Abstract
Heavy metals are environmental contaminants that are hazardous to soil, water, animals, and human health. Recently, the problem of heavy metal treatment in soil after mining has been studied by many scientists both at laboratory and field. The application of native plants in treating heavy metals (Phytoremediation) in the soil after mining is interesting, appreciated, and highly applicable because of its friendliness to the polluted environment, cost-saving, improving the landscape and environment, and favorable for long-term implementation. The primal objective of this study was to examine the tolerance of native plants to lead (Pb) accumulation in soil. The plant samples and soil samples were taken analyzed for Pb concentration. Research results showed that, all three species of plants had the ability to grow and develop well in the environment with the concentrations of Pb. The average Pb accumulated was the highest in Lantana camara at 3.38 mg/kg, followed by Eleusine indica and Aglaonema muntifolium at 1.67 mg/kg and1.37 mg/kg, respectively. Based on the enrichment coefficients for stem-leaves and root values, plant was separated into two group including hyperaccumulator (Aglaonema muntifolium) and phytostabilizer (Lantana camara and Eleusine indica). Our study suggested the effectiveness of some native plant species in treating Pb contaminants in soil, this result can be applied in mitigating the effect of heavy metals caused by mining on environmental and human health.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Mine activities have been considered as the main hazardous land uses that lead to the serious consequences on terrestrial ecosystems, particularly because of the long-lasting contamination and risks from heavy metals generated and released into the environment (Hye et al. 2008; Mileusnić et al. 2014). Metals are released from mining sites via activities including mining-milling, ore concentrating, refining processes, and disposal of tailings, and waste rock (Hien et al. 2012). Indeed, the high level of heavy metal derived from the weathering processes of minerals, tailings and waste rock, then concentrated in soil resources and water resources neighboring to mining sites has been reported in many of previous studies (Lee et al. 2001; Israel et al. 2004; Aparajita et al. 2006). The impacts of heavy metal on human health are of concern as the cause of cytotoxicity, mutagenicity, and carcinogenicity (Lim and Schoenung 2010).
In recent decades, the remediation to reduce the concentration of heavy metal in soil and their risks to human health has become a worldwide environmental goal (Sivarajasekar and Baskar 2014). The treatment of heavy metal contaminated soils is complicated and often incomplete because the properties of the soil are changed when associated with heavy metals. Many physio-chemical methods have been selected to treat soil contaminated with heavy metals such as soil washing, concreting, chemical precipitation, thermal desorption, redox, adsorption, incineration, and solidification (Traina et al. 2007; Karthik et al. 2016; Muthusaravanan et al. 2018). Conventional techniques for pollution control can be very effective in solving the contamination, however, on the other hand, these methods have certain disadvantages because of the high cost, high energy consumption, large quantities of wastes that require disposal, and the high potential of contaminants exposure for surrounding residents (Moeller 2005; Vidalli 2005). This situation raises the need for a more eco-friendly and effective approach to rectify polluted soil.
Currently, the method of using plants to treat heavy metals in the soil (Phytoremedation) has been emphasized as an effective and potential strategy for cleaning soil polluted by heavy metals (Edgar et al. 2982). This remediation is outweighed as it provides cheaper, high efficiency, and environmentally acceptable technology for the bioremediation of contaminated soil (Glick 2010; Eslamian 2016). One of the mechanisms of phytoremediation is phytoaccumulation, which is the process of contaminants absorption by plants, the absorbed contaminants then being accumulated in shoots, leaves, and other plant parts (Muthusaravanan et al. 2018; Rashid et al. 2014). This is the best method to be able to remove the pollutant from the soil and then sequester it without destroying the structure and fertility of the soil (Ghosh and Singh 2005). Indeed, numerous of previous studies have focused on the effectiveness of phytoremediation. For instance, (Conesa et al. 2007) reported that Zygophyllum fabago can accumulate Pb concentration in soil at 750 mg/kg in the shoots. In another study, (Altinozlu et al. 2012) found that in the polluted area where the soil contains of 2000–3000 mg Ni per kg soil, Isatis pinnatiloba has the ability to absorb and accumulate Ni contaminant up to 1441 mg/kg in the above-ground plant parts. The exceptional ability of metal accumulation was also found in Noccaea caerulescens, this species can accumulate up to 26,000 mg/kg Zn without any symptom of injuries (Brown et al. 1995).
