Abstract
The hand is complex and used in many functions, including eating and communication. To control the hand accurately the brain needs information about the position, velocities and forces around each joint, which is provided by proprioception. Despite being studied for over a century, there is much to learn about this enigmatic sense. The first part of this chapter summarises the key historical debates and the evidence that shaped the current view of proprioception. The main part then highlights recent evidence that has profoundly changed the understanding of proprioception. One recent development is the discovery that the firing rates of muscles spindles depend upon the contraction history of the muscle and that it is possible for muscle spindles to become insensitive to movement of the joint, remaining quiet during small joint movements. This alters the perceived position of joints. Other experiments show that illusions of joint movement can be induced by stretching the skin, providing evidence that slowly adapting cutaneous receptors contribute to movement sense. However, at least at finger joints, it seems that rapidly adapting cutaneous receptors interfere with the detection of the direction of movement. Recent evidence reveals illusions of altered joint position and movement with voluntary efforts during paralysis and anaesthesia. Thus command signals generated by the brain provide direct information about joint position and movement. Other experiments using anaesthesia have shown key roles of muscle receptors in generating the body maps stored by the brain. Together this recent work shows that the textbook view of proprioception needs revision.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The hand has a remarkable structure with more than 15 joints and more than 20 muscles which can move them. It is positioned with seeming ease to perform a wide range of essential tasks from manipulation and communication to grooming and eating. The sensory and motor mechanisms which permit this have long interested scientists and clinicians. One overarching contribution on the sensory side is proprioception. The present chapter reviews some background to this topic and recent developments. It is not coincidental that many of the mechanisms subserving proprioception have been exposed in psychophysical studies of the human hand.
The brain generates movement by activation of skeletal muscles. Activation of a skeletal muscle causes it to pull on the bones, causing them to move relative to each other. However, the brain can only make skeletal muscles shorten, it cannot lengthen them directly. Thus each muscle can only move a joint in one direction. To move the joint back to where it started a second opposing, or antagonist, muscle is required. Furthermore many joints contain multiple degrees of freedom and therefore require more than two muscles to control them. Some joints are controlled by remote muscles. Muscles located in the forearm move the fingers, and their tendons cross several joints to reach the fingertip. Even simple movements require the control of multiple joints, so clearly controlling movement of the skeleton is a complex task.
To perform movements accurately the brain requires information about the position, velocity and forces around each joint. Perception of this information is known as proprioception. This term is used now to refer to the sensation of any movements and forces that occur within or are imposed on the body. Proprioception is provided via two broad mechanisms. The first is a feedback mechanism and involves information being transduced in the peripheral parts of the body and sent to the brain. This is afferent information and it is generated by sensory receptors located in the muscles and skin as well as the joints themselves. Sensory receptors in the muscles, called muscle spindles, transduce information about the length and rate of change of length of the muscles while Golgi tendon organs, located mostly in the musculo-tendinous junction, signal the forces generated by muscles. Some slowly adapting receptors in the skin signal stretch of the skin around a joint as the joint moves. Similarly, joint receptors signal stretch of the joint capsule. The second mechanism involves the brain using a motor command signal and a stored model to generate information about how the body responds to the efferent command signals sent to the muscles. Where the term afferent refers to information moving towards the brain, the term efferent refers to information associated with generation of motor output, often termed corollary discharge or efference copy (see Sect. 3.4). The term efferent is used here because the proprioceptive information is derived from an efferent signal, although once derived, the information may stay in the brain.
How proprioceptive information is generated and used by the brain has been studied for more than a century and has often been a source of controversy. However, despite the long history, there is still a lot that is unknown, and there have been important recent developments in the understanding of proprioceptive mechanisms. Over the history of research into proprioception many of the key experiments have been performed in the hand [e.g. 1–3] and the hand continues to play an important role in the study of proprioception.
2 History
2.1 Muscle Spindles Versus Joint Receptors
The idea of having a sense that signals the position and movements of the limbs goes back at least as far as Bell in the 1830s [4] and even early on there was controversy about whether this sense was based on afferent signals [e.g. 5–7] or centrally-generated signals [e.g. 8]. Sherrington’s [7] ‘muscle sense’ favoured afferent information, specifically from muscle receptors, such as muscle spindles. Muscle spindles do not detect joint angle as such, but the length and rate of change of length of the skeletal muscles. If the length of all the muscles around a joint is known then the position of the joint and velocity of joint movements can be determined. Sherrington’s view became dominant in the early 1900s [9–11] but muscle receptors subsequently fell out of favour as contributors to sensation. By the 1950s joint receptors were seen as the dominant source of information about joint position and movement. This view was supported by the extensive work of Skoglund [12]. Further support came from the discovery of neurons in the sensory cortex that responded to joint movement and joint probing [13], combined with evidence that muscle receptors did not project into the cortex [14]. The latter result was eventually proved wrong and was due to the class of anaesthetics used in animal experiments at the time [15].
Muscle spindles again became the favoured source for the sense of joint position and movement after experiments by Goodwin, McCloskey and Matthews [16] showed that vibration of the muscle’s tendon induced illusions of altered joint position and joint movement. Muscle spindles are known to be sensitive to low-amplitude vibration (frequency ~80 Hz, amplitude ~1 mm) [e.g. 17, 18]. Furthermore illusions of movement are produced when the muscle is stretched by pulling on an exposed tendon without any movement of the joint [19, 20, cf. 21] or when the muscle afferents are stimulated electrically [22]. This strong evidence of a role for muscle spindles was accompanied by evidence that joint receptors were not ideal as transducers of joint position and movement. It was shown that joint receptors were usually not good at encoding joint angle in cats [23] or in the human hand [24]. Furthermore, they produced ambiguous signals because their output was often similar at both ends of the joint range [23]. In addition, after total hip replacement surgery, which removes the joint capsule and presumably all joint receptors, position sense at the hip is still intact [25, 26]. Similar results have been found for the knee [27] and the shoulder [28]. Currently it is thought that muscle spindles are the dominant afferent source of information about the position and movement of the joints, and that joint receptors are important for signalling the extremes of joint range.
