Abstract
Purpose of review
Statins are drugs developed to treat hypercholesterolemia. Its use in patients with liver disease has been limited because one of its potential and most feared side effects is hepatotoxicity. However, there is robust evidence that supports the safety of statins in this population in the absence of severe liver dysfunction. In this review, we will summarize the efficacy and safety of statins in cirrhosis.
Recent findings
Statins are effective in the treatment of dyslipidemia in patients with liver disease, because of their pleiotropic properties. These properties are independent of their effect on cholesterol levels, such as improving endothelial dysfunction or having antioxidant, antifibrotic, anti-inflammatory, antiproliferative, antiangiogenic, proapoptotic, or immunomodulation properties. Statins have been studied in other areas such as in treatment of portal hypertension, prevention of hepatocellular carcinoma, and/or protection against ischemia/reperfusion injury.
Summary
Approved indications for statins in patients with cirrhosis are those of the general population, including dyslipidemia and increased cardiovascular risk. Compensated cirrhosis is not a contraindication. In patients with decompensated cirrhosis, statins should be prescribed with extreme caution at low doses, and with frequent monitoring of creatinine phosphokinase levels in order to detect adverse events in a timely fashion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase commonly known as statins are drugs originally developed to treat hypercholesterolemia. However, its use in patients with chronic liver disease has been limited because one of its potential and most feared side effects is hepatotoxicity.
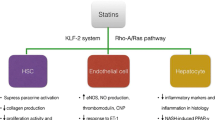
The so-called pleiotropic effects of statins, which are properties independent of their effect on cholesterol levels such as improvement of endothelial dysfunction and antioxidant, antifibrotic, anti-inflammatory, antiproliferative, antiangiogenic, proapoptotic, or immunomodulation effects, have made them the drug class most extensively assessed for drug repurposing, and hepatology is not the exception (Fig. 1) [1,2,3,4,5].
In this review, we will summarize the efficacy and safety of statins in chronic liver diseases and cirrhosis, taking into account both their lipid-lowering and their pleiotropic effects. A summary of the main studies evaluating the use of statins in patients with chronic liver disease can be found in Table 1.
Safety of Statins in Patients with Liver Disease
Myositis and hepatotoxicity are two of the most well-known side effects attributed to statins. The most common liver adverse event with their use consists of an asymptomatic and non-clinically significant elevation of the aminotransferase levels (alanine aminotransferase [ALT], aspartate aminotransferase [AST]) in up to 3% of patients [32]. This usually occurs within the first week of their use and tends to be transient even if patients continue with the treatment. This phenomenon is most probably due to an alteration in the permeability of the hepatocyte membrane that leads to a leak of liver enzymes, rather than to damage to the hepatocyte per se. This is supported by the fact that liver biopsies done in these patients do not show significant abnormalities. Only 3% or less of patients will have a persistent elevation of aminotransferases [32, 33].
In a study from the Drug-Induced Liver Injury Network (DILIN) of the USA, drug-induced significant toxicity [ALT or AST > 5 times the upper limit of normal (ULN) and/or alkaline phosphatase > 2 times ULN], statins were implicated in 1.8% of cases of drug-induced liver injury [34]. Importantly, four patients with statin-induced injury developed acute liver failure, of which one patient died, although this patient had alcohol-induced cirrhosis.
In another study of 133 patients with acute liver failure, statins were deemed responsible of 4.5% of cases [35]. The risk of hepatotoxicity related to statins is variable, but it has been estimated in 1 of every 3700 to 11,000 users. The mechanism of liver toxicity is an idiosyncratic reaction, which most commonly occurs during the first months of use, but the latency has been reported of up to 10 years in some cases. Liver toxicity seems to be a class effect, but the statins most commonly implicated are atorvastatin, simvastatin, and fluvastatin [36]. In the case of atorvastatin, the risk of developing significant elevation of aminotransferases (> 3 ULN) is 0.3%. However, in patients receiving high doses (≥ 80 mg), this can increase up to 2.3%. As mentioned above, most of the elevations in liver enzymes are transient, asymptomatic, and not clinically relevant. Nonetheless, atorvastatin can be implicated in clinically significant liver toxicity in 1 of 3000 to 5000 users, usually showing a cholestatic or mixed pattern in the liver biochemistry tests, though a hepatocellular pattern can also be seen in some cases. There are case reports of atorvastatin-induced autoimmune hepatitis [37].
