Abstract
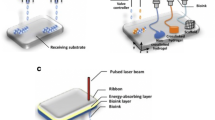
Three-dimensional (3D) printing is a type of additive manufacturing that works by the application of material inks layer by layer using data from computer-aided design (CAD) to help to place the ink in a predefined place, thus producing a highly accurate product even with complex geometry. The goal in using 3D bioprinting is to develop a biological scaffold that resembles the desired tissue to be replaced, including the cells and the growth factors, in a specific spatial relationship. The developments in bone tissue engineering (BTE) and 3D bioprinting are revolutionizing osseous craniofacial reconstructive surgery. This chapter aims to describe 3D bioprinting of biomaterial and bioceramic scaffolds for bone tissue engineering and maxillofacial reconstructive surgery.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Additive manufacturing
- Layer by layer
- Bioprinting
- Biological scaffold
- Bioactive glass
- Calcium phosphate
- Hydroxyapatite
- Mesenchymal stem cells
- Induced pluripotent stem cells
- Exosome
- Biomimetics
- Self-assembly
2.1 Introduction
Three-dimensional (3D) printing is a type of additive manufacturing that was first invented in 1984 for engineering and industrial purposes. It works by the application of material inks layer by layer using data from computer-aided design (CAD) to help to place the ink in a predefined place, thus producing a highly accurate product even with complex geometry [1, 2]. The technology found its way to the health sector through dentistry when additive manufacturing was used to print a solid block of dental implants, crowns, and bridges from a biocompatible and bioinert material that does not elicit an immune reaction [3].
Scientists were overly ambitious realizing the precision of the end-product when 3D printing was used. They decided to unleash the power of 3D printing and use it for medicinal purposes to bioprint tissues. The first bioprinting attempt was undertaken early in 1988, using an inkjet printer depositing cell drops on-demand approach. The goal in using 3D bioprinting is to develop a biological scaffold that resembles the desired tissue to be replaced, including the cells and the growth factors, in a specific spatial relationship. It is a customizable, patient-specific solution meeting the patient’s need at a macro level (i.e., shape and size), and on a micro level resembling patients’ tissue structure and architecture [4, 5]. The development in bone tissue engineering and 3D bioprinting also aims to solve the crisis in the shortage in organs needed for transplantation [6].
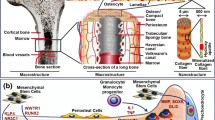
Tissue loss in the craniofacial region can occur due to a craniofacial genetic deformity, trauma, or surgical excision as a treatment of tissue malignancy [7]. Facial disfigurement has a severe negative impact on individuals, both socially and psychologically, and requires rapid, precise, and aesthetic rebuilding producing a functional, harmonious, and symmetrical face [8]. Osseous craniofacial reconstruction traditionally employs a graft harvested from the iliac crest or the ribs, which serve as the bridge needed to direct the 3D bone growth (osseoconduction), as well as inducing the differentiation and the recruiting of osteoblasts (osseoinduction) into the injured area to promote bone healing [9]. However, placing a graft is not without risk; autogenous bone grafting carries the risk of morbidity (pain in the donor site, neuralgia, blood loss, or infection), while the allogenic bone graft is associated with the possibility of transmitting infection or eliciting an immune reaction [10]. Moreover, facial reconstruction using a bone graft does not always provide aesthetic results due to the anatomical complexity of bone, soft tissue, and the hollow cavities in the face. 3D bioprinting, on the other hand, may provide a more precise alternative that fits the defects, reducing the need to count on the surgeon’s ability to harvest or carve the graft to fit the surface.
2.2 Bone Tissue Engineering
Bone tissue engineering has received much attention in the last few decades, and it showed tremendous progress due to the improved understanding of bone biology, along with the advances in the biomaterials. It focuses on:
-
(a)
Developing biomaterials that can provide the same physical and biological properties as natural bone [11].
-
(b)
Producing scaffolds from these biomaterials, having the same architecture and topography that ensure nutrient and oxygen passage, micro-vessels, and nerve ingrowth, as well as regulating the stem cell differentiation down the osteogenic fate [12, 13].
-
(c)
Incorporating mesenchymal stem cells (MSC) that are directed toward differentiating into osteogenic cell lineage [14].
-
(d)
Incorporating bone growth factors; bone morphogenic proteins (BMP), insulin-like growth factor-2 (IGF-1), vascular endothelial growth factors (VEGF), and others that enhance osteogenesis [15].
2.3 Biomaterials in Bone Tissue Engineering
Bone is composed of 60–70% inorganic phase in the form of hydroxyapatite (Ca10(PO4)6(OH)2), while the organic phase is mostly formed of collagen type I with some other proteins and growth factors. The simplicity of the natural bone composition enabled the progress in bone tissue engineering. Biomaterials used to fabricate scaffolds should be biocompatible, biodegradable to be replaced by the newly generated bone, and bioactive to enhance bone regeneration, having physical strength and mechanical properties, which enable it to support the load the natural bone is supporting [16]. Examples of biomaterials used in bone tissue engineering include demineralized bone matrix as well as a number of bioceramics and bioglasses.
