Abstract
Ants live in complex societies that organize their activities, as all animal societies, mostly by means of communication. While chemical communication via pheromones is ubiquitous in ants, increasing evidence points at the use of stridulatory vibrations not only as modulators of chemical communication signals but also as releasers of context-specific behaviors. Leaf-cutting ants, particularly those of the genus Atta, have extensively been investigated concerning both physiological and behavioral aspects of their vibrational communication, and they therefore provide the most comprehensive example of the use of vibrational signals as organizers of social behavior in ants. In this chapter, I summarize pioneer, early studies on signal production, response sensitivity thresholds, and function of stridulation in Atta leaf-cutting ants. I then follow with more recent laboratory and field investigations highlighting different contexts, both outside and inside the nest, in which leaf-cutting ants employ mechanical communication to coordinate their behaviors. These encompass cutting behavior and recruitment of nestmates, leaf transport, leaf processing inside the nest, nest excavation, and underground waste disposal. It will be argued that the response to stridulatory signals in leaf-cutting ants, despite their elementary and unitary character, strongly depends on the social context in which the receivers are situated.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Ant colonies are highly organized societies without central control, which rely on communication signals from different modalities for the organization of their tasks (Hölldobler and Wilson 1990). It has often been argued that vibrations are less common than other communication signals, particularly pheromones. This view may be true, yet rests on the facts that research on pheromones and particularly ant pheromones has a long tradition (Karlson and Butenandt 1959; Wilson and Bossert 1963), and also that only few studies have experimentally addressed the use of vibrational signals in ants, as compared to the bulk of literature on pheromone communication (Morgan 2008). Ants are well known to communicate via tactile displays, pheromones, and the substrate-borne components of their mechanical signals, thus making them tempting model systems for the emerging field of Biotremology (Hill and Wessel 2016). Communication via mechanical signals is in fact common in social insects (Hunt and Richard 2013), and not only new examples in different social insect species accumulated over the years but also new contexts for which the specific use and function of vibrational communication were clarified.
For ants in general, three mechanisms of sound production have been described so far: knocking or “drumming”, scratching, and stridulation (e.g., Sharp 1893; Markl and Fuchs 1972; Rohe and Rupprecht 2001), and the produced mechanical signals mediate alarm, food recruitment, interruption of mating, and may also modulate the receiver’s response to communication signals of other modalities. First reports of sound-producing ants trace back to the 1880s (Forbes 1881; Peal 1881) and first detailed morphological descriptions of both the stridulatory apparatus and chordotonal organs in ants were also published at that time (Lubbock 1877; Graber 1881). The presence of chordotonal organs and the ability to produce human-audible sounds led to the hypothesis that ants may be sensitive to both the airborne- and substrate-borne components of the produced signals. Experimental studies, however, have so far provided no evidence that ants respond to airborne vibrations (Fielde and Parker 1904; Autrum 1936; Haskins and Enzmann 1938). More recent claims that ants can hear stridulatory signals produced by nestmates as near-field sound (Hickling and Brown 2000) were challenged by calculations of the amplitude of the near-field particle oscillation around a stridulating ant, and by comparisons with the sensitivity threshold of the ant’s sensory receptors described so far. The calculated amplitude was at least 50 times lower than the sensitivity threshold, a fact that precludes the perception of near-field sounds (Roces and Tautz 2001). So far, there is no compelling evidence that ants may use airborne sounds for communication, as is the case for substrate-borne drumming (Markl and Fuchs 1972) and stridulatory vibrations (Roces et al. 1993).
Leaf-cutting ants form one of the most complex insect societies, characterized by extremely large colony sizes, marked polymorphism and task allocation among workers, and their underground agriculture, i.e., the maintenance of gardens of a symbiotic fungus that represents the main food source for the developing brood (Weber 1972; Fröhle and Roces 2009; Roces and Bollazzi 2009). To organize their social behaviors, leaf-cutting ants rely very strongly on chemical signals, particularly during recruitment to newly discovered plants (Kleineidam et al. 2007; Hölldobler and Wilson 2011). In fact, the very first ant trail pheromone chemically identified was that of the leaf-cutting ant Atta texana (Tumlinson et al. 1971), with workers showing graded responses to changes in pheromone concentration (Robinson et al. 1974). Besides chemical communication, the production of stridulatory vibrations by leaf-cutting ants was recognized already at the end of the 19th and the beginning of the twentieth century in Atta cephalotes and Atta fervens (actually Atta texana), respectively (Sharp 1893; Wheeler 1903), although their function remained initially elusive until the discovery of one of their functions, namely alarm communication (Markl 1965).
Together with Bert Hölldobler, we have reviewed several aspects of the behavioral ecology of stridulatory communication in leaf-cutting ants several years ago (Hölldobler and Roces 2001), particularly emphasizing the production of vibrational signals during foraging and their use in the context of multimodal communication. Since then, a few studies on stridulation in leaf-cutting and other fungus-growing ants have been published. It is not because of the admittedly reduced number of following studies on the topic that a review on vibrational communication in leaf-cutting ants appears worth writing. More exciting appears to be the fact that recent studies revealed the use of stridulatory signals during foraging under natural field conditions and also inside the nest during the organization of both collective nest excavation and underground disposal of colony refuse. Leaf-cutting ants emerged, as I outline in this chapter, as the ant group for which studies on vibrational communications highlighted both mechanistic aspects such as signal production and sensitivity thresholds, as well as adaptive aspects such as a number of functions of stridulatory vibrations and the context-specificity of responses to them.