2.2 Cutaneous Receptors
When joint movement occurs, the skin around the joint is stretched and thus cutaneous receptors, usually thought of as being simply used for the sense of touch, are able to provide proprioceptive information. This was first noted in 1929 by Adrian and Umrath [29] but not until 1979 was it shown that cutaneous receptors in the human hand respond to the movement of nearby joints [30]. Hulliger and colleagues found that rapidly adapting type two (RA-II) receptors and slowly adapting type two (SA-II) receptors were the most responsive to joint movement. In addition SA-II afferent nerve fibres encoded the static position of a joint. This fitted with previous evidence that SA-II afferents responded to skin stretch and were directionally sensitive [31]. These studies demonstrated that signals from SA-II receptors were capable of contributing to proprioception because they produced stable firing rates that depended upon the amount of skin stretch around a joint. However a problem with cutaneous afferent signals is that the same afferents also respond to skin stretch that is not related to movement of position of the joint. This means that cutaneous signals must undergo some processing if they are to be used for proprioception.
While it was clear that cutaneous receptors could contribute to proprioception, it was not straightforward to determine if they actually had an important role. One problem was that the experimental techniques that remove cutaneous signals, for example blocking the digital nerves of the finger with local anaesthetic, usually also removed the signals from joint receptors. Some authors assumed that skin receptors did not contribute directly to proprioception when they blocked the digital nerves to study the contribution of joint receptors [1, 32, see also 2]. This separation is less difficult in the knee and thus Clark et al. [33] believed they were able to independently block skin and joint afferents using local anaesthetic. They suggested that skin receptors did not contribute to static position sense, but they noted that that the ability to match limb position degraded with the removal of cutaneous input. They suggested that signals from the skin may be more important for movement sense in the hand than for proximal joints. Further studies suggested that cutaneous receptors contributed to limb position and movement sense [21, 34], but opposition continued [35]. One problem at the time was that it was not clear if cutaneous signals provided a specific signal of limb position and/or movement or whether they provided central facilitation to aid in the decoding of muscle and joint receptor input [2, 36, 37]. This issue has been addressed in recent studies which are discussed in Sect. 3.2.
2.3 Tendon Organs
Proprioception also includes the ability to perceive force and heaviness, the history of which has been less controversial than the senses of limb position and movement [38]. The sense of force refers to the ability to perceive the force that is generated by the muscles and its primary receptor is the Golgi tendon organ. Tendon organs are located mostly near the musculo-tendinous junction [39] and their firing rates are closely related to muscle force [18, 40]. However they are more sensitive to force produced by an actively contracting muscle than passive forces [41]. In addition to tendon organs, information about force can be obtained from cutaneous receptors [42] because when a limb moves and applies force to something the skin is compressed or sheared in the region of contact. A third source of information about force is the centrally generated motor command which is directly related to the signal driving the muscle to contract. This signal may increase when a muscle is effectively weakened [43]. However, this information is not ideal because it is corrupted by factors, such as muscle fatigue, that change the relationship between motor command output and muscle force output. This is where the sense of effort is distinguished from the sense of force. An example of the divergence of the sense of force and effort can be seen when carrying a heavy suitcase some distance. Over time the suitcase feels heavier, even though its weight has not changed. This is because the loaded muscles are fatiguing and therefore have to be driven harder to maintain the force required to lift the suitcase. The increase in motor command required to drive the muscles is perceived as increased effort and interpreted as increased heaviness of the suitcase. Central signals of motor command are thought to be a major source of the sense of effort, however it is possible that muscle spindles provide input as well [44].
It is common to break proprioception into ‘sub-senses’ to simplify investigation. In the history presented above it was broken into senses of limb position, limb movement, force and effort. All proprioceptive signals provide information simultaneously when a movement is made. Some receptors, such as muscle spindles and tendon organs, are specialised to signal one type of information. This does not mean that they only provide that information. As an example, tendon organs signal force, but could also detect lengthening during muscle contraction because if the muscle shortens they will be stretched. Likewise, muscle spindles could also signal muscle force. Because they are ‘co-activated’ by fusimotor drive during voluntary contractions, they also fire more when the muscle contracts (see Sect. 3.1). Although it is possible to focus and perceive the angle, velocity or force at one joint, this is not how we generally perceive our body. For example, we are consciously aware of the position of our hands, but we do not perceive the information from individual joint and muscles, we simply perceive the position of the hand. This emphasises that proprioceptive information is combined into a synthesised representation of the body, and we perceive that representation which is updated continuously by sensory information.
3 Recent Developments
3.1 Muscle Spindles
When skeletal muscle contracts and shortens it might be expected that the muscle spindles would also shorten, and therefore fall slack. However this is overcome by the structure of the muscle spindles which comprise small muscle fibres inside a spindle-shaped capsule. These intrafusal muscle fibres have their own motor supply, the fusimotor system. Fusimotor activation acts to shorten the muscle spindles and thus, ideally to ensure that they are at an appropriate length to signal changes in muscle length. However the presence of the fusimotor system also creates its own complications for the muscle spindle signal. The contractile parts of the intrafusal muscle fibres are predominantly located at both ends of the spindles. This means that when the fusimotor system is activated both ends of the intrafusal muscle fibres shorten and the centre of the fibres, where the afferent nerve terminals are located, is stretched. During an isometric contraction there is little change in total muscle length (i.e. the length of the muscle–tendon unit), although muscle fibres would shorten slightly. However, the fusimotor system is usually activated along with the rest of the muscle [45–49, cf. 50, 51] and this will stretch the muscle spindle endings and increase their firing rate. Hence despite the actual muscle length remaining relatively constant, the spindle signal during an isometric contraction should indicate that the muscle is lengthening, which is consistent with a movement or change in position opposite to the direction of the contraction. In the 1970s, it was suggested that we do not perceive such a change because the part of the muscle spindle signal that is due to the fusimotor activation is subtracted out using a corollary of the motor command [16, 20, 37]. However there is no evidence that this is the case. In fact recent studies have shown that we do perceive a change in joint position during an isometric contraction [52, 53], although the change is in the opposite direction to that expected from the fusimotor activation of muscle spindles. So while fusimotor activation can assist in putting muscle spindles at an appropriate length to indicate muscle length accurately, it also corrupts the signals and makes them potentially ambiguous. In situations in which the muscle spindle signal from the agonist muscles is ambiguous, the signal from muscle spindles in the passive antagonist muscle could be very useful [54].