2.3.1 Demineralized Bone Matrix
These are allografts treated with chemical acid to demineralize as well as removing the inorganic component of the graft, leaving the matrix proteins, mainly collagen I and bone growth factor BMP, and are then treated with radiation to decrease the possibility of eliciting an immune reaction [17]. Demineralized bone matrix has been used for decades in clinical applications, and has shown tremendous success being osteoconductive and osseoinductive, but because the end-product is in a powder form, making it is difficult to handle during surgery, which consequently has limited its use [18]. Solutions implemented to ease the manipulation of the powder were based on using the powder mixed with a viscous carrier to enable it to condense and pack into bony defects [17].
Wagner et al. reported using demineralized bone matrix for mandibular reconstruction by wrapping it in an acellular dermal matrix to confine the demineralized bone matrix paste and placing it over a bent plate [19]. The patients were followed up for five years and showed evidence of bone healing. In a recent study, Driscoll et al. used demineralized bone matrix mixed with hydroxyapatite crystals in different ratios in a 3D printer to print scaffolds for spinal repair, and it was tested in rat models [20]. The preclinical studies showed successful fusion, with the developed biomaterial being a hybrid encompassing the osseoinductive properties of the demineralized bone matrix carrying the bone growth factor along with the osteoconductive properties of the hydroxyapatite.
2.3.2 Bioceramics and Bioglasses
These are inorganic oxides including hydroxyapatite, calcium phosphate, tricalcium phosphate (TCP), and calcium silicate. They are considered bioactive as they bond to bone and elicit osteogenesis [21].
2.3.2.1 Hydroxyapatite
This makes the bulk of the bone composition, thus, it has been studied extensively as a bone substitute because its composition resembles natural bone. Both calcium and phosphate ions present in hydroxyapatite promote bone regeneration. Calcium ions stimulate osteoblasts by activating ERK1/2 pathways, which protect them from apoptosis, as well as having a central role in bone maturation by deposition in immature bone [22]. Phosphate ions activate the IGF-1 pathway in osteoblasts, which is implicated in cell survival, growth, and protein synthesis [23]. Besides, it is osseoinductive and osseoconductive, making it ideal to be a synthetic bone substitute. But, it is organized in a highly arranged crystalline microstructure that hinders it from degrading, and it also inherits a low compressive and tensile strength making it brittle when loaded [24]. To reduce the brittleness of hydroxyapatite, Mukherjee et al. investigated the effect of adding carbon nanotubes (CNT) to hydroxyapatite and found that it increased the fracture toughness of the scaffold. They tested the scaffold on animal models and found that the addition of CNT was biologically safe with no toxicity shown in either the liver or kidney, but with enhanced bone regeneration on the implanted site [25]. However, the data was stated to be preliminary and incomplete to proceed onto clinical studies.
In relations to the 3D printing of hydroxyapatite, Seitz et al. were able to use hydroxyapatite powder sprayed with a polymeric binder dissolved in water to ensure ink flow to produce a porous scaffold with fully interconnected channels sixteen years ago, which are further compacted after printing in a 1250 °C furnace to remove and achieve binder pyrolysis [26]. Six years ago, Shao et al. proposed the use of 3D-gel printing (3DGP) instead of regular 3D printing with the use of hydroxyethylmethacrylate (HEMA) gelation system to produce a flowable slurry. Their new system has the advantage of being appropriate to a wide range of materials from metal to ceramics, keeping the cost low while achieving high printing efficiency, producing complex shapes due to the flow of the slurry. They used it with stainless steel, zirconia, and hydroxyapatite, and tested the fabricated scaffolds and their mechanical properties [27,28,29]. Most studies on the fabrication of hydroxyapatite scaffolds were carried out using biomaterials only, without embedding cells within the scaffold and the seeding of cells occurred after fabrication, thus it should not be confused with bioinks, which incorporate both biomaterials and cells [30].
2.3.2.2 Tricalcium Phosphate
In 1920, Albee was the first to report that rhombohedral β-form, β-tricalcium phosphate (β-TCP), enhances osteogenesis [31]. Tricalcium phosphate is composed of calcium and phosphate ions just like hydroxyapatite, which renders it to have the same effect on osteoblasts resulting in bone regeneration. Gao et al. showed the in vivo osteogenic potential of the tricalcium phosphate granules placed in a titanium porous scaffold and implanted in a femur defect on animal models [32].
In contrast to hydroxyapatite, tricalcium phosphate has a crystalline structure that is not highly organized, which makes it more susceptible to resorption and degradation, which is an ideal property for a scaffold material [33]. Ishikawa et al. compared the mechanical properties and recorded the histological findings of the newly generated bone when tricalcium phosphate and hydroxyapatite were used [34]. The study confirmed tricalcium phosphate has higher solubility than hydroxyapatite, which explains why more bone is found around the implanted tricalcium phosphate than the hydroxyapatite.
Degradation of the tricalcium phosphate is a desired property when constructing a scaffold, but degradation of the material should be coordinated with the speed of the osteogenesis process. To adjust tricalcium phosphate degradation, it has been doped with mineral oxides like magnesium oxide (MgO) and strontium oxide (SrO), which affect the crystalline orientation of the tricalcium phosphate and make it less soluble and alter both the mechanical and biological properties of the tricalcium phosphate [35]. Banerjee et al. confirmed slower degradation of the implanted MgO/SrO-doped β-TCP than pure β-TCP on animal models [36]. They also showed that the doped implant had more cell attachment, which increases cell differentiation and proliferation. Analysis of osteocalcin and type I collagen inside the implants indicated faster osteogenesis and remodeling. Recently, Gu et al. used Mg-doped tricalcium phosphate and 3D-printed an interconnected-pores scaffold with mechanical properties close to bone [37]. They further seeded the scaffold with MSCs derived from bone marrow and umbilical cord and showed that both osteogenesis and angiogenesis were enhanced. In an animal model, Kim et al. transplanted 3D-printed scaffolds from a composite of tricalcium phosphate and polycaprolactone polymer and used it to repair the maxilla in a dog after resecting a tumor with success [38].
