Abstract
Terpenes are the largest and most diverse group of naturally occurring compounds found in plants. They can be classified according to the number of isoprene units, the most common being monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), and triterpenes (C30). Besides being the principal constituents of essential oils and playing fundamental roles in plants, many terpenes are extensively used in pharmaceutical and industrial applications ranging from flavours to fragrances and medicines. Several studies have already demonstrated the diversity of terpenes’ biological properties, including cancer chemopreventive effects, antimicrobial, antiviral, analgesic, anti-inflammatory, antifungal, antiparasitic, and other activities. This chapter compiles the various terpenes isolated from plants, their sources, biological activities and beneficial health effects, mechanism of action, extraction and applications, and the future perspective for using the terpenes as lead molecules in several areas of the industry.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
From a structural and chemical perspective, terpenes are the largest and most diverse class of natural compounds, including more than 50,000 known molecules (Cox-Georgian et al. 2019; Pasquini et al. 2021; Torres-Fajardo and Higuera-Piedrahita 2021). They originate from mevalonic acid and are composed almost entirely of carbon, oxygen, and hydrogen. Their presence in different parts of the plant (leaves, flowers, stems, buds, fruits, pods, seeds, roots, and barks) contributes to its aroma, colour, and flavour (Cox-Georgian et al. 2019; Uwineza and Waśkiewicz 2020). Structural diversity in terpenoids from natural products arises from the coupling chemistry employed to link C5 isoprenoid precursors. Moreover, different terpenes or derived compounds have been described to perform distinct functions ranging from primarily structural (such as cholesterol in maintaining the membrane structure) to more functional ones such as carotenoid pigments or gibberellin a plant-derived terpene responsible for the regulation of cell growth and defence (Mostofian et al. 2020; Wang et al. 2019a). In addition, due to their allelopathic effects, terpenes have a natural role in attracting pollinators, healing plants’ injured tissues from herbivore attacks and naturally repelling insects and parasites (War et al. 2012; Maffei 2010; Nejia et al. 2013). Despite terpenes being defined as hydrocarbons having the five-carbon isoprene unit as their building block (Fig. 5.1), many authors use the term ‘terpenes’ more broadly to include also the terpenoids. These terpenes have their carbon skeleton modified by oxidation and rearrangement since methyl groups are generally moved or removed when oxygen atoms are added to the hydrocarbon molecules. Therefore, terpenes are simple hydrocarbons while terpenoids contain oxygen in distinct functional groups, such as alcohols, aldehydes, ketones, acids, esters, and ethers, increasing terpene’s chemical and functional diversity (Pasquini et al. 2021; Mewalal et al. 2017; Mosquera et al. 2021).
Most of the natural terpenes have the general formula (C5H8)n, and their thermal decomposition gives isoprene as one of the products (Fig. 5.1) (Mewalal et al. 2017; Pichersky and Raguso 2018; Caputi and Aprea 2011; Hanuš and Hod 2020). They can be classified based on the value of n (number of isoprene units) or the number of carbon atoms present in the structure. Mono- and sesquiterpenes (C10 and C15, respectively) and their derivatives are highly volatile compounds and frequently the main constituents of essential oils (EOs). At the same time, di- and triterpenes (C20 and C30, respectively) and their derivatives are more complex compounds being less volatile or even assumed as non-volatile. They are found in EOs at low concentrations, mainly from plant gums and resins (Bicas et al. 2009; Haberstroh et al. 2018; Yáñez-Serrano et al. 2018). Terpenes can also be classified as acyclic with an open structure, cyclic with one ring structure, and bi-, tri-, and tetra-cyclic with two, three, and four rings.
Plants synthesise diverse compounds unique to this class of chemicals and contribute to the vast number of existing terpenes and terpenoids. Some plants store or emit these compounds and deposit them in specific organs, such as resin ducts, performing a defensive function or emitting immediately after their biosynthesis. Abiotic and biotic stress factors such as drought, extreme temperature, pollution, or pathogen attack can interfere and rearrange the biosynthesis and emission of terpenes. However, the response may depend on the stressor type and intensity (Pasquini et al. 2021; Haberstroh et al. 2018; Blanch et al. 2007).
Besides their fundamental role in plants, many terpenes are widely used in pharmaceutical and industrial applications ranging from flavours to fragrances (Cox-Georgian et al. 2019). Extensive application of chemical techniques and biological tests has led to the identification, characterisation, and extraction of significant components of broad interest, especially concerning the recovery of terpenes with particular interest for cosmetic and other industries (Salha et al. 2021). Several studies have already demonstrated the diversity of their biological properties, including cancer chemopreventive effects, antimicrobial, antiviral, analgesic, anti-inflammatory, antifungal, antiparasitic, and other activities (Yang et al. 2020). Recently, there has been an increased interest in these compounds obtained from industries' crops since some specific terpenes have been identified as specialties biofuels (Mewalal et al. 2017; Greay and Hammer 2011; Guimarães et al. 2014; Tetali 2019).
This chapter compiles some of the major roles of terpenes and terpenoids isolated from plants. For different compounds, their biological activities and beneficial health effects are emphasised. Moreover, general aspects regarding biosynthesis and extraction are also included.
2 Chemistry and Biosynthesis of Terpenes
2.1 Classification
These compounds are part of the secondary metabolism of plant species and consist of isoprene units: 2-methylbuta-1,3-diene, (C5H8) (Fig. 5.1) linked to each other in numerous ways, most commonly by head to the tail arrangement but other arrays can be found (Bicas et al. 2009; Rubulotta and Quadrelli 2019).
According to the number of isoprene units, terpenes can be classified as hemiterpenes when having a single isoprene unit (C5), monoterpenes (C10) when two isoprene units are linked, sesquiterpenes (C15), diterpenes (C20), sesterterpenes (C25), triterpenes (C30), and tetraterpenes (C40) (Table 5.1 and Fig. 5.1). When more than eight isoprene units are connected, they are named polyterpenes (Torres-Fajardo and Higuera-Piedrahita 2021; Guimarães et al. 2014; Tetali 2019; Rubulotta and Quadrelli 2019; Harman-Ware 2020a). The isopropyl part of 2-methylbutane is defined as the head and the ethyl residue as the tail. In mono-, sesqui-, di-, and sesterterpenes, the isoprene units are linked from head-to-tail; tri- and tetraterpenes contain one tail-to-tail connection the centre (Pichersky and Raguso 2018). Examples of these compounds are shown in Fig. 5.2.
2.2 Biosynthesis
Overall, all terpenes originate, in part, from the C5 substrate dimethylallyl pyrophosphate (DMAPP) and isopentenyl diphosphate (IPP), typically by initially condensing DMAPP with one or more IPP molecules. The type of compound formed is associated with the synthesis pathway (Petrović et al. 2019). Tri- and sesqui-terpenes are most likely synthesised from the precursors produced in the mevalonate pathway (MVA) in the cytoplasm, while hemi-, mono-, di-, and tetra-terpenes are generally synthesised from the precursors produced in the methylerythritol 4-phosphate (MEP) pathway in the chloroplast (Fig. 5.3) (Mewalal et al. 2017; Tetali 2019). Both pathways use IPP and its respective isomer DMAPP to form the isoprene unit (C5). The main difference is that, while in the MVA pathway, the activated building blocks are synthesised from acetyl-CoA, in the MEP pathway, they are obtained from pyruvate with glyceraldehyde 3-phosphate. Molecules of IPP and DMAPP condense to form geranyl pyrophosphate (GPP) with 10 carbons, and this reaction is catalysed by GPP synthase. Then, a molecule of IPP will react with the GPP to form a molecule of farnesyl pyrophosphate (FPP), presenting 15 carbons. The FPP can further react with a molecule of IPP and form a geranylgeranyl pyrophosphate (GGPP) with 20 carbons. Each combination will cause the release of pyrophosphate. Overall, these compounds are considered linear precursors of all terpenes (Mewalal et al. 2017; Rubulotta and Quadrelli 2019; Harman-Ware 2020a; Petrović et al. 2019).
3 Extraction Technologies
In general, extraction methods can be classified as conventional and non-conventional. The first group encompasses maceration, Soxhlet, steam distillation, and hydrodistillation; they require long extraction times, high amounts of solvents, and high energy costs. The second group encompasses supercritical fluid extraction, ultrasound-assisted extraction, and microwave-assisted extraction. Non-conventional methods are considered eco-friendly as they are associated with lower energy costs and the avoidance of toxic solvents and therefore have a lower environmental impact.
3.1 Conventional Extraction Methods for Low Molecular Weight (LMW) Terpenes
LMW terpenes (mono- and sesquiterpenes) are the main constituents of essential oil (Tongnuanchan and Benjakul 2014). The techniques most frequently used for their extraction are steam distillation and hydrodistillation, as both are considered the main methods to obtain essential oil from different anatomical parts of plants (Salha et al. 2021).
3.1.1 Steam Distillation
Steam distillation is one of the ancient and officially approved methods for isolating essential oils from plant materials (Li et al. 2014) being used in commercial-scale production due to the low cost associated with the process (Giacometti et al. 2018). In this conventional type of extraction, the plant material is exposed to a distillation process with boiling water without the water entering in contact with the plant material. Heat plays a fundamental role in this method since the steam passes through the sample, destroying its morphology and releasing the volatile aromatic compounds, which are then carried by vapour that is then condensed, resulting in a separation of phases of distilled water-essential oil (Tongnuanchan and Benjakul 2014; Pateiro et al. 2018). Steam distillation can also be carried out under pressure, depending on the characteristics of the sample (Li et al. 2014). This method is used to create fine perfumes, obtain distillates that refine the flavours and aroma of food and drinks, and produce medicines from plants (Salha et al. 2021). Different studies reveal that steam distillation can extract significant quantities of terpenes, leading to good responses on bioactive assays (Salha et al. 2021; El Kharraf et al. 2021; Ragab et al. 2019).
3.1.2 Hydrodistillation
Hydrodistillation is a variant of steam distillation. Instead of using steam that passes through the sample, it relies on a solid–liquid maceration of plant material in the water that is heated to ebullition. The heat allows the release of molecules of interest/volatile compounds present in the plant cells, which are carried by water vapour in an azeotropic mixture and collected at the end of the process (Tongnuanchan and Benjakul 2014). This process obtains two phases, namely water and an oil-rich phase. Hydrodistillation with Clevenger® apparatus is described in the European Pharmacopoeia as a method adequate for volatile oil extraction from different herbal products such as medicinal plants and dried spices (Li et al. 2014; Giacometti et al. 2018; Tavakolpour et al. 2017). This method is one of the most widely used at laboratory scale, being described in several studies to extract essential oil from different plant matrices (Fagbemi et al. 2021; Jafari-Sales and Pashazadeh 2020; Baker et al. 2021).
3.1.3 Enfleurage method
Enfleurage is the oldest perfumery process commonly employed in the south of France. It is a traditional method of extraction of flowers’ essential oil by their contact with odourless cold fat or vegetable oil. In this way, the essential oil and aromas are absorbed by the fat/oil (Ali et al. 2015), preserving the essential oil from hydrolysis during the extraction (Oktavianawati et al. 2019). Enfleurage extraction is generally a lengthy process since it requires three days to be complete. Moreover, the fat may be liquefied several times to enhance its organoleptic characteristics. The subsequent addition of alcohol to the fat/oil allows the absorbed essential oil to recover (Ali et al. 2015). Enfleurage has a greater yield of extraction when compared to other methods (Paibon et al. 2011). Formerly it was used for extracting essential oil of all flowers, but modern methods have shown that better results can be obtained more economically (Picot-Allain et al. 2021). However, some exceptions still remain such as the cases of jasmine (Jasminum L.) and tuberose (Agave amica L.), which do not tolerate other extraction methods (Barrales-Cureño et al. 2021; Roopashree and Naik 2019; Poucher 1993).
3.2 Conventional Extraction Methods for High Molecular Weight (HMW) Terpenes
Diterpenes, triterpenes, esters, and waxes are examples of high molecular weight molecules present in plant material. Di- and triterpenes are considered semi- or non-volatile terpenes compared with mono- and sesquiterpenes (LMW). Therefore, non-polar organic solvents are commonly used to extract HMW terpenes (Wang et al. 2014). However, for pharmaceutical, cosmetic, and food industries, the utilisation of organic solvents is limited and maximum residues are strictly defined for the allowed solvents (Choi and Verpoorte 2019). The maceration and Soxhlet extraction methods are used traditionally to obtain gums and resins containing terpenes of high molecular weight (Bensebia et al. 2016). However, cold press extraction is also described in the literature as an industrial method for extracting HMW terpenes (Putnik et al. 2017; Chemat et al. 2012).
3.2.1 Cold Press Extraction
Considered an old but evergreen extraction method, the cold press is commonly used in the industrial field to extract oil from different matrices (Chemat et al. 2012). The cold pressing method consists of the physical process of crushing citrus peels, in which the essential oil is present. With the mechanical action of crushing, the oil is released and transferred to a natural sponge, from which it is finally removed. For essential oil extraction, the citrus genus is the most suitable for this type of operation. The oil obtained by this method adds better fruit odour characteristics than other oil extraction methods (Ali et al. 2015).
3.2.2 Maceration, Heat-Assisted Extraction, and Soxhlet Extraction
Maceration is a common solid–liquid extraction used in the nutraceutical field (Chemat et al. 2012). In this extraction method, the sample is soaked and agitated to increase cell permeability, releasing the compounds of interest, such as terpenes (Chia et al. 2020). This process is performed at ambient temperature or in the cold, and compounds are extracted based on diffusion and mass-transfer phenomena. However, to increase the extraction efficiency, the temperature can be applied. In that case, the process is called heat-assisted extraction (HAE) (Rocchetti et al. 2019). The constant agitation in dynamic maceration or heat-assisted extraction increases the solubilisation of the compounds of interest, generating higher extraction yields. Using different solvents or their mixtures can facilitate the mass transfer of the compounds of interest (Garcia-Vaquero et al. 2020). Soxhlet extraction occurs by adding the sample into a cellulose container inserted into the extraction chamber, situated on top of a collecting flask and under a reflux condenser, making this approach a hybrid continuous-discontinuous technique (Fernandez-Pastor et al. 2017; López-Bascón and Luque de Castro 2020).
The use of volatile non-polar solvents and high extraction times are generally perceived as disadvantages of these techniques (de Melo et al. 2020). In addition, these extraction methods have demonstrated poor selectivity. Another aspect being considered is that HAE and Soxhlet frequently use high temperatures, which can degrade thermo-labile bioactive compounds (Palmieri et al. 2020). However, there is the possibility of coupling the Soxhlet apparatus to other equipment to improve the extraction efficiencies such as ultrasound extraction and (Subramanian et al. 2016) microwave-assisted Soxhlet (Prados-Rosales et al. 2002) though the most used is still extraction by convection heat.
Because of the mentioned drawbacks of these methodologies, the search for more environmentally friendly extractions has driven the development of new extraction technologies aiming to reduce the use of toxic solvents and energy costs while improving the extracts' quality and yield.
3.3 Novel Non-Conventional Extraction Technologies
In order to reduce energy costs, waste generation, and the use of environmentally friendly solvents, new extraction technologies are being considered viable alternatives for the future (Fig. 5.4), being less aggressive than conventional technologies and many times more profitable in terms of extraction yield. Green extraction methods such as supercritical fluid extraction, ultrasound-assisted extraction (UAE), and microwave-assisted extraction (MAE) have increased the efficiency of the extraction processes of terpenes in general (Bensebia et al. 2016).
3.3.1 Supercritical Fluid Extraction (SFE)
Supercritical Fluid Extraction (SFE) is a suitable and efficient technology considered an environmentally friendly process. SFE allows the processing of plant material at low temperatures, limiting thermal degradation and avoiding toxic solvents (Tongnuanchan and Benjakul 2014). Supercritical fluids such as CO2, propane, butane, or ethylene are used as solvents for various applications. CO2 is the supercritical solvent of choice in the extraction of flavour and fragrance compounds since it is colourless, odourless, safe, pure, cost-effective, non-flammable, and recyclable at relatively low pressures near room temperature. Higher CO2 density (above 500 kg/m3) favours the yield of extraction of diterpenes, triterpenes, esters, and waxes (Jasna et al. 2009; Reverchon et al. 1995). In short, the solvent is forced to pass through a metallic filter submitted to cooling through heat exchangers. The cooled solvent is then pressurised using a high-pressure pump. In this stage, a co-solvent like ethanol may be mixed to enhance the solubilisation of compounds with greater polarity (Capuzzo et al. 2013). The solvent flows through the feedstock to extract its soluble compounds in supercritical conditions. Subsequently, the extract is precipitated in the separation vessel due to depressurisation (Priyanka 2018).
