Abstract
The pericardium is an important structure, and there are many diseases that affect the pericardium and the heart. Often, surgery is required for drainage or removal of the pericardium, but techniques are not standardized, and there is controversy, especially with regard to treatment of constrictive pericarditis. This paper reviews surgical methods for the treatment of inflammatory and constrictive pericarditis and presents early and late outcome of operation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction and surgical anatomy
The pericardium is a passive but important cardiac structure that covers all four cardiac chambers and has openings for incoming and outgoing vessels, the superior and inferior venae cavae, pulmonary arteries, and aorta. The pericardium is composed of two layers: the visceral and parietal pericardium. The visceral pericardium reflects back near the origins of the great vessels, becoming continuous with and forming the inner layer of the parietal pericardium. Normally, the pericardium is a mechanical barrier that isolates the heart from other structures, and there is no communication between heart and adjacent organs aside from major blood vessels. Thus, the heart is completely surrounded by pericardium, and the space between the visceral and parietal layers contains fluid that minimizes friction from the beating heart. The pericardium has only limited elasticity, and most diseases are related to increased pericardial fluid or pericardial constriction.
Pathophysiology of pericardial disease
The hemodynamic influence of pericardial disease is related to the potential limitation of diastolic filling. Cardiac tamponade and constrictive pericarditis have the same pathophysiology, which is reduced or limited stroke volume due to decreased cardiac preload. Inflammation of the pericardium can lead to pain, cough, or hiccups. It may also cause generalized symptoms, such as fever, chills, rash, or weight loss. Theses systemic symptoms may result from inflammatory pericarditis per se, and also from systemic inflammatory disease such as connective tissue disease or malignancy. From the surgical standpoint, pericardial pathology can be classified as non-constrictive or constrictive and tumor.
Non-constrictive pericardial disease
Clinically important signs and symptoms related to excessive pericardial fluid may be caused by bleeding, inflammation, or infection. Traumatic injury (penetrating wounds, catheter-based procedures) and sequelae of cardiac surgery can cause bleeding into the pericardial space and produce cardiac tamponade. Inflammation or infection also can produce pericardial fluid. Excessive pericardial fluid may increase intrapericardial pressure, which, in turn, limits cardiac filling. Thus, intra-atrial pressure is increased, and ventricular preload and cardiac output are reduced. The rate of accumulation of fluid in the pericardium may have an important influence on hemodynamic effects. The pericardium has little capacity to stretch acutely, but a great capacity to stretch over a longer period of time. Thus, the acute accumulation of fluid in the pericardial space may be tolerated poorly. Because a slow rate of fluid accumulation allows time for the pericardium to distend, a greater amount of fluid is required to produce tamponade symptoms in chronic conditions [1, 2].
Constrictive pericarditis
The loss of pericardial elasticity is the primary pathophysiology of constrictive pericarditis. Various processes such as cardiac surgery, inflammation, infection, and mediastinal radiation can produce pericardial constriction. Among patients presenting for surgical treatment, the frequency of the various etiologies depends on locale. For example, in regions of the world where tuberculosis is prevalent, tuberculous pericarditis may be the most common cause of constriction leading to pericardiectomy [3].
In the United States and other Western countries, many patients with constrictive pericarditis have no history of antecedent infection and are presumed to have had subclinical viral infections that result in late constriction; often such cases are termed “idiopathic” when a specific cause is not identifiable. Thus, in many surgical series, idiopathic category constitutes the largest group of patients undergoing pericardiectomy for constrictive pericarditis. However, among surgical patients for whom a specific diagnosis can be made, prior cardiac surgery is now the most common inciting cause of constriction (Fig. 1) [4–6].
Diagnostic and therapeutic overview of relapsing pericarditis. CMR cardiac magnetic resonance imaging, CRP C-reactive protein, CT computed tomography, ESR erythrocyte sedimentation rate, NSAID nonsteroidal anti-inflammatory drug, WBC white blood cell count. *Corticosteroids should not be routinely used initially unless there is a rheumatologic etiology or NSAIDs and colchicine are contraindicated. Adapted from Mayo Clin Proc. 2010;85(6):572–593
Adhesions between the parietal and visceral pericardium are expected following intrapericardial surgery, but subsequent development of pericardial constriction is uncommon, and the mechanism is unknown. It may be related to intraoperative manipulation of the heart, inflammatory reaction from the cardiopulmonary bypass machine, or retained hematoma or fluid. Among patients with constrictive pericarditis following cardiac surgery, the most common previous operation is coronary artery bypass. This may reflect the frequency of this procedure relative to other cardiac operations or there may be an association with the degree of manipulation of the heart during initial operation.
