Abstract
Native plant species growing on metal contaminated soil at the foot of the Legadembi tailings dam were selected to evaluate their phytoremediation potential. For this purpose, soil, aboveground tissues, and roots of plant samples were analyzed for the concentrations of Zn, Cu, Ni, Pb, and Cd. The bioaccumulation and transfer of metals were evaluated in terms of translocation factor (TF), bioconcentration factor (BCF), and biological accumulation coefficient (BAC). The results showed that most of the species were efficient to take up and translocate more than one trace element (TE) from roots to shoots. Argemone mexicana L., Rumex nepalensis Spreng., Cyperus alopecuroides Rottb., and Schoenoplectu sconfusus (N.E.Br.) Lye showed potential for phytoextraction of Cu, while R. nepalensis and C. alopecuroides can accumulate in their above-ground parts and are suitable for phytoextraction of Ni. Rumex nepalensis, C. alopecuroides, and Typha latifolia L. have the ability for phytostabilization of Zn metal. Findings suggest concentrations of some metals in plants’ tissue showed above the normal range which suggests their potential use in phytoremediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pollution by heavy metals due to natural processes and anthropogenic activities poses a significant threat to the environment (Ameh and Aina 2020; Bai et al. 2023; Younis 2018). Heavy metals are non-biodegradable (Roychoudhury and Bhowmik 2023), and the accumulation of these metals in soils has potential risk in the food chain and thus intimidates human and animal health (Ighariemu et al. 2023). Tailings from gold mining release potentially toxic elements (Zhao et al. 2021) and are a serious source of soil pollution with public health implications (Gallego and Fernández-Caliani 2023).
Phytoremediation is the use of metal accumulating plants to remove or reduce heavy metals in an economical and environmentally friendly technique (Ameh and Aina 2020; Akhtar et al. 2023). It is the natural capability of certain plants to accumulate and degrade contaminants in water and soil (Herrera-Cabrera et al. 2022; Sladkovska et al. 2022). Some previous studies but not limited to (Ameh and Aina 2020; Chang et al. 2018; Oladoye et al. 2022) have shown that phytoremediation technology is the most effective approach to alleviating heavy metal contaminated soil. Phytoremediation is a process that includes phytoextractors, phytostabilizer, and hyperaccumulator plants. Phytoextraction is the removal of heavy metals by aboveground parts of the plant (Chen et al. 2023). Plants can remove trace metals from soil and accumulate them in their shoots, which can then be harvested (Wechtler et al. 2019). Phytostabilization is the plant’s capability to resist high metal concentrations and to extract or immobilize them within the roots (Ke et al. 2021; Yongpisanphop et al. 2020). Hyperaccumulators are defined as plants that can accumulate high concentrations of metals in their above-ground tissues (Sytar et al. 2021; Van der Pas and Ingle 2019).
Remediation of soil contaminated by heavy metals is necessary to halt the associated risks, make the land resource available for agricultural production, and enhance food security (Oladoye et al. 2022; Qin et al. 2021). The success of remedial action in phytoremediation technology is determined by the accessibility and mobility of heavy metals in the soil. Phytoavailability of heavy metals is influenced by a variety of soil parameters (pH, redox potential, clay, and organic matter contents) and plant biomass yield (Antoniadis et al. 2017). Translocation factor (TF), bioconcentration factor (BCF), and biological accumulation coefficient (BAC) can be used to determine and identify plants that are suitable for phytoremediation (Dung et al. 2023; Wang et al. 2021; Zanganeh et al. 2022).
Some plants growing on metal-contaminated soil can accumulate considerable amounts of heavy metals in their roots and shoots. Many plant species that have become heavy metal tolerant are those grown in contaminated sites (Alsherif et al. 2022; Sainger et al. 2011). Previous studies have shown that Legadembi gold mine tailing dams are contaminated with several heavy metals (Mengistu et al. 2022). The selection of native plants from such contaminated area can accumulate heavy metals and plays a vital role in phytoremediation (Ahmad et al. 2022; Niu et al. 2022). The aim of this study is therefore to determine the concentration of heavy metals in native plants grown on the soil around tailing dams and compare the concentration of metals in the tissues of the plant and the soil. This research also assesses, identifies, and recommends plant species that are suitable for phytoremediation purposes.