Despite the current efforts to develop plant base remediation, the selection of suitable plants that can show advanced performance in phytoremediation is still critical and remains certain difficulties (Pan et al. 2019). The adaptation of plant to the environmental conditions of target regions is remaining as the drawback for the success of the phytoremediation strategy (Mahar et al. 2016). Native plants therefore should be an important aspect in treating heavy metal pollution using phytoremediation. Native plant species are often more resistant to changes in their habitat, they perform better in terms of survival, growth, and reproduction compared to exotic species, behind, they are more tolerant to metal pollution in specified soil conditions (Frérot et al. 2006; Yoon et al. 2006; Antosiewicz et al. 2008). Thus, the assessment of native plants that grow naturally around mining and mineral processing areas in treating heavy metal pollution becomes essential.
Vietnam is a developing country, located in Southeast Asia. In recent decades, the rapid industrialization and development of a market for environmental services had led to many issues of environmental protection and sustainable development, among them, the mining waste disposal program became one of the Vietnamese government’s strategic priorities (Xuan et al. 2013). Indeed, Vietnam has been ranked as the third-largest mineral producer among Southeast Asian countries (Khoi 2014). The northern part of Vietnam has abundant of Pb and Zinc with a total ore reserve of up to 97 million tons and a lot of metalliferous mines have been established (Tran et al. 2009). Along with the growth of the Vietnamese economy contributed by mining, there are the risks of high contents of heavy metals in waste dumps and soil around mining areas (e.g., Chu Ngoc et al. 2009; Anh et al. 2011; Xuan et al. 2017). Therefore, the monitoring of native plants phytoremediation in soil pollution is needed especially in the vicinity of mining areas in Vietnam. The goal of this study was to examine the tolerance of native plants to lead (Pb) accumulation in soil in northeastern Vietnam. To this end, the concentration of lead in soil at the study site was determined and the ability to absorb and accumulate lead of native plants were examined. Based on the results of this study, we proposed solutions to apply plants in the treatment of lead pollution in soil due to mineral mining.
15.2 Study Site and Method
15.2.1 Study Site
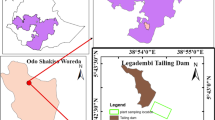
This study was conducted in Vang Danh coal mine, which is located in Uong Bi city, Quang Ning province, northeastern Vietnam (20° 58′ N, 106° 41′ E, Fig. 15.1a–c). Vang Danh coal mine is owned by Vang Danh Coal Joint Stock Company-a member of Vietnam National Coal-Mineral Industries Holding Corporation Limited. Vang Danh is known as the largest underground coal mine in Vietnam's coal industry, the area of the mine site is 2000 ha. The climate is warm and temperate with a mean annual temperature of 22.2 °C and an average annual rainfall of 1600 mm (Son et al. 2019). The rainy season occurs from June to August, accounting for 60% of annual rainfall, while the dry season is usually from November to April. The coal-bearing strata mostly combine of Conglomerate, sandstone, siltstone, claystone, clay-coal, and coal seams (Dung and Quang 2021). The native plant species of the study site mainly belong to Fabaceae, Moraceae, Araceae, Verbenaceae, Poaceae, Polypodiaceae, and Piperaceae. The mining area has been established since 1964. The extent of coal mining has been increased for both open pit and underground mines. Coal mining brings back huge economic benefits, however, the heavy metal pollution in the soil caused by mining activities poses a big threat to the environment and human health (Hai 2007).
15.2.2 Sampling and Analyzing of Soil and Plant Species
Three different areas in the coal mine site were selected for sampling and analysis (Fig. 15.1b). These three areas were located surrounding the mine dump and were covered by a large number of native species. The detailed field survey of soil and native plants was conducted in August/2021.