3.3.2 Microwave-Assisted Extraction (MAE)
Microwave is a non-contact heat source that can achieve more effective and selective heating, resulting in a distillation process that is completed in minutes instead of hours as in conventional extractions (Li et al. 2014). Microwaves are electromagnetic spectrum radiation ranging in frequency from 300 MHz (radio radiation) to 300 GHz. The principal mechanism of microwave equipment is based on two principles. The first is related to the interaction of electric and magnetic fields, resulting in dielectric and magnetic losses leading to heating. The second principle refers to the dipole rotation that occurs when dipole molecules try to align themselves with the alternating electric field in a microwave-produced medium, with heat being produced due to this rotation (Vinatoru et al. 2017).
Microwave extraction is a technique that aims to achieve higher efficiency, which is given by its selective heating. The microwave extraction technique may or may not be accompanied by solvent addition to the extraction. In addition to the frequency used in the extraction and the type of solvent (or the absence of solvent), parameters such as temperature, time, and sample mass are essential in determining the extraction yield. Moreover, besides being used in microwave-assisted hydrodistillation for the extraction of essential oils, it also allows the combination with conventional organic solvents for the extraction of less volatile terpenes (Liu et al. 2018; Sarfarazi et al. 2020). The short extraction time and the possibility of low MAE temperatures make this an excellent technique for extracting terpenes from plants (Isidore et al. 2021).
3.3.3 Ultrasound-Assisted Extraction (UAE)
Ultrasound-assisted extraction is a solid–liquid extraction that uses waves with ultrasonic frequencies between 20 and 100 kHz, which enhances the extraction of bioactive compounds from plants (Wen et al. 2018). Waves at these frequencies generate cavitation and vibration, which are responsible for creating cycles of expansion and compression, causing the formation of bubbles that promote and facilitate cellulose destruction and the release of compounds of interest into the extraction solvent (Isidore et al. 2021; Villalva et al. 2021). In addition to frequency, the propagation of ultrasonic waves is related to power (W). Despite UAE not requiring the use of high temperatures commonly associated with other extraction methodologies, temperature and extraction time are also considered relevant parameters in this method (Garcia-Perez et al. 2021; Turrini et al. 2021; Dzah et al. 2020). To date, UAE has proven to be a valuable extraction technique for obtaining terpenes present in the essential oils of botanical species (Chemat et al. 2012; Santos et al. 2019; Munekata et al. 2020).
3.3.4 Enzymatic-Assisted Extraction
Enzyme-assisted extraction has been intensively studied in the last decade. Since plants have a cell wall composed of resistant polysaccharides that can interfere in extracting bioactive compounds and essential oils (Tirgarian et al. 2019). Enzymes like cellulase, xylanase, and pectinase can degrade cell wall components, enabling more efficient extraction of bioactive compounds and improving the bioactive content of essential oils and extracts. Influential factors that determine the extraction efficiency of enzymatic-assisted extraction include solvent pH, reaction temperature, enzyme concentration, and enzyme type (Miljanović et al. 2020). Some authors have considered that enzymatic treatment also promotes changes in the molecular structure of compounds by converting phytochemicals into final products with higher bioavailability and bioactivities (Kitrytė et al. 2018; Martins et al. 2016a).
3.3.5 Micelle-Mediated Extraction
Also known as cloud-point extraction, it is a surfactant-based extraction technique. The micelle-mediated extraction procedure involves forming hydrophobic species by complexation with an organic ligand in a surfactant medium (Paleologos et al. 2005). The solution becomes cloudy and separates in two phases, i.e. aqueous phase and surfactant rich coacervate phase (Chatterjee et al. 2017). The distribution of solute is more in the surfactant rich phase due to solubilisation in the micelles, thereby leading to its extraction. Therefore, several studies have focused on the choice of the surfactant and chelating agent. Nonionic surfactants are the most common surfactants in cloud-point extraction (Hinze and Pramauro 1993).
3.4 Separation of Terpenes
Essential oils are complex mixtures as they are frequently composed of several terpenes and terpenoids. Because some terpenes are prone to oxidation phenomena, thus contributing to off-flavours and limiting essential oil’s application in food, cosmetic, and pharmaceutical industries, they are frequently submitted to deterpenation (i.e. separation of terpenes from terpenoids) to maintain the quality of the final product. The separation of terpenes significantly improves the oil's stability, solubility, and storage requirements, thus allowing for their industrial applications (Ozturk et al. 2018).
3.5 Identification and Quantification Techniques
Various methods to identify and quantify terpenes have been widely studied. Most often, those require the use of techniques such as gas chromatography (GC), high-performance liquid chromatography (HPLC), capillary electrophoresis, thin-layer chromatography, and SFE chromatography to separate the compounds of interest (Kumar et al. 2016; Xu et al. 2018a). Jiang et al. (2016) proposed different protocols for the extraction/isolation and analysis of terpenoids according to their polarity and size. The analysis was mainly based on chromatographic techniques, such as HPLC and GC. Among those, GC coupled to mass spectrometry detection (GC-MS) is considered the gold-standard technique being the most frequently used to identify volatile terpenes (Louw 2021). On the other hand, for less volatile compounds, such as triterpenes, LC-MS-MS is preferred as an alternative to GC-MS due to its remarkable capacity in the identification of compounds, with the advantages of being also faster and requiring minimum sample preparation (Garg et al. 2020).
4 Pharmacological Activities
The presence of diverse functional groups such as phenols, esters, aldehydes, ketones, and alcohols in secondary metabolites produced plants are related to their variety of biological effects due to different action sites available (Masyita et al. 2022; Boukhatem 2020; Singh and Sharma 2015). Many of these bioactive compounds are volatiles obtained in the form of essential oils. Individually or as a mixture of compounds, they have been used in folk medicine for their disinfectant and preservative effects since ancient times. Nowadays, different terpenes and terpenoids are also used as active ingredients in modern pharmaceuticals (Pasquini et al. 2021; Yang et al. 2020; Petrović et al. 2019; Paduch et al. 2007). Many of these novel drugs are based on the knowledge of folk medicine. Two of the most successful terpenes in the market today are used against malaria (artemisinin and its derivatives) (Krishna et al. 2008) and different cancers (taxol and its derivatives) (Cox-Georgian et al. 2019; Yared and Tkaczuk 2012). Several studies have been demonstrating the bioactivity of different terpenes, including those related to antitumour, anti-inflammatory, antiviral, antimicrobial, antiparasitic, antioxidant, antidepressant, antidiabetic, and against cardiovascular diseases, among other pharmacological activities (Cox-Georgian et al. 2019; Yang et al. 2020; Paduch et al. 2007). Additionally, they can be employed as a natural alternative to ward off insects for their anti-insect properties (Salha et al. 2021; Petrović et al. 2019; Singh and Sharma 2015). It has been estimated that the market of terpene-based pharmaceuticals grows every year, with sales of several million dollars (Wang et al. 2005; Kim et al. 2019). Accordingly, the research on the biological potential of terpene-core compounds is of increasing interest to develop new drugs in contemporary medicine. Several terpenes and/or their derivatives presenting biological activity, namely mono-, sesquiterpenes, and their derivatives, are frequently found as complex mixtures in different essential oils. Therefore, many studies have focused on separating and isolating these compounds towards a deep knowledge of their specific activities. These studies also showed that the combination of specific compounds could lead to improved results concerning biological potential, probably due to a synergic effect (Boukhatem 2020; Singh and Sharma 2015). Moreover, it is essential to highlight that those different enantiomers will have different biological potentials, and the configuration should be considered (Zhu et al. 2017). Some of the most common terpenes and their described biological potential are compiled in Tables 5.1, 5.2, 5.3, and 5.4.
4.1 Monoterpenes with Relevant Pharmacological Activities
Figure 5.5 shows the chemical structures of different monoterpenes that have been associated with relevant biological activities. Several studies so far have evidenced a correlation between the antitumour activity of some essential oils and their monoterpene composition, showing that these compounds can play an important role in cancer prevention and treatment. The antitumoural activity can occur through multiple mechanisms in different steps, such as preventing the interaction of carcinogens in the initiation stage, inhibiting the development and migration of cancer cells, and inducing cancer cell apoptosis and, therefore, tumour regression. According to Paduch et al. (2007), the post-translational isoprenylation of proteins that regulate the growth of cells is the most important mechanism influenced by monoterpenes. Up until now, various works have reported that different monoterpenes exhibit antitumour activity. Limonene and perillyl alcohol, as well as their derivatives and stereoisomers, have been described to have a potent antitumour activity (da Silva et al. 2021) that allows the apoptosis of different tumours, including prostate, pancreas, breast, colon, and liver, both in vitro (Rabi and Bishayee 2009; Shi and Gould 2002; Bardon et al. 2002; Crowell 1999) and in vivo tests, specifically in nude mice (Lu et al. 2004; Gould et al. 1994) and human patients with early and advanced cancer (Boukhatem 2020; Vigushin et al. 1998; Miller et al. 2013; Zielińska-Błajet and Feder-Kubis 2020; Chebet et al. 2021). Also, by inducing apoptosis and cell cycle arrest, thymoquinone, naturally found in Nigella sativa (black cumin), exhibits activity against breast, skin, brain, bile duct, and non-small cell lung cancers both in studies in vitro (cancer cell lines) and in vivo (rats) (Majdalawieh et al. 2017; Khader and Eckl 2014). The main compound from Cannabis sativa essential oil, β-myrcene, presents a significant antiproliferative action against some cancer cell lines, such as breast and human lung carcinoma and leukaemia (Bai and Tang 2020; Tomko et al. 2020; Surendran et al. 2021). In a study with camphene isolated from Piper cernuum (Piperaceae), the growth of a subcutaneous tumour was inhibited in both human tumour cell lines and B16F10-Nex2 murine melanoma, suggesting its promising role in tumour therapy (Girola et al. 2015). Another study demonstrated that carvone could reduce pulmonary adenoma and fore-stomach cancer formation against different cell lines (Patel and Thakkar 2014; Pina et al. 2022). Geraniol has been demonstrated to have both preventive and therapeutic potential against different types of tumours (lung, colon, prostate, pancreatic, and liver) when tested in different cancer cell lines but also in vivo using rats (Cho et al. 2016; Galle et al. 2014; Carnesecchi et al. 2001; Kim et al. 2011; Burke et al. 1997). As demonstrated by different studies, this can be due to the inhibition of the expression of the HMG-CoA (3-hydroxy-3-methylglutaryl coenzyme A) reductase gene in tumour cells. Therefore, geraniol has been suggested as a potential multitarget drug in chemotherapy since it can intervene in different processes such as cell proliferation, apoptosis, autophagy, and metabolism (Yang et al. 2020). By inhibiting the NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) activity, terpineol and its isomers have shown antitumoural activity when tested in cell lines (breast, lung, prostate, ovarian, and leukaemia) (Khaleel et al. 2018; Hassan et al. 2010). Similarly, the isomers thymol and carvacrol have also evidenced interesting antitumour properties. Several studies in vitro and in vivo have shown that thymol and carvacrol induced apoptosis, cytotoxicity, cell cycle arrest, and antimetastatic activity, and also displayed different antiproliferative effects and inhibition of signalling pathways (Sampaio et al. 2021; Islam et al. 2019; Fan et al. 2015). Linalool, a monoterpene found in many aromatic plants, has also demonstrated a high potential as an antitumoural compound since it promotes autophagy and apoptosis of tumour cells (prostate, breast, colorectal, and liver) either in mice or in vitro using different cell lines (Yang et al. 2020; Iwasaki et al. 2016; Pan and Zhang 2019; Chang and Shen 2014; Cerchiara et al. 2015; Xiu-Bin et al. 2015). In addition to its antitumoural effects, linalool and its derivatives also have anti-inflammatory activity since they can relieve the symptoms of inflammation by affecting the nuclear translocation of NF-κB and related pathways (Petrović et al. 2019; Ma et al. 2015). According to Ma et al. (2015), linalool inhibits acute lung inflammation in mice by producing interleukin-6 (IL-6), IL-1β, IL-8, tumour necrosis factor-alpha (TNF-α), and monocyte chemoattractant protein-1 (MCP-1), which are involved in neuroinflammatory processes and several diseases. Other monoterpenes such as 1,8-Cineole, the main compound of Eucalyptus sp., and terpinen-4-ol, the main compound of Melaleuca alternifolia, have also demonstrated anti-inflammatory activity as they can suppress the production of several pro-inflammatory substances such as prostaglandins, tumour necrosis factor-alpha (TNF-α), and interleukin 1 beta (IL-1β) (Salha et al. 2021; Paduch et al. 2007). Paeoniflorin and its derivatives can inhibit the production of inflammatory factor nitric oxide (NO), interleukin 6 (IL-6), and TNF-α induced by lipopolysaccharides (Yang et al. 2020; Singh and Sharma 2015). The reduction of pro-inflammatory interleukins production is also the mechanism of action of α-pinene, explaining the anti-inflammatory activity of plants whose essential oil is rich in this compound, such as pine trees (Kim et al. 2015a). Besides the mentioned monoterpenes, borneol, β-myrcene, citronellol are also known for their anti-inflammatory activities (Salha et al. 2021; Paduch et al. 2007).
Terpenes have shown several other interesting biological properties, such as neuroprotection, and some are being studied to treat depression. According to Cox-Georgian et al. (2019), antidepressant properties are evidenced for linalool and pinene (active principles of Litsea glaucescens, Tagetes lucida and flowers of lavender) by interacting with the serotonin-1A receptors (5HT1A) of the serotonergic pathway (Bonilla-Jaime et al. 2015). When inhaled, the monoterpenes car-3-ene, borneol, verbenol, and pinocarveol have also evidenced antidepressant properties (Pasquini et al. 2021). Some studies showed that myrcene, linalool, citronellol, 1,8-cineole, α- and γ-terpinene have neuroprotective functions owing to their antioxidant effect (Salha et al. 2021; Petrović et al. 2019; Singh and Sharma 2015). Furthermore, borneol can act as an essential neuroprotective agent against Alzheimer’s disease due to its free radical scavenging activity (Yang et al. 2020). Several studies indicate that β-myrcene, menthol, camphor, citronellol, terpineol and its isomers, linalool and linalyl acetate, have analgesic or sedative properties. Therefore, menthol is frequently used topically as an analgesic agent, with the advantage of presenting antipruritic, counterirritant, and antiseptic activity. Similarly, camphor is traditionally used in different preparations, including liniments and cream formulations (Cox-Georgian et al. 2019; Salha et al. 2021; Singh and Sharma 2015; Paduch et al. 2007). Several monoterpenes, including terpineol and its isomers, 1,8-cineole, d-limonene, pinene, nerol, and geranial, as well as the essential oils containing them, have shown antimicrobial activity, which is most probably related to the lipophilic structure of these compounds (Pasquini et al. 2021). These compounds can easily pass through lipid bilayers, causing morphological changes in the cell membrane and cytoplasm, resulting in the leakage of intracellular material (Carson and Riley 1995). Especially terpinen-4-ol, 1,8-cineole and linalool possess antibacterial activity against bacteria isolated from the oral cavity, skin, and respiratory tract (Pasquini et al. 2021; Zengin and Baysal 2014; Wang et al. 2019b). The antimicrobial potential of menthol, particularly against Escherichia coli and Candida albicans has also been demonstrated (Gupta et al. 2021). Carvone and perillaldehyde were also able to inhibit the growth of C. albicans, with the former being effective against Listeria monocytogenes, Enterococcus faecium, and Escherichia coli (Yang et al. 2020; Singh and Sharma 2015).
