Abstract
Contamination of heavy metals from mining causes major environmental problems all over the world, representing threats to soil resources as well as human health. Phytoremediation can be potentially used to remediate metal-contaminated areas. This study aims to assess the extent of metal accumulation by plants found in two mine tailings area in Aletai in the northwest part of China. In present study, shoots and roots of the 19 plant species and the associated soil samples were collected. The concentrations of elements (Pb, Cu, Zn and Cd) of collected samples were then analyzed using flame atomic absorption spectrometry. The results showed that the concentrations of Pb, Cu, Zn and Cd in total mine tailings soil varied from 311 to 1162, 74 to 3251, 1618 to 4815, and 3.37 to 34 mg/kg, those in the plants ranged from 7.35 to 512, 20.4 to 873, 18.7 to 1279, and 0.37 to 47.6 mg/kg, respectively. Although no plant species were identified as metal hyperaccumulators, among those plant species collected from the two mine tailings, Spiraea media Schmidt was most suitable for phytostabilization of Cu (BCF = 6.77). Polygonum aviculare has the potential as a hyperaccumulator, especially in phytoextraction for Cu. Karelinia caspica (Pall.) Less was most effective species for phytostabilization of Cd. These results suggested that some native plants growing on mine tailings area may have the potential for phytoremediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution is a concern of environmental issues. Heavy metal contaminated soil through the food chain to the human or animal, serious harm to human health (Yoon et al. 2006; Gall et al. 2015). Along with the rapid development of industrialization and the unreasonable activities of human beings, such as mining, chemical sewage, dust, waste gas, agricultural fertilizer and other heavy metals through various ways enter the environment, resulting in heavy metal pollution (Selene et al. 2003; Atafar et al. 2010). Waste released from mines causes major environmental problems all over the world (Nouri et al. 2011). In these metals, lead, copper, zinc and cadmium are usually present together in many minerals, which can cause pollution surrounding environment of the mining area. Physical, chemical and biological methods are available to remediate soils contaminated by heavy metals (Cao et al. 2002; Zhang et al. 2015). Drainage also plays an important role to improve soil environment for sustainable agriculture (Valipour 2014a, b, 2015a, b; Valipour et al. 2015). Furthermore, phytoremediation removing the environmental pollution by plants has the advantages of cost-effective, sustainable, does not destroy the soil ecological environment, can be carried out in the polluted area in situ remediation (Singh et al. 2003; Davari et al. 2015). One of the strategies of phytoremediation is phytostabilization, i.e. certain heavy metal contaminants in soils can be concentrated and contained in the root zone of the plants. This process is not to degrade but to reduce the mobility of the contaminant and prevent its migration to the deeper soil. Another application of phytoremediation is phytoaccumulation, also called as phytoextraction, which refers to the extraction of metals by plant roots from contaminated soil and then translocating them to aboveground shoots (Morikawa and Erkin 2003). In this approach, metals accumulated in harvestable parts of the plant can be simply restored from the ash that is produced after drying, and ashing of these harvestable parts (Garbisu and Alkorta 2001).
At present, the criteria used for hyperaccumulation vary per metal, ranging from 100 mg/kg dry mass for Cd, to 1000 mg/kg for Co, Cu, Cr, Pb and Ni, to 10,000 mg/kg for Mn and Zn (Baker and Brooks 1989; Martínez et al. 2006). Bioconcentration factors (BCF) and translocation factors (TF) are more than one in the plants, which have the potential to be used in phytoextraction, while the BCF is greater than one, TF less than one in the plants have the potential for phytostabilization (Yoon et al. 2006).
Over the past few decades, scientists have discovered a large number of metal bioaccumulation potential plant species. More than 500 species of plants are hyperaccumulators of metals, and they can accumulate high concentrations of metal and transfer to their aboveground. There is evidence that plants such as Brassica juncea acts as hyperaccumulator for Ni, Pteris vittata is for As, Ipomea alpine is for Cu and Thlaspi caerulescens is for Zn and Cd (Sarma 2011). Arabidopsis thaliana (Delhaize 1996), B. juncea (Bennett et al. 2003), and Vetiveria zizanioides (Shu et al. 2002) have been successfully used for phytoremediation of mine tailings (Ernst 2005; Mendez and Maie 2008). Sedum alfredii is identified as hyperaccumulator for Zn and Cd in China (Yang et al. 2002, 2004), which occurs on both metalliferous and non-metalliferous soils. There has been a continuing interest in searching for native plants that are tolerant to metal pollutants.