Soil samples were collected by using the stainless-steel sampler (Diameter: three cm, Height: 20 cm). The samplings were processed following a mixed sampling method (Vietnam Standard 2005). In general, three soil samples including topsoil and subsoil within the top 20 cm were taken, the total amount of each sample was 200 g. The samples then were air-dried, sieved to < two mm, and stored in plastic bags.
The selection of plants for accessing the ability to absorb and accumulate Pb in soil was based on plant coverage of the site as well as plant health. The three most common distributed plant species that grow directly in the coal field of the study area were monitored, including Aglaonema muntifolium, Lantana camara, and Eleusine indica (Fig. 15.1d–f). These plant species associated with soil samples were collected in the same area (Fig. 15.1b). Each plant was divided into three parts, which are root, stem, and leaves. The vegetable samples were stored in plastic bags after being manually washed with tap water and rinsed with deionized water three times to remove dust.
For the chemical analysis, all the soil and plant samples were sent to the Mekong Institute of Science and Technology in Hanoi. The concentration of Pb in soil was determined by flame atomic absorption spectrometry analysis (TCVN 6496:2009). This method is based on atomic absorption spectrometry of elemental concentrations in Aqua Regia sample extracts prepared in accordance with ISO 11466. Used 217.0 nm wavelength is used and the flame type is oxidizing air. Whereas the Atomic absorption spectrometric method was applied to examining Pb concentration in plants after dry ashing (AOAC 999.11). The test portion is dried and then ashed at 450 °C with increasing temperature. Add six M hydrochloric acid solution and evaporate to dryness. The residue was dissolved in 0.1 M nitric acid solution, the analytical samples were determined by flame atomic absorption spectrometry using a graphite furnace.
Bioconcentration factor (BCF), bioaccumulation factor (BAF) and translocation factor (TF) were used in data analyzing (Yoon et al. 2006; Caille et al. 2005; Zu et al. 2005). BCF is calculated as the ratio between the Pb content in the roots with the total Pb content in the original soil, while BAF is calculated by considering Pb concentration in stem-leaves with respect to soil Pb concentrations. The BCF and BAF accumulation coefficient reflects the ability of plants to accumulate Pb from soil to plants and is used to assess the potential for pollution treatment of plant species (Ghosh and Singh 2005). TF is the ratio between the Pb content in the stem—leaves with the corresponding content in the roots. This index evaluates the ability to transport Pb from the roots to the leaves (Zhang et al. 2002).
Analysis of variance (ANOVA) was performed to determine statistical differences between Pb concentration in soil, plant parts, BCF, BAF, and TF values with a confidence level of 0.95 (P < 0.05), using SPSS for Windows, version 20.
15.3 Result and Discussion
15.3.1 Biomass of Plants
The result of fresh biomass of sampling plant species was present in Table 15.1. For all species in three sampling areas, the biomass of stem-leaves was higher compared to that of roots (Table 15.1; Fig. 15.2). The average above-ground biomass of A. muntifolium and L. camara was comparable at 11 g, whereas there was a slightly different in root biomass of these two species at four and five g, respectively. The lowest biomass was found in E. indica, the average biomass of stem-leaves and roots in turn were seven g and two g (Fig. 15.2). Among of three sampling areas, area three had the highest total plant biomass, which was 70% higher than the biomass of area one and 34% higher than the biomass of area two (Table 15.1).
15.3.2 Concentration of Pb in Soil and in Native Plant Species
The mean Pb concentration in soil samples of the study site was 54 ± 18 mg/kg (Table 15.1). Soil Pb concentration in this study was within the Vietnamese standard for Pb concentration in soil (QCVN 03: 2015). There were significant differences among Pb concentrations in three sampling areas with the p-value < 0.05. The contents of Pb detected in order of smallest to largest were 34.8 mg/kg in area one, 55.7 mg/kg in area two, and 70.8 mg/kg in area three (Table 15.1).