Antifungal activity was also evidenced by α-terpinene, which exhibited activity similar to commercial drugs, and by some terpenoids such as citronellal, citronellol, nerol, geraniol, borneol, and geranial (Salha et al. 2021; Paduch et al. 2007; Abbaszadeh et al. 2014). Moreover, monoterpene mixtures, such as the one including terpinen-4-ol, α-pinene, β-pinene, 1,8-cineole linalool, and α-terpineol, have also the capacity of limiting fungi development (Marei et al. 2012; Wang et al. 2019c).
In addition, paeoniflorin, a monoterpene glycoside isolated from the root bark of Paeonia suffruticosa, showed in vitro antifungal activity against Candida albicans and was effective against carbapenem-resistant Klebsiella pneumoniae, which are emerging and highly resistant pathogens that can have a significant impact in clinical practice but also in food production (Qian et al. 2020). Besides their interest as antibacterial and antifungal compounds, some volatile terpenes are known for their antiviral potential. Among them are 1,8 cineole, thymol, α-terpineol, α-and γ-terpinene, carvone, carveol, α- and β-pinene, camphor, β-ocimene, p-cymene, citral, and isoborneol. This last compound has great antiviral activity against Herpes simplex Virus-1 (HSV-1) by inhibiting the virus replication and the glycosylation of viral proteins (Cox-Georgian et al. 2019; Pasquini et al. 2021). Another relevant activity associated with monoterpenes is their antiparasitic activity. For example, d-limonene, α-pinene, and β-pinene have antiplasmodial properties, especially against Plasmodium falciparum (Petrović et al. 2019; Boukhatem 2020), while thymol and menthol have anti-leishmanial and trypanocidal activity (Luna et al. 2019). The antiparasitic functions of terpenes are related to their interaction with Fe (II) groups of the infected erythrocytes, releasing free radicals that can kill parasites (Rodrigues Goulart et al. 2004). Monoterpenes are also considered very interesting as natural anti-insect compounds. Besides its anti-inflammatory, antioxidant, and analgesic activities, citronellol, neral, and geranial, as well as thymol and menthol and their derivatives, have anti-insect properties and thus can be potential alternatives to synthetic insecticides (Reis et al. 2016; Al Dawsari and Alam 2022).
4.2 Sesquiterpenes with Relevant Pharmacological Activities
The chemical structures of some relevant sesquiterpenes are shown in Fig. 5.6. Several sesquiterpenes, such as caryophyllene, caryophyllene oxide, germacrene, and patchoulene, have been attracting attention due to their interesting biological activity, including anti-inflammatory, analgesic, antiparasitic, and antitumour properties. A pharmacological study revealed that β-caryophyllene isolated from the leaves of M. koenigii exhibited promising antimalarial activity against the chloroquine-sensitive strain of Plasmodium sp. (Kamaraj et al. 2017). Additionally, several studies highlighted its anti-inflammatory and tissue-protective properties by modulating numerous signalling pathways and inhibiting inflammatory mediators, including cytokines (Jha et al. 2021). Its isomer humulene, or α-caryophyllene, given orally or by aerosol, exhibited anti-inflammatory properties in a murine model by reducing the inflammatory mediators (Rogerio et al. 2009). Caryophyllene oxide is also known for its similar properties since it evidenced anti-inflammatory activity in vivo and central and peripheral analgesia (Chavan et al. 2010). β-patchoulene also showed relevant anti-inflammatory activity in mice models with acute inflammation (Zhang et al. 2016a) being able to mediate a gastroprotective effect against the oxidative stress associated with a gastric ulcer, both in vitro and in vivo (Cox-Georgian et al. 2019). Other terpenoids with anti-inflammatory therapeutic potential are parthenolides (from Tanacetum parthenium L.), which suppress inflammation by inhibiting the activity of NF-κB (Mathema et al. 2012).
Besides having anti-inflammatory activity, β-caryophyllene can reduce alcohol-induced liver injury and has demonstrated neuroprotective properties against Parkinson's disease by acting on specific G-protein-coupled receptors, namely the cannabinoid CB2R, which is a receptor that reduces neuropathic pain and glial upregulation (Viveros-Paredes et al. 2017; Sharma et al. 2015; Navarro et al. 2016). Farnesene has two significant isomers, α and β, the former having four stereoisomers and the last two (Tang et al. 2021). (E)-β-farnesene is a typical volatile component of many higher plants, being also an alarm pheromone in several insect species with the capacity of inducing a flee response in aphids (Bhatia et al. 2015; Zhang et al. 2017). Besides that, neuroprotective effects against H2O2-induced cell death in primary cultured cortical neurons have been demonstrated in previous studies (Turkez et al. 2014). Farnesol, an intermediate in the biosynthesis of farnesene, has been shown to inhibit tumorigenesis in animal models suggesting that it functions as a chemopreventative and antitumour agent in vivo (Joo and Jetten 2010). Similarly, β-eudesmol, a hydroxylated sesquiterpene, presented antitumour activity using in vitro and in vivo experimental models (Ma et al. 2008; Srijiwangsa et al. 2018). Moreover, this compound can inhibit angiogenesis, as demonstrated in mice implanted with subcutaneous Matrigel plugs and in mice adjuvant-induced granuloma (Small 1992). Other sesquiterpenes such as helenalin (Arnica sp.), eupalinin (Eupatorium chinense L.), inuviscolide (Inula viscosa L.), angeloylenolin (Centipeda minima L.) were also reported as antitumour agents by promoting autophagy in cancer cells, inhibiting the proliferation by cell cycle arrest and apoptosis (Petrović et al. 2019; Paduch et al. 2007). A similar mechanism of action mediated via G2/M cell cycle arrest and promotion of apoptosis was reported for germacrone, which showed an antitumour effect on a human hepatoma cell line (He et al. 2019). Bisabolol, a compound widely used in pharmaceutical and cosmetic industries, and its isomers α-bisabolol and epi-α-bisabolol have evidenced antitumour effects against pancreatic cancer by inducing apoptosis and suppressing Akt (Protein kinase B) activation (Seki et al. 2011; Maurya et al. 2014). Moreover, Raut, Karuppayil (Raut and Karuppayil 2014) suggested that α-(-)-bisabolol may be a useful therapeutic candidate for treating skin inflammation. Another study suggests the use of α-bisabolol and nerolidol as potential antifungal agents against Trichophyton spp. (de Medeiros et al. 2021). This compound also shows potential to be incorporated in oral healthcare products since it evidenced antimicrobial activity against Solobacterium moorei, a Gram-positive bacteria associated with halitosis (Forrer et al. 2013).
Besides its antitumoural activity, germacrone also possesses antiviral activity as it was shown to inhibit influenza virus, porcine parvovirus, feline calicivirus, and porcine reproductive and respiratory syndrome virus replication. Germacrone has inhibitory effects on influenza A virus subtype H1N1 (H1N1), Influenza A virus subtype H3N2 (H3N2), and influenza B viruses in the early stages of the viral cycle and can protect mice from a fatal infection (He et al. 2019).
The artemisinins are a class of sesquiterpene lactones extracted from the sweet wormwood plant (Artemisia annua), being a successful example of drugs available in the market (Eckstein-Ludwig et al. 2003). They are potent antimalarial drugs since they can kill all stages of the malaria parasite (Plasmodium falciparum) and are thus widely used worldwide. The action mechanism of the artemisinin is thought to occur through haem-dependent activation of an endoperoxide bridge that occurs within the parasite’s food vacuole (Ismail et al. 2016). Recently, artemisinin and its derivatives have attracted attention. Several in vivo studies confirmed that they have significant therapeutic effects on metabolic diseases, especially diabetes, by promoting insulin secretion and protecting pancreatic ß cells (Jiang et al. 2020).
4.3 Diterpenes with Relevant Pharmacological Activities
The chemical structures of some relevant dizer-enes are shown in Fig. 5.7. This class of compounds shows significant biological activities, including anti-inflammatory, anticancer, analgesic, antimicrobial, and antifungal activities. Some diterpenes also have cardiovascular activity, such as forskolin (Salehi et al. 2019), marrubenol (El Bardai et al. 2004), and kaurene (Ambrosio et al. 2006). They cause relaxation of smooth muscles in the walls of blood vessels by inhibiting Ca2+ channels (Salehi et al. 2019). Likewise, tanshinone IIA, an active ingredient isolated from the rhizome of Salvia miltiorrhiza Bunge, can act on Ca2+ influx and increase endothelial nitric oxide synthase protein expression and phosphorylation, leading to vasodilatation and blood pressure reduction (Shang et al. 2012). Recently, it has also been suggested that tanshinone IIA can prevent the formation of atherosclerosis and the damage and hypertrophy of the heart (Wang et al. 2017; Chen and Xu 2014). Due to the low intestinal absorption of this lipophilic compound, sodium tanshinone IIA sulfonate has been developed, aiming to increase its bioavailability (Shang et al. 2012).
Kaurenoic and pimaradienoic acids, isolated from the roots of Viguiera sp., have been evaluated on vascular smooth muscle contractility of rat aorta and showed pronounced antispasmodic and relaxant activity (Tirapelli et al. 2005). Resiniferatoxin, a vanilloid isolated from the Euphorbia resinifera latex, is used in clinical trials for bladder hyperreflexia and diabetic neuropathy (Appendino and Szallasi 1997).
Andrographolide, a diterpene-lactone isolated from herbaceous plant Andrographis paniculata, has promissory effects for treating diabetes (Nugroho et al. 2013). It also has potent antibacterial activity against most Gram-positive bacteria, showing interesting inhibitory properties on P. aeruginosa and S. aureus biofilms (Zhang et al. 2020).
The diterpenoid stevioside is an active ingredient isolated from Stevia rebaudiana (Bertoni) Hemsl. It possesses insulinotropic, glucagonostatic, and antihyperglycaemic effects, therefore acting as an antihyperglycaemic agent and inducing blood pressure reduction (Gregersen et al. 2004; Jeppesen et al. 2000). It has been shown that stevioside and the aglucon steviol potentiate insulin secretion in a glucose-dependent way in vitro (Gu et al. 2019). A clinical trial using stevia supplementation on glycaemic control improved cardiometabolic risk in diabetic patients (Rashad et al. 2019).
Paclitaxel is a tetracyclic diterpenoid isolated from Taxus sp, a known anticancer drug currently used in medicine to treat several kinds of tumours. Paclitaxel and its analogue docetaxel act as an antimitotic agent by binding to the microtubules (Yared and Tkaczuk 2012; Galletti et al. 2007).
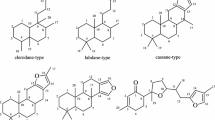
The abietane-type diterpenoid ferruginol also displays antitumour properties in human ovarian cancer and malignant melanoma cell models by inhibiting cell migration and inducing apoptosis. Nevertheless, up until now, there is only one commercial drug composed of abietane-derived diterpenoids, ecabet sodium, which is used to treat reflux oesophagitis and peptic ulcer disease (González-Cardenete et al. 2021). In addition, abietane analogues such as ferruginol and dehydroabietinol exhibit inhibitory activity in vitro towards some Mycobacterium species and have been shown to have antiparasitic activity in the antipromastigote assay (González-Cardenete et al. 2021). Triptolidenol, triptolide, tripterine, and triptonide, which are isolated from Tripterygium wilfordii, are effective in inflammation and auto-immune disorders. Its primary mechanism consists in inhibiting the production of inflammatory cytokines (Jin et al. 2021).
The diterpene pepluanone, isolated from Euphorbia peplus, reduced inflammatory processes by inhibiting cell signalling (Barile et al. 2007), while putranjivain A, isolated from Euphorbia jolkini, demonstrated an antiviral effect against Herpes simplex virus-2 (HSV-2) in Vero cells by inhibiting cell penetration (Cheng et al. 2004). Andrographolide also showed promising antiviral activity due to inhibiting Chikungunya virus infection and reduced virus production (Wintachai et al. 2015). Other diterpenes with very interesting biological activity are the ginkgolides which show antagonistic activity towards platelet-activating factors, resulting in antithrombotic effects (Cui et al. 2019). A clinical study showed that ginkgolide effectively improved neurological deficit after recombinant tissue plasminogen activator therapy in acute ischaemic stroke patients (Zhang et al. 2021).
4.4 Triterpenes with Relevant Pharmacological Activities
The chemical structures of some relevant triterpenes are shown in Fig. 5.8. The majority of triterpenes found in nature are cyclic triterpenes that can be further converted to various metabolites, including saponins. Saponins are not as abundant as most low molecular weight terpenes. However, this compound class presents a large diversity since they present different active sites allowing for the glycosylation of saponin aglycones (Dinda et al. 2010). Among saponins for which broad-spectrum pharmacological bioactivities have been reported, cucurbitacins are the most widely known (Alghasham 2013). Different studies have shown that these compounds, present in plants from the Cucurbitaceae family and used in traditional Chinese medicine, have different biological properties with particular relevance for their anticancer, anti-inflammatory, and hepatoprotective activity (Jing et al. 2020; Liu et al. 2021). Cucurbitacins exhibit anticancer activity through a variety of mechanisms, including the promotion of apoptosis through the Janus kinase/signal transducer and activator of transcription (JAK/STAT3) and Mitogen-Activated Protein Kinase (MAPK) pathways, the promotion of cell cycle arrest by inhibiting cyclins and by inhibiting the invasion and migration of cancer cells (Jing et al. 2020). These highly oxidised tetracyclic triterpenes have shown synergistic effects when combined with some drugs and also have been proposed as potential chemotherapy before the onset of lung cancer (Jing et al. 2020; Liu et al. 2021).
Lupeol, a compound found in a wide range of fruits, vegetables, and medicinal herbs, manifests chemopreventive effects and, at the same time, shows direct antioxidant activity in vitro and on animal models due to decreased lipid peroxidation and increased enzymatic and non-enzymatic antioxidants (Chaturvedi et al. 2008; Palanimuthu et al. 2012).
Ursolic acid can induce tumour cell apoptosis and has apparent antitumour effects on the NCI-H292 human lung cancer cells in vitro (Chen et al. 2019). In another in vitro study with human osteosarcoma (143B cells), apoptosis was induced by inactivating Wnt/β-catenin signalling through upregulating p53 (Zhang et al. 2016b).
Another natural triterpenoid, pomolic acid (isolated from Chrysobalanus icaco and Licania tomentosa), is cytotoxic against K562 cell line (human erythroleukaemia) and possesses anti-multidrug resistance properties (Fernandes et al. 2003).
Jenisseensosides (glucosides of quillaic acid) isolated from the plant Silene jenisseensis and Silene fortunei, can stimulate the proliferation of the Jurkat cell line when in low concentrations, while in higher doses, they regress to apoptosis (Lacaille-Dubois et al. 1997; Gaidi et al. 2002). Avicins, saponins isolated from Acacia victoriae, have preventive effects against H-ras gene mutation and changes in carcinogenesis (Hanausek et al. 2001).
Oleanolic acid and its isomer, ursolic acid, are pentacyclic triterpenoid compounds isolated from plants that frequently occur simultaneously in nature, either in free acid form or as an aglycone precursor of triterpenoid saponins. Because they share similar structural features, they show similar pharmacological activities (Jesus et al. 2015). Oleanolic and ursolic acid have shown immunomodulatory activity induced by activating macrophages infected in vitro with Mycobacterium tuberculosis as they passed from an M2-like (macrophages anti-inflammatory) phenotype to a M1-like phenotype (macrophages pro-inflammatory) characterised by high production of TNF-α and reactive oxygen species (ROS), and low production of Transforming Growth Factor Beta (TGF-β), resulting in the control and elimination of the intracellular mycobacteria (López-García et al. 2015). In another study, a clinical trial was performed with adolescents that ingested olive oil enriched with oleanolic acid to obtain postprandial triglyceride-rich lipoproteins that were further incubated with THP-1 (monocyte-like cell line) derived macrophages, showing that this compound has a high potential to prevent metabolic syndrome due to its anti-inflammatory activity (Fernández-Aparicio et al. 2021).
Ginsenosides are a group of glycosylated triterpenes constituents of ginseng that show anti-inflammatory activity both in vitro and animal models. Its mechanism of action is related to the inhibition of the NF-κB signalling pathway, suppressing the production of pro-inflammatory mediators and enzymes such as TNF-α, inducible Nitric Oxide Synthase (iNOS), and cyclooxygenase-2 (COX2) (Kim et al. 2017).