In the case of simvastatin, the most common liver pattern of liver injury is hepatocellular. Significant elevation of aminotransferase levels is present in 1% of cases of long-term users, though clinically significant hepatotoxicity is uncommon. There are also case reports of simvastatin-induced acute liver failure or autoimmune hepatitis [38].
Patients with baseline abnormalities in liver biochemistry tests, for example because of non-alcoholic fatty liver disease (NAFLD) or chronic hepatitis C virus (HCV) infection, including patients with compensated cirrhosis, do not have a higher risk of statin-induced liver injury when compared to the general population. Therefore, if a patient with a chronic liver disease or with preexistent alterations in liver biochemistry tests has an indication for statins (e.g., hypercholesterolemia), there is no reason to refrain prescribing them [36, 39,40,41].
Current recommendations of the Food and Drug Administration consist in testing baseline AST and ALT when initiating statins, but it is no longer recommended to monitor them periodically, unless there is an additional clinical indication for monitoring AST/ALT levels, as this strategy has not shown to be effective in detecting or preventing liver injury [42••].
Decompensated cirrhosis is a different scenario, it seems that as liver function worsens, there is a reduced expression of the solute carrier organic anion transporter family member 1B (SLCO1B) in the hepatocytes, a membrane transporter that modulates the uptake of statins. This leads to an increase in statin serum concentrations with the subsequent increase in the risk of adverse events, including myopathy and hepatotoxicity [43, 44]. In the Bleeding Prevention with Simvastatin (BLEPS) study, a randomized clinical trial (RCT) that assessed simvastatin as a variceal bleeding secondary prophylaxis strategy, 3% (2/69) of the patients that received simvastatin (40 mg) developed rhabdomyolysis, which is a much higher frequency than that reported in general population. Of note, the two patients that developed this complication had decompensated cirrhosis with a bilirubin > 5 mg/dl [6••]. Additionally, in this same study, the use of simvastatin was associated with increased survival in patients with class Child-Pugh A/B cirrhosis, but not in Child-Pugh C [6••, 42••].
Statins and Treatment of Portal Hypertension
Only 40% of the patients that are started on non-selective beta blockers (NSBBs) for primary prophylaxis of variceal bleeding due to portal hypertension achieve a hemodynamic response. In the case of secondary prophylaxis, the 2-year rebleeding rate with standard therapy (NSBBs plus endoscopic banding) is of 30%; therefore, there is room for improvement in the management of these patients. Treatment of portal hypertension should aim at improving all elements implicated in its physiopathology: liver injury, liver function, fibrogenesis, increased intrahepatic vascular resistance, and the splanchnic vasodilation. Statins are an attractive therapeutic alternative because they are capable of modifying the intrahepatic vascular resistance, and studies in experimental models suggest that they can also impact the fibrogenesis [45].
Based on positive results of the effects of simvastatin in portal hypertension in animal models [5, 46], Zafra et al., a proof of concept study, demonstrated that simvastatin administration decreased hepatic vascular resistance, likely through an increase in liver nitric oxide production [7]. In a subsequent RCT, 55 patients with cirrhosis were randomized to receive simvastatin or placebo and had baseline hepatic venous pressure gradient (HVPG) measurement after one month of treatment [8]. Simvastatin induced a reduction of 8.3% in the HVPG. Importantly, this benefit was an additive to that of the NSBBs. Patients that were on NSBBs had a reduction of 11% compared to 5.9% of those that were not on NSBBs. In addition to this, simvastatin improved liver perfusion and function as shown by an improvement in the indocyanine green clearance test. In a similar three-month RCT of 34 patients with cirrhosis randomized to simvastatin or placebo, patients on simvastatin achieved a clinically relevant reduction of the HVPG (reduction of ≥ 20% or to < 12 mmHg) in 55% of patients [9].
Finally, in a prospective study of 100 patients with cirrhosis, esophageal varices, and an HVPG > 12 mmHg, the efficacy of simvastatin was evaluated in those without hemodynamic response to carvedilol (NSBB β1, β2, and an alpha adrenergic receptor blocker α1). Of the 100 patients, 38 had no hemodynamic response and were started on simvastatin (40 mg qd). Hemodynamic response was achieved in 16 patients [10]. This result is particularly interesting because it would mean that with the combination of carvedilol/simvastatin, 80% of patients could achieve hemodynamic response. Nonetheless, a major drawback of the study is that it did not include a control group.