Mediastinal radiation for the treatment of mediastinal malignancy causes inflammation and fibrosis of all mediastinal structures including the pericardium [7]. These associated myocardial, valvular, and coronary problems influence the outcome of pericardiectomy for constrictive pericarditis related to prior radiation [4, 8, 9]. Inflammatory and infectious pericarditis may also evolve into constrictive physiology.
In cardiac tamponade, the filling of the heart is limited at the level of atria by external compression. However, in pericardial constriction, ventricles have limited diastolic function due to the lack of pericardial compliance. In early diastole, the ventricles will be filled with blood and enlarged at some degree, and diastolic filling will be limited by the noncompliant pericardium. Thus, the early diastolic phase is normal, but from the middle of diastole, all four cardiac chambers will have the same pressure. This is so-called square root sign in cardiac catheterization.
The primary pathophysiologic finding in constrictive pericarditis is limited stroke volume and limited cardiac output. However, clinical symptoms are also caused by systemic and pulmonary venous congestion. As is true with other causes of right-heart failure, systemic venous congestion from constrictive pericarditis can lead to ascites, peripheral edema, and hepatic congestion. Pulmonary venous congestion can cause pleural effusion and subsequently pleural thickening and lung entrapment. Chronically elevated diastolic pressure may also lead to atrial fibrillation and tricuspid valve regurgitation.
Pericardial tumor
Although there are several benign tumors and cysts of the pericardium, those are not usually clinically significant. The most common manifestation of pericardial malignancy is malignant pericardial effusion, and this, in turn, is most often caused by metastatic cancer (breast, lung, etc.) [10, 11]. However, primary or metastatic tumors can also present with the findings of constrictive physiology [12, 13]. Primary malignancy from the pericardium is rare, most often due to mesothelioma.
Diagnosis of pericardial disease
Symptoms of pericardial disease are mainly related to decreased cardiac output or inability to increase cardiac output, or systemic venous congestion. These symptoms include dyspnea, peripheral swelling, renal or hepatic dysfunction, etc. There may be high jugular venous pressure or Kussmaul’s sign. The chest X-ray may reveal cardiomegaly when pericardial effusion is present or pericardial calcification in some patients with constrictive pericarditis. Also, computed tomography and MRI can demonstrate pericardial effusion or thicken or calcified pericardium suggesting constrictive pericarditis. However, confirmative studies for pericardial disease are echocardiography and cardiac catheterization.
Traditionally cardiac catheterization has been the gold standard to diagnose pericardial disease, because of characteristic wave forms and equalization of ventricular end-diastolic pressures. Echocardiography is advantageous because it is noninvasive and also because it can demonstrate characteristic Doppler patterns that may be diagnostic for pericardial disease. Thus, in many patients, echocardiography has replaced cardiac catheterization as the confirmatory study for diagnosis of pericardial disease. We reserve cardiac catheterization for situations where echocardiographic data are ambiguous. Details in diagnostic modalities are discussed in another chapter.
Surgical management of pericardial disease
Mediastinal exploration
Any type of catheter-based cardiac procedure can cause cardiac injury by chamber perforation [14]. If the injury is minimal and detected early, accumulated blood can be drained by catheter, and surgical exploration is not needed [15]. However, if there is uncontrollable bleeding, or if there is concomitant cardiac pathology that requires intracardiac repair, immediate surgery for mediastinal exploration is indicated.
Cardiac tamponade after a cardiac surgical procedure can be corrected by either pericardiocentesis or mediastinal re-exploration. In the acute setting after cardiac surgery, mediastinal re-exploration is favored rather than pericardiocentesis because the source of bleeding can be easily identified and repaired. If the patient has severe coagulopathy, the sternotomy can be left open to prevent recurrent tamponade until the coagulopathy is corrected. However, pericardiocentesis is still a viable option in a subacute phase of cardiac surgery [16]. Postcardiotomy pericardial effusions occur in approximately 1.5 % of patients, and pericardiocentesis is necessary in approximately half of these patients.
Pericardial window
When there is an excessive amount of pericardial fluid, pericardiocentesis under echocardiography can be done safely in most cases [15]. Because the success rate of pericardiocentesis seems to be improving, the need for surgical drainage and creation of a pericardial window are decreasing. If the location or character of the effusion is not suitable for aspiration by needle or catheter, then surgical drainage can be performed. With this surgical approach, the pericardial tissue biopsy can be done at the same time. Drainage of the pericardial space is performed through either directly into the subxiphoid area or indirectly into the pleural space or peritoneal cavity after pericardiotomy. Many surgeons prefer the subxiphoid or transpleural approach to the transperitoneal approach.
Piehler et al. [10] found that the chance of reoperation was high in the patients who had a window procedure. Thus, our current practice is pericardiectomy through a small anterolateral thoracotomy rather than window creation if surgical drainage of the pericardial effusion is necessary.