Studies have revealed that ginsenosides can play a relevant role in treating diabetes and ameliorate insulin resistance in the liver. Experimental and clinical data emerge to support the antidiabetic efficacy of ginsenosides attributed to their anti-inflammatory, antioxidant, and antihyperglycaemic activities, therefore, supporting the use of Panax ginseng in traditional medicine as an adjuvant to treat diabetes mellitus (Shao et al. 2020). In addition to their anti-inflammatory effects, ginsenosides also have an antioxidant effect. They can protect cardiomyocytes from oxidative damage caused by internal and external oxidants (Sarhene et al. 2021) and act on other cardiovascular diseases by regulating vascular function, inhibiting cardiomyocyte hypertrophy, and inhibiting thrombosis (Lee and Kim 2014).
Anti-inflammatory activity has also been ascribed to α-amarin, a pentacyclic terpene that exhibited this activity in rat models by reducing neutrophil infiltration and pro-inflammatory cytokine production of acute periodontitis (Holanda Pinto et al. 2008).
Other pentacyclic triterpenoids, such as boswellic acids isolated from the gum resin of Boswellia sp. (in particular cetyl-11-keto-ß-boswellic acid and 11-keto-ß-boswellic acid), have also evidence the same activity (Ammon 2016). Among other mechanisms of action, boswellic acids can act by inhibiting leukotriene synthesis and, to a less extent, prostaglandin synthesis. Various preclinical and clinical studies have established that these compounds exhibit substantial potential in managing inflammatory such as diminishing inflammation in osteoarthritis and colitis and helping control the brain oedema associated with radiotherapy of cerebral tumours (Takahashi et al. 2012; Hamidpour et al. 2013). In addition, it has been demonstrated that boswellic acids have an inhibitory and apoptotic effect in vitro against the cellular growth of leukaemia HL-60 cells, inhibiting the synthesis of DNA and RNA in a dose-dependent manner (Shao et al. 1998).
Different triterpenes also play a role in microorganism growth inhibition or elimination. Oleanolic acid and its derivatives present a specific inhibitory effect on Staphylococcus aureus, including methicillin-resistant strains, Streptococcus mutans, Listeria monocytogenes, Enterococcus faecium, and Enterococcus faecalis by destroying the cell membranes of the bacteria (Blanco-Cabra et al. 2019; Kim et al. 2015b). They were also active against species of mycoplasma, and Gram-positive and Gram-negative bacteria also have anti-human immunodeficiency virus (anti-HIV) activity (Khwaza et al. 2018).
Likewise, many studies have shown that glycyrrhizin, a saponine from liquorice (Glycyrrhiza glabra) has potent effects in inhibiting Gram-positive and Gram-negative bacteria, such as Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Bacillus subtilis as well as the yeast Candida albicans (Karahan et al. 2016; Wang et al. 2015; Martins et al. 2016b). Glycyrrhizin has also demonstrated antiviral activity, with the possible mechanism of action being the decrease of the expression of genes of virulence. Glycyrrhizin has anti-HSV-1 and anti-HSV-2 activities in vivo and in vitro; therefore, can significantly inhibit virus replication and reduce the number of infectious virus particles (Huan et al. 2021). It also effectively inhibited the Severe Acute Respiratory Syndrome-Associated Coronavirus (SARS-CoV) virus replication through cellular signalling pathways such as protein kinase C (Cinatl et al. 2003). Moreover, it is proposed that the long-term administration of glycyrrhizin protects patients with chronic hepatitis C from developing hepatocellular cancer. It improves the outcomes of interferon administration due to a synergistic effect (Ashfaq et al. 2011).
Antiviral activity has also been evidenced by moronic acid and betulonic acid, two compounds extracted from the plant Rhus javanica that showed a potent in vitro inhibitory effect on Herpes Simplex Virus-1 (HSV-1). Betulinic acid and its derivatives also have anti-human immunodeficiency virus activity (Huang et al. 2018) being considered the earliest discovered pentacyclic triterpenoid compound with anti-HIV activity (Kumar et al. 2018; Aiken and Chen 2005). Additionally, they have been shown to inhibit the replication of vesicular stomatitis virus and encephalomyocarditis virus (Cavalcante et al. 2020; Meira et al. 2019).
Notoginsenoside ST-4i, isolated from the Chinese herb Panax notoginseng, demonstrated in vitro inhibitory activities against HSV-1 and Herpes Simplex Virus-2 (HSV-2) (Pei et al. 2011).
Lupenone and its saponin showed in vitro viral plaque inhibitory effects against HSV-1 and HSV-2 (Xu et al. 2018b). Lupenone isolated of rhizoma musae (the root of Musa basjoo Sied. et Zucc.) also has antidiabetic activity by inhibition of α-amylase and α-glucosidase in diabetic mice (Xu et al. 2021). Table 5.5. describes the activities and examples of plant sources for relevant triterpenes.
5 Terpene Applications, Challenges, and Future Perspectives

5.1 Application
Besides playing a fundamental role in plants, terpenes and their derivatives find diverse applications in several industries, such as food, pharmaceutical, flavours, fragrances, and biofuels (Bicas et al. 2009; Tetali 2019; Harman-Ware 2020b). Herbs and spices have been used since ancient times to preserve food due to their antimicrobial and insecticidal properties ascribed to their composition in terpenes (Gottardi et al. 2016). These properties were probably the basis of their initial use in ancient medicine and supported their continued use of mouthwashes, cough medicines, disinfectants, and insect repellents. More complex structural terpenes frequently show other properties of interest that have been taken into an advantage for their health benefits (Guimarães et al. 2019; Papada et al. 2018; Bhavaniramya et al. 2019). In this regard, taxol and vinblastine are used as drugs in cancer treatment. In contrast, others mimic animal hormones such as diosgenin, a sterol synthesised from terpenoid precursors and present in the Mexican yam (Dioscorea mexicana), has been used to produce progesterone for birth control pills and other medicinal steroids (Demain and Vaishnav 2011; Jesus et al. 2016). The most important use of terpenes by humans is for consumption since they are required to produce essential molecules, such as vitamin A synthesised from β-carotene, an abundant plant terpene (Wagner and Elmadfa 2003; Grassmann 2005). More commonly, terpenes in foods affect our eating experience. Terpenoid pigments, such as astaxanthin and lycopene, are heavily used in the food industry as natural colours, for they have a large influence on the acceptability of many foods (Mata-Gómez et al. 2014). Volatile terpenoid compounds impart flavour to foods via their detection by the olfactory system (Schieber and Wüst 2020). For example, the ginger flavour is caused by nootkatone (Srinivasan 2017), and zingiberene imparts a grapefruit flavour (El Hadi et al. 2013). Many herbs used as fresh plant material contain volatile organic compounds (VOCs) that are used in the preparation of flavours in beverages. For example, a soda contains more than one citrus oil (e.g., lemon, lime, orange, neroli) (Ameh and Obodozie-Ofoegbu 2016), already in the preparation of cola flavours are used significant amounts of spice oils such as cinnamon (Buglass and Caven-Quantrill 2012). In terms of alcoholic spirits, specifically for gin, the aroma stems from the use of botanicals during the distillation process, whereby individual aroma notes are imparted by constituent odour-active VOCs predominant compounds, whereby monoterpenes were the most frequently identified compounds, assigned mainly to juniper and coriander oil (Buck et al. 2020). More recently, studies have reported that some terpenes have adequate properties, such as viscosity, flash point, high energy density, among others, that make them good candidates for speciality biofuels, capable of supplementing or even replacing current petroleum-derived fuels (Mewalal et al. 2017; Donoso et al. 2022; Donoso et al. 2021). In the last years, the most promising examples include the monoterpenes limonene and β-phellandrene (Lei et al. 2021), the sesquiterpenes farnesene and bisabolene (Gupta and Phulara 2015) and the diterpenes phytene and cambrene (Scown et al. 2021). Although plant terpenes represent a potential source of alternative and sustainable energy, their implementation as commercial biofuels is compromised by the low yield of specific terpenes in plants (Jiang et al. 2016). Nevertheless, commercial-scale recovery of plant terpenes from some plants such as pine, citrus, and eucalyptus is already taking place and together with the recent advances of biotechnological tools such as genome editing, can pave the way for effective use of terpenes as a feedstock for alternative biofuels (Beller et al. 2015).
5.2 Challenges and Future Perspectives in the Terpenes Industry
Terpenes are compounds of great industrial interest with a wide range of applications. However, there is still a long way between the characterisation and isolation of terpenes and their extraction and application on an industrial scale (Jiang et al. 2016). There are resources throughout nature that could be discovered with applications of human interest. Secondary metabolites from plant matrices, especially terpenes, are still an underexplored source of compounds, with only a small proportion being investigated. Diverse genetic and climatic factors influence the synthesis of secondary plant metabolites (Pang et al. 2021). Biotyping factors such as plant competition, pollinators, viruses, bacteria and predators, and abiotic conditions such as soil type, hydric or climatic stress, high light intensity, among others, alter the production of terpenes in plants. Besides climatic and biotyping factors, there are also difficulties related to botanical species' slow growth, low yield, and incomplete knowledge of biosynthesis mechanisms (Dudareva et al. 2013). Therefore, developing a model that can standardise the production of terpenes in plants under different environmental conditions is still a challenge for the industry. However, the biosynthesis of many natural products is still poorly understood, and this hampers the ability to engineer microbial hosts to produce these compounds (Beutler 2009). The anticancer agent paclitaxel (taxol) biosynthesis is an excellent example of this problem. The compound is derived from the bark of the slow-growing evergreen Pacific yew tree (Taxus brevifolia), and the bark is the only part of the plant that is used commercially. The bark is harvested sustainably, but it is still a limited resource. In addition, the complex biosynthesis of paclitaxel makes it challenging to produce, resorting to recombination techniques and microbial fermentation engineering. A potential solution to this problem may be genetically modified plants (Croteau et al. 2006; Zhu and Chen 2019). This technology would involve constructing a metabolic pathway to produce the natural product in the microbial frame and incorporate the necessary genes into the organism's genome (Heskes et al. 2018). From a future perspective, a better understanding between the action of molecules, the synergy or antagonism of compounds, and the proof of the biological activity in vivo is of paramount importance, with the synthetic production of terpenes being a potentially viable solution in the future for terpenes supply and the development of industries that have these compounds as raw material.
References
Abbaszadeh S, Sharifzadeh A, Shokri H, Khosravi AR, Abbaszadeh A (2014) Antifungal efficacy of thymol, carvacrol, eugenol and menthol as alternative agents to control the growth of food-relevant fungi. J Mycol Med 24(2):e51–ee6. https://doi.org/10.1016/j.mycmed.2014.01.063
Aiken C, Chen CH (2005) Betulinic acid derivatives as HIV-1 antivirals. Trends Mol Med 11(1):31–36. https://doi.org/10.1016/j.molmed.2004.11.001
Al Dawsari MM, Alam P (2022) Disruption impact of citronella and menthol insecticides on adults behavior and hemocytes morphology in the red palm weevil Rhynchophorus ferrugineus “Oliver” (Coleoptera: Curculionidae). Sci Prog 105(1):368504221079437. https://doi.org/10.1177/00368504221079437
Alghasham AA (2013) Cucurbitacins - a promising target for cancer therapy. Int J Health Sci 7(1):77–89. https://doi.org/10.12816/0006025
Ali B, Al-Wabel NA, Shams S, Ahamad A, Khan SA, Anwar F (2015) Essential oils used in aromatherapy: a systemic review. Asian Pac J Trop Biomed 5(8):601–611. https://doi.org/10.1016/j.apjtb.2015.05.007
Al-Salihi SAA, Alberti F (2021) Naturally occurring terpenes: a promising class of organic molecules to address influenza pandemics. Nat Prod Bioprospect 11(4):405–419. https://doi.org/10.1007/s13659-021-00306-z
Ambrosio SR, Tirapelli CR, da Costa FB, de Oliveira AM (2006) Kaurane and pimarane-type diterpenes from the Viguiera species inhibit vascular smooth muscle contractility. Life Sci 79(10):925–933. https://doi.org/10.1016/j.lfs.2006.05.015
Ameh SJ, Obodozie-Ofoegbu O (2016) Chapter 11 - Essential oils as flavors in carbonated cola and citrus soft drinks. In: Preedy VR (ed) Essential oils in food preservation, flavor and safety. Academic Press, San Diego, pp 111–121
Ammon HP (2016) Boswellic acids and their role in chronic inflammatory diseases. Adv Exp Med Biol 928:291–327. https://doi.org/10.1007/978-3-319-41334-1_13
Appendino G, Szallasi A (1997) Euphorbium: modern research on its active principle, resiniferatoxin, revives an ancient medicine. Life Sci 60(10):681–696. https://doi.org/10.1016/S0024-3205(96)00567-X
Ashfaq UA, Masoud MS, Nawaz Z, Riazuddin S (2011) Glycyrrhizin as antiviral agent against Hepatitis C Virus. J Transl Med 9(1):112. https://doi.org/10.1186/1479-5876-9-112
Bai X, Tang J (2020) Myrcene exhibits antitumor activity against lung cancer cells by inducing oxidative stress and apoptosis mechanisms. Nat Prod Commun 15(9). https://doi.org/10.1177/1934578x20961189
Baker H, Levieille G, Jackson S (2021) Hydrodistillation assay and essential oil optimising yield study – a comparative analysis of essential oil content of Irish Grown and commercially sourced Chamomile Matricaria recutita. Planta Med 87(15):PC8