There is only one RCT that has evaluated the effects of statins in patients with cirrhosis assessing clinical endpoints. This was a phase III RCT with a 2-year follow-up in which 147 patients were randomized to simvastatin or placebo after an episode of acute variceal bleeding. All patients received standard secondary prophylaxis (banding and NSBBs). The primary endpoint was a composite variable of rebleeding and/or death. Simvastatin did not improve the rate of rebleeding. However, there was a significant improvement in survival with simvastatin, mainly related to a decrease in bleeding and sepsis-related death [6••]. Due to the fact that mortality was not the primary endpoint of this study, these results need further validation in new RCTs with clinical endpoints.
Statins for the Treatment of Dyslipidemia in Patients with Chronic Liver Disease
Patients with cirrhosis, as any other patient, can have hyperlipidemia and increased cardiovascular risk, making them candidates to pharmacological treatment with statins. As a matter of fact, patients with NAFLD and HCV have an increased risk of premature atherosclerosis, which is independent of the traditional cardiovascular risk factors [47]. Also, up to 27% of patients with end-stage liver disease listed for liver transplant have significant coronary artery disease [48]. It is also worth mentioning that patients with cholestatic liver diseases have a high prevalence of hypercholesterolemia. In this section, we will review the evidence regarding the safety and utility of statins in these conditions [49].
Chronic Cholestatic Liver Diseases
Despite the fact that there is a high prevalence of hypercholesterolemia in patients with chronic cholestatic diseases, such as primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC), treatment with statins is controversial due to the fact that these patients do not seem to have an increased cardiovascular risk when compared to the general population [49]. Pharmacological treatment is usually recommended only in the presence of additional cardiovascular risk factors such as obesity, smoking, hypertension, diabetes, or family history of premature cardiovascular disease [49]. There is evidence from non-controlled studies and with a small number of patients that suggests statins are safe and effective for the treatment of hyperlipidemia in patients with PBC, which can have a prevalence of hypercholesterolemia of up to 58%. Therefore, these patients should be started on statins whenever there is a clinical indication [11,12,13,14,15, 50].
Non-alcoholic Fatty Liver Disease
Patients with NAFLD have an increased frequency of the different components of the metabolic syndrome, including dyslipidemia of in 20 to 80% of patients. As in other clinical scenarios, abnormal liver biochemistry tests should not preclude the prescription of statins in these patients, as the most common cause of death in this group of patients is cardiovascular disease [51].
The use of statins as a therapeutic strategy for NAFLD, either steatosis or steatohepatitis, is not supported with strong evidence. In a systematic review of the Cochrane Collaboration of statins in NAFLD, after applying inclusion criteria for the selection of the studies, it was found that only two were eligible, both with high risk of bias and only one of them with histopathological outcomes [16]. As a result, no meta-analysis or firm conclusions could be made. A more recent study evaluated 20 patients with biopsy-proven steatohepatitis that received rosuvastatin for 12 months. At the end of follow-up, a new liver biopsy was performed, and there was resolution of steatohepatitis in 19 of the 20 patients. Unfortunately, there was no control group, and this greatly limits the interpretation of the results [17••]. There is a need for further RCTs assessing the role of statins in NAFLD.
Statins in Chronic Hepatitis
Chronic HCV Infection
There is biologic plausibility behind the study of statins in chronic HCV. The HCV circulates as a lipoviral particle, and similar to apolipoproteins, it contains ApoE and ApoB on its surface, promoting its entry to the liver cells after binding to the low-density lipoprotein (LDL) cholesterol and to the scavenger receptor class B member 1 (SRB1). In order for the HCV to achieve a successful replication, it has to upregulate the host transcription of genes implicated in lipid metabolism. Consequently, the inhibition of cholesterol synthesis could have therapeutic implications by interfering with viral replication [52, 53].