Pericardial window: subxiphoid approach
The subxiphoid approach is used by some surgeons for the treatment of postoperative or infectious pericardial effusion. If the patient had recent median sternotomy, subxiphoid drainage can be done via a small extension of a previous incision or occasionally without an additional incision. If the patient has an infectious pericardial effusion, the subxiphoid approach may decrease the risk of pleural empyema.
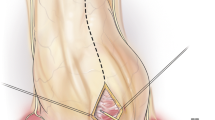
Because single lung ventilation is not necessary, subxiphoid pericardiotomy can be done by general anesthesia, monitored anesthesia care, or even simple local anesthesia [17]. After a lower vertical chest incision, the rectus abdominis muscle is divided through linea Alba. The xiphoid process is exposed and excised or retracted. After incision on the pericardium, the pericardial space is drained, and a single 24–32 French chest tube is placed.
Pericardial window: transpleural approach
The subxiphoid pericardial window is used mainly for temporary relief of pericardial effusion, and a transpleural pericardial window may be a better option when persistent or recurrent pericardial effusion is expected [18]. The transpleural pericardial window, whether performed open through a small incision or with thoracoscopic instruments, allows drainage into the pleural space as well as access for pericardiectomy.
This procedure is performed by either lateral thoracotomy or video-assisted thoracoscopy. The thoracoscopic approach has the advantage of being minimally invasive, but the method requires single lung ventilation. Decision on the right or left side depends on the clinical situation. Generally, a left-side approach is favored because most patients have levocardia, and more pericardium is present in the left side. With either a small thoracotomy or a minimally invasive incision, pericardial drainage includes wide pericardiectomy to prevent re-accumulation of effusion that may occur after creation of a small pericardial window [10]. The procedure is completed by insertion of one or two chest tubes in the pleural space.
Pericardiectomy
Indication of pericardiectomy
Pericardiectomy is usually performed for constrictive pericarditis. However, operation may also be helpful for relapsing inflammatory pericarditis. Most of patients with relapsing pericarditis improve with medical therapy, but some patients continue to have intolerable symptoms or complications of corticosteroid use that impairs quality of life [19]. First-line medical treatment for symptom relief has included NSAIDs and colchicine, and colchicine appears to be effective in preventing recurrences [19, 20]. Corticosteroid use has been controversial and has been recommended only when first-line treatment has failed [21]. Immunosuppressive therapy has generally been used when the underlying cause of pericarditis is autoimmune or rheumatologic [22]. However, pericardiectomy should be considered in patients with severe relapsing pericarditis in whom an adequate drug treatment has failed (Fig. 1) [22].
Classically, constrictive pericarditis has been considered as irreversible. Therefore, once the diagnosis of constrictive pericarditis is made in patients with symptoms of heart failure, pericardiectomy is advised. Even though it may be rare, transient constrictive pericarditis has been reported [23, 24]. Haley et al. [23] reported that 36 patients who were diagnosed as constrictive pericarditis recovered without pericardiectomy. They suggested a trial of anti-inflammatory therapy for acutely developing constrictive pericarditis. If a patient has more chronic (more than 6 months) or worsening symptoms not responding to medical therapy, pericardiectomy should be performed (Fig. 2).
Diagnostic and therapeutic overview of constrictive pericarditis. ACE angiotensin-converting enzyme, ECG electrocardiography, IVC inferior vena cava, JVP jugular venous pressure, LV left ventricle, NSAID nonsteroidal anti-inflammatory drug, RV right ventricle. Adapted from Mayo Clin Proc. 2010;85(6):572–593
Extent of pericardiectomy
Before describing the surgical techniques, we should define and clarify terms used for pericardiectomy. We define complete pericardiectomy as removal of the whole pericardium overlying the heart and great vessels except for the pericardium posterior to the left atrium in the oblique sinus. In practice, a small strip of pericardium remains beneath the phrenic nerves. The term radical pericardiectomy has been used to describe removal of the anterior pericardium (phrenic nerve-to-phrenic nerve), the diaphragmatic pericardium, and pericardium posterior to the left phrenic nerve. Anterior pericardiectomy is defined as removing only the anterior portion of pericardium from the right to left phrenic nerve. If operation is undertaken for the treatment of recurrent inflammatory pericarditis, the goal of the procedure is removal of as much pericardium as possible including that overlying the atria. In patients with constrictive physiology, complete pericardiectomy can sometimes be done, but the primary objective is relief of constriction over the left and right ventricles, and the pericardium overlying atria does not have to be removed.