Bardon S, Foussard V, Fournel S, Loubat A (2002) Monoterpenes inhibit proliferation of human colon cancer cells by modulating cell cycle-related protein expression. Cancer Lett 181(2):187–194. https://doi.org/10.1016/s0304-3835(02)00047-2
Barile E, Fattorusso E, Ialenti A, Ianaro A, Lanzotti V (2007) Paraliane and pepluane diterpenes as anti-inflammatory agents: first insights in structure–activity relationships. Bioorg Med Chem Lett 17(15):4196–4200. https://doi.org/10.1016/j.bmcl.2007.05.072
Barrales-Cureño HJ, Salgado-Garciglia R, López-Valdez LG, Reynoso-López R, Herrera-Cabrera BE, Lucho-Constantino GG et al (2021) Use of secondary metabolites from medicinal and aromatic plants in the fragrance industry. In: Aftab T (ed) Medicinal and aromatic plants. Springer, Cham, pp 669–690
Beller HR, Lee TS, Katz L (2015) Natural products as biofuels and bio-based chemicals: fatty acids and isoprenoids. Nat Prod Rep 32(10):1508–1526. https://doi.org/10.1039/c5np00068h
Bensebia O, Bensebia B, Allia K, Barth D (2016) Supercritical CO2 extraction of triterpenes from rosemary leaves: kinetics and modelling. Sep Sci Technol 51(13):2174–2182. https://doi.org/10.1080/01496395.2016.1202977
Beutler JA (2009) Natural products as a foundation for drug discovery. Curr Protoc Pharmacol 46:9.11.1–9.11.21. https://doi.org/10.1002/0471141755.ph0911s46
Bhatia V, Maisnam J, Jain A, Sharma KK, Bhattacharya R (2015) Aphid-repellent pheromone E-β-farnesene is generated in transgenic Arabidopsis thaliana over-expressing farnesyl diphosphate synthase2. Ann Bot 115(4):581–591. https://doi.org/10.1093/aob/mcu250
Bhavaniramya S, Vishnupriya S, Al-Aboody MS, Vijayakumar R, Baskaran D (2019) Role of essential oils in food safety: antimicrobial and antioxidant applications. Grain Oil Sci Technol 2(2):49–55. https://doi.org/10.1016/j.gaost.2019.03.001
Bicas JL, Dionísio AP, Pastore GM (2009) Bio-oxidation of terpenes: an approach for the flavor industry. Chem Rev 109(9):4518–4531. https://doi.org/10.1021/cr800190y
Blanch J-S, Peñuelas J, Llusià J (2007) Sensitivity of terpene emissions to drought and fertilization in terpene-storing Pinus halepensis and non-storing Quercus ilex. Physiol Plant 131(2):211–225. https://doi.org/10.1111/j.1399-3054.2007.00944.x
Blanco-Cabra N, Vega-Granados K, Moya-Andérico L, Vukomanovic M, Parra A, Álvarez de Cienfuegos L et al (2019) Novel oleanolic and maslinic acid derivatives as a promising treatment against bacterial biofilm in nosocomial infections: an in vitro and in vivo study. ACS Infect Dis 5(9):1581–1589. https://doi.org/10.1021/acsinfecdis.9b00125
Bonilla-Jaime H, Guadarrama-Cruz G, Alarcon-Aguilar FJ, Limón-Morales O, Vazquez-Palacios G (2015) Antidepressant-like activity of Tagetes lucida Cav. is mediated by 5-HT(1A) and 5-HT(2A) receptors. J Nat Med 69(4):463–470. https://doi.org/10.1007/s11418-015-0909-5
Boukhatem MN (2020) The amazing use of essential oils and their related terpenes as natural preservatives to improve the shelf life of food. Food Sci Nutr Technol 5(2). https://doi.org/10.23880/fsnt-16000215
Buck N, Goblirsch T, Beauchamp J, Ortner E (2020) Key aroma compounds in two bavarian gins. Appl Sci 10(20):7269
Buglass AJ, Caven-Quantrill DJ (2012) Applications of natural ingredients in alcoholic drinks. In: Baines D, Seal R (eds) Natural food additives, ingredients and flavourings, vol 16. Woodhead Publishing, London, pp 358–416
Burke YD, Stark MJ, Roach SL, Sen SE, Crowell PL (1997) Inhibition of pancreatic cancer growth by the dietary isoprenoids farnesol and geraniol. Lipids 32(2):151. https://doi.org/10.1007/s11745-997-0019-y
Caputi L, Aprea E (2011) Use of terpenoids as natural flavouring compounds in food industry. Recent Pat Food Nutr Agric 3(1):9–16. https://doi.org/10.2174/2212798411103010009
Capuzzo A, Maffei ME, Occhipinti A (2013) Supercritical fluid extraction of plant flavors and fragrances. Molecules 18(6):7194–7238
Carnesecchi S, Schneider Y, Ceraline J, Duranton B, Gosse F, Seiler N et al (2001) Geraniol, a component of plant essential oils, inhibits growth and polyamine biosynthesis in human colon cancer cells. J Pharmacol Exp Ther 298(1):197
Carson CF, Riley TV (1995) Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J Appl Bacteriol 78(3):264–269. https://doi.org/10.1111/j.1365-2672.1995.tb05025.x
Cavalcante BRR, Aragão-França LS, Sampaio GLA, Nonaka CKV, Oliveira MS, Campos GS et al (2020) Betulinic acid exerts cytoprotective activity on Zika Virus-infected neural progenitor cells. Front Cell Infect Microbiology 10. https://doi.org/10.3389/fcimb.2020.558324
Cerchiara T, Straface SV, Brunelli E, Tripepi S, Gallucci MC, Chidichimo G (2015) Antiproliferative effect of linalool on RPMI 7932 human melanoma cell line: ultrastructural studies. Nat Prod Commun 10(4):547–549
Chang M-Y, Shen Y-L (2014) Linalool exhibits cytotoxic effects by activating antitumor immunity. Molecules 19(5):6694–6706
Chatterjee S, Jain A, De S (2017) Effect of different operating conditions in cloud point assisted extraction of thymol from Ajwain (Trachyspermum Ammi L.) seeds and recovery using solvent. J Food Sci Technol 54(13):4353–4361. https://doi.org/10.1007/s13197-017-2906-z
Chaturvedi PK, Bhui K, Shukla Y (2008) Lupeol: connotations for chemoprevention. Cancer Lett 263(1):1–13. https://doi.org/10.1016/j.canlet.2008.01.047
Chavan MJ, Wakte PS, Shinde DB (2010) Analgesic and anti-inflammatory activity of Caryophyllene oxide from Annona squamosa L. bark. Phytomedicine 17(2):149–151. https://doi.org/10.1016/j.phymed.2009.05.016
Chebet JJ, Ehiri JE, McClelland DJ, Taren D, Hakim IA (2021) Effect of d-limonene and its derivatives on breast cancer in human trials: a scoping review and narrative synthesis. BMC Cancer 21(1):902. https://doi.org/10.1186/s12885-021-08639-1
Chemat F, Vian MA, Cravotto G (2012) Green extraction of natural products: concept and principles. Int J Mol Sci 13(7):8615–8627. https://doi.org/10.3390/ijms13078615
Chen Z, Xu H (2014) Anti-inflammatory and immunomodulatory mechanism of Tanshinone IIA for atherosclerosis. Evid Based Complement Alternat Med 2014:267976. https://doi.org/10.1155/2014/267976
Chen CJ, Shih YL, Yeh MY, Liao NC, Chung HY, Liu KL et al (2019) Ursolic acid induces apoptotic cell death through AIF and Endo G release through a mitochondria-dependent pathway in NCI-H292 human lung cancer cells in vitro. In Vivo 33(2):383–391. https://doi.org/10.21873/invivo.11485
Cheng H-Y, Lin T-C, Yang C-M, Wang K-C, Lin L-T, Lin C-C (2004) Putranjivain A from Euphorbia jolkini inhibits both virus entry and late stage replication of herpes simplex virus type 2 in vitro. J Antimicrob Chemother 53(4):577–583. https://doi.org/10.1093/jac/dkh136
Chia VV, Pang SF, Gimbun J (2020) Mass spectrometry analysis of auxiliary energy-induced terpenes extraction from Andrographis Paniculata. Ind Crop Prod 155:112828. https://doi.org/10.1016/j.indcrop.2020.112828
Cho M, So I, Chun JN, Jeon J-H (2016) The antitumor effects of geraniol: modulation of cancer hallmark pathways (Review). Int J Oncol 48(5):1772–1782. https://doi.org/10.3892/ijo.2016.3427
Choi YH, Verpoorte R (2019) Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Curr Opin Food Sci 26:87–93. https://doi.org/10.1016/j.cofs.2019.04.003
Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW (2003) Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 361(9374):2045–2046. https://doi.org/10.1016/S0140-6736(03)13615-X
Cox-Georgian D, Ramadoss N, Dona C, Basu C (2019) Therapeutic and medicinal uses of terpenes. In: Joshee N, Dhekney SA, Parajuli P (eds) Medicinal plants: from farm to pharmacy. Springer, Cham, pp 333–359
Croteau R, Ketchum RE, Long RM, Kaspera R, Wildung MR (2006) Taxol biosynthesis and molecular genetics. In: Phytochemistry reviews: proceedings of the Phytochemical Society of Europe, vol 5. Springer, New York, pp 75–97. https://doi.org/10.1007/s11101-005-3748-2
Crowell PL (1999) Prevention and therapy of cancer by dietary monoterpenes. J Nutr 129(3):775S–778S. https://doi.org/10.1093/jn/129.3.775S
Cui J, Hu LA, Shi W, Cui G, Zhang X, Zhang Q-W (2019) Design, synthesis and anti-platelet aggregation activity study of Ginkgolide-1,2,3-triazole derivatives. Molecules 24(11):2156
da Silva CEH, Gosmann G, de Andrade SF (2021) Limonene and perillyl alcohol derivatives: synthesis and anticancer activity. Mini Rev Med Chem 21(14):1813–1829. https://doi.org/10.2174/1389557521666210212150504
de Medeiros CAC, Pinto ÂV, de Oliveira JC, Silva GS, Arrua JMM, Lima IO et al (2021) Evaluating the antifungal activity of α-Bisabolol in association with NaCl on fusarium oxysporum in maize grains. Curr Microbiol 78(2):604–610. https://doi.org/10.1007/s00284-020-02313-8
de Melo MMR, Carius B, Simões MMQ, Portugal I, Saraiva J, Silva CM (2020) Supercritical CO2 extraction of V. vinifera leaves: influence of cosolvents and particle size on removal kinetics and selectivity to target compounds. J Supercrit Fluids 165:104959. https://doi.org/10.1016/j.supflu.2020.104959
Demain AL, Vaishnav P (2011) Natural products for cancer chemotherapy. Microb Biotechnol 4(6):687–699. https://doi.org/10.1111/j.1751-7915.2010.00221.x
Dinda B, Debnath S, Mohanta BC, Harigaya Y (2010) Naturally occurring triterpenoid saponins. Chem Biodivers 7(10):2327–2580. https://doi.org/10.1002/cbdv.200800070
Donoso D, García D, Ballesteros R, Lapuerta M, Canoira L (2021) Hydrogenated or oxyfunctionalized turpentine: options for automotive fuel components. RSC Adv 11(30):18342–18350. https://doi.org/10.1039/D1RA03003E
Donoso D, Bolonio D, Ballesteros R, Lapuerta M, Canoira L (2022) Hydrogenated orange oil: a waste derived drop-in biojet fuel. Renew Energy 188:1049–1058. https://doi.org/10.1016/j.renene.2022.02.078
Dudareva N, Klempien A, Muhlemann JK, Kaplan I (2013) Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol 198(1):16–32. https://doi.org/10.1111/nph.12145
Dzah CS, Duan Y, Zhang H, Wen C, Zhang J, Chen G et al (2020) The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: a review. Food Biosci 35:100547. https://doi.org/10.1016/j.fbio.2020.100547
Eckstein-Ludwig U, Webb RJ, Van Goethem ID, East JM, Lee AG, Kimura M et al (2003) Artemisinins target the SERCA of Plasmodium falciparum. Nature 424(6951):957–961. https://doi.org/10.1038/nature01813
El Bardai S, Hamaide M-C, Lyoussi B, Quetin-Leclercq J, Morel N, Wibo M (2004) Marrubenol interacts with the phenylalkylamine binding site of the L-type calcium channel. Eur J Pharmacol 492(2):269–272. https://doi.org/10.1016/j.ejphar.2004.04.007
El Hadi MAM, Zhang F-J, Wu F-F, Zhou C-H, Tao J (2013) Advances in fruit aroma volatile research. Molecules (Basel, Switzerland) 18(7):8200–8229. https://doi.org/10.3390/molecules18078200
El Kharraf S, Faleiro ML, Abdellah F, El-Guendouz S, El Hadrami EM, Miguel MG (2021) Simultaneous hydrodistillation-steam distillation of Rosmarinus officinalis, Lavandula angustifolia and Citrus aurantium from Morocco, major Terpenes: impact on biological activities. Molecules 26(18):5452
Fagbemi KO, Aina DA, Olajuyigbe OO (2021) Soxhlet extraction versus hydrodistillation using the clevenger apparatus: a comparative study on the extraction of a volatile compound from Tamarindus indica seeds. Sci World J 2021:5961586. https://doi.org/10.1155/2021/5961586
Fan K, Li X, Cao Y, Qi H, Li L, Zhang Q et al (2015) Carvacrol inhibits proliferation and induces apoptosis in human colon cancer cells. Anticancer Drugs 26(8):813–823. https://doi.org/10.1097/cad.0000000000000263
Fernandes J, Castilho RO, da Costa MR, Wagner-Souza K, Coelho Kaplan MA, Gattass CR (2003) Pentacyclic triterpenes from Chrysobalanaceae species: cytotoxicity on multidrug resistant and sensitive leukemia cell lines. Cancer Lett 190(2):165–169. https://doi.org/10.1016/S0304-3835(02)00593-1
Fernández-Aparicio Á, Perona JS, Castellano JM, Correa-Rodríguez M, Schmidt-RioValle J, González-Jiménez E (2021) Oleanolic acid-enriched olive oil alleviates the Interleukin-6 overproduction induced by postprandial triglyceride-rich lipoproteins in THP-1 macrophages. Nutrients 13(10):3471
Fernandez-Pastor I, Fernandez-Hernandez A, Perez-Criado S, Rivas F, Martinez A, Garcia-Granados A et al (2017) Microwave-assisted extraction versus Soxhlet extraction to determine triterpene acids in olive skins. J Sep Sci 40(5):1209–1217. https://doi.org/10.1002/jssc.201601130
Fiore C, Eisenhut M, Krausse R, Ragazzi E, Pellati D, Armanini D et al (2008) Antiviral effects of glycyrrhiza species. Phytother Res 22(2):141–148. https://doi.org/10.1002/ptr.2295
Forrer M, Kulik EM, Filippi A, Waltimo T (2013) The antimicrobial activity of alpha-bisabolol and tea tree oil against Solobacterium moorei, a Gram-positive bacterium associated with halitosis. Arch Oral Biol 58(1):10–16. https://doi.org/10.1016/j.archoralbio.2012.08.001
Gaidi G, Miyamoto T, Laurens V, Lacaille-Dubois M-A (2002) New acylated triterpene saponins from silene fortunei that modulate lymphocyte proliferation. J Nat Prod 65(11):1568–1572. https://doi.org/10.1021/np020105a
Galle M, Crespo R, Rodenak Kladniew B, Montero Villegas S, Polo M, de Bravo MG (2014) Suppression by geraniol of the growth of A549 human lung adenocarcinoma cells and inhibition of the mevalonate pathway in culture and in vivo: potential use in cancer chemotherapy. Nutr Cancer 66(5):888–895. https://doi.org/10.1080/01635581.2014.916320
Galletti E, Magnani M, Renzulli ML, Botta M (2007) Paclitaxel and docetaxel resistance: molecular mechanisms and development of new generation taxanes. ChemMedChem 2(7):920–942. https://doi.org/10.1002/cmdc.200600308
Garcia-Perez P, Xiao J, Munekata PES, Lorenzo JM, Barba FJ, Rajoka MSR et al (2021) Revalorization of almond by-products for the design of novel functional foods: an updated review. Foods 10(8):1823
Garcia-Vaquero M, Rajauria G, Tiwari B (2020) Chapter 7 - Conventional extraction techniques: solvent extraction. In: Torres MD, Kraan S, Dominguez H (eds) Sustainable seaweed technologies. Elsevier, New York, pp 171–189
Garg A, Sharma R, Dey P, Kundu A, Kim HS, Bhakta T et al (2020) Chapter 11 - Analysis of triterpenes and triterpenoids. In: Sanches Silva A, Nabavi SF, Saeedi M, Nabavi SM (eds) Recent advances in natural products analysis. Elsevier, New York, pp 393–426