In China, there are a large number of metal mines in the process of mining caused serious pollution of metals, mainly due to lead, copper, zinc and cadmium pollution around the land (Lan et al. 1992; Wong 2003). Our investigation found that there are many actively growing plants in the soil contaminated by metallic elements, especially in mine tailing areas, at Ashel copper–zinc mine and Kirk Tall lead–zinc mine in Aletai, Xinjiang of China. Compared with other plants, because of the strong growth and stress tolerance of native plants, it is more advantageous to remediate heavy metal contaminated environment. Heavy metals can cause severe phytotoxicity, and may act as a powerful force for the evolution of the tolerant plant populations. Therefore, it is possible to identify metal-tolerant plant species from natural vegetation in mine tailing areas contaminated with various metallic elements.
Traditional physical and chemical methods in the remediation of heavy metal contaminated soils may bring the second pollution, and finding a safe, environment-friendly hyperaccumulator for phytoremediation become a very interesting and meaningful research. In the present study, soil and plant samples were analyzed to investigate the Pb, Cu, Zn and Cd accumulation capability of 37 native species collected from the two mine tailings. The concentrations of Pb, Cu, Zn and Cd of samples on the mine tailing areas were determined by flame atomic absorption spectrometry (FAAS). Furthermore, the BCF and TF were compared for identifying the hyperaccumulator. The results of this study should provide insights for finding the potential plant of phytoremediation to remediate metal-contaminated areas.
Materials and methods
Site characterization
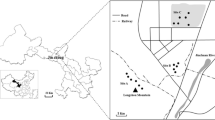
The plant and soil samples used in this study were collected from two mines, Ashel copper–zinc mine (latitude: 48°16′N, longitude: 86°22′E) and Kirk Tall lead–zinc mine (latitude: 47°20′N, longitude: 89°12′E), located in Aletai, Xinjiang of China. Ashel copper–zinc mine is famous in China for its high Cu content in mining deposits, the altitude 842 m, rainfall around 170 mm, maximum of temperature 39 °C, average annual air temperature 4 °C. Kirk Tall lead–zinc mine has the largest lead–zinc deposit in Xinjiang, the altitude 923 m, rainfall around 158 mm, maximum of temperature 38.7 °C, average annual air temperature 1.9 °C. Typical vegetation is temperate desert vegetation, including Chenopodiaceae, Compositae, Rosaceae, Brassicaceae, Poaceae, Polygonaceae.
Plant sampling and analysis
Plant samples, together with the associated soil samples were collected in the surrounding areas of mine tailing at Ashel copper–zinc mine and Kirk Tall lead–zinc mine in June. 5–6 plants were collected randomly within the sampling area for each species. At Ashel copper–zinc mine tailing, the collected samples consisted of 29 species and 15 families, of which 4 species belonged to Polygonaceae, 6 species belonged to Poaceae, and 4 species belonged to Compositae, forming the dominant components in the metal-polluted area. At Kirk Tall lead–zinc mine tailing, the collected 17 species belongs to 8 families, of which 5 species belonged to Compositae, 3 species belonged to Poaceae, forming the dominant component in the area.
After collection, the plants were divided into shoots and roots. Plants samples were washed with running tap water and rinsed with deionized water to remove any soil particles adhered to the plant surfaces, then oven dried (70 °C) for 48 h to constant weight. For analyzing of metal concentration, the dried tissues were weighed and ground into powder and then sieved by 2-mm stainless steel sieve. Metal contents analysis (Pb, Cu, Zn and Cd) of the plant samples was carried out by acid digestion [HNO3/HClO4 (4:1, v/v) (Deng et al. 2004)] followed by the measurement for the concentration of each element using flame atomic absorption spectrometry (FAAS) (HITACHI, Z-2000, Japan).
