Abstract
Tissue engineering and regenerative medicine (TERM) research has advanced significantly with the development of three-dimensional (3D) printing technology. Specifically, 3D bioprinting provides additional benefits and improvement to 3D printing by incorporating the ability to include cells into the process, creating a cell-laden ink, termed bioink. By incorporating cells in conjunction with the superior temporospatial precision of 3D printing, scientists and clinicians have the potential to print live tissues with sophisticated structures and microarchitectures. While 3D bioprinting is still in its early phase, with limited preclinical animal and human studies, it has been proposed as a promising tool for TERM research in many areas of medicine and dentistry, including craniofacial, oral, and dental (DOC) tissue regeneration. This chapter aims to provide a comprehensive overview of major concepts in 3D bioprinting including its armamentarium, types of bioprinters, bioprinting process, clinical applications in craniofacial regeneration, limitations, and future perspectives.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- 3D bioprinting
- 3D printing
- Additive manufacturing
- Tissue engineering
- Regenerative medicine
- Craniofacial tissue regeneration
- Periodontal regeneration
- Pulp regeneration
- Dentistry
- Oral surgery
10.1 Introduction
Three-dimensional (3D) bioprinting technology has progressed at a rapid pace since its invention in the 1980s. Charles W. Hull (Chuck Hull) had first described the 3D printing technique under the name of stereolithography. Since then, multiple different techniques and methods have emerged. These techniques all aim for the same objective: to create 3D structures that mimic the external and internal structures of the anatomic sites, to provide scaffolds for cell attachment and migration, and to initiate tissue regeneration. This concept of 3D bioprinting, combined with the advancement of tissue engineering, has been proposed as a promising strategy to reconstruct and replace damaged tissues and diseased organs in many areas of medicine and dentistry, including craniofacial tissue regeneration [1]. In particular, 3D bioprinting technology allows for precise manufacturing of biocompatible scaffolds with complex 3D architectures using cell sources and other biomaterials [2].
Craniofacial tissues have highly complex 3D architectures with sophisticated multicellular interactions. Due to this complexity, complete regeneration of craniofacial structures from congenital malformations, trauma, and resective surgeries is extremely challenging. Despite the advances in the field of craniofacial reconstruction, conventional regenerative strategies still have difficulty mimicking the complex architectures and the biological interactions of this anatomical site [2]. To date, the development and advancement of 3D bioprinting technology are still in its early phase. In fact, 3D bioprinting is mainly used in research settings, and its clinical application has been limited by its ability to mostly fabricate simple homogeneous tissues as opposed to heterogeneous tissues in clinical settings [3].
Currently, reconstruction of extensive or complex craniofacial defects requires local or regional flap, or sometimes microvascular transfer of free flaps as the gold standard treatment [4]. These reconstructive procedures have significant limitations including donor site morbidity as well as size mismatch to the recipient site, leading to compromised aesthetics and function [5]. Therefore, 3D bioprinting technology in combination with tissue engineering strategies presents a promising alternative to the current reconstruction techniques. The aim of this chapter is to provide a comprehensive overview of major concepts in 3D bioprinting including the bioprinting process, armamentarium, types of bioprinters, clinical application in craniofacial regenerative medicine, limitations, and future perspectives.
10.2 3D Bioprinting Process
The basic process of 3D bioprinting in craniofacial regeneration can be classified into three phases including pre-bioprinting phase, bioprinting phase, and post-bioprinting phase (Fig. 10.1).
3D bioprinting process: The basic process of 3D bioprinting in craniofacial regeneration can be classified into three phases including (1) pre-bioprinting phase, (2) bioprinting phase, and (3) post-bioprinting phase. The pre-bioprinting phase involves (1) 3D digital imaging and computer-assisted design; (2) biomaterial selection and bioink preparation; (3) cell selection, isolation, culture, and preparation [6]. The post-bioprinting phase involves tissue construct maturation in a bioreactor, and in vivo transplantation [6]
10.2.1 Pre-bioprinting Phase
The pre-bioprinting phase involves (1) digital imaging and computer-assisted design; (2) biomaterial selection and bioink preparation; and (3) cell selection, isolation, culture, and preparation [6].
First, digital imaging of the defect or structure to be replaced is acquired via cone beam computed tomography (CBCT), computed tomography (CT), or magnetic resonance imaging (MRI). These imaging modalities are the most commonly used for medical and dental application of 3D bioprinting [7]. After imaging, the Digital Imaging and Communications in Medicine (DICOM) files are processed with computer-assisted design (CAD) softwares. In addition, tomographic reconstruction is performed to achieve segmented 2D images for the layer-by-layer 3D bioprinting process. Subsequently, Standard Triangle/Tesselation Language (STL) files are generated and sent to the bioprinter [3].
Biomaterial and bioink selection is another crucial part of the pre-bioprinting phase and is determined by the type of 3D printer used as well as specific mechanical, rheological, and biological requirements of the final tissue construct or organ discussed in Sect. 10.4 [8].
Prior to the bioprinting phase, isolation, expansion, and quality assessment of the desired cells represent another important step. The different types of cells including their sources, characteristics, and advantages are described in Sect. 10.3.1. It is important to ensure that cells have adequate viability, proliferative, differentiation, and extracellular matrix production potential. In addition, cells can be supplemented with biologics and growth factors-enriched culture media to enhance cell viability, proliferation, and differentiation. Currently, only two growth factors are approved by the Food and Drug Administration (FDA) for clinical applications in craniofacial regeneration including human recombinant platelet-derived growth factor-BB (rhPDGF-BB) and human recombinant bone morphogenetic protein-2 (rhBMP-2). Additional biologics include fibroblast growth factor (FGF), insulin-like growth factor (IGF), transforming growth factor beta (TGF-B), and vascular endothelial growth factor (VEGF). Finally, other culture medium supplements can be used to potentiate cell viability and growth including vitamins, hormones, and other macronutrients (Fig. 10.2) [9].
Cell preparation for 3D bioprinting: Key components of cellular preparation for 3D bioprinting include (1) growth factors (2) biomaterials (3) cell source (4) growth medium (5) vitamins. Achieving an optimal combination of the above elements allow desired cells to proliferate, differentiate, and synthesize extracellular matrix to enhance the quality of the 3D bioprinted tissue construct or organ
10.2.2 Bioprinting Phase
The bioprinting phase involves the deposition of bioink, cells, and signaling molecules with a bioprinter to form a tissue construct. Bioink and cells are prepared and transferred to their respective cartridges, installed in the printer, and the bioprinting process is initiated to print 3D structures with specific microarchitecture. The main types of bioprinters, their advantages, and respective mechanisms of action are reviewed in Sect. 10.4.
10.2.3 Post-bioprinting Phase
The post-bioprinting phase is key to ensuring the manufacturing of reliable 3D tissue constructs with appropriate structural integrity and biological function [7]. One important component of this phase is tissue maturation. The transfer of 3D bioprinted tissue constructs into an incubator or bioreactor allows for enhanced survival, maturation, vascularization, and remodeling prior to in vivo implantation [3]. Recent advances in bioreactor design enable convective nutrient transport, creation of microgravity environment, and compression for dynamic mechanical stimulation [6]. Once the tissue construct or bioprinted organ is ready to be used, a surgical team will perform the surgical implantation or transplantation in animals or patients to address the clinical problem.
10.3 3D Bioprinting Armamentarium
Tissue engineering combines the field of biology and engineering to develop functional substitutes for damaged tissues. The creation of functional tissue engineered constructs requires three main components termed “Tissue Engineering Triad,” which includes (1) cells, (2) scaffold, and (3) regulators [10]. 3D bioprinting utilizes the principles of tissue engineering and combines these three key tissue building blocks with spatial precision to enhance tissue structure, architecture, and functionality.
10.3.1 Cellular Component
The first and most important component of the tissue engineering triad is the cells [10]. They are the fundamental building blocks that reconstitute the 3D bioprinted tissue construct and/or organ. Many factors must be considered during cell selection for 3D bioprinting. Ideally, the user should be able to control the proliferative properties of the cells as excessive or insufficient proliferation can lead to complications. This is commonly evident in multicellular constructs that overgrow and develop a necrotic core due to hypoxia. Additionally, researchers should be able to predict or control the timing for cell proliferation [6]. Also, cells must be able to withstand the mechanical and physiological stresses associated with 3D bioprinting as cell viability can be affected by stresses such as sheer forces, changes in temperature and pH, and the presence of chemicals, toxins, and enzymes [6, 11]. Finally, cell viability may also be greatly altered by the bioprinting technique and properties of the scaffold material selected to contain and support the cells.