In vitro studies have shown that statins have anti-HCV activity, and among the different types of statins, the one with the most potent antiviral activity seems to be fluvastatin, followed by atorvastatin and simvastatin [54]. Based on this assumption, Sheridan et al. [55] randomized 60 patients with HCV to fluvastatin and/or omega 3 fatty acids or no active treatment and assessed the impact of these strategies on the viral load. The study was negative and there was no association between these therapeutic strategies and changes in viremia after three months of treatment. Similar results were found in the study by Simon et al. [56] that evaluated 543 patients with chronic HCV, of whom 29 were chronic users of statins.
Information of the effects of statins on clinical outcomes in patients with chronic HCV comes from observational studies. In a retrospective study of 7248 patients with chronic HCV that received antiviral treatment and had a minimum follow-up of 2 years, a significant association was found between the use of statins (3347 patients of the sample) and a decrease in fibrosis progression. Moreover, the use of statins was associated with the possibility of achieving sustained viral response (SVR) and with a decreased risk of developing hepatocellular carcinoma (HCC). It is worth mentioning that there were significant baseline differences between the groups, so despite the fact that the authors adjusted for potential confounders with Cox proportional hazard models, the results should be interpreted with caution, as these were not RCTs [26]. Likewise, in a study of 9135 patients with chronic HCV, statins were associated with a reduced risk of fibrosis progression, and of HCC [18]. In another study of 2747 patients with HCV-induced cirrhosis that used a propensity score matching strategy to compensate for the potential confounding by indication bias, a significant association was found between the use of statins and a reduced risk of death and liver decompensation [19••]. A recent study in Asian population that included 298 patients with HCV-related cirrhosis also found that statins were associated with a decreased liver decompensation rate [22]. Moreover, retrospective observational studies from the era of the HCV treatment with interferon, ribavirin, and boceprevir found significant association between statins and the rates of SVR [20, 21]. The relevance of this information in the era of direct acting antivirals with SVR higher than 90% is uncertain, and in fact, it is possible that the interaction between the new treatments and statins may increase the concentration of the latter, with the potential of risk of adverse events [53].
Chronic HBV Infection
There is less evidence of the use of statins in patients with chronic HBV. Even though some in vitro studies suggested an anti-HBV effect [57], this has not been translated in clinical practice. However, large cohort studies in Asian population have shown an association between statins and a decreased risk of HCC, both in patients with and without cirrhosis. In addition to this, they also seem to decrease the rate of progression to cirrhosis, and in patients with cirrhosis, they have been associated with a reduced risk of liver decompensation and death [22,23,24,25, 58].
Statins and Prevention of HCC
The pleiotropic effects of statins, such as their antiangiogenic properties, make them an attractive option for cancer prevention. In terms of HCC, a recent meta-analysis reported a 37% risk reduction of developing HCC in patients taking statins, though there was significant heterogeneity between the studies. Of note, the statistical association was lost in a sub-analysis that included only RCT and was free of significant heterogeneity [28]. As mentioned above, this association has also been studied in patients with chronic HCV and HBV [22,23,24, 26], in whom a dose-response relationship was suggested. One study in Asian populations not included in the previous meta-analysis also proposed this association [27]. In order to confirm this association, a long-term follow-up RCT with an adequate number of patients would be needed.
Other Potential Benefits of Statins in Patients with Liver Disease
Data from observational studies suggest that statins could modify the natural history of cirrhosis. In a retrospective analysis of 243 patients with cirrhosis (70% child A), whom 81 were on statins, there was a significant association between the use of statins and improved survival, and decreased risk of liver decompensation [30], essentially replicating the findings of a previous study in patients with chronic HCV [19••]. Moreover, a recent meta-analysis including most of the aforementioned studies showed a significant association between statins and a lower risk of liver decompensation and death in patients with cirrhosis [59••].
Statins have also been associated with a decreased risk of incident infections. Statins are capable of fostering the activity of phagocytes [60], and in an animal model, simvastatin was able to attenuate the liver endothelial dysfunction induced by endotoxemia [61]. In the same line, a retrospective study of patients with cirrhosis that used propensity score matching reported an inverse association between statins and infectious-related hospitalizations. Importantly, when comparing the results of patients on statins with those taking other lipid-lowering agents, no differences were found, so it cannot be ruled out that the findings in fact are due to features associated with patients that receive treatment for hyperlipidemia [31]. Moreover, in the BLEPS study, the association between simvastatin and decreased mortality was mainly due to a reduction in infections and bleeding-related deaths [6••].