2 Vibrational Communication during Foraging
2.1 Stridulation as Short-Range Recruitment Signal
Ants stridulate by raising and lowering their gasters, so that a cuticular file located on the first gastric tergite is rubbed against a scraper situated on the preceding third abdominal segment (postpetiole), whereby a series of so-called “chirps” synchronized with the gaster movements are produced (Spangler 1967). In Atta cephalotes leaf-cutting ants, a single chirp is produced while the gaster is moving up, which is much stronger than the chirp produced during its downward movement (Markl 1968; Roces et al. 1993), as observed in other fungus-growing ants of the genus Trachymyrmex (Carlos et al. 2014), and also in seed-harvester ants (Spangler 1967). Sometimes the scraper may not contact the cuticular file during the downward movement, so that no chirp would be produced (Markl 1968; Carlos et al. 2014). Each chirp is composed of a sequence of clicks that results from the impact of the scraper against each ridge of the cuticular file (pars stridens), which comprises, depending of ant body size, ca. 40–100 ridges (Markl 1968; Kermarrec et al. 1976; Carlos et al. 2014). The duration of each single upwards and downwards chirp, therefore, appears to directly depend on the number of ridges impacted on the file during the stridulatory movements, since there is so far no evidence that ants modulate the duration of each single chirp by changing the speed of their gaster movements (which is exactly what blood-sucking bugs do to produce distinct, context-specific vibrational signals by rubbing the tip of their proboscis against the groove of the prosternal file: single chirps of alarm stridulations are roughly four times longer than those of male-deterring stridulations; Roces and Manrique 1996). Nor that the observed variability in chirp duration and chirp repetition rate in leaf-cutting ants (Carlos et al. 2018), as it will be outlined below, conveys information for recipient workers.
Leaf-cutting ant workers stridulate whenever they are prevented from moving freely. This may occur, for instance, when part of the colony is confined by a cave-in of the nest: buried workers stridulate and attract nestmates, which subsequently engage in rescue digging (Markl 1965, 1967). Stridulation was therefore considered to serve primarily as an alarm and underground distress signal, and although early studies have discussed the evolution of stridulatory communication in ants for this particular context (Markl 1973), a recent detailed phylogenetical comparison regarding the presence of the stridulatory organ among arboreal and ground-nesting ant genera disproved the view that stridulation in ants first evolved as an underground signal to alert nestmates for rescue digging (Golden and Hill 2016).
In the 1990s, during my postdoctoral stage at the Department of Behavioral Physiology and Sociobiology of the University of Würzburg, headed by Bert Hölldobler, we discovered a hitherto unknown function of stridulation in ants, which also illustrated the significance of context-specific responses to communication signals: Atta cephalotes workers stridulated when they cut an attractive leaf. Nearby workers responded to the stridulatory vibrations transmitted through the plant material by orienting towards the source of the vibrations. As it often occurs in science, the discovery of stridulation during cutting was accidental. At that time, I was particularly interested in the organization of foraging behavior in leaf-cutting ants, and addressed questions related to fragment-size selection, communication signals, and the energetics of leaf-cutting (Roces and Hölldobler 1994; Roces and Lighton 1995). To characterize the mandible movements during cutting, I videotaped ants on a small rotating platform while cutting pieces of scented Parafilm as “pseudoleaves,” a simple method I developed to standardize the mechanical and chemical properties of the material to be cut (Roces 1990). Fascinated by the use of a very strong macro lens and high-speed recording, I was curious not only about the mandible movements, but also about the position of the legs and body axis while cutting, and displaced the focus of the camera lens back and forth regularly. I noted that a number of workers displayed dorsoventral motions with their gasters while cutting, with a pattern nearly identical to that performed by restrained stridulating workers. We verified that leaf-cutting ants produced stridulation signals while cutting by employing noninvasive laser-Doppler vibrometry. For that, we offered several leaves of privet individually pinned to the foraging table of a laboratory colony of Atta cephalotes, and as soon as a worker started to cut at the leaf edge, we carefully removed the leaf from the nest table and pinned it on a vibration-buffered table, while the ant continued with its cutting behavior. The laser beam was focused on a small white spot (2 mm diameter), painted on the leaf ca. 2 cm away from the cutting site. When a worker stridulated during cutting, vibrational signals were recorded on the leaf surface, which also traveled along the stem for several centimeters. They consisted of long series of chirps, similar to those observed during alarm vibrations in the same species (Markl 1968; Masters et al. 1983), repeated at a rate that varied between 2 and 20 chirps per second (Roces et al. 1993). Stridulations, markedly attenuated as compared to those recorded while cutting (Fig. 17.1), could also be recorded after the workers finished the cut and stood freely on the leaf with their loads.
Drawing of an Atta worker stridulating by moving its gaster up and down while cutting a leaf. The arrow denotes the gaster movements during signal production (top). Stridulatory signals produced by a single worker of the leaf-cutting ant Atta cephalotes engaged in cutting, recorded as velocity of the leaf’s vibration via laser-Doppler-vibrometry (bottom). Measurements were performed on the leaf surface, approximately 2 cm away from the cutting site. On the left, substrate-borne vibrations transmitted mostly through the mandibles while cutting. “Up” and “down” denote the direction of the gaster’s movement. On the right, vibrations transmitted into the substrate only through the legs, when the worker finished its cut and stood on the leaf. Drawing by Griselda Roces, with permission. Bottom part of the figure from Roces et al. (1993), modified
Not all ants stridulated while cutting. However, the probability to stridulate, yet not the repetition rate of the signal, strongly depended on the quality of the leaf being cut. When presented with leaves of two different grades of toughness, i.e., thin and thick privet leaves, which strongly differed in their acceptance, significantly more ants stridulated while cutting the more attractive thin leaves. When the quality of the two kinds of leaves was increased by coating them with sugar, almost all workers stridulated while cutting, irrespective of the different mechanical properties of the leaves (Roces et al. 1993).