The results of the Pb concentration in plant species grown in coal mine site were presented in Table 15.1. The total concentration of Pb in L. camara ranged from 1.62 to 5.26 mg/kg, whereas, in A. muntifolium and E. indica these values fluctuated from 0.61 to 1.99 mg/kg and 0.86 to 2.84 mg/kg, respectively. In general, the highest concentration of Pb was found in L. camara. The concentration of Pb in plants varied among sampling areas, all monitored species in area one contained the lowest amount of Pb, whereas the concentration of Pb in area three was consistently the highest for all sampling plant species (Table 15.1).
Identifying the tolerance plant that can adapt to local climate and soil condition is essential for soil remediation, thus native plant species have been selected because of their suitability. In this study, none of the visible toxicity symptoms, such as whitish-brown chlorosis, or young leaves deformation were observed in the monitored species. All the studied plants grew normally and exhibited tolerance to Pb contamination. Pb concentrated in the different parts of the plant varied among species (Figs. 15.3 and 15.4). The average concentration of Pb in the stem was the lowest in all species at 0.62 mg/kg in L. camara, 0.33 mg/kg in A. muntifolium and 0.13 mg/kg in E. indica (Fig. 15.3). Pb concentrated in roots was higher than that in the leaves in L. camara and E. indica. In particular, the average level of Pb in root and leaves of L. camara were 1.72 mg/kg and 1.04 mg/kg, respectively. Average root and leaves Pb values of E. indica were 1.15 mg/kg and 0.39 mg/kg, respectively. In A. muntifolium root and leaves Pb concentrations were similar at 0.51 mg/kg and 0.53 mg/kg, respectively (Fig. 15.3). In general, the concentration of Pb in plants in this study was significantly lower than that in previous studies of (Pan et al. 2019; Nguyen et al. 2009; Petelka et al. 2019), however, results from monitored plants were higher compared to Petelka et al. (2019), suggesting the influence of plant species, the environmental concentrations of heavy metals, and other environmental factors to the concentration of heavy metals in plants.
L. camara and E. indica contained the higher amount of Pb in the root parts, which account for 50–70% of the total Pb accumulated. Plants with these characteristics are highlighted to be the potential plant for phytostabilization purposes (Mendez and Maier 2008; Ali et al. 2013). The high concentration of Pb in roots is possibly related to the features of the root system. The study by Pinto et al. (2015) has pointed out that the dense and tough root system is also important for absorbing metals in contaminated soil. L. camara and E. indica have a notably tough root system, this characteristic can enhance the ability to absorb and accumulate Pb, thus making these species the good candidates for Pb phytostabilization (Pan et al. 2019). In the contrary, A. muntifolium exhibited the higher concentration of Pb in the stem-leaves than in the root, suggesting a high transportability for metals from the root to the above-ground parts. Besides the capacity of metal uptake via the xylems, atmospheric deposition of Pb may be another factor that resulted in the high concentrations of stem and leaves (Temmerman et al. 2015).
15.3.3 The Effectives of Native Plants Remediation in Pb Treatment
The BAF, BCF, and TF are the key factors in evaluating and qualifying the heavy metal hyperaccumulator (Pan et al. 2019), in this study, these factors were used to evaluate the phytoremediation potential of plants.
The BAF values of plants ranged from 0.005 to 0.042 and the accumulation coefficient BCF in the root part varied from 0.007 to 0.036 (Table 15.1; Fig. 15.5). L. camara had the highest ability to absorb and accumulate Pb with the average BAF and BCF were 0.029 and 0.031, respectively. The average value of BAF and BCF in A. muntifolium in turn were 0.015 and 0.001. E. indica had the lowest BAF value among species, which was 0.008 and the BCF value was 0.021 for average (Table 15.1).
The TF value was found to be the highest in A. muntifolium, with all the measurement values larger than one, the average TF value for this species was 1.606. TF value was followed by L. camara at 0.952 and E. indica at 0.410 for average. The significant difference in the TF value of A. muntifolium and E. indica with p-value < 0.05.