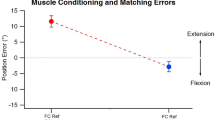
Another problem with the muscle spindle signal is that it depends on the history of contraction of the muscle. Skeletal muscle has thixotropic properties in that after contraction, or even after a period of rest at a set length, the behaviour of the crossbridges means that the muscle fibres have a resistance to length changes [55]. Hence, length changes imposed on passive muscle are met with some stiffness. The result is that a muscle contracted at a long length and then passively shortened may fall slack (Fig. 1) and a muscle contracted at a short length and then passively stretched may initially resist the increase in length. These thixotropic properties extend to the intrafusal muscle fibres of the muscle spindles [56–58] so that the state of the spindle endings and their firing rate in a passive muscle depend on the previous muscle contraction and any subsequent passive movement. The effects of muscle spindle thixotropy on the sense of joint position are large enough to produce illusions of altered joint position [e.g. 57, 59]. While the role of muscle spindles in the sense of joint position and the sense of joint movement is well established, they are not ideal detectors of muscle length or rate of change of muscle length. Activation of the fusimotor system can make the spindle signal ambiguous in active muscle and the thixotropic properties of the intrafusal fibres can make the signal ambiguous in passive muscle. Furthermore the initial response of spindle afferents to stretch is reset a short time after the last change in length [60]. Despite being accepted as the primary detector of joint position and movement, muscle spindles seem poorly suited to the task. However this is typical of proprioceptive signals, none are ideal and all have shortcomings, but they all combine to provide an accurate sense of what the body is doing.
The effect of thixotropy on passive muscle. The dotted arms show the position at which an isometric contraction was made. In (a) the elbow was extended after the contraction while biceps and triceps were relaxed. This action stretched biceps and shortened triceps, but because of the splinting caused by stable crossbridges the triceps falls slack. In this situation any passive lengthening or contraction of triceps will be taken up by the slack. A similar situation is depicted in b, the elbow has been passively flexed after an isometric contraction at a more extended position. This causes the biceps to fall slack. The intrafusal muscle fibres of the muscle spindles are affected by the same thixotropic property and spindles that fall slack will lower their firing rate and may go silent. Muscle spindles that are stretched (biceps in a and triceps in b) will increase their firing rates. The situation depicted in a is known as flexion conditioning and b is known as extension conditioning. The figure is adapted from [83] with permission
Muscle spindles have generally been thought of as receptors for muscle length and rate of change of muscle length. Recent work investigating the firing of muscle afferents in the hand during ‘natural’ grasping movements has shown that muscle spindles may also encode the second derivative of muscle length, that is acceleration [61, 62]. Dimitriou and Edin also suggested that muscle length and velocity information is not simple to extract from muscle spindle signals in active muscle and that information about fusimotor activation and the ongoing load properties may be necessary. These authors have gone on to show that muscle spindles are also capable of acting as forward sensory models and predicting future kinematic states of their parent muscle [63]. Forward models are important in motor control because the ability to predict motor outcomes allows faster adaptation and correction ‘on-the-fly’.
3.2 Cutaneous Receptors
Studies that blocked the nerves containing cutaneous afferents impaired the sense of limb position in the hand. However it was not known if signals from cutaneous receptors signalled position and movement directly or whether they simply provided facilitation to the signal from other receptors. More recently it has been shown that a facilitatory role is not likely because removing the input from the digital nerves of the middle finger did not impair movement detection of the proximal interphalangeal joint of the adjacent index finger, and adding input did not improve it [64]. However this study also showed that an electrical or natural cutaneous stimulus applied to the adjacent finger did impair movement detection, suggesting that there is an interaction between cutaneous input and proprioception. Edin and Johansson [65] suggested that afferents from skin receptors contributed to the perception of movement. They demonstrated that illusions of finger movement could be induced by manually stretching the skin around the proximal interphalangeal joint in a way that mimicked how it would normally be stretched during a movement of that joint. Similar illusions were shown for the metacarpophalangeal joint by Collins and Prochazka [3], who also produced these illusions by electrically stimulating the skin on the back of the hand. More recently, skin stretch was shown to produce illusions of movement of the finger, elbow and knee (Fig. 2) [66], although illusions in the hand occurred in more subjects than illusions at proximal joints. This suggests that perhaps skin receptors are most important for movement detection in the hand. Cutaneous receptors have a high density in the hand [67] which gives them the potential to provide greater, more accurate input. Furthermore the accuracy of muscle spindles is compromised when a muscle spans multiple joints [68], whereas cutaneous receptors in the fingers may provide more localised information about which joint moved.
Illusions of altered joint position induced by tendon vibration and stretch of the skin around joints of the index finger. Vibration results in errors in the perception of joint angle at the metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints of the index finger. Skin stretch can also induce these illusions. The effect of skin stretch is enhanced by simultaneous vibration and is greater at the PIP joint which is distal compared to the MCP joint. * indicates a result that is significantly different from the vibration only result (p < 0.05). This figure is adapted from [66] with permission
Another recent development in proprioception concerns whether some input from the cutaneous receptors can interfere with proprioception. Very low-amplitude (20–50 μm peak-to-peak) high-frequency (300 Hz) vibration favours input from Pacinian corpuscles, while lower frequency (30 Hz) favours input from Meissner corpuscles. Low-amplitude, high-frequency stimulation applied to either the middle finger or the thenar eminence reduces the ability to detect movements of the proximal interphalangeal joint in the index finger [69]. This reduction in the ability to detect movement is not seen with a lower frequency stimulus, which suggests that input from Pacinian corpuscles can interfere with movement detection in the hand. If the digital nerves of the adjacent finger are blocked, removing input from skin and joint receptors, there is no impairment produced by Pacinian corpuscle input (Fig. 3) [70]. Furthermore, removal of the muscle receptor contribution makes no difference to the effect of the high frequency vibration. The evidence from these two studies shows that cutaneous receptors can interfere with proprioception in the hand.