In 1986, another strategy was proposed to adjust the solubility of tricalcium phosphate in physiological conditions by combining hydroxyapatite with tricalcium phosphate in different ratios to achieve the best physical and mechanical properties for the desired load-bearing application referred to as biphasic calcium phosphate [39]. Increasing the hydroxyapatite content in the biphasic calcium phosphate leads to a more stable material, while increasing the tricalcium phosphate results in a material that is more soluble, thus, it can be easily tailored. Liu et al. 3D-printed scaffolds using biphasic calcium phosphate and examined the in vivo behavior using rabbit calvarial defects which showed an increase in osteogenesis and high bone density [40].
2.3.2.3 Calcium Phosphate Cement
This cement was accidentally invented in the 1980s by the American Dental Association Health Foundation Paffenbarger Research Centre (ADAHF-PRC) who were trying to develop a cement to treat and remineralize early dental caries. A mixture of tetra calcium phosphate, dicalcium phosphate anhydrous, and dicalcium phosphate dihydrate with water was found to rapidly produce hydroxyapatite. A decade after that, the FDA approved calcium phosphate cement for clinical use, and since then a tremendous number of studies have been conducted [41]. The cement was found to promote osteogenesis, being osteoconductive, and most importantly it is injectable, which makes it easier for clinical use. Injecting the material into the site of surgery will allow it to mould into the shape of the deformity without the need for further drilling at the surgical site to match the size and shape of the scaffold. Yu et al. reported the success of calcium phosphate cement in bone regeneration when they performed an in vivo study in which injectable calcium phosphate cement was implanted into a femoral condyle defects of rabbits [42]. Lin et al. cultured three types of cells: induced pluripotent stem cells (iPSC), human umbilical vein endothelial cells (HUVECs), and pericytes; into scaffolds made from calcium phosphate cement and implanted them into cranial defects created on rats [43]. They found that the tri-culture group had elevated angiogenic and osteogenic markers, and mineralization.
2.3.2.4 Bioactive Glass
These are silicate-based ceramics composed of silicon dioxide, calcium oxide, phosphorus oxide, potassium oxide, magnesium oxide, and boric oxide. The composition and percentage of these oxides vary, but the key component, silicate, always constitutes 45–52% of its weight [44]. Bioglasses possess the capability to form a strong chemical bond with the bone tissue that is created through the polycondensation of a silicone-rich layer on the surface of the bioactive glass due to ion exchange between ions in the physiological fluid and leaching of ions from the surface of the bioglass [44]. Moreover, the electronegative silicone-rich layer on the surface is considered osseoinductive as it adsorbs protein that in turn attracts macrophages and MSCs [44].
As a bone substitute material, bioactive glass proved its worthiness in an in vivo animal study carried out by Moimas et al. where bioactive glass implanted into tibial defects were found to be completely resorbed in six months and be replaced by bone tissue [45]. It was shown that the composition of the bioactive glass affects the union chemical reaction and stimulation of cells to promote osteogenesis. Bioactive glass went through optimization of its formula, and 45S4 was invented composed of 45% SiO2, 24.5% Na2O, 24.5% CaO, 6% P2O5 (wt%), characterized by a high amount of Na2O and CaO, which make the surface of the material very bioactive [46]. Scaffolds made from 45S5 were found by Detsch et al. to drive umbilical cord-derived MSCs down the osteogenic differentiation pathway [47].
Recently, the development of the sol–gel method, adding ammonia to the sol phase to transform it into a gel and then freeze-dry it, produced 58S bioactive glass composing of 60 mol.% SiO2, 36 mol.% CaO and 4 mol.% P2O5. 58S bioactive glass has the benefit of achieving a homogeneous biomaterial compared to the melting method used originally where phosphate becomes volatile at high temperature [48]. Wheeler et al. compared in vivo bone regeneration capacity between 45S4 and 58S scaffolds after implantation within critical-sized distal femoral cancellous bone defects in a rabbit model and the results showed that the 58S degraded much quicker but was able to form bone earlier than 45S4 at 4 weeks, which is normalized at 12 weeks [49].
The 3D printing of scaffolds composed of bioactive glass have been investigated in a number of in vitro and in vivo studies El-Rashidy et al. comprehensively reviewed the in vivo studies undertaken on the regeneration of bone with 3D-printed bioactive glass scaffolds [50]. Recently, Kolan et al. compared the osteogenic potential of bioactive glass scaffolds made by 3D printing with and without the use of BMP after implantation into cranial defects in rats [51]. Their study concluded that the addition of BMP to the scaffold greatly enhanced bone regeneration.
2.4 Cells in Bone Tissue Engineering
3D bioprinting includes both biomaterial and cells in the bioink to fabricate a scaffold. Ideal biomaterials for bone substitutes should stimulate the seeded stem cells to differentiate into osteoblasts responsible for the bone regeneration. Gao et al. proposed different molecular mechanisms by which biomaterials interact with stem cells to promote osteogenesis [14]. The exact process is not known, but they postulated that phosphorus, magnesium, and strontium ions released from the biomaterial activate the BMP pathway and increase the concentration of the calcitonin gene-related peptide. The following section describes the various types of cells used in bone tissue engineering.