Due to their complexity and intricacy, more than one type of cells is required to adequately reconstruct the desired tissue and/or organ. For example, in alveolar bone regeneration, osteoblasts, osteoclasts, and osteocytes are required for bone repair and remodeling; epithelial cells and fibroblasts provide structural and barrier functions; and endothelial cells form vasculature to support osteogenesis [6, 12, 13]. In addition, stem cells and progenitor cells are required to provide the tissue construct with self-renewing abilities. Stem cells are undifferentiated cells that have the potential to divide indefinitely and give rise to various cell lineages. In contrast, progenitor cells have limited proliferative capabilities and determined set of cell fates and thus can only differentiate into certain cell types. One particularly important type of stem cells is the mesenchymal stem cells (MSCs). As many craniofacial structures are derived from MSCs, they are an integral component to craniofacial regeneration [14].
Cells may be categorized based on their differentiation potential or source. Firstly, cells may be selected based on their differentiation capabilities: undifferentiated stem cells {totipotent, pluripotent [e.g., embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs)], multipotent stem cells (e.g., MSCs, oligopotent, or omnipotent stem cells)}, and differentiated somatic cells [15]. Secondly, cells may also be selected based on their sources: endogenous cells from the donor or exogenous cells from another organism. Exogenous cells may pose immunogenicity challenges [16]. Lastly, in light of 3D bioprinting, cells may also be selected as single cells or larger clusters of cells termed spheroids and organoids. This subsection will explore the use of single cells and multicellular constructs for 3D bioprinting of craniofacial tissue.
10.3.1.1 Single Cells
Single cells are particularly useful in 3D bioprinting to creating vascular channels and capillaries that are composed of a single layer of endothelial cells. In addition, stem cells and progenitor cells from various sources are used in 3D bioprinting. The most common source of stem cells and progenitor cells for craniofacial regeneration come from the bone marrow. Although bone marrow-derived stem and progenitor cells have been extensively used in tissue engineering and regenerative medicine, their harvest is rather invasive and involves bone marrow aspiration from long bones or iliac crests, which may lead to patient discomfort and morbidity.
Since the discovery and characterization of multipotent mesenchymal stem cells (MSCs) from the bone marrow, MSCs from other tissues have been identified and characterized including umbilical cord blood, adipose tissues, and dental tissues. These MSCs are capable of differentiating into cell lineages including osteogenic, chondrogenic, myogenic, and adipogenic [17]. From the early 2000s, significant progress has been made toward identifying different human MSC-like stem/progenitor cells from dental and oral sources. These cells include periodontal ligament stem cells (PDLSCs), dental pulp stem cells (DPSCs), stem cells from exfoliated deciduous teeth (SHED), stem cells from apical papilla (SCAP), dental follicle progenitor cells (DFPCs), and gingiva-derived MSCs (GMSCs) [18,19,20,21,22,23]. Dental stem cells have the advantage of being easily accessible, thus avoiding the need for invasive harvest in comparison to BM-derived MSCs. Together, this group of cells represents a promising cell source for 3D bioprinting for craniofacial regeneration.
Other cell types are useful in 3D bioprinting of functional craniofacial tissue and structures including salivary glands, nerves, and vasculature; three examples are highlighted in this section. To begin with, exogenous salivary gland stem cells may be used in 3D bioprinting as a building block for functional salivary organoids [24, 25]. In addition, in a preclinical animal study, Zhang et al. demonstrated that human gingiva-derived MSCs (GMSCs) can be differentiated into both neuronal and Schwann-like cells and be used in 3D bioprinting to generate nerve constructs that promoted nerve regeneration and functional recovery in bridge segmental defects in rat facial nerves [26]. Finally, human umbilical vein endothelial cells (HUVECs) may be used in combination with MSCs to induce the formation of pre-vascular networks leading to improved cell viability and proliferation [27].
10.3.1.2 Multicellular Constructs
Fabricating 3D multicellular constructs grown in suspension is an alternative to growing cells in a monolayer fashion. In fact, cells tend to aggregate in clusters and form 3D constructs termed spheroids and organoids. While both spheroids and organoids are well-organized multicellular structures, there are a few defining features that differentiate the two types of constructs. On one hand, spheroids are derived from cell line monoculture, have transient cell organization, and only represent a component of tissue. They are difficult to maintain long term and depend on cell-cell and cell-environment interactions to proliferate and survive. On the other hand, organoids are heterogeneous multilineage constructs derived from stem cells and/or progenitor cells, which possess the ability to differentiate and self-renew. Consequently, organoids can better recapitulate organ physiological parameters and can be maintained in culture for a longer period of time [28, 29]. Ultimately, cells grown in 3D cultures will have increased cell-cell and cell-extracellular matrix (cell-ECM) interactions compared to cells cultured in a monoplane orientation [28]. Thus, 3D cellular models are more physiologically relevant and biologically applicable to 3D bioprinting tissue engineered constructs.
Another advantage of using spheroids and organoids in 3D bioprinting is that they require fewer amount of scaffolding material to support the cells, thus applicable to “scaffold-free printing.” More specifically, spheroids and organoids can synthesize and secrete their native ECM; thus, only a minimal amount of scaffold is required their initial formation and subsequent bioprinting. The benefits of multicellular constructs include reduced costs and efforts associated with the fabrication of cell-laden hydrogels, enhanced biocompatibility, and physiological relevance as the cell construct secretes native ECM; thus, the use of exogenous materials is reduced [2, 28, 29].
Craniofacial structures pose challenges in tissue reconstruction due its various multicellular interactions and complex anatomical features [2]. By harnessing the power of spheroids and organoids in 3D bioprinting, researchers have the ability to print homotypic and heterotypic multicellular constructs with higher spatial resolution and density and, thus, may be able to recreate complex tissues such as vascularized bone, cartilage, periodontium, and whole teeth. Specific clinical applications of 3D bioprinting are reviewed in detail in Sect. 10.5 [2].
10.3.2 Biomaterials
The next component to the tissue engineering triad is the biomaterial scaffolds. This component encompasses all natural and synthetic biomaterials, or a combination of both, used to provide structural support and a favorable microenvironment for cells. Biomaterials can be engineered for tunable release of regulators such as growth factors (GF).
Biomaterials used during the bioprinting process to encapsulate cells are termed bioink [3]. Bioinks can serve as cell encapsulation material to provide cells with protection. In addition, bioinks can be printed onto acellular biomaterial ink scaffolds with higher rigidity, which provides the construct with higher structural integrity. Bioinks are typically composed of cell-laden hydrogels consisting of natural or synthetic materials. In contrast, acellular biomaterial ink scaffolds can be composed of a wider selection of materials depending on desired properties and its intended use [30, 31]. Researchers and clinicians may select a biomaterial based on its rheological, mechanical, chemical, and biological properties, which should ultimately reflect the target organ or tissue’s native physiological environment. These properties may include pH levels, biocompatibility, immunogenicity, cytotoxicity, degradation rate, inductivity, stiffness, viscoelasticity, and strength. Other properties that may be considered include the material’s tunability, reproducibility, cost, availability, printability, and complexity of use [32, 33].
The main advantage of 3D bioprinted scaffolds compared to 3D printed scaffolds is its micron-level precision of cell positioning throughout the scaffold. This characteristic enables researchers and clinicians to create a more desirable and viable scaffold for tissue reconstruction [1]. However, in comparison to the traditional acellular 3D printing method, 3D bioprinting requires additional considerations due to the presence of cells. These considerations include cell positioning, the degree of heat generated, sheering forces, maximum compressive moduli of the biomaterial, and their respective impact on cell viability. Additional printing parameters may affect cell viability and proliferation such as vibrating frequencies, voltage, and mechanical impact during the printing process [1, 31,32,33,34]. As a result, some printing techniques and materials may not be suitable for 3D bioprinting of living tissue constructs.
