of breath with activity; some may be short of breath with lying down. When these symptoms are present, patients need a thorough evaluation. CXR and CT scans are some of the initial tests which may be used. Constrictive physiology is best diagnosed by measuring specific heart pressures using a catheter placed through the groin into the heart (cardiac catheterization).
Pericardiectomy, or removal of the pericardium, is indicated once the diagnosis of constrictive pericarditis has been established. True constrictive pericarditis is irreversible and surgical resection is the only effective treatment. The management of recurring pericarditis relies on exercise restriction and medicines such as NSAIDS, colchicine, and/or corticosteroids. Patients with little physiologic effects and significant comorbidities should be delayed until more significant symptoms occur. This is especially true in the case of radiation-induced pericarditis. Unfortunately, the radiation effects do not stop at the pericardium. The myocardium, the heart muscle itself, is often affected as well. The chest wall and lungs can also be damaged with radiation. In the 20 % of patients that develop constrictive pericarditis secondary to radiation therapy, the operative mortality is high (21 %) and the postoperative 5-year survival is very low (1 %).
Pericardiectomy Technique and Outcomes
Pericardiectomy for constrictive pericarditis corrects the hemodynamic abnormalities and can produce dramatic clinical improvement. Pericardiectomy is performed under general anesthesia. The technique is still an area of considerable controversy. The heart-lung machine is usually on standby and surgeons will reserve it for extremely difficult dissections, reoperations, or if concomitant intracardiac surgery is required. The two main incisions are either through a median sternotomy, which is an incision going through the middle of the sternum, or a thoracotomy (i.e., left thoracotomy or bilateral anterior thoracotomies), which is an incision on either side through the rib spaces. Most commonly the median sternotomy is used. Ultimately the goal is to do as complete a resection as safely possible, with removal of the pericardium covering both ventricles, both atria, and both vena cava. Chest tubes are left in place after surgery to drain residual fluid.
Pericardiectomy for constrictive pericarditis has a mortality rate of 6–12 %. Overall survival is related to the duration of symptoms. If the indication for surgery was established early, long-term survival after pericardiectomy corresponds to that of the general population. Several factors have been found to be independent predictors of overall survival. These include: constriction caused by radiation, age, congestive heart failure, and kidney function.
Complications of pericardiectomy include direct myocardial injury during dissection, which can lead to cardiac failure and bleeding. Failure to achieve complete resection may result in suboptimal hemodynamic changes, but the extent of dissection heavily depends on being able to safely remove the pericardium. A serious condition called low cardiac output syndrome can develop after the dense covering is removed from the surface of the heart. This can cause the heart to overfill and lead to poor blood flow out of the heart and organ dysfunction of the kidneys and liver. This syndrome may be treated with intravenous medications or more significant cardiac support systems.
References
Harken AH, Hall AW, Hammond FL. In: Baue AE, editor. The pericardium in Glenn’s thoracic and cardiovascular surgery. 6th ed. Stamford: Appleton and Lange; 1996. p. 2299–310.
Spodick DH. Macrophysiology, microphysiology, and anatomy of the pericardium: a synopsis. Am Heart J. 1992;124:1046–51.
Stoller JK, Shaw C, Matthay RA. Enlarging atypically located pericardial cyst: recent experience ad literature review. Chest. 1986;89(3):402–6.
Maisch B, Seferovic PM, Ristic AD, et al. Guidelines on the diagnosis and management of pericardial diseases. Executive summary. Eur Heart J. 2004;25:587–610.
Weder W, Klotz HP, Segesser LV, Larguader F. Thoracoscopic resection of a pericardial cyst. J Thorac Cardiovasc Surg. 1994;107:313.
Burke A, Virmani R. Tumors of the heart and great vessels. In: Atlas of tumor pathology, third Series, Fascicle 16. Washington DC, Armed Forces Institute of Pathology, 1995;181–207.
Luk A, Ahn E, Vaideeswar P, Butany III JW. Pericardial tumors. Semin Diagn Pathol. 2008;25:47–53.
Cottrill CM, Tamaren J, Hall B. Sternal defects associated with congenital pericardial and cardiac defects. Cardiol Young. 1998;8(1):100–4.