There is still debate, however, regarding the extent of pericardiectomy for constrictive pericarditis. Although radical pericardiectomy is ideal for constrictive pericarditis, anterior pericardiectomy is relatively easy to perform and is advocated by many surgeons. The rationale for this approach is that anterior pericardiectomy improves constrictive hemodynamics in most patients, and the procedure avoids the technical difficulty of exposing the inferior (diaphragmatic) and left lateral (posterior to the left phrenic nerve) surfaces of the ventricles. The theoretical merit of radical pericardiectomy comes from the anatomy of heart and pericardium. Although the anterior portion is not a small part of the overall pericardium, the diaphragmatic and posterior surfaces of the pericardium still cover quite a large portion of the right and left ventricles (Fig. 2), and anterior pericardiectomy (Fig. 3) does not address these areas (Fig. 4). After anterior pericardiectomy, the base of the heart and the posterolateral aspect of the left ventricle are still encased in constricting layers. Abnormal hemodynamic result after incomplete pericardiectomy was shown by Kloster et al. [25], and we have observed persistent or recurrent symptoms in patients who have had this lesser procedure (Figs. 5, 6).
Nataf et al. [26] reported that anterior pericardiectomy was effective and sufficient to achieve a good clinical result without recurrence, but the follow-up of their patients was relatively limited. In their study, there were seven patients who previously underwent incomplete pericardiectomy, and it is not clear what the indication was for surgery initially and what procedure was carried out for those patients. In most large series of pericardiectomy for constrictive pericarditis, the standard technique was radical pericardiectomy [3, 5, 6, 27].
In contrast, Chowdhury et al. [3] retrospectively compared the surgical results between total (radical) and anterior pericardiectomy and found that surgical outcome of the total pericardiectomy was better than the partial pericardiectomy. Indeed, in this series, risk of death was 4.5 times higher in patients undergoing partial pericardiectomy compared to total pericardiectomy, and at mean follow-up of 18 years, actuarial survival was 84 % following radical pericardiectomy compared to 74 % for patients having anterior pericardiectomy (P = 0.004). They concluded that total (radical) pericardiectomy has lower perioperative and late mortality and confers significant long-term advantage by providing superior hemodynamics; these improved results appear to be independent of the etiology of constrictive pericarditis.
Interestingly, Chowdhury used cardiopulmonary bypass in only 7 (2 %) out of 338 radical pericardiectomy patients, and it is clear that cardiopulmonary bypass is not necessary for all patients undergoing radical pericardiectomy. Although cardiopulmonary bypass and systemic heparinization may increase the risk of bleeding, decompression of the heart with extracorporeal circulation facilitates dissection and allows manipulation of the ventricles. This is especially important in operations performed in patients with patent bypass grafts. Further, with cardiopulmonary bypass, any injury to the myocardium is more easily repaired. Another theoretical advantage is that during cardiopulmonary bypass, the patient’s total blood volume is drained into the reservoir, and after pericardiectomy, intravascular volume is adjusted to maintain adequate cardiac output and perfusion. Often a large volume of blood remains in the cardiotomy reservoir indicating preoperative volume overload. Performing pericardiectomy without extracorporeal circulation in such patients might lead to ventricular distension once the constriction is removed. Therefore, we have been liberal in use of cardiopulmonary bypass when pericardial dissection is difficult.
A final important point in the performance of pericardiectomy is adequate removal of any residual epicardial constriction. Frequently, initial dissection will create a plane that allows relatively easy dissection of considerable thickness of pericardium with minimal bleeding. If, however, this plane is above the epicardial constricting layer, the patient will have persistent constriction and symptoms. Adequate epicardial dissection results in visible expansion of the ventricles after removal of the pericardium. In some cases where epicardial constriction is especially difficult to dissect and where there is risk of injury to epicardial coronary arteries near the base of the heart, scoring the epicardium with the waffle-like pattern may allow expansion of the myocardium with reduced risk of coronary and myocardial injury [28].
Tricuspid valve regurgitation
Tricuspid valve regurgitation may be an important complicating feature of constrictive pericarditis, or it may develop late in patients who have undergone pericardiectomy. Gongora et al. [5] reported that tricuspid valve regurgitation of more than a moderate degree was present in 21 % of patients preoperatively. The presence of significant tricuspid regurgitation is a risk factor for overall mortality and rarely improves with pericardiectomy alone. Because functional tricuspid regurgitation indicates right ventricular dysfunction, ongoing right ventricular failure after pericardiectomy will affect long-term outcome. It seems reasonable, therefore, to perform an adjunctive tricuspid valve repair in patients who have moderate or worse tricuspid valve regurgitation at the time of pericardiectomy. Importantly, intraoperative transesophageal echocardiography should be used to assess the tricuspid valve after pericardiectomy because we have observed worsening tricuspid valve regurgitation with expansion of the right ventricle immediately after operation.