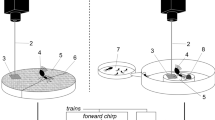
The observed relationship between leaf quality and stridulation prompted the idea that stridulations by foraging leaf-cutting ants may attract workers to the cutting site, acting therefore as short-range recruitment signals. This idea was explored in the laboratory by presenting foraging workers with a choice between two stems of privet: a “stridulating” (test) stem and a “silent” (control) one, and by recording their choice when walking towards a harvesting site. Each stem was attached to a membrane of a loudspeaker that served as a vibrator, and stridulations were played back to one of the sides, which was changed after a single worker was tested (Fig. 17.2). Putative chemical cues that could affect the workers’ choice were excluded. It was observed that significantly more ants chose the vibrating stem as compared to the silent side, clearly indicating that substrate-borne stridulatory vibrations alone, in the absence of recruitment pheromones, can act as short-range recruitment signals (Roces et al. 1993).
Set-up to present workers in the context of foraging with a binary choice between two stems of privet. Single stems could be marked with pheromones or not, depending on the posed question, and/or vibrated using play backs of either synthetic or natural stridulatory signals, which were transmitted to the vibrators. A shield in front of them precluded air movements that might disturb ants. Drawing by Griselda Roces (with permission; modified from Roces et al. 1993)
An additional line of evidence based on field studies also indicated that stridulatory vibrations alone can serve as short-range recruitment signals. The grasslands of the South American Gran Chaco are inhabited by a number of ant species. Colonies of the grass-cutting ant Atta vollenweideri build conspicuous conical or ellipsoidal-shaped mounds that are exposed to seasonal floods (Pielström and Roces 2014) and maintain a system of superficial trunk trails that lead to harvesting areas where workers mainly cut grass fragments (Röschard and Roces 2003a) for the maintenance of their underground fungus gardens. To address the question of whether workers engaged in cutting grass fragments stridulate and attract nestmates to join at the cutting site, we monitored colonies located at the Biological Reserve “El Bagual” (Formosa, Argentina) and recorded stridulations produced by workers while cutting the grass Paspalum intermedium, a densely tufted, yearlong green and perennial grass that may reach up to 100 cm in height. During foraging, workers climb on a single grass blade until reaching the grass tip, turn down and up 2–3 times after having walked very short distances, and finally start to cut across the grass width, which results in the selection of a longish fragment that is either carried directly to the nest or dropped to the ground for further transport (Röschard and Roces 2003b). It is unlikely that workers deposit a pheromone while walking up a long grass blade before the initiation of a cut, as no typical trail-marking behavior, i.e., dragging or tipping the gaster on the grass blade while walking, can be observed. By gently attaching an accelerometer to the grass blade being cut, stridulations produced by roughly 90% of workers engaged in cutting were recorded. Vibrations produced by the mechanism of stridulating traveled along the blades for distances up to 80 cm, attenuating at an average of 0.6 dB/cm. Playbacks of the recorded stridulations were alone sufficient to attract nearby workers, which readily climbed the vibrating grass blade, in a choice experiment. As in the laboratory experiments described in this section, single workers approaching a Paspalum plant adjacent to a trunk trail, i.e., those in the foraging context, were confronted with a choice between a vibrating and a silent grass blade, both experimentally removed from a distant plant and therefore not pheromone-marked. Stridulations were played back via a vibrator attached to one of the blades (Fig. 17.3; unpublished results). It was observed that significantly more ants chose the vibrating grass blade, providing the first evidence that substrate-borne stridulatory vibrations are used under natural conditions and can act as short-range recruitment signals alone, in the absence of recruitment pheromones.
Number of recruited workers of the leaf-cutting ant Atta vollenweideri that chose between a silent and a vibrating grass blade in the field, as shown in the drawing on the right upper corner. On the left, an experiment with the two grass blades silent and not marked with trail pheromone, to control for potential side biases in the setting (n = 60). On the right, non-marked grass blades were also offered, one of them vibrating using a playback of natural stridulatory signals (“V” in the drawing denotes the vibrator used for playbacks) (n = 70). Statistics: “**” indicates that the distribution of single choices differs statistically from the ratio 1:1, after a log-likelihood G-test for goodness of fit to the ratio 1:1; “ns” indicates that choices were not significantly different from the ratio 1:1. Drawing by Malu Obermayer, with permission
2.2 Stridulatory Signals in the Presence of Pheromones
The use of stridulatory signals during food recruitment is also known for other ant species, in which vibrations act as modulators of chemical signals and not as releasers of specific behaviors (Hölldobler 1999). Ants of the genera Aphaenogaster and Messor lay pheromone trails and produce stridulatory vibrations during recruitment of nestmates to attractive food sources. The chemical recruitment is enhanced by the presence of stridulatory vibrations (Markl and Hölldobler 1978; Hahn and Maschwitz 1985; Baroni-Urbani et al. 1988), but there is no evidence that vibrations alone trigger a recruitment response, as is the case in leaf-cutting ants.
The extent to which pheromones and stridulatory vibrations may act in conjunction in leaf-cutting ants was explored in the laboratory. The experimental set-up (Fig. 17.2; see Sect. 17.2.1) enabled us to combine the two communication modalities, stridulatory vibrations, and trail pheromone, and also to provide them as competing signals in a binary choice. Facing a choice between a “silent” stem marked with synthetic trail pheromone (a solution of 0.05 ng/μl 4-methylpyrrole-2-carbolylic acid in hexane, applied to produce a pheromone trail with 1 μl of the solution over 5 cm) and an unmarked “stridulating” stem, significantly more workers preferred the chemically-marked, yet silent stem (Hölldobler and Roces 2001). Similar results were obtained for an artificial trail laid with a pheromone solution diluted by one order of magnitude, which is known to still attract foragers (Robinson et al. 1974), and also for natural trails laid on the experimental stem by a single loaded forager, indicating that the use of the synthetic main component of the Atta trail pheromone does not invalidate the approach. When both stems were marked with trail pheromone but only one was vibrating, however, the “stridulating” stem was markedly preferred (Hölldobler and Roces 2001). Clearly, outgoing Atta foragers rely more on recruitment pheromones than on substrate-borne stridulations, yet the response to the recruitment pheromone is much higher when the chemical signal is combined with vibrational signals. Under natural conditions, pheromone trails may lead ants, for instance to a tree crown, but it appears unlikely that trails may end up in the close vicinity of particular leaves to be cut. Successful workers stridulating while cutting may therefore attract nearby nestmates solely via the stridulatory vibrations transmitted through the plant, which may be locally used to orient to the actual cutting site.