Soil analysis
0–20 cm soil layers were collected from the root zoon of each location. After the soil sample sending to the laboratory, the large stones and plant debris were removed, oven dried for 72 h to constant weight, and sieved through a 2-mm mesh. For analyzing of total metals, 1 g of soil samples were digested with 15 ml of concentrated HNO3/HCl/HClO4 (1:2:2) mixture on a thermo block and then were diluted with 5 ml of 0.2 % HNO3 (Liu et al. 2008). The total concentrations of the metal (Pb, Cu, Zn and Cd) were determined in the acid extracts using FAAS.
Statistical analysis
The ability of plants to tolerate and accumulate metallic elements is useful for phytostabilization and phytoextraction purpose, which was measured by using bioconcentration factor (BCF) and translocation factor (TF) defined as the ratio of metal concentration in plant roots to soils ([Metal]Root/[Metal]Soil) and the ratio of metal concentration in plant shoots to roots ([Metal]Shoot/[Metal]Root), respectively (Yoon et al. 2006). The Microsoft Excel 2010 statistical program package was used for statistical analysis of data.
Results
Metal concentrations in soils
Soils metal concentrations of eight collected soil samples at two mine tailings are listed in Table 1. At Ashel copper–zinc mine tailing, the soil was mainly contaminated with Cu and Zn. Total Cu concentrations in the soil samples collected from the site were variable, ranging from 1361 at site 4 to 3251 mg/kg at site 2. For Zn, the lowest concentration is 2641 mg/kg at site 5, and the highest concentration is 4815 mg/kg at site 2. Although the Ashel copper–zinc mine tailing was predominantly contaminated with Cu and Zn in this study, it also contained elevated concentrations of Pb and Cd, ranging from 311 to 1162 mg/kg for Pb, and from 3.37 to 16.74 mg/kg for Cd. At Kirk Tall lead–zinc mine, the soil was mainly contaminated with Pb and Zn. Total Pb concentrations in the soil samples collected from the site were variable, ranging from 369 at site 8 to 863 mg/kg at site 7. For Zn, the lowest concentration is 1618 mg/kg at site 8, and the highest concentration is 2919 mg/kg at site 7.
Pb concentration in plants
In this study, 19 selected plant samples of 37 species were collected from 8 locations at the two mine tailings. Total Pb concentrations in the plants ranged from 7.35 to as high as 512 mg/kg, with the maximum value in the roots of Karelinia caspica (Pall.) Less from site 7. In addition, the roots of Bromus squarrosus Linn (388 mg/kg), Stipa capillata Linn (490 mg/kg) and Spiraea media Schmidt (308 mg/kg), the shoots of Polygonum aviculare L. (329 mg/kg) also contained high amounts of Pb. None of the plant species accumulated Pb above 1000 mg/kg in the shoots, the criteria for a hyperaccumulator (Baker and Brooks 1989) (Table 2).
Cu concentration in plants
Copper concentration in the plants varied from 20.4 to 873 mg/kg (Table 3), with the maximum value in the shoots of Polygonum aviculare L. from site 8. None of plant species accumulated Cu above 1000 mg/kg. Furthermore, the roots of Astragalus membranaceus Fisch. (519 mg/kg), and Achnatherum splendens Nevski. (554 mg/kg), the shoots of Polygonum patulum M. (485 mg/kg), Bromus squarrosus Linn. (505 mg/kg) and Stipa capillata Linn. (552 mg/kg) also contained high amounts of Cu. Moreover, Spiraea media Schmidt has high Cu concentrations in both the shoots (407 mg/kg) and roots (549 mg/kg).
Zn and Cd concentrations in plants
The content of Zn in the samples ranged from 18.75 to 1279 mg/kg (Table 4), with the maximum value in the shoots of Karelinia caspica (Pall.) Less from site 7. Besides, the roots of Bromus squarrosus Linn (699 mg/kg), Leymus secalinus (Georgi) Tzvel. (431 mg/kg), and Carex microglochin Wahl. (416 mg/kg), the shoots of Polygonum aviculare L. (608 mg/kg), and Leymus secalinus (Georgi) Tzvel. (316 mg/kg) also contained high amounts of Zn.
