Abstract
Chronic liver injuries evolve from benign and manageable dysfunction to life-threatening end-stage liver disease with severe complications especially due to portal hypertension. Still liver transplantation remains the only and final curative option for end-stage liver disease, while several drugs have been repurposed for the treatment of complications of cirrhosis in this setting. The demand for organ transplants exceeds by far the supply and therefore those treatment options are urgently needed. But the evidence is not always as encouraging and of high enough good quality.
One example is statins, primarily cholesterol-lowering drugs, but broadly used for primary or secondary prevention of cardiovascular diseases. The beneficial effects of statins are mediated by the so-called pleiotropic mechanisms on inflammation, fibrosis, endothelial function, thrombosis, coagulation, and contraction of contractile cells to improve portal hypertension. However, concerns remain about the efficacy and safety of some statins due to their potential hepatotoxic risks and as of now, these risks impede broader use of statins in the treatment of chronic liver diseases.
This chapter tries to comprehensively describe the mechanisms by which statins may improve pathophysiological processes and summarize the clinical experience of statin use in the treatment of liver cirrhosis.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
General Underlying Mechanisms of Statins
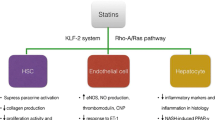
Statins are a class of competitive inhibitors of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme for endogenous cholesterol, and isoprenoid synthesis in the mevalonate pathway. Its inhibition leads to decreased production of precursors and subsequently reduction of cholesterol biosynthesis [1] (Fig. 23.1).
The so-called pleiotropic effects are mediated by the reduction of isoprenoids, such as Farnesyl-pyrophosphate (FPP) and Geranylgeranyl-pyrophosphate (GGPP), necessary for the activation of small GTPases like Rho- and Ras-proteins (Fig. 23.1). Statins diversely inhibit RhoA and Rac1 prenylation, modulating endothelial Nitric Oxide Synthase (eNOS), NO availability and enhancing stability of eNOS mRNA [2] (Fig. 23.1). This statin-dependent eNOS restoration can be also mediated by increased Krüppel-like factor 2 (KLF2) expression [3]. Similar effects were observed in hepatic injury followed by fibrosis and cirrhosis. Moreover, statins decrease oxidative stress in the liver and enhance eNOS expression and activity by inhibiting RhoA membrane association [2, 4]. Taken together, inhibition of GTPase prenylation, restoration of eNOS, and NO availability are the key roles of statins in the improvement of vascular and endothelial function (Fig. 23.1).
But also, the decreased cholesterol synthesis itself is beneficial several-fold. Decreased cholesterol level leads to an increase of Sterol Regulatory Element-Binding Proteins (SREBP), which act as transcription factors for the LDL receptor that induces higher plasma LDL clearance due to an increased LDL receptor-mediated uptake and after lysosomal degradation of LDL [5]. Also, the decreased hepatic triglyceride synthesis is possibly associated with activated peroxisome proliferator-activated receptor α (PPARα) and increased β-oxidation activity [5] (Fig. 23.1). Moreover, statins seem to beneficially modify PPARγ activity, which attenuates the production of tumor necrosis factor α (TNFα), interleukins 1-beta (IL1β) and 6 (IL6) and C-reactive protein (CRP) [6, 7].
In summary, due to various mechanisms, dependent or not on the cholesterol levels, statins modify pathological conditions as outlined in the following section.