Materials and methods
Study area
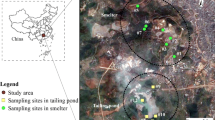
The study area is located 500 km south of Addis Ababa in Oromia National Regional State, Southern Ethiopia (Fig. 1). The gold mine site extends over 18.26 km2 with an average elevation of 2000 m above sea level. The longitude and latitude of the mine site are 38.885526° and 5.729509°. There are two rainy periods (bimodal rainfall), March to May and September to November. The annual rainfall of the area varies from 394 to 1748 mm with an average of 1150 mm. Plant and soil sampling were carried out on August 2021 which is out of the rainy season (in dry period) to avoid washing of the metals. Legadembi gold Mine PLC commenced primary gold production by open pit mining method in the late 1980s. The gold deposit of the area is associated with ores like pyrite, pyrrhotite, chalcopyrite, galena, sphalerite, and others. Heavy metals in soils, sediments, vegetation, and water bodies are due to these sulfide minerals. These heavy metals enter the receiving environment through both natural and anthropogenic activities (Getaneh and Alemayehu 2006).
Tailings produced by the processing plant are disposed of in a dedicated Tailings Disposal Facility, comprising a valley with a rock-fill dam structure. The supernatant water from this dam is decanted through a culvert structure into the second dam. The total length of the tailings dam is about 1 km. Plant species dominate the area adjacent to the Legadembi tailings dam (Fitamo and Leta 2010) despite the high concentration of heavy metals. Unpublished report in 2018 undertaken on the chemical management of Legadembi Gold mine PLC listed some grass and herbaceous families dominating the foot of the tailings dam. The downstream tailing dams, in dam 2 and dam 3, are dominated by grass and herbaceous families such as Cyperaceae, Typhaceae, and Alismataceae. This report suggested the plants are indicators of pollution and also have some additional attenuation function for the heavy metals released from the tailings dam.
Sampling and analysis
The success of remedial action in phytoremediation is determined by the accessibility of heavy metals in the soil (Antoniadis et al. 2017). Characterizing the area in terms of heavy metal pollution was done by sampling soils from the dry beach of the tailings dam as well as from downstream of the dam. Since the tailings storage facility is currently active, tailing soil samples were collected from depth of 50 cm which is higher than the depth for other soil sampling, which was 20 cm from the ground surface. Furthermore, the sampling of tailing soil at the depth of 50 cm is directly related to physical, chemical, and mineralogical properties of the tailings (Cesar et al. 2022). Composite samples of 500 g for each soil samples were stored in properly labeled polyethylene zip-lock bags and transported to the laboratory for analysis.
Studying plant species grown in mining areas is useful for the restoration of heavy metals pollution (Niu et al. 2022). In the previous study, Fitamo and Leta (2010) pointed out that 57% of plants at foot of the Legadembi tailing dam are dominated by the Cyperaceae family. Three extensively grown grass species dominating the swamps and stream bank downstream of the Legadembi tailings dam were selected based on their availability. Two herbaceous plant species were chosen among other 7 species, based on their high growth rate, production of more above-ground biomass, and highly branched root system (Ali et al. 2013). Photographs of the plant species were taken, and plant identification was carried out at the National Herbarium, Addis Ababa University.
A total of twenty-five individual plants for each species (two for taxonomy purposes and three of them for heavy metal analysis) were collected from the foot of the Legadembi tailing dam bounded by 5.7078°N–5.7137°N and 38.9027°E–38.9082°E. Plants were uprooted, washed in tap water, and rinsed with deionized water. Above-ground tissues were analyzed for the grass species, while leaves were analyzed for the other two herbaceous plants (Table 1). The soil from the diameter of the rhizosphere around each plant was collected at 0–20 cm depth and thoroughly homogenized before packing in polyethylene containers (Nirola et al. 2016). The plant samples were divided into their roots and leaf or above-ground tissues, oven dried at 70 °C for 48 h to a constant weight, and then finely ground (Usman et al. 2012). Equal subsamples of soil were collected from each sample, mixed thoroughly, and homogenized to make a composite. The homogenized soil samples were then dried in an oven. The dried soil samples were grounded, sieved, well-labeled, and stored in polyethylene bags. Each plant, soil, and tailing soil sample (0.5 g) were digested according to EPA method 3051A (EPA 1996), in a High-Performance Microwave Digestion System (ETHOS UP milestone).
Quality control was achieved by analyzing duplicates and after every 5 samples; a calibration standard was analyzed to test the response and efficiency of the instrument. The metal contents were within ± 10% relative percent deviation of the standards. The accuracy was verified with known standards, and the recovery of the tested samples varied from 81.75 to 107.25%. Both plant and soil samples were analyzed for heavy metals: Pb, Ni, Zn, Cu, and Cd using the Agilent Technologies 4200 Microwave Plasma Atomic Emission Spectrometer (MP-AES).