The concentration of Cd in the plants varied from 0.37 to 47.6 mg/kg (Table 5), the maximum value was found in the root of Karelinia caspica (Pall.) Less; none of plant species accumulated Cd above 100 mg/kg. The results of Cadmium concentration in the samples showed the lower variability than that of Zn concentration of the tested plants.
Accumulation and translocation of metals in plants
Both BCF and TF can be used to estimate a plant’s potential for phytoremediation purpose. Among the selected 19 plants samples, Karelinia caspica (Pall.) Less collected from site 7 of tailing area in Kirk Tall lead–zinc mine has the maximum Pb concentration (512 mg/kg), but BCF for Pb is only 0.59 (Table 6), which does not accord with the characteristics of hyperaccumulator.
Similar to Pb, none of plant species accumulated Cu above 1000 mg/kg (Table 3). Spiraea media Schmidt (BCF = 6.77) shows the greatest BCF value than others for Cu (Table 6), but its TF value is less than one. Table 6 shows that, Polygonum aviculare L. (BCF = 1.01; TF = 11.72) and Achnatherum splendens Nevski. (BCF = 3.34; TF = 1.31) have the greatest BCFs and TFs values than others. However, the concentrations of Cu in the roots and shoots of the plants are below 1000 mg/kg.
The highest Zn concentration of the tested plants was 1279 mg/kg, which was tested in the roots of Karelinia caspica (Pall.) Less (Table 4). But its BCF value is only 0.44, which was the highest BCF value found in tested plants (Table 6). The largest TF value of Zn was found in Polygonum aviculare L. (TF = 7.33).
As for Cd, Table 6 also shows that Polygonum patulum M. (TF = 2.15) and Polygonum aviculare L. (TF = 1.5) have greater TF value than one. However, the concentrations of Cd in the shoots are relatively lower (2–6.69 mg/kg). As shown in Tables 5 and 6, Karelinia caspica (Pall.) Less (47.6 mg/kg in the root, BCF = 1.38) was found to have a potential for Cd accumulation.
Discussion
In the present study, the contaminated fields were located in two mine tailings. Soil samples were highly contaminated with Pb, Cu, Zn and Cd due to mining activities. Based on total fractions in soil, 100–400 mg/kg Pb, 60–125 mg/kg Cu, 70–400 mg/kg Zn, and 3–8 mg/kg Cd considered toxic to plants have been reported (Deng et al. 2004). The metal (Pb, Cu, Zn and Cd) contents in the surrounding area of mine tailings greatly exceed these ranges (Table 1); therefore, 19 plant species grown in these contaminated sites showed high metal tolerance.
Normal and phytotoxic concentrations of Pb, Zn and Cu were reported (Shi et al. 2011; Yoon et al. 2006), which were 0.5–10 and 30–300 mg/kg for Pb, 3–30 and 20–100 mg/kg for Cu, and 10–150 and >100 mg/kg for Zn. Almost all collected plant species showed higher heavy metal concentration than the normal or phytotoxic levels. These results indicated that the species grown in the mine tailings were tolerant to these metals with varying degrees.
Metal concentration in plants growing in uncontaminated soils were 0.5–10 mg/kg for Pb, whereas the highest metal concentration in plants growing in contaminated soils has been reported as 1506 mg/kg for Pb (Yoon et al. 2006). The present study shows that plants grown in the Pb-contaminated areas usually contained higher concentrations of the metal than that of the uncontaminated soils (Table 2). In our study, although some plants have the ability to accumulate Pb (Table 2), such as Karelinia caspica (Pall.) Less (512 mg/kg in root), Bromus squarrosus Linn (388 mg/kg), Stipa capillata Linn (490 mg/kg), the Pb concentrations in roots and shoots of all tested plants were below 1000 mg/kg. Stoltz and Greger (2002) reported a similar range of 3.4–920 mg/kg of Pb concentrations in the wetland plant species collected from mine tailings. The results indicated that low mobility of Pb from the soil to the roots and shoots.