VanSon JAM, Danelson GK, Callahan JA. Congenital absence of the pericardium: displacement of the heart associated with tricuspid insufficiency. Ann Thorac Surg. 1993;56:1405.
Meunier JP, Lopez S, Teboul J, et al. Total pericardial defect: risk factor for traumatic aortic type A dissection. Ann Thorac Surg. 2002;74(1):266.
Risher WH, Rees AD, Oschner JL, McFadden PM. Thoracoscopic resection of pericardium for symptomatic congenital pericardial defect. Ann Thorac Surg. 1993;56:1390.
Loebe M, Meskhishvili V, Weng Y, et al. Use of polytetrafluoroetylene surgical membrane as a pericardial substitute in the correction of congenital heart defects. Tex Heart Inst J. 1993;20(3):213–7.
Arom KV, Richardson JD, Webb G, Grover FL, Trinkle JK. Subxiphoid pericardial window in patients with suspected traumatic pericardial tamponade. Ann Thorac Surg. 1977;23(6):545–9.
Andrade-Alegre R, Mon L. Subxiphoid pericardial window in the diagnosis of penetrating cardiac trauma. Ann Thorac Surg. 1994;58(4):1139–41.
Brewster SA, Thirlby RC, Syder 3rd WH. Subxiphoid pericardial window and penetrating cardiac trauma. Arch Surg. 1988;123(8):937–41.
Huang YK, Lu MS, Liu KL, Liu EH, Chu JJ Tsai FC, Lin PJ. Traumatic pericardial effusion: impact of diagnostic and surgical approaches. Resuscitation. 2010;81(12):1682–6.
Holmes Jr DR, Nishimura R, Fountain R, Turi ZG. Iatrogenic pericardial effusion and tamponade in the percutaneous intracardiac intervention era. JACC Cardiovasc Interv. 2009;2(8):705–17.
Hsu LF, Jais P, Hocini M, et al. Incidence and prevention of cardiac tamponade complicating ablation for atrial fibrillation. Pacing Clin Electrophysiol. 2005;28 Suppl 1:S106–9.
Mahapatra S, Bybee KA, Bunch TJ, et al. Incidence and predictors of cardiac perforation after permanent pacemaker placement. Heart Rhythm. 2005;2:907–11.
Spodick DH. Acute cardiac tamponade. N Engl J Med. 2003;349:684–90.
Holt JP, Rhode EA, Kines H. Pericardial and ventricular pressure. Circ Res. 1960;8:1171–80.
Ashikhmina EA, Schaff HV, Sinak LJ, Li Z, Dearani JA, Suri RM, Park SJ, Orszulak TA, Sundt RM. Pericardial effusion after cardiac surgery: risk factors, patient profiles, and contemporary management. Ann Thorac Surg. 2010;89:112–8.
Boyd WD, Tyberg JV, Cox JL. A review of the current status of pericardial closure following cardiac surgery. Expert Rev Cardiovasc Ther. 2012;10(9):1109–18.
Hoit BD. Management of effusive and constrictive pericardial heart disease. Circulation. 2002;105:2939–42.
Chen EP, Miller JI. Modern approaches and use of surgical treatment for pericardial disease. Curr Cardiol Rep. 2002;4:41–6.
Cho YH, Schaff HV. Surgery for pericardial disease. Heart Fail Rev. 2013;18:375–87.
Becit N, Unlu Y, Ceviz M, Kocogullari CU, Kocak H, Gurlertop Y. Subxiphoid pericardiostomy in the management of pericardial effusions: case series analysis of 368 patients. Heart. 2005;91:785–90.
Mirhosseini SM, Fakhri M, Mozaffary A, Lotfaliany M, Behzadnia N, Aval ZA, Ghiasi SMS, Boloursaz MR, Masjedi MR. Risk factors affecting the survival rate in patients with symptomatic pericardial effusion undergoing surgical intervention. Intract Cardiovasc Thorac Surg. 2013;16:495–500.
Azam S, Hoit BD. Treatment of pericardial disease. Cardiovasc Ther. 1999;1(1):79–89.
Palacios IF. Pericardial effusion and tamponade. Curr Treat Options Cardiovasc Med. 1999;1(1):79–89.