It is suggested that bioinks should have minimal incorporation of synthetic biopolymers to minimize unwanted changes and effects on cells [31]. However, natural biomaterials often have significantly lower mechanical strength compared to synthetic biomaterials and thus cannot be used to create certain craniofacial tissues with high mechanical strength requirements. For example, craniofacial bone has a compressive moduli between 100 MPa and 20 GPa. While alginate is highly biocompatible and fibrin is highly biologically active, both materials have very low compressive moduli (~5 kPa). Comparatively, while the use of synthetic materials such as polyethylene glycol (PEG) with cells may be less favorable, PEG confers higher physical and mechanical strength (~300–350 kPa) needed for harder tissues or areas of high stress such as bone and teeth. These obstacles can be overcome by using natural-synthetic composite bioink and/or simultaneously using 3D printing and bioprinting techniques together [1].
The various biomaterials used in 3D bioprinting have been categorized into the following five categories: natural materials, synthetic materials, bioactive ceramics and cements, metals, and hybrids and composites. It is important to note that there are hundreds of biomaterials being researched, and even more when considering the possible combinations of materials used to create composite gels and scaffolds. Thus, this section will provide an overview of the most commonly used materials and notable composites (Table 10.1).
10.3.2.1 Natural Materials
The main advantage of natural material is its bioactivity and ability to induce cellular activity. For instance, researchers have used protein-based natural biomaterials such as collagen, elastin, laminin, fibrin, fibronectin, and gelatin as scaffolds to mimic the cell’s native ECM, which can enhance cell differentiation, proliferation, and migration. Previous studies on the use of ECM-like scaffolds and 3D bioprinting have demonstrated, both in vitro and in vivo, the successful fabrication of various tissue engineered constructs including skin, bone, and cartilage, cardiovascular tissue, hepatic tissue, neuronal tissue, and cornea tissue [46]. Furthermore, there is increasing interest in the use of more complex protein scaffolds containing more than one type of protein substrate such as decellularized ECM (dECM) and decellularized bone matrix (DBM). These materials are natural ECM that have been cleared of native cells, debris, and other immunogenic components leaving intact structure and microarchitectures composed of collagen, adhesive proteins, growth factors, proteoglycans, and glycosaminoglycans (GAGs). Subsequently, dECM can be reseeded with desired cells. In the case of 3D bioprinting, dECM can be further processed into bioinks to be bioprinted [47, 48]. Other protein-based natural materials used in 3D bioprinting include albumin, keratin, and silk fibers.
Natural biomaterials can also be carbohydrate-based (alginate, chitin, chitosan, cellulose, starch, glycosaminoglycans (GAGs), and hyaluronic acid) as shown in Table 10.1 [3, 35, 39, 49]. While some natural carbohydrate-based materials are not found in the human body (e.g., alginate, cellulose, and chitin), their unique properties including biocompatibility, affordability, and accessibility make them excellent biomaterial scaffold candidates for research [50, 51]. However, a major drawback of using naturally occurring materials is the variability in material composition depending on its source. Consequently, this can affect reproducibility and reliability, thus the quality of the research [51].
10.3.2.2 Synthetic Materials
Synthetic materials unlike natural materials are much more reproducible due to its controlled manufacturing conditions. As a result, their use in biomedical research provides more consistent and reliable data [32]. Another advantage of synthetic materials is their superior physical properties such as higher mechanical strength, compressive moduli, and stress-bearing capabilities. Furthermore, many synthetic materials such as polylactic acid (PLA), polyglycolic acid (PGA), and polylactic-co-glycolic acid (PLGA) are thermoplastic and thus can be easily manipulated into desired shapes and microstructures [52]. However, the main concern of synthetic materials is its degradation byproducts. For example, PLA and PGA produce carbon dioxide as they degrade and thus can lead to hypercapnia, an acidic environment, and consequently necrosis of proximal tissue. Another concern with synthetic material stems from its common bioinert property, which can result in rejection of the material in vivo [34].
Other synthetic materials commonly used for 3D bioprinting include poly-ɛ-caprolactone (PCL), polyethylene glycol (PEG), polyethylene glycol dimethacrylate (PEGDMA), porous polyethylene (PPE), polymerization of methyl methacrylate (PMMA), polyether urethane (PU), polyetherketoneketone (PEKK), and polyetheretherketone (PEEK) [3, 35, 39, 49]. Here, we will further classify synthetic materials into two subgroups, biodegradable and nonbiodegradable (Table 10.1).
10.3.2.3 Bioactive Ceramics and Cements
Bioactive ceramics and cements are great candidates for use in 3D bioprinting due to their chemical properties resembling the mineral components of natural bone. Typically, this group of biomaterials exhibits excellent biocompatibility, high mechanical stiffness, brittleness, low elasticity, and slow degradation rate. However, its most notable advantage is its osteoinductive property, hence its popular use in bone regeneration [32, 34]. The most commonly used bioactive ceramics are those with a mineral phase composed of calcium and phosphate, such as hydroxyapatite (HA), β-tricalcium phosphate (β-TCP), biphasic calcium phosphate (BCP), and calcium phosphate. Other types of ceramics or cements include calcium carbonates, calcium sulfates, calcium silicate, silicon, bioactive glasses, zirconia, and aluminum oxide [3, 35, 53,54,55]. Some ceramics such as bioactive glass or HA can be further improved with the incorporation of silicone which can promote angiogenesis and bone ingrowth. Other researchers have explored the addition of metallic ions such as copper and/or cobalt into bioactive glass which has been shown to induce angiogenesis [3]. The major drawbacks to using bioactive ceramics are its brittleness and porous property which makes it difficult to sustain high mechanical loading required for bone remodeling [34]. Here we subcategorize bioactive ceramics and cements into two groups: calcium phosphate-based and non-calcium phosphate-based.
10.3.2.4 Metals
Metals are the last group of materials being used in tissue engineering. Metals are generally incorporated into bioinks to increase its stiffness, processability, and printability [56]. Metals used in bioprinting include gold, zinc oxide, iron, stainless steel, titanium alloys, and cobalt alloys. Advantages that metals have to offer are its superior mechanical properties, bioinert, and nondegradable which allow for it to last a long time, even in high-stress areas such as bone and teeth [3, 32, 53,54,55]. While most metals are nondegradable, there is increasing research on biodegradable metals such as magnesium alloys and iron alloys [43].
It has been suggested that metal-based scaffolds can cause stress shielding due to its higher relatively higher elastic modulus which can result in bone resorption and therefore leaving subjects prone to implant failures [3, 54]. To date, titanium-based constructs are the most widely used metal for craniofacial reconstruction due to its biocompatibility, high strength-to-weight ratio, elastic modulus, nonabsorbable characteristic, and potential for bone ingrowth [36].
10.3.2.5 Hybrids and Composites
Due to each type of material having their own unique set of advantages and disadvantages, there is increasing interest in exploring and using hybrid or composite scaffolds, which are biomaterials comprised of multiple phases and materials [34]. In general, composites have higher biological capacity because it is comprised of two or more materials, where one material’s weakness is supported by the strength of another material. Depending on the intended use or desired properties, researchers may combine bioactive ceramics with synthetic or natural biomaterials, or more commonly, they may combine synthetic biomaterials with natural biomaterials. Researchers may even combine materials of the same category, such as a protein phase with a carbohydrate phase from the natural biomaterial category. This allows researchers to create ideal bioinks or scaffolds that would otherwise be not viable when used alone. For example, synthetic materials may often create local acidity through its byproducts as it degrades, in addition to being bioinert. Conversely, natural materials have excellent bioactivity, though it lacks mechanical properties. By combining a synthetic material such as PEG with a natural material such as collagen, a cell-inductive scaffold with improved mechanical properties can be created [35].
10.3.3 Regulators
The third and final component to the tissue engineering triad is the regulators, which consists of signaling molecules, notably growth factors (GFs). These biological molecules signal cells to undergo proliferation, morphogenesis, differentiation, migration, and survival [57]. While GFs exist naturally in the human body, for the purpose of tissue engineering, an exogenous source is also required. They are typically incorporated into scaffolds and are released as the material degrades. The release of GFs should be controlled spatiotemporally to adequately guide proper cellular growth, differentiation, morphology, and function. The release of GFs should also be steady as to prevent unwanted diffusion and therefore unwanted outcomes [57, 58]. A gradient of GF diffusion in conjunction with physical contact of ECM and other inductive cues establishes the microenvironment necessary to induce these effects on the embedded cells.