Copper is essential for plants growth, but it will cause toxic effects when accumulation level of Cu in shoots or leaves exceeds 20 mg/kg (Borkert et al. 1998). Several authors have investigated the accumulation of Cu by native plants species on contaminated sites (Liu et al. 2008; Nouri et al. 2009). Liu et al. (2008) investigated 19 plants growing on contaminated sites in Pb mine area, Heqing County, Yunnan Province; the highest Cu in shoots of Polygonum rude was approximately 570 mg/kg. The values of Cu accumulation in this study were all higher than 20 mg/kg. The highest Cu concentration in the shoot of Polygonum aviculare L. was 873 mg/kg (Table 3). The majority of Cu values in this study were below or similar to the above-mentioned Cu concentration.
Zinc is an essential element for all plants. Comparing with the threshold of zinc toxicity (>100 mg/kg) to plants, Zn accumulation by some of the plants studied exceeded the critical level. The results showed that some plant species could tolerate excess Zn in their shoots. As for Cd, Karelinia caspica (Pall.) Less was the only species accumulating Cd (47.6 mg/kg in root) higher than the toxic levels (10 mg/kg, Macnicol and Beckett 1985) in their tissues. Metal concentration above the level of toxicity in some species indicated that internal metal detoxification tolerance mechanisms might exist in these plants.
Phytoremediation is defined as the use of green plants to remove, contain, or render environmental contaminants harmless. One reason for using plants for remediation concerns the relatively low cost and maintenance requirements (Cunningham and Berti 1993). Phytoremediation has been used in mined soil restoration since these soils are sources of air and water pollution using phytoestabilization and phytoextraction techniques (Wong 2003). The ability of different plants to take up metals from soils and transfer it to the shoots is compared with the levels of BCF and TF. Hyperaccumulators can take up and translocate metals to their aboveground. The TF value of the plant, especially the BCF value of less than one, is not suitable for phytoextraction (Fitz and Wenzel 2002). In this study, the plant species collected from the contaminated sites differed greatly in their capacity of metal accumulation. Based on the average BCFs of the tested plant samples from two mine tailings (Table 6), plant roots were most efficient in accumulating Cu (BCF = 1.44), followed by Cd (0.43), Pb (0.32), and Zn (0.12). Based on the average TFs, the tested plants were most efficient in translocating Cu (TF = 3.04), followed by Zn (1.57), Pb (0.96), and Cd (0.82). For the tested metals, the plants growing on the mine tailing site were most efficient in taking up and translocating Cu. Since Cu is essential nutrients for plant systems, higher translocation from roots to shoots is understandable.
Plants with bioconcentration factor greater than one and translocation factor less than one (BCF >1 and TF <1) have the potential for phytostabilization (Yoon et al. 2006). Phytostabilization can be used to minimize migration of contaminants in soils. This process relies on a principle that plant roots have the ability to alter environmental conditions via root exudates. Plants can immobilize metals through absorption and accumulation by roots, adsorption onto roots (Susarla et al. 2002). Although no metal hyperaccumulators were found in our study, some interesting observations were noted. Karelinia caspica (Pall.) Less could take up 47.6 mg/kg of Cd (BCF = 1.38) in their roots (Table 5), which might be considered as potential species of Cd phytostabilization. Examples of such plants in our study also included Spiraea media Schmidt for Cu (Table 6). Spiraea media Schmidt had high Cu concentrations in both the shoots (407 mg/kg) and roots (549 mg/kg), with 6.77 of BCF and 0.74 of TF, which has potential to be used for phytostabilization. By taking advantage of metal-tolerant plant species for stabilizing contaminants in soil, particularly metals, it could also provide improved conditions for natural attenuation or stabilization of contaminants in the mine tailings. Phytostabilization might be the most appropriate way to remediate mine tailings and other metal contaminated lands.
BCF and TF are more than one of the plants, which have the potential to be used in phytoextraction. At the same time, according to the different capacities of metal uptake, species with the ability of accumulating relatively high metal concentrations in the aboveground tissues could be good candidates for phytoextraction. Phytoextraction aims at decreasing the metal concentration in soil via removal by the plant. Polygonum aviculare L. with 1.01 of BCF and 11.72 of TF has potential to be used for phytoextraction, which is not hyperaccumulator for Cu because the accumulating concentrations in the root and shoot are lower than 1000 mg/kg. However, as shown in Table 3, the concentration of Cu in the shoot of Polygonum aviculare L. was 873 mg/kg, which is close to the criteria of hyperaccumulator. Considering contained higher amounts of Cu (873 mg/kg) in Polygonum aviculare L. and its BCF and TF are more than one, it is concluded that it has the potential to act as a hyperaccumulator. In addition, Achnatherum splendens Nevski with 3.34 of BCF and 1.31 of TF has potential to be used for phytoextraction.