Phytoremediation efficiency
The TF value is an indicator for the plant’s ability to transport metal in the upper parts of the plant (Khan et al. 2022). It is defined as the ratio of metal concentration in above-ground tissues to the metal concentration in roots (Ameh and Aina 2020). Bioconcentration factor (BCF) is the ratio of metal concentration in the root to the metal concentration in the soil (Yoon et al. 2006). Plant species with TF greater than BCF could be used in phytoextraction, while plant species with BCF greater than 1 and low TF could be considered as candidates for phytostabilization (Berkaoui et al. 2022). And biological accumulation coefficient (BAC) is the concentration of metals in plant shoots divided by the metal concentration in soil (Khan et al. 2022). It was used to calculate the plants’ ability to accumulate TE compared to the soil substrate (Jeddi and Chaieb 2018). In this study, the TF, BCF, and BAC values for Pb, Cu, Ni, and Zn were calculated by the following equations;
Statistical analysis
Arithmetic means, variance, and standard deviation were performed including analysis of variance (ANOVA) test to analyze differences between variables using the STATA14.1 software. The statistical significance was confirmed when P < 0.05.
Results and discussion
Metals levels in soil
The average values of all the studied heavy metals in the soil samples, except for nickel, were above the concentrations of shale values (Table 2). However, concentrations of all the heavy metals in soil samples were below the national soil quality standards. The variation coefficients (CV) for the studied heavy metals in the increasing order were Pb (34.7%) < Cu (54.6%) < Zn (190.2%). The data demonstrates that the variability of these measurements could be classified under a large concentration variation (Belimov et al. 2003). This indicates that there were wide variations in the concentrations of the heavy metals in the study area, which could have been caused by the mining activities. The larger the variation coefficient means, the more the abnormal distribution of the heavy metal contents and the greater the anthropogenic activities (Zhao et al. 2021). Zinc is the most unevenly distributed in soil samples compared to other heavy metals, with a CV value of 190.2%.
The concentrations of Zn, Cu, and Pb were 1.16, 1.07, and 1.31 times higher than the threshold values set under the shale average respectively. This shows the soil in the study area is slightly polluted compared to the shale average. The concentrations of Cd and Ni in the soil samples of the study area were below the detection limit. However, 0.5 to 22.5 mg/kg of Ni was detected in plant tissues grown above the soil. This could be due to the efficient transfer of Ni from the soil to the plant root (Ameh and Aina 2020). The highest lead (Pb) concentration in soil samples was 40 mg/kg. This value is 4 times lower than the concentration of Pb in the upstream tailings soil. It might be due to the least mobility of Pb among other heavy metals (Al-Qahtani 2012), and it is categorized as a non-essential element.
The concentrations of the studied heavy metals in the tailings soil were all above the average shale values. The concentrations of Zn, Cu, Ni, Pb, and Cd in tailings soil were 4.2, 2.9, 3.2, 7.5, and 13.3 times, respectively, above the average shale values (Table 3). The increased heavy metal concentrations in the tailing dam might be due to the association of the gold-ore with pyrite (FeS2), chalcopyrite (CuFeS2), galena (PbS), sphalerite (ZnS), violarite (FeNiS4), and other ores (Zenebe 2006) which are potential contributors to the metal pollution in the tailings dam. In relation to physicochemical parameters, the mean pH of soil collected beneath each plant species is 7.3 ± 0.12 which does not show variations as such (Table 4).
Metal concentration in plant tissues
Unilateral F-test on the accumulation of each metal separately in the plant parts (Table 2) indicated that the accumulation of heavy metals in the shoot is more significant for Zn followed by Cu, Pb, and Ni with F-values: 0.005, 0.027, 0.057, and 0.129, respectively, whereas the accumulation of heavy metals in the root is more significant for Zn followed by Pb, Cu, and Ni with F-values: 0.019, 0.058, 0.079, and 0.203, respectively.
The metal concentrations in the roots and shoots showed similar variations of the CV values with decreasing order: Ni > Pb > Cu > Zn (Table 2). The CV values in shoot and roots were (117.1, 84.2, 65.4, and 39.1) and (146.9, 85, 95.2, and 61.2), respectively. Nickel showed the highest concentration of variation with CV values of 146.9% for the root part. The variation in the concentration level of Ni showed the impact of pollution is from anthropogenic activities rather than lithogenic ones (Adesuyi et al. 2018). Cadmium was not detected in all the studied plant species.
Cyperus alopecuroides recorded 113.5 mg/kg of Zn metal in its above-ground parts followed by T. latifolia with 108 mg/kg. Compared to the other heavy metals, A. mexicana, R. nepalensis, and S. sconfusus also accumulated a higher amount of Zn in its roots and shoots. The present study showed the plants accumulate by far higher amount of zinc compared to the shoot of Nymphaea lotus (10.43 mg/kg) reported by Adesuyi et al. (2018), which was a study conducted on 14 wetland plants of Lagos Lagoon, Nigeria. Maximum zinc concentration of 220 mg/kg in the root of E. glaucophyllum was reported by Jeddi and Chaieb (2018) which is still lower than 291.5 mg/kg by C. alopecuroides of the current study. For all plant species, the concentrations of Zn were higher in roots than in shoots (Fig. 2) which show low mobility of Zn from roots to shoots (Tripathi and Misra 2013). The current study revealed the selected plants are not hyperaccumulators for zinc but accumulated a good amount in roots and shoots. Hyperaccumulators for zinc metal are defined as those plants that accumulate > 10,000 mg kg−1 of Zn (Wechtler et al. 2019).