Animal models suggest that statins could blunt the ischemia-reperfusion-induced liver injury in grafts with steatosis in the context of liver transplantation [62], and they could have a role in the treatment of hepatopulmonary syndrome [63]. In one experimental study in rats that underwent hemorrhage shock and subsequent resuscitation, simvastatin was capable of reducing liver necrosis and was associated with improved survival. The rationale underlying this finding is that part of the liver injury is promoted by a dysregulation in the production of nitric oxide that can be overcome by statins [64]. Likewise, in a cirrhosis model in rats, pretreatment with simvastatin blunted the liver injury after hemorrhage—resuscitation [65]. Moreover, a retrospective analysis of 851 patients admitted to an intensive care unit found that previous use of statins was a protective factor for ischemic hepatitis, further supporting the evidence described in animal models [29].
There are also experimental models that have assessed the use of statins to improve liver fibrosis with encouraging results [66]. These animal studies should be replicated in humans, and this could be the ground basis for the design RCTs with clinical endpoints in specific subpopulations.
Conclusions
At present, the only approved indications for statins in patients with cirrhosis are those of the general population (i.e., dyslipidemia, increased cardiovascular risk). There is a special need for new RCT with an adequate number of patients to demonstrate that the pleiotropic effects of statins can modify clinically significant endpoints in patients with cirrhosis, since the current evidence comes mostly from observational studies or clinical trials with a limited number of patients [67].
The presence of abnormal liver biochemistry tests, chronic liver disease, and compensated cirrhosis should not be seen as contraindication for the use of statins, if the classical indications are present. However, in patients with decompensated cirrhosis, statins should be prescribed with extreme caution at low doses, and with frequent monitoring of creatinine phosphokinase levels in order to detect adverse events in a timely fashion.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Cabrera L, Abraldes J. Statins: The panacea of cirrhosis? Curr Hepatol Rep. 2016;15:1–7.
Moreno M, Ramalho LN, Sancho-Bru P, Ruiz-Ortega M, Ramalho F, Abraldes JG, et al. Atorvastatin attenuates angiotensin II-induced inflammatory actions in the liver. Am J Physiol Gastrointest Liver Physiol. 2009;296(2):G147–56.
Marrone G, Maeso-Diaz R, Garcia-Cardena G, Abraldes JG, Garcia-Pagan JC, Bosch J, et al. KLF2 exerts antifibrotic and vasoprotective effects in cirrhotic rat livers: behind the molecular mechanisms of statins. Gut. 2015;64(9):1434–43.
Bosch J, Abraldes JG, Fernandez M, Garcia-Pagan JC. Hepatic endothelial dysfunction and abnormal angiogenesis: new targets in the treatment of portal hypertension. J Hepatol. 2010;53(3):558–67.
Abraldes JG, Rodriguez-Vilarrupla A, Graupera M, Zafra C, Garcia-Caldero H, Garcia-Pagan JC, et al. Simvastatin treatment improves liver sinusoidal endothelial dysfunction in CCl4 cirrhotic rats. J Hepatol. 2007;46(6):1040–6.
•• Abraldes JG, Villanueva C, Aracil C, Turnes J, Hernandez-Guerra M, Genesca J, et al. Addition of simvastatin to standard therapy for the prevention of variceal rebleeding does not reduce rebleeding but increases survival in patients with cirrhosis. Gastroenterology. 2016;150(5):1160–70 e3. Prospective study that showed a lower mortality in patients that received statins.
Zafra C, Abraldes JG, Turnes J, Berzigotti A, Fernandez M, Garca-Pagan JC, et al. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology. 2004;126(3):749–55.
Abraldes JG, Albillos A, Banares R, Turnes J, Gonzalez R, Garcia-Pagan JC, et al. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology. 2009;136(5):1651–8.
Pollo-Flores P, Soldan M, Santos UC, Kunz DG, Mattos DE, da Silva AC, et al. Three months of simvastatin therapy vs. placebo for severe portal hypertension in cirrhosis: a randomized controlled trial. Dig Liver Dis. 2015;47(11):957–63.
Wani ZA, Mohapatra S, Khan AA, Mohapatra A, Yatoo GN. Addition of simvastatin to carvedilol non responders: a new pharmacological therapy for treatment of portal hypertension. World J Hepatol. 2017;9(5):270–7.