While the above examples refer to the combined use of vibrations and pheromones in the foraging context, the two communication modalities are also used in the alarm context (Markl 1967), in which workers release alarm pheromones and stridulate whenever they are prevented from moving freely, for instance during a nest cave-in. But even in the foraging context, alarm pheromones may be released and impact on the response of ants to stridulatory vibrations. Hager et al. (2017) recently explored the influence of an alarm pheromone on directional sensitivity to vibrations in leaf-cutting ants. They used Citral as alarm compound, a monoterpene from the mandibular gland of Atta sexdens leaf-cutting ants that deters workers (Blum et al. 1968; Roces 1994), yet the focus of their elegant study was on the mechanisms underlying the directional vibration sensing. The authors discovered that workers use time differences as small as 0.1 ms in the arrival of a vibration between contralateral legs to turn and orient to the signal source. Regarding the influence of alarm pheromone perception on directional sensitivity, ants exposed to a low dose of Citral still preferred the side from which a vibrational signal arrived first. However, ants showed no side preferences, i.e., no time-of-arrival-based directional sensitivity, for higher doses of Citral. Ants in the foraging context, therefore, appear to ignore time-based directional cues when exposed to high doses of an alarm pheromone (Hager et al. 2017). The fact that ants still showed directional sensitivity when exposed to a low dose of Citral may not necessarily indicate that vibrations and alarm pheromones serve as multimodal communication signals in this context; it is possible that such a low Citral dose is below threshold and therefore elicits no change in the expected response of workers.
We have asked a different question regarding the effect of Citral as an alarm pheromone on the ants’ response to stridulations, which extends the findings by Hager et al. (2017) outlined in this section. Namely, whether the presence of Citral changes the context from foraging to alarm and prompts workers to orient to a source of stridulatory vibrations in a choice situation. We used again the experimental choice set-up (Fig. 17.2; see Sect. 17.2.1) to present workers with a choice between stems marked with Citral that were combined with presence or absence of stridulatory vibrations, as follows. First, we explored whether foraging workers coming from the nest would orient to a source of Citral and show alarm behaviors, as expected if the presence of the Citral leads to a context change. Ants presented with a choice between two non-vibrating stems, one marked with Citral and the other non-marked, clearly preferred the marked one (Fig. 17.4, left; unpublished results). Workers ran over the marked stem with increased velocity and opened mandibles, showing that Citral was perceived and elicited a change of contexts, from foraging to alarm. Secondly, a control series with both non-vibrating stems marked with Citral evinced no side bias in the experimental set-up (Fig. 17.4, middle; unpublished results). Finally, when the two stems were marked with Citral and only one of them vibrated, workers strongly preferred the vibrating one (Fig. 17.4, right; unpublished results), which indicates that in the context of alarm, elicited by the perception of Citral, workers show clear directional alarm responses to a source of stridulatory vibrations.
Number of recruited workers of the leaf-cutting ant Atta cephalotes that chose between two stems of privet in a laboratory setting. These were marked with alarm pheromones and/or vibrated, depending on the posed question. On the left, an experiment presenting a non-marked vs. a Citral-marked stem, both silent (n = 60). In the middle, both stems were silent and marked with Citral, to control for potential side biases in the setting (n = 60). On the right, both stems were marked with Citral, and one of them vibrated via playbacks of natural stridulatory signals (n = 60). Statistics: “**” indicates that the distribution of single choices differs statistically from the ratio 1:1, after a log-likelihood G-test for goodness of fit to the ratio 1:1; “ns” indicates that choices were not significantly different from the ratio 1:1
If alarm stridulations can be released at the cutting site and so communicate danger, the question arose whether workers would be able to differentiate such alarm stridulations from cutting stridulations, i.e., those produced, for instance, by nearby workers engaged in cutting that may have not noticed the danger. So far, there is no evidence that ants can extract information from the repetition rate of stridulations, or its temporal modulation. Alarm and cutting stridulations do not differ in the structure of their single chirps, yet in their repetition rate. While alarm stridulations are monotonically repeated at almost invariant rates around 5 to 7 chirps per second (Markl 1965, 1968), cutting stridulations occur most often when the mandibles are closing, reaching rates up to 10–15 chirps/s over 2–3 seconds, and not at all in the brief pauses between the single bites (Tautz et al. 1995).
To investigate whether foraging ants may distinguish between a source of alarm stridulation and one of cutting stridulations, we used the choice set-up (Fig. 17.2; see Sect. 17.2.1) and presented workers with a choice between them, using play backs from natural stridulations. Workers showed no preference for the offered stridulations when both stems were marked with synthetic trail pheromone (Fig. 17.5, left; unpublished results). The same was the case when both stems were not marked with trail pheromone; although, a slight, yet not statistically significant tendency towards a preference for cutting stridulations can be recognized (Fig. 17.5, right; unpublished results). Direct observations provided no evidence for alarm behaviors, such as hectic runs with opened mandibles, in those ants, roughly one-half of which chose the stem with alarm vibrations, i.e., ants did not appear to have changed their context upon their perception. Taken together, the differences in the signal repetition rate between alarm and stridulatory vibrations, at least for ants in the foraging context, convey no context-specific information. Interestingly, Crematogastar ants also produce different stridulatory vibrations in the contexts of alarm and fluid feeding, and vibrations in the feeding context even differ depending on the size of the food droplet (Masoni et al. 2021). Future playback experiments focusing on the behavior of receiver ants, as discussed by the authors, will elucidate whether stridulations indeed provide contextual information and function as graded food-recruitment signals.