Surgical technique
Anesthetic considerations are the same as other routine cardiac procedures except for the use of short-acting muscle relaxants. It is helpful to have minimal paralysis during dissection near the nerves. Identification of the phrenic nerve, especially the left phrenic nerve, may be difficult due to excessive fat, edema, inflammation, and scarring from previous cardiac surgery. Careful dissection using the electrocautery at a low energy setting or use of a nerve can be helpful to identify the phrenic nerve when this anatomic structure is obscured by scar tissue or fat. Transesophageal echocardiography is used routinely to evaluate change in cardiac size and function and, specifically, to assess the tricuspid valve as discussed above [5, 29, 30].
Pericardiectomy can be performed through a median sternotomy, left anterolateral, or bilateral thoracotomy. Complete pericardiectomy, which only leaves the pericardium posterior to the left atrium in the oblique sinus, may be difficult through left anterolateral thoracotomy. Because bilateral thoracotomy carries more risk of respiratory problems after surgery, it is only useful for redo surgery or when anterolateral thoracotomy is already performed and its exposure is not sufficient. Thus, the most popular approach is median sternotomy, which is familiar to most surgeons and permits central cannulation for cardiopulmonary bypass and excellent exposure for the anterior and right sides of heart.
The potential advantage of the anterolateral thoracotomy is access to the posterior portion of the pericardium, which may be difficult to expose through the sternotomy unless cardiopulmonary bypass is used. If the anterolateral approach is used and cardiopulmonary bypass is necessary, cannulation can be performed through the femoral artery and vein or through the axillary artery. The disadvantage of this approach is inadequate access to the right side of the heart if intracardiac repair is necessary. However, it provides excellent exposure of left side of the heart and minimizes risk of cardiac injury in patients who have had previous sternotomy. For the recurrent inflammatory pericarditis, the whole pericardium, except the part of pericardium posterior to the right atrium, should be removed. Therefore, median sternotomy is favored rather than left anterolateral thoracotomy. For constrictive pericarditis, either median sternotomy or left lateral thoracotomy is good, and the incision can be decided by the surgeon’s preference and possibility of concomitant cardiac surgery. Median sternotomy seems to be more popular than anterolateral thoracotomy. Steps in the operation for pericardiectomy are illustrated in Figs. 2, 3, 4. An illustrative case is presented in Figs. 7, 8, 9.
We have a low threshold for using cardiopulmonary bypass to facilitate dissection in patients undergoing pericardiectomy for constrictive pericarditis. Hemodynamic support with cardiopulmonary bypass allows considerable manipulation of the heart to expose the diaphragmatic surface and the portion of pericardium posterior to the left phrenic nerve, and this is also helpful in dissecting epicardial constriction. Removal of heavily calcified pericardium and areas where pericardium penetrates into the myocardium is greatly facilitated by cardiac decompression during extracorporeal circulation. Our technique is simple using ascending aortic cannulation and two-stage single venous cannulation if there is no intracardiac pathology. The heart is allowed to beat rhythmically, and aortic occlusion is unnecessary.
When hemodynamically important tricuspid regurgitation is present, we use occlusive bicaval cannulation to permit exposure of the tricuspid valve for repair. A tack vent is placed on aspiration in the ascending aorta, and we use a short period of cardioplegic arrest during exploration of the right atrium when any interatrial communications are closed, and the tricuspid valve is assessed. For most patients, a simple de Vega annuloplasty is sufficient to correct tricuspid valve regurgitation. When tricuspid valve replacement is necessary, we excise the anterior leaflet and place our initial sutures in the leaflet and annular tissue near the penetrating bundle of His during the period of aortic occlusion. After these sutures are placed, the aortic cross-clamp is released and the remainder of the procedure is performed with the heart beating.
As mentioned previously, identification of the proper plane of dissection between the constricting pericardium and the epicardium can be challenging, especially when there is heavy calcification that invades the myocardium. Special care should be taken to avoid injury to the epicardial coronary arteries, and when there is particular concern about epicardial injury near the base of the heart, the constricting layers can be incised in a waffle-like pattern. Other adjunctive measures include use of a harmonic scalpel for dissection and use of the ultrasonic aspirating device, which fragments calcium deposits. Both of these tools avoid electrical stimulation of the heart.
Some surgeons believe that the diaphragmatic pericardium should not be removed because doing so might injure the diaphragm and its function. However, the diaphragm is muscular structure, and pericardium is a membranous structure adjacent to the diaphragm. In our experience, those can be separated without difficulty. The central portion of the diaphragm is a fibrous, relatively thin structure, and defects in the fibrous portion are occasionally created during dissection; however, these can be repaired directly or patched with bovine pericardium.