There are several types of GFs that are being used in craniofacial regeneration research. These include transforming growth factor beta (TGF-β), fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), insulin-like growth factor (IGF), and bone morphogenic proteins (BMP-2,4,6,7) [35, 59]. While many GFs show promising results in vivo and in vitro, there are currently only two GFs that are FDA-approved for clinical use: recombinant human PDGF-BB (rhPDGF-BB) and recombinant human BMP-2 (rhBMP-2).
PDGF has several isoforms (PDGF-A, PDGF-B, PDGF-C, and PDGF-D) and becomes active when it dimerizes. These dimeric isoforms include PDGF-AA, PDGF-BB, PDGF-AB, PDGF-CC, and PDGF-DD. PDGF-BB has the highest activity as it is capable of binding all dermic forms of PDGF receptors and thus is the isoform that has translated into clinical use. In 2005, Nevins et al. reported in a pivotal randomized control trial study involving 180 subjects the clinical application of rhPDGF-BB in periodontal tissue regeneration, more specifically, its effectiveness in inducing radiographic bone fill, and clinical attachment level gain, and reduction in probing depth when used in conjunction with β-TCP [59,60,61].
BMPs are the second FDA-approved GF for clinical use. Currently, the only BMP isoform approved by the FDA for clinical use is rhBMP-2 (InFUSE Bone Graft®, Medtronic and Wyeth). rhBMP-2 is infused in an absorbable collagen scaffold and is capable of guiding bone regeneration via inducing MSCs to differentiate into osteoblasts. rhFGF-2 is also noteworthy as it currently used for periodontal regeneration in Japan, and has shown to have beneficial outcomes in patients with lower limb ischemia [55, 59].
10.4 3D Bioprinter Technology
10.4.1 Inkjet 3D Bioprinting
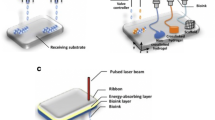
Inkjet bioprinting uses thermal or piezoelectric processes in the nozzle head to dispense droplets of bioink (Fig. 10.3a). The thermal induced inkjet nozzles pass a current through a resistor to create a bubble by vaporizing the nearby fluid, and therefore building up pressure in the nozzle head resulting in droplet ejection. The piezoelectric inkjet nozzles apply voltage to the piezo element to create a pulse, which produces volumetric changes in the nozzle head resulting in droplet ejection. The droplet ejection process from the piezoelectric nozzle head allows for more control of the droplet shape and size and has a greater tolerability for heat-sensitive materials (such as cells) when compared to the thermal induced nozzle head, but the vibrational frequencies from the piezoelectric process can cause cell membrane damage [31, 62]. Thermal inkjet printers have advantages in availability, higher print speed, and lower cost of parts fabrication [62]. When compared to other bioprinting technologies, inkjet bioprinting has advantages in high print speeds, low cost, and wide availability [11, 63].
Types of 3D bioprinting technologies: (a) Inkjet 3D bioprinting. Droplets dispensed by thermal or piezoelectric processes in the nozzle head. (b) Extrusion 3D bioprinting. The bioink is extruded through a nozzle due to pneumatic or mechanical (piston/screw driven) pressure. (c) Laser-assisted 3D bioprinting. A laser is focused on an absorbing substrate to generate pressure that propel cell-containing bioink onto a collector substrate. (Reproduced with permission from Murphy and Atala, 2014 [6])
Several considerations must be made with regard to cell viability when choosing to use inkjet-based bioprinters. While a wide range of bioinks can be used with inkjet bioprinting (including various combinations of cells, ceramics, polymers, and proteins), a limitation however in the type of bioink used is the viscosity requirement: to prevent the continuous flow of the material once a droplet is ejected and to prevent high ejection pressures (which can damage the cells), a low viscosity fluid between 3.5 and 12 mPa s is required [64]. This limitation is achieved by using low concentration solutions, which can increase the possibilities of cells drying and dying, and a low viscosity fluid will have greater difficulty in forming larger 3D structures [11, 31, 65]. The mechanical impact of the cells leaving the nozzle head and hitting the collector surface also affects cell viability. Another limitation is that cell aggregation within the bioink can affect droplet formation and trajectory, resulting in the poor precision of bioink droplet placement and potentially affecting the distribution of cells in the final construct [65]. Despite these considerations, observations of inkjet-based bioprinting have reported good cell viabilities (over 90%), and a resolution of greater than 50 μm [11, 31, 65].
10.4.2 Light-Assisted 3D Bioprinting
Light-assisted 3D bioprinting includes stereolithography (SLA) and laser-induced forward transfer (LIFT). While selective laser sintering (SLS) is another light-based 3D printing technology, it is not compatible for bioprinting due to its methodology of melting the polymer (and subjecting cells to high temperatures) to create a 3D construct [2]. Light-assisted 3D printing is noncontact and nozzle free and therefore has the advantage that materials with higher viscosities can be used (1–300 mPa s) without the issue of nozzle clogging [64].
SLA, more specifically, consists of directing a light source (UV or visible light) over a photopolymerizable fluid (Fig. 10.3b). Once a layer of the polymer is completed, the printing platform is lowered to allow for photopolymerization of a new layer. An advantage of SLA printing is its high fabrication accuracy and low printing time [66]. The resolution and cell viability are >50 μm and >85%, respectively [65]. The materials that are used for SLA consist of photocrosslinkable hydrogels such as those containing an acryloyl or alkenyl functional group, or a photoinitiator such as lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) or the benzophenone/tertiary amine system [67]. A major cell viability consideration for SLA bioprinting is the exposure to laser energy, which can be harmful to cells [65, 66]. Regardless, the specific material requirements of being photocrosslinkable are a disadvantage of the SLA methodology due to a lack of compatible materials, and the potential cytotoxicity to cells due to the photoinitiators that are added to the hydrogels [63].
LIFT consists of three main components: a light source, a ribbon (transparent glass, metal, and bioink), and a collection plate. The light source vaporizes the metal layer and creates a high-pressure bubble resulting in the production of bioink droplets that are deposited onto the collection plate (Fig. 10.3c). As with inkjet printing, LIFT is droplet-based and has similar considerations for cell viability such as the mechanical impact of the bioink hitting the collector surface, and the less accurate positioning of cells [31]. An advantage of LIFT is high precision and resolution (>20 μm) with high cell viability (>95%); however, LIFT is often costly and time-consuming due to the use of high viscosity materials which are required to obtain a highly precise shape [64, 66, 68]. The materials that have been used with LIFT include polymers, ceramics, proteins, and cells of varying viscosities (in contrast to the low viscosity requirement of inkjet printing) [69].
10.4.3 Extrusion 3D Bioprinting
Extrusion 3D printing is the most commonly used 3D bioprinting technique and is a pressure-driven system. The bioink is continuously extruded (in contrast to the droplet-based system in inkjet and LIFT) through a nozzle due to pneumatic or mechanical (piston/screw driven) pressure. A more complex construct can be created by using multiple nozzles, each carrying different a bioink [70]. Fused deposition modeling is a type of extrusion printing that heats and melts the material as it is extruded through the nozzle. It can be used to create scaffolds for tissue engineering. However, this technique is unable to bioprint cells due to high temperatures reached during the printing process.
The considerations for cell viability when choosing to use extrusion-based bioprinters include dispensing pressure and shear stress. While the pressure-assisted system in extrusion printing allows for the printing of very high cell densities, higher viscosity fluids, and more homogenous cell distributions, shear stress is a factor that can affect cell viability and is increased as the viscosity of the fluid is increased [31]. Furthermore, as the dispensing pressure increases, there is greater cellular distortion, all of which can result in low cell viability (>40%) [66]. In addition, the absence of droplet control (compared to inkjet and LIFT) results in a lower resolution (>100 μm) [65]. The higher viscosity bioinks used in extrusion printing can include natural polymers such as collagen, gelatin, alginate, hyaluronic acid, as well as synthetic polymers such as polyvinyl alcohol (PVA) and polyethylene glycol (PEG) [71].
The types of 3D bioprinting technologies are summarized in Table 10.2.
10.5 3D Bioprinting Clinical Applications
Although various 3D printing methods are widely applied to the manufacturing of biocompatible scaffolds and constructs to support complex functional living tissue in clinical trials, the use of 3D bioprinting to generate functional craniofacial tissues remains at an experimental stage. This section reviews key areas for clinical application of 3D bioprinting at the tooth level, periodontal support tissue level, craniofacial, and maxillofacial tissue level.