Rubin RH, Moellering RC. Clinical, microbiological and therapeutic aspects of purulent pericarditis. Am J Med. 1975;59:68.
Mills SA, Julian S, Holliday RH, Vinten-Johansen J, Case LD, Hudspeth AS, Tucker WY, Cordell AR. Subxiphoid pericardial window for pericardial effusive disease. J Cardiovasc Surg (Torino). 1989;30(5):768–73.
O’Connor CJ, Tuman KJ. The intraoperative management of patients with pericardial tamponade. Anesthesiol Clin. 2010;28(1):87–96.
Moores DWO, Allen KB, Faber LP, Dziuban SW, Gillman DJ, Warren WH, Ilves R, Lininger L. Subxiphoid pericardial drainage for pericardial tamponade. J Thorac Cardiovasc Surg. 1995;109:546–52.
Allen KB, Faber LP, Warren WH, Shaar CJ. Pericardial effusion: subxiphoid pericardiostomy versus percutaneous catheter drainage. Ann Thorac Surg. 1999;67:437–40.
Dosios T, Theakos N, Angouras D, Asimacopoulos P. Risk factors affecting the survival of patients with pericardial effusion submitted to subxiphoid pericardiostomy. Chest. 2003;124:242–6.
McDonald JM, Meyer BF, Guthrie TJ, Battafarano RJ, Cooper JD, Patterson GA. Comparison of open subxiphoid pericardial drainage with percutaneous catheter drainage for symptomatic pericardial effusion. Ann Thorac Surg. 2003;76:811–6.
Wang HJ, Hsu KL, Chiang FT, Tseng CD, Tseng YZ, Liau CS. Technical and prognostic outcomes of double-balloon pericardiotomy for large malignancy-related pericardial effusions. Chest. 2002;122:893–9.
Cullinane CA, Paz IB, Smith D, Carter N, Grannis Jr FW. Prognostic factors in the surgical management of pericardial effusion in the patient with concurrent malignancy. Chest. 2004;125:1328–34.
Roberts JR, Kaiser LR. Pericardial procedures. In: Kaiser LR, Kron IR, Spray TL, editors. Mastery of cardiothoracic surgery. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 1998. p. 221–9.
Saltzman AJ, Paz YE, Rene AG, Green P, Hassanin A, Argenziano MG, Rabbani L, Dangas G. Comparision of surgical pericardial drainage with percutaneous catheter drainage for pericardial effusion. J Invasive Cardiol. 2012;24(11):590–3.
Rafique AM, Patel N, Biner S, Eshaghian S, Mendoza F, Cercek B, Siegel RJ. Frequency of recurrence of pericardial tamponade in patients with extended versus nonextended pericardial catheter drainage. Am J Cardiol. 1820–1825;2011:108.
Georghiou GP, Stamler A, Sharoni E, Fichman-Horn S, Berman M, Vidne BA, Saute M. Video-assisted thoracoscopic pericardial window for diagnosis and management of pericardial effusions. Ann Thorac Surg. 2005;80:607–10.
O’Brien PKH, Kucharczuk JC, Marshall B, Friedberg JS, Chen Z, Kaiser LR, Shrager JB. Comparative study of subxiphoid versus video-thoracoscopic pericardial “Window”. Ann Thorac Surg. 2005;80:2013–9.
Neragi-Miandoab S, Linden PA, Ducko CT, Bueno R, Richards WG, Sugarbaker DJ, Jaklitsch MT. VATS pericardiotomy for patients with known malignancy and pericardial effusion: survival and prognosis of positive cytology and metastatic involvement of the pericardium: a case control study. Int J Surg. 2008;6:110–4.
Muhammad MIA. The pericardial window: is a video-assisted thoracoscopy approach better than a surgical approach? Interact Cardiovasc Thorac Surg. 2011;12:174–8.
Harken AH, Hammond GL, Edmunds Jr LH. Pericardial diseases in cardiac surgery in the adult. New York: McGraw Hill; 1997. p. 1303–17.
Talreja DR, Edwards WD, Danielson GK, et al. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation. 2003;108:1852–7.