2.4.1 Mesenchymal Stem Cells (MSCs )
These are used for their pluripotency, ability to differentiate into osteoblasts, and immune modulative effect [52]. They can also be derived from a variety of sources ranging from bone marrow, umbilical cord, placenta, dental pulp, adipose tissue, and other sources [52]. Injecting MSCs derived from adipose tissue along mandibular fracture lines were found to enhance osseointegration and bone quality, as well as promoted bone healing as observed in the study by Castillo-Cardiel et al. [53]. In 2016, a study by Chamieh et al. discovered that implanting collagen scaffolds seeded with dental pulp-derived MSCs into calvarial defects in rats resulted in accelerated bone regeneration compared to rats having a collagen scaffold with no seeded cells, demonstrated by evaluating the variations in bone density and through histological examination [54]. Fahimipour et al. reported in a recent article the utilization of the bioprinter to bioprint collagen matrix to mimic the extracellular matrix of natural bone with the MSC and BMP [55]. The matrix has the benefit of confining BMP as well as preventing it from escaping the scaffold, which is known to cause ectopic bone formation or osteomas [56]. The 3D printing was used again to 3D print a scaffold that represents the mineralized part of the bone, which is then used to support the MSC-BMP collagen matrix. It was found that using this method enhanced MSCs seeding, and proliferation while the availability of BMP enhanced the osteogenic potential of the MSCs [55]. A recent report by Dong et al. showed that the presence of osteoclasts is crucial for bone regeneration as well as osteoblasts [57]. In their study, a proteomic analysis was performed, and mass spectrometry was used to identify proteins secreted in extracellular matrix. The analysis showed the presence of more than 608 protein presents, among which two proteins are known to be secreted by pre-osteoclasts, CXCL12 and IGFBP5 proteins, both are responsible for MSC cells’ migration and osteogenic differentiation, respectively [57]. They confirmed their hypothesis by implanting scaffolds made from decalcified bone matrix seeded with co-cultured MSCs and pre-osteoclasts into femur defects in rats showing significant enhancements in bone regeneration compared to implanting scaffolds seeded with MSCs only.
2.4.2 Induced Pluripotent Stem Cells (iPSCs)
These are another exciting source of cells that can differentiate into any cell type, mimicking embryonic stem cells. However, with the ethical dilemma that has risen by extracting embryonic stem cells, which results in the destruction of human embryos, motivated scientists to look for other sources of cells that have the same pluripotency [58]. To circumvent this issue, iPSCs were produced by Takahashi et al. in 2007 by transducing four factors: Oct3/4, Sox2, Klf4, and c-Myc, present in embryonic stem cells, in fibroblast turning them into cells mimicking pluripotency [59]. Xie et al. investigated the osteogenic differentiation of iPSCs seeded on a scaffold made from a composite of hydroxyapatite-chitosan-collagen and found the proliferation of iPSCs into osteoblasts and an increase in bone protein secretion [60]. Moreover, they implanted these scaffolds into cranial defects of animal models, and compared the density of bone with the scaffold seeded with iPSC and other seeded with MSCs and found that the iPSCs scaffold has nearly double the bone density than when MSCs were used alone.
The osteogenic differentiation of iPSCs was studied by a number of research groups [61]. A study by Kao et al. discovered that resveratrol has a supporting effect on the osteogenic differentiation of iPSCs [62]. Later, a study by Ji et al. examined the osteogenic differentiation of human iPSCs regulated by nano-hydroxyapatite/chitosan/gelatine 3D scaffolds with nano-hydroxyapatite in different ratios [63]. Investigation was also carried out to reprogram iPSCs to functional osteoblasts using only the small molecule exogenous adenosine [64]. However, iPSCs still carry the potential of tumorigenicity and teratoma formation, which still limits its use clinically, and further investigation should be conducted to optimize its use and safety [65].
2.4.3 Exosome
Recently, increasing interest was diverted into cell-free therapies after the discovery that MSCs cause tissue regeneration due to its paracrine effect. This approach carries the benefit of avoiding tumorigenicity, resistance to apoptosis, triggering an immune response, and genetic instability, which are all present in MSCs utilization [66]. It will also permit the repeated injections or administration of the therapy without the fear of accumulation of cells in non-targeted tissue, especially the lungs [67].
The cell-free approach uses the exosomes, which are membrane-bound vesicles, produced by endosomes in the cell containing a specific cargo either: micro-RNA, messenger-RNA, proteins, or other biomolecules, and get excreted outside the cell to be communicated into another cell [68]. Exosomes are produced by most cell types as a way of communication and crosstalk between cells. Exosomes from MSCs regulate the paracrine effect that enhances the regeneration of tissues [69]. Several studies have been conducted and showed the potential of using exosomes for bone regeneration. Lu et al. extracted exosomes from adipose-derived MSCs and used a TNF-α pre-conditioned medium, which was found to positively promote osteogenesis and bone repair [70]. Zhao et al. proposed that exosomes extracted from bone marrow-derived MSCs and co-cultured with osteoblasts, result in the activation of the MAPK pathway on the osteoblasts, which is important for the cell cycle and growth, and results in their proliferation, thus promoting bone regeneration [71].
More importantly, Diomede et al. demonstrated the ability of an implanted 3D-printed scaffold to heal calvarial defect in rats that is composed of a polymer polylactic acid (PLA), seeded with exosomes and gingiva-derived MSCs [72]. Furthermore, Zhang et al. also worked extensively on exosomes and in one of their study, they showed that a scaffold made with tricalcium phosphate combined with exosomes derived from iPSCs healed calvarial defects on rats via activating the PI3K/Akt signalling pathway [73]. In a later study, they used exosomes derived from umbilical cord-derived MSCs combined in hydrogel and transplanted at the femoral fracture site in the animal model. They found that implanted exosomes promoted angiogenesis, which in turn enhanced fracture healing [74]. Although the results of these studies are promising, still, a consensus on exosome extraction and purification has not been achieved which is important in translational medicine.
2.5 3D Bioprinting Approaches
In the process of bioprinting, deposition of both the biomaterial and the cells occur simultaneously. 3D bioprinting is achieved by one or a combination of the following strategies.
2.5.1 Biomimicry
This is a straightforward approach using the bioprinter to replicate the original architecture of the tissue, thereby providing the right environmental factors that guide cells to differentiate into the right type of cells. This approach of bioprinting is extremely dependent on the material ink used to construct the scaffold. A scaffold is the parallel of the extracellular matrix, that should be able to provide the chemical and physiological cues important for cell viability, differentiation, and expansion [75]. Scaffold biomaterials should be biocompatible, permeable to nutrients, having adequate stiffness to withstand loading and deformation while at the same time, able to undergo degradation at the same pace that allows the growth of new bone tissue and eventually replaces the scaffold [76]. All these requirements are crucial in choosing the most ideal scaffold bioink and they are also the primary factors that determines the success of the printed scaffold. After bioprinting, a bioreactor is used to regulate environmental factors such as the oxygen, temperature, nutrient diffusion, and the gravitational force needed for cell infiltration to the depth of the printed scaffold [77].
2.5.2 Self-assembly Approach
This is a scaffold-free approach that eliminates the need for scaffold biomaterials and mitigating the difficulties faced using the scaffold. The approach adopts the same embryological development process which utilizes interaction and signals between adjacent cells and their extracellular matrix to self-organize into the tissue intended for engineering [78]. High-density initial cell seeding ensures cell–cell interaction, resulting in cell producing their own extracellular matrix and forming cell aggregates in the form of spheres or sheets, and carries the advantage of efficiency to produce tissues faster than using scaffold bioink. Various methods are used to form these cell aggregates from magnetic levitation, hanging drop, hydrogel microwell, and others, each with its pros and cons [79]. Spheroids and sheets are then used in a 3D printer to form the engineered tissue. The advantage of using this approach is the ability to use different types of cells as well as regulating their ratios. This allowed for the co-culturing endothelial cells with MSCs, which promotes angiogenesis in the final construct, while the MSCs differentiate into the desired cell type [80]. Yamasaki et al. created a scaffold-free construct from adipose tissue-derived MSCs by using the needle array 3D printing method and implanted them into femoral defects of pigs which showed enhanced osteochondral regeneration [81]. Recently, Heo et al. described a method to 3D print spheroid aggregates, made from human umbilical vein endothelial cells (HUVECs) and MSCs and called it the aspiration-assisted bioprinting (AAB) technique, in which they showed that it allowed for better and more precise positioning of the spheroids to produce scaffold-free bone tissue [82].
2.5.3 3D Bioprinting in Bone Tissue Engineering and Craniofacial Reconstruction
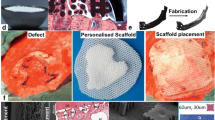
3D bioprinting is offering an exciting future for bone tissue engineering and craniofacial reconstruction, but the technology is still in its early stages. Few studies were carried out or are currently in progress that shows promising results. In 2014, Goh et al. implanted a polycaprolactone scaffold fabricated by 3D printing in sockets of newly extracted teeth to preserve the height of maxillary and mandibular ridges [83]. In the same year, a Chinese team, led by Zhang who worked extensively in BTE, published the results of their clinical trial on 23 female patients reconstructing the mandibular angle after ostectomy [84]. They demonstrated that using 3D bioprinting titanium scaffolds, led to greater bone regeneration, shorter operation time, and better aesthetic results. In 2015, Sumida et al. published the results of their clinical trial of implanting 3D printed scaffolds for maxillary and mandibular ridges in 13 patients without randomization and reported favorable outcomes [85]. 3D printing is also used by neurosurgeons for the correction of calvarial defects after resecting brain tumours. Kilstrom et al. reported in 2019 the results of using 3D printing to fabricate calcium phosphate-titanium reinforced scaffolds implanted on the skull of 52 patients with the intention to promote bone regeneration and osteointegration [86].
A search in the clinical trial government website (www.clinicaltrial.gov) in March 2021 revealed the presence of 342 clinical trials with different statuses, when searching MSCs and bone regeneration, of which 6 trials are concerned with bone regeneration in the craniomaxillofacial region, listed in Table 2.1.
At the same time, only 4 studies are concerned with using 3D printing for the correction of bone defects in the craniomaxillofacial region, listed in Table 2.2.
2.6 Concluding Remarks
Developing bone tissue engineering is important, as the need for bone implants increases due to increasing population, increasing facial injuries, orthognathic surgeries, tumors, and craniofacial deformities. Translation of this technology would be the only solution to treat large defects and non-union fractures and when technology is combined with 3D printing, it allows potentially more aesthetic facial reconstruction and reduced surgery time. However, the technology needs further investigation to optimize the biomaterial to ensure both optimal osteogenesis and angiogenesis to enable vascularization of the scaffolds. Biomaterials used should also provide the mechanical properties needed for the implanted site, as bone engineered to be implanted in a load-bearing bone should be different from scaffolds created for non-load bearing bone. Enhancing 3D printing technology enables it to provide scaffolds exactly mimicking the natural bone with the highest resolution. Also, the ease and availability of the biomaterial, 3D printer, and expertise in hospital settings should be discussed to allow its translation directly to patients.
References
Khorram Niaki M, Nonino F (2017) Additive manufacturing management: a review and future research agenda. Int J Prod Res 55:1419–1439
Wohlers T, Gornet T (2014) History of additive manufacturing. http://www.wohlersassociates.com/history2016.pdf. Accessed 12 Apr 2021
Javaid M, Haleem A (2019) Current status and applications of additive manufacturing in dentistry: a literature-based review. J Oral Biol Craniofac Res 9:179–185
Murphy SV, De Coppi P, Atala A (2020) Opportunities and challenges of translational 3D bioprinting. Nat Biomed Eng 4:370–380
Mandrycky C, Wang Z, Kim K et al (2016) 3D bioprinting for engineering complex tissues. Biotechnol Adv 34:422–434
Abouna GM (2008) Organ shortage crisis: problems and possible solutions. Transplant Proc 40:34–38
Zhang W, Yelick PC (2018) Craniofacial tissue engineering. Cold Spring Harb Perspect Med 8:a025775
Singh VP, Moss TP (2015) Psychological impact of visible differences in patients with congenital craniofacial anomalies. Prog Orthod 16:5
Elsalanty ME, Genecov DG (2009) Bone grafts in craniofacial surgery. Craniomaxillofac Trauma Reconstr 2:125–134
Sohn HS, Oh JK (2019) Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater Res 23:9
Wang W, Yeung KWK (2017) Bone grafts and biomaterials substitutes for bone defect repair: a review. Bioact Mater 2:224–247
Cun X, Hosta-Rigau L (2020) Topography: a biophysical approach to direct the fate of mesenchymal stem cells in tissue engineering applications. Nanomaterials 10:2070
Chen X, Fan H, Deng X et al (2018) Scaffold structural microenvironmental cues to guide tissue regeneration in bone tissue applications. Nanomaterials 8:960
Gao C, Peng S, Feng P et al (2017) Bone biomaterials and interactions with stem cells. Bone Res 5:17059
De Witte TM, Fratila-Apachitei LE, Zadpoor AA et al (2018) Bone tissue engineering via growth factor delivery: from scaffolds to complex matrices. Regen Biomater 5:197–211
Hutmacher DW (2000) Scaffolds in tissue engineering bone and cartilage. Biomaterials 21:2529–2543
Zhang H, Yang L, Yang XG et al (2019) Demineralized bone matrix carriers and their clinical applications: an overview. Orthop Surg 11:725–737
Gruskin E, Doll BA, Futrell FW et al (2012) Demineralized bone matrix in bone repair: history and use. Adv Drug Deliv Rev 64:1063–1077
Wagner JD, Wagner JP, Wagner SA (2017) Mandibular defect reconstruction utilizing preoperative 3D modeling, plate pre-bending and a novel triad graft: a case report. Stomatological Dis Sci 1:97–102
Driscoll JA, Lubbe R, Jakus AE et al (2020) 3D-printed ceramic-demineralized bone matrix hyperelastic bone composite scaffolds for spinal fusion. Tissue Eng Part A 26:157–166
Ribas RG, Schatkoski VM, do Amaral Montanheiro TL et al (2019) Current advances in bone tissue engineering concerning ceramic and bioglass scaffolds: a review. Ceram Int 45:21051–21061
Danciu TE, Adam RM, Naruse K et al (2003) Calcium regulates the PI3K-Akt pathway in stretched osteoblasts. FEBS Lett 536:193–197
Guntur AR, Rosen CJ (2013) IGF-1 regulation of key signaling pathways in bone. Bonekey Rep 2:437
Mondal S, Pal U (2019) 3D hydroxyapatite scaffold for bone regeneration and local drug delivery applications. J Drug Deliv Sci Technol 53:101131
Mukherjee S, Nandi SK, Kundu B et al (2016) Enhanced bone regeneration with carbon nanotube reinforced hydroxyapatite in animal model. J Mech Behav Biomed Mater 60:243–255
Seitz H, Rieder W, Irsen S et al (2005) Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater 74:782–788
Shao H, He J, Lin T et al (2019) 3D gel-printing of hydroxyapatite scaffold for bone tissue engineering. Ceram Int 45:1163–1170
Shao H, Zhao D, Lin T et al (2017) 3D gel-printing of zirconia ceramic parts. Ceram Int 43:13938–13942
Ren X, Shao H, Lin T et al (2016) 3D gel-printing—an additive manufacturing method for producing complex shape parts. Mater Des 101:80–87
Groll J, Burdick JA, Cho DW et al (2018) A definition of bioinks and their distinction from biomaterial inks. Biofabrication 11:013001
Albee FH (1920) Studies in bone growth: triple calcium phosphate as a stimulus to osteogenesis. Ann Surg 71:32–39
Gao P, Zhang H, Liu Y et al (2016) Beta-tricalcium phosphate granules improve osteogenesis in vitro and establish innovative osteo-regenerators for bone tissue engineering in vivo. Sci Rep 6:23367
Bohner M, Santoni BLG, Döbelin N (2020) β-tricalcium phosphate for bone substitution: synthesis and properties. Acta Biomater 113:23–41
Ishikawa K, Miyamoto Y, Tsuchiya A et al (2018) Physical and histological comparison of hydroxyapatite, carbonate apatite, and β-tricalcium phosphate bone substitutes. Materials 11:1993
Gallo M, Le Gars SB, Douillard T et al (2019) Effect of grain orientation and magnesium doping on β-tricalcium phosphate resorption behavior. Acta Biomater 89:391–402
Banerjee SS, Tarafder S, Davies NM et al (2010) Understanding the influence of MgO and SrO binary doping on the mechanical and biological properties of beta-TCP ceramics. Acta Biomater 6:4167–4174
Gu Y, Zhang J, Zhang X et al (2019) Three-dimensional printed Mg-doped β-TCP bone tissue engineering scaffolds: effects of magnesium ion concentration on osteogenesis and angiogenesis in vitro. Tissue Eng Regen Med 16:415–429
Kim SE, Shim KM, Jang K et al (2018) Three-dimensional printing-based reconstruction of a maxillary bone defect in a dog following tumor removal. In Vivo 32:63–70
Daculsi G (1998) Biphasic calcium phosphate concept applied to artificial bone, implant coating and injectable bone substitute. Biomaterials 19:1473–1478
Liu F, Liu Y, Li X et al (2019) Osteogenesis of 3D printed macro-pore size biphasic calcium phosphate scaffold in rabbit calvaria. J Biomater Appl 33:1168–1177
Chow LC, Takagi S (2001) A natural bone cement-a laboratory novelty led to the development of revolutionary new biomaterials. J Res Natl Inst Stand Technol 106:1029–1033
Yu L, Li Y, Zhao K et al (2013) A novel injectable calcium phosphate cement-bioactive glass composite for bone regeneration. PLoS One 8:e62570
Zhang C, Hu K, Liu X et al (2017) Novel hiPSC-based tri-culture for pre-vascularization of calcium phosphate scaffold to enhance bone and vessel formation. Mater Sci Eng C Mater Biol Appl 79:296–304
Välimäki VV, Aro HT (2006) Molecular basis for action of bioactive glasses as bone graft substitute. Scand J Surg 95:95–102
Moimas L, Biasotto M, Di Lenarda R et al (2006) Rabbit pilot study on the resorbability of three-dimensional bioactive glass fibre scaffolds. Acta Biomater 2:191–199
Fiume E, Barberi J, Verné E et al (2018) Bioactive glasses: from parent 45S5 composition to scaffold-assisted tissue-healing therapies. J Funct Biomater 9:24
Detsch R, Alles S, Hum J et al (2015) Osteogenic differentiation of umbilical cord and adipose derived stem cells onto highly porous 45S5 Bioglass®-based scaffolds. J Biomed Mater Res A 103:1029–1037
Vuong BX, Hiep DT (2019) Bioactive glass 58S prepared using an innovation sol-gel process. Process Appl Ceram 13:98–103
Wheeler DL, Eschbach EJ, Hoellrich RG et al (2000) Assessment of resorbable bioactive material for grafting of critical-size cancellous defects. J Orthop Res 18:140–148
El-Rashidy AA, Roether JA, Harhaus L et al (2017) Regenerating bone with bioactive glass scaffolds: a review of in vivo studies in bone defect models. Acta Biomater 62:1–28
Kolan KCR, Huang YW, Semon JA et al (2020) 3D-printed biomimetic bioactive glass scaffolds for bone regeneration in rat calvarial defects. Int J Bioprint 6:274
Liu C, Zhang H, Tang X et al (2018) Mesenchymal stem cells promote the osteogenesis in collagen-induced arthritic mice through the inhibition of TNF-α. Stem Cells Int 2018:4069032
Castillo-Cardiel G, López-Echaury AC, Saucedo-Ortiz JA et al (2017) Bone regeneration in mandibular fractures after the application of autologous mesenchymal stem cells, a randomized clinical trial. Dent Traumatol 33:38–44
Chamieh F, Collignon AM, Coyac BR et al (2016) Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci Rep 6:38814
Fahimipour F, Dashtimoghadam E, Mahdi Hasani-Sadrabadi M (2019) Enhancing cell seeding and osteogenesis of MSCs on 3D printed scaffolds through injectable BMP2 immobilized ECM-Mimetic gel. Dent Mater 35:990–1006
Blackwood KA, Bock N, Dargaville TR et al (2012) Scaffolds for growth factor delivery as applied to bone tissue engineering. Int J Polym Sci. https://doi.org/10.1155/2012/174942
Dong R, Bai Y, Dai J et al (2020) Engineered scaffolds based on mesenchymal stem cells/preosteoclasts extracellular matrix promote bone regeneration. J Tissue Eng 11:2041731420926918
Lo B, Parham L (2009) Ethical issues in stem cell research. Endocr Rev 30:204–213
Takahashi K, Tanabe K, Ohnuki M et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Xie J, Peng C, Zhao Q et al (2016) Osteogenic differentiation and bone regeneration of iPSC-MSCs supported by a biomimetic nanofibrous scaffold. Acta Biomater 29:365–379
Csobonyeiova M, Polak S, Zamborsky R et al (2017) iPS cell technologies and their prospect for bone regeneration and disease modeling: a mini review. J Adv Res 8:321–327
Kao CL, Tai LK, Chiou SH et al (2010) Resveratrol promotes osteogenic differentiation and protects against dexamethasone damage in murine induced pluripotent stem cells. Stem Cells Dev 19:247–258
Ji J, Tong X, Huang X et al (2016) Patient-derived human induced pluripotent stem cells from gingival fibroblasts composited with defined nanohydroxyapatite/chitosan/gelatin porous scaffolds as potential bone graft substitutes. Stem Cells Transl Med 5:95–105
Kang H, Shih YR, Nakasaki M et al (2016) Small molecule-driven direct conversion of human pluripotent stem cells into functional osteoblasts. Sci Adv 2:e1600691
Gorecka J, Kostiuk V, Fereydooni A et al (2019) The potential and limitations of induced pluripotent stem cells to achieve wound healing. Stem Cell Res Ther 10:87
Barkholt L, Flory E, Jekerle V et al (2013) Risk of tumorigenicity in mesenchymal stromal cell-based therapies–bridging scientific observations and regulatory viewpoints. Cytotherapy 15:753–759
Boltze J, Arnold A, Walczak P et al (2015) The dark side of the force—constraints and complications of cell therapies for stroke. Front Neurol 6:155
de la Torre GC, Goreham RV, Bech Serra JJ et al (2018) “Exosomics”—a review of biophysics, biology and biochemistry of exosomes with a focus on human breast milk. Front Genet 9:92
Nikfarjam S, Rezaie J, Zolbanin NM et al (2020) Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. J Transl Med 18:449
Lu Z, Chen Y, Dunstan C et al (2017) Priming adipose stem cells with tumor necrosis factor-alpha preconditioning potentiates their exosome efficacy for bone regeneration. Tissue Eng Part A 23:1212–1220
Zhao P, Xiao L, Peng J et al (2018) Exosomes derived from bone marrow mesenchymal stem cells improve osteoporosis through promoting osteoblast proliferation via MAPK pathway. Eur Rev Med Pharmacol Sci 22:3962–3970
Diomede F, Gugliandolo A, Cardelli P et al (2018) Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: a new tool for bone defect repair. Stem Cell Res Ther 9:104
Zhang J, Liu X, Li H et al (2016) Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res Ther 7:136
Zhang Y, Hao Z, Wang P et al (2019) Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif 52:e12570.
Kim TG, Shin H, Lim DW (2012) Biomimetic scaffolds for tissue engineering. Adv Funct Mater 22:2446–2468
Velasco MA, Narváez-Tovar CA, Garzón-Alvarado DA (2015) Design, materials, and mechanobiology of biodegradable scaffolds for bone tissue engineering. Biomed Res Int 2015:729076.
Nikolova MP, Chavali MS (2019) Recent advances in biomaterials for 3D scaffolds: a review. Bioact Mater 4:271–292
Bishop ES, Mostafa S, Pakvasa M et al (2017) 3-D bioprinting technologies in tissue engineering and regenerative medicine: current and future trends. Genes Dis 4:185–195
Khoshnood N, Zamanian A (2020) A comprehensive review on scaffold-free bioinks for bioprinting. Bioprinting 19:e00088
Ovsianikov A, Khademhosseini A, Mironov V (2018) The synergy of scaffold-based and scaffold-free tissue engineering strategies. Trends Biotechnol 36:348–357
Yamasaki A, Kunitomi Y, Murata D et al (2919) Osteochondral regeneration using constructs of mesenchymal stem cells made by bio three-dimensional printing in mini-pigs. J Orthop Res 37:1398–1408
Heo DN, Ayan B, Dey M et al (2020) 3D bioprinting of co-cultured osteogenic spheroids for bone tissue fabrication. bioRxiv. https://doi.org/10.1101/2020.06.16.155143
Goh BT, Teh LY, Tan DB et al (2015) Novel 3D polycaprolactone scaffold for ridge preservation—a pilot randomised controlled clinical trial. Clin Oral Implants Res 26:271–277
Shen C, Zhang Y, Li Q et al (2014) Application of three-dimensional printing technique in artificial bone fabrication for bone defect after mandibular angle ostectomy. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 28:300–303
Sumida T, Otawa N, Kamata YU et al (2015) Custom-made titanium devices as membranes for bone augmentation in implant treatment: clinical application and the comparison with conventional titanium mesh. J Craniomaxillofac Surg 43:2183–2188
Kihlström Burenstam Linder L, Birgersson U, Lundgren K et al (2019) Patient-specific titanium-reinforced calcium phosphate implant for the repair and healing of complex cranial defects. World Neurosurg 122:e399–e407
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Salah, M., Naini, F.B., Tayebi, L. (2022). 3D Printing and Bioprinting of Biomaterials and Bioceramic Scaffolds: Clinical Outcomes and Implications in Bone Tissue Engineering and Maxillofacial Reconstructive Surgery. In: Choi, A.H., Ben-Nissan, B. (eds) Innovative Bioceramics in Translational Medicine II. Springer Series in Biomaterials Science and Engineering, vol 18. Springer, Singapore. https://doi.org/10.1007/978-981-16-7439-6_2
Download citation
DOI: https://doi.org/10.1007/978-981-16-7439-6_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-7438-9
Online ISBN: 978-981-16-7439-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)