The effect of high-frequency cutaneous vibration (designed to activate Pacinian corpuscles) on movement detection at the proximal interphalangeal joint (PIP) of the index finger (test digit). Movement detection was measured as the number of correct detections of the direction of movement at the PIP joint out of 40 trials, during three conditions: (1) vibration of the index finger, (2) vibration of the middle finger and (3) vibration of the anaesthetised middle finger. The dark lines and symbols show the group mean ± SEM. The grey lines show each of the nine subjects from each condition. There was a significant (asterisk, p < 0.05) decrease in the incidence of correct detection of movement direction during vibration of the index finger and vibration of the adjacent middle finger compared to control trials (C). No change occurred when vibration was applied to an anaesthetised finger. Reproduced from [70] with permission
3.3 Pain
Pain is another factor that has been shown to interfere with proprioception. Studies done on the elbow flexor muscles have shown that muscle pain induced by eccentric muscle damage or injection of hypertonic saline causes subjects to make errors when matching the force produced by the painful muscle with a force generated by the other arm [71–73]. Pain in the skin over the muscle also resulted in subjects making force matching errors [72]. Another study of the hand investigated the effect of muscle and skin pain on detection of movement of the thumb. Experimentally induced pain in the flexor pollicis longus muscle which moves the thumb, or in the skin of the thumb, impaired movement detection at the distal joint of the thumb [74]. However pain in the skin over the flexor pollicis longus muscle, or in the flexor carpi radialis muscle, which does not act on the thumb, did not impair movement detection in the thumb. One concept emerging from the work is that while pain may have some generalised effects on motor and sensory performance, nociceptive inputs may also exert specific effects on aspects of proprioception.
3.4 Motor Commands
If the brain is responsible for causing a movement, it can theoretically monitor the commands that were issued to enact that movement. Using that information and knowledge gained from experience about how the body responds, the brain could determine the outcome of its motor commands. For example, during a voluntary flexion of the index finger, the brain could know that the index finger is flexed because the muscle, skin and joint receptors are signalling finger flexion, or it could know that the finger is flexed because it commanded the finger to flex. The concept that we are consciously aware of the drive to our muscle may be as old as the 1500s [75], but it became more widely known in the late 1800s [8, 10]. In the 1950s it was proposed that corollary discharges [76] or efference copies [77] of the motor command interacted with afferent information to compensate for, or subtract, the corrupting influences of self-generated action on afferent information. From the 1970s until recently it was speculated that this was the only role of central motor commands in the sense of limb position and movement. That is, corollary discharges allowed subtraction of the increased firing of muscle spindles caused by fusimotor activation, but did not provide a direct signal of position or velocity [16, 36–38].
Recently it was shown that when subjects have one set of elbow flexors weakened by fatigue or eccentric muscle damage, they make errors in matching the angle of one arm, placed by the experimenter, with the other arm in the absence of vision [78–80]. It was proposed that subjects used the amount of effort required to hold their arm against gravity as a cue for the angle of their elbow, rather than afferent signals. Further experiments in the hand have shown that during anaesthesia and paralysis induced by ischaemic block, if subjects are asked to make a voluntary effort with their wrist muscles they perceive the hand to become displaced in the same direction as the effort [81]. For example, if a subject makes an effort into flexion, they report their wrist to be more flexed. Furthermore the size of the perceived displacement grades with the level and duration of the voluntary effort [82]. This result is consistent with a motor command signal directly influencing the sense of limb position. Similar, although smaller, illusions of displacement also occur when subjects who have all proprioceptive signals intact perform isometric contractions with their wrist muscles [52, 53].
The work described above is focused on the role of central motor commands in signalling joint position. The sense of joint movement is distinctly different from the sense of joint position. Joint position sense is concerned with signalling the static position of the limbs, whereas movement sense is more concerned with signalling velocity. A recent study, again performed in the hand, has shown that in addition to contributing to limb position sense, central motor command signals also contribute to the sense of limb movement and velocity (Fig. 4) [82].
Movements of a phantom hand during voluntary efforts. In our recent study [96] subjects had their forearm wrist and hand anaesthetised and paralysed by a cuff around the upper arm. When the block was complete subjects were asked to make voluntary efforts with their paralysed and anaesthetised wrist and indicate with a pointer any movements that they perceived. The filled hands (index and middle finger are shown) represent the actual position that the subjects’ hand was in. The blue outlined hands show the mean size of the phantom movements indicated by subjects during a voluntary effort that was 1 s long. The red outlined hand shows the mean size of the phantom movements reported by subjects during a 5-second voluntary effort. The speeds of the movements produced by the four voluntary efforts shown are ranked by the terms slow, medium, fast and fastest and these varied from 11s−1 to 35s−1. Subjects indicated that they perceived movements of their phantom hand which were bigger when they made stronger efforts or longer efforts and faster if they made stronger efforts. Adapted from [96] with permission
In contrast to these studies, it has been shown that when illusions of altered joint position are produced by voluntary efforts about the elbow, they can be removed by manipulating the firing rates of muscle spindles using conditioning contractions of the muscle [83]. This suggests that the effects of voluntary efforts on position sense may be due to changes in the firing rates of muscle spindles that occur during the muscle contractions that result from the efforts. However, at the wrist, an effect of the voluntary efforts is present even when the muscle spindle firing rates are controlled by careful conditioning of the muscles [53]. It is not yet clear why the effect of motor commands on position sense is apparently different at the elbow and the wrist. There may be a difference in position sense between the two joints, but it may also be due to a methodological difference. The elbow experiments involved a bilateral matching task in which one arm was used to indicate the position of the other. However, in the wrist experiments the subject indicated the position of their wrist with a pointer. In the two-arm task there may be an interaction between perception of the two arms that does not exist between one wrist and a pointer. Alternatively the wrist experiments may involve an effect related to the process of matching the position of an object in the visual space with the position of a joint. This conflict of results needs to be resolved in order to fully understand how central motor command signals contribute to the sense of limb position.