Conclusions
This study was performed to screen plants growing on Ashel copper–zinc mine and Kirk Tall lead–zinc mine tailings to determine their potential for metal accumulation. Only species with both BCFs and TFs greater than one have the potential to be used for phytoextraction. Among the 19 selected plant samples screened, no plant species were identified as metal hyperaccumulators. However, several plants had BCFs and/or TFs greater than one. Among those plant species collected from the mining tailing, Spiraea media Schmidt was considered as the most effective species for phytostabilization of Cu. Polygonum aviculare has the potential to as a hyperaccumulator, especially in phytoextraction for Cu. Karelinia caspica (Pall.) Less was most effective species for phytostabilization of Cd. The potential use of these plant species in phytoremediation, especially for use in mine tailing areas, needs to be further investigated.
References
Atafar Z, Mesdaghinia A, Nouri J, Homaee M, Yunesian M, Ahmadimoghaddam M, Mahvi AH (2010) Effect of fertilizer application on soil heavy metal concentration. Environ Monit Assess 160:83–89
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyperaccumulate metallic elements—a review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126
Bennett LE, Burkhead JL, Hale KL, Terry N, Pilon M, Pilon-Smits EAH (2003) Analysis of transgenic Indian mustard plants for phytoremediation of metal-contaminated mine tailings. J Environ Qual 32:432–440
Borkert CM, Cox FR, Tucker MR (1998) Zinc and copper toxicity in peanut, soybean, rice and corn in soil mixtures. Commun Soil Sci Plant Anal 29:2991–3005
Cao X, Ma LQ, Chen M, Singh SP, Harris WG (2002) Impacts of phosphate amendments on lead biogeochemistry in a contaminated site. Environ Sci Technol 36:5296–5304
Cunningham SD, Berti WR (1993) Remediation of contaminated soils with green plants: an overview. In Vitro Cell Dev Biol 29:207–212
Davari M, Homaee M, Rahnemaie R (2015) An analytical deterministic model for simultaneous phytoremediation of Ni and Cd from contaminated soils. Environ Sci Pollut Res 22:4609–4620
Delhaize E (1996) A metal-accumulator mutant of Arabidopsis thaliana. Plant Physiol 111:849–855
Deng H, Ye ZH, Wong MH (2004) Accumulation of lead, zinc, copper and cadmium by 12 wetland plant species thriving in metal-contaminated sites in China. Environ Pollut 132:29–40
Ernst WHO (2005) Phytoextraction of mine wastes—options and impossibilities. Chem Erde 65(S1):29–42
Fitz WJ, Wenzel WW (2002) Arsenic transformations in the soil–rhizosphere–plant system: fundamentals and potential application of phytoremediation. J Biotechnol 99:259–278
Gall JE, Boyd RS, Rajakaruna N (2015) Transfer of heavy metals through terrestrial food webs: a review. Environ Monit Assess 187:201–222
Garbisu C, Alkorta I (2001) Phytoextraction: a cost-effective plant-based technology for the removal of metals from the environment. Bioresour Technol 77:229–236
Lan CY, Chen GZ, Li LC, Wong MH (1992) Use of cattails in treating wastewater from a Pb/Zn mine. Environ Manag 16:75–80
Liu XH, Gao YT, Sardar K, Duan G, Chen AK, Ling L, Zhao L, Liu ZH, Wu XK (2008) Accumulation of Pb, Cu, and Zn in native plants growing on contaminated sites and their potential accumulation capacity in Heqing, Yunnan. J Environ Sci 20:1469–1474
Macnicol RD, Beckett PHT (1985) Critical tissue concentration of potentially toxic elements. Plant Soil 85:107–129
Martínez M, Bernal P, Almela C, Vélez D, García-Agustín P, Serrano R, Navarro-Aviňό J (2006) An engineered plant that accumulates higher levels of heavy metals than Thlaspi caerulescens, with yields of 100 times more biomass in mine soils. Chemosphere 64:478–485