10.5.1 Dental Pulp and Whole-Tooth Regeneration
The dental pulp is a highly vascularized and innervated tissue enclosed within the root canal that plays a crucial role in providing sensation, nutrition, and innervation to the tooth [74]. After trauma, dental caries, and iatrogenic exposure of the pulp, there is an unmet clinical need to regenerate the pulp and reestablish innervation and vascularization. The ultimate goal of dental pulp regeneration is the formation of reparative dentin, vascular supply, and pulp neurotization [75].
Current strategies in pulp regeneration have been largely unsuccessful, although researchers are exploring the use of hydrogels to support dental pulp stem cells (DPSCs), mimic the native pulp chamber microenvironment, and recapitulate cell proliferation and differentiation into functional tissue [76]. However, the main limitation of this strategy, consisting of simple scaffolds loaded with cells and growth factors, is the inability to control multicellular spatial orientation, and subsequent cellular interactions and function [77].
With 3D bioprinting, researchers can achieve enhanced spatial control by printing cells to specific locations in the tissue-engineered construct to achieve desired cellular interactions. In addition, the use of bioink with tunable mechanical properties, optimized rheological properties to enhance printability, and inclusion of growth factors may further potentiate cell function. For the generation of vascularized constructs mimicking the human dental pulp, extrusion-based bioprinting is the preferred method. In these methods, sacrificial template material composed of dissolvable or removal material can be extruded and subsequently replaced with a cell-laden hydrogel or aggregate of cells to create vascular channels [74].
Currently, there is a lack of evidence supporting the use of 3D bioprinting for regenerative endodontics in patients [75]. Several in vitro studies have made progress toward developing biomaterials and bioinks that allow for control of stem cells and endothelial cells to promote pulp regeneration. For instance, Khayat et al. (2017) developed a photocrosslinkable GelMA hydrogel to encapsulate hDPSCs/HUVECs to promote revascularization and regenerate human dental pulp tissue [78]. Similarly, Yu et al. (2019) demonstrated that alginate/gelatin scaffold hydrogel is suitable for growth of hDPSCs [79]. Researchers have also combined extracellular matrix-derived scaffolds with natural polymers to develop a novel bioink with cytocompatibility and natural odontogenic capacity. The hydrogel consisting of alginate and dentin matrix was shown to have the ability to enhance odontogenic differentiation of stem cells from the apical papilla. In addition, 3D bioprinting was used to induce odontoblast at specific positions by localizing growth factors between the pulp tissue and wall of the pulp cavity [80]. To further enhance cell differentiation, growth factors can be conjugated to the biomaterial scaffold. Park et al. (2020) demonstrate that a bone morphogenetic protein (BMP) peptide-tethered GelMA-based bioink formulation can accelerate the differentiation of hDPCs in a 3D bioprinted dental construct [81]. Together, development of 3D bioprinting technology and its main components, including the bioprinters and bioinks, will enable predictable dental pulp regeneration and accelerate its clinical translation to ultimately help treating patients [74].
When it comes to whole-tooth regeneration, two strategies have been proposed: (1) reconstruction of tooth germ and autologous transplantation and (2) 3D printing of tooth mimicking tissue-engineered constructs [75]. 3D printing has been applied to fabricating anatomically mimicking human molar and rat incisal scaffolds with PCL and HA with interconnecting microchannels. Upon stimulation with stromal-derived factor 1 (SDF-1) and bone morphogenetic protein 7 (BMP-7), PDL and new bone regeneration were demonstrated in the rat model [82]. In the future, 3D bioprinting technology will boost the precise and controlled manufacturing of bioengineered teeth to one day benefit patients in the clinical arena as a biomimetic dental implant.
10.5.2 Periodontal Regeneration
Periodontal regeneration is the regeneration of tooth supporting structures including periodontal ligament (PDL), cementum, and alveolar bone, lost due to periodontal disease. Notably, key advances in the field of periodontal tissue engineering in developing biomaterial scaffolds, enhancing growth factor delivery systems, and optimizing cell delivery systems paved the road for these elements to be integrated with 3D bioprinting [75, 82,83,84].
Previously, 3D printing has been demonstrated to be an effective approach in periodontal tissue engineering due to its ability to manufacture scaffolds with precision. Polyphasic biomaterial scaffolds composed of three distinct compartments were developed using 3D printing to guide various periodontal ligament fiber orientations to mimic native periodontal attachment apparatus [85,86,87,88,89]. In addition, growth factors release from polymeric scaffolds can be tuned spatially and temporally to promote the optimal growth of cementum, PDL, and bone [90].
When it comes to clinical application, Rasperini et al. (2015) pioneered the first in human use of a 3D-printed bioresorbable polycaprolactone (PCL) scaffold adapted to the patient’s periodontal defect in combination with human platelet growth-derived growth factor (rh-PDGF-BB) to stimulate periodontal regeneration. Although the long-term follow-up showed graft failure, this study contributed significantly toward the clinical translation of 3D printing for periodontal tissue engineering. The authors proposed areas of improvement including the use of fast resorbing material with highly porous structure, which may contribute to improved tissue ingrowth and vascularization [91].
The main drawback of 3D printing is that it only allows control over the external properties of the scaffolds, and macroarchitecture of the printer construct, but does not allow precise distribution of individual cells. More specifically, stem and progenitor cells may be seeded onto the scaffold but cannot penetrate the scaffold uniformly [75].
Although 3D bioprinting technology is not currently used clinically for periodontal regeneration, it offers several advantages worth investigating. 3D bioprinting allows deposition of single cells or multicellular constructs to precise locations and enables the use of a wide range of biomaterial and bioinks that can be functionalized with growth factors.
Recent progress has been made to utilize 3D bioprinting for periodontal tissue engineering. Notably, several bioinks were optimized for 3D bioprinting of constructs with PDLSCs including gelatin-methacryloyl (GelMA), GelMA/PEG, and sodium alginate (SA)/gelatin (Gel)/nano-hydroxyapatite (na-HA) to ensure cell viability, proliferation, and differentiation [92,93,94]. In addition, the influence of bioprinting parameters including photoinitiator concentration, UV exposure, pressure, and dispensing needle diameter were fine-tuned [93]. The next step in periodontal tissue engineering research would be to explore the use of 3D bioprinting to fabricate biomimetic polyphasic scaffolds with various cells deposited precisely into each compartment and stimulated with specific growth factors [95, 96].
10.5.3 Craniofacial and Maxillofacial Regeneration
10.5.3.1 Craniomaxillofacial Bone
Craniomaxillofacial bone defects are common and result from trauma, tumor resection, infection, or congenital malformation. In addition, alveolar bone resorption after tooth loss may result in atrophic maxillary and mandibular ridge and maxillary sinus pneumatization that require reconstructive surgery [97]. Regeneration of craniofacial bone defect is challenging due to the complexity of the anatomical structures, bone biomechanics, and microenvironment. 3D bioprinting has been used to generate heterogeneous tissue-engineered bone constructs with customized architecture, cellular composition, and growth factor incorporation [98, 99].
Currently, the implementation of personalized scaffolding technologies for craniofacial bone regeneration shows promise for clinical translation. With advances in 3D bioprinting to allow for fabrication of personalized biomaterial matrices functionalized with biologics or genes with precise and spatially controlled delivery of cells, patients with debilitating bone defects will benefit from this transformative technology. Additional preclinical animal studies and human clinical trials with long-term results are needed to ensure safety and efficacy of this technology for routine use in clinical practice [100].
10.5.3.2 Cartilage
Cartilaginous tissues in the craniofacial area primarily consist of the temporomandibular joint (TMJ) disc, the auricular cartilage, and the nasal cartilage [101]. 3D printing has been used to mimic the 3D architecture of these cartilages. Previous studies have used extrusion 3D printing to fabricate cell-laden hydrogels using various natural and synthetic polymers to encapsulate chondrocytes and MSCs capable of synthesizing native cartilaginous ECM [102].
Several biomaterials have been studied as bioink to regenerate cartilaginous tissue including GelMA, alginate, collagen, and PCL [103,104,105,106]. For instance, GelMA in combination with hyaluronic acid and co-deposition with thermoplastics such as PCL may allow engineered constructs to match native human cartilage mechanical and geometrical properties [103].