3.5 Body Representation
We can focus on the position and velocity of a single joint, but do not generally perceive the position of the body this way. The proprioceptive information about the position, velocity and forces at each joint are combined into a ‘representation’ of the body. For example, if the arm is held out in front of the body, rather than perceiving a shoulder angle, elbow angle, wrist angle and finger angles we tend to perceive the arm, as a whole, in a posture. This view is supported by evidence that subjects are better at judging the orientation of their limbs [84] and limb segments [85] rather than joint angles. Body representations must be updated continuously to remain accurate as the body is moved around. However the body representation does not only store information about movements, forces and joint positions.
To know the position of the fingertips, the brain must know the angles of all the joints in the hand and arm, but must also know the distances between joints. There are no receptors that provide information about the length of the body segments, so presumably this information is provided by vision and experience. Information about body segments can be updated less frequently because while the length of body segments is not constant, it changes very slowly. Even adolescent boys only grow a maximum of 80 to 100 mm per annum [86, 87].
Body representations can also be manipulated. Anaesthesia of a body part blocks its sensory information from reaching the brain, but perception of the body part does not cease. Instead perception of the body part continues as a ‘phantom’ limb. Phantom limbs can occur after amputation [88], but can also be induced experimentally using local anaesthetic, or by inflating a cuff around the limb to above arterial pressure to produce an ischaemic block. When the hand or finger is anaesthetised, subjects report that the anaesthetised body part feels larger [89–91]. This change in perceived size has been suggested to be due to the loss of small-diameter afferent nerve fibres [90], but must also involve large-diameter afferents because changes in perceived size are reported when large fibres are becoming blocked but small fibres are still mostly intact [91]. Changes in perceived position of joints in the hand also occur if the block is applied using a cuff around the upper arm which slowly paralyses and anaesthetises the hand, wrist and forearm. As the large-diameter afferent nerves, which carry proprioceptive and touch information, cease to function, subjects report that the fingers become more flexed or more extended [91]. The direction of these changes depends upon the actual position of the subject’s hand before the block. An initially flexed hand leads to perceived extension and an extended hand to perceived flexion. The final position is not the same, suggesting that there is no ‘default’ position for a phantom and perhaps the body representation. Phantom limbs can also be moved. If subjects are asked to make voluntary efforts with their wrist muscles after they have been paralysed and anaesthetised, subjects report movement (see above) of the phantom in the same direction as the effort [82]. The amplitude and velocity of the movement grades with the level of effort. The amplitude also grades with the duration of the effort.
Another body representation that can be manipulated is the map of which ‘parts’ belong to the body. We know that our body belongs to us without needing to ‘interrogate’ the body part in question. Presumably this sense of body ownership is generated from sensory information, and senses such as touch and proprioception must play an important role because they do not signal remote events but exclusively those events occurring in or on the body. With careful manipulation of sensory information subjects will perceive an artificial rubber hand as belonging to their body [92]. One method of doing this is to cover the subject’s real hand so that they cannot see it and place the rubber hand where they can see it. The subject then watches the rubber hand while an experimenter synchronously strokes both the rubber hand and the subject’s real hand in the same location. Once the illusion of ownership of the rubber hand is induced the subject will report that they feel the stroking on the rubber hand rather than their own hand. They will have physiological responses to threats made against the rubber hand [93] and the temperature of their real hand will drop [94] suggesting that the real hand is being neglected in favour of the adopted rubber hand. The rubber hand illusion does not require vision to be established [95]. It is also possible to establish a similar illusion in the finger with proprioceptive movement signals in the absence of tactile signals [97]. Congruent movements are applied to the proximal interphalangeal joint of the subject’s index finger and a plastic finger. The plastic finger is visible to the subject and is set up to appear visually as though it is part of a false arm that could be the subject’s arm. The subject’s finger is not visible and both its digital nerves have been blocked with local anaesthetic, which excludes skin and joint receptors, but leaves intact the muscle receptors in the forearm muscles that control the finger. When an illusion of ownership over the plastic finger is established, and subjects are asked to judge the position of their finger in space, they report the position as close to the plastic finger, rather than close to their actual finger (see Fig. 5), suggesting that the illusion of ownership has biased their proprioception.
Perceived height of the subject’s index finger following simultaneous passive movement of the subject’s finger and a plastic finger. The diagram shows the perceived elevation of the index finger (median and interquartile range) above the table on which the subject’s hand was resting. Zero represents the level of the table. During the control condition (shown by the whole hand) the passive movement of the subject’s finger and the plastic finger were unrelated to each other (incongruent). For the ‘blocked congruent passive movement’ condition (shown by the disembodied finger) the digital nerves of the subject’s index finger were blocked with local anaesthetic. Also the plastic finger was coupled to the subject’s index finger so that movement of one was reproduced by the other. That is movement was congruent between the subject’s finger and the plastic finger Subjects perceived their finger to be at significantly different elevations in the two conditions (p < 0.005). Adapted from [97] with permission
4 Conclusions
The direction of research into proprioception has changed. In the past, the focus was on determining which afferent signals contribute and the information they provide. Therefore, a lot is known about the peripheral receptors and their individual contributions to proprioception. However little is known about how multiple proprioceptive signals, afferent and efferent, interact with each other and combine to produce a coherent perception of the body and its actions. It is also unknown how stored information is generated and combined with the dynamic signals to produce continuously updated body representations through which we perceive our body. Recent research has begun to focus on these key points and exposing these mechanisms will be important for understanding proprioception. The human hand has been an important body part for studies on proprioception in the past and it continues to be important in current research.