The BAF, BCF, and TF of plant species varied among the sample sites, suggesting the diversity in heavy metal tolerance, absorption and translocation varied with genotypes. These results are possibly explained by the environmental condition and the characteristic of the plant itself. The ability to absorb and accumulate heavy metals in plants depends on many factors, for example, the bioavailability of metal within the rhizosphere (Petelka et al. 2019), the characteristic of the root systems that determine how heavy metals are absorbed and fixed within the root cells (Muthusaravanan et al. 2018), xylem loading capacity and cellular tolerance to toxic metals of the plant (Pan et al. 2019). Plant species that had a BAF, BCF, or TF smaller than one are unsuitable for phytoextraction, which is the withdrawal of heavy metals by the harvestable parts of roots, stems, and leaves (Kumar et al. 1995; Fitz and Wenzel 2002). Based on the result, none of the studied species is recommended for phytoextraction. TF indicates the efficiency of a plant in translocating heavy metals from roots to aerial parts (Zhang et al. 2002). TF larger than one also indicates that a large portion of Pb was transported from root to stem and leaves then accumulated in the above-ground parts of plants. The low TF value from the plant might relate to the metal toxicity that can damage photosynthetic activity, chlorophyll synthesis, and antioxidant enzymes (Chaabani et al. 2017). When the value of TF is larger than one, the plant is a hyperaccumulator, and if TF is smaller than one, the plant is a phytostabilizer (Yoon et al. 2006). The result of the study indicated that A. muntifolium was the hyperaccumulator with a higher concentration of Pb in stem-leaves than in the root part, whereas L. camara and E. indica were phytostabilizer.
The summary of Pb concentration and translocation of various plant species from other studies was presented in Table 15.2. The amount of Pb content in different plant parts of the three studied species was significantly lower compared to that from some of the previous studies (Pan et al. 2019; Chang et al. 2018), this result was attributable to the 50–1000 times lower in Pb concentration in soil from our study site (Table 15.2). Whereas, the values of bioaccumulation factor, bioconcentration factor, and translocation factor of this study were within the range of previous studies. Most of the plant species contained more metals in their roots than in the above-ground parts and can be classified as phytostabilizer. The ability of the plant to absorb and translocate heavy metals into the plant system is strongly determined by their solubility and complexity (Chaabani et al. 2017; Rungwaa et al. 2013). Pb contaminated in soil being absorbed and uptaken by plants occurs either passively with the water flow taken by roots or actively through the plasma membrane of root epidermal cells (Nouri et al. 2011).
In screening the suitable and effective plant for phytoremediation, besides the characteristics of accumulation or fixation of metals by plants, there are some other botanical features, such as abundance, a high growth rate, and good biomass yields (Ali et al. 2013) that should be considered. In general, L. camara and A. muntifolium are two species of plants with relatively large biomass, fast growth, and easy cultivation. Especially, A. muntifolium is hyperaccumulators, this plant can live well in soils contaminated with heavy metals and accumulate unusually high concentrations of metals in their leaves. Therefore, L. camara and A. muntifolium might be selected for Pb treatment in coal mine soil. E. indica is not a super-accumulator of Pb, however, this species can grow and develop in the mine site and can accumulate Pb, suggesting that it is a potential Pb-processing species in the soil. Behind, with the high amount of Pb content in the root, E. indica is an effective material for phytostabilization with a high potential to minimize the concentration of Pb in the soil while reducing the risk of entry into the food chain.
15.4 Conclusion
The concentration of Pb in soil and dominant native plant species was investigated from Vang Danh coal mine to determine the potential capacity of these plants for phytoremediation. The concentration of Pb in soil samples varied from 35 to 71 mg/kg, and these values were within the Vietnamese standard for Pb concentration in soil. The results indicated that plant native species in the study site could grow intolerance to Pb contaminants. L. camara was found to have the highest Pb accumulated at 3.38 mg/kg for average. The concentrations of Pb were lower for E. indica and A. muntifolium at 1.67 mg/kg and1.37 mg/kg, respectively. None of the studied species appeared to be suitable for phytoextraction. Based on the monitoring, the plants with high enrichment coefficients but translocation factor smaller than one like L. camara and E. indica could be appropriate for phytostabilization, while A. muntifolium, characterized by the translocation factor larger than one and the small biological absorption coefficient can be considered for the potential application to hyperaccumulation. The phytoremediation potential of native plant species should be investigated further for building better heavy metal remediation practices in the mine site in Vietnam to mitigate the negative impacts of mining on the environment and human health.