Although 3D printing technology is not currently used clinically, it has been applied to regenerate TMJ discs in animal models. Using a micro-precise spatiotemporal delivery system with heterogeneous fibrocartilaginous matrix and region-dependent viscoelastic properties, Taradfer et al. (2016) have demonstrated significant healing of perforated TMJ discs in a rabbit model [107, 108]. In addition, 3D printing and sacrificial layer technology were applied to regenerate both the auricular cartilage and adipose tissue using PCL and cell-laden hydrogel. This study showcases that the aforementioned technique can be used to regenerate tissues and organs with complex morphology and multiple types of cells in addition to enhancing cartilage growth with chondrocyte adipose-derived stem cell co-culture [70, 109].
Several key challenges remain in the field of cartilage regeneration, which may be addressed using 3D bioprinting. Future research aimed at mimicking structural and biomechanical properties of cartilage combined with precise deposition of bioink, and cells will enhance integration of native cartilage to the tissue engineered cartilage.
10.5.3.3 Salivary Gland
Salivary gland hypofunction with subjective xerostomia is a clinical condition caused by radiotherapy for head and neck cancers and other systemic conditions such as Sjogren’s syndrome. Consequently, saliva output is greatly reduced putting patients at risk of rampant dental caries, impaired speech, mastication, and swallowing. Despite various therapeutic strategies to repair and regeneration salivary glands and regain salivary flow, this remains an unmet clinical need. Recently, 3D bioprinting has been used to fabricate an innervated salivary gland (SG) like organoid from hDPSC and implanted into an ex vivo model. After implantation, the SG-like organoid significantly stimulated epithelial and neuronal growth in the damaged SG. This is an important step toward the regeneration of salivary gland to treat patients with radiotherapy-induced and Sjogren syndrome-induced xerostomia [25].
10.5.3.4 Nerve
Peripheral facial nerve injuries lead to dysfunction of facial muscles, impaired sensation, and painful neuropathies. Reconstruction of these nerve defects has been commonly performed using autologous nerve graft, which may be hindered by donor site morbidity and limited availability of donor nerves [110]. Recently, a novel scaffold-free 3D bioprinting approach was successfully used to fabricate nerve constructs by using GMSC spheroids, which were implanted and promoted the repair and regeneration of rat facial nerve defects [26]. This is a promising step toward using an easily accessible, minimally invasive source of stem cells that can be used in conjunction with 3D bioprinting to address the increasing clinical demand for nerve repair and regeneration.
10.6 Limitations and Areas of Research
Despite considerable advances in the recent years, the field of 3D bioprinting remains in the early stages of development. Most studies have been performed in vitro followed by a limited amount of in vivo animal studies. Significant work remains before 3D bioprinting technology can be predictably applied to address unmet clinical needs in craniofacial regenerative medicine and enter the clinical arena.
Several key areas of improvement and future research are critical at the level of the bioprinters, bioinks, and cell sources to ensure the scalability and clinical application of 3D bioprinting (Table 10.3). First, faster printing speed must be achieved in order to manufacture tissues and organs of clinically relevant size in a time efficient manner. Second, printing resolution must be enhanced to better biomimic the native tissue microarchitecture, which promotes the functionality of the printed tissue. Third, the ability to predictably print microvasculature must be developed in order to maintain high cell viability of printed tissues over a long period of time allowing the construct to be integrated in vivo. Finally, new generations of bioinks with tunable mechanical, rheological, and biological properties must be formulated in order to achieve a fine balance between tissue printability, structure, and function to support larger 3D printer organs for clinical use [6, 111].
However, significant progress must be made in preclinical animal studies and human clinical trials before widespread adoption to address unmet medical needs in the clinical arena. For 3D bioprinting to be approved by regulatory authorities (i.e., FDA), large animal preclinical studies demonstrating safety and efficacy combined with human clinical studies with long-term follow-up are required.
10.7 Future Perspectives and Summary
With rapid advances in 3D bioprinting, interdisciplinary collaboration between biologists, engineers, and clinicians is crucial to spearhead this powerful technology to overcome clinical challenges and resolve unmet clinical needs in craniofacial regeneration.
In summary, 3D bioprinting has the potential to limit the use of animals in drug discovery and testing; reduce the need to harvest autologous tissues to repair and regenerate craniofacial, oral, and dental defects; decrease the risk of rejection; and enhance the generation of artificial craniofacial tissues and organs such as salivary glands. With the emergence of novel techniques including 4D bioprinting using smart and programmable materials to guide tissue regeneration, the future of craniofacial regenerative medicine is promising for both patients and clinicians.
References
Nyberg EL, Farris AL, Hung BP, Dias M, Garcia JR, Dorafshar AH, et al. 3D-printing technologies for craniofacial rehabilitation, reconstruction, and regeneration. Ann Biomed Eng. 2017;45(1):45–57.
Obregon F, Vaquette C, Ivanovski S, Hutmacher DW, Bertassoni LE. Three-dimensional bioprinting for regenerative dentistry and craniofacial tissue engineering. J Dent Res. 2015;94(9_Suppl):143S–52S.
Dwivedi R, Mehrotra D. 3D bioprinting and craniofacial regeneration. J Oral Biol Craniofac Res. 2020;10(4):650–9.
Mustoe TA, Corral CJ. Soft tissue reconstructive choices for craniofacial reconstruction. Clin Plast Surg. 1995;22(3):543–54.
Urken ML, Weinberg H, Buchbinder D, Moscoso JF, Lawson W, Catalano PJ, et al. Microvascular free flaps in head and neck reconstruction: report of 200 cases and review of complications. Arch Otolaryngol Head Neck Surg. 1994;120(6):633–40.
Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–85.
Shafiee A, Atala A. Printing technologies for medical applications. Trends Mol Med. 2016;22(3):254–65.
Skardal A, Atala A. Biomaterials for integration with 3-D bioprinting. Ann Biomed Eng. 2015;43(3):730–46.
Phillippi JA, Miller E, Weiss L, Huard J, Waggoner A, Campbell P. Microenvironments engineered by inkjet bioprinting spatially direct adult stem cells toward muscle- and bone-like subpopulations. Stem Cells. 2008;26(1):127–34.
Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–6.
Kačarević ŽP, Rider PM, Alkildani S, Retnasingh S, Smeets R, Jung O, et al. An introduction to 3D bioprinting: possibilities, challenges and future aspects. Materials. 2018;11(11):2199.
Pirraco RP, Marques AP, Reis RL. Cell interactions in bone tissue engineering. J Cell Mol Med. 2010;14(1–2):93–102.
Fishero B, Kohli N, Das A, Christophel J, Cui Q. Current concepts of bone tissue engineering for craniofacial bone defect repair. Craniomaxillofac Trauma Reconstr. 2015;8(1):23–30.
Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, et al. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85(11):966–79.
Abou Neel EA, Chrzanowski W, Salih VM, Kim H-W, Knowles JC. Tissue engineering in dentistry. J Dent. 2014;42(8):915–28.
Warren SM, Fong KD, Chen CM, Loboa EG, Cowan CM, Lorenz HP, et al. Tools and techniques for craniofacial tissue engineering. Tissue Eng. 2003;9(2):187–200.
Huang GT-J, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88(9):792–806.
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci. 2000;97(25):13625–30.
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81(8):531–5.
Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18(4):696–704.
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci. 2003;100(10):5807–12.
Seo B-M, Miura M, Gronthos S, Mark Bartold P, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364(9429):149–55.
Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34(2):166–71.
Emmerson E, Knox SM. Salivary gland stem cells: a review of development, regeneration and cancer. Genesis. 2018;56(5):e23211.
Adine C, Ng KK, Rungarunlert S, Souza GR, Ferreira JN. Engineering innervated secretory epithelial organoids by magnetic three-dimensional bioprinting for stimulating epithelial growth in salivary glands. Biomaterials. 2018;180:52–66.
Zhang Q, Nguyen PD, Shi S, Burrell JC, Cullen DK, Le AD. 3D bio-printed scaffold-free nerve constructs with human gingiva-derived mesenchymal stem cells promote rat facial nerve regeneration. Sci Rep. 2018;8(1):6634.
Heo DN, Hospodiuk M, Ozbolat IT. Synergistic interplay between human MSCs and HUVECs in 3D spheroids laden in collagen/fibrin hydrogels for bone tissue engineering. Acta Biomater. 2019;95:348–56.
Zhuang P, Sun AX, An J, Chua CK, Chew SY. 3D neural tissue models: from spheroids to bioprinting. Biomaterials. 2018;154:113–33.