Del Puppo M, Galli Kienle M, Crosignani A, Petroni ML, Amati B, Zuin M, et al. Cholesterol metabolism in primary biliary cirrhosis during simvastatin and UDCA administration. J Lipid Res. 2001;42(3):437–41.
Kurihara T, Akimoto M, Abe K, Ishiguro H, Niimi A, Maeda A, et al. Experimental use of pravastatin in patients with primary biliary cirrhosis associated with hypercholesterolemia. Clin Ther. 1993;15(5):890–8.
Ritzel U, Leonhardt U, Nather M, Schafer G, Armstrong VW, Ramadori G. Simvastatin in primary biliary cirrhosis: effects on serum lipids and distinct disease markers. J Hepatol. 2002;36(4):454–8.
Stojakovic T, Claudel T, Putz-Bankuti C, Fauler G, Scharnagl H, Wagner M, et al. Low-dose atorvastatin improves dyslipidemia and vascular function in patients with primary biliary cirrhosis after one year of treatment. Atherosclerosis. 2010;209(1):178–83.
Stojakovic T, Putz-Bankuti C, Fauler G, Scharnagl H, Wagner M, Stadlbauer V, et al. Atorvastatin in patients with primary biliary cirrhosis and incomplete biochemical response to ursodeoxycholic acid. Hepatology. 2007;46(3):776–84.
Eslami L, Merat S, Malekzadeh R, Nasseri-Moghaddam S, Aramin H. Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2013;12:CD008623. https://doi.org/10.1002/14651858.CD008623.pub2.
•• Kargiotis K, Athyros VG, Giouleme O, Katsiki N, Katsiki E, Anagnostis P, et al. Resolution of non-alcoholic steatohepatitis by rosuvastatin monotherapy in patients with metabolic syndrome. World J Gastroenterol. 2015;21(25):7860–8. This article shows that further investigation is needed regarding the role of statins in the treatment of NAFLD.
Simon TG, Bonilla H, Yan P, Chung RT, Butt AA. Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: results from ERCHIVES. Hepatology. 2016;64(1):47–57.
•• Mohanty A, Tate JP, Garcia-Tsao G. Statins are associated with a decreased risk of decompensation and death in veterans with hepatitis c-related compensated cirrhosis. Gastroenterology. 2016;150(2):430–40 e1. One of the largest observational studies showing a benefit of statins in patients with cirrhosis.
Harrison SA, Rossaro L, Hu KQ, Patel K, Tillmann H, Dhaliwal S, et al. Serum cholesterol and statin use predict virological response to peginterferon and ribavirin therapy. Hepatology. 2010;52(3):864–74.
Manzano-Robleda Mdel C, Ornelas-Arroyo V, Barrientos-Gutierrez T, Mendez-Sanchez N, Uribe M, Chavez-Tapia NC. Boceprevir and telaprevir for chronic genotype 1 hepatitis C virus infection. A systematic review and meta-analysis. Ann Hepatol. 2015;14(1):46–57.
Chang FM, Wang YP, Lang HC, Tsai CF, Hou MC, Lee FY, et al. Statins decrease the risk of decompensation in hepatitis B virus- and hepatitis C virus-related cirrhosis: a population-based study. Hepatology. 2017;66:896–907.
Chen CI, Kuan CF, Fang YA, Liu SH, Liu JC, Wu LL, et al. Cancer risk in HBV patients with statin and metformin use: a population-based cohort study. Medicine (Baltimore). 2015;94(6):e462.
Hsiang JC, Wong GL, Tse YK, Wong VW, Yip TC, Chan HL. Statin and the risk of hepatocellular carcinoma and death in a hospital-based hepatitis B-infected population: a propensity score landmark analysis. J Hepatol. 2015;63(5):1190–7.
Huang YW, Lee CL, Yang SS, Fu SC, Chen YY, Wang TC, et al. Statins reduce the risk of cirrhosis and its decompensation in chronic hepatitis B patients: a nationwide cohort study. Am J Gastroenterol. 2016;111(7):976–85.
Butt AA, Yan P, Bonilla H, Abou-Samra AB, Shaikh OS, Simon TG, et al. Effect of addition of statins to antiviral therapy in hepatitis C virus-infected persons: results from ERCHIVES. Hepatology. 2015;62(2):365–74.