The concentration of Cu in the roots of the plant species varied from 6 to 61 mg/kg. Typha latifolia accumulated the highest, while S. sconfusus accumulated the lowest Cu in its roots. The level of Cu accumulation in shoots ranged from 11 mg/kg in A. mexicana to 36.5 mg/kg in C. alopecuroides. The amount of Cu accumulated by the studied plant species is lower compared to other similar studies undertaken by Ameh and Aina (2020) and Chang et al. (2018) which recorded 318.00 to 6217 mg/kg and 41.36 mg/kg to 251.07 mg/kg, respectively. However, the results of the accumulated Cu in the present study are roughly similar to the one reported by Kumar et al. (2013) which was 28.7 to 45.47 mg/kg. From the studied plant species, A. mexicana and T. latifolia accumulated higher concentrations of Cu in their roots while R. nepalensis, and C. alopecuroides accumulated higher Cu concentration in their shoots. Hyperaccumulators are those plants that accumulate > 1000 mg kg−1 of copper metal (Sainger et al. 2011). The accumulation of Ni in roots of plant species ranged from 1.0 to 22.5 mg/kg. The T. latifolia accumulated the highest value of 22.5 mg/kg in the roots. This value is higher than 6.6 mg/kg reported by Chang et al. (2018) for J. bufonius roots and also 3.58 mg/kg found in the root of S. pruinosa, another study by El-Amier et al. (2017). The concentration of Ni in shoots of plant species ranged from 0 to 10 mg/kg. The largest concentration of Ni in the shoot was recorded by T. latifolia, while the lowest concentration in the shoot was seen in S. sconfusus, which was not detectable. The value recorded in the shoot was lower than a similar study done by Chang et al. (2018) for W. nubigena plant which was 26.8 mg/kg Ni concentration. For this study, the lowest concentration in the shoot indicated not-detectable and is also similar to the study conducted by Tripathi and Misra (2013) for Cassia tora L. plant.
The concentration of Pb in the root varied between not-detectable to 6 mg/kg. The highest concentration of Pb was shown by T. latifolia, whereas it was not detectable in R. nepalensis. The highest value recorded in this study is lower than 189 mg/kg of Pb in Nicotina glauca reported by Santos-Jallath et al. (2012). The concentration of Pb in shoots of plant species ranged from not-detectable to 3 mg/kg for S. sconfusus and R. nepalensis, respectively. Compared to another similar study, Kumar et al. (2013) have reported 242.39 mg/kg Pb in the root of C. procera plant species. This value is by far greater than the current study which is 3 mg/kg for R. nepalensis plant. Cadmium was not detected in all the plant tissues and soil collected under the roots of each plant.
In general, the concentrations of Zn and Pb in the studied plants’ shoot were in normal ranges according to Kabata-Pendias and Pendias (2001). However, Cu showed concentrations in shoot above the normal range for all plant species, while C. alopecuroides and T. latifolia accumulated Ni more than the normal range in their shoots about 3.5 and 5 times, respectively. Moreover, among standards for hyper accumulator plants, a plant accumulating metals in shoot 10–500 times as much as those in normal plant can be considered as a hyperaccumulator (Sainger et al. 2011). However, in the current study, no plant species can be considered as a hyperaccumulator based on this standard.
Pair wise comparison of metals in plant parts (shoot and root) among the selected five plant species were tested using ANOVA (P < 0.05), followed by post hoc analysis using Tukey’s honestly significant difference (HSD) as showed in Table 5. Similar trends were observed in the accumulation of pairs of heavy metals in the shoot and root parts. The plants’ ability in accumulating a pair of heavy metals, which are present together, in both shoots and roots are differed significantly in decreasing order: Zn-Pb > Zn-Ni > Zn-Cu > Cu-Pb > Cu-Ni > Ni-Pb. The ANOVA result was significant for Zn-Pb, Zn-Ni, and Zn-Cu pairs, indicating significant differences in accumulating pairs of these heavy metals in the shoot and root parts of the plants species.
Similarly, ANOVA following Tukey’s post hoc test among plant parts and soil of the plant species (Table 6) showed there is no such significant difference between plant parts in accumulating Zn, Cu, and Ni. However, Pb showed a significant difference in soil verses root and soil verses shoot with (t = 6.68, P < 0.000) and (t = 7.17, P < 0.000) respectively. This might indicate toxicity of Pb for some of the selected plant species.