Zanoni M, Cortesi M, Zamagni A, Arienti C, Pignatta S, Tesei A. Modeling neoplastic disease with spheroids and organoids. J Hematol Oncol. 2020;13(1):97.
Gungor-Ozkerim PS, Inci I, Zhang YS, Khademhosseini A, Dokmeci MR. Bioinks for 3D bioprinting: an overview. Biomater Sci. 2018;6(5):915–46.
Adhikari J, Roy A, Das A, Ghosh M, Thomas S, Sinha A, et al. Effects of processing parameters of 3D bioprinting on the cellular activity of bioinks. Macromol Biosci. 2021;21(1):2000179.
Tevlin R, McArdle A, Atashroo D, Walmsley GG, Senarath-Yapa K, Zielins ER, et al. Biomaterials for craniofacial bone engineering. J Dent Res. 2014;93(12):1187–95.
Datta P, Ozbolat V, Ayan B, Dhawan A, Ozbolat IT. Bone tissue bioprinting for craniofacial reconstruction. Biotechnol Bioeng. 2017;114(11):2424–31.
O’Brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today. 2011;14(3):88–95.
Thrivikraman G, Athirasala A, Twohig C, Boda SK, Bertassoni LE. Biomaterials for craniofacial bone regeneration. Dent Clin N Am. 2017;61(4):835–56.
Maroulakos M, Kamperos G, Tayebi L, Halazonetis D, Ren Y. Applications of 3D printing on craniofacial bone repair: a systematic review. J Dent. 2019;80:1–14.
Christopher B, Patrick L. Biopolymers. In: Standard handbook of biomedical engineering & design. New York: McGraw-Hill Education; 2003.
Qu H, Fu H, Han Z, Sun Y. Biomaterials for bone tissue engineering scaffolds: a review. RSC Adv. 2019;9:11.
Visscher DO, Farré-Guasch E, Helder MN, Gibbs S, Forouzanfar T, van Zuijlen PP, et al. Advances in bioprinting technologies for craniofacial reconstruction. Trends Biotechnol. 2016;34(9):700–10.
Juhasz JA, Best SM. Bioactive ceramics: processing, structures and properties. J Mater Sci. 2012;47(2):610–24.
Fahmy MD, Jazayeri HE, Razavi M, Masri R, Tayebi L. Three-dimensional bioprinting materials with potential application in preprosthetic surgery. J Prosthodont. 2016;25(4):310–8.
Wen Y, Xun S, Haoye M, Baichuan S, Peng C, Xuejian L, et al. 3D printed porous ceramic scaffolds for bone tissue engineering: a review. Biomater Sci. 2017;5(9):1690–8.
Poologasundarampillai G, Nommeots-Nomm A. 3 - Materials for 3D printing in medicine: metals, polymers, ceramics, hydrogels. In: Kalaskar DM, editor. 3D printing in medicine. Sawston, UK: Woodhead Publishing; 2017. p. 43–71.
Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012;30(10):546–54.
Yazdimamaghani M, Razavi M, Vashaee D, Moharamzadeh K, Boccaccini AR, Tayebi L. Porous magnesium-based scaffolds for tissue engineering. Mater Sci Eng C. 2017;71:1253–66.
Osidak EO, Kozhukhov VI, Osidak MS, Domogatsky SP. Collagen as bioink for bioprinting: a comprehensive review. Int J Bioprint. 2020;6(3):270.
Chen G, Lv Y. Decellularized bone matrix scaffold for bone regeneration. In: Turksen K, editor. Decellularized scaffolds and organogenesis: methods and protocols. New York, Springer; 2018. p. 239–54.
Heath DE. A review of decellularized extracellular matrix biomaterials for regenerative engineering applications. Regen Eng Transl Med. 2019;5(2):155–66.
Akter F. Chapter 2 - Principles of tissue engineering. In: Akter F, editor. Tissue engineering made easy. San Diego: Academic Press; 2016. p. 3–16.
Deepthi S, Venkatesan J, Kim S-K, Bumgardner JD, Jayakumar R. An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering. Int J Biol Macromol. 2016;93:1338–53.
Shams S, Silva EA. Chapter 4 - Bioengineering strategies for gene delivery. In: Fernandes TG, Diogo MM, Cabral JMS, editors. Engineering strategies for regenerative medicine. San Diego: Academic Press; 2020. p. 107–48.
Kim B-S, Baez CE, Atala A. Biomaterials for tissue engineering. World J Urol. 2000;18(1):2–9.
Yang S, Leong K-F, Du Z, Chua C-K. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001;7(6):679–89.
Neumann A, Kevenhoerster K. Biomaterials for craniofacial reconstruction. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2009;8:Doc08.
Tollemar V, Collier ZJ, Mohammed MK, Lee MJ, Ameer GA, Reid RR. Stem cells, growth factors and scaffolds in craniofacial regenerative medicine. Genes Dis. 2016;3(1):56–71.
Mobaraki M, Ghaffari M, Yazdanpanah A, Luo Y, Mills DK. Bioinks and bioprinting: a focused review. Bioprinting. 2020;18:e00080.
Chen F-M, Zhang M, Wu Z-F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2010;31(24):6279–308.
Bittner SM, Guo JL, Mikos AG. Spatiotemporal control of growth factors in three-dimensional printed scaffolds. Bioprinting. 2018;12:e00032.
Kuroda Y, Kawai T, Goto K, Matsuda S. Clinical application of injectable growth factor for bone regeneration: a systematic review. Inflamm Regen. 2019;39(1):20.
Nevins M, Giannobile WV, McGuire MK, Kao RT, Mellonig JT, Hinrichs JE, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol. 2005;76(12):2205–15.
Colciago A, Celotti F, Casati L, Giancola R, Castano SM, Antonini G, et al. In vitro effects of PDGF isoforms (AA, BB, AB and CC) on migration and proliferation of SaOS-2 osteoblasts and on migration of human osteoblasts. Int J Biomed Sci. 2009;5(4):380–9.
Shirazi SFS, Gharehkhani S, Mehrali M, Yarmand H, Metselaar HSC, Adib Kadri N, et al. A review on powder-based additive manufacturing for tissue engineering: selective laser sintering and inkjet 3D printing. Sci Technol Adv Mater. 2015;16(3):033502.
Rider P, Kačarević ŽP, Alkildani S, Retnasingh S, Barbeck M. Bioprinting of tissue engineering scaffolds. J Tissue Eng. 2018;9:204173141880209.
Jeong H-J, Nam H, Jang J, Lee S-J. 3D bioprinting strategies for the regeneration of functional tubular tissues and organs. Bioengineering. 2020;7(2):32.
Zheng Z, Eglin D, Alini M, Richards GR, Qin L, Lai Y. Visible light-induced 3D bioprinting technologies and corresponding bioink materials for tissue engineering: a review. Engineering. 2020;7:966–78.
Bishop ES, Mostafa S, Pakvasa M, Luu HH, Lee MJ, Wolf JM, et al. 3-D bioprinting technologies in tissue engineering and regenerative medicine: current and future trends. Genes Dis. 2017;4(4):185–95.
Kumar H, Kim K. Stereolithography 3D bioprinting. Methods Mol Biol. 2020;2140:93–108.
Guillotin B, Souquet A, Catros S, Duocastella M, Pippenger B, Bellance S, et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials. 2010;31(28):7250–6.
Li J, Chen M, Fan X, Zhou H. Recent advances in bioprinting techniques: approaches, applications and future prospects. J Transl Med. 2016;14(1):271.
Lee J-S, Hong JM, Jung JW, Shim J-H, Oh J-H, Cho D-W. 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication. 2014;6(2):024103.
Tappa K, Jammalamadaka U. Novel biomaterials used in medical 3D printing techniques. J Funct Biomater. 2018;9(1):17.
Crook JM, editor. 3D bioprinting: principles and protocols, Methods in molecular biology, vol. 2140. New York: Springer; 2020.
Jiang T, Munguia-Lopez JG, Flores-Torres S, Kort-Mascort J, Kinsella JM. Extrusion bioprinting of soft materials: an emerging technique for biological model fabrication. Appl Phys Rev. 2019;6(1):011310.
Bertassoni LE. Progress and challenges in microengineering the dental pulp vascular microenvironment. J Endod. 2020;46(9S):S90–100.
Ma Y, Xie L, Yang B, Tian W. Three-dimensional printing biotechnology for the regeneration of the tooth and tooth-supporting tissues. Biotechnol Bioeng. 2019;116(2):452–68.