References
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—concepts and applications. Chemosphere 91(7):869–881
Altinozlu H, Karagoz A, Polat T, Unver I (2012) Nickel hyperaccumulation by natural plants in Turkish serpentine soils. Turk J Bot 36:269–280
Anh BTK, Kim DD, Van TT, Kien NT, Anh DT (2011) Phytoremediation potential of indigenous plants from Thai Nguyen province, Vietnam. J Environ Biol 32:257–262
Antosiewicz DM, Escudĕ-Duran C, Wierzbowska E, Skłodowska A (2008) Indigenous plant species with the potential for the phytoremediation of arsenic and metals contaminated soil. Water Air Soil Pollut 193(1–4):197–210
Aparajita B, Joyanto R, Gunnar J, Prosun B, Magnus M (2006) Environmental assessment of abandoned mine tailings in Adak, Vasterbotten district (northern Sweden). Appl Geochem 21(10):1760–1780
Brown SL, Chaney RL, Angle JS, Baker AJM (1995) Zinc and cadmium uptake by hyperaccumulator Thlaspi caerulescens and metal tolerant Silene vulgaris grown on sludge-amended soils. Environ Sci Technol 29(6):1581–1585
Caille N, Zhao FJ, McGrath SP (2005) Comparison of root absorption translocation and tolerance of arsenic in the hyperaccumulator Pteris vittata and the nonhyperaccumulator Pteris tremula. New Phytol 165:755–761
Chaabani S, Abdelmalek-Babbou C, Ahmed HB, Chaabani A, Sebei A (2017) Phytoremediation assessment of native plants growing on Pb-Zn mine site in Northern Tunisia. Environ Earth Sci 76:585
Chandra SK, Kamala CT, Chary NS, Balaram V, Garcia G (2005) Potential of Hemidesmus indicus for phytoextraction of lead from industrially contaminated soils. Chemosphere 58:507–514
Chang KJ, Gonzales MJ, Ponce O, Ramírez L, León V, Torres A, Corpus M, Loayza-Muro R (2018) Accumulation of heavy metals in native Andean plants: potential tools for soil phytoremediation in Ancash (Peru). Environ Sci Pollut Res 25(34):33957–33966
Chu Ngoc K, Nguyen VN, Nguyen DB, Le TS, Tanaka S, Kang Y (2009) Arsenic and heavy metal concentrations in agricultural soils around tin and tungsten mines in the Dai Tu district, N. Vietnam. Water Air Soil Pollution 197:75–89
Conesa HM, Faz Á, Arnaldos R (2007) Initial studies for the phytostabilization of a mine tailing from the Cartagena-La union mining District (SE Spain). Chemosphere 66:38–44
De Temmerman L, Waegeneers N, Ruttens A, Vandermeiren K (2015) Accumulation of atmospheric deposition of As, Cd and Pb by bush bean plants. Environ Pollut 199:83–88
Dung LT, Quang DH (2021) Field investigation of face spall in moderate strength coal seam at Vang Danh Coal Mine Vietnam. VNU J Sci Earth Environ Sci 37(2):107–105
Edgar VN, Fabián Fl, Mario PCJ, Ileana VR (2021) Coupling plant biomass derived from phytoremediation of potential toxic-metal-polluted soils to bioenergy production and high value by-products—a review. Apply Sci 11:2982
Eslamian S (2016) Urban water reuse handbook. CRC Press
Fitz WJ, Wenzel WW (2002) Arsenic transformation in the soil–rhizosphere–plant system, fundamentals and potential application of phytoremediation. J Biotechnol 99(3):259–278
Frérot H, Lefèbvre C, Gruber W, Collin C, Santos AD, Escarré J (2006) Specific Interactions between local metallicolous plants improve the phytostabilization of mine soils. Plant Soil 282(1–2):53–65
Ghosh M, Singh SP (2005) A review on phytoremediation of heavy metals and utilization of its byproducts. Appl Ecol Environ Res 3(1):1–18