Statins in Cardiovascular Diseases and Interaction with Liver Disease
Statins also decrease Low-Density Lipoprotein (LDL) cholesterol levels, which are pro-atherogenic. For this reason, statins have become the standard of care in the treatment of diabetes and Cardiovascular Diseases (CVD) to decrease or even reverse atherosclerosis. However, liver diseases, they are often underused even in high-risk patients [8]. Especially in CVD, several studies have shown a clear effect of statins on inflammation. Besides the Pravastatin Inflammation/CRP Evaluation (PRINCE) study, also another study demonstrated that statins improve inflammation and decrease interleukin-6 (IL-6) levels, the main regulator of CRP [6]. Furthermore, statins directly inhibit the expression of major histocompatibility complex class II molecules by interferon g (IFN-g) in CD4+ helper T cells (TH1 cells), leading to a shift toward anti-inflammatory TH2 cell actions and beneficially modifying atherosclerosis [9]. This anti-inflammatory effect is extremely important for the liver disease since especially in the last years’ systemic inflammation has been identified as a marker of disease progression [10] and is persistent besides portal hypertension [11], with strong effects on other organs leading to dysfunction [12, 13]. This has also been recently demonstrated by the PREDICT study, in which portal hypertension and systemic inflammation are the two main mechanisms leading to acute decompensation of liver cirrhosis [14].
Moreover, as outlined above statins in addition improve eNOS and NO in the endothelium, which decreases leukocytes’ chemotaxis, adhesion, and inflammation, key mechanisms aggravating atherosclerosis [9]. The decreased infiltration of plaques by macrophages and downregulation of proteolytic enzymes are associated also with decreasing NADPH oxidase isoform 2 (NOX2) [15]. While statins might inhibit the immune cells activity, they increase the number of circulating endothelial progenitor cells, which are important in the neovascularization of ischemic tissue, thereby contributing to the restoration of endothelial function [16]. Endothelial function is extremely important and differently regulated in portal hypertension and cirrhosis not only in the liver but also outside [17]. Endothelial function in cirrhosis with portal hypertension is impaired, promoting vasoconstriction in the liver and sustained vasodilation in the splanchnic region [17].
Furthermore, statins elicit anti-thrombotic effects by down-regulating platelet CD40L, by inhibiting tissue factor activity and thrombin generation [5, 9]. A growing number of studies suggest the importance of Intrahepatic microvascular thrombosis for fibrosis progression and portal hypertension. This observation connecting thrombosis, liver cirrhosis, and portal hypertension was first described as “parenchymal extinction” in pathological specimens of human liver cirrhosis [18]. While the development and progression of splanchnic venous thrombotic events implicate disease progression in cirrhosis, their pathogenesis remains unclear. According to Virchow’s triad, coagulation/platelets and vascular wall are the drivers of thrombosis. Even in patients with TIPS, when the flow is restored and, to a large extent, also the shear stress due to portal hypertension, the prothrombotic milieu is increased, probably due to platelet activation [19].
Recent data demonstrate that statins have an important role in the microbiota and their use may be associated with less gut dysbiosis [20]. It is known that microbiota influences progression of the different diseases. In addition, it may also be a driver of the development of portal hypertension and decompensation of liver cirrhosis [21, 22]. The translocated bacteria or bacterial components drive systemic inflammation and potentially also thrombosis and thereby may aggravate the progression of liver disease and development of complications [19, 23].
Adverse Effects and Hepatotoxicity
Although similar mechanisms as in CVD are involved in the development and progression of liver disease, caution is required due to hepatotoxicity. Hepatic cells make a considerable contribution to cholesterol production and therefore are a major target of statins. The pharmacological activity and the hepatic metabolism of statins depend on their molecular structure and physical properties such as lipophilicity, solubility, and absorption. Simvastatin, lovastatin, fluvastatin, and atorvastatin are metabolized by cytochrome P450, while pravastatin, rosuvastatin, and pitavastatin remain almost unaffected by any hepatic metabolic processes.
The effect of statins on aminotransferase levels in the treatment of cardiovascular diseases was investigated in several studies with contradictory results. This inconsistency may be explained by pharmacogenetics and differences in the statins used. Although statins are generally well-tolerated, reports about statin-induced liver injury can be found mainly for atorvastatin and simvastatin. However, this might be coincidental since these two statins are also the two most commonly prescribed ones [24].