Metal accumulation and translocation in plant species
The bioaccumulation of metals in shoots, roots, and concentration ratio values of the five plant species were shown in Table 2. The TF, BCF, and BAC were used to evaluate the ability of studied plants to uptake metals from the soils and translocating them to the shoots. Plant species with TF and BCF greater than 1 are suitable for phytoextraction of heavy metals (Pilon-Smits 2005; Yoon et al. 2006), while those plant that shows TF and BCF less than 1 are unsuitable for phytoextraction (Fitz and Wenzel 2002). From the studied plant species, the highest TF and BCF values (3.00 and 14.58) were recorded in Rumex nepalensis Spreng. and Cyperus alopecuroides Rottb., respectively. The lowest TF and BCF value, 0, was observed by Schoenoplectus confusus (N.E.Br.) Lye for Ni metal.
Biological factors
The ANOVA result was significant (Table 7), indicating significant differences in translocation among plant parts and soil. TF values > 1 (1.24, 2.84, 2.52, and 1.58) for Cu metal were shown by A. mexicana, R. nepalensis, C. alopecuroides, and S. sconfusus, respectively. This implies the plants’ ability to accumulate Cu metal in their above-ground parts and which makes them suitable for phytoextraction. In addition, TF values > 1 (1.5 and 2.33) for Ni metal were recorded by R. nepalensis and C. alopecuroides which shows the plant’s ability to accumulate Ni in its shoots. In general, the plants which show TF > 1 is an indicator for the plant’s ability to transport metal in the upper parts of the plant (Khan et al. 2022) and can be used for phytoextraction (Waris et al. 2022). All plant species except R. nepalensis have shown TF value for Pb < 1, which indicates these plants can accumulate Pb but cannot translocate it to their shoots. That means they have potential as Pb stabilizers and hence are excluders (Ameh and Aina 2020). In addition, the translocation of Pb in a plant is usually small due to its toxicity (Yoon et al. 2006). For most plant species when TF values for Zn decrease, TF values for Ni increase and vice versa. This shows that the absence of essential nutrients like Zn in the soil might be making the plant accumulate non-essential nutrients like Ni (Annan et al. 2010). Of the studied plant species, only S. sconfusus showed TF, BCF, and BAC values > 1 which makes the plant species suitable for phytoexrtaction for Zn metal. This finding agreed with the study undertaken by Ameh and Aina (2020). Based on the average TF of all plant species, the ability of plant shoots in translocating the heavy metals is in the order of TF: Cu > Ni > Pb > Zn.
Rumex nepalensis, C. alopecuroides, and T. latifolia had a high BCF (8.47, 14.58, and 5.75 respectively) and low TF (0.58, 0.39, and 0.85 respectively) for Zn, showing potential for phytostabilization of this metal (Ameh and Aina 2020; Berkaoui et al. 2022). For Ni and Pb metals, all plant species have shown a BCF value of less than 1, which indicates a low accumulation of the plants in their roots, whereas BCF values of Zn are much higher than Cu, Ni, and Pb. This shows the high ability of these plants to accumulate Zn in their roots compared to other metals. Based on the average BCF of all plant species (Table 7), the capacity of the plant in accumulating heavy metals in their roots becomes Zn > Cu > Pb. Except A. mexicana, all plant species have showed BAC values greater than 1 for Zn metal. It shows a special capacity of the plants to extract and transfer metals from soil to the plant shoot. These plant species can be used for phytoextraction of metals (Sabir et al. 2022).
Conclusion
Results of this research indicated that all plant species are accumulators for the studied heavy metals. The mean concentrations of Zn, Cu, and Pb in soil samples were higher than the shale average values. Although there are no hyperaccumulators, some plant species have accumulated a high amount in roots and above-ground parts which makes them suitable for phytoextraction and phytostabilization. TF values > 1 (1.24, 2.84, 2.52, and 1.58) for Cu metal were shown by A. mexicana, R. nepalensis, C. alopecuroides, and S. sconfusus, respectively. In addition, TF values > 1 (1.5 and 2.33) for Ni metal were recorded by R. nepalensis and C. alopecuroides. These imply the plants’ ability to accumulate Cu and Ni metal in their above-ground parts and make them suitable for phytoextraction. All plant species