Postoperative management
Generally, the postoperative course of patients who undergo pericardiectomy for effusive or relapsing pericarditis is smooth, and risks of complications are very low. The course of patients who undergo pericardiectomy for chronic constrictive pericarditis is highly variable and depends upon the degree of organ dysfunction and heart failure, present preoperatively. Indeed, some patients have continued venous congestion if there is underlying cardiomyopathy as sometimes occurs in patients with radiation-induced constriction [6, 31]. The other issue is undiagnosed ventricular systolic dysfunction which may become evident after pericardiectomy and may influence recovery [32–34].
Surgical outcome
There have been variable results for pericardiectomy for non-constrictive inflammatory pericarditis [27, 35, 36]. Fowler et al. [35] reported that pericardiectomy was successful in relieving symptoms in only 2 of 9 patients, but they did not describe their surgical technique, and it is possible that residual pericardium was responsible for recurrent symptoms. Better results were described by Hatcher et al. [36] who had only 2 non-responders (and 2 partial responders) among 24 patients who survived surgery. Their surgical technique was complete pericardiectomy. Again, in treating inflammatory pericarditis, even small amounts of residual pericardium may lead to symptoms, and if less aggressive pericardiectomy is performed, there will be a higher chance of recurrence of symptoms. In some other patients, systemic disease and pleuritis may persist even though pericarditis is eliminated.
The results of pericardiectomy for constrictive pericarditis are more consistent in spite of technical variances. Most large studies show a low rate of recurrence of heart failure symptoms and relatively good overall survival. In most series, the favored surgical technique was radical pericardiectomy [3, 5, 6, 27], and there is only one report claiming effectiveness of anterior pericardiectomy [26]. However, at our clinic, we have seen patients who did not respond to anterior pericardiectomy and have performed completion pericardiectomy. In the current era, in-hospital mortality is reported as about 5–10 % [3, 5, 6, 26, 27].
Old age, functional class, etiology of constriction, signs and symptoms of right-heart failure, and various echocardiographic parameters have been suggested as risk factors for survival [4–6]. The strong negative influence of old age and poor preoperative functional class on early and late outcome suggests that early diagnosis and pericardiectomy may increase survival. However, as illustrated in Fig. 10, the most important variable in long-term survival is the etiology of constriction. Patients with constrictive pericarditis due to radiation injury have markedly reduced late survival, and late survival is also reduced in patients who have underlying coronary or valvular heart disease [6]. Most postradiation patients have myocardial fibrosis, restrictive cardiomyopathy, coronary artery disease, and valvular heart disease [7, 37]. The dissection is also quite challenging, and this makes radical pericardiectomy more difficult [38]. Their recovery is also complicated by poor lung function and chest wall fibrosis. Chowdhury et al. [3] showed that less aggressive pericardiectomy is a risk factor of overall survival. Thus, radical pericardiectomy for constrictive pericardiectomy is strongly recommended.
Theoretically, radical pericardiectomy should cure symptoms of constrictive pericarditis, and in earlier reviews from our institution, survival of patients following pericardiectomy for constrictive pericarditis was similar to that of an age- and gender-matched population [39]. In a more recent surgical series, however, we have treated more patients with radiation-induced heart disease and previous cardiac surgery, and survival of this cohort is inferior to the general population [4]. Not only is survival reduced in these high risk patients, but approximately one-third of the patients will experience recurrent class III or IV symptoms. Senni et al. [31] found that echocardiographic parameters of diastolic function were abnormal in 43 % of patients after pericardiectomy for constrictive pericarditis. The mechanism of delayed or incomplete recovery after pericardiectomy is unclear. It was suggested that delayed improvement and persistent symptoms of constriction are most commonly the result of imperfect or incomplete decortication [40, 41]. However, autopsy findings have indicated that myocardial atrophy and fibrosis are present in constrictive pericarditis [42, 43]. Muscle atrophy may be related to prolonged pericardial compression and appears uniformly throughout the myocardium. Different mechanisms could produce myocardial fibrosis, such as direct subepicardial penetration, impairment of coronary blood flow, and concomitant myocardial and pericardial processes (radiation-induced cardiac diseases or autoimmune disease). Therefore, residual impairment of left ventricular systolic or diastolic function may be due to myocardial involvement. Many authors have reported that longer duration and a more severe degree of symptoms were linked with poor surgical outcome [31, 39, 40, 44]. These support the hypothesis that long periods of myocardial compression contribute to a “remodeling” process of the ventricles with greater involvement of myocardium and more severe symptoms. Thus, it is likely that many patients with long-standing constrictive pericarditis and especially patients with radiation-induced constrictive pericarditis may have associated myocardial fibrosis and restrictive cardiomyopathy. Radical pericardiectomy in such patients will eliminate the constrictive component, but patients may have residual impairment due to underlying cardiomyopathy. Finally, there is a rare occurrence of exuberant scar tissue formation after pericardiectomy that can lead to recurrent symptoms [45].