Number of recruited workers of the leaf-cutting ant Atta cephalotes that chose between two stems of privet in a laboratory setting. These offered playbacks of either alarm stridulations (single chirps repeated at a rate of 5–7 chirps/s) or cutting stridulations, the repetition rate of which is strongly modulated over time, reaching up to 10–15 chirps/s. See Sect. 17.2.2 for further details. On the left, both stems were marked with synthetic trail pheromone (a solution of 0.05 ng/μl 4-methylpyrrole-2-carbolylic acid in hexane, applied to produce a pheromone trail with 1 μl of the solution over 5 cm) (n = 60). On the right, the stems were not marked (n = 60). Statistics: “**” indicates that the distribution of single choices differs statistically from the ratio 1:1, after a log-likelihood G-test for goodness of fit to the ratio 1:1; “ns” indicates that choices were not significantly different from the ratio 1:1
2.3 Stridulation as Communication Signal Between Leaf Carriers and Hitchhikers
In a foraging column of leaf-cutting ants, minim workers, which are unable to cut leaf fragments because of their small body size, often “hitchhike” on leaf fragments carried by foragers back to the nest. At the cutting site and along the foraging trail, minim workers usually walk around or stand with opened mandibles near workers engaged in cutting, and often investigate loaded nestmates by briefly climbing onto the carrier and its load. It has been demonstrated that they defend loaded workers against parasitic Phorid flies that attempt to oviposit on the ants’ bodies (Eibl-Eibesfeldt and Eibl-Eibesfeldt 1967; Feener and Moss 1990).
In the laboratory, we investigated the cues used by potential hitchhikers of the leaf-cutting ant Atta cephalotes to locate leaf carriers and explored whether vibrational signals produced by carriers may attract minim workers for hitchhiking. Three different lines of evidence demonstrated that hitchhikers and leaf carriers communicate by using plant-borne stridulatory vibrations produced by the latter (Roces and Hölldobler 1995). Firstly, the repetition rate of the stridulations produced by foragers as they maneuvered the leaf fragment into the carrying position immediately before carriage, when hitchhiking usually takes place, significantly increased as compared to the rate while cutting. Even workers that did not stridulate during cutting were observed to stridulate as they loaded up the fragment. Secondly, stridulations played back on leaves, in the absence of cutting workers, were highly attractive for minim workers, which spent significantly longer times on “stridulating” than on “silent” leaves. Finally, hitchhiking occurred more often in leaf carriers that foraged on stridulating than on silent leaves, indicating that the presence of stridulations motivated minims to search for loaded workers in order to climb and to be carried back to the nest.
In Sect. 17.2.2 we indicated that even though the repetition rate of the chirps differs between alarm and cutting stridulations, foraging workers showed no preference for one of them when facing a choice. It is an open question whether the temporal distribution of the chirps during stridulations encoded information, the meaning of which could be decoded by the recipient ant. If this would be the case, the observed higher repetition rate of stridulations produced immediately after the cut in the context of hitchhiking, up to three times (Roces and Hölldobler 1995), may convey information tailored to hitchhikers, an idea that remains to be investigated.
As depicted in Fig. 17.1, stridulations produced by workers standing on a leaf can only be transmitted to the substrate through the worker’s legs, showing considerable attenuation in comparison with those transmitted mostly through the mandibles when a worker is actually cutting a leaf fragment. Their amplitudes average 20 nm, 4–5 times lower than those recorded during the cutting activity (Roces et al. 1993), yet they are above the sensitivity threshold measured electrophysiologically in leg nerves of Atta workers (1.3 nm in forelegs of minor workers, which are 4–5 times more sensitive than middle and hind legs; Markl 1970). Stridulations by walking foragers can therefore only be detected from a maximal distance of 2–3 cm. Interestingly, minim workers are on average 3–4 times more sensitive to substrate-borne vibrations than larger workers (Markl 1970), making them considerably more responsive to leg-transmitted vibrations than their larger nestmates.
2.4 Stridulation: Mechanical Support during Cutting or Communication Signal?
It has been hypothesized that stridulatory vibrations may aid soil manipulation in ants engaged in digging, by loosening aggregated soil particles while workers press their mandibles into the soil (Spangler 1973). The question arose, whether stridulations may also mechanically facilitate the cutting process, irrespective of their function as signals, in the manner of a vibrating knife that accelerates and thereby stiffens the material to be cut. To answer this question, we performed a detailed video analysis of the temporal relation between mandible movements and stridulations recorded via laser vibrometry (Tautz et al. 1995). Stridulation generated complex vibrations of the mandibles, particularly of the cutting mandible that is pulled against the leaf tissue, and which appeared to stiffen the material to be cut. Ants did not stridulate continuously; stridulation occurred most often when the cutting mandible was moved through the plant tissue. Force measurements of detached mandibles pushed against tender and tough leaves clearly indicated that vibrations facilitated a smoother cut through tender leaf tissue (Tautz et al. 1995). Interestingly, the vibratome-mode of cutting leaves inspired applied research on bionic applications for minimally invasive surgery, with the development of a surgical cutting tool with flexible ultrasonic transmission (Qiao et al. 2002); although, the feasibility of such an approach remains to be verified. A recent study on the stridulatory organ in Atta cephalotes (Yao et al. 2018), which extends and complements a very detailed, 50-years-old study on biophysical aspects of vibrational signaling in the same species (Markl 1968), provides detailed information about the mechanical properties of the file-scraper device and also theoretical arguments for a future development of bionic vibrating surgical instruments.