except R. nepalensis have shown TF value for Pb < 1, which indicates the studied plant species are excluder for Pb metal due to its toxicity. Rumex nepalensis, C. alopecuroides, and T. latifolia had a high BCF (8.47, 14.58, and 5.75 respectively) and low TF (0.58, 0.39, and 0.85 respectively) for Zn, showing their potential for phytostabilization of this metal. The collected plant species have capability to transfer metals from roots to shoots as well as stabilize metals in their roots and could be used for the phytoremediation of multi-metals contaminated soil. Our findings suggest concentrations of Cu in shoot showed above the normal range for all plant species, while C. alopecuroides and T. latifolia accumulated Ni beyond the normal range in their shoots.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Adesuyi A, Njoku K, Akinola M, Jolaoso A (2018) Biomonitoring of heavy metals level in wetland plants of Lagos Lagoon, Nigeria. J Appl Sci Environ Manage 22(9):1489. https://doi.org/10.4314/jasem.v22i9.22
Ahmad I, Gul I, Irum S, Manzoor M, Arshad M (2022) Accumulation of heavy metals in wild plants collected from the industrial sites—potential for phytoremediation. Int J Environ Sci Technol 20(2):1–12
Akhtar MS, Hameed A, Aslam S, Ullah R, Kashif A (2023) Phytoremediation of metal-contaminated soils and water in Pakistan: a review. Water Air Soil Pollut 234(1):1–21
Ali H, Khan E, Anwar M (2013) Phytoremediation of heavy metals — concepts and applications. Chemosphere 91(7):869–881
Al-Qahtani KM (2012) Assessment of heavy metals accumulation in native plant species from soils contaminated in Riyadh City, Saudi Arabia. Life Sci 9(2):32
Alsherif EA, Al-Shaikh TM, AbdElgawad H (2022) Heavy metal effects on biodiversity and stress responses of plants inhabiting contaminated soil in Khulais, Saudi Arabia. Biology 11(2):164
Ameh EG, Aina DO (2020) Search for autochthonous plants as accumulators and translocators in a toxic metal-polluted coal mine soil in Okaba. Nigeria Sci Afr 10:1–17. https://doi.org/10.1016/j.sciaf.2020.e00630
Annan K, Isaac KA, Cindy A, Asare-Nkansah S, Marcel TB (2010) Profile of heavy metals in some medicinal plants from Ghana commonly used as components of herbal formulations. Pharmacogn Res 2(1):41–44
Antoniadis V, Levizou E, Shaheen SM, Sik Y, Sebastian A, Baum C, Prasad MNV, Wenzel W, Rinklebe J (2017) Trace elements in the soil-plant interface: phytoavailability, translocation, and phytoremediation–A review Vasileios. Earth-Sci Rev. https://doi.org/10.1016/j.earscirev.2017.06.005
Bai Z, Wu F, He Y, Han Z (2023) Pollution and risk assessment of heavy metals in Zuoxiguo antimony mining area, southwest China. Environ Pollut Bioavail 35(1):1–11
Belimov AA, Safronova VI, Tsyganov VE, Borisov AY, Andrei P (2003) Genetic variability in tolerance to cadmium and accumulation of heavy metals in pea (Pisum sativum L.). Euphytica 131:25–35. https://doi.org/10.1023/A:1023048408148
Berkaoui M, El Adnani M, El Hakkou R, Ouhammou A, Bendaou N, Smouni A (2022) Assessment of the transfer of trace metals to spontaneous plants on abandoned pyrrhotite mine: potential application for phytostabilization of phosphate wastes. Plants 11(179):1–13. https://doi.org/10.3390/plants11020179
Cesar R, Arruda F, Ramiro V, Faria R, Barcelos D, Pontes F, Passos F, Kaiser K, Maria A, Serrano A, Abreu L, Siqueira D, Teixeira M, Vezzone M, Polivanov H, Castilhos Z (2022) Deposition of gold mining tailings in tropical soils: metal pollution and toxicity to earthworms. J Soils Sediments 22:547–558. https://doi.org/10.1007/s11368-021-03105-8
Chang KJ, Gonzales MJ, Ponce O, Ramírez L, León V, Torres A, Corpus M, Loayza-Muro R (2018) Accumulation of heavy metals in native Andean plants: potential tools for soil phytoremediation in Ancash (Peru). Environ Sci Pollut Res 25(34):33957–33966. https://doi.org/10.1007/s11356-018-3325-z
Chen X, Feng J, Mou H, Liang Z, Ding T, Chen S, Li F (2023) Utilization of indole acetic acid with Leucadendron rubrum and Rhododendron pulchrum for the phytoremediation of heavy metals in the artificial soil made of municipal sewage sludge. Toxics 11(1):43
Dung BX, Anh TN, Linh NTM (2023) Assessment of Lead (Pb) Accumulation in native plants growing on coal mine site in Northeastern Vietnam. In International Conference on Geo-Spatial Technologies and Earth Resources (pp. 237–252). Springer, Cham