Future directions
Thoracoscopic or robot-assisted partial or anterior pericardiectomy has been reported, but these methods may not be applicable for patients with chronic constrictive pericarditis, especially where there is extensive calcification that often penetrates into the myocardium.
The large number of patients who have undergone cardiac surgery or mediastinal irradiation during the past two decades might be expected to increase the number of patients who have constrictive pericarditis as a late complication, and this appears to be true in our institutional experience (Fig. 11). It is exactly these patients who present the greatest challenges to the clinician in diagnosis and in surgical treatment. Heightened awareness of the possibility of pericardial constriction in patients with right-heart failure who have had previous surgery should lead to early evaluation by echocardiography and/or hemodynamic catheterization and early consideration of surgical exploration.
References
Hancock EW (1979) Cardiac tamponade. Med Clin North Am 63:223–237
Alpert MA, Ravenscraft MD (2003) Pericardial involvement in end-stage renal disease. Am J Med Sci 325:228–236
Chowdhury UK, Subramaniam GK, Kumar AS et al (2006) Pericardiectomy for constrictive pericarditis: a clinical, echocardiographic, and hemodynamic evaluation of two surgical techniques. Ann Thorac Surg 81:522–529
Ling LH, Oh JK, Schaff HV et al (1999) Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation 100:1380–1386
Gongora E, Dearani JA, Orszulak TA, Schaff HV, Li Z, Sundt TM (2008) Tricuspid regurgitation in patients undergoing pericardiectomy for constrictive pericarditis. Ann Thorac Surg 85:163–170 (discussion 70-1)
Bertog SC, Thambidorai SK, Parakh K et al (2004) Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol 43:1445–1452
Veinot JP, Edwards WD (1996) Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol 27:766–773
Handa N, McGregor CGA, Danielson GK et al (2001) Valvular heart operation in patients with previous mediastinal radiation therapy. Ann Thoracic Surg 71:1880–1884
Handa N, McGregor CGA, Danielson GK et al (1999) Coronary artery bypass grafting in patients with previous mediastinal radiation therapy. J Thorac Cardiovasc Surg 117:1136–1143
Piehler JM, Pluth JR, Schaff HV, Danielson GK, Orszulak TA, Puga FJ (1985) Surgical management of effusive pericardial disease. Influence of extent of pericardial resection on clinical course. J Thorac Cardiovasc Surg 90:506–516
Luk A, Ahn E, Vaideeswar P, Butany JW III (2008) Pericardial tumors. Semin Diagn Pathol 25:47–53
Porter D, Jadoon M, McGrogan D, Nzewi O (2011) Occult malignancy presenting as constrictive pericarditis. Interact Cardiovasc Thorac Surg 12:1046–1047
Llewellyn MJ, Atkinson MW, Fabri B (1987) Pericardial constriction caused by primary mesothelioma. Br Heart J 57:54–57
Holmes DR Jr, Nishimura R, Fountain R, Turi ZG (2009) Iatrogenic pericardial effusion and tamponade in the percutaneous intracardiac intervention era. JACC 2:705–717
Tsang TS, Enriquez-Sarano M, Freeman WK et al (2002) Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc 77:429–436
Ashikhmina EA, Schaff HV, Sinak LJ et al (2010) Pericardial effusion after cardiac surgery: risk factors, patient profiles, and contemporary management. Ann Thorac Surg 89:112–118
Becit N, Ünlü Y, Ceviz M, Koçoğullari CU, Koçak H, Gürlertop Y (2005) Subxiphoid pericardiostomy in the management of pericardial effusions: case series analysis of 368 patients. Heart 91:785–790
O’Brien PKH, Kucharczuk JC, Marshall MB et al (2005) Comparative study of subxiphoid versus video-thoracoscopic pericardial “window”. Ann Thorac Surg 80:2013–2019
Soler-Soler J, Sagrista-Sauleda J, Permanyer-Miralda G (2004) Relapsing pericarditis. Heart 90:1364–1368
Imazio M, Bobbio M, Cecchi E et al (2005) Colchicine as first-choice therapy for recurrent pericarditis: results of the CORE (COlchicine for REcurrent pericarditis) trial. Arch Intern Med 165:1987–1991
Imazio M, Brucato A, Adler Y et al (2007) Prognosis of idiopathic recurrent pericarditis as determined from previously published reports. Am J Cardiol 100:1026–1028
Maisch B, Seferovic PM, Ristic AD et al (2004) Guidelines on the diagnosis and management of pericardial diseases executive summary; the task force on the diagnosis and management of pericardial diseases of the european society of cardiology. Eur Heart J 25:587–610