Giacometti J, Bursać Kovačević D, Putnik P, Gabrić D, Bilušić T, Krešić G et al (2018) Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: a review. Food Res Int 113:245–262. https://doi.org/10.1016/j.foodres.2018.06.036
Girola N, Figueiredo CR, Farias CF, Azevedo RA, Ferreira AK, Teixeira SF et al (2015) Camphene isolated from essential oil of Piper cernuum (Piperaceae) induces intrinsic apoptosis in melanoma cells and displays antitumor activity in vivo. Biochem Biophys Res Commun 467(4):928–934. https://doi.org/10.1016/j.bbrc.2015.10.041
González-Cardenete MA, Rivas F, Basset R, Stadler M, Hering S, Padrón JM et al (2021) Biological profiling of semisynthetic C19-functionalized ferruginol and sugiol analogues. Antibiotics 10(2):184
Gottardi D, Bukvicki D, Prasad S, Tyagi AK (2016) Beneficial effects of spices in food preservation and safety. Front Microbiol 7:1394. https://doi.org/10.3389/fmicb.2016.01394
Gould MN, Moore CJ, Zhang R, Wang B, Kennan WS, Haag JD (1994) Limonene chemoprevention of mammary carcinoma induction following direct in situ transfer of v-Ha-ras1. Cancer Res 54(13):3540–3543
Grassmann J (2005) Terpenoids as plant antioxidants. Vitam Horm 72:505–535. https://doi.org/10.1016/s0083-6729(05)72015-x
Greay SJ, Hammer KA (2011) Recent developments in the bioactivity of mono- and diterpenes: anticancer and antimicrobial activity. Phytochem Rev 14:1–6
Gregersen S, Jeppesen PB, Holst JJ, Hermansen K (2004) Antihyperglycemic effects of stevioside in type 2 diabetic subjects. Metab Clin Exp 53(1):73–76. https://doi.org/10.1016/j.metabol.2003.07.013
Gu W, Rebsdorf A, Anker C, Gregersen S, Hermansen K, Geuns JMC et al (2019) Steviol glucuronide, a metabolite of steviol glycosides, potently stimulates insulin secretion from isolated mouse islets: studies in vitro. Endocrinol Diabetes Metab 2(4):e00093. https://doi.org/10.1002/edm2.93
Guimarães AG, Serafini MR, Quintans-Júnior LJ (2014) Terpenes and derivatives as a new perspective for pain treatment: a patent review. Expert Opin Therap Pat 24(3):243–265. https://doi.org/10.1517/13543776.2014.870154
Guimarães AC, Meireles LM, Lemos MF, Guimarães MCC, Endringer DC, Fronza M et al (2019) Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 24(13):2471
Guo Y, Baschieri A, Amorati R, Valgimigli L (2021) Synergic antioxidant activity of γ-terpinene with phenols and polyphenols enabled by hydroperoxyl radicals. Food Chem 345:128468. https://doi.org/10.1016/j.foodchem.2020.128468
Gupta P, Phulara SC (2015) Metabolic engineering for isoprenoid-based biofuel production. J Appl Microbiol 119(3):605–619. https://doi.org/10.1111/jam.12871
Gupta A, Jeyakumar E, Lawrence R (2021) Strategic approach of multifaceted antibacterial mechanism of limonene traced in Escherichia coli. Sci Rep 11(1):13816. https://doi.org/10.1038/s41598-021-92843-3
Haberstroh S, Kreuzwieser J, Lobo-do-Vale R, Caldeira MC, Dubbert M, Werner C (2018) Terpenoid emissions of two mediterranean woody species in response to drought stress. Front Plant Sci 9. https://doi.org/10.3389/fpls.2018.01071
Hamidpour R, Hamidpour S, Hamidpour M, Shahlari M (2013) Frankincense ( rǔ xiāng; boswellia species): from the selection of traditional applications to the novel phytotherapy for the prevention and treatment of serious diseases. J Tradit Complement Med 3(4):221–226. https://doi.org/10.4103/2225-4110.119723
Hanausek M, Ganesh P, Walaszek Z, Arntzen CJ, Slaga TJ, Gutterman JU (2001) Avicins, a family of triterpenoid saponins from Acacia victoriae (Bentham), suppress H-ras mutations and aneuploidy in a murine skin carcinogenesis model. Proc Natl Acad Sci 98(20):11551–11556. https://doi.org/10.1073/pnas.191363198
Hanuš LO, Hod Y (2020) Terpenes/Terpenoids in Cannabis: are they important? Med Cannabis Cannabinoids 3(1):25–60. https://doi.org/10.1159/000509733
Harman-Ware AE (2020a) Conversion of terpenes to chemicals and related products. In: Santillan-Jimenez MCAE (ed) Chemical catalysts for biomass upgrading. Wiley, Hoboken, NJ
Harman-Ware AE (2020b) Conversion of terpenes to chemicals and related products. In: Chemical catalysts for biomass upgrading. Wiley, Hoboken, NJ, pp 529–568
Hassan SB, Gali-Muhtasib H, Goransson H, Larsson R (2010) Alpha terpineol: a potential anticancer agent which acts through suppressing NF-κB signalling. Anticancer Res 30(6):1911–1919
He W, Zhai X, Su J, Ye R, Zheng Y, Su S (2019) Antiviral activity of germacrone against pseudorabies virus in vitro. Pathogens 8(4):258
Heskes AM, Sundram TCM, Boughton BA, Jensen NB, Hansen NL, Crocoll C et al (2018) Biosynthesis of bioactive diterpenoids in the medicinal plant Vitex agnus-castus. Plant J 93(5):943–958. https://doi.org/10.1111/tpj.13822
Hinze WL, Pramauro E (1993) A critical review of surfactant-mediated phase separations (cloud-point extractions): theory and applications. Crit Rev Anal Chem 24(2):133–177. https://doi.org/10.1080/10408349308048821
Holanda Pinto SA, Pinto LM, Cunha GM, Chaves MH, Santos FA, Rao VS (2008) Anti-inflammatory effect of alpha, beta-Amyrin, a pentacyclic triterpene from Protium heptaphyllum in rat model of acute periodontitis. Inflammopharmacology 16(1):48–52. https://doi.org/10.1007/s10787-007-1609-x
Huan C, Xu Y, Zhang W, Guo T, Pan H, Gao S (2021) Research progress on the antiviral activity of glycyrrhizin and its derivatives in liquorice. Front Pharmacol 12:680674. https://doi.org/10.3389/fphar.2021.680674
Huang QX, Chen HF, Luo XR, Zhang YX, Yao X, Zheng X (2018) Structure and anti-HIV activity of betulinic acid analogues. Curr Med Sci 38(3):387–397. https://doi.org/10.1007/s11596-018-1891-4
Isidore E, Karim H, Ioannou I (2021) Extraction of phenolic compounds and terpenes from Cannabis sativa L. by-products: from conventional to intensified processes. Antioxidants 10(6):942
Islam MT, Khalipha ABR, Bagchi R, Mondal M, Smrity SZ, Uddin SJ et al (2019) Anticancer activity of thymol: a literature-based review and docking study with emphasis on its anticancer mechanisms. IUBMB Life 71(1):9–19. https://doi.org/10.1002/iub.1935
Ismail HM, Barton V, Phanchana M, Charoensutthivarakul S, Wong MH, Hemingway J et al (2016) Artemisinin activity-based probes identify multiple molecular targets within the asexual stage of the malaria parasites Plasmodium falciparum 3D7. Proc Natl Acad Sci U S A 113(8):2080–2085. https://doi.org/10.1073/pnas.1600459113
Ivanova-Petropulos V, Mitrev S, Stafilov T, Markova N, Leitner E, Lankmayr E et al (2015) Characterisation of traditional Macedonian edible oils by their fatty acid composition and their volatile compounds. Food Res Int 77:506–514. https://doi.org/10.1016/j.foodres.2015.08.014
Iwasaki K, Zheng Y-W, Murata S, Ito H, Nakayama K, Kurokawa T et al (2016) Anticancer effect of linalool via cancer-specific hydroxyl radical generation in human colon cancer. World J Gastroenterol 22(44):9765–9774. https://doi.org/10.3748/wjg.v22.i44.9765
Jafari-Sales A, Pashazadeh M (2020) Study of chemical composition and antimicrobial properties of Rosemary (Rosmarinus officinalis) essential oil on Staphylococcus aureus and Escherichia coli in vitro. Int J Life Sci Biotechnol 3(1):62–69. https://doi.org/10.38001/ijlsb.693371
Jasna I, Sonja Đ, Milka J, Vlatka V, Nada B, Slobodan P et al (2009) Supercritical carbon dioxide extraction of antioxidants from rosemary (Rosmarinus officinalis L.) and sage (Salvia officinalis L.). J Serbian Chem Soc 74(7):717–732. https://doi.org/10.2298/JSC0907717I
Jeppesen PB, Gregersen S, Poulsen CR, Hermansen K (2000) Stevioside acts directly on pancreatic β cells to secrete insulin: Actions independent of cyclic adenosine monophosphate and adenosine triphosphate—sensitivie K+-channel activity. Metab Clin Exp 49(2):208–214. https://doi.org/10.1016/S0026-0495(00)91325-8
Jesus JA, Lago JHG, Laurenti MD, Yamamoto ES, Passero LFD (2015) Antimicrobial activity of oleanolic and ursolic acids: an update. Evid Based Complement Alternat Med 2015:620472. https://doi.org/10.1155/2015/620472
Jesus M, Martins APJ, Gallardo E, Silvestre S (2016) Diosgenin: recent highlights on pharmacology and analytical methodology. J Anal Methods Chem 2016:4156293. https://doi.org/10.1155/2016/4156293
Jha NK, Sharma C, Hashiesh HM, Arunachalam S, Meeran MN, Javed H et al (2021) β-Caryophyllene, a natural dietary CB2 receptor selective cannabinoid can be a candidate to target the trinity of infection, immunity, and inflammation in COVID-19. Front Pharmacol 12. https://doi.org/10.3389/fphar.2021.590201
Jiang Z, Kempinski C, Chappell J (2016) Extraction and analysis of terpenes/terpenoids. Curr Protoc Plant Biol 1:345–358. https://doi.org/10.1002/cppb.20024
Jiang Y-Y, Shui J-C, Zhang B-X, Chin J-W, Yue R-S (2020) The potential roles of artemisinin and its derivatives in the treatment of type 2 diabetes mellitus. Front Pharmacol 11:585487. https://doi.org/10.3389/fphar.2020.585487
Jin J, Zhou M, Wang X, Liu M, Huang H, Yan F et al (2021) Triptolidenol, isolated from Tripterygium wilfordii, disrupted NF-κB/COX-2 pathway by targeting ATP-binding sites of IKKβ in clear cell renal cell carcinoma. Fitoterapia 148:104779. https://doi.org/10.1016/j.fitote.2020.104779
Jing S, Zou H, Wu Z, Ren L, Zhang T, Zhang J et al (2020) Cucurbitacins: bioactivities and synergistic effect with small-molecule drugs. J Funct Foods 72:104042. https://doi.org/10.1016/j.jff.2020.104042
Joo JH, Jetten AM (2010) Molecular mechanisms involved in farnesol-induced apoptosis. Cancer Lett 287(2):123–135. https://doi.org/10.1016/j.canlet.2009.05.015
Kamaraj C, Balasubramani G, Siva C, Raja M, Balasubramanian V, Raja RK et al (2017) Ag nanoparticles synthesized using β-caryophyllene isolated from Murraya koenigii: antimalarial (Plasmodium falciparum 3D7) and anticancer activity (A549 and HeLa cell lines). J Clust Sci 28(3):1667–1684. https://doi.org/10.1007/s10876-017-1180-6
Karahan F, Avsar C, Ozyigit II, Berber I (2016) Antimicrobial and antioxidant activities of medicinal plant Glycyrrhiza glabra var. glandulifera from different habitats. Biotechnol Biotechnol Equip 30(4):797–804. https://doi.org/10.1080/13102818.2016.1179590
Khader M, Eckl PM (2014) Thymoquinone: an emerging natural drug with a wide range of medical applications. Iran J Basic Med Sci 17(12):950–957
Khaleel C, Tabanca N, Buchbauer G (2018) α-Terpineol, a natural monoterpene: a review of its biological properties. Open Chem 16(1):349–361. https://doi.org/10.1515/chem-2018-0040
Khwaza V, Oyedeji OO, Aderibigbe BA (2018) Antiviral activities of oleanolic acid and its analogues. Molecules 23(9). https://doi.org/10.3390/molecules23092300
Kim S-H, Bae HC, Park E-J, Lee CR, Kim B-J, Lee S et al (2011) Geraniol inhibits prostate cancer growth by targeting cell cycle and apoptosis pathways. Biochem Biophys Res Commun 407(1):129–134. https://doi.org/10.1016/j.bbrc.2011.02.124
Kim DS, Lee HJ, Jeon YD, Han YH, Kee JY, Kim HJ et al (2015a) Alpha-pinene exhibits anti-inflammatory activity through the suppression of MAPKs and the NF-κB pathway in mouse peritoneal macrophages. Am J Chin Med 43(4):731–742. https://doi.org/10.1142/s0192415x15500457
Kim S, Lee H, Lee S, Yoon Y, Choi K-H (2015b) Antimicrobial action of oleanolic acid on Listeria monocytogenes, Enterococcus faecium, and Enterococcus faecalis. PLoS One 10(3):e0118800. https://doi.org/10.1371/journal.pone.0118800
Kim JH, Yi Y-S, Kim M-Y, Cho JY (2017) Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J Ginseng Res 41(4):435–443. https://doi.org/10.1016/j.jgr.2016.08.004
Kim WS, Shalit ZA, Nguyen SM, Schoepke E, Eastman A, Burris TP et al (2019) A synthesis strategy for tetracyclic terpenoids leads to agonists of ERβ. Nat Commun 10(1):2448. https://doi.org/10.1038/s41467-019-10415-6
Kitrytė V, Bagdonaitė D, Rimantas VP (2018) Biorefining of industrial hemp (Cannabis sativa L.) threshing residues into cannabinoid and antioxidant fractions by supercritical carbon dioxide, pressurized liquid and enzyme-assisted extractions. Food Chem 267:420–429. https://doi.org/10.1016/j.foodchem.2017.09.080
Krishna S, Bustamante L, Haynes RK, Staines HM (2008) Artemisinins: their growing importance in medicine. Trends Pharmacol Sci 29(10):520–527. https://doi.org/10.1016/j.tips.2008.07.004
Kumar A, Dubey RK, Kant K, Sasmal D, Ghosh M, Sharma N (2016) Determination of Deltamethrin in mice plasma and immune organs by simple reversed-phase HPLC. Acta Chromatogr 28(2):193–206. https://doi.org/10.1556/1326.2016.28.2.4
Kumar P, Bhadauria AS, Singh AK, Saha S (2018) Betulinic acid as apoptosis activator: molecular mechanisms, mathematical modeling and chemical modifications. Life Sci 209:24–33. https://doi.org/10.1016/j.lfs.2018.07.056
Lacaille-Dubois MA, Hanquet B, Cui ZH, Lou ZC, Wagner H (1997) Jennisseensosides C and D, biologically active acylated triterpene saponins from Silene jenisseensis. Phytochemistry 45(5):985–990. https://doi.org/10.1016/s0031-9422(97)00087-3
Lee CH, Kim J-H (2014) A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res 38(3):161–166. https://doi.org/10.1016/j.jgr.2014.03.001
Lei D, Qiu Z, Qiao J, Zhao G-R (2021) Plasticity engineering of plant monoterpene synthases and application for microbial production of monoterpenoids. Biotechnol Biofuels 14(1):147. https://doi.org/10.1186/s13068-021-01998-8
Li Y, Fabiano-Tixier A-S, Chemat F (2014) Essential oils: from conventional to green extraction. In: Li Y, Fabiano-Tixier A-S, Chemat F (eds) Essential oils as reagents in green chemistry. Springe, Cham, pp 9–20
Liu Z, Deng B, Li S, Zou Z (2018) Optimization of solvent-free microwave assisted extraction of essential oil from Cinnamomum camphora leaves. Ind Crop Prod 124:353–362. https://doi.org/10.1016/j.indcrop.2018.08.016
Liu M, Yan Q, Peng B, Cai Y, Zeng S, Xu Z et al (2021) Use of cucurbitacins for lung cancer research and therapy. Cancer Chemother Pharmacol 88(1):1–14. https://doi.org/10.1007/s00280-021-04265-7
López-Bascón MA, Luque de Castro MD (2020) Chapter 11 - Soxhlet extraction. In: Poole CF (ed) Liquid-phase extraction. Elsevier, New York, pp 327–354
López-García S, Castañeda-Sanchez JI, Jiménez-Arellanes A, Domínguez-López L, Castro-Mussot ME, Hernández-Sanchéz J et al (2015) Macrophage activation by ursolic and oleanolic acids during mycobacterial infection. Molecules 20(8):14348–14364