El-Amier AYM, Alghanem SM, Alzuaibr F (2017) Bioaccumulation and translocation of heavy metals from coastal soil by wild halophytes. Am J Environ Protect 5(2):52–60. https://doi.org/10.12691/env-5-2-4
EPA, US (1996). Method 3050B: acid digestion of sediments, sludges, and soils. Environ Prot Agency 2:3–5
Fitamo D, Leta S (2010) Assessment of plants growing on gold mine wastes for their potential to remove heavy metals from contaminated soils. Int J Environ Stud 67(5):705–724. https://doi.org/10.1080/00207233.2010.513587
Fitz WJ, Wenzel WW (2002) Arsenic transformations in the soil-rhizosphere-plant system: fundamentals and potential application to phytoremediation. J Biotechnol 99(3):259–278. https://doi.org/10.1016/S0168-1656(02)00218-3
Gallego L, Fernández-Caliani JC (2023) Pyrite ore cargo spills as a source of soil pollution and ecological risk along the abandoned railway corridors of the Tharsis and Rio Tinto mines (Spain). Environ Monit Assess 195(1):1–15
Getaneh W, Alemayehu T (2006) Metal contamination of the environment by placer and primary gold mining in the Adola region of southern Ethiopia. Environ Geol 50(3):339–352. https://doi.org/10.1007/s00254-006-0213-5
Herrera-Cabrera BE, López-Valdez LG, Cetina Alcalá VM, Montiel-Montoya J, Sánchez Herrera LM, Ocaño Higuera VM, de la Rosa-Montoya CR, Barrales-Cureño HJ (2022) Phytoremediation capacity of medicinal plants in soils contaminated with heavy metals. environmental challenges and medicinal plants: Sustainable production solutions under adverse conditions (pp. 409–431). Cham: Springer International Publishing
Ighariemu V, Wegwu MO, Chuku LC (2023) Evaluation of heavy metals and health risk assessment of shellfish contaminated in Santa Barbara River, Niger Delta, Nigeria. Current Research in Interdisciplinary Studies 2(1):1–20
Jeddi K, Chaieb M (2018) Evaluation of the potential of Erodium glaucophyllum L. for phytoremediation of metal-polluted arid soils. Environ Sci Pollut Res 25(36):36636–36644. https://doi.org/10.1007/s11356-018-3561-2
Kabata-Pendias A, Pendias H (2001) Trace elements in soils and plant. CRC Press, Boca Raton
Ke T, Guo G, Liu J, Zhang C, Tao Y, Wang P, Chen L (2021) Improvement of the Cu and Cd phytostabilization efficiency of perennial ryegrass through the inoculation of three metal-resistant PGPR strains. Environ Pollut 271:116314
Khan N, Ali K, Ezaz M, Khan H, Jones DA (2022) Phytoaccumulation of heavy metals by sodom apple (Calotropis procera (Aiton) W. T. Aiton) along an urban–rural gradient. Appl Sci 12(3):1003. https://doi.org/10.3390/app12031003
Kumar N, Bauddh K, Kumar S, Dwivedi N, Singh DP, Barman SC (2013) Accumulation of metals in weed species grown on the soil contaminated with industrial waste and their phytoremediation potential. Ecol Eng 61:491–495. https://doi.org/10.1016/j.ecoleng.2013.10.004.10(1080/15226514),pp.1146224,2016
Mengistu G, Sahilu G, Alakangas L, Mulat W, Kloos H (2022) Assessment of heavy metal pollution associated with tailing dam in gold mining area, Southern Ethiopia. Geosyst Eng 25(1-2):1–11
Nirola R, Megharaj M, Aryal R, Naidu R (2016) Screening of metal uptake by plant colonizers growing on abandoned copper mine in Kapunda, South Australia. Int J Phytorem 18(4):399–405. https://doi.org/10.1080/15226514.2015.1109599
Niu X, Jia Y, Wu X, Wang S, Hou J, Zhang W (2022) Phytoremediation potential of indigenous plants growing in soils affected by mine activities in Gejiu City, Yunnan Province. Int J Phytoremediation 25 (5):1–9
Oladoye PO, Olowe OM, Asemoloye MD (2022) Phytoremediation technology and food security impacts of heavy metal contaminated soils: a review of literature. Chemosphere 288:132555
Pilon-Smits E (2005) Phytoremediation. Annu Rev Plant Biol 56:15–39. https://doi.org/10.1146/annurev.arplant.56.032604.144214
Qin G, Niu Z, Yu J, Li Z, Ma J, Xiang P (2021) Soil heavy metal pollution and food safety in China: effects, sources and removing technology. Chemosphere 267:129205
Roychoudhury A, Bhowmik R (2023) Nanoremediation of heavy metals in agricultural soil. In Agricultural and environmental nanotechnology: Novel Technologies and their Ecological Impact (pp. 433-450). Singapore: Springer Nature Singapore
Sabir M, Baltrėnaitė-Gedienė E, Ditta A, Ullah H, Kanwal A, Ullah S, Faraj TK (2022) Bioaccumulation of heavy metals in a soil–plant system from an open dumpsite and the associated health risks through multiple routes. Sustainability 14(20):13223