The question of whether statins have a hepatotoxic effect is considerably more relevant in patients with acute or chronic liver dysfunction. In a retrospective cohort study, lovastatin showed no increased risk of adverse hepatic effects in a total of 93,106 patients with liver disease. Another prospective randomized, double-blind, placebo-controlled multicenter trial, investigated the safety of high-dose pravastatin in chronic liver disease. After 36 weeks of treatment, alanine aminotransferase (ALT) levels were even lower in the pravastatin-treated group [25]. Furthermore, HMG-CoA reductase inhibitors were also found to be safe in patients after liver transplantation [26].
Few studies revealed that statin hepatotoxicity is a rare condition and might mimic an autoimmune phenotype of liver injury [27, 28]. However, in patients with chronic kidney diseases, the incidence of severe adverse events seems to be higher [29]. However, in patients with decompensated liver cirrhosis simvastatin seems to elicit rhabdomyolysis and hepatotoxic effects at the dose of 40 mg daily [30]. Myopathy, and less common rhabdomyolysis, are known adverse effects of statin and are rare in normal circumstances—about 2–3 cases/year per 100,000 patients treated [31]. Again, in another cirrhosis trial these adverse events were relatively frequent [27]. The reasons might be related to the dose of statins, genetic predisposition (e.g., SCLO1B1 polymorphism), but also alcoholic etiology of liver disease, being the most prevalent in this study [27]. This was again confirmed in a small uncontrolled Phase IIa study [32].
In a recent meta-analysis on Pharmacokinetics (PK), cardiovascular outcomes, and safety profiles of statins in cirrhosis, the authors conclude that rosuvastatin and pitavastatin showed minimal PK changes, while atorvastatin caused more pronounced PK changes in Child-Pugh A cirrhosis, while no data was available for the most used simvastatin [33]. Yet, simvastatin 40 mg had a pooled frequency for rhabdomyolysis of 2%, and incidence 40-fold higher than that reported in non-cirrhotic patients, while there was no rhabdomyolysis observed in patients on simvastatin 20 mg, atorvastatin 20 mg, or pravastatin 40 mg. In the experience so far published, no overt liver failure was reported. Still, in most cases, the benefits of statins outweigh any potential hepatotoxic risks [34, 35]. Another option unexplored in clinics is the use of novel statin drugs, as suggested by using a compound containing atorvastatin and a NO-donor [36], which significantly reduced myopathy in an animal model.
Distinct and Common Mechanisms of Statins in Liver Diseases
CVD in many patients is associated with Metabolic Syndrome (MS), which is the common ground for the development of NAFLD and NASH. Statins have been considered for treatment in NASH, and in recent years they have been generally evaluated as safe—even at high doses—leading to a wider use in patients [25, 37,38,39]. Nevertheless, studies assessing the beneficial effects of statins are scarce, mostly investigating only a small number of patients with different endpoints. These studies showed that statin therapy either attenuates inflammation and steatosis [40] or shows a trend toward decreased fibrosis while other studies found no change in fibrosis [40, 41]. The different study outcomes may be due to the different statins used in the respective trials. While atorvastatin at 10 mg/day for 24 months elicited positive effects in NASH, simvastatin 20 mg/day over 12 months had no effect in a similar cohort of patients [41]. Moreover, genetic predispositions were disregarded by most of these studies and may provide additional explanations for the different outcomes regarding fibrosis. For example, a large multicenter study revealed that statin use in high-risk patients is beneficial, except in patients carrying the PNPLA3 I148M risk alleles [42]. Thus, genetic screening may be advisable for NAFLD patients to ascertain the optimal therapy for each patient.
Recent studies revealed improvements in NASH and MS after statin treatment [42, 43]. Statins decrease LDL cholesterol levels in serum, and as a result, oxidized LDL levels play an important role in NASH. As highlighted in Fig. 23.1, statin therapy leads to decreased hepatic steatosis by decreasing LDL and activating SREBPs, PPARα, and β-oxidation [5]. However, the anti-inflammatory effect of statins in NAFLD and NASH is partly attributed to the activation of PPARγ and subsequent downregulation of pro-inflammatory mediators [6, 7]. Additionally, the inhibition of small GTPase prenylation and diminished downstream signaling contribute to the anti-inflammatory features of statins [7]. Recent experimental NASH studies further suggest a beneficial impact of statins in fibrosis. They inhibit the paracrine signaling of hepatocytes on Hepatic Stellate Cells (HSC), thereby inhibiting Hepatic StellateCells (HSC) activation and fibrogenesis during experimental NASH.