Glick BR (2010) Using soil bacteria to facilitate phytoremediation. Biotechnol Adv 28:367–374
Hai TQ (2007) Spatial organization for rational land use and environmental protection in Uong Bi Town by functional sub-areas. VNU J Sci Earth Sci 23:88–95
Hien NTT, Yoneda M, Nakayama A, Matsui Y, Hai HT, Pho NV, Quang NH (2012) Environmental contamination of Arsenic and heavy metals around Cho Dien Lead and Zinc Mine Vietnam. J Water Environ Technol 10(3):253–265
Hye SL, Jin SL, Hyo TC, Manfred S (2008) Heavy metal contamination and health risk assessment in the vicinity of the abandoned Songcheon Au-Ag mine in Korea. J Geochem Explor 196:223–230
Israel R, Letica C, Javie C, Fernando D, Marcos M (2004) Arsenic and heavy metal pollution of soil, water and sediment in a semi-arid climate mining area in Mexico. Water Air Soil Pollut 152:129–152
Karthik V, Saravanan K, Sivarajasekar N, Suriyanarayanan N (2016) Utilization of biomass from Trichoderma harzianum for the adsorption of reactive red dye. Ecol Environ Conserv J 22:435–440
Khoi NN (2014) Mineral resources potential of Vietnam and current state of mining activity. Appl Environ Res 36(1):37–46
Kumar PB, Dushenkov V, Motto H, Raskin I (1995) Phytoextraction—the use of plants to remove heavy metals from soils. Environ Sci Technol 29(5):1232–1238
Lee CG, Chon HT, Jung MC (2001) Heavy metal contamination in the vicinity of the Daduk Au-Ag-Pb-Zn mine in Korea. Appl Geochem 16:1377–1368
Lim SR, Schoenung JM (2010) Human health and ecological toxicity potentials due to heavy metal content in waste electronic devices with flat panel displays. J Hazard Mater 9:177–251
Mahar A, Wang P, Ali A, Awasthi MK, Lahori AH, Wang Q, Li R, Zhang Z (2016) Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: a review. Ecotoxicol Environ Saf 126:111–121
Mendez MO, Maier RM (2008) Phytostabilization of mine tailings in arid and semiarid environments-an emerging remediation technology. Environ Health Perspect 116(3):278–283
Mileusnić M, Mapani BS, Kamona AF, Ružičić S, Mapaure I, Chimwamurombe PM (2014) Assessment of agricultural soil contamination by potentially toxic metals dispersed from improperly disposed tailings, Kombat mine, Namibia. J Geochem Explor 144:409–420
Moeller DW (2005) Environmental health, 3rd edn. Harvard University Press, Cambridge, MA
Muthusaravanan S, Sivarajasekar N, Vivek JS, Paramasivan T, Naushad M, Prakashmaran J, Gayathri V, Al-Duaij OK (2018) Phytoremediation of heavy metals: mechanisms, methods and enhancements. Environm Chem Lett 16:1339–1359
Nguyen TH, Sakakibara M, Sano S, Hori RS, Sera K (2009) The potential of eleocharis acicularis for phytoremediation: case study at an abandoned mine site. Clean—Soil Air Water 37(3):203–208
Nouri J, Lorestani B, Yousefi N, Khorasani N, Hasani AH, Seif F, Cheraghi M (2011) Phytoremediation potential of native plants grown in the vicinity of Ahangaran lead–zinc mine (Hamedan, Iran). Environ Earth Sci 62:639–644
Pan P, Lei M, Qiao P, Zhou G, Wan X, Chen T (2019) Potential of indigenous plant species for phytoremediation of metal(loid)-contaminated soil in the Baoshan mining area. Environ Sci Pollut Res 26:23583–23592
Petelka J, Abraham J, Bockreis A, Deikumah JP, Zerbe S (2019) Soil heavy metal(loid) pollution and phytoremediation potential of native plants on a former gold mine in Ghana. Water Air Soil Pollut 230:267