Mendez MO, Maie RM (2008) Phytoremediation of mine tailings in temperate and arid environments. Rev Environ Sci Biotechnol 7:47–59
Morikawa H, Erkin ÖC (2003) Basic processes in phytoremediation and some applications to air pollution control. Chemosphere 52:1553–1558
Nouri J, Khorasani N, Lorestani B, Karami M, Hassani AH, Yousefi N (2009) Accumulation of heavy metals in soil and uptake by plant species with phytoremediation potential. Environ Earth Sci 59:315–323
Nouri J, Lorestani B, Yousefi N, Khorasani N, Hasani AH, Seif F, Cheraghi M (2011) Phytoremediation potential of native plants grown in the vicinity of Ahangaran lead–zinc mine (Hamedan, Iran). Environ Earth Sci 62:639–644
Sarma H (2011) Metal hyperaccumulation in plants: a review focusing on phytoremediation technology. J Environ Sci Technol 4:118–138
Selene CH, Chou J, De Rosa CT (2003) Case studies—Arsenic. Int J Hyg Environ Health 206:381–386
Shi X, Zhang XL, Chen GC, Chen YT, Wang L, Shan XQ (2011) Seedling growth and metal accumulation of selected woody species in copper and lead/zinc mine tailings. J Environ Sci 23:266–274
Shu WS, Xia HP, Zhang ZQ, Lan CY, Wong MH (2002) Use of vetiver and three other grasses for revegetation of Pb/Zn mine tailings: field experiment. Int J Phytorem 4:47–57
Singh OV, Labana S, Pandey G, Budhiraja R, Jain RK (2003) Phytoremediation: an overview of metallic ion decontamination from soil. Appl Microbiol Biotechnol 61:405–412
Stoltz E, Greger M (2002) Accumulation properties of As, Cd, Cu, Pb and Zn by four wetland plant species growing on submerged mine tailings. Environ Exp Bot 47:271–280
Susarla S, Medina VF, McCutcheon SC (2002) Phytoremediation: an ecological solution to organic contamination. Ecol Eng 18:647–658
Valipour M (2014a) Drainage, waterlogging, and salinity. Arch Agron Soil Sci 60:1625–1640
Valipour M (2014b) Future of the area equipped for irrigation. Arch Agron Soil Sci 60:1641–1660
Valipour M (2015a) A comprehensive study on irrigation management in Asia and Oceania. Arch Agron Soil Sci 61:1247–1271
Valipour M (2015b) Future of agricultural water management in Africa. Arch Agron Soil Sci 61:907–927
Valipour M, Ahmadi MZ, Raeini-Sarjaz M, Sefidkouhi MAG, Shahnazari A, Fazlola R, Darzi-Naftchali A (2015) Agricultural water management in the world during past half century. Arch Agron Soil Sci 61:657–678
Wong MH (2003) Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils. Chemosphere 50:775–780
Yang XE, Long XX, Ni WZ, Fu CX (2002) Sedum alfredii H: a new Zn hyperaccumulating plant first found in China. Chin Sci Bull 47:1634–1637
Yang XE, Long XX, Ye HB, He ZL, Calvert DV, Stoffella PJ (2004) Cadmium tolerance and hyperaccumulation in a new Zn-hyperaccumulating plant species (Sedum alfredii Hance). Plant Soil 259:181–189
Yoon J, Cao XD, Zhou QX, Ma LQ (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368:456–464
Zhang J, Zhang GL, Cai DQ, Wu ZY (2015) Immediate remediation of heavy metal (Cr(VI)) contaminated soil by high energy electron beam irradiation. J Hazard Mater 285:208–211
Acknowledgments
This research was supported by the Grant (No. 201416102) from the Xinjiang Uygur Autonomous Region high tech research and development project of China. The authors would like to thank Dr. Hong Wang for assistance in plant identification and Mr. Minshan Shi for his assistance in chemical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Z., Hamuti, A., Abdulla, H. et al. Accumulation of metallic elements by native species thriving in two mine tailings in Aletai, China. Environ Earth Sci 75, 781 (2016). https://doi.org/10.1007/s12665-016-5594-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-5594-5