Abstract
Fusarium infections result in reduced maize grain (Zea mays L.) yields and notable impacts on human and animal health. Research involving natural products to control fungi in food is a promising alternative. Combinations of α-bisabolol (AB) and sodium chloride (NaCl) may suggest the use of lower effective concentrations of the drugs. This study aimed to evaluate the antifungal potential of AB associated with NaCl against Fusarium oxysporum strains isolated from maize. Minimum inhibitory concentrations (MICs) values of AB and NaCl were determined by microdilution, and an association study was performed (checkerboard). Effects on fungal mycelial growth (poisoned substrate technique) and a maize grain contamination model were analyzed. AB presented MIC values ranging from 128 and 1024 μg/mL; NaCl inhibited fungal growth at 16,384 μg/mL. The AB/NaCl association study revealed synergism by decreasing inhibitory concentrations by eight times. In corn kernels, AB and NaCl, whether isolated (at MIC) or in association (at sub-inhibitory concentrations), significantly inhibited in vitro mycelial growth (P < 0.05). Further analysis of liquid from a canned maize sample also revealed the fungistatic effects of the compounds associations (P < 0.05). The results confirm the antifungal potential of AB, whether isolated or in association with NaCl to control F. oxysporum in maize.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maize (Zea mays L.) is the largest agricultural crop in the world. In Brazil, maize is one of the most important crops, with the country being among the main producers and consumers in the world. With its good nutritional composition (starch, oil, balanced proteins, and carotenoids), this cereal plays an important role in human and animal health [1]. Maize, configured as a food with economic and cultural relevance, is present in many traditional culinary preparations in Brazil, especially in the Northeast [2].
A great number of fungi attack and interfere in maize grain development, and research emphasizing the microbiological safety of this cereal is thus relevant [3]. Fusarium oxysporum brings more than 100 host-specific strains, many of which have worldwide distributions [4]. A major concern in maize production is rot, caused by Fusarium spp., which results in yield losses, reduction of seed quality and the accumulation of mycotoxins in the harvested grains [5]. The most common Fusarium mycotoxin groups are trichothecenes, zearalenone, and fumonisins; for often being detected in maize, and owing to their toxicity and carcinogenicity in humans and animals, they have received wide attention [6].
The use of salts, such as sodium chloride (NaCl), for microbial control has been practiced for years. NaCl reduces water activity in food, and (through osmotic stress and ion toxicity) inhibits fungal growth [7]. However, high salt or sodium intake is associated with elevated blood pressure. Meta-analysis has revealed the magnitude of blood pressure reduction when lowering sodium intake and a dose–response relationship in hypertensive older populations [8]. Reducing salt intake is a worthy objective for advancing public health worldwide [7]. In fact, reports on microbial tolerance or adaptation, suggest that reducing sodium in food can also effect food safety [9].
To avoid problems related to high sodium intake when fighting fungal contamination, one alternative is to use natural vegetable products, since they are environmentally safe and easily biodegradable. In addition, they can increase the antifungal effectiveness of conventional products like salts [10]. User concern with other preservatives has resulted in increased use of natural antimicrobials such as essential oils and their compounds [11]. Plant-based compounds such as essential oils and terpenes are well tested for their bio-efficacy in the management of wide range of fungal diseases in plants.
The monocyclic sesquiterpene alcohol α-bisabolol (AB) is found in a variety of aromatic plant essential oils: such as Matricaria chamomilla, Eremanthus erythropappus, Smyrniopsis aucheri, and Salvia runcinata [12]. A number of pharmacological and biological effects have been reported for AB, including antimicrobial activity [13]. Yet the scientific literature lacks information on the antifungal potential of AB against maize contaminating fungi such as F. oxysporum. Therefore, we have evaluated the protective potential of AB (whether isolated or combined with NaCl) on maize grain contamination by F. oxysporum.
Materials and Methods
Drugs
α-Bisabolol and NaCl were purchased from Sigma-Aldrich® (Brazil). Solutions were freshly prepared for the tests by dissolving AB first in dimethylsulfoxide (DMSO) and NaCl in sterilized distilled water to obtain a concentration of 1024 µg/mL. From this concentration, dilutions were performed to achieve a concentration of 1 µg/mL using RPMI 1640 medium (Sigma-Aldrich®, Brasil).
Fungal Inocula
The strains of F. oxysporum 73, 105, 132, 134, 136, 141 isolated from maize grains were adequately identified [14]. The fungal strains belong to the Colección de Cultivos de Microorganismos de la Universidad Nacional de Assunción (Paraguay). The fungi were grown in potato dextrose agar (Difco®) at 28 °C for 7 days. The fungal inocula were obtained in sterile saline (0.85% NaCl). The resulting mixture of conidia and hyphae fragments was transferred to sterile test tubes. After stirring for 15 s, each inoculum rested for 3 min and the supernatant was collected. Turbidity of the final inocula was adjusted to 5 × 106 CFU/mL, at a wavelength of 530 nm, and transmission adjusted to 68–70% in a UV-5100 Spectrophotometer [15].
Minimum Inhibitory Concentration (MIC)
MIC values of the drugs test were determined against Fusarium strains by microdilution technique using 96-well flat bottom micro-titer plates [15]. To each row of the plate was added 100 µL of the diluted test drugs in RPMI 1640 (Sigma-Aldrich®-Brazil). To each well of the plate was added 100 µL of a previously prepared inoculum diluted in RPMI 1640 at a ratio of 1:50. A fungal control was performed by replacing the test drug using sterile saline (growth control). A sterility control was also performed, using only the culture medium, without the inoculum. A control with DMSO (0.5%) was also performed. The plates were sealed and incubated at 28 °C for 7 days. MIC was the lowest concentration of drugs capable of inhibiting observed fungal growth in the wells.
Association Studies
The effect of the association of AB with NaCl was determined by the checkerboard technique, using 96 well flat bottom micro-titer plates. For this, different concentrations of the test drugs in RPMI 1640 were used (8 × MIC, 4 × MIC, 2 × MIC, 1 × MIC, 1/2 MIC, 1/4 MIC, 1/8 MIC). An aliquot of 50 μL of NaCl was then added to the wells of the plate in a vertical sense, and then 50 μL of a specific AB dilution was added in the horizontal direction of the plate. Finally, 100 μL of inoculum was added. The plates were sealed and incubated at 28 °C for 7 days for MIC readings. In the context of evaluating the activity of the drug associations, the fractional inhibitory concentration index (FICI) was calculated as the sum of: FICA + FICB, where A represents AB; and B represents NaCl. The FICA = (MICA combined)/(MICA alone), while the FICB = (MICB combined)/(MICB alone). The FICI was interpreted in the following way: synergism (<0.5), additivity (0.5–1), indifference (>1 and <4), or antagonism (>4) [16].
Effects on Mycelial Growth
The effects of the test drugs on the radial mycelial growth of F. oxysporum 134 were analysed using the poisoned substrate technique. Briefly, a 5 mm diameter was cut from the 7-day-old fungal culture grown on potato dextrose agar (Difco®) and placed onto the petri plates with 10 mL of Sabouraud dextrose agar and drugs-test as follows: AB (MIC), NaCl (MIC), AB (1/8 MIC) + NaCl (1/8 MIC), AB(1/4 MIC) + NaCl (1/4 MIC), AB (1/2 MIC) + NaCl (1/2 MIC). A control without drugs was performed. All the plates were incubated at 28 °C for 7 days. The diameter of the radial growth of the fungi was recorded daily up to 7 days [17].
Effects on Contamination in Maize Grains
Two groups of maize grains were used: grains for human consumption provided by the Department of Agriculture (Cuité, Brazil) and grains for animal consumption provided by the National Supply Company (Brazil). The healthy maize grains were dried at 40 °C for 48 h to reach approximately 14% moisture. Only grains that did not appear to be diseased were used. The grains (300 g) were packed into Erlenmeyers and autoclaved for 15 min at 121 °C [18]. Initially, the autoclaved grains were immersed for 1 min in 200 μL of the fungal inoculum (106 CFU/mL) of F. oxysporum 134, and then ten grains were transferred to sterile tubes contained drugs-test as follows: AB (8 × MIC, 4 × MIC, 2 × MIC, 1 × MIC, 1/2 MIC, 1/4 MIC, 1/8 MIC), NaCl (8 × MIC, 4 × MIC, 2 × MIC, 1 × MIC, 1/2 MIC, 1/4 MIC, 1/8 MIC), and associations of AB and NaCl (MIC + MIC, 1/2 MIC + 1/2 MIC, 1/4 MIC + 1/4 MIC, 1/8 MIC + 1/8 MIC). In the control groups, sterile distilled water was added. The solutions covered all the grains in tubes during the experiment (canning liquid). Finally, the tubes were then incubated at 28 °C for 7 days and visual analysis was performed. After that, we determined the incidence of contaminated maize grains (%). The contaminated grains showed mycelial growth on the surface. After this step, the canning liquid was analyzed in order to verify if the fungal structures present were viable. For this, 10 µL of the canning liquid from each tube was transferred onto the petri plates with 10 mL of Sabouraud dextrose agar and incubated at 28 °C for 7 days for analysis of fungal growth (CFU/mL) [19].
Statistical Analysis
All assays were performed in triplicate. MIC values obtained were the same in the replicates, then the results were expressed in modal values. The results of mycelial growth, contamination in maize grains and canned liquid tests were expressed as mean ± standard deviation (SD). Statistical evaluation of the results was done using the unpaired t test to determine significant differences, with a value of P < 0.05.
Ethical Statement
We registered our research with the Genetic Heritage Management Council (SisGen, in Portuguese) under number A848F8C. Also, a patent was registered in Brazil (2018) from these results, with code BR 1020180743910.
Results
The MIC values for AB and NaCl against strains of F. oxysporum are presented in Table 1. Both compounds inhibited the growth of the tested strains. However, AB presented lower MIC values (ranging from 128 and 1024 μg/mL) than NaCl which inhibited fungal growth at 16,384 μg/mL. The control (absence of drugs) permitted the growth of all F. oxysporum strains tested, proving fungal viability. At the concentration tested (0.5%), DMSO did not prevent fungal growth.
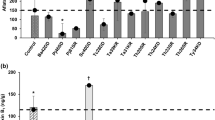
An association study for AB and NaCl was also performed for evaluating changes in their respective MIC values against F. oxysporum 134. The purpose was to verify the inhibitory efficacy of sub-MIC concentrations of AB and NaCl in combination. The results revealed an eightfold decrease in MIC values for both AB and NaCl. This behavior is characterized as synergism (FICI = 0.25). Considering that the drug combinations effectively inhibited fungal growth in sub-inhibitory concentrations, these same values were later used in mycelial growth tests with artificial maize grain contamination. The results of the mycelial growth assays are presented in Fig. 1. By the last analysis day (Day 5), AB, whether isolated (at its MIC) or in association with NaCl, (at sub-inhibitory concentrations) prevented mycelial growth as compared to the control (P < 0. 05). Preventing mycelial growth was not observed for NaCl alone (at its MIC) (P > 0.05).
Figure 2 presents the results for the inhibitory effects of the test drugs on maize grains artificially contaminated with F. oxysporum 134. The treatments with AB (1/8 MIC) + NaCl (1/8 MIC), and AB (1/4 MIC) + NaCl (1/4 MIC) were unable to inhibit F. oxysporum development (P > 0.05) (data not presented). However, we noted that AB (MIC) whether isolated or in association with NaCl (at MIC and 1/2 MIC) inhibited F. oxysporum 134 development in maize for human consumption when compared to the control (P < 0.05) (Fig. 2a). In maize for animal consumption (Fig. 2b), we observed that only the AB/NaCl associations (at MIC and 1/2 MIC) were effective compared to the control (P < 0.05).
The canning liquid was also analyzed for CFU/mL (Fig. 3). The results indicated that whether isolated or in association, both products significantly reduced viable fungal cell numbers as compared to the control (P < 0.05). It can thus be said that they presented a fungistatic effect.
Discussion
Ensuring microbiological safety in cereals such as maize is an essential issue in food science, and much needed for handling and storage. Synthetic fungicidal agents and salts have been used as chemical preservatives in foods [20]. In agricultural practices, fungicidal treatment against Fusarium brings great benefits to grain production. Synthetic fungicides are useful for controlling fungal diseases in food plants; azoles are the most effective group in preventing Fusarium spp. and reducing mycotoxin levels in maize grains [21]. However, prolonged use of fungicides can induce harmful effects in human health and increase environmental pollution [22].
NaCl is one of the most widely used additives in food processing. It has several functions, including impacts on physical and sensory properties. However, with the immense contribution of processed and canned products to daily diets, sodium intake can exceed recommended daily intake levels. A reformulation of food products to reduce salt intake by the population and address the associated chronic disease burden must be considered [23].
Against this background, reductions in salt over time have been accompanied in studies investigating substitutive strategies that neither interfere in salt's antimicrobial power nor compromise consumer acceptance concerning physical and sensory aspects [24]. A promising approach to control Fusarium spp. colonization and mycotoxin contamination in cereals is to use bioactive plant metabolites. Thus, essential oils and their components, which can allay users' concerns about synthetic preservatives have received increasing attention as natural antimicrobials [11].
In this context, our results showed the potential anti-Fusarium activity of AB alone and in combination with NaCl (Table 1, Fig. 1). Similar to Fusarium fungi, Aspergillus species are relevant fungal contaminants of maize grains. Depending on the storage conditions, these fungi might grow and subsequently produce mycotoxins, representing a health hazard to both animals and humans. Previously, AB inhibited the viability of A. flavus, A. fumigatus, A. niger, A. terreus, F. oxysporum, F. solani, and F. verticillioides conidia in fungicidal in vitro assay [25].
In this study, the in vitro antifungal potential of AB against strains of F. oxysporum, in much lower concentrations than those of NaCl was evidenced. Further, when we combined the drugs in an in vitro model, synergistic effect was observed (Checkerboard). The purpose of the AB/NaCl association was to analyze whether there would be an increase in the drugs' effects, with subsequent decreases in MIC values. Synergy is identified when two compounds increase each other's effectiveness by more than the sum of their single-agent responses [26]. In fact, we confirmed that the drugs, when they were tested in the associated form, presented inhibitory effects on the growth of F. oxysporum in lower concentrations (eight times lower), concerning their respective MICs. The results were evidenced in vitro modeling of mycelial growth and in tests with maize grains artificially contaminated with Fusarium (Figs. 2, 3).
Microbial survival at high salt concentrations is energetically expensive because the microorganism must balance its cytoplasm osmotic with its medium. Thus, membrane activity is essential to achieve osmotic balance [27]. It is plausible to believe that the association between AB and NaCl may be synergistic. The antifungal activity of essential oils and their terpene components reveals the plasma membrane as a principal cellular target. Essential oils and their constituents damage microorganism membranes, causing proton pump collapse and electron transport chain breakdown [28].
Advanced studies have reported possible mechanisms involved in the antifungal activity of AB in particular. AB interferes in the fungal cell membrane functions as a result of blocking ergosterol biosynthesis [29]. A previous study showed that AB inhibits A. fumigatus growth via affecting ∆24-sterol methyltransferase, a crucial enzyme in ergosterol biosynthetic pathway [13]. These findings demonstrate that AB can act as a fungal membrane chemosensitizer, damaging resistance mechanisms such as efflux pumps. For instance, AB potentiated the action of griseofulvin (antifungal drug, against strains of dermatophytes by checkerboard method [30].
Essential oils and their components in food matrices bring changes to a food's sensory properties. For this reason, to reduce overall concentrations, many studies are investigating intelligent combining of different compounds that might maintain their antimicrobial properties [31]. Other reports regarding the use of natural product associations with antifungal potential in food preservation are found in the literature that reveal both reductions in MIC values and effective modulation of microbial resistance [32, 33]. According to [34], a product's antifungal bioactivity is often lower in food matrices (even when in associations) than when measured in vitro. Specific food components such as fats, carbohydrates, proteins, salts, and even pH can reduce the antimicrobial effect. It is also known that even though terpenes are lipophilic, high concentrations of fats and proteins in foods can provide microorganisms with protection [31, 35].
Conclusions
Our results provide evidence that AB, whether isolated or associated with NaCl, is a potential alternative for F. oxysporum control in maize. Evaluating its synergistic effects in other food matrices to expand its applicability will also be necessary. Besides, since these are aromatic oil components, possible modulation of sensory aspects must also be considered and investigated. Finally, we conclude that AB-NaCl represents a promising strategy for pre- and post-harvest management of maize to minimize fungal contamination, contributing to food security and food production systems.
References
Augusto De Miranda R, Martinho A, Lício A (2014) Diagnóstico dos Problemas e Potencialidades da Cadeia Produtiva do Milho no Brasil. Embrapa Milho e Sorgo, Sete Lagoas
Correa AAS, Quinzani SSP, Peixoto DV, Silveira Corrêa TC (2016) A tradição do milho: o ingrediente base da cozinha caipira e das festas juninas. Ágora 18:99. https://doi.org/10.17058/agora.v18i1.6917
Lanubile A, Maschietto V, Borrelli VM et al (2017) Molecular basis of resistance to fusarium ear rot in maize. Front Plant Sci 8:1774. https://doi.org/10.3389/fpls.2017.01774
Gordon TR (2017) Fusarium oxysporum and the Fusarium Wilt Syndrome. Annu Rev Phytopathol 55:23–39. https://doi.org/10.1146/annurev-phyto-080615-095919
Qin PW, Xu J, Jiang Y et al (2020) Survey for toxigenic Fusarium species on maize kernels in China. World Mycotoxin J 13:213–224. https://doi.org/10.3920/wmj2019.2516
Hassan ZU, Al Thani R, Balmas V et al (2019) Prevalence of Fusarium fungi and their toxins in marketed feed. Food Control 104:224–230. https://doi.org/10.1016/j.foodcont.2019.04.045
Blesa E, Aliño M, Barat JM et al (2008) Microbiology and physico-chemical changes of dry-cured ham during the post-salting stage as affected by partial replacement of NaCl by other salts. Meat Sci 78:135–142. https://doi.org/10.1016/j.meatsci.2007.07.008
Huang L, Trieu K, Yoshimura S et al (2020) Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials. BMJ 368:m315. https://doi.org/10.1136/bmj.m315
Harper NM, Getty KJK (2012) Effect of salt reduction on growth of listeria monocytogenes in meat and poultry systems. J Food Sci. https://doi.org/10.1111/j.1750-3841.2012.02975.x
Al-Samarrai G, Singh H, Syarhabil M (2012) Evaluating eco-friendly botanicals (natural plant extracts) as alternatives to synthetic fungicides. Ann Agric Environ Med 19:673–676
Pandey AK, Kumar P, Singh P et al (2017) Essential oils: sources of antimicrobials and food preservatives. Front Microbiol 7:2161. https://doi.org/10.3389/fmicb.2016.02161
Kamatou GPP, Viljoen AM (2010) A review of the application and pharmacological properties of α-bisabolol and α-bisabolol-rich oils. J Am Oil Chem Soc 87:1–7. https://doi.org/10.1007/s11746-009-1483-3
Jahanshiri Z, Shams-Ghahfarokhi M, Asghari-Paskiabi F et al (2017) α-Bisabolol inhibits Aspergillus fumigatus Af239 growth via affecting microsomal ∆24-sterol methyltransferase as a crucial enzyme in ergosterol biosynthesis pathway. World J Microbiol Biotechnol 33:1–8. https://doi.org/10.1007/s11274-017-2214-9
Leslie JF, Summerell BA, Bullock S (2006) The Fusarium Laboratory Manual. Wiley-Blackwell, Ames
Clinical and Laboratory Standard Iinstitute (2017) M38 reference method for broth dilution antifungal susceptibility testing of filamentous fungi, 3rd edn. Clinical and Laboratory Standard Iinstitute, Wayne
Doern CD (2014) When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J Clin Microbiol 52:4124–4128. https://doi.org/10.1128/JCM.01121-14
Silva KVS, Lima MIO, Cardoso GN et al (2017) Inibitory effects of linalool on fungal pathogenicity of clinical isolates of Microsporum canis and Microsporum gypseum. Mycoses 60:387–393. https://doi.org/10.1111/myc.12606
Dambolena JS, Zunino MP, López AG et al (2010) Essential oils composition of Ocimum basilicum L. and Ocimum gratissimum L. from Kenya and their inhibitory effects on growth and fumonisin production by Fusariumverticillioides. Innov Food Sci Emerg Technol 11:410–414. https://doi.org/10.1016/j.ifset.2009.08.005
Menniti AM, Gregori R, Neri F (2010) Activity of natural compounds on Fusarium verticillioides and fumonisin production in stored maize kernels. Int J Food Microbiol 136:304–309. https://doi.org/10.1016/j.ijfoodmicro.2009.10.008
Coban HB (2020) Organic acids as antimicrobial food agents: applications and microbial productions. Bioprocess Biosyst Eng 43:569–591. https://doi.org/10.1007/s00449-019-02256-w
Ferrigo D, Raiola A, Causin R (2016) Fusarium toxins in cereals: occurrence, legislation, factors promoting the appearance and their management. Molecules 21:627. https://doi.org/10.3390/molecules21050627
Butt UR, Naz R, Nosheen A et al (2019) Changes in pathogenesis-related gene expression in response to bioformulations in the apoplast of maize leaves against Fusarium oxysporum. J Plant Interact 14:61–72. https://doi.org/10.1080/17429145.2018.1550217
Inguglia ES, Zhang Z, Tiwari BK et al (2017) Salt reduction strategies in processed meat products: a review. Trends Food Sci Technol 59:70–78. https://doi.org/10.1016/j.tifs.2016.10.016
Jaenke R, Barzi F, McMahon E et al (2017) Consumer acceptance of reformulated food products: a systematic review and meta-analysis of salt-reduced foods. Crit Rev Food Sci Nutr 57:3357–3372. https://doi.org/10.1080/10408398.2015.1118009
Lucca AJD, Pauli A, Schilcher H et al (2011) Fungicidal and bactericidal properties of bisabolol and dragosantol. J Essent Oil Res 23:47–54. https://doi.org/10.1080/10412905.2011.9700457
Caesar LK, Cech NB (2019) Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2. Nat Prod Rep 36:869–888. https://doi.org/10.1039/c9np00011a
Oren A (2011) Thermodynamic limits to microbial life at high salt concentrations. Environ Microbiol 13:1908–1923
Raut JS, Karuppayil SM (2014) A status review on the medicinal properties of essential oils. Ind Crops Prod 62:250–264. https://doi.org/10.1016/j.indcrop.2014.05.055
Pauli A (2006) α-Bisabolol from chamomile: a specific ergosterol biosynthesis inhibitor? Int J Aromather 16:21–25. https://doi.org/10.1016/j.ijat.2006.01.002
Oliveira JC, de Vasconcelos PÂ, de Medeiros CAC et al (2020) The sensitivity modifying activity of nerolidol and α-bisabolol against Trichophyton spp. Indian J Microbiol 60:505–510. https://doi.org/10.1007/s12088-020-00895-2
Perricone M, Arace E, Corbo MR et al (2015) Bioactivity of essential oils: a review on their interaction with food components. Front Microbiol. https://doi.org/10.3389/fmicb.2015.00076
Cherrat L, Espina L, Bakkali M et al (2014) Chemical composition and antioxidant properties of Laurus nobilis L. and Myrtus communis L. essential oils from Morocco and evaluation of their antimicrobial activity acting alone or in combined processes for food preservation. J Sci Food Agric 94:1197–1204. https://doi.org/10.1002/jsfa.6397
Danielli LJ, Pippi B, Soares KD et al (2017) Chemosensitization of filamentous fungi to antifungal agents using Nectandra Rol. ex Rottb. species essential oils. Ind Crops Prod 102:7–15. https://doi.org/10.1016/j.indcrop.2017.03.013
Tyagi AK, Gottardi D, Malik A, Guerzoni ME (2014) Chemical composition, in vitro anti-yeast activity and fruit juice preservation potential of lemon grass oil. LWT Food Sci Technol 57:731–737. https://doi.org/10.1016/j.lwt.2014.02.004
Macwan SR, Dabhi BK, Aparnathi KD, Prajapati JB (2016) Essential oils of herbs and spices: their antimicrobial activity and application in preservation of food. Int J Curr Microbiol Appl Sci 5:885–901. https://doi.org/10.20546/ijcmas.2016.505.092
Acknowledgements
The authors would like to thank the Department of Agriculture (Cuité, Brazil) and National Supply Company (Brazil) for supplying the maize grains.
Author information
Authors and Affiliations
Contributions
FOP and IOL conceived and designed the experiments; CACM, AVP, and JCO performed the experiments and analyzed data; CACM and GSS wrote the paper; GSS and JMMA executed the article editing. JMMA contributed to the critical reading of the manuscript and experiments with maize. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
No conflict of interest declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Medeiros, C.A.C., Pinto, Â.V., de Oliveira, J.C. et al. Evaluating the Antifungal Activity of α-Bisabolol in Association with NaCl on Fusarium oxysporum in Maize Grains. Curr Microbiol 78, 604–610 (2021). https://doi.org/10.1007/s00284-020-02313-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02313-8