References
K.A. Provins, The effect of peripheral nerve block on the appreciation and execution of finger movements. J. Physiol. 143, 55–67 (1958)
S.C. Gandevia, D.I. McCloskey, Joint sense, muscle sense, and their combination as position sense, measured at the distal interphalangeal joint of the middle finger. J. Physiol. 260, 387–407 (1976)
D.F. Collins, A. Prochazka, Movement illusions evoked by ensemble cutaneous input from the dorsum of the human hand. J. Physiol. 496, 857–871 (1996)
C. Bell, The Hand. Its Mechanism and Vital Endowments as Evincing Design (William Pickering, London, 1833)
D. Ferrier, The Functions of the Brain (Smith Elder & Co., London, 1876)
W. James, The Principles of Psychology, vol. 1 (Dover Publications, Inc., New York, 1890)
C. Sherrington, The muscular sense, in Text Book of Physiology, vol. 2, ed. by E. Schafer (Pentland, Edinburgh, 1900), pp. 1002–1025
H. von Helmholtz, Helmholtz’s Treatise on Physiological Optics, vol. 3 (Optical Society of America, Menasha, 1867)
W.B. Pillsbury, Does the sensation of movement originate in the muscle? Am. J. Psychol. 12, 346–353 (1901)
J.H. Jackson, L. Paton, On some abnormalities of ocular movements. Lancet 173, 900–905 (1909)
J.E. Winter, The sensation of movement. Psychol. Rev. 19, 374–385 (1912)
S. Skoglund, Anatomical and physiological studies of knee joint innervation in the cat. Acta. Physiol. Scand. 36(Supp 124), 1–101 (1956)
V. Mountcastle, T. Powell, Central nervous mechanisms subserving position sense and kinesthesis. B. Johns Hopkins Hosp. 105, 173–200 (1959)
G. Brindley, P. Merton, The absence of position sense in the human eye. J. Physiol. 153, 127–130 (1960)
A.K. McIntyre, U. Proske, J.A. Rawson, Cortical projection of afferent information from tendon organs in the cat. J. Physiol. 354, 395–406 (1984)
G.M. Goodwin, D.I. McCloskey, P.B.C. Matthews, The contribution of muscle afferents to kinæsthesia shown by vibration induced illusion of movement and by the effects of paralysing joint afferents. Brain 95, 705–748 (1972)
M.C. Brown, I. Engberg, P.B.C. Matthews, The relative sensitivity to vibration of muscle receptors of the cat. J. Physiol. 192, 773–800 (1967)
D. Burke, K.E. Hagbarth, L. Lofstedt, B.G. Wallin, The responses of human muscle spindle endings to vibration of non-contracting muscles. J. Physiol. 261, 673–693 (1976)
P. B. C. Matthews, A. Simmonds, Sensations of finger movement elicited by pulling upon flexor tendons in man, in Proceedings of the Physiological Society, London, 1974, pp. 27–28
D.I. McCloskey, S. Gandevia, E.K. Potter, J.G. Colebatch, Muscle sense and effort: motor commands and judgments about muscular contractions. Adv. Neurol. 39, 151–167 (1983)
E. Moberg, The role of cutaneous afferents in position sense, kinaesthesia, and motor function of the hand. Brain 106, 1–19 (1983)
S.C. Gandevia, Illusory movements produced by electrical stimulation of low-threshold muscle afferents from the hand. Brain 108, 965–981 (1985)
P. Burgess, F. Clark, Characteristics of knee joint receptors in the cat. J. Physiol. 203, 317–335 (1969)
D. Burke, S. Gandevia, G. Macefield, Responses to passive movement of receptors in joint, skin and muscle of the human hand. J. Physiol. 402, 347–361 (1988)
P. Grigg, G. Finerman, L. Riley, Joint-position sense after total hip replacement. J. Bone Joint Surg. 55-A, 1016–1025 (1973)
Y. Ishii, T. Tojo, K. Terajima, S. Terashima, J.E. Bechtold, Intracapsular components do not change hip proprioception. J. Bone Joint Surg. 81-B, 345–348 (1999)
Y. Ishii, K. Terajima, S. Terashima, J.E. Bechtold, R.S. Laskin, Comparison of joint position sense after total knee arthroplasty. J. Arthroplasty 12, 541–545 (1997)
F. Cuomo, M.G. Birdzell, J.D. Zuckerman, The effect of degenerative arthritis and prosthetic arthroplasty on shoulder proprioception. J. Shoulder Elb. Surg. 14, 345–348 (2005)
E.D. Adrian, K. Umrath, The impulse discharge from the Pacinian corpuscle. J. Physiol. 68, 139–154 (1929)
M. Hulliger, E. Nordh, A.E. Thelin, A.B. Vallbo, The responses of afferent fibres from the glabrous skin of the hand during voluntary finger movements in man. J. Physiol. 291, 233–249 (1979)
M. Knibestöl, Stimulus-response functions of slowly adapting mechanoreceptors in the human glabrous skin area. J. Physiol. 245, 63–80 (1975)
K. Browne, J. Lee, P.A. Ring, The sensation of passive movement at the metatarso-phalangeal joint of the great toe in man. J. Physiol. 126, 448–458 (1954)
F.J. Clark, K.W. Horch, S.M. Bach, G.F. Larson, Contributions of cutaneous and joint receptors to static knee-position sense in man. J. Neurophysiol. 42, 877–888 (1979)
S.C. Gandevia, L.A. Hall, D.I. McCloskey, E.K. Potter, Proprioceptive sensation at the terminal joint of the middle finger. J. Physiol. 335, 507–517 (1983)
P.R. Burgess, J.Y. Wei, F.J. Clark, J. Simon, Signaling of kinesthetic information by peripheral sensory receptors. Annu. Rev. Neurosci. 5, 171–187 (1982)
D.I. McCloskey, Kinaesthetic sensibility. Physiol. Rev. 58, 763–820 (1978)
P.B.C. Matthews, Where does Sherrington’s “muscular sense” originate? Muscles, joints, corollary discharges? Annu. Rev. Neurosci. 5, 189–218 (1982)