Sainger PA, Rajesh D, Sainger M, Kaushik A, Singh RP (2011) Assessment of heavy metal tolerance in native plant species from soils contaminated with electroplating effluent. Ecotoxicol Environ Saf 74(8):2284–2291. https://doi.org/10.1016/j.ecoenv.2011.07.028
Santos-Jallath J, Castro-Rodríguez A, Huezo-Casillas J, Torres-Bustillos L (2012) Arsenic and heavy metals in native plants at tailings impoundments in Queretaro, Mexico. Phys Chem Earth 37–39:10–17. https://doi.org/10.1016/j.pce.2011.12.002
Sladkovska T, Wolski K, Bujak H, Radkowski A, Sobol Ł (2022) A review of research on the use of selected grass species in removal of heavy metals. Agronomy 12(10):2587
Sytar O, Ghosh S, Malinska H, Zivcak M, Brestic M (2021) Physiological and molecular mechanisms of metal accumulation in hyperaccumulator plants. Physiol Plant 173(1):148–166
Tripathi A, Misra DR (2013) Bioaccumulation of Pb, Ni And Zn in some plant species growing in and around a municipal waste dumpsite at Allahabad, India. J Solid Waste Technol and Manag 39(1):1–12. https://doi.org/10.5276/JSWTM.2013.1
Turekian KK, Haven N, Hans K, Universitat WMD (1961) Distribution of the elements in some major units of the Earth’s crust. Geol Soc Am Bull 72:175–192
Usman ARA, Lee SS, Awad YM, Lim KJ, Yang JE, Ok YS (2012) Soil pollution assessment and identification of hyperaccumulating plants in chromated copper arsenate (CCA) contaminated sites, Korea. Chemosphere 87(8):872–878. https://doi.org/10.1016/j.chemosphere.2012.01.028
Van der Pas L, Ingle RA (2019) Towards an understanding of the molecular basis of nickel hyperaccumulation in plants. Plants 8(1):11
Wang J, Wang P, Zhao Z, Huo Y (2021) Uptake and concentration of heavy metals in dominant mangrove species from Hainan Island, South China. Environ Geochem Health 43(4):1703–1714. https://doi.org/10.1007/s10653-020-00717-w
Waris M, Baig JA, Talpur FN, Kazi TG, Afridi HI (2022) An environmental field assessment of soil quality and phytoremediation of toxic metals from saline soil by selected halophytes. J Environ Health Sci Eng. https://doi.org/10.1007/s40201-022-00800-7
Wechtler L, Laval-Gilly P, Bianconi O, Walderdorff L, Bonnefoy A, Falla-Angel J, Henry S (2019) Trace metal uptake by native plants growing on a brownfield in France: zinc accumulation by Tussilago farfara L. Environ Sci Pollut Res 26(35):36055–36062. https://doi.org/10.1007/s11356-019-06892-3
Yongpisanphop J, Babel S, Kruatrachue M, Pokethitiyook P (2020) Pb phytostabilization by fast-growing trees inoculated with Pb-resistant plant growth-promoting endophytic bacterium. Pollution 6(4):923–934
Yoon J, Cao X, Zhou Q, Ma LQ (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368(2–3):456–464. https://doi.org/10.1016/j.scitotenv.2006.01.016
Younis YM (2018) Monitoring the anthropogenic and geochemical environment surrounding the Butana (Sudan) drinking water sources via the determination of heavy metals composition of the soil, streams sediments and gold mining tailings in the Wet Season (III). BJSTR 9(4):51–64. https://doi.org/10.26717/bjstr.2018.09.001821
Zanganeh F, Heidari A, Sepehr A, Rohani A (2022) Bioaugmentation and bioaugmentation–assisted phytoremediation of heavy metal contaminated soil by a synergistic effect of cyanobacteria inoculation, biochar, and purslane (Portulaca oleracea L.). Environ Sci Pollut Res 29(4):6040–6059
Zenebe S (2006) Environmental impacts of gold mining on waters and sediments of Legadembi Area A. Dissertation, Addis Ababa Unversity
Zhao G, Li X, Zhu J, Zhao X, Zhang J, Zhai J (2021) Pollution assessment of potentially toxic elements (PTEs) in soils around the Yanzhuang gold mine tailings pond, Pinggu county, Beijing, China. Int J Environ Res 18(14):7240. https://doi.org/10.3390/ijerph18147240
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data analysis, interpretation, and correction of grammatical errors were performed by Gera Techane, Geremew Sahilu, and Worku Mulat. The first draft of the manuscript was prepared by Gera Techane, and all authors commented on the manuscript. All authors read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Elena Maestri
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mengistu, G.T., Sahilu, G., Mulat, W. et al. Assessment of native plants for their potential to remove trace metals around Legadembi tailings dam, Southern Ethiopia. Environ Sci Pollut Res 30, 55615–55624 (2023). https://doi.org/10.1007/s11356-023-26349-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26349-y