Additionally to the hepatic and general metabolic improvement which are known to be tightly linked to chronic viral hepatitis C (HCV) infection, statins might exert a direct anti-replicative effect on HCV [44] Previous small-scale studies investigated the effect of statin monotherapy and revealed only mild antiviral effects [45], while cohort studies could confirm the benefit [46]. Especially in combination with direct-acting antiviral agents statins may have an added value to the antiviral efficacy and mitigate the progression of HCV-related diseases, such as cirrhosis or HCC [47, 48]. Additionally, HCV patients with compensated cirrhosis under statin treatment seem to have a lower risk for decompensation as well as lower mortality [49]. This is a rather decreasing indication and will probably not play a significant role in the future as shown recently [50]; other studies report a highly significant decrease in HCC risk resulting from statin use [51, 52]. The data from the Electronically Retrieved Cohort of HCV Infected Veterans (ERCHIVES) database shows a dose-dependent reduction of fibrosis progression, going along with the decreasing HCC incidence. Remarkably, reduction of HCC incidence was about 47% in all treated patients. Also, this study clearly showed differences in statin efficacy, whereby atorvastatin and fluvastatin had the strongest effects on fibrosis progression, as well as HCC incidence [51]. Nonetheless, it remains uncertain whether these effects are due to fibrosis reduction, direct effects on HCC progression or a combination of both.
Statins decrease not only the risk of development of liver cirrhosis but also may alter the hepatic resistance. Cirrhosis, the common end-stage of chronic liver injury, is characterized by profound liver remodeling and portal hypertension. Portal hypertension in cirrhosis arises by a mechanically increased intrahepatic resistance by narrowing of hepatic outflow. An additional dynamic component of increased intrahepatic resistance is dominated by an imbalance of vascular tone-regulating pathways, showing a shift towards vasocontraction [17]. Furthermore, RhoA and Rho-kinase signaling are responsible for the increased tone of the hepatic vasculature contributing to the activation of HSC, the major contributor to ECM synthesis upon chronic liver injury. Statins modulate the mechanic and the dynamic intrahepatic pathways [17]. Both pathways represent targets of statin therapy in liver cirrhosis with portal hypertension (Fig. 23.1). Atorvastatin inhibits the translocation of RhoA and thus the activity of Rho-kinase. This effect decreases collagen production and hepatic stellate cell activation in early fibrosis as well as proliferation, cytokine production, and contraction of activated hepatic stellate cells in cirrhosis. Importantly, statins might induce senescence in activated HSC leading to a decreased turnover of these highly active cells. Simultaneously, statins improve endothelial dysfunction by the upregulated activity of eNOS and NO availability in cirrhotic livers and further decrease portal pressure [2, 53, 54].
In several studies, acute and chronic effects of statins on portal pressure, complications, or overall outcome of patients with cirrhosis were investigated (Table 23.1). Statins seem to significantly decrease hepatic vascular resistance in cirrhosis with portal hypertension, in addition to the extrahepatic effect of beta-blockers [4, 55]. Another study, so far published only as an abstract confirmed the beneficial effects of statins, even in patients identified as non-responder to non-selective beta-blockers [56]. However, this was not observed in a randomized placebo-controlled trial in the primary prophylaxis setting [57]. Simvastatin may decrease portal pressure by around 10% after only 1 month [4]. The same group intended to show a decrease in rebleeding during the secondary prophylaxis but were unable to demonstrate a lower number of variceal bleeds [27]. However, and most interestingly statins improved overall survival in this study [27]. A meta-analysis summarizing the effect of statins on lowering portal pressure and the related clinical effects defined as the risk of variceal hemorrhage demonstrated a clear overall portal pressure lowering effect, while showing only a tendency for a decreased risk of variceal hemorrhage [58].