Haley JH, Tajik AJ, Danielson GK, Schaff HV, Mulvagh SL, Oh JK (2004) Transient constrictive pericarditis: causes and natural history. J Am Coll Cardiol 43:271–275
SagristÁ-Sauleda J, Permanyer-Miralda G, Candell-Riera J, Angel J, Soler-Soler J (1987) Transient cardiac constriction: an unrecognized pattern of evolution in effusive acute idiopathic pericarditis. Am J Cardiol 59:961–966
Kloster FE, Crislip RL, Bristow JD, Herr RH, Ritzmann LW, Griswold HE (1965) Hemodynamic studies following pericardiectomy for constrictive pericarditis. Circulation 32:415–424
Nataf P, Cacoub P, Dorent R et al (1993) Results of subtotal pericardiectomy for constrictive pericarditis. Eur J Cardiothorac Surg 7:252–255 (discussion 5–6)
DeValeria PA, Baumgartner WA, Casale AS et al (1991) Current indications, risks, and outcome after pericardiectomy. Ann Thorac Surg 52:219–224
Yamamoto N, Ohara K, Nie M, Torii S, Inoue N, Miyaji K (2011) For what type of constrictive pericarditis is the waffle procedure effective? Asian Cardiovasc Thorac Ann 19:115–118
Johnson TL, Bauman WB, Josephson RA (1993) Worsening tricuspid regurgitation following pericardiectomy for constrictive pericarditis. Chest 104:79–81
Nakamura T, Masai T, Yamauchi T et al (2008) Successful surgical management for severe mitral regurgitation unmasked after pericardiectomy for chronic constrictive pericarditis. Ann Thorac Surg 86:1994–1996
Senni M, Redfield MM, Ling LH, Danielson GK, Tajik AJ, Oh JK (1999) Left ventricular systolic and diastolic function after pericardiectomy in patients with constrictive pericarditis: Doppler echocardiographic findings and correlation with clinical status. J Am Coll Cardiol 33:1182–1188
Homsi M, Mahenthiran J, Vaz D, Sawada SG (2007) Reduced right ventricular systolic function in constrictive pericarditis indicates myocardial involvement and persistent right ventricular dysfunction and symptoms after pericardiectomy. J Am Soc Echocardiogr 20:1417.e1–1417.e7
Schofield RS, Shoemaker SB, Ryerson EG, Cooper GR, Spotnitz WD (2004) Left ventricular dysfunction after pericardiectomy for constrictive pericarditis. Ann Thorac Surg 77:1449–1451
Homsi M, Alsayed L, Aldeiri M, Halal A, Feigenbaum H, Sawada S (2011) Prevalence and prognostic value of right ventricular systolic dysfunction in patients with constrictive pericarditis undergoing pericardiectomy. J Am Coll Cardiol 57:E304
Fowler NO, Harbin AD III (1986) Recurrent acute pericarditis: follow-up study of 31 patients. J Am Coll Cardiol 7:300–305
Hatcher CR Jr, Logue RB, Logan WD Jr, Symbas PN, Mansour KA, Abbott OA (1971) Pericardiectomy for recurrent pericarditis. J Thorac Cardiovasc Surg 62:371–378
Brosius FC III, Waller BF, Roberts WC (1981) Radiation heart disease: analysis of 16 young (aged 15 to 33 years) necropsy patients who received over 3,500 rads to the heart. Am J Med 70:519–530
Ni Y, von Segesser LK, Turina M (1990) Futility of pericardiectomy for postirradiation constrictive pericarditis? Ann Thorac Surg 49:445–448
McCaughan BC, Schaff HV, Piehler JM et al (1985) Early and late results of pericardiectomy for constrictive pericarditis. J Thorac Cardiovasc Surg 89:340–350
Somerville W (1968) Constrictive pericarditis. With special reference to the change in natural history brought about by surgical intervention. Circulation 38:102–110
Culliford AT, Lipton M, Spencer FC (1980) Operation for chronic constrictive pericarditis: do the surgical approach and degree of pericardial resection influence the outcome significantly? Ann Thorac Surg 29:146–152
Dines DE, Edwards JE, Burchell HB (1958) Myocardial atrophy in constrictive pericarditis. Proc Staff Meet Mayo Clin 33:93–99
Levine HD (1973) Myocardial fibrosis in constrictive pericarditis. Electrocardiographic and pathologic observations. Circulation 48:1268–1281
Tirilomis T, Unverdorben S, von der Emde J (1994) Pericardectomy for chronic constrictive pericarditis: risks and outcome. Eur J Cardiothorac Surg 8:487–492
Elmistekawy EM, Veinot JP, Dennie CJ, Rubens FD (2011) Recurrent cardiac constriction after complete pericardiectomy. Heart Lung Circ 20:766–768
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cho, Y.H., Schaff, H.V. Surgery for pericardial disease. Heart Fail Rev 18, 375–387 (2013). https://doi.org/10.1007/s10741-012-9338-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-012-9338-7