We have speculated, based on our results on mechanical facilitation and on the evidence that stridulations act as recruitment signals, on the evolution of stridulatory vibrations in leaf-cutting ants, arguing that stridulations may have been first used to mechanically facilitate the cutting of leaves, and subsequently may have evolved via ritualization to serve as short-range recruitment signals (Tautz et al. 1995). However, a number of observations were in conflict with the idea that the use of stridulation is related to the mechanical problem of leaf cutting. Increasing the sugar content of the leaves, for example, without changing their physical traits, i.e., increasing leaf palatability, led to a significant increase in the probability to stridulate while cutting, as indicated above (Roces et al. 1993). Secondly, tender leaves, the cutting of which is mechanically facilitated by the stridulations, are highly preferred by leaf-cutting ants anyway, and significantly more nestmates are recruited when ants harvest tender leaves (Roces and Hölldobler 1994). Admittedly, these observations could be interpreted in a converse way, as follows: leaf-cutting ants actually stridulate to attract nestmates, and the mechanical facilitation during cutting of tender leaves represents a by-product of recruitment communication.
We designed an experimental study to distinguish between these two competing hypotheses, i.e., foragers stridulate to mechanically support their cutting behavior, or the mechanical facilitation represents an epiphenomenon derived from the use of stridulation as a recruitment signal. For that, leaf-cutting ant foragers were presented with tender leaves of invariant mechanical properties, and the production of stridulation was evaluated in the following situations, which were aimed to modulate the thresholds at which recruitment communication is initiated. Firstly, leaves were smeared with plant secondary compounds to reduce their palatability. Secondly, harvesting deprivation was simulated, since lack of leaves over several days is known to increase the intensity of chemical recruitment (Roces and Hölldobler 1994). Finally, workers were given unfamiliar leaves after a period of feeding with familiar leaves. Unfamiliar leaves, when palatable, are strongly preferred by leaf-cutting ants (Cherrett 1972) and this “novelty effect” markedly increases the intensity of chemical recruitment (Roces and Hölldobler 1994). If stridulation is primarily used for communication irrespective of the mechanical support during cutting, the occurrence of stridulation would directly depend on the workers’ foraging motivation, i.e., it would be modulated by leaf palatability, colony starvation, etc., as known for chemical recruitment signals (Roces 1993; Roces and Núñez 1993).
As in the analysis of hitchhiking behavior (see Sect. 17.2.3), three different lines of evidence supported the hypothesis that leaf-cutting ants stridulate during cutting in order to recruit nestmates and that the observed mechanical facilitation of cutting represents an epiphenomenon of recruitment communication (Roces and Hölldobler 1996). Firstly, by keeping constant the mechanical properties of the leaves, and by reducing their palatability by coating them with tannin, it was shown that the number of stridulating workers decreased with decreasing leaf palatability, an observation that is inversely comparable to the positive effect on stridulation caused by a sugar coating (Roces et al. 1993). Secondly, no workers at all stridulated while cutting tender leaves after intense feeding and “harvesting satiation”, when no recruitment of nestmates appears to be necessary. The percentage of stridulating workers increased over the period of harvesting deprivation, reaching its maximum of 100% five days after the last feeding event. Thirdly, whatever kind of leaves used to initially feed the colony until satiation, significantly more workers stridulated when cutting unfamiliar leaves of similar physical features. Such a novelty effect caused by attractive, unfamiliar leaves strongly supports the idea that stridulation is produced as a recruitment signal, independent of the leaves’ mechanical properties, and that the observed mechanical facilitation during cutting is likely an epiphenomenon of recruitment communication (Roces and Hölldobler 1996).
3 Vibrational Communication inside the Nest
3.1 Stridulatory Signals and the Organization of Collective Digging
Leaf-cutting ants of the genus Atta excavate the largest and likely most complex nests among ants (Jonkman 1980; Moreira et al. 2004; Bollazzi et al. 2012), with several thousands of underground chambers, mostly for rearing their fungus garden, and for waste disposal. The external nest mound does not solely result from a passive accumulation of the excavated soil, because workers import material to reinforce and stabilize the construction, and in some species, they also build structures on the top of several central nest openings that function as ventilation turrets (Kleineidam et al. 2001). Both the underground cavities and the turrets are structures that emerge from collective digging and building activities that are decentrally organized.
How individual ants coordinate their activities to create functional structures is poorly understood, and only a few studies have experimentally addressed the mechanisms underlying the organization of collective building in leaf-cutting ants (Bollazzi and Roces 2007, 2010; Fröhle and Roces 2009; Cosarinsky and Roces 2012; Römer and Roces 2014, 2015; Halboth and Roces 2017).
One well-known mechanism involved in the organization of collective responses in social insects is the stigmergy, a term coined by Grassé (1959) during his pioneer studies on nest building in termites. Stigmergy describes the process by which an animal responds to the product of earlier building work done by nestmates, without the need of a worker-worker interaction, and its response then amplifies the former stimulus that subsequently triggers a stronger response, and so on. As such, stigmergy represents a form of indirect communication among workers via the structure being built, without the involvement of communication signals.
While stigmergic responses may occur during nest building in leaf-cutting ants, we asked whether communication via vibrational signals mediated the organization of their collective digging. Steffen Pielström, a brilliant doctoral student in my lab, explored the occurrence of stridulation in digging workers of the leaf-cutting ant Atta vollenweideri. He designed a number of ingenious laboratory experiments to investigate whether stridulatory vibrations guide workers when searching for a site to initiate their excavations, and if workers in the social context of nest enlargement stridulate depending on their space demands and potential needs for additional recruitment of nestmates. He discovered that beyond the use of vibrational signals in the contexts of food recruitment and alarm communication (see Sects. 17.2.1 and 17.2.2), leaf-cutting ant workers stridulate while engaged in digging and attract nestmates to join excavation activity at the site (Pielström and Roces 2012; commented by Sendova-Franks 2012).