S.C. Gandevia, Kinesthesia: roles for afferent signals and motor command, in Handbook on Integration of Motor, Circulatory, Respiratory and Metabolic Control during Exercise, ed. by L. Rowell, J. Shepherd (American Physiological Society, Bethesda, 1996), pp. 128–172
D. Barker, The innervation of mammalian skeletal muscle, in Myotatic, Kinesthetic and Vestibular Mechanisms, ed. by A.V.S. de Reuck, J. Knight (J. & A. Churchill Ltd., London, 1967)
Å.B. Vallbo, Discharge patterns in human muscle spindles afferents during isometric voluntary conrtactions. Acta. Physiol. Scand. 80, 552–566 (1970)
J. Houk, E. Henneman, Responses of Golgi tendon organs to active contractions of the soleus muscle of the cat. J. Neurophysiol. 30, 466–481 (1967)
D.I. McCloskey, Muscular and cutaneous mechanisms in the estimation of the weights of grasped objects. Neuropsychologia 12, 513–520 (1974)
G. Holmes, The symptoms of acute cerebellar injuries due to gunshot injuries. Brain 40, 461–535 (1917)
B.L. Luu, B.L. Day, J.D. Cole, R.C. Fitzpatrick, The fusimotor and reafferent origin of the sense of force and weight. J. Physiol. 589, 3135–3147 (2011)
R. Granit, The functional role of the muscle spindle’s primary end organs. P. Roy. Soc. Med. 61, 69–78 (1968)
K.-E. Hagbarth, Å.B. Vallbo, Discharge characteristics of human muscle afferents during muscle stretch and contraction. Exp. Neurol. 22, 674–694 (1968)
Å.B. Vallbo, Human muscle spindle discharge during isometric voluntary contractions. Amplitude relations between spindle frequency and torque. Acta. Physiol. Scand. 90, 319–336 (1974)
D. Burke, K.E. Hagbarth, L. Lofstedt, B.G. Wallin, The responses of human muscle spindle endings to vibration during isometric contraction. J. Physiol. 261, 695–711 (1976)
A. Prochazka, M. Gorassini, Ensemble firing of muscle afferents recorded during normal locomotion in cats. J. Physiol. 507, 293–304 (1998)
S.C. Gandevia, L. Wilson, P.J. Cordo, D. Burke, Fusimotor reflexes in relaxed forearm muscles produced by cutaneous afferents from the human hand. J. Physiol. 479, 499–508 (1994)
E. Ribot-Ciscar, V. Hospod, J.-P. Roll, J.-M. Aimonetti, Fusimotor drive may adjust muscle spindle feedback to task requirements in humans. J. Neurophysiol. 101, 633–640 (2009)
J.L. Smith, M. Crawford, U. Proske, J.L. Taylor, S.C. Gandevia, Signals of motor command bias joint position sense in the presence of feedback from proprioceptors. J. Appl. Physiol. 106, 950–958 (2009)
L.D. Walsh, J.L. Smith, S.C. Gandevia, J.L. Taylor, The combined effect of muscle contraction history and motor commands on human position sense. Exp. Brain Res. 195, 603–610 (2009)
I. Di Giulio, C.N. Maganaris, V. Baltzopoulos, I.D. Loram, The proprioceptive and agonist roles of gastrocnemius, soleus and tibialis anterior muscles in maintaining human upright posture. J. Physiol. 587, 2399–2416 (2009)
U. Proske, D.L. Morgan, J.E. Gregory, Thixotropy in skeletal muscle and in muscle spindles: a review. Prog. Neurobiol. 41, 705–721 (1993)
D.L. Morgan, A. Prochazka, U. Proske, The after-effects of stretch and fusimotor stimulation on the responses of primary endings of cat muscle spindles. J. Physiol. 356, 465–477 (1984)
J.E. Gregory, D.L. Morgan, U. Proske, Aftereffects in the responses of cat muscle spindles and errors of limb position sense in man. J. Neurophysiol. 59, 1220–1230 (1988)
L.R. Wilson, S.C. Gandevia, D. Burke, Increased resting discharge of human spindle afferents following voluntary contractions. J. Physiol. 488, 833–840 (1995)
A. Wise, J. Gregory, U. Proske, The effects of muscle conditioning on movement detection thresholds at the human forearm. Brain Res. 735, 125–130 (1996)
P.B.C. Matthews, Mammalian Muscle Receptors and their Central Actions (Edward Arnold Ltd., London, 1972)
M. Dimitriou, B.B. Edin, Discharges in human muscle spindle afferents during a key-pressing task. J. Physiol. 586, 5455–5470 (2008)
M. Dimitriou, B.B. Edin, Discharges in human muscle receptor afferents during block grasping. J. Neurosci. 28, 12632–12642 (2008)
M. Dimitriou, B.B. Edin, Human muscle spindles act as forward sensory models. Curr. Biol. 20, 1763–1767 (2010)
K.M. Refshauge, D.F. Collins, S.C. Gandevia, The detection of human finger movement is not facilitated by input from receptors in adjacent digits. J. Physiol. 551, 371–377 (2003)
B.B. Edin, N. Johansson, Skin strain patterns provide kinesthetic information to the human central nervous system. J. Physiol. 487, 243–251 (1995)
D.F. Collins, K.M. Refshauge, G. Todd, S.C. Gandevia, Cutaneous receptors contribute to kinesthesia at the index finger, elbow, and knee. J. Neurophysiol. 94, 1699–1706 (2005)
R.S. Johansson, A.B. Vallbo, Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J. Physiol. 286, 283–300 (1979)
D.L. Sturnieks, J.R. Wright, R.C. Fitzpatrick, Detection of simultaneous movement at two human arm joints. J. Physiol. 585, 833–842 (2007)
N.S. Weerakkody, D.A. Mahns, J.L. Taylor, S.C. Gandevia, Impairment of human proprioception by high-frequency cutaneous vibration. J. Physiol. 581, 971–980 (2007)