Besides decreased portal pressure, cirrhotic patients under statins may also benefit from improved liver function. Interestingly, statin effects are enhanced with increased severity of portal hypertension [59]. In addition, simvastatin improved survival in patients after variceal hemorrhage suggesting multiple beneficial effects of statins. As demonstrated in an animal model [60], the severity of hemorrhage might be also lower in patients receiving statins. Even acute decompensation induced artificially in animal models using LPS could be prevented by the use of statins [61]. This was also retrospectively observed in a large set of patients in the US, presented as abstract so far [62].
Summary/Conclusion
Statins exhibit pleiotropic effects in many liver diseases. One of these effects is the inhibition of isoprenoid synthesis as a consequence of decreased HMG-CoA reductase activity since the resulting modulated GTPase activity plays a major role in the treatment of most chronic liver diseases (Fig. 23.1).
Statins are cost-effective and generally well-tolerated by patients and the benefits of statin treatment in most patients outweigh their potential hepatotoxic risk. Especially in patients with severe chronic liver injury and high risk of CVD, statin treatment is very promising since it not only prevents the development of CVD but also could help to prevent the progression of liver fibrosis to cirrhosis and the development of HCC, decrease portal pressure, lower inflammation and the related acute decompensation and even ACLF. Therefore, reasons for statin use in chronic liver diseases are more convincing than reasons against, rendering statin treatment a definite advantage.
Abbreviations
- BDL:
-
Bile duct ligation
- CRP:
-
C-reactive protein
- CVD:
-
Cardiovascular disease
- ECM:
-
Extracellular matrix
- eNOS:
-
Endothelial nitric oxide synthase
- FPP:
-
Farnesyl-pyrophosphate
- GGPP:
-
Geranylgeranyl-pyrophosphate
- HCC:
-
Hepatocarcinoma
- HCV:
-
Viral hepatitis C
- HMG-CoA:
-
3-Hydroxy-3-methyl-glutaryl-coenzyme A
- HSC:
-
Hepatic stellate cell
- KLF2:
-
Kruppel-like factor 2
- MS:
-
Metabolic syndrome
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- NOX2:
-
Nicotinamide adenine dinucleotide phosphate oxidase isoform 2
- PNPLA3:
-
Patatin-like phospholipase domain-containing protein 3
- PPARα:
-
Activated peroxisome proliferator-activated receptor alpha
- SREBP:
-
Sterol regulatory element binding proteins
- UDCA:
-
Ursodeoxycholic acid
References
Schierwagen R, Uschner FE, Magdaleno F, Klein S, Trebicka J. Rationale for the use of statins in liver disease. Am J Physiol Gastrointest Liver Physiol. 2017;312:G407–12.
Trebicka J, Hennenberg M, Laleman W, Shelest N, Biecker E, Schepke M, Nevens F, et al. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/rho-kinase and activation of endothelial nitric oxide synthase. Hepatology. 2007;46:242–53.
Marrone G, Shah VH, Gracia-Sancho J. Sinusoidal communication in liver fibrosis and regeneration. J Hepatol. 2016;65:608–17.
Abraldes JG, Rodriguez-Vilarrupla A, Graupera M, Zafra C, Garcia-Caldero H, Garcia-Pagan JC, Bosch J. Simvastatin treatment improves liver sinusoidal endothelial dysfunction in CCl4 cirrhotic rats. J Hepatol. 2007;46:1040–6.
Goldstein JL, Brown MS. A century of cholesterol and coronaries: from plaques to genes to statins. Cell. 2015;161:161–72.
Gruzdeva O, Uchasova E, Dyleva Y, Akbasheva O, Karetnikova V, Barbarash O. Early effects of treatment low-dose atorvastatin on markers of insulin resistance and inflammation in patients with myocardial infarction. Front Pharmacol. 2016;7:324.
Schierwagen R, Maybuchen L, Hittatiya K, Klein S, Uschner FE, Braga TT, Franklin BS, et al. Statins improve NASH via inhibition of RhoA and Ras. Am J Physiol Gastrointest Liver Physiol. 2016;311:G724–33.
Blais P, Lin M, Kramer JR, El-Serag HB, Kanwal F. Statins are underutilized in patients with nonalcoholic fatty liver disease and dyslipidemia. Dig Dis Sci. 2016;61:1714–20.
Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399–402.
Trebicka J, Amoros A, Pitarch C, Titos E, Alcaraz-Quiles J, Schierwagen R, Deulofeu C, et al. Addressing profiles of systemic inflammation across the different clinical phenotypes of acutely decompensated cirrhosis. Front Immunol. 2019;10:476.
Trebicka J, Reiberger T, Laleman W. Gut-liver Axis links portal hypertension to acute-on-chronic liver failure. Visc Med. 2018;34:270–5.
Praktiknjo M, Monteiro S, Grandt J, Kimer N, Madsen JL, Werge MP, William P, et al. Cardiodynamic state is associated with systemic inflammation and fatal acute-on-chronic liver failure. Liver Int. 2020;40:1457–66.
Monteiro S, Grandt J, Uschner FE, Kimer N, Madsen JL, Schierwagen R, Klein S, et al. Differential inflammasome activation predisposes to acute-on-chronic liver failure in human and experimental cirrhosis with and without previous decompensation. Gut. 2021;70(2):379–87.
Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, Giovo I, et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020;73:842–54.
Pignatelli P, Carnevale R, Pastori D, Cangemi R, Napoleone L, Bartimoccia S, Nocella C, et al. Immediate antioxidant and antiplatelet effect of atorvastatin via inhibition of Nox2. Circulation. 2012;126:92–103.
Oikonomou E, Siasos G, Zaromitidou M, Hatzis G, Mourouzis K, Chrysohoou C, Zisimos K, et al. Atorvastatin treatment improves endothelial function through endothelial progenitor cells mobilization in ischemic heart failure patients. Atherosclerosis. 2015;238:159–64.
Iwakiri Y, Trebicka J. Portal hypertension in cirrhosis: pathophysiological mechanisms and therapy. JHEP Rep. 2021;3:100316.
Wanless IR, Wong F, Blendis LM, Greig P, Heathcote EJ, Levy G. Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension. Hepatology. 1995;21:1238–47.
Queck A, Carnevale R, Uschner FE, Schierwagen R, Klein S, Jansen C, Meyer C, et al. Role of portal venous platelet activation in patients with decompensated cirrhosis and TIPS. Gut. 2020;69:1535–6.
Vieira-Silva S, Falony G, Belda E, Nielsen T, Aron-Wisnewsky J, Chakaroun R, Forslund SK, et al. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature. 2020;581:310–5.
Trebicka J, Bork P, Krag A, Arumugam M. Utilizing the gut microbiome in decompensated cirrhosis and acute-on-chronic liver failure. Nat Rev Gastroenterol Hepatol. 2021;18:167–80.
Trebicka J, Macnaughtan J, Schnabl B, Shawcross DL, Bajaj JS. The microbiota in cirrhosis and its role in hepatic decompensation. J Hepatol. 2021;75(Suppl 1):S67–81.
Schierwagen R, Alvarez-Silva C, Madsen MSA, Kolbe CC, Meyer C, Thomas D, Uschner FE, et al. Circulating microbiome in blood of different circulatory compartments. Gut. 2019;68:578–80.
Bjornsson ES. Hepatotoxicity of statins and other lipid-lowering agents. Liver Int. 2017;37:173–8.
Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R, Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46:1453–63.
Martin JE, Cavanaugh TM, Trumbull L, Bass M, Weber F Jr, Aranda-Michel J, Hanaway M, et al. Incidence of adverse events with HMG-CoA reductase inhibitors in liver transplant patients. Clin Transpl. 2008;22:113–9.
Abraldes JG, Villanueva C, Aracil C, Turnes J, Hernandez-Guerra M, Genesca J, Rodriguez M, et al. Addition of simvastatin to standard therapy for the prevention of Variceal Rebleeding does not reduce Rebleeding but increases survival in patients with cirrhosis. Gastroenterology. 2016;150:1160–70. e1163
Russo MW, Hoofnagle JH, Gu J, Fontana RJ, Barnhart H, Kleiner DE, Chalasani N, et al. Spectrum of statin hepatotoxicity: experience of the drug-induced liver injury network. Hepatology. 2014;60:679–86.
Palmer SC, Craig JC, Navaneethan SD, Tonelli M, Pellegrini F, Strippoli GF. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2012;157:263–75.
Pose E, Napoleone L, Amin A, Campion D, Jimenez C, Piano S, Roux O, et al. Safety of two different doses of simvastatin plus rifaximin in decompensated cirrhosis (LIVERHOPE-SAFETY): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Gastroenterol Hepatol. 2020;5:31–41.
Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, Blumenthal R, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–61.
Munoz AE, Pollarsky F, Marino M, Cartier M, Miguez C, Vazquez H, Alvarez D, et al. Safety of chronic simvastatin treatment in patients with decompensated cirrhosis: many adverse events but no liver injury. Dig Dis Sci. 2021;66:3199–208.
Sung S, Al-Karaghouli M, Kalainy S, Cabrera Garcia L, Abraldes JG. A systematic review on pharmacokinetics, cardiovascular outcomes and safety profiles of statins in cirrhosis. BMC Gastroenterol. 2021;21:120.
Bjornsson E, Jacobsen EI, Kalaitzakis E. Hepatotoxicity associated with statins: reports of idiosyncratic liver injury post-marketing. J Hepatol. 2012;56:374–80.
Pose E, Trebicka J, Mookerjee RP, Angeli P, Gines P. Statins: old drugs as new therapy for liver diseases? J Hepatol. 2019;70:194–202.
Rodriguez S, Raurell I, Torres-Arauz M, Garcia-Lezana T, Genesca J, Martell M. A nitric oxide-donating statin decreases portal pressure with a better toxicity profile than conventional statins in cirrhotic rats. Sci Rep. 2017;7:40461.
Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126:1287–92.
Chalasani N. Statins and hepatotoxicity: focus on patients with fatty liver. Hepatology. 2005;41:690–5.
Pastori D, Polimeni L, Baratta F, Pani A, Del Ben M, Angelico F. The efficacy and safety of statins for the treatment of non-alcoholic fatty liver disease. Dig Liver Dis. 2015;47:4–11.
Ekstedt M, Franzen LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: a histopathological follow-up study. J Hepatol. 2007;47:135–41.
Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: a randomized placebo-controlled trial. J Clin Gastroenterol. 2009;43:990–4.
Dongiovanni P, Petta S, Mannisto V, Mancina RM, Pipitone R, Karja V, Maggioni M, et al. Statin use and non-alcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63:705–12.
Eslami L, Merat S, Malekzadeh R, Nasseri-Moghaddam S, Aramin H. Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2013:CD008623.
Kapadia SB, Chisari FV. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc Natl Acad Sci U S A. 2005;102:2561–6.
Bader T, Fazili J, Madhoun M, Aston C, Hughes D, Rizvi S, Seres K, et al. Fluvastatin inhibits hepatitis C replication in humans. Am J Gastroenterol. 2008;103:1383–9.
Butt AA, Yan P, Bonilla H, Abou-Samra AB, Shaikh OS, Simon TG, Chung RT, et al. Effect of addition of statins to antiviral therapy in hepatitis C virus-infected persons: results from ERCHIVES. Hepatology. 2015;62:365–74.
Simon TG, King LY, Zheng H, Chung RT. Statin use is associated with a reduced risk of fibrosis progression in chronic hepatitis C. J Hepatol. 2015;62:18–23.
Yang YH, Chen WC, Tsan YT, Chen MJ, Shih WT, Tsai YH, Chen PC. Statin use and the risk of cirrhosis development in patients with hepatitis C virus infection. J Hepatol. 2015;63:1111–7.
Mohanty A, Tate JP, Garcia-Tsao G. Statins are associated with a decreased risk of Decompensation and death in veterans with hepatitis C-related compensated cirrhosis. Gastroenterology. 2016;150:430–440.e431.
Gu W, Hortlik H, Erasmus H-P, Schaaf L, Zeleke Y, Uschner FE, Ferstl P, et al. Diagnosis of cirrhosis is associated with premature death in hospital admissions. Lancet Reg Health Eur. 2021;12:100240.
Simon TG, Bonilla H, Yan P, Chung RT, Butt AA. Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: results from ERCHIVES. Hepatology. 2016;64:47–57.
Singh S, Singh AG, Singh PP, Murad MH, Iyer PG. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:620–9.
Trebicka J, Hennenberg M, Odenthal M, Shir K, Klein S, Granzow M, Vogt A, et al. Atorvastatin attenuates hepatic fibrosis in rats after bile duct ligation via decreased turnover of hepatic stellate cells. J Hepatol. 2010;53:702–12.
Klein S, Klosel J, Schierwagen R, Korner C, Granzow M, Huss S, Mazar IG, et al. Atorvastatin inhibits proliferation and apoptosis, but induces senescence in hepatic myofibroblasts and thereby attenuates hepatic fibrosis in rats. Lab Investig. 2012;92:1440–50.
Zafra C, Abraldes JG, Turnes J, Berzigotti A, Fernandez M, Garca-Pagan JC, Rodes J, et al. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology. 2004;126:749–55.
Alvarado-Tapias E, Ardévol A, Pavel O, Montanes R, Murzi M, Oblitas Susanibar E, Poca M, et al. Hemodynamic effects of Carvedilol plus simvastatin in cirrhosis with portal hypertension and no-response to βBlockers: a double-blind randomized trial. Hepatology. 2016;64:74A.
Vijayaraghavan R, Jindal A, Arora V, Choudhary A, Kumar G, Sarin SK. Hemodynamic effects of adding simvastatin to Carvedilol for primary prophylaxis of Variceal bleeding: a randomized controlled trial. Am J Gastroenterol. 2020;115:729–37.
Wan S, Huang C, Zhu X. Systematic review with a meta-analysis: clinical effects of statins on the reduction of portal hypertension and variceal haemorrhage in cirrhotic patients. BMJ Open. 2019;9:e030038.
Pollo-Flores P, Soldan M, Santos UC, Kunz DG, Mattos DE, da Silva AC, Marchiori RC, et al. Three months of simvastatin therapy vs. placebo for severe portal hypertension in cirrhosis: a randomized controlled trial. Dig Liver Dis. 2015;47:957–63.
Meireles CZ, Pasarin M, Lozano JJ, Garcia-Caldero H, Gracia-Sancho J, Garcia-Pagan JC, Bosch J, et al. Simvastatin attenuates liver injury in rodents with biliary cirrhosis submitted to hemorrhage/resuscitation. Shock. 2017;47:370–7.
Tripathi DM, Vilaseca M, Lafoz E, Garcia-Caldero H, Viegas Haute G, Fernandez-Iglesias A, Rodrigues de Oliveira J, et al. Simvastatin prevents progression of acute on chronic liver failure in rats with cirrhosis and portal hypertension. Gastroenterology. 2018;155:1564–77.
Mahmud N, Chapin S, Goldberg DS, Reddy KR, Kaplan DE. Statins are associated with reduced development of acute-on-chronic liver failure in a large national cohort of patients with cirrhosis. J Hepatol. 2022;76(5):1100–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this paper
Cite this paper
Trebicka, J. (2022). Statins in Compensated and Decompensated Cirrhosis: Approaching the Bedside. In: de Franchis, R. (eds) Portal Hypertension VII. Springer, Cham. https://doi.org/10.1007/978-3-031-08552-9_23
Download citation
DOI: https://doi.org/10.1007/978-3-031-08552-9_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-08551-2
Online ISBN: 978-3-031-08552-9
eBook Packages: MedicineMedicine (R0)