It was observed that isolated workers readily stridulated while excavating in moist soil, producing chirps not only while actually working the material with their mandibles, but also in advance, before they started to mechanically interact with the soil (Pielström and Roces 2012). When leaving the site carrying a soil pellet, most workers ceased stridulating. Signals produced by single workers were, as expected, damped over distance, and the measured attenuation rate suggested that signals might be detected up to a distance of 6 cm. The possibility that digging stridulation is used as recruitment signal to attract other workers was explored in a choice experiment offering different locations where workers may initiate digging. Results clearly indicated that workers were more likely to dig close to a source of stridulatory vibrations than at alternative sites, with a probability that positively correlated with the intensity of the vibrations produced at that location (Pielström and Roces 2012).
In the social context of nest enlargement, workers excavating a tunnel that were allowed to suddenly break into an existing chamber gradually discontinued their signal production when nest space was available; although, the removal of excavated soil pellets continued at a constant rate for at least 3 hours after the decrease in stridulation rate. It is unclear whether excavation continued while workers decreased their stridulation rate, or whether digging activity decreased yet soil transport continued because of prior accumulation of soil pellets inside the chamber (Pielström and Roces 2012). In any case, stridulations recorded in the social context of regular nest excavation to relocate and maintain the symbiotic fungus support the idea that they are used under natural conditions to coordinate, and likely control, the dynamics of collective nest building.
To investigate whether the stridulation by digging workers may be related to the mechanical properties of the material to be removed, stridulations produced by Atta vollenweideri workers digging in soils of different moistures were recorded (Pielström and Roces 2014). It was observed that stridulation rates were slightly yet significantly lower, the higher the soil water content. It is unclear whether the observed decrease in repetition rates has a communicative meaning, or it occurs because of a different handling of moist materials by digging workers. As discussed in Sects. 17.2.2 and 17.2.3, the repetition rate of stridulatory vibrations appears to convey no specific information, at least in the foraging context, which makes it unlikely that the slight differences in repetition rates between dry and moist soils have a meaning for recruited workers. Regarding a potential mechanical aid during excavation, as suggested by Spangler (1973) for seed-harvester ants, we discussed different possible interpretations for the observed relationship between soil moisture and stridulation rates (Pielström and Roces 2014). Firstly, if we assume that workers may adjust their stridulatory behavior based on the actual mechanical properties of the soil, lower stridulation rates in moist soils, with poor transmission properties, may be adaptive because workers would stridulate less when the environment is poorly suitable for vibrational recruitment. Conversely, high stridulation rates in dry soils could be a reaction to materials harder to excavate in, so that workers may increase their efforts to recruit additional workers. Finally, the effect of soil moisture on stridulatory behavior could also be interpreted without any communicative function, i.e., excavation of dryer, harder soils would require more stridulatory vibrations to mechanically support the removal of soil particles, a possibility that cannot be ruled out and remains, as the other possibilities, as an open question for future research (Pielström and Roces 2014).
Taken together, stridulation signals produced while digging are used for communication as short-range recruitment signals that attract nestmates, which react by digging close to stridulating ants and therefore amplify the digging process (Pielström and Roces 2012). Results revealed a new context in which stridulations are used by leaf-cutting ants, and provided the first experimental evidence that communication signals are used to spatially organize collective nest building in social insects.
3.2 Stridulatory Signals and Underground Waste Disposal
Fungus cultivation by colonies of leaf-cutting ants produces copious amounts of waste, which is mostly composed of decaying fungus and plant material, discarded leaf fragments, and dead ants. There are two species-specific modes of waste disposal in leaf-cutting ants, either outside the nest in above ground piles, or in underground chambers. Colonies of Atta laevigata dispose of their waste in large underground chambers, with workers showing preferred values of temperature and air humidity across the soil profile, yet not of CO2 levels, for the deposition of their waste (Römer et al. 2019). Once the first waste particles are disposed of at a suitable underground free space, for instance inside a tunnel end or an empty cavity, workers that are carrying additional waste particles orient towards waste volatiles and drop their loads at the site in a stigmergic response (Römer and Roces 2019), resulting in the deposition and accumulation of large amounts of refuse at a single location.
The task of underground waste disposal is expected to be tightly associated with the task of digging, which creates empty space for waste deposition. Free space may trigger waste deposition at the site, causing a concomitant reduction in space that may prompt workers to dig and generate additional space. Such a hypothetical regulatory feedback loop may control the final size of the waste chamber, as it is known for the control of the size of fungus chambers in leaf-cutting ants (Fröhle and Roces 2009; Römer and Roces 2014, 2015). The question arose, whether waste-carrying workers may also locate a dumpsite to drop their loads by responding to the stridulatory vibrations produced by workers engaged in excavation, besides the use of environmental and olfactory cues for orientation.
Baris Düdükcü, a bright Master’s student in my lab, recently investigated the organization of underground waste disposal by the leaf-cutting ant Atta laevigata, focusing on the question of whether waste-carrying workers orient towards a digging site and drop their waste particles there because of the presence of stridulatory vibrations produced by digging workers (Düdükcü 2018). In laboratory colonies, waste removal was triggered by initially adding waste particles in the close vicinity of a fungus garden. Workers readily picked up waste particles and walked towards a deposition site in an empty plastic chamber, the base of which offered a binary choice between one side providing stridulatory vibrations, and the other side being silent. The base of the deposition chamber consisted of two adjacent, identical plastic plates separated by a thin segment of rubber, which prevented vibrations from one side from traveling to the adjacent side. Below each side of the deposition chamber, an empty box, which served as a control, or a box partially filled with moist clay as digging chamber, were tightly attached. Workers from a separate fungus chamber gained access to the covered box containing clay and initiated digging there, with their stridulations being transmitted through the box walls to the “upper floor”, i.e., to the base of the deposition chamber. Consequently, the deposition chamber represented for waste-carrying workers a uniform empty space, yet one side of its base vibrated, and the other side was silent. By scoring individual choices made only by the first waste-loaded workers in independent assays, to avoid social influences and stigmergic responses, Düdükcü (2018) showed that significantly more depositions of waste particles, approximately 90% of them, occurred on the side of the deposition chamber where stridulations were provided. Stridulations evidently attracted waste-loaded workers and appear therefore to help coordinate the activities of workers engaged in two associated tasks, namely digging and waste disposal, thus providing evidence for their function in a hitherto unknown context inside the underground nest.