N.S. Weerakkody, J. Taylor, S. Gandevia, The effect of high-frequency cutaneous vibration on different inputs subserving detection of joint movement. Exp. Brain Res. 197, 347–355 (2009)
U. Proske et al., Force-matching errors after eccentric exercise attributed to muscle soreness. Clin. Exp. Pharmacol. P 30, 576–579 (2003)
N. Weerakkody, P. Percival, B. Canny, D. Morgan, U. Proske, Force matching at the elbow joint is disturbed by muscle soreness. Somatosens. Mot. Res. 20, 27–32 (2003)
U. Proske et al., Force matching errors following eccentric exercise. Hum. Movement Sci. 23, 365–378 (2004)
N.S. Weerakkody, J.S. Blouin, J.L. Taylor, S.C. Gandevia, Local subcutaneous and muscle pain impairs detection of passive movements at the human thumb. J. Physiol. 586, 3183–3193 (2008)
H.C. Bastian, The “muscular sense”; its nature and cortical localisation. Brain 10, 5–137 (1887)
R.W. Sperry, Neural basis of the spontaneous optokinetic response produced by visual inversion. J. Comp. Physiol. Psych. 43, 482–489 (1950)
E. von Holst, Relations between the central nervous system and the peripheral organs. B. J. Anim. Behav. 2, 89–94 (1954)
L.D. Walsh, C.W. Hesse, D.L. Morgan, U. Proske, Human forearm position sense after fatigue of elbow flexor muscles. J. Physiol. 558, 705–715 (2004)
T.J. Allen, U. Proske, Effect of muscle fatigue on the sense of limb position and movement. Exp. Brain Res. 170, 30–38 (2006)
L.D. Walsh, T.J. Allen, S.C. Gandevia, U. Proske, The effect of eccentric exercise on position sense at the human forearm in different postures. J. Appl. Physiol. 100, 1109–1116 (2006)
S.C. Gandevia, J.L. Smith, M. Crawford, U. Proske, J.L. Taylor, Motor commands contribute to human position sense. J. Physiol. 571, 703–710 (2006)
L.D. Walsh, S.C. Gandevia, J.L. Taylor, Illusory movements of a phantom hand grade with the duration and magnitude of motor commands. J. Physiol. 588, 1269–1280 (2010)
G.E. Ansems, T.J. Allen, U. Proske, Position sense at the human forearm in the horizontal plane during loading and vibration of elbow muscles. J. Physiol. 576, 445–455 (2006)
J.F. Soechting, Does position sense at the elbow reflect a sense of elbow joint angle or one of limb orientation? Brain Res. 248, 392–395 (1982)
C.J. Worringham, G.E. Stelmach, Z.E. Martin, Limb segment inclination sense in proprioception. Exp. Brain Res. 66, 653–658 (1987)
J.M. Tanner, Growth at Adolescence, 2nd edn. (Blackwell Scientific Publications, Oxford, 1962)
J. Visser, R.H. Geuze, A.F. Kalverboer, The relationship between physical growth, the level of activity and the development of motor skills in adolescence: differences between children with DCD and controls. Hum. Movement Sci. 17, 573–608 (1998)
V.S. Ramachandran, D. Rogers-Ramachandran, Phantom limbs and neural plasticity. Arch. Neurol. 57, 317–320 (2000)
S.C. Gandevia, C.M.L. Phegan, Perceptual distortions of the human body image produced by local anaesthesia, pain and cutaneous stimulation. J. Physiol. 514, 609–616 (1999)
X. Paqueron, M.E. Gentili, J.C. Willer, P. Coriat, B. Riou, Time sequence of sensory changes after upper extremity block: swelling sensation is an early and accurate predictor of success. Anesthesiology 101, 162–168 (2004)
N. Inui, L.D. Walsh, J.L. Taylor, S.C. Gandevia, Dynamic changes in the perceived posture of the hand during ischaemic anaesthesia of the arm. J Physiol 589, 5775–5784 (2011)
M. Botvinick, J. Cohen, Rubber hands ‘feel’ touch that eyes see. Nature 391, 756 (1998)
H.H. Ehrsson, K. Wiech, N. Weiskopf, R.J. Dolan, R.E. Passingham, Threatening a rubber hand that you feel is yours elicits a cortical anxiety response. Proc. Nat. Acad. Sci. USA 104, 9828–9833 (2007)
G.L. Moseley, N. Olaf, A. Venema, S. Don, M. Wijers, A. Gallace, C. Spence, Psychologically induced cooling of a specific body part caused by the illusory ownership of an artificial counterpart. Proc. Nat. Acad. Sci. USA 105, 13169–13173 (2008)
H.H. Ehrsson, N.P. Holmes, R.E. Passingham, Touching a rubber hand: feeling of body ownership is associated with activity in multisensory brain areas. J. Neurosci. 25, 10564–10573 (2005)
L.D. Walsh, S.C. Gandevia, J.T. Taylor, Phantom hands: a window into how we perceive limb position and movement. Physiol. News 81, 34–36 (2010)
L.D. Walsh, G.L. Moseley, J.L. Taylor, S.C. Gandevia, Proprioceptive signals contribute to the sense of body ownership. J. Physiol. 589, 3009–3021 (2011)
Acknowldegments
The authors and their labs are supported by funding from the National Health and Medical Research Council (of Australia).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Walsh, L.D., Taylor, J.L., Gandevia, S.C. (2014). Proprioceptive Mechanisms and the Human Hand. In: Balasubramanian, R., Santos, V. (eds) The Human Hand as an Inspiration for Robot Hand Development. Springer Tracts in Advanced Robotics, vol 95. Springer, Cham. https://doi.org/10.1007/978-3-319-03017-3_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-03017-3_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-03016-6
Online ISBN: 978-3-319-03017-3
eBook Packages: EngineeringEngineering (R0)