Lai SW, Liao KF, Lai HC, Muo CH, Sung FC, Chen PC. Statin use and risk of hepatocellular carcinoma. Eur J Epidemiol. 2013;28(6):485–92.
Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144(2):323–32.
Drolz A, Horvatits T, Michl B, Roedl K, Schellongowski P, Holzinger U, et al. Statin therapy is associated with reduced incidence of hypoxic hepatitis in critically ill patients. J Hepatol. 2014;60(6):1187–93.
Kumar S, Grace ND, Qamar AA. Statin use in patients with cirrhosis: a retrospective cohort study. Dig Dis Sci. 2014;59(8):1958–65.
Motzkus-Feagans C, Pakyz AL, Ratliff SM, Bajaj JS, Lapane KL. Statin use and infections in veterans with cirrhosis. Aliment Pharmacol Ther. 2013;38(6):611–8.
•• Jose J. Statins and its hepatic effects: newer data, implications, and changing recommendations. J Pharm Bioallied Sci. 2016;8(1):23–8. Complete review mainly focused on the safety of statins from the liver perspective
Black DM, Bakker-Arkema RG, Nawrocki JW. An overview of the clinical safety profile of atorvastatin (lipitor), a new HMG-CoA reductase inhibitor. Arch Intern Med. 1998;158(6):577–84.
Russo MW, Hoofnagle JH, Gu J, Fontana RJ, Barnhart H, Kleiner DE, et al. Spectrum of statin hepatotoxicity: experience of the drug-induced liver injury network. Hepatology. 2014;60(2):679–86.
Reuben A, Koch DG, Lee WM. Acute Liver Failure Study G. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52(6):2065–76. https://doi.org/10.1002/hep.23937.
Bjornsson ES. Hepatotoxicity of statins and other lipid-lowering agents. Liver Int. 2017;37(2):173–8.
Alla V, Abraham J, Siddiqui J, Raina D, Wu GY, Chalasani NP, et al. Autoimmune hepatitis triggered by statins. J Clin Gastroenterol. 2006;40(8):757–61.
http://livertox.nih.gov/.Accessed September 23, 2017.
Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126(5):1287–92.
Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R, et al. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46(5):1453–63.
Onofrei MD, Butler KL, Fuke DC, Miller HB. Safety of statin therapy in patients with preexisting liver disease. Pharmacotherapy. 2008;28(4):522–9.
•• Bays H, Cohen DE, Chalasani N, Harrison SA, The National Lipid Association’s Statin Safety Task F. An assessment by the Statin Liver Safety Task Force: 2014 update. J Clin Lipidol. 2014;8(3 Suppl):S47–57. Specific recommendations of a panel of experts about the liver safety of statins; the strength of the recommendations and quality of evidence are given.
Calderon RM, Cubeddu LX, Goldberg RB, Schiff ER. Statins in the treatment of dyslipidemia in the presence of elevated liver aminotransferase levels: a therapeutic dilemma. Mayo Clin Proc. 2010;85(4):349–56.
Theile D, Haefeli WE, Seitz HK, Millonig G, Weiss J, Mueller S. Association of liver stiffness with hepatic expression of pharmacokinetically important genes in alcoholic liver disease. Alcohol Clin Exp Res. 2013;37(Suppl 1):E17–22.
Bosch J, Abraldes JG, Groszmann R. Current management of portal hypertension. J Hepatol. 2003;38(Suppl 1):S54–68.
Trebicka J, Hennenberg M, Laleman W, Shelest N, Biecker E, Schepke M, et al. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology. 2007;46(1):242–53.
Targher G, Bertolini L, Padovani R, Rodella S, Arcaro G, Day C. Differences and similarities in early atherosclerosis between patients with non-alcoholic steatohepatitis and chronic hepatitis B and C. J Hepatol. 2007;46(6):1126–32.
Berzigotti A, Erice E, Gilabert R, Reverter E, Abraldes JG, Garcia-Pagan JC, et al. Cardiovascular risk factors and systemic endothelial function in patients with cirrhosis. Am J Gastroenterol. 2013;108(1):75–82.
Longo M, Crosignani A, Battezzati PM, Squarcia Giussani C, Invernizzi P, Zuin M, et al. Hyperlipidaemic state and cardiovascular risk in primary biliary cirrhosis. Gut. 2002;51(2):265–9.
Sorokin A, Brown JL, Thompson PD. Primary biliary cirrhosis, hyperlipidemia, and atherosclerotic risk: a systematic review. Atherosclerosis. 2007;194(2):293–9.
Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5(5):1544–60.
Syed GH, Amako Y, Siddiqui A. Hepatitis C virus hijacks host lipid metabolism. Trends Endocrinol Metab. 2010;21(1):33–40.
Bosch J, Forns X. Therapy Statins and liver disease: from concern to ‘wonder’ drugs? Nat Rev Gastroenterol Hepatol. 2015;12(6):320–1.
Ikeda M, Abe K, Yamada M, Dansako H, Naka K, Kato N. Different anti-HCV profiles of statins and their potential for combination therapy with interferon. Hepatology. 2006;44(1):117–25.
Sheridan DA, Bridge SH, Crossey MM, Felmlee DJ, Fenwick FI, Thomas HC, et al. Omega-3 fatty acids and/or fluvastatin in hepatitis C prior non-responders to combination antiviral therapy—a pilot randomised clinical trial. Liver Int. 2014;34(5):737–47.
Simon TG, King LY, Zheng H, Chung RT. Statin use is associated with a reduced risk of fibrosis progression in chronic hepatitis C. J Hepatol. 2015;62(1):18–23.
Bader T, Korba B. Simvastatin potentiates the anti-hepatitis B virus activity of FDA-approved nucleoside analogue inhibitors in vitro. Antivir Res. 2010;86(3):241–5.
Tsan YT, Lee CH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol. 2012;30(6):623–30.
•• Kim RG, Loomba R, Prokop LJ, Singh S. Statin use and risk of cirrhosis and related complications in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2017;15:1521–30. Meta-analysis that reinforces the concept that statins may probably decrease mortality in patients with cirrhosis.
Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292(5519):1160–4.
La Mura V, Pasarin M, Meireles CZ, Miquel R, Rodriguez-Vilarrupla A, Hide D, et al. Effects of simvastatin administration on rodents with lipopolysaccharide-induced liver microvascular dysfunction. Hepatology. 2013;57(3):1172–81.
Gracia-Sancho J, Garcia-Caldero H, Hide D, Marrone G, Guixe-Muntet S, Peralta C, et al. Simvastatin maintains function and viability of steatotic rat livers procured for transplantation. J Hepatol. 2013;58(6):1140–6.
Chang CC, Wang SS, Hsieh HG, Lee WS, Chuang CL, Lin HC, et al. Rosuvastatin improves hepatopulmonary syndrome through inhibition of inflammatory angiogenesis of lung. Clin Sci (Lond). 2015;129(6):449–60.
Relja B, Lehnert M, Seyboth K, Bormann F, Hohn C, Czerny C, et al. Simvastatin reduces mortality and hepatic injury after hemorrhage/resuscitation in rats. Shock. 2010;34(1):46–54.
Meireles CZ, Pasarin M, Lozano JJ, Garcia-Caldero H, Gracia-Sancho J, Garcia-Pagan JC, et al. Simvastatin attenuates liver injury in rodents with biliary cirrhosis submitted to hemorrhage/resuscitation. Shock. 2017;47(3):370–7.
Trebicka J, Hennenberg M, Odenthal M, Shir K, Klein S, Granzow M, et al. Atorvastatin attenuates hepatic fibrosis in rats after bile duct ligation via decreased turnover of hepatic stellate cells. J Hepatol. 2010;53(4):702–12.
Abraldes JG, Burak KW. STAT order: should patients with chronic liver disease be prescribed statins to prevent fibrosis progression and hepatocellular carcinoma? Hepatology. 2016;64(1):13–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Carlos Moctezuma-Velázquez declares that he has no conflict of interest. Juan Abraldes declares that he has no conflict of interest. Aldo Montano-Loza declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Juan G. Abraldes and Aldo J. Montano-Loza are co-senior authors
This article is part of the Topical Collection on Liver
Rights and permissions
About this article
Cite this article
Moctezuma-Velázquez, C., Abraldes, J.G. & Montano-Loza, A.J. The Use of Statins in Patients With Chronic Liver Disease and Cirrhosis. Curr Treat Options Gastro 16, 226–240 (2018). https://doi.org/10.1007/s11938-018-0180-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11938-018-0180-4