Pinto AP, Varennes AD, Fonseca R, Teixeira DM (2015) In: Ansari AA et al (eds) Phytoremediation: management of environmental contaminats phytoremediation of soils contaminated with heavy metals: techniques and strategies, vol 2015. Springer, pp 133–155
Rashid A, Mahmood T, Mehmood F, Khalid A, Saba B, Batool A, Riaz A (2014) Phytoaccumulation, competitive adsorption and evaluation of chelators-metal interaction in lettuce plant. Environ Eng Manag J 13(10):2583–2592
Rungwaa S, Arpab G, Sakulasc H, Harakuwed A, Timie D (2013) Phytoremediation-an eco-friendly and sustainable method of heavy metal removal from closed mine environments in Papua New Guinea. Prog Earth Planet Sci 6:269–277
Sivarajasekar N, Baskar R (2014) Adsorption of basic red 9 on activated waste Gossypium hirsutum seeds: process modeling, analysis and optimization using statistical design. J Ind Eng Chem 20:2699–2709
Son NP, Anh NT, Hoi NT, Thuy NT (2019) Applying remote sensing images to establish saline soil map: case study in Uong Bi city, Quang Ninh province. J Geodesy Cartography 39:28–33
Traina G, Morselli L, Adorno GP (2007) Electrokinetic remediation of bottom ash from municipal solid waste incinerator. Electrochim Acta 52:3380–3385
Tran AT, Tran TH, Ngo TP, Pham TD, Bui AN, Nguyen VY, Vu VV, Tran QH, Pham NC (2009) Lead-Zinc mineralization in North Vietnam. In: Abstract with programs CD-ROM, 59th annual society of resource geology, June 24–26, Tokyo, Japan
Tua TV, Kiên NT, Anh DT, Kim DD (2011) Nghiên cứu khả năng chống chịu và hấp thu chì pb, zn của dương xỉ Pteris vittata l. Tạp Chí Khoa Học Và Công Nghệ 49(4):101–109
Vidalli M (2005) Bioremediation: an overview. Pure Appl Chem 73(7):1167–1172
Vietnam Standard (2005) Soil quality—sampling. Part 2 guidance on soil sampling technique: TCVN 7538-2:2005
Xuan PT, Tran TA, Doan TTT, Hoang TTN, Pham TD, Nguyen TL, Nguyen VP (2017) Environmental issues of mining activities in Tay Nguyen. Vietnam J Earth Sci 37(2):139–147
Xuan PT, Phoa NV, Gas’kovab OL, Bortnikova SB (2013) Geochemistry of surface waters in the vicinity of open pit mines at the cay cham deposit, Thai Nguyen Province, Northern Vietnam. Geochem Int 51(11):931–938
Xue L, Liu J, Shi S, Wei Y, Chang E, Gao M, Chen L, Jiang Z (2014) Uptake of heavy metals by native herbaceous plants in an antimony mine (Hunan, China). Clean: Soil Air Water 42:81–87
Yoon J, Cao X, Zhou Q, Ma LQ (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368(2):456–464
Zhang WH, Cai Y, Tu C, Ma QL (2002) Arsenic speciation and distribution in an arsenic hyperaccumulating plant. Sci Total Environ 300(1–3):167–177
Zu YQ, Li Y, Chen JJ, Chen HY, Qin L, Christian S (2005) Hyperaccumulation of Pb, Zn and Cd in herbaceous plants grown on lead–zinc mining area in Yunnan China. Environ Int 31:755–762
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Dung, B.X., Anh, T.N., Linh, N.T.M. (2023). Assessment of Lead (Pb) Accumulation in Native Plants Growing on Coal Mine Site in Northeastern Vietnam. In: Vo, P.L., Tran, D.A., Pham, T.L., Le Thi Thu, H., Nguyen Viet, N. (eds) Advances in Research on Water Resources and Environmental Systems. GTER 2022. Environmental Science and Engineering. Springer, Cham. https://doi.org/10.1007/978-3-031-17808-5_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-17808-5_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-17807-8
Online ISBN: 978-3-031-17808-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)