Karram T, Rinkevitch D, Markiewicz W. Poor outcome in radiation- induced constrictive pericarditis. Int J Radiat Oncol Biol Phys. 1993;25(2):329–31.
Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation. 1999;100(13):1380–6.
Ufuk Y, Kestelli M, Yilik L, et al. Recent surgical experience in chronic constrictive pericarditis. Tex Heart Inst J. 2003;30(1):27–30.
Bertog SC, Thambidorai SK, Parakh K, Schoenhagen P, Ozduran V, Houghtaling PL, et al. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol. 2004;43:1445–52.
Szabó G, Schmack B, Bulut C, Soós P, Weymann A, Stadtfeld S, Karck M. Constrictive pericarditis: risks, aetiologies and outcomes after total pericardiectomy: 24 years of experience. Eur J Cardiothorac Surg. 2013;44(6):1023–8.
George TJ, Arnaoutakis GJ, Beaty CA, Kilic A, Baumgartner WA, Conte JV. Contemporary etiologies, risk factors, and outcomes after pericardiectomy. Ann Thorac Surg. 2012;94(2):445–51.
Gopaldas RR, Dao TK, Caron NR, Markley JG. Predictors of in-hospital complications after pericardiectomy: a nationwide outcomes study. J Thorac Cardiovasc Surg. 2013;145:1227–33.
Tokuda Y, Miyata H, Motomura N, Araki Y, Oshima H, Usui A, Takamoto S, Japan Adult Cardiovascular Database Organization. Outcome of pericardiectomy for constrictive pericarditis in Japan: a nationwide outcome study. Ann Thorac Surg. 2013;96(2):571–6.
Lin Y, Zhou M, Xiao J, Wang B, Wang Z. Treating constrictive pericarditis in a chinese single-center study: a five-year experience. Ann Thorac Surg. 2012;94(4):1235–40.
DeValeria PA, Baumgartner WA, Casale AS, et al. Current indications, risks, and outcome after pericardiectomy. Ann Thorac Surg. 1991;52(2):219–24.
Senni M, Redfield MM, Ling LH, et al. Left ventricular systolic and diastolic function after pericardiectomy in patients with con- strictive pericarditis: doppler echocardiographic findings and correlation with clinical status. J Am Coll Cardiol. 1999;33(5):1182–8.
Seifert FC, Miller DC, Oesterle SN, Oyer PE, Stinson EB, Shumway NE. Surgical treatment of constrictive pericarditis: analysis of outcome and diagnostic error. Circulation. 1985;72:II264–73.
Nataf P, Cacoub P, Dorent R, et al. Results of subtotal pericardiectomy for constrictive pericarditis. Eur J Cardiothorac Surg. 1993;7:252–5; discussion: 255–6.
Aagaard MT, Haraldsted VY. Chronic constrictive pericarditis treated with total pericardiectomy. Thorac Cardiovasc Surg. 1984;32:311–4.
Kang SH, Song JM, Kim M, Choo SJ, Chung CH, Kang DH, Song JK. Prognostic predictors in pericardiectomy for chronic constrictive pericarditis. J Thorac Cardiovasc Surg. 2014;147(2):598–605.
Sunday R, Robinson LA, Bosek V. Low cardiac output complicating pericardiectomy for pericardial tamponade. Ann Thorac Surg. 1999;67:228–31.
Chowdhury UK, Subramaniam GK, Kumar AS, Airan B, Singh R, Talwar S, et al. Pericardiectomy for constrictive pericarditis: a clinical, echocardiographic, and hemodynamic evaluation of two surgical techniques. Ann Thorac Surg. 2006;81:522–9.
Cho YH, Schaff HV, Dearani JA, Daly RC, Park SJ, Li Z, Oh JK. Completion pericardiectomy for recurrent constrictive pericarditis: importance of timing of recurrence on late clinical outcome of operation. Ann Thorac Surg. 2012;93(4):1236–40.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Balaram, S.K., Teng, A., Praeger, J. (2014). Surgical Management of Pericardial Disease. In: Herzog, E. (eds) Management of Pericardial Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-06124-5_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-06124-5_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-06123-8
Online ISBN: 978-3-319-06124-5
eBook Packages: MedicineMedicine (R0)