4 Behavioral Contexts and the Evolution of Stridulatory Communication in Leaf-Cutting Ants
Since the pioneer and detailed studies on stridulation in leaf-cutting ants by Markl (1965, 1967, 1968, 1970), it has been hypothesized that stridulation in ants first evolved as an underground alarm signal to attract nestmates for rescue digging (Markl 1973). Markl’s hypothesis was based on a correlative study of nesting ecology, mode of colony foundation, and presence of a stridulatory organ in workers of all ant subfamilies. It is important to indicate that stridulation is not ubiquitous among ants; it is only found in ants belonging to four (out of 17) subfamilies: Myrmeciinae, Myrmicinae, Ponerinae, and Pseudomyrmecinae. By studying 1354 ant species belonging to 205 genera, Markl (1973) argued that a hypothesis correlating the evolution of stridulation production with nesting ecology in ants may explain major patterns of the occurrence of the stridulatory organ in ants, yet he recognized that many exceptions did not fit into the outlined arguments, and therefore advanced his arguments as a working hypothesis. As indicated in Sect. 17.2.1, a recent detailed phylogenetic study comparing the occurrence of stridulatory organs in arboreal and ground-dwelling ants did not provide supportive evidence for the hypothesis that stridulation in ants first evolved as an underground signal to alert nestmates for rescue digging. Workers of a large proportion of ant genera considered primarily arboreal have a stridulatory organ, and those from a number of completely subterranean ant genera possess no organ (Golden and Hill 2016).
The above arguments make it therefore unlikely that the stridulatory organ in ants first evolved to mechanically facilitate digging, without any communicative function, as proposed by Spangler (1973). Not only do leaf-cutting ants stridulate while digging for communicative purpose (see Sect. 17.3.1; Pielström and Roces 2012, 2014), but also Solenopsis fire ants were observed to stridulate during nest excavation in the laboratory (Rauth and Vinson 2006). Although different functions were hypothesized for the stridulations of fire ants (i.e., the mechanical facilitation and compression of soil particles, the potential assessment of the tunnel wall thickness, much like an acoustic imaging tool does, and communication to attract nestmates to the digging site; Rauth and Vinson 2006), no one of these has so far been experimentally demonstrated.
Besides the context of alarm and rescue digging, stridulation in leaf-cutting ants is used in several additional contexts to attract nestmates to the sender ant, as described in this chapter, and may therefore serve different functions. We have demonstrated that in Atta leaf-cutting ants, stridulations are used as a short-range recruitment signal, both under controlled laboratory conditions and in the field, and that trail and alarm pheromones modulate the response of receiver ants to them. In addition, stridulation produced by leaf carriers serves as a communication signal to attract minor nestmates as hitchhikers. Inside the nest, leaf-cutting ants stridulate while digging and attract nestmates to the site, and waste carriers orient to stridulating nestmates to find digging sites to dispose of their waste particles. In the alarm context, Attine ants (genus Trachymyrmex) were observed to stridulate when attacked by army ants (LaPolla et al. 2002), and newly-mated queens of the leaf-cutting ant Acromyrmex striatus appear to stridulate while walking on the floor searching for a suitable place to initiate the founding of a nest (Diehl-Fleig and Lucchese 1992), perhaps as aposematic signaling to deter potential predators (Masters 1979). Finally, leaf-cutting ants also stridulate inside the nest during further cutting, licking, and shredding of the collected plant tissue to be incorporated into their fungus gardens. The signals recorded on the incorporated plant fragments during those processes showed high variability in both signal amplitude and repetition rates. Considering that only a few records of single workers stridulating while performing a given task were obtained, and that in most of them 3–5 workers processed the leaf fragment at the same time and may have stridulated simultaneously, it appears difficult to correlate a given signal amplitude with a specific behavior, as aimed by the authors (Carlos et al. 2018). Understanding the use of stridulatory vibrations in the context of leaf processing and fungus tending remains a challenge for future research.
In the foraging context, it has recently been reported that leaf-cutting ants appear to use their vibrational sense to detect substrate-borne waves produced by the impact of rain droplets on the substrate, and upon perception of such “rain signals,” walk faster to the nest in order to avoid losing the carried fragments because of potential wetting (Farji-Brener et al. 2018). This behavior is intriguing, since loaded ants returning to the nest are expected to link the perception of likely varying substrate-borne vibrations with a specific threat, such as the rain, not experienced directly. In field colonies, researchers monitored the speed of single loaded workers before, during, and after a simulation of rainfall generated by dropping water from a watering can onto a large leaf, the stem of which was buried 10 cm beside the foraging trail. It was assumed, yet not measured, that the vibrations generated on the leaf surface would travel through both the leaf stem and the soil, and be perceived as substrate-borne vibrations by workers on their way to the nest. Intriguingly, loaded workers increased their walking speed up to 30%, yet not during the actual simulation of rainfall, but thereafter. Since ants typically respond to disturbances with increases in walking speed, it remains an open question whether the observed increase in speed after the simulation represented a specific response aimed at returning earlier to the nest to avoid rainfall, or a non-specific alarm response triggered by the vibrations. Regrettably, measurements were not validated, for instance with control records of walking speed, in workers confronted with vibrational stimuli not related to rainfall, such as alarm stridulations, or in workers before and during actual rainfalls. A crucial control experiment would have also recorded putative changes in speed of unloaded workers running towards the foraging patch, and not to the nest. If outgoing workers respond to vibrations as indicators of rain, they would either turn back to the nest or maintain, but not necessarily increase, their speed toward the foraging patch. A potential increase in speed, on the other hand, would indicