Abstract
As the main substance in some traditional Chinese medicines, cucurbitacins have been used to treat hepatitis for decades in China. Currently, the use of cucurbitacins against cancer and other diseases has achieved towering popularity among researchers worldwide, as detailed in this review with summarized tables. Numerous studies have reported the potential tumor-killing activities of cucurbitacins in multiple aspects of human malignancies. Continuous research on its anticancer activity mechanisms also brings a glimmer of light to the treatment of patients with lung cancer. In line with the promising roles of cucurbitacins against cancer, through various molecular signaling pathways, it is justifiable to propose the use of cucurbitacins as a potential mainline chemotherapy before the onset and after the diagnosis of lung cancers. Here, this article mainly summarized the findings about the biological functions and underlying mechanisms of cucurbitacins on lung cancer pathogenesis and treatment. In addition, we also discussed the safety and efficacy of their application for further research and even clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is a worldwide public health problem and has been a major cause of mortality in recent years [1,2,3]. According to the report of global cancer statistics, there are approximately 2.1 million new cases of lung cancer diagnosed each year, and 1.8 million patients died of lung cancer in 2018 [4,5,6]. In clinical practice, the treatments that are available for lung cancer include surgical resection, radiotherapy and chemotherapy drugs [7, 8]. Specific treatment is mainly based on the diagnosis and clinical staging of patients [9]. However, the status quo is that the clinical screening of lung cancer is not accessible for each patient. As a result, patients are usually diagnosed at advanced stages. At the same time, the 5-year survival rate of lung cancer, which continues to decrease as the disease stage progresses, with comprehensive consideration of various factors, only varies from 4–17% [10, 11]. Even though some emerging therapeutic strategies, including targeted therapy and immunotherapy, have exhibited momentous clinical benefits [7, 10, 12], some patients do not show durable remission, and some tumor cells have been refractory to response with the anti-cancer drugs [10, 13, 14].
Therefore, to further prolong the life expectancy of these patients, and better improve their quality of life, the progress of research regarding more sophisticated diagnostic methods and more effective therapeutic drugs for early lung cancer needs to be sped up.
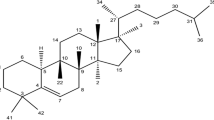
Cucurbitacins, natural products originally derived from Cucurbitaceae, have been shown to possess strong antitumor activity by modulating multiple signaling pathways in vivo and in vitro [15,16,17]. With several unique advantages, such as lower toxicity and fewer side effects, these compounds could more promptly be moved into clinical practice [18,19,20]. Currently, besides the Cucurbitaceae, cucurbitacins can also be extracted from various families of plants worldwide [18, 21]. In terms of structural composition, except for the nucleus skeleton of tetracyclic cucurbitane, cucurbitacins carry different oxygen-containing functional groups in different positions. There are approximately 12 different classes of cucurbitacins, divided from A to T with over 200 derivatives [21]. After years of accumulation, a growing contingency of researchers have confirmed their activity against several human pathological processes, for example, the anticancer effects, anti-inflammatory action, immunomodulatory capacities, etc. [15, 22,23,24]. Emerging evidence documents that cucurbitacin has a certain degree of inhibitory effect on a variety of tumors [25,26,27]. Nowadays, the research advancement of cucurbitacins in various human cancers have been reviewed in several papers [18, 28,29,30,31,32]. Especially, a recent review has summarized the potential anti-cancer properties in breast cancer [33]. However, the detailed biological functions and regulatory mechanisms of cucurbitacins in the occurrence and development of lung cancer are poorly summarized. Recently, its potent antagonized efficacy in lung cancer attracts the attention of researchers and has been well-identified in different experiments (Tables 1, 2). Therefore, as a promising antitumor drug, the detailed roles of cucurbitacins in lung cancer research and treatment are worthy of further research. To date, the major mechanisms of action involve apoptosis induction, cell cycle arrest, cytoskeleton regulation and so on [21, 34,35,36,37,38]. Although a large number of reports exist regarding the effective anticancer functions of cucurbitacins, their detailed mechanisms have not been fully elucidated so far.
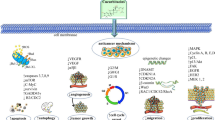
In this article, we mainly summarize the application of cucurbitacins in lung cancers, focusing on the therapeutic functions and related biological mechanisms (Fig. 1). The isolated cucurbitacin components with attractive anticancer activity for lung cancer have been reported to include A, B, D, E, I, Q, IIa and their derivatives. Furthermore, the safety problems are also discussed for the purpose of clinical application in the future.
Cucurbitacin B
Cucurbitacin B (CuB) is one of the most active and popular cucurbitacins studied. It is widely distributed in a variety of plants, mainly in the form of glycosides. CuB and its derivatives, extracted from different parts of different plants, showed significant cytotoxicity to different lung cancer cells, including A549, SK-LU1 and so on [39, 40]. Although the roles of CuB have not yet been elucidated clearly, relevant mechanisms and targets that have been discovered deserve our attention.
Epidermal growth factor receptor (EGFR), which is normally overexpressed in various cancers, is a key target for lung cancer therapy, especially for non-small-cell lung cancer (NSCLC) [41,42,43]. For patients who carry a sensitizing mutation in EGFR, tyrosine kinase inhibitors (TKIs) are recommended first [6, 44, 45]. A recent study has found that CuB could directly suppress EGFR signaling through the lysosomal pathway both in vitro and in vivo, which is distinct from TKIs [46]. Consequently, CuB successfully impeded cell migration and invasion in the gefitinib-resistant (GR) NSCLC EGFR/ERK pathway. The CIP2A/PP2A/Akt axis was verified to play a dominant role in CuB-induced cell proliferation inhibition through a series of studies by Liu PF’s group [46].
Indeed, it was also reported that CuB inhibited tumor growth and cell colony formation regardless of their EGFR expression in different studies [47,48,49]. Another possible reason for this attractive result might, in part, stem from the inhibitory effect of CuB on the downstream molecules of EGFR. Khan N et al. [48] revealed that CuB suppressed PI3K/Akt/mTOR and signal transducer and activator of transcription 3 (STAT3) signaling both in EGFR-mutant and EGFR-wild-type lung cancer cells, leading to growth inhibition along with G2-phase cell cycle blocking. As the downstream molecules of EGFR, STATs have been recognized as promising targets for cancer treatment as well [50]. Existing evidence indicated that Janus kinase (JAK)-STAT3 signaling played a significant role in promoting cancer progression, with effects on tumor cell proliferation, survival and invasion [51,52,53]. As early as 2004, it was found that CuB inhibited the JAK-STATs pathway in vitro and in vivo and induced apoptosis and tumor growth inhibition [54]. Li YM’s group revealed that CuB suppressed cell proliferation and induced caspase-related apoptosis through the STAT3 pathway, along with cytochrome c release and Bcl-2 reduction. Upregulation and activation of STAT1, the important effector in IFN-γ signaling, further enhanced the antitumor effects of CuB [55]. Similar to STAT3, Akt is also closely related to cell survival and proliferation and is commonly overexpressed in cancer cells [56, 57]. Treatment with CuB was found to result in decreased viability and improved apoptosis of lung cancer cells by causing inhibition of PI3K/Akt/mTOR signaling pathway [48]. To sum up, CuB may provide an opportunity to overcome the common clinical problems of EGFR-TKI therapy in lung cancer, that is, poor sensitivity and drug resistance [41, 46]. Aside from the EGFR/Akt pathway mentioned above, CuB exhibited significant inhibitory effects on the migratory and invasive abilities of NSCLC cells in vitro and in vivo, with attenuation of the canonical Wnt/β-catenin signaling pathway [46, 58]. Given that the migration and invasion potentials in lung cancer often predict progression and recurrence [59, 60], CuB has good prospects in inhibiting the progression of lung cancer tumors and enhancing the treatment response.
It is worthy to note that EGFR-wild-type and EGFR-mutant NSCLC cells exhibited differential expression of sestrin-3 with the treatment of CuB [48]. In Khan N’s studies, the connection between sestrin-3 and the AMPKα/mTOR1 axis was verified, which was partly involved in the CuB-induced growth attenuation in H1659 (EGFR-mutant). The elevated protein and mRNA expression of sestrin-3 resulted in inhibition of the mTOR1 complex and its downstream molecules through phosphorylation of AMPKα. This provided a novel sight for a potential treatment mechanism of CuB. The role that CuB-induced setrin-3 downregulation played in A549 cells (EGFR-mutant) was not clearly explained. Recent research unveiled the role of sestrin-3 in cellular redox balance as an antioxidant against the production of ROS, which was ignored by Khan N. As a result, it was speculated that the inhibition of sestrin-3 may be related to an increase in ROS production as a potential anticancer mechanism of CuB. In fact, ROS production was observed in A549 cells by Guo J [61], which was consistent with the putative results. Nevertheless, validation is still needed in further research, along with mechanistic insight into the different effects of CuB on sestrin-3. As was evident in Guo J’s experiments, CuB induced DNA damage along with the promotion of the ATM-Chk1-Cdc25C-Cdk1 cascade in A549 mediated by reactive oxygen species (ROS) formation, which induced G2/M cell cycle arrest [61].
As a redox regulator of tumor pathophysiology, ROS upregulation promotes cancer occurrence and development and is therefore proposed to be a promising target [62, 63]. Beyond that, intracellular thiols and glutathione (GSH) are other redox regulators in cancer cells. CuB was tested to see if it could interrupt the cellular redox balance in NSCLC cells through the downregulation of protein thiols and the GSH/GSSG ratio starting from a 0.1 μM concentration [49], but no marked change in ROS was observed at 3 h. Given that ROS formation was observed after 24 h of CuB treatment [61], it was suggested that the effects on ROS may be lagging, and CuB may interact directly with thiols. In addition, the cytotoxicity of CuB based on thiols and GSH specifically manifested as a reduction in cell viability and induction of G2/M cell cycle arrest and mitochondrial apoptosis. Furthermore, the epigenetic analysis indicated that CuB suppressed the activities of DNA methyltransferases (DNMTs) and histone deacetylases (HDACs) and facilitated histone acetyltransferases (HATs), which led to the upregulation of some key tumor suppressor genes (p16, p21) and concurrent downregulation of some key tumor promoter genes (hTERT) [64]. However, CuB negatively regulated the protein expression pertaining to oncogenes (c-MYC, K-RAS) in H1299 without significant alteration of the mRNA expression. Hence, it provides a novel perspective for the chemotherapeutic potential of CuB for patients with cancer.
Izabella TS et al. [40] synthesized a new derivation of CuB, namely, 2-deoxy-2-amine-cucurbitacin E (DACE). DACE has an amino moiety in C2 instead of the hydroxyl group, compared to CuB, which brings out a nucleophilic, basic and hydrogen bond donor/acceptor group. In addition, the mechanism of action was changed along with an optimized solubility and bioavailability via the alteration of hybridization from sp3 to sp2 in C2. In the presence of DACE, the activation of the EGFR, STAT3, PI3K/Akt and ERK pathways was inhibited in A549 cells, similar to the inhibition seen with CuB. Of note, the Ras/Raf/MEK pathway was considered a key signaling pathway of DACE-induced cytotoxicity in vitro and in vivo. As illustrated in various NIH3T3 model cell lines, DACE exhibited selectivity toward malignant cells that were transduced with RAF or RAS.
Cucurbitacin E
Cucurbitacin E (CuE) is a well-known antifeedant, and shows strong antitumor activity in various cancers both in vitro and in vivo [65,66,67,68]. A recent study from Cheng SE’s group has identified CuE as a promising STAT3 inhibitor that displays attractive cytotoxicity on human lung cancer A549 cells [69]. In this article, CuE successfully inhibited ATPγS-induced expression of COX-2 mRNA and PGE2 generation through its downregulation of STAT3, which provided a new prospective treatment for chronic lung pathologies.
In addition, with regard to mechanisms in lung cancer research, increasing studies have demonstrated that CuE attenuated cell proliferation mediated by inhibition of the Wnt/β-Catenin signaling axis along with upregulation of the tumor suppressor Menin [70]. In addition, with the activation of caspase family proteins, Unc-51-like kinase 1 (ULK-1) phosphorylation and BECN1 upregulation in 95D cancer cells, CuE treatment induced apoptosis and autophagy by weakening the Akt/mTOR pathway. Further results were obtained when an accumulation of LC3II and CuE-induced cytotoxicity were enhanced by pretreatment with the autophagy inhibitors chloroquine and bafilomycin. Moreover, it is noteworthy that these functions were mediated by ROS generation [71]. One recent study indicated that CuE inhibits the migration and invasion of H2030-BrM3 and PC9-BrM3 cells through the Yes-associated protein (YAP) signaling pathway, among which, both H2030-BrM3 with KRAS mutation and PC9-BrM3 with EGFR mutation are prone to metastasize. Especially in the H2030-BrM3 murine model, CuE successfully reduced brain metastasis through the downregulation of YAP. In addition to these direct effects, CuE also had a negative impact on YAP modulation by interfering with the EGFR/MAPK/ERK axis [72]. It was reported that the EGFR/MAPK/ERK pathway influenced CTGF and CYR61 expression, the downstream genes of the Hippo/YAP pathway [73]. Altogether, this research provided new insight for the treatment of metastatic lung cancer.
Cucurbitacin I
Cucurbitacin I (CuI), also called JSI-124, was first extracted from plants that belonged to cucurbitaceae and was also found in cruciferae [67, 74]. The first time that CuI was thought to be a possible agent for lung cancer treatment was when its anticancer activity was identified in A549 cells and in a xenograft model where it was identified as a targeted JAK/STAT3 inhibitor [75]. By screening the NCI Structural Diversity Set comprising a library of 1,992 compounds, Blaskovich MA et al. [75] uncovered that CuI was highly selective for the JAK/STAT3 signaling pathway by functioning on STAT3 DNA-binding activity precisely and had no obvious effect on other oncogenic pathways such as Akt, ERK1/2 and JNK. For example, CuI treatment of human lung adenocarcinoma Calu-1 cells, which lack constitutive STAT3 activation, showed no clear impact on cellular apoptosis in vitro or on corresponding xenograft tumor growth. On account of its pharmacological targeting of STAT3, subsequent research on CuI against lung cancer was mainly focused on the modulation of STAT signaling. Like CuB, CuI dose-dependently blocked STAT3 signaling but promoted STAT1 signaling, possibly by disrupting actin filaments [76]. The findings indicated that the actin filaments physically interacted with STAT3 in A549 cells and thus regulated STAT3 phosphorylation through two different signaling complexes, the IL-6 receptor complex and the focal adhesion complex. Meanwhile, actin filaments could also significantly promote STAT1 dephosphorylation by physically interacting with STAT1. Thus, these data demonstrated the interesting roles of CuI on the actin filament-mediated STAT signaling regulation. In addition, the compound CuI also displays anticancer stem cell properties, especially in NSCLC-derived CD133 + cells by deactivating the STAT3 signaling. CuI successfully inhibited the proliferation of CD133 + cells and promoted their differentiation into CD133- cells with lower tumorigenicity and radio-resistance, to significantly improve the therapeutic effects [77].
To date, apart from STAT3, other recent works reported that CuI could act as an inhibitor of p21-activated kinase 1 (PAK1), which can promote proliferation and invasion in multiple solid tumors [78, 79]. CuI treatment resulted in A549 cell growth arrest by blocking phosphorylated PAK1 [80]. CuI was also verified to promote pro-death autophagy by inhibiting ERK activation and the downstream STAT3 phosphorylation level in A549 cells [81]. Based on autophagy induction, the triggered cell death and apoptosis with CuI treatment have been enhanced to some extent. In fact, similar to the findings from Blaskovich MA’s group [75], they also further revealed that CuI treatment had no remarkable effect on the PI3K/Akt signaling cascade. However, CuI inhibits p-ERK expression, and this is absolutely the opposite of Blaskovich MA’s results. Comparing the details of the two experiments, the discrepancy may arise from the distinct duration of CuI treatment. Nevertheless, there were no explanations regarding CuI-induced phosphorylation of ERK at early timepoints.
In addition, some inconsistent functions of CuI on PI3K/Akt/mTOR modulation have been found recently. When CuI acts at a very low concentration (50 nM), it could markedly attenuate the phosphorylated AKT and p70S6K pathway, at last leading to cell growth inhibition of human NSCLC A549 cells [82]. These contradictory results might be due to different standards; one considered the level of phosphorylation only, while the other chose the ratio of phosphorylation to dephosphorylation or dephosphorylation only, which needs to be well-clarified in further studies.
Other cucurbitacins
In addition to CuB, CuE and CuI mentioned above, other independent research teams have discovered cucurbitacin compounds that also have pivotal functions against the pathological behaviors of human lung cancers, including cucurbitacin A, D, E, Q, IIa, etc.
Cucurbitacin A (CuA), mainly isolated from Cucumis species with a narrow distribution, is identified as a potential PI3K/Akt/mTOR signaling suppressor in A549 cells [64]. Specifically, CuA treatment blocked the cell cycle progression in a dose- and time-dependent manner, triggering cell proliferation inhibition and apoptosis induction in A549 lung cancer cells [83].
Cucurbitacin D (CuD) is one of the most common derivatives from Cucurbitaceae and has strong antitumor properties [54, 67, 84]. Jacquot C. and her group found that CuD triggered CDK1 overexpression at the transcriptional level, leading to G1-phase cell cycle arrest, succedent apoptosis induction, and eventually irreversible growth inhibition in NSCLC-N6 cells [85]. In addition, Ku JM et al. [86] further explained the molecular mechanisms behind CuD’s actions against lung cancer. In H1299 cells, CuD administration obviously induced cell apoptosis incidence by increasing the apoptosis markers (cleaved caspases and increased proapoptotic protein Bcl-2). Further exploration indicated that CuD inhibited ErbB3 signaling, which could bind many downstream signaling proteins correlated with cancer progression and prognosis. More importantly, it was worth noting that trichosanthes, the main derivatives from CuD, successfully inhibited the growth of patient-derived tumor cells [86, 87].
Cucurbitacin Q (CuQ) has been discovered as a more selective inhibitor that disrupts STAT3 without significant effects on other carcinogenic biomarkers in A549 cells, especially JAK [54]. However, more in-depth studies are needed to further clarify its specific role in disrupting STAT3 signaling.
Cucurbitacin IIa (CuIIa), also called hemslecin A, is the major bioactive cucurbitacin in Hemsleya amabilis and has been verified to have antitumor effects in different cancers, including lung cancer [88, 89]. Unlike the other cucurbitacins, CuIIa could not cause death in H1299 cells by regulating the JAK/STAT3 pathway [90]. However, a recent kinase inhibition assay indicated that CuIIa could directly act as a potential EGFR-TKI with an IC50 value of 1.455 nM [91]. Due to its evident antagonistic effects on EGFR oncogenic signaling, CuIIa successfully induced cell apoptosis and cell-cycle arrest in A549 lung cancer cells [91]. In addition, the CuIIa homolog, CuIIb, could also exhibit more potent activity against A549 cells [92].
Characterization of the concentration and structure
Collectively, the effective concentration of cucurbitacins in vitro ranges from 10 nM ~ 200 μM [58, 83]. At the nanomolar level, it was reported that CuB inhibited tumor cells stemness and angiogenesis via the canonical Wnt/β-catenin axis [70], induced ATM-dependent DBS through ROS [61] and regulated epigenetic alteration, although it was clearly stated that STAT3 did not change significantly. CuI had an impact on the PI3K/Akt/p70S6K pathway [82] and PAK1 beginning at a 50 nM concentration [80]. In addition, CuE was declared to augment the level of ROS [71] and inhibit the YAP signaling pathway [72]. It was worth noting that the lagging effects on ROS mentioned above compared to the interaction between thiol and CuB may be due to its insignificant impact at high concentrations of CuB [61]. These cucurbitacins functioned on different targets at 0.1 to 1 μM, while CuA blocked the PI3K/Akt/mTOR axis from 40 μM, CuQ inhibited STAT3 with an IC50 of 3.77 ± 1.7 μM in A549 cells [54], and CuIIa attenuated EGFR from 40 μM [91]. However, another study showed that CuA could inhibit the cell proliferation of A549 cells with an IC50 of 0.4 ± 0.013 μM [93]. On account of insufficient evidence, further research may be needed to ascertain whether CuA functions at a lower dose.
Given the characteristics of these cucurbitacins, the different active concentrations may partly derive from structural differences such as highly oxidized tetracyclic triterpenoids. A lanostane skeleton with multiple substituents, including hydroxyls at C-16 and C-20, carbonyls at C-11 and C-22, and a methyl at C-9 rather than C-10 in lanostane, along with an unsaturated double bond at position 5 was the common structure. Among these cucurbitacins that exhibited antitumor activity in lung cancer, the discrepancies were mainly embodied in C-1, 2, 3, 2 3, 24, and 25. Compared with others, CuB seems to have the most typical traits, which represented the majority of substitutions. Lang KL et al. pointed out that the cytotoxic potency of cucurbitacins in A549 cells was related to multivariate factors, among which, the electrophilicity of molecules played a pivotal role, according to multivariate SAR and QSAR analyses of cucurbitacin derivatives. The derivatives that carried an amino (compound 32, also known as DACE) or bromine (compound 34) at C-2 exhibited the most potent cytotoxicity, among 43 compounds, in A549 cells. On this foundation, further studies that focused on DACE proposed that the double bond, along with the amino substitution that formed a conjugated structure, and the Michael acceptor (α, β-unsaturated ketone) in the side chain should be noticed [40]. Likely, CuIIa, whose conjugated 23,24 olefinic bond was saturated, functioned at high concentrations, which supported the importance of the Michael acceptor. Meanwhile, CuIIa was unable to inhibit phosphorylation of STAT3 in A549 cells even though it could promote the expression of STAT3 in H1299 cells due to the absence of a 23, 24 double bond. Notably, the special side chain of CuIIa made its combination with EGFR more stable [90]. According to Sun J’s study, the absence of 3-carboxy in CuQ, which was replaced by a hydroxyl, led to higher selectivity towards STAT3 without any obvious effects on JAK2, while the presence of 11-hydroxyl in CuA eliminated its anti-STAT3 activity [54]. Furthermore, Lang and his group hinted at the importance of a 25-acetoxy group [93], which seemed limited after considering the cytotoxicity of CuI and CuD.
Safety and efficiency
The toxicity of cucurbitacins and related derivatives has been reported for a long time. Garg S et al. concluded that CuB caused some poisoning events up until 2018 [18]. In addition, six other kinds of cucurbitacins were recognized as acutely toxic, with the exception of CuE, which was an irritant, according to the Laboratory Chemical Safety Summary Datasheet (LCSS). The median lethal dose (LD50) of CuB in mice was 14 mg/kg (oral route), 2.73 mg/kg (intramuscular route), and 1 mg/kg (subcutaneous route) [94]. The experiments performed in mice with lung tumors found that low doses of CuB, such as 0.1 mg/kg (intraperitoneal), successfully impeded tumor growth. It was confusing that no toxicity reaction was observed in H1299 xenograft mice with 1 mg/kg intraperitoneal CuB [49], which may depend on the purity of CuB or differences in the model. Furthermore, it was reported that the IC50 of CuB against 16-HBE, a kind of human bronchial epithelial-like cell, was 4.23 ± 0.81 μM, which was much higher than its active concentration in lung cancer cells [46]. With regard to CuD, it exhibited similar LD50 values in rats as CuB, namely, 8.2 mg/kg (oral), 3.4 mg/kg (subcutaneous), and 1.3 mg/kg (intraperitoneal) [95]. However, the active dose for lung cancer in vivo remains unclear. CuA, I, Q, and IIa lacked relevant toxicity data in mice, although there were no side effects mentioned in the A549 xenograft model or in a Lewis lung carcinoma mouse with 1 mg/kg intraperitoneal CuQ [54] or 15 mg/kg intravenous CuIIa, respectively [90]. However, one kind of adverse reaction, edema, was reported in an A549 xenograft model [75] but not in an NSCLC-derived CD133-positive-xenograft model [77]. Ultimately, it was attractive that CuE could inhibit brain metastasis and improve the survival time of H2030‐BrM3 murine cells after 0.2 mg/kg intraperitoneal CuE [72], whose LD50 was as high as 340 mg/kg (oral) [96]. All in all, the active dose and lethal dose of different types of cucurbitacin are not the same, which may be associated with the diversity in structure.
Cucurbitacins are originally derived from Chinese traditional herbal plants that are used in dietary supplements and in medicine [97]. These compounds have shown various bioactivities against human disorders with the development of modern science and technology. On account of their attractive anti-inflammatory, anti-tumor and hepatoprotective effects, several Chinese medical patents based on cucurbitacins have been approved by the China Food and Drug Administration as an adjuvant treatment strategy for patients with chronic hepatitis and primary liver cancer [98]. Similarly, hemsleyadine tablets, which contain CuIIa and IIb as the main ingredients, have been used clinically to treat bacillary dysentery, enteritis and acute tonsillitis for a period of time [99, 100]. In Italy, a topical preparation for treating mono- or bilateral exudative otitis media in children, called Sinuclean Nebules, is already on the market. It is a saline solution with 45 mg of various cucurbitacins from Ecballium elaterium, including CuB, CuD, CuI and CuE [101]. In addition, a clinical trial examining the effect of CuB in patients with lung cancer indicated that oral use of CuB at 120 μg three times a day, i.e. approximately 6 μg/kg after conversion according to the standard human weight of 60 kg [102], can effectively decrease the frequency of immature myeloid cells (imCs); this may represent a deficiency of antitumor immunity in advanced patients [103]. And there were no serious adverse reactions reported in these patients. Nevertheless, because of this trial did not directly explore the anti-tumor effects of CuB, more clinical trials were needed to explore whether the dose in cells and animals can provide similar clinical benefit in lung cancer patients. As illustrated in Table 2, the experimental dose range of most cucurbitacins in mouse is 0.1–1 mg/kg. According to the conversion formula recommended by the practice guidance [102], Human Equivalent Dose (mg/kg) = Animal dose (mg/kg) × (Animal Km ÷ Human Km), the human equivalent dose range is 0.008–0.081 mg/kg, i.e. 8–81 μg/kg, which may be used as the recommended dose for future clinical trials evaluating the anti-tumor functions of cucurbitacins. In addition, Km in the conversion formula is a correction factor that is calculated by dividing the average body weight of species to its body surface area. As reported by US Food and Drug Administration, the Km values in mouse and human are 3 and 37, respectively. Evidently, these evaluations will shed more light on how to properly treat infections or cancer with cucurbitacins without inducing apparent and serious adverse effects at recommended concentrations. Even so, more investigations on the clinical application of cucurbitacin-based therapies are required to reveal the details regarding their safety and efficacy.
In addition, to elucidate the underlying biological functions of cucurbitacins, specific and sensitive detection methods need to be developed. Evaluating the pharmacokinetics and pharmacodynamics would afford information on the development of cucurbitacin-based therapy. As early as 2006, a group from Canada established a promising method for quantitative analysis of CuI in rat plasma based on liquid chromatography/mass spectrometry (LC–MS) [104]. Subsequently, other cucurbitacin compounds have been quantitatively analyzed with LC–MS methods in the plasma of different species [105, 106], for example, rhesus monkeys [99]. In addition, Wang Z’s group conducted several experiments to test the pharmacokinetic parameters of CuB and CuE in rat plasma by UHPLC-MS/MS [98, 107], and they found that the absolute oral bioavailability of CuB and CuE was very low (only approximately 10%). Surprisingly, with a high volume of distribution, these compounds were widely distributed in several organs including the lung, spleen and kidney [105]. However, the major pathways of CuB metabolism still require detailed clarification.
Bioavailability is another barrier to the use of plant-derived chemopreventive agents [17, 108,109,110]. Scientists put forward some attempts seeking the optimum carrier to improve the bioavailability of cucurbitacins. Micelles, including poly(ethylene oxide)-block-poly(ε-caprolactone) (PEO-b-PCL) and poly(ethylene oxide)-block-poly (α-benzyl carboxylate ε-caprolactone) (PEO-b-PBCL), were evaluated as the solubilizers and delivery vehicles for CuI and CuB [111, 112]. These drug conjugates make CuI and CuB more soluble, thus ameliorating their antitumor activity. Moreover, Lv Q and his group reported another similar mucoadhesive buccal film micelle as an effective carrier for CuB delivery [113]. Recently, a novel phospholipid complex carried CuB and not only ameliorated its permeability but also improved the targeted killing effect on cholangiocarcinoma cells to a certain extent [114]. According to the latest research, polymer nanoparticles modified with collagen peptides (CuB-MMs-CPs) have been developed to evidently increase the cellular uptake and transportation of CuB. The animal experiment involving rapid-growing rats further verified the significantly increased tumor inhibition caused by CuB-MMs-CPs [115]. In summary, choosing nanomicelles as a potential cucurbitacin carrier could be an effective strategy to overcome the difficulties in bioavailability.
To date, although there is a certain theoretical basis for the safety and efficacy of cucurbitacin-based therapy, these studies provide only limited information due to a lack of reliable clinical evidence. Thus, more studies are needed as a reference for its approval for extensive clinical application.
Perspective on cucurbitacins
As novel compounds extracted from a number of plant families all over the world, cucurbitacins have been used in the diet and in medicine for a long time. For instance, Goya containing CuI grows in Okinawa, Japan, while Ibervillea sonorae-containing CuIIb is a kind of traditional Mexican medicine [116]. Referring to earlier research, the anti-lung cancer activity of CuD from Sloanea zuliaensis was first reported around 2003 [117]. After that time, CuB and its derivatives, which were isolated from different plants, also showed significant cytotoxicity against different lung cancer cells [93, 118, 119].
Currently, finding novel intervention strategies for lung cancer that can overcome treatment failure in the clinic is becoming urgent [120, 121]. Natural agents with emerging cytotoxicity tend to attract more attention, due to their economic superiority and multitarget effects compared to synthetic products. A number of plant-derived molecules have been selected for further research, including cucurbitacins [20, 29, 122]. Abou-Salim MA et al. [123] designed innovative nitric oxide-donating cucurbitacin-inspired estrone analogs (NO-CIEAs) and suggested that NO-CIEAs exhibited more potent sensitization activity to cancer chemotherapy. More evidence has indicated that CuB and CuD derivatives both exhibited significant synergistic anticancer effects on human lung cancers in vivo and in vitro when used in combination with known chemotherapy drugs, such as paclitaxel [124] and cisplatin [86, 125]. It was remarkable that the cucurbitacins could function even at 0.005 μM, further ensuring the safety of the treatment. In addition, targeting of STAT3 signaling by CuI significantly enhanced the chemoradiosensitivity in CD133-positive cells isolated from patients with lung cancer [77]. Above all, further investigation of these issues may help identify more promising strategies to enhance the benefits of therapeutic response in the treatment of patients with lung cancer.
Conclusion
As novel natural tetracyclic triterpenoid compounds, cucurbitacins display a wide range of biological effects. Moreover, with significant cytotoxic properties, cucurbitacins possess very potent effects toward a number of cancer cells. Herein, we summarized the pharmacological principles and mechanisms of action of different cucurbitacins in lung cancer research. Furthermore, the possibility of cucurbitacins actually entering clinical use for the treatment of lung cancer is also discussed in this article. The compounds CuA, B, D, E, I, Q and IIa were well-summarized as potential anticancer agents with different mechanisms in lung cancer. They successfully inhibited tumor growth and induced cell apoptosis and cell cycle arrest, with no obvious toxicity for normal lung tissues. Furthermore, these compounds could impair cell migration, which occurs in aggressive malignancy and has a negative influence on the chemotherapy response. The evidence mentioned above highlights the preponderance of cucurbitacins as promising agents for lung cancer prevention, based on existing favorable evidence of their safety and efficacy. Nevertheless, for use in clinical practice, more clinical trials focused on cucurbitacins as mainline targeted anticancer therapies for lung cancer, either as independent effectors or as supplements, are warranted.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Liu X, Liu B, Li R, Wang F, Wang N, Zhang M, Bai Y, Wu J, Liu L, Han D, Li Z, Feng B, Zhou G, Wang S, Zeng L, Miao J, Yao Y, Liang B, Huang L, Wang Q, Wu Y (2020) miR-146a-5p plays an oncogenic role in NSCLC via suppression of TRAF6. Front Cell Dev Biol 8:847. https://doi.org/10.3389/fcell.2020.00847
Mamdani H, Jalal SI (2020) Histone deacetylase inhibition in non-small cell lung cancer: hype or hope? Front Cell Dev Biol 8:582370. https://doi.org/10.3389/fcell.2020.582370
Kuerban K, Gao X, Zhang H, Liu J, Dong M, Wu L, Ye R, Feng M, Ye L (2020) Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta Pharm Sin B 10(8):1534–1548. https://doi.org/10.1016/j.apsb.2020.02.002
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Qian J, Chen R, Zhao R, Han Y, Yu Y (2020) Comprehensive molecular characterizations of chinese patients with different subtypes of lung squamous cell carcinoma. Front Oncol 10:607130. https://doi.org/10.3389/fonc.2020.607130
Zhang M, Tian J, Wang R, Song M, Zhao R, Chen H, Liu K, Shim JH, Zhu F, Dong Z, Lee MH (2020) Dasatinib inhibits lung cancer cell growth and patient derived tumor growth in mice by targeting LIMK1. Front Cell Dev Biol 8:556532. https://doi.org/10.3389/fcell.2020.556532
Herbst RS, Morgensztern D, Boshoff C (2018) The biology and management of non-small cell lung cancer. Nature 553(7689):446–454. https://doi.org/10.1038/nature25183
Xu Z, Yan Y, Xiao L, Dai S, Zeng S, Qian L, Wang L, Yang X, Xiao Y, Gong Z (2017) Radiosensitizing effect of diosmetin on radioresistant lung cancer cells via Akt signaling pathway. PLoS ONE 12(4):e0175977. https://doi.org/10.1371/journal.pone.0175977
Rami-Porta R, Call S, Dooms C, Obiols C, Sanchez M, Travis WD, Vollmer I (2018) Lung cancer staging: a concise update. Eur Respir J. https://doi.org/10.1183/13993003.00190-2018
Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr, Wu YL, Paz-Ares L (2017) Lung cancer: current therapies and new targeted treatments. Lancet 389(10066):299–311. https://doi.org/10.1016/S0140-6736(16)30958-8
Yan Y, Xu Z, Qian L, Zeng S, Zhou Y, Chen X, Wei J, Gong Z (2019) Identification of CAV1 and DCN as potential predictive biomarkers for lung adenocarcinoma. Am J Physiol Lung Cell Mol Physiol 316(4):L630–L643. https://doi.org/10.1152/ajplung.00364.2018
Osmani L, Askin F, Gabrielson E, Li QK (2018) Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin Cancer Biol 52(Pt 1):103–109. https://doi.org/10.1016/j.semcancer.2017.11.019
Lim ZF, Ma PC (2019) Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J Hematol Oncol 12(1):134. https://doi.org/10.1186/s13045-019-0818-2
Yang S, Zhang Z, Wang Q (2019) Emerging therapies for small cell lung cancer. J Hematol Oncol 12(1):47. https://doi.org/10.1186/s13045-019-0736-3
Attard E, Martinoli MG (2015) Cucurbitacin E, an experimental lead triterpenoid with anticancer, immunomodulatory and novel effects against degenerative diseases. A Mini-Rev Curr Top Med Chem 15(17):1708–1713. https://doi.org/10.2174/1568026615666150427121331
Lin X, Farooqi AA (2020) Cucurbitacin mediated regulation of deregulated oncogenic signaling cascades and non-coding RNAs in different cancers: spotlight on JAK/STAT, Wnt/beta-catenin, mTOR, TRAIL-mediated pathways. Semin Cancer Biol. https://doi.org/10.1016/j.semcancer.2020.10.012
Ranjan A, Ramachandran S, Gupta N, Kaushik I, Wright S, Srivastava S, Das H, Srivastava S, Prasad S, Srivastava SK (2019) Role of phytochemicals in cancer prevention. Int J Mol Sci. https://doi.org/10.3390/ijms20204981
Garg S, Kaul SC, Wadhwa R (2018) Cucurbitacin B and cancer intervention: chemistry, biology and mechanisms (Review). Int J Oncol 52(1):19–37. https://doi.org/10.3892/ijo.2017.4203
Hussain SS, Kumar AP, Ghosh R (2016) Food-based natural products for cancer management: is the whole greater than the sum of the parts? Semin Cancer Biol 40–41:233–246. https://doi.org/10.1016/j.semcancer.2016.06.002
Wei J, Yan Y, Chen X, Qian L, Zeng S, Li Z, Dai S, Gong Z, Xu Z (2019) The roles of plant-derived triptolide on non-small cell lung cancer. Oncol Res 27(7):849–858. https://doi.org/10.3727/096504018X15447833065047
Cai Y, Fang X, He C, Li P, Xiao F, Wang Y, Chen M (2015) Cucurbitacins: a systematic review of the phytochemistry and anticancer activity. Am J Chin Med 43(7):1331–1350. https://doi.org/10.1142/S0192415X15500755
Ramezani M, Rahmani F, Dehestani A (2017) Comparison between the effects of potassium phosphite and chitosan on changes in the concentration of cucurbitacin E and on antibacterial property of Cucumis sativus. BMC Complement Altern Med 17(1):295. https://doi.org/10.1186/s12906-017-1808-y
Wang W, Zhao X, Hu H, Chen D, Gu J, Deng Y, Sun J (2010) Galactosylated solid lipid nanoparticles with cucurbitacin B improves the liver targetability. Drug Deliv 17(3):114–122. https://doi.org/10.3109/10717540903580176
Yang DK, Kim SJ (2018) Cucurbitacin I protects H9c2 cardiomyoblasts against H2O2-induced oxidative stress via protection of mitochondrial dysfunction. Oxid Med Cell Longev 2018:3016382. https://doi.org/10.1155/2018/3016382
Mao D, Liu AH, Wang ZP, Zhang XW, Lu H (2019) Cucurbitacin B inhibits cell proliferation and induces cell apoptosis in colorectal cancer by modulating methylation status of BTG3. Neoplasma 66(4):593–602. https://doi.org/10.4149/neo_2018_180929N729
Niu Y, Sun W, Lu JJ, Ma DL, Leung CH, Pei L, Chen X (2016) PTEN activation by DNA damage induces protective autophagy in response to cucurbitacin B in hepatocellular carcinoma cells. Oxid Med Cell Longev 2016:4313204. https://doi.org/10.1155/2016/4313204
Zhang T, Li J, Dong Y, Zhai D, Lai L, Dai F, Deng H, Chen Y, Liu M, Yi Z (2012) Cucurbitacin E inhibits breast tumor metastasis by suppressing cell migration and invasion. Breast Cancer Res Treat 135(2):445–458. https://doi.org/10.1007/s10549-012-2175-5
Chen X, Bao J, Guo J, Ding Q, Lu J, Huang M, Wang Y (2012) Biological activities and potential molecular targets of cucurbitacins: a focus on cancer. Anticancer Drugs 23(8):777–787. https://doi.org/10.1097/CAD.0b013e3283541384
Hussain H, Green IR, Saleem M, Khattak KF, Irshad M, Ali M (2019) Cucurbitacins as anticancer agents: a patent review. Recent Pat Anticancer Drug Discov 14(2):133–143. https://doi.org/10.2174/1574892813666181119123035
Lee DH, Iwanski GB, Thoennissen NH (2010) Cucurbitacin: ancient compound shedding new light on cancer treatment. Sci World J 10:413–418. https://doi.org/10.1100/tsw.2010.44
Patlolla JM, Rao CV (2012) Triterpenoids for cancer prevention and treatment: current status and future prospects. Curr Pharm Biotechnol 13(1):147–155. https://doi.org/10.2174/138920112798868719
Rios JL, Andujar I, Escandell JM, Giner RM, Recio MC (2012) Cucurbitacins as inducers of cell death and a rich source of potential anticancer compounds. Curr Pharm Des 18(12):1663–1676. https://doi.org/10.2174/138161212799958549
Ateba SB, Mvondo MA, Ngeu ST, Tchoumtchoua J, Awounfack CF, Njamen D, Krenn L (2018) Natural terpenoids against female breast cancer: a 5 year recent research. Curr Med Chem 25(27):3162–3213. https://doi.org/10.2174/0929867325666180214110932
Deng C, Zhang B, Zhang S, Duan C, Cao Y, Kang W, Yan H, Ding X, Zhou F, Wu L, Duan G, Shen S, Xu G, Zhang W, Chen M, Huang S, Zhang X, Lv Y, Ling T, Wang L, Zou X (2016) Low nanomolar concentrations of cucurbitacin-I induces G2/M phase arrest and apoptosis by perturbing redox homeostasis in gastric cancer cells in vitro and in vivo. Cell Death Dis 7:e2106. https://doi.org/10.1038/cddis.2016.13
Duangmano S, Sae-Lim P, Suksamrarn A, Patmasiriwat P, Domann FE (2015) Corrigendum to “Cucurbitacin B causes increased radiation sensitivity of human breast cancer cells via G2/M cell cycle arrest.” J Oncol 2015:486850. https://doi.org/10.1155/2015/486850
Guo J, Zhao W, Hao W, Ren G, Lu J, Chen X (2014) Cucurbitacin B induces DNA damage, G2/M phase arrest, and apoptosis mediated by reactive oxygen species (ROS) in leukemia K562 cells. Anticancer Agents Med Chem 14(8):1146–1153. https://doi.org/10.2174/1871520614666140601220915
Roopa L, Akshai PS, Pravin Kumar R (2020) Connecting the dots in the mechanism of action of cucurbitacin E (CurE)—path analysis and steered molecular dynamics reveal the precise site of entry and the passage of CurE in filamentous actin. J Biomol Struct Dyn 38(3):635–646. https://doi.org/10.1080/07391102.2019.1593243
Gabrielsen M, Schuldt M, Munro J, Borucka D, Cameron J, Baugh M, Mleczak A, Lilla S, Morrice N, Olson MF (2013) Cucurbitacin covalent bonding to cysteine thiols: the filamentous-actin severing protein Cofilin1 as an exemplary target. Cell Commun Signal 11:58. https://doi.org/10.1186/1478-811X-11-58
Mallick MN, Khan W, Parveen R, Ahmad S, Sadaf NMZ, Ahmad I, Husain SA (2017) Exploring the cytotoxic potential of triterpenoids-enriched fraction of bacopa monnieri by implementing in vitro, in vivo, and in silico approaches. Pharmacogn Mag 13(Suppl 3):S595–S606. https://doi.org/10.4103/pm.pm_397_16
Silva IT, Carvalho A, Lang KL, Dudek SE, Masemann D, Duran FJ, Caro MS, Rapp UR, Wixler V, Schenkel EP, Simoes CM, Ludwig S (2015) In vitro and in vivo antitumor activity of a novel semisynthetic derivative of cucurbitacin B. PLoS ONE 10(2):e0117794. https://doi.org/10.1371/journal.pone.0117794
Lopez Sambrooks C, Baro M, Quijano A, Narayan A, Cui W, Greninger P, Egan R, Patel A, Benes CH, Saltzman WM, Contessa JN (2018) Oligosaccharyltransferase inhibition overcomes therapeutic resistance to EGFR tyrosine kinase inhibitors. Cancer Res 78(17):5094–5106. https://doi.org/10.1158/0008-5472.CAN-18-0505
Zhou S, Yan Y, Chen X, Wang X, Zeng S, Qian L, Wei J, Yang X, Zhou Y, Gong Z, Xu Z (2019) Roles of highly expressed PAICS in lung adenocarcinoma. Gene 692:1–8. https://doi.org/10.1016/j.gene.2018.12.064
Guo Y, Song J, Wang Y, Huang L, Sun L, Zhao J, Zhang S, Jing W, Ma J, Han C (2020) Concurrent genetic alterations and other biomarkers predict treatment efficacy of EGFR-TKIs in EGFR-mutant non-small cell lung cancer: a review. Front Oncol 10:610923. https://doi.org/10.3389/fonc.2020.610923
Bironzo P, Di Maio M (2018) A review of guidelines for lung cancer. J Thorac Dis 10(Suppl 13):S1556–S1563. https://doi.org/10.21037/jtd.2018.03.54
Ettinger DS, Aisner DL, Wood DE, Akerley W, Bauman J, Chang JY, Chirieac LR, D’Amico TA, Dilling TJ, Dobelbower M, Govindan R, Gubens MA, Hennon M, Horn L, Lackner RP, Lanuti M, Leal TA, Lilenbaum R, Lin J, Loo BW, Martins R, Otterson GA, Patel SP, Reckamp K, Riely GJ, Schild SE, Shapiro TA, Stevenson J, Swanson SJ, Tauer K, Yang SC, Gregory K, Hughes M (2018) NCCN guidelines insights: non-small cell lung cancer, version 5.2018. J Natl Compr Canc Netw 16(7):807–821. https://doi.org/10.6004/jnccn.2018.0062
Liu P, Xiang Y, Liu X, Zhang T, Yang R, Chen S, Xu L, Yu Q, Zhao H, Zhang L, Liu Y, Si Y (2019) Cucurbitacin B induces the lysosomal degradation of EGFR and suppresses the CIP2A/PP2A/Akt signaling axis in gefitinib-resistant non-small cell lung cancer. Molecules. https://doi.org/10.3390/molecules24030647
Zhang M, Bian ZG, Zhang Y, Wang JH, Kan L, Wang X, Niu HY, He P (2014) Cucurbitacin B inhibits proliferation and induces apoptosis via STAT3 pathway inhibition in A549 lung cancer cells. Mol Med Rep 10(6):2905–2911. https://doi.org/10.3892/mmr.2014.2581
Khan N, Jajeh F, Khan MI, Mukhtar E, Shabana SM, Mukhtar H (2017) Sestrin-3 modulation is essential for therapeutic efficacy of cucurbitacin B in lung cancer cells. Carcinogenesis 38(2):184–195. https://doi.org/10.1093/carcin/bgw124
Kausar H, Munagala R, Bansal SS, Aqil F, Vadhanam MV, Gupta RC (2013) Cucurbitacin B potently suppresses non-small-cell lung cancer growth: identification of intracellular thiols as critical targets. Cancer Lett 332(1):35–45. https://doi.org/10.1016/j.canlet.2013.01.008
Li J, Li Q, Li D, Shen Z, Zhang K, Bi Z, Li Y (2020) Long non-coding RNA MNX1-AS1 promotes progression of triple negative breast cancer by enhancing phosphorylation of Stat3. Front Oncol 10:1108. https://doi.org/10.3389/fonc.2020.01108
Yu H, Lee H, Herrmann A, Buettner R, Jove R (2014) Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer 14(11):736–746. https://doi.org/10.1038/nrc3818
Liao J, Chen Z, Yu Z, Huang T, Hu D, Su Y, He Z, Zou C, Zhang L, Lin X (2020) The role of ARL4C in erlotinib resistance: activation of the Jak2/Stat 5/beta-catenin signaling pathway. Front Oncol 10:585292. https://doi.org/10.3389/fonc.2020.585292
Zhang Y, Lee D, Brimer T, Hussaini M, Sokol L (2020) Genomics of peripheral T-cell lymphoma and its implications for personalized medicine. Front Oncol 10:898. https://doi.org/10.3389/fonc.2020.00898
Sun J, Blaskovich MA, Jove R, Livingston SK, Coppola D, Sebti SM (2005) Cucurbitacin Q: a selective STAT3 activation inhibitor with potent antitumor activity. Oncogene 24(20):3236–3245. https://doi.org/10.1038/sj.onc.1208470
Li YM, Yu JM, Liu ZY, Yang HJ, Tang J, Chen ZN (2019) Programmed death ligand 1 indicates pre-existing adaptive immune response by tumor-infiltrating CD8(+) T cells in non-small cell lung cancer. Int J Mol Sci. https://doi.org/10.3390/ijms20205138
Dai S, Yan Y, Xu Z, Zeng S, Qian L, Huo L, Li X, Sun L, Gong Z (2017) SCD1 confers temozolomide resistance to human glioma cells via the Akt/GSK3beta/beta-catenin signaling axis. Front Pharmacol 8:960. https://doi.org/10.3389/fphar.2017.00960
Chen Y, Chen C, Zhang X, He C, Zhao P, Li M, Fan T, Yan R, Lu Y, Lee RJ, Khan MW, Sarfraz M, Ma X, Yang T, Xiang G (2020) Platinum complexes of curcumin delivered by dual-responsive polymeric nanoparticles improve chemotherapeutic efficacy based on the enhanced anti-metastasis activity and reduce side effects. Acta Pharm Sin B 10(6):1106–1121. https://doi.org/10.1016/j.apsb.2019.10.011
Shukla S, Sinha S, Khan S, Kumar S, Singh K, Mitra K, Maurya R, Meeran SM (2016) Cucurbitacin B inhibits the stemness and metastatic abilities of NSCLC via downregulation of canonical Wnt/beta-catenin signaling axis. Sci Rep 6:21860. https://doi.org/10.1038/srep21860
Aboubakar Nana F, Vanderputten M, Ocak S (2019) Role of focal adhesion kinase in small-cell lung cancer and its potential as a therapeutic target. Cancers (Basel). https://doi.org/10.3390/cancers11111683
Wang X, Adjei AA (2015) Lung cancer and metastasis: new opportunities and challenges. Cancer Metastasis Rev 34(2):169–171. https://doi.org/10.1007/s10555-015-9562-4
Guo J, Wu G, Bao J, Hao W, Lu J, Chen X (2014) Cucurbitacin B induced ATM-mediated DNA damage causes G2/M cell cycle arrest in a ROS-dependent manner. PLoS ONE 9(2):e88140. https://doi.org/10.1371/journal.pone.0088140
Kong H, Chandel NS (2018) Regulation of redox balance in cancer and T cells. J Biol Chem 293(20):7499–7507. https://doi.org/10.1074/jbc.TM117.000257
Yin Y, Chen F (2020) Targeting human MutT homolog 1 (MTH1) for cancer eradication: current progress and perspectives. Acta Pharm Sin B 10(12):2259–2271. https://doi.org/10.1016/j.apsb.2020.02.012
Shukla S, Khan S, Kumar S, Sinha S, Farhan M, Bora HK, Maurya R, Meeran SM (2015) Cucurbitacin B alters the expression of tumor-related genes by epigenetic modifications in NSCLC and inhibits NNK-induced lung tumorigenesis. Cancer Prev Res (Phila) 8(6):552–562. https://doi.org/10.1158/1940-6207.CAPR-14-0286
Liu Y, Yang H, Guo Q, Liu T, Jiang Y, Zhao M, Zeng K, Tu P (2020) Cucurbitacin E inhibits Huh7 hepatoma carcinoma cell proliferation and metastasis via suppressing MAPKs and JAK/STAT3 pathways. Molecules. https://doi.org/10.3390/molecules25030560
Yang P, Liu W, Fu R, Ding GB, Amin S, Li Z (2020) Cucurbitacin E chemosensitizes colorectal cancer cells via mitigating TFAP4/Wnt/beta-catenin signaling. J Agric Food Chem. https://doi.org/10.1021/acs.jafc.0c05551
Chen JC, Chiu MH, Nie RL, Cordell GA, Qiu SX (2005) Cucurbitacins and cucurbitane glycosides: structures and biological activities. Nat Prod Rep 22(3):386–399. https://doi.org/10.1039/b418841c
Hsu YC, Huang TY, Chen MJ (2014) Therapeutic ROS targeting of GADD45gamma in the induction of G2/M arrest in primary human colorectal cancer cell lines by cucurbitacin E. Cell Death Dis 5:e1198. https://doi.org/10.1038/cddis.2014.151
Cheng SE, Lee IT, Lin CC, Wu WL, Hsiao LD, Yang CM (2013) ATP mediates NADPH oxidase/ROS generation and COX-2/PGE2 expression in A549 cells: role of P2 receptor-dependent STAT3 activation. PLoS ONE 8(1):e54125. https://doi.org/10.1371/journal.pone.0054125
Feng H, Zang L, Zhao ZX, Kan QC (2014) Cucurbitacin-E inhibits multiple cancer cells proliferation through attenuation of Wnt/beta-catenin signaling. Cancer Biother Radiopharm 29(5):210–214. https://doi.org/10.1089/cbr.2014.1614
Ma G, Luo W, Lu J, Ma DL, Leung CH, Wang Y, Chen X (2016) Cucurbitacin E induces caspase-dependent apoptosis and protective autophagy mediated by ROS in lung cancer cells. Chem Biol Interact 253:1–9. https://doi.org/10.1016/j.cbi.2016.04.028
Hsu PC, Tian B, Yang YL, Wang YC, Liu S, Urisman A, Yang CT, Xu Z, Jablons DM, You L (2019) Cucurbitacin E inhibits the yesassociated protein signaling pathway and suppresses brain metastasis of human nonsmall cell lung cancer in a murine model. Oncol Rep 42(2):697–707. https://doi.org/10.3892/or.2019.7207
Klein R, Stiller S, Gashaw I (2012) Epidermal growth factor upregulates endometrial CYR61 expression via activation of the JAK2/STAT3 pathway. Reprod Fertil Dev 24(3):482–489. https://doi.org/10.1071/RD10335
Huang X, Renwick JA, Sachdev-Gupta K (1993) Oviposition stimulants and deterrents regulating differential acceptance ofIberis amara by Pieris rapae andP. napi oleracea. J Chem Ecol 19(8):1645–1663. https://doi.org/10.1007/BF00982298
Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM (2003) Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res 63(6):1270–1279
Guo H, Kuang S, Song QL, Liu M, Sun XX, Yu Q (2018) Cucurbitacin I inhibits STAT3, but enhances STAT1 signaling in human cancer cells in vitro through disrupting actin filaments. Acta Pharmacol Sin 39(3):425–437. https://doi.org/10.1038/aps.2017.99
Hsu HS, Huang PI, Chang YL, Tzao C, Chen YW, Shih HC, Hung SC, Chen YC, Tseng LM, Chiou SH (2011) Cucurbitacin I inhibits tumorigenic ability and enhances radiochemosensitivity in nonsmall cell lung cancer-derived CD133-positive cells. Cancer 117(13):2970–2985. https://doi.org/10.1002/cncr.25869
Ito M, Codony-Servat C, Codony-Servat J, Llige D, Chaib I, Sun X, Miao J, Sun R, Cai X, Verlicchi A, Okada M, Molina-Vila MA, Karachaliou N, Cao P, Rosell R (2019) Targeting PKCiota-PAK1 signaling pathways in EGFR and KRAS mutant adenocarcinoma and lung squamous cell carcinoma. Cell Commun Signal 17(1):137. https://doi.org/10.1186/s12964-019-0446-z
Wang S, Wang SY, Du F, Han Q, Wang EH, Luo EJ, Liu Y (2019) Knockdown of PAK1 inhibits the proliferation and invasion of non-small cell lung cancer cells through the ERK pathway. Appl Immunohistochem Mol Morphol. https://doi.org/10.1097/PAI.0000000000000803
Nguyen BC, Be Tu PT, Tawata S, Maruta H (2015) Combination of immunoprecipitation (IP)-ATP_Glo kinase assay and melanogenesis for the assessment of potent and safe PAK1-blockers in cell culture. Drug Discov Ther 9(4):289–295. https://doi.org/10.5582/ddt.2015.01041
Ni Y, Wu S, Wang X, Zhu G, Chen X, Ding Y, Jiang W (2018) Cucurbitacin I induces pro-death autophagy in A549 cells via the ERK-mTOR-STAT3 signaling pathway. J Cell Biochem 119(7):6104–6112. https://doi.org/10.1002/jcb.26808
Zhu X, Huang H, Zhang J, Liu H, Ao R, Xiao M, Wu Y (2018) The anticancer effects of cucurbitacin I inhibited cell growth of human nonsmall cell lung cancer through PI3K/AKT/p70S6K pathway. Mol Med Rep 17(2):2750–2756. https://doi.org/10.3892/mmr.2017.8141
Wang WD, Liu Y, Su Y, Xiong XZ, Shang D, Xu JJ, Liu HJ (2017) Antitumor and apoptotic effects of cucurbitacin a in a-549 lung carcinoma cells is mediated via G2/M cell cycle arrest and M-Tor/Pi3k/Akt signalling pathway. Afr J Tradit Complement Altern Med 14(2):75–82. https://doi.org/10.21010/ajtcam.v14i2.9
Sikander M, Malik S, Chauhan N, Khan P, Kumari S, Kashyap VK, Khan S, Ganju A, Halaweish FT, Yallapu MM, Jaggi M, Chauhan SC (2019) Cucurbitacin D reprograms glucose metabolic network in prostate cancer. Cancers (Basel). https://doi.org/10.3390/cancers11030364
Jacquot C, Rousseau B, Carbonnelle D, Chinou I, Malleter M, Tomasoni C, Roussakis C (2014) Cucurbitacin-D-induced CDK1 mRNA up-regulation causes proliferation arrest of a non-small cell lung carcinoma cell line (NSCLC-N6). Anticancer Res 34(9):4797–4806
Ku JM, Hong SH, Kim HI, Kim MJ, Kim SK, Kim M, Choi SY, Park J, Kim HK, Kim JH, Seo HS, Shin YC, Ko SG (2020) Synergistic anticancer effect of combined use of Trichosanthes kirilowii with cisplatin and pemetrexed enhances apoptosis of H1299 non-small-cell lung cancer cells via modulation of ErbB3. Phytomedicine 66:153109. https://doi.org/10.1016/j.phymed.2019.153109
Ni L, Zhu X, Gong C, Luo Y, Wang L, Zhou W, Zhu S, Li Y (2015) Trichosanthes kirilowii fruits inhibit non-small cell lung cancer cell growth through mitotic cell-cycle arrest. Am J Chin Med 43(2):349–364. https://doi.org/10.1142/S0192415X15500238
Chen XB, Chen GY, Liu JH, Lei M, Meng YH, Guo DA, Liu X, Hu LH (2014) Cytotoxic cucurbitane triterpenoids isolated from the rhizomes of Hemsleya amabilis. Fitoterapia 94:88–93. https://doi.org/10.1016/j.fitote.2014.01.014
Wu J, Wu Y, Yang BB (2002) Anticancer activity of Hemsleya amabilis extract. Life Sci 71(18):2161–2170. https://doi.org/10.1016/s0024-3205(02)02013-1
Boykin C, Zhang G, Chen YH, Zhang RW, Fan XE, Yang WM, Lu Q (2011) Cucurbitacin IIa: a novel class of anti-cancer drug inducing non-reversible actin aggregation and inhibiting survivin independent of JAK2/STAT3 phosphorylation. Br J Cancer 104(5):781–789. https://doi.org/10.1038/bjc.2011.10
Zhang J, Song Y, Liang Y, Zou H, Zuo P, Yan M, Jing S, Li T, Wang Y, Li D, Zhang T, Wei Z (2019) Cucurbitacin IIa interferes with EGFR-MAPK signaling pathway leads to proliferation inhibition in A549cells. Food Chem Toxicol 132:110654. https://doi.org/10.1016/j.fct.2019.110654
Torres-Moreno H, Marcotullio MC, Velazquez C, Ianni F, Garibay-Escobar A, Robles-Zepeda RE (2020) Cucurbitacin IIb, a steroidal triterpene from Ibervillea sonorae induces antiproliferative and apoptotic effects on cervical and lung cancer cells. Steroids 157:108597. https://doi.org/10.1016/j.steroids.2020.108597
Chen C, Qiang S, Lou L, Zhao W (2009) Cucurbitane-type triterpenoids from the stems of Cucumis melo. J Nat Prod 72(5):824–829. https://doi.org/10.1021/np800692t
Cucurbitacin B | C32H46O8—PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/5281316#section=Toxicological-Information. Accessed 2020/6/8
Edery H, Schatzberg-Porath G, Gitter S (1961) Pharmaco-dynamic activity of elatericin (cucurbitacin D). Arch Int Pharmacodyn Ther 130:315–335
Cucurbitacin E | C32H44O8—PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/5281319#section=Toxicity. Accessed 2020/6/8
Hong SH, Ku JM, Lim YS, Lee SY, Kim JH, Cheon C, Ko SG (2020) Cucurbitacin D overcomes gefitinib resistance by blocking EGF binding to EGFR and inducing cell death in NSCLCs. Front Oncol 10:62. https://doi.org/10.3389/fonc.2020.00062
Wang Z, Zhu W, Gao M, Wu C, Yang C, Yang J, Wu G, Yang B, Kuang H (2017) Simultaneous determination of cucurbitacin B and cucurbitacin E in rat plasma by UHPLC-MS/MS: a pharmacokinetics study after oral administration of cucurbitacin tablets. J Chromatogr B Analyt Technol Biomed Life Sci 1065–1066:63–69. https://doi.org/10.1016/j.jchromb.2017.09.024
Bai M, Li HL, He JC, He GH, Feng EF, Liu YQ, Shi PP, Xu GL (2014) Development and validation of an LC-ESI-MS/MS method for the quantitation of hemslecin A in rhesus monkey plasma and its application in pharmacokinetics. Biomed Chromatogr 28(3):385–390. https://doi.org/10.1002/bmc.3032
Wang Y, Zhao GX, Xu LH, Liu KP, Pan H, He J, Cai JY, Ouyang DY, He XH (2014) Cucurbitacin IIb exhibits anti-inflammatory activity through modulating multiple cellular behaviors of mouse lymphocytes. PLoS ONE 9(2):e89751. https://doi.org/10.1371/journal.pone.0089751
Varricchio A, De Lucia A, Varricchio AM, Della Volpe A, Mansi N, Pastore V, Ciprandi G (2017) Sinuclean Nebules treatment in children suffering from otitis media with effusion. Int J Pediatr Otorhinolaryngol 94:30–35. https://doi.org/10.1016/j.ijporl.2017.01.001
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7(2):27–31. https://doi.org/10.4103/0976-0105.177703
Lu P, Yu B, Xu J (2012) Cucurbitacin B regulates immature myeloid cell differentiation and enhances antitumor immunity in patients with lung cancer. Cancer Biother Radiopharm 27(8):495–503. https://doi.org/10.1089/cbr.2012.1219
Molavi O, Shayeganpour A, Somayaji V, Hamdy S, Brocks DR, Lavasanifar A, Kwon GS, Samuel J (2006) Development of a sensitive and specific liquid chromatography/mass spectrometry method for the quantification of cucurbitacin I (JSI-124) in rat plasma. J Pharm Pharm Sci 9(2):158–164
Hunsakunachai N, Nuengchamnong N, Jiratchariyakul W, Kummalue T, Khemawoot P (2019) Pharmacokinetics of cucurbitacin B from Trichosanthes cucumerina L. in rats. BMC Complement Altern Med 19(1):157. https://doi.org/10.1186/s12906-019-2568-7
Fiori GML, D’Agate S, Rocha A, Pereira AMS, Della Pasqua O, Lopes NP (2017) Development and validation of a quantification method for cucurbitacins E and I in rat plasma: application to population pharmacokinetic studies. J Pharm Biomed Anal 144:99–105. https://doi.org/10.1016/j.jpba.2017.02.021
Wang S, Guan X, Zhong X, Yang Z, Huang W, Jia B, Cui T (2016) Simultaneous determination of cucurbitacin IIa and cucurbitacin IIb of Hemsleya amabilis by HPLC-MS/MS and their pharmacokinetic study in normal and indomethacin-induced rats. Biomed Chromatogr 30(10):1632–1640. https://doi.org/10.1002/bmc.3733
Dong XD, Zhang M, Ma X, Wang JQ, Lei ZN, Teng QX, Li YD, Lin L, Feng W, Chen ZS (2020) Bruton’s tyrosine kinase (BTK) inhibitor RN486 overcomes ABCB1-mediated multidrug resistance in cancer cells. Front Cell Dev Biol 8:865. https://doi.org/10.3389/fcell.2020.00865
Metti S, Gambarotto L, Chrisam M, Baraldo M, Braghetta P, Blaauw B, Bonaldo P (2020) The polyphenol pterostilbene ameliorates the myopathic phenotype of collagen VI deficient mice via autophagy induction. Front Cell Dev Biol 8:580933. https://doi.org/10.3389/fcell.2020.580933
Uchida Y, Ferdousi F, Zheng YW, Oda T, Isoda H (2020) Global gene expression profiling reveals isorhamnetin induces hepatic-lineage specific differentiation in human amniotic epithelial cells. Front Cell Dev Biol 8:578036. https://doi.org/10.3389/fcell.2020.578036
Garg SM, Vakili MR, Molavi O, Lavasanifar A (2017) Self-associating poly(ethylene oxide)-block-poly(alpha-carboxyl-epsilon-caprolactone) drug conjugates for the delivery of STAT3 inhibitor JSI-124: potential application in cancer immunotherapy. Mol Pharm 14(8):2570–2584. https://doi.org/10.1021/acs.molpharmaceut.6b01119
Molavi O, Ma Z, Mahmud A, Alshamsan A, Samuel J, Lai R, Kwon GS, Lavasanifar A (2008) Polymeric micelles for the solubilization and delivery of STAT3 inhibitor cucurbitacins in solid tumors. Int J Pharm 347(1–2):118–127. https://doi.org/10.1016/j.ijpharm.2007.06.032
Lv Q, Shen C, Li X, Shen B, Yu C, Xu P, Xu H, Han J, Yuan H (2015) Mucoadhesive buccal films containing phospholipid-bile salts-mixed micelles as an effective carrier for cucurbitacin B delivery. Drug Deliv 22(3):351–358. https://doi.org/10.3109/10717544.2013.876459
Cheng L, Xu PH, Shen BD, Shen G, Li JJ, Qiu L, Liu CY, Yuan HL, Han J (2015) Improve bile duct-targeted drug delivery and therapeutic efficacy for cholangiocarcinoma by cucurbitacin B loaded phospholipid complex modified with berberine hydrochloride. Int J Pharm 489(1–2):148–157. https://doi.org/10.1016/j.ijpharm.2015.04.024
Tang L, Fu L, Zhu Z, Yang Y, Sun B, Shan W, Zhang Z (2018) Modified mixed nanomicelles with collagen peptides enhanced oral absorption of cucurbitacin B: preparation and evaluation. Drug Deliv 25(1):862–871. https://doi.org/10.1080/10717544.2018.1425773
Clericuzio M, Mella M, Vita-Finzi P, Zema M, Vidari G (2004) Cucurbitane triterpenoids from Leucopaxillus gentianeus. J Nat Prod 67(11):1823–1828. https://doi.org/10.1021/np049883o
Rodriguez N, Vasquez Y, Hussein AA, Coley PD, Solis PN, Gupta MP (2003) Cytotoxic cucurbitacin constituents from Sloanea zuliaensis. J Nat Prod 66(11):1515–1516. https://doi.org/10.1021/np0303106
Clericuzio M, Tabasso S, Bianco MA, Pratesi G, Beretta G, Tinelli S, Zunino F, Vidari G (2006) Cucurbitane triterpenes from the fruiting bodies and cultivated mycelia of Leucopaxillus gentianeus. J Nat Prod 69(12):1796–1799. https://doi.org/10.1021/np060213n
Kongtun S, Jiratchariyakul W, Kummalue T, Tan-ariya P, Kunnachak S, Frahm AW (2009) Cytotoxic properties of root extract and fruit juice of Trichosanthes cucumerina. Planta Med 75(8):839–842. https://doi.org/10.1055/s-0029-1185455
Tartarone A, Giordano P, Lerose R, Rodriquenz MG, Conca R, Aieta M (2017) Progress and challenges in the treatment of small cell lung cancer. Med Oncol 34(6):110. https://doi.org/10.1007/s12032-017-0966-6
Visconti R, Morra F, Guggino G, Celetti A (2017) The between now and then of lung cancer chemotherapy and immunotherapy. Int J Mol Sci. https://doi.org/10.3390/ijms18071374
Wu G, Yan Y, Zhou Y, Duan Y, Zeng S, Wang X, Lin W, Ou C, Zhou J, Xu Z (2020) Sulforaphane: expected to become a novel anti-tumor compound. Oncol Res. https://doi.org/10.3727/096504020X15828892654385
Abou-Salim MA, Shaaban MA, Abd El Hameid MK, Elshaier Y, Halaweish F (2019) Design, synthesis and biological study of hybrid drug candidates of nitric oxide releasing cucurbitacin-inspired estrone analogs for treatment of hepatocellular carcinoma. Bioorg Chem 85:515–533. https://doi.org/10.1016/j.bioorg.2019.01.068
Marostica LL, de Barros ALB, Oliveira J, Salgado BS, Cassali GD, Leite EA, Cardoso VN, Lang KL, Caro MSB, Duran FJ, Schenkel EP, de Oliveira MC, Simoes CMO (2017) Antitumor effectiveness of a combined therapy with a new cucurbitacin B derivative and paclitaxel on a human lung cancer xenograft model. Toxicol Appl Pharmacol 329:272–281. https://doi.org/10.1016/j.taap.2017.06.007
Marostica LL, Silva IT, Kratz JM, Persich L, Geller FC, Lang KL, Caro MS, Duran FJ, Schenkel EP, Simoes CM (2015) Synergistic antiproliferative effects of a new cucurbitacin B derivative and chemotherapy drugs on lung cancer cell line A549. Chem Res Toxicol 28(10):1949–1960. https://doi.org/10.1021/acs.chemrestox.5b00153
Acknowledgements
The study was supported by the National Natural Science Foundation of China (82002239, 81803035, 81703036), and the Natural Science Foundation of Hunan Province, China (2020JJ5934, 2019JJ50932).
Author information
Authors and Affiliations
Contributions
ML, QY, SZ, ZX, ZG and YY were the main authors of the manuscript; ML and ZX contributed to the design and format of figures and tables; BP, YC, and YY revised the manuscript. All authors were responsible for the manuscript writing and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, M., Yan, Q., Peng, B. et al. Use of cucurbitacins for lung cancer research and therapy. Cancer Chemother Pharmacol 88, 1–14 (2021). https://doi.org/10.1007/s00280-021-04265-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-021-04265-7