Louw S (2021) Recent trends in the chromatographic analysis of volatile flavor and fragrance compounds: annual review 2020. Anal Sci Adv 2(3-4):157–170. https://doi.org/10.1002/ansa.202000158
Lu XG, Zhan LB, Feng BA, Qu MY, Yu LH, Xie JH (2004) Inhibition of growth and metastasis of human gastric cancer implanted in nude mice by d-limonene. World J Gastroenterol 10(14):2140–2144. https://doi.org/10.3748/wjg.v10.i14.2140
Luna EC, Luna IS, Scotti L, Monteiro AFM, Scotti MT, de Moura RO et al (2019) Active essential oils and their components in use against neglected diseases and arboviruses. Oxidative Med Cell Longev 2019:6587150. https://doi.org/10.1155/2019/6587150
Ma EL, Li YC, Tsuneki H, Xiao JF, Xia MY, Wang MW et al (2008) Beta-eudesmol suppresses tumour growth through inhibition of tumour neovascularisation and tumour cell proliferation. J Asian Nat Prod Res 10(1-2):159–167. https://doi.org/10.1080/10286020701394332
Ma J, Xu H, Wu J, Qu C, Sun F, Xu S (2015) Linalool inhibits cigarette smoke-induced lung inflammation by inhibiting NF-κB activation. Int Immunopharmacol 29(2):708–713. https://doi.org/10.1016/j.intimp.2015.09.005
Maffei ME (2010) Sites of synthesis, biochemistry and functional role of plant volatiles. South Afr J Bot 76(4):612–631. https://doi.org/10.1016/j.sajb.2010.03.003
Majdalawieh AF, Fayyad MW, Nasrallah GK (2017) Anti-cancer properties and mechanisms of action of thymoquinone, the major active ingredient of Nigella sativa. Crit Rev Food Sci Nutr 57(18):3911–3928. https://doi.org/10.1080/10408398.2016.1277971
Marei GIK, Abdel Rasoul MA, Abdelgaleil SAM (2012) Comparative antifungal activities and biochemical effects of monoterpenes on plant pathogenic fungi. Pestic Biochem Physiol 103(1):56–61. https://doi.org/10.1016/j.pestbp.2012.03.004
Martins IM, Roberto BS, Blumberg JB, Chen CYO, Macedo GA (2016a) Enzymatic biotransformation of polyphenolics increases antioxidant activity of red and white grape pomace. Food Res Int 89:533–539. https://doi.org/10.1016/j.foodres.2016.09.009
Martins N, Ferreira ICFR, Henriques M, Silva S (2016b) In vitro anti-Candida activity of Glycyrrhiza glabra L. Ind Crop Prod 83:81–85. https://doi.org/10.1016/j.indcrop.2015.12.029
Masyita A, Mustika Sari R, Dwi Astuti A, Yasir B, Rahma Rumata N, Emran TB et al (2022) Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem X 13:100217. https://doi.org/10.1016/j.fochx.2022.100217
Mata-Gómez LC, Montañez JC, Méndez-Zavala A, Aguilar CN (2014) Biotechnological production of carotenoids by yeasts: an overview. Microb Cell Factories 13:12. https://doi.org/10.1186/1475-2859-13-12
Mathema VB, Koh YS, Thakuri BC, Sillanpää M (2012) Parthenolide, a sesquiterpene lactone, expresses multiple anti-cancer and anti-inflammatory activities. Inflammation 35(2):560–565. https://doi.org/10.1007/s10753-011-9346-0
Maurya AK, Singh M, Dubey V, Srivastava S, Luqman S, Bawankule DU (2014) α-(-)-bisabolol reduces pro-inflammatory cytokine production and ameliorates skin inflammation. Curr Pharm Biotechnol 15(2):173–181. https://doi.org/10.2174/1389201015666140528152946
Meira CS, Santos EDS, Santo RFE, Vasconcelos JF, Orge ID, Nonaka CKV et al (2019) Betulinic acid derivative BA5, attenuates inflammation and fibrosis in experimental chronic Chagas Disease cardiomyopathy by inducing IL-10 and M2 polarization. Front Immunol 10. https://doi.org/10.3389/fimmu.2019.01257
Mewalal R, Rai DK, Kainer D, Chen F, Külheim C, Peter GF et al (2017) Plant-derived terpenes: a feedstock for specialty biofuels. Trends Biotechnol 35(3):227–240. https://doi.org/10.1016/j.tibtech.2016.08.003
Miljanović A, Bielen A, Grbin D, Marijanović Z, Andlar M, Rezić T et al (2020) Effect of enzymatic, ultrasound, and reflux extraction pretreatments on the yield and chemical composition of essential oils. Molecules 25(20):4818
Miller JA, Lang JE, Ley M, Nagle R, Hsu CH, Thompson PA et al (2013) Human breast tissue disposition and bioactivity of limonene in women with early-stage breast cancer. Cancer Prev Res (Phila) 6(6):577–584. https://doi.org/10.1158/1940-6207.capr-12-0452
Mosquera MEG, Jiménez G, Tabernero V, Vinueza-Vaca J, García-Estrada C, Kosalková K et al (2021) Terpenes and terpenoids: building blocks to produce biopolymers. Sustain Chem 2(3):467–492
Mostofian B, Johnson QR, Smith JC, Cheng X (2020) Carotenoids promote lateral packing and condensation of lipid membranes. Phys Chem Chem Phys 22(21):12281–12293. https://doi.org/10.1039/D0CP01031F
Munekata PES, Alcántara C, Žugčić T, Abdelkebir R, Collado MC, García-Pérez JV et al (2020) Impact of ultrasound-assisted extraction and solvent composition on bioactive compounds and in vitro biological activities of thyme and rosemary. Food Res Int 134:109242. https://doi.org/10.1016/j.foodres.2020.109242
Navarro G, Morales P, Rodríguez-Cueto C, Fernández-Ruiz J, Jagerovic N, Franco R (2016) Targeting cannabinoid CB2 receptors in the central nervous system. Medicinal chemistry approaches with focus on neurodegenerative disorders. Front Neurosci 10. https://doi.org/10.3389/fnins.2016.00406
Nejia H, Severine C, Jalloul B, Mehrez R, Stephane CJ (2013) Extraction of essential oil from Cupressus sempervirens: comparison of global yields, chemical composition and antioxidant activity obtained by hydrodistillation and supercritical extraction. Nat Prod Res 27(19):1795–1799. https://doi.org/10.1080/14786419.2012.755680
Nugroho AE, Lindawati NY, Herlyanti K, Widyastuti L, Pramono S (2013) Anti-diabetic effect of a combination of andrographolide-enriched extract of Andrographis paniculata (Burm f.) Nees and asiaticoside-enriched extract of Centella asiatica L. in high fructose-fat fed rats. Indian J Exp Biol 51(12):1101–1108
Oktavianawati I, Letisya N, Citra P, Utari DP, Winata INA, Handayani W et al (2019) Essential oil composition of rose flowers from Karangpring Village Jember district extracted by distillation and enfleurage. Jurnal ILMU DASAR 2. https://doi.org/10.19184/jid.v20i2.8995
Ozturk B, Esteban J, Gonzalez-Miquel M (2018) Deterpenation of citrus essential oils using glycerol-based deep eutectic solvents. J Chem Eng Data 63(7):2384–2393. https://doi.org/10.1021/acs.jced.7b00944
Paduch R, Kandefer-Szerszeń M, Trytek M, Fiedurek J (2007) Terpenes: substances useful in human healthcare. Arch Immunol Ther Exp 55(5):315. https://doi.org/10.1007/s00005-007-0039-1
Paibon W, Yimnoi CA, Tembab N, Boonlue W, Jampachaisri K, Nuengchamnong N et al (2011) Comparison and evaluation of volatile oils from three different extraction methods for some Thai fragrant flowers. Int J Cosmet Sci 33(2):150–156. https://doi.org/10.1111/j.1468-2494.2010.00603.x
Palanimuthu D, Baskaran N, Silvan S, Rajasekaran D, Manoharan S (2012) Lupeol, a bioactive triterpene, prevents tumor formation during 7,12-dimethylbenz(a)anthracene induced oral carcinogenesis. Pathol Oncol Res POR 18(4):1029–1037. https://doi.org/10.1007/s12253-012-9541-9
Paleologos EK, Giokas DL, Karayannis MI (2005) Micelle-mediated separation and cloud-point extraction. TrAC Trends Anal Chem 24(5):426–436. https://doi.org/10.1016/j.trac.2005.01.013
Palmieri S, Pellegrini M, Ricci A, Compagnone D, Lo SC (2020) Chemical composition and antioxidant activity of Thyme, Hemp and coriander extracts: a comparison study of Maceration, Soxhlet, UAE and RSLDE techniques. Foods 9(9):1221
Pan W, Zhang G (2019) Linalool monoterpene exerts potent antitumor effects in OECM 1 human oral cancer cells by inducing sub-G1 cell cycle arrest, loss of mitochondrial membrane potential and inhibition of PI3K/AKT biochemical pathway. J BUON 24(1):323–328
Pang Z, Chen J, Wang T, Gao C, Li Z, Guo L et al (2021) Linking plant secondary metabolites and plant microbiomes: a review. Front Plant Sci 12. https://doi.org/10.3389/fpls.2021.621276
Papada E, Gioxari A, Brieudes V, Amerikanou C, Halabalaki M, Skaltsounis AL et al (2018) Bioavailability of terpenes and postprandial effect on human antioxidant potential. An open-label study in healthy subjects. Mol Nutr Food Res 62(3):1700751. https://doi.org/10.1002/mnfr.201700751
Park SN, Lim YK, Choi MH, Cho E, Bang IS, Kim JM et al (2018) Antimicrobial mechanism of oleanolic and ursolic acids on Streptococcus mutans UA159. Curr Microbiol 75(1):11–19. https://doi.org/10.1007/s00284-017-1344-5
Pasquini D, Detti C, Ferrini F, Brunetti C, Gori A (2021) Polyphenols and terpenes in mediterranean plants: an overview of their roles and possible applications. Italus Hortus 28(1):3–31. https://doi.org/10.26353/j.itahort/2021.1.0331
Pateiro M, Barba FJ, Domínguez R, Sant'Ana AS, Mousavi Khaneghah A, Gavahian M et al (2018) Essential oils as natural additives to prevent oxidation reactions in meat and meat products: a review. Food Res Int 113:156–166. https://doi.org/10.1016/j.foodres.2018.07.014
Patel PB, Thakkar VR (2014) L-carvone induces p53, caspase 3 mediated apoptosis and inhibits the migration of breast cancer cell lines. Nutr Cancer 66(3):453–462. https://doi.org/10.1080/01635581.2014.884230
Pei Y, Du Q, Liao P-Y, Chen Z-P, Wang D, Yang C-R et al (2011) Notoginsenoside ST-4 inhibits virus penetration of herpes simplex virus in vitro. J Asian Nat Prod Res 13(06):498–504. https://doi.org/10.1080/10286020.2011.571645
Petrović J, Stojković D, Soković M (2019) Chapter 8 - Terpene core in selected aromatic and edible plants: natural health improving agents. In: Ferreira ICFR, Barros L (eds) Advances in food and nutrition research. Academic Press, New York, pp 423–451
Pichersky E, Raguso RA (2018) Why do plants produce so many terpenoid compounds? New Phytol 220(3):692–702. https://doi.org/10.1111/nph.14178
Picot-Allain C, Mahomoodally MF, Ak G, Zengin G (2021) Conventional versus green extraction techniques—a comparative perspective. Curr Opin Food Sci 40:144–156. https://doi.org/10.1016/j.cofs.2021.02.009
Pina LTS, Serafini MR, Oliveira MA, Sampaio LA, Guimarães JO, Guimarães AG (2022) Carvone and its pharmacological activities: a systematic review. Phytochemistry 196:113080. https://doi.org/10.1016/j.phytochem.2021.113080
Poehland BL, Carté BK, Francis TA, Hyland LJ, Allaudeen HS, Troupe N (1987) In vitro antiviral activity of dammar resin triterpenoids. J Nat Prod 50(4):706–713. https://doi.org/10.1021/np50052a022
Poucher WA (1993) The production of natural perfumes. In: Perfumes, cosmetics and soaps. Springer, Dordrecht
Prados-Rosales RC, Herrera MC, Luque-Garcia JL, Luque de Castro MD (2002) Study of the feasibility of focused microwave-assisted Soxhlet extraction of N-methylcarbamates from soil. J Chromatogr A 953(1):133–140. https://doi.org/10.1016/S0021-9673(02)00118-8
Priyanka KS (2018) Influence of operating parameters on supercritical fluid extraction of essential oil from turmeric root. J Clean Prod 188:816–824. https://doi.org/10.1016/j.jclepro.2018.04.052
Putnik P, Bursac Kovacevic D, Rezek Jambrak A, Barba FJ, Cravotto G, Binello A et al (2017) Innovative “green” and novel strategies for the extraction of bioactive added value compounds from citrus wastes: a review. Molecules 22(5). https://doi.org/10.3390/molecules22050680
Qian W, Zhang J, Wang W, Wang T, Liu M, Yang M et al (2020) Antimicrobial and antibiofilm activities of paeoniflorin against carbapenem-resistant Klebsiella pneumoniae. J Appl Microbiol 128(2):401–413. https://doi.org/10.1111/jam.14480
Rabi T, Bishayee A (2009) d-Limonene sensitizes docetaxel-induced cytotoxicity in human prostate cancer cells: generation of reactive oxygen species and induction of apoptosis. J Carcinog 8:9. https://doi.org/10.4103/1477-3163.51368
Ragab TIM, El Gendy ANG, Saleh IA, Esawy MA (2019) Chemical composition and evaluation of antimicrobial activity of the origanum majorana essential oil extracted by microwave-assisted extraction, conventional hydro-distillation and steam distillation. J Essent Oil Bear Plants 22(2):563–573. https://doi.org/10.1080/0972060X.2019.1611486
Rashad NM, Abdelsamad MAE, Amer AM, Sitohy MZ, Mousa MM (2019) The impact of stevioside supplementation on glycemic control and lipid profile in patients with type 2 diabetes: a controlled clinical trial. Egypt J Internal Med 31(1):22–30. https://doi.org/10.4103/ejim.ejim_68_18
Raut JS, Karuppayil SM (2014) A status review on the medicinal properties of essential oils. Ind Crop Prod 62:250–264. https://doi.org/10.1016/j.indcrop.2014.05.055
Reis SL, Mantello AG, Macedo JM, Gelfuso EA, da Silva CP, Fachin AL et al (2016) Typical monoterpenes as insecticides and repellents against stored grain pests. Molecules (Basel, Switzerland) 21(3):258. https://doi.org/10.3390/molecules21030258
Reverchon E, Taddeo R, Porta GD (1995) Extraction of sage oil by supercritical CO2: influence of some process parameters. J Supercrit Fluids 8(4):302–309. https://doi.org/10.1016/0896-8446(95)90005-5
Rocchetti G, Blasi F, Montesano D, Ghisoni S, Marcotullio MC, Sabatini S et al (2019) Impact of conventional/non-conventional extraction methods on the untargeted phenolic profile of Moringa oleifera leaves. Food Res Int 115:319–327. https://doi.org/10.1016/j.foodres.2018.11.046
Rodrigues Goulart H, Kimura EA, Peres VJ, Couto AS, Aquino Duarte FA, Katzin AM (2004) Terpenes arrest parasite development and inhibit biosynthesis of isoprenoids in Plasmodium falciparum. Antimicrob Agents Chemother 48(7):2502–2509. https://doi.org/10.1128/AAC.48.7.2502-2509.2004
Rogerio AP, Andrade EL, Leite DFP, Figueiredo CP, Calixto JB (2009) Preventive and therapeutic anti-inflammatory properties of the sesquiterpene alpha-humulene in experimental airways allergic inflammation. Br J Pharmacol 158(4):1074–1087. https://doi.org/10.1111/j.1476-5381.2009.00177.x
Roopashree K, Naik D (2019) Advanced method of secondary metabolite extraction and quality analysis. J Pharmacogn Phytochem 8(3):1829–1842
Rubulotta G, Quadrelli EA (2019) Chapter 11 - Terpenes: a valuable family of compounds for the production of fine chemicals. In: Albonetti S, Perathoner S, Quadrelli EA (eds) Studies in surface science and catalysis. Elsevier, New York, pp 215–229
Salehi B, Staniak M, Czopek K, Stępień A, Dua K, Wadhwa R et al (2019) The therapeutic potential of the Labdane Diterpenoid Forskolin. Appl Sci 9(19):4089
Salha GB, Abderrabba M, Labidi J (2021) A status review of terpenes and their separation methods. Rev Chem Eng 37(3):433–447. https://doi.org/10.1515/revce-2018-0066