Yang J, Yuan G, Chen Z. Pulp regeneration: current approaches and future challenges. Front Physiol. 2016;7:58.
Tao O, Wu DT, Pham HM, Pandey N, Tran SD. Nanomaterials in craniofacial tissue regeneration: a review. Appl Sci. 2019;9(2):317.
Khayat A, Monteiro N, Smith EE, Pagni S, Zhang W, Khademhosseini A, et al. GelMA-encapsulated hDPSCs and HUVECs for dental pulp regeneration. J Dent Res. 2017;96(2):192–9.
Yu H, Zhang X, Song W, Pan T, Wang H, Ning T, et al. Effects of 3-dimensional bioprinting alginate/gelatin hydrogel scaffold extract on proliferation and differentiation of human dental pulp stem cells. J Endod. 2019;45(6):706–15.
Athirasala A, Tahayeri A, Thrivikraman G, França CM, Monteiro N, Tran V, et al. A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry. Biofabrication. 2018;10(2):024101.
Park JH, Gillispie GJ, Copus JS, Zhang W, Atala A, Yoo JJ, et al. The effect of BMP-mimetic peptide tethering bioinks on the differentiation of dental pulp stem cells (DPSCs) in 3D bioprinted dental constructs. Biofabrication. 2020;12(3):035029.
Kim JH, Park CH, Perez RA, Lee HY, Jang JH, Lee HH, et al. Advanced biomatrix designs for regenerative therapy of periodontal tissues. J Dent Res. 2014;93(12):1203–11.
Ivanovski S, Vaquette C, Gronthos S, Hutmacher DW, Bartold PM. Multiphasic scaffolds for periodontal tissue engineering. J Dent Res. 2014;93(12):1212–21.
Liu J, Ruan J, Weir MD, Ren K, Schneider A, Wang P, et al. Periodontal bone-ligament-cementum regeneration via scaffolds and stem cells. Cell. 2019;8(6):537.
Park CH, Rios HF, Jin Q, Bland ME, Flanagan CL, Hollister SJ, et al. Biomimetic hybrid scaffolds for engineering human tooth-ligament interfaces. Biomaterials. 2010;31(23):5945–52.
Park CH, Rios HF, Jin Q, Sugai JV, Padial-Molina M, Taut AD, et al. Tissue engineering bone-ligament complexes using fiber-guiding scaffolds. Biomaterials. 2012;33(1):137–45.
Vaquette C, Fan W, Xiao Y, Hamlet S, Hutmacher DW, Ivanovski S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials. 2012;33(22):5560–73.
Park CH, Rios HF, Taut AD, Padial-Molina M, Flanagan CL, Pilipchuk SP, et al. Image-based, fiber guiding scaffolds: a platform for regenerating tissue interfaces. Tissue Eng Part C Methods. 2014;20(7):533–42.
Park CH, Kim K-H, Lee Y-M, Giannobile WV, Seol Y-J. 3D printed, microgroove pattern-driven generation of oriented ligamentous architectures. Int J Mol Sci. 2017;18(9):1927.
Lee CH, Hajibandeh J, Suzuki T, Fan A, Shang P, Mao JJ. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng Part A. 2013;20(7–8):1342–51.
Rasperini G, Pilipchuk SP, Flanagan CL, Park CH, Pagni G, Hollister SJ, et al. 3D-printed bioresorbable scaffold for periodontal repair. J Dent Res. 2015;94(9 Suppl):153S–7S.
Ma Y, Ji Y, Huang G, Ling K, Zhang X, Xu F. Bioprinting 3D cell-laden hydrogel microarray for screening human periodontal ligament stem cell response to extracellular matrix. Biofabrication. 2015;7(4):044105.
Thattaruparambil Raveendran N, Vaquette C, Meinert C, Samuel Ipe D, Ivanovski S. Optimization of 3D bioprinting of periodontal ligament cells. Dent Mater. 2019;35(12):1683–94.
Tian Y, Liu M, Liu Y, Shi C, Wang Y, Liu T, et al. The performance of 3D bioscaffolding based on a human periodontal ligament stem cell printing technique. J Biomed Mater Res A. 2020; https://doi.org/10.1002/jbm.a.37114.
Vaquette C, Saifzadeh S, Farag A, Hutmacher DW, Ivanovski S. Periodontal tissue engineering with a multiphasic construct and cell sheets. J Dent Res. 2019;98(6):673–81.
Staples RJ, Ivanovski S, Vaquette C. Fibre guiding scaffolds for periodontal tissue engineering. J Periodontal Res. 2020;55(3):331–41.
Nguyen TT, Wu DT, Ramamoorthi M, Syrbu J, Tran SD. 17 - Scaffolds for maxillary sinus augmentation. In: Mozafari M, Sefat F, Atala A, editors. Handbook of tissue engineering scaffolds, vol. 1. Sawston, UK: Woodhead Publishing; 2019. p. 369–86.
Zhang L, Yang G, Johnson BN, Jia X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019;84:16–33.
Genova T, Roato I, Carossa M, Motta C, Cavagnetto D, Mussano F. Advances on bone substitutes through 3D bioprinting. Int J Mol Sci. 2020;21(19):7012.
Yu N, Nguyen T, Cho YD, Kavanagh NM, Ghassib I, Giannobile WV. Personalized scaffolding technologies for alveolar bone regenerative medicine. Orthod Craniofac Res. 2019;22(Suppl 1):69–75.
Lin Y, Lin H, Ramamoorthi M, Wu DT, Zhang Z, Tran SD. 21 - Scaffolds for temporomandibular joint disc engineering. In: Mozafari M, Sefat F, Atala A, editors. Handbook of tissue engineering scaffolds, vol. 1. Sawston, UK: Woodhead Publishing; 2019. p. 437–55.
Tao O, Kort-Mascort J, Lin Y, Pham HM, Charbonneau AM, ElKashty OA, et al. The applications of 3D printing for craniofacial tissue engineering. Micromachines. 2019;10(7):480.
Schuurman W, Levett PA, Pot MW, van Weeren PR, Dhert WJA, Hutmacher DW, et al. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol Biosci. 2013;13(5):551–61.
Rhee S, Puetzer JL, Mason BN, Reinhart-King CA, Bonassar LJ. 3D bioprinting of spatially heterogeneous collagen constructs for cartilage tissue engineering. ACS Biomater Sci Eng. 2016;2(10):1800–5.
Park SH, Yun BG, Won JY, Yun WS, Shim JH, Lim MH, et al. New application of three-dimensional printing biomaterial in nasal reconstruction. Laryngoscope. 2017;127(5):1036–43.
Messaoudi O, Henrionnet C, Bourge K, Loeuille D, Gillet P, Pinzano A. Stem cells and extrusion 3D printing for hyaline cartilage engineering. Cell. 2020;10(1):2.
Tarafder S, Koch A, Jun Y, Chou C, Awadallah MR, Lee CH. Micro-precise spatiotemporal delivery system embedded in 3D printing for complex tissue regeneration. Biofabrication. 2016;8(2):025003.
Legemate K, Tarafder S, Jun Y, Lee CH. Engineering human TMJ discs with protein-releasing 3D-printed scaffolds. J Dent Res. 2016;95(7):800–7.
Morrison RJ, Nasser HB, Kashlan KN, Zopf DA, Milner DJ, Flanangan CL, et al. Co-culture of adipose-derived stem cells and chondrocytes on three-dimensionally printed bioscaffolds for craniofacial cartilage engineering. Laryngoscope. 2018;128(7):E251–7.
Volk GF, Pantel M, Guntinas-Lichius O. Modern concepts in facial nerve reconstruction. Head Face Med. 2010;6(1):25.
Yu J, Park SA, Kim WD, Ha T, Xin Y-Z, Lee J, et al. Current advances in 3D bioprinting technology and its applications for tissue engineering. Polymers (Basel). 2020;12(12):2958.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wu, D.T., Pham, H.M., Tao, O., Wu, K.Y., Tran, S.D. (2022). Bioprinting Applications in Craniofacial Regeneration. In: Chaudhari, P.K., Bhatia, D., Sharan, J. (eds) 3D Printing in Oral Health Science. Springer, Cham. https://doi.org/10.1007/978-3-031-07369-4_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-07369-4_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-07368-7
Online ISBN: 978-3-031-07369-4
eBook Packages: MedicineMedicine (R0)