Sampaio LA, Pina LTS, Serafini MR, Tavares DDS, Guimarães AG (2021) Antitumor effects of carvacrol and thymol: a systematic review. Front Pharmacol 12:702487. https://doi.org/10.3389/fphar.2021.702487
Santos KA, Gonçalves JE, Cardozo-Filho L, da Silva EA (2019) Pressurized liquid and ultrasound-assisted extraction of α-bisabolol from candeia (Eremanthus erythropappus) wood. Ind Crop Prod 130:428–435. https://doi.org/10.1016/j.indcrop.2019.01.013
Sarfarazi M, Jafari SM, Rajabzadeh G, Galanakis CM (2020) Evaluation of microwave-assisted extraction technology for separation of bioactive components of saffron (Crocus sativus L.). Ind Crop Prod 145:111978. https://doi.org/10.1016/j.indcrop.2019.111978
Sarhene M, Ni JY, Duncan ES, Liu Z, Li S, Zhang J et al (2021) Ginsenosides for cardiovascular diseases; update on pre-clinical and clinical evidence, pharmacological effects and the mechanisms of action. Pharmacol Res 166:105481. https://doi.org/10.1016/j.phrs.2021.105481
Schieber A, Wüst M (2020) Volatile phenols-important contributors to the aroma of plant-derived foods. Molecules 25(19):4529. https://doi.org/10.3390/molecules25194529
Scown CD, Baral NR, Yang M, Vora N, Huntington T (2021) Technoeconomic analysis for biofuels and bioproducts. Curr Opin Biotechnol 67:58–64. https://doi.org/10.1016/j.copbio.2021.01.002
Seki T, Kokuryo T, Yokoyama Y, Suzuki H, Itatsu K, Nakagawa A et al (2011) Antitumor effects of α-bisabolol against pancreatic cancer. Cancer Sci 102(12):2199–2205. https://doi.org/10.1111/j.1349-7006.2011.02082.x
Shang Q, Xu H, Huang L (2012) Tanshinone IIA: a promising natural cardioprotective agent. Evid Based Complement Alternat Med 2012:716459. https://doi.org/10.1155/2012/716459
Shao Y, Ho CT, Chin CK, Badmaev V, Ma W, Huang MT (1998) Inhibitory activity of boswellic acids from Boswellia serrata against human leukemia HL-60 cells in culture. Planta Med 64(4):328–331. https://doi.org/10.1055/s-2006-957444
Shao J-W, Jiang J-L, Zou J-J, Yang M-Y, Chen F-M, Zhang Y-J et al (2020) Therapeutic potential of ginsenosides on diabetes: from hypoglycemic mechanism to clinical trials. J Funct Foods 64:103630. https://doi.org/10.1016/j.jff.2019.103630
Sharma C, Sadek B, Goyal SN, Sinha S, Kamal MA, Ojha S (2015) Small molecules from nature targeting G-protein coupled cannabinoid receptors: potential leads for drug discovery and development. Evid Based Complement Alternat Med 2015:238482. https://doi.org/10.1155/2015/238482
Shi W, Gould MN (2002) Induction of cytostasis in mammary carcinoma cells treated with the anticancer agent perillyl alcohol. Carcinogenesis 23(1):131–142. https://doi.org/10.1093/carcin/23.1.131
Singh B, Sharma RA (2015) Plant terpenes: defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech 5(2):129–151. https://doi.org/10.1007/s13205-014-0220-2
Siqueira HDS, Neto BS, Sousa DP, Gomes BS, da Silva FV, Cunha FVM et al (2016) α-Phellandrene, a cyclic monoterpene, attenuates inflammatory response through neutrophil migration inhibition and mast cell degranulation. Life Sci 160:27–33. https://doi.org/10.1016/j.lfs.2016.07.008
Small EW (1992) Method of moments and treatment of nonrandom error. Methods Enzymol 210:237–279. https://doi.org/10.1016/0076-6879(92)10013-4
Srijiwangsa P, Ponnikorn S, Na-Bangchang K (2018) Effect of β-Eudesmol on NQO1 suppression-enhanced sensitivity of cholangiocarcinoma cells to chemotherapeutic agents. BMC Pharmacol Toxicol 19(1):32. https://doi.org/10.1186/s40360-018-0223-4
Srinivasan K (2017) Ginger rhizomes (Zingiber officinale): a spice with multiple health beneficial potentials. PharmaNutrition 5(1):18–28. https://doi.org/10.1016/j.phanu.2017.01.001
Subramanian R, Subbramaniyan P, Noorul Ameen J, Raj V (2016) Double bypasses soxhlet apparatus for extraction of piperine from Piper nigrum. Arab J Chem 9:S537–SS40. https://doi.org/10.1016/j.arabjc.2011.06.022
Surendran S, Qassadi F, Surendran G, Lilley D, Heinrich M (2021) Myrcene—what are the potential health benefits of this flavouring and aroma agent? Front Nutr 8. https://doi.org/10.3389/fnut.2021.699666
Takahashi M, Sung B, Shen Y, Hur K, Link A, Boland CR et al (2012) Boswellic acid exerts antitumor effects in colorectal cancer cells by modulating expression of the let-7 and miR-200 microRNA family. Carcinogenesis 33(12):2441–2449. https://doi.org/10.1093/carcin/bgs286
Tang R, Wen Q, Li M, Zhang W, Wang Z, Yang J (2021) Recent advances in the biosynthesis of farnesene using metabolic engineering. J Agric Food Chem 69(51):15468–15483. https://doi.org/10.1021/acs.jafc.1c06022
Tavakolpour Y, Moosavi-Nasab M, Niakousari M, Haghighi-Manesh S, Hashemi SMB, Mousavi KA (2017) Comparison of four extraction methods for essential oil from Thymus daenensis Subsp. Lancifolius and chemical analysis of extracted essential oil. J Food Process Preserv 41(4):e13046. https://doi.org/10.1111/jfpp.13046
Tetali SD (2019) Terpenes and isoprenoids: a wealth of compounds for global use. Planta 249(1):1–8. https://doi.org/10.1007/s00425-018-3056-x
Tirapelli CR, Ambrosio SR, Coutinho ST, de Oliveira DC, da Costa FB, de Oliveira AM (2005) Pharmacological comparison of the vasorelaxant action displayed by kaurenoic acid and pimaradienoic acid. J Pharm Pharmacol 57(8):997–1004. https://doi.org/10.1211/0022357056578
Tirgarian B, Farmani J, Milani JM (2019) Enzyme-assisted aqueous extraction of oil and protein hydrolysate from sesame seed. J Food Meas Charac 13(3):2118–2129. https://doi.org/10.1007/s11694-019-00132-5
Tomko AM, Whynot EG, Ellis LD, Dupré DJ (2020) Anti-cancer potential of cannabinoids, terpenes, and flavonoids present in cannabis. Cancers (Basel) 12(7):1985. https://doi.org/10.3390/cancers12071985
Tongnuanchan P, Benjakul S (2014) Essential oils: extraction, bioactivities, and their uses for food preservation. J Food Sci 79(7):R1231–R1249. https://doi.org/10.1111/1750-3841.12492
Torres-Fajardo R, Higuera-Piedrahita R (2021) Actividad antihelmíntica in vivo de terpenos y aceites esenciales en pequeños rumiantes. Revista MVZ Córdoba 26(3). https://doi.org/10.21897/rmvz.2317
Turkez H, Sozio P, Geyikoglu F, Tatar A, Hacimuftuoglu A, Di Stefano A (2014) Neuroprotective effects of farnesene against hydrogen peroxide-induced neurotoxicity in vitro. Cell Mol Neurobiol 34(1):101–111. https://doi.org/10.1007/s10571-013-9991-y
Turrini F, Beruto M, Mela L, Curir P, Triglia G, Boggia R et al (2021) Ultrasound-assisted extraction of Lavender (Lavandula angustifolia Miller, Cultivar Rosa) solid by-products remaining after the distillation of the essential oil. Appl Sci 11(12):5495
Uwineza PA, Waśkiewicz A (2020) Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules 25(17):3847
Vigushin DM, Poon GK, Boddy A, English J, Halbert GW, Pagonis C et al (1998) Phase I and pharmacokinetic study of D-limonene in patients with advanced cancer. Cancer Research Campaign Phase I/II Clinical Trials Committee. Cancer Chemother Pharmacol 42(2):111–117. https://doi.org/10.1007/s002800050793
Villalva M, Santoyo S, Salas-Pérez L, MdlN S-S, Rodríguez García-Risco M, Fornari T et al (2021) Sustainable extraction techniques for obtaining antioxidant and anti-inflammatory compounds from the lamiaceae and asteraceae species. Foods 10(9):2067
Vinatoru M, Mason TJ, Calinescu I (2017) Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal Chem 97:159–178. https://doi.org/10.1016/j.trac.2017.09.002
Viveros-Paredes JM, González-Castañeda RE, Gertsch J, Chaparro-Huerta V, López-Roa RI, Vázquez-Valls E et al (2017) Neuroprotective effects of β-Caryophyllene against Dopaminergic neuron injury in a Murine model of Parkinson’s disease induced by MPTP. Pharmaceuticals (Basel) 10(3):60. https://doi.org/10.3390/ph10030060
Wagner K-H, Elmadfa I (2003) Biological relevance of terpenoids overview focusing on mono-, di- and tetraterpenes. Ann Nutr Metab 47(3):95–106
Wang G, Tang W, Bidigare RR (2005) Terpenoids as therapeutic drugs and pharmaceutical agents. In: Zhang L, Demain AL (eds) Natural products: drug discovery and therapeutic medicine. Humana Press, Totowa, NJ, pp 197–227
Wang Q, Sohrabi R, Tholl D (2014) Analysis of diterpenes and triterpenes from plant foliage and roots. Methods Mol Biol 1153:149–159. https://doi.org/10.1007/978-1-4939-0606-2_10
Wang L, Yang R, Yuan B, Liu Y, Liu C (2015) The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm Sin B 5(4):310–315. https://doi.org/10.1016/j.apsb.2015.05.005
Wang B, Ge Z, Cheng Z, Zhao Z (2017) Tanshinone IIA suppresses the progression of atherosclerosis by inhibiting the apoptosis of vascular smooth muscle cells and the proliferation and migration of macrophages induced by ox-LDL. Biol Open 6(4):489–495. https://doi.org/10.1242/bio.024133
Wang Q, Quan S, Xiao H (2019a) Towards efficient terpenoid biosynthesis: manipulating IPP and DMAPP supply. Bioresour Bioprocess 6(1):6. https://doi.org/10.1186/s40643-019-0242-z
Wang C-Y, Chen Y-W, Hou C-Y (2019b) Antioxidant and antibacterial activity of seven predominant terpenoids. Int J Food Prop 22(1):230–238. https://doi.org/10.1080/10942912.2019.1582541
Wang K, Jiang S, Pu T, Fan L, Su F, Ye M (2019c) Antifungal activity of phenolic monoterpenes and structure-related compounds against plant pathogenic fungi. Nat Prod Res 33(10):1423–1430. https://doi.org/10.1080/14786419.2017.1419232
War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S et al (2012) Mechanisms of plant defense against insect herbivores. Plant Signal Behav 7(10):1306–1320. https://doi.org/10.4161/psb.21663
Wen C, Zhang J, Zhang H, Dzah CS, Zandile M, Duan Y et al (2018) Advances in ultrasound assisted extraction of bioactive compounds from cash crops – a review. Ultrason Sonochem 48:538–549. https://doi.org/10.1016/j.ultsonch.2018.07.018
Wintachai P, Kaur P, Lee RC, Ramphan S, Kuadkitkan A, Wikan N et al (2015) Activity of andrographolide against chikungunya virus infection. Sci Rep 5:14179. https://doi.org/10.1038/srep14179
Xiu-Bin S, Shao-Mei W, Tao L, Yong-qing Y (2015) Anticancer activity of linalool terpenoid: apoptosis induction and cell cycle arrest in prostate cancer cells. Trop J Pharm Res 14(4):619–625. https://doi.org/10.4314/tjpr.v14i4.9
Xu C, Wang B, Pu Y, Tao J, Zhang T (2018a) Techniques for the analysis of pentacyclic triterpenoids in medicinal plants. J Sep Sci 41(1):6–19. https://doi.org/10.1002/jssc.201700201
Xu F, Huang X, Wu H, Wang X (2018b) Beneficial health effects of lupenone triterpene: a review. Biomed Pharmacother 103:198–203. https://doi.org/10.1016/j.biopha.2018.04.019
Xu F, Wang X, Wei X, Chen T, Wu H (2021) Explore the active ingredients and mechanisms in Musa basjoo pseudostem juice against diabetes based on animal experiment, gas chromatography-mass spectrometer and network pharmacology. Comb Chem High Throughput Screen. https://doi.org/10.2174/1386207324666210827112233
Yáñez-Serrano AM, Fasbender L, Kreuzwieser J, Dubbert D, Haberstroh S, Lobo-do-Vale R et al (2018) Volatile diterpene emission by two Mediterranean Cistaceae shrubs. Sci Rep 8(1):6855. https://doi.org/10.1038/s41598-018-25056-w
Yang W, Chen X, Li Y, Guo S, Wang Z, Yu X (2020) Advances in pharmacological activities of terpenoids. Nat Prod Commun 15(3). https://doi.org/10.1177/1934578x20903555
Yared JA, Tkaczuk KH (2012) Update on taxane development: new analogs and new formulations. Drug Des Dev Therapy 6:371–384. https://doi.org/10.2147/dddt.s28997
Zengin H, Baysal AH (2014) Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 19(11):17773–17798. https://doi.org/10.3390/molecules191117773
Zhang Z, Chen X, Chen H, Wang L, Liang J, Luo D et al (2016a) Anti-inflammatory activity of β-patchoulene isolated from patchouli oil in mice. Eur J Pharmacol 781:229–238. https://doi.org/10.1016/j.ejphar.2016.04.028
Zhang RX, Li Y, Tian DD, Liu Y, Nian W, Zou X et al (2016b) Ursolic acid inhibits proliferation and induces apoptosis by inactivating Wnt/β-catenin signaling in human osteosarcoma cells. Int J Oncol 49(5):1973–1982. https://doi.org/10.3892/ijo.2016.3701
Zhang J-P, Qin Y-G, Dong Y-W, Song D-L, Duan H-X, Yang X-L (2017) Synthesis and biological activities of (E)-β-farnesene analogues containing 1,2,3-thiadiazole. Chin Chem Lett 28(2):372–376. https://doi.org/10.1016/j.cclet.2016.10.030
Zhang L, Bao M, Liu B, Zhao H, Zhang Y, Ji X et al (2020) Effect of andrographolide and its analogs on bacterial infection: a review. Pharmacology 105(3–4):123–134. https://doi.org/10.1159/000503410
Zhang X, Zhong W, Ma X, Zhang X, Chen H, Wang Z et al (2021) Ginkgolide with intravenous alteplase thrombolysis in acute ischemic stroke improving neurological function: a multicenter, cluster-randomized trial (GIANT). Front Pharmacol 12. https://doi.org/10.3389/fphar.2021.792136
Zhu L, Chen L (2019) Progress in research on paclitaxel and tumor immunotherapy. Cell Mol Biol Lett 24(1):40. https://doi.org/10.1186/s11658-019-0164-y
Zhu Y, Shao CY, Lv HP, Zhang Y, Dai WD, Guo L et al (2017) Enantiomeric and quantitative analysis of volatile terpenoids in different teas (Camellia sinensis). J Chromatogr A 1490:177–190. https://doi.org/10.1016/j.chroma.2017.02.013
Zhu BJ, Qian ZQ, Yang HR, Li RX (2021) Tripterine: a potential anti-allergic compound. Curr Pharm Biotechnol 22(1):159–167. https://doi.org/10.2174/1389201021666200327163322
Zielińska-Błajet M, Feder-Kubis J (2020) Monoterpenes and their derivatives-recent development in biological and medical applications. Int J Mol Sci 21(19). https://doi.org/10.3390/ijms21197078
Acknowledgments
The authors are grateful to the Foundation for Science and Technology (FCT, Portugal) for financial support through national funds FCT/MCTES to CIMO (UIDB/00690/2020). L. Barros thank the national funding by FCT through the institutional scientific employment programme-contract for their contract, while S. A. Heleno thank FCT through the individual scientific employment programme-contracts (CEECIND/03040/2017). R. Sprea also thank to FCT for his Ph.D. grant (2020.08092.BD).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Xavier, V. et al. (2023). Terpenes. In: Carocho, M., Heleno, S.A., Barros, L. (eds) Natural Secondary Metabolites. Springer, Cham. https://doi.org/10.1007/978-3-031-18587-8_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-18587-8_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-18586-1
Online ISBN: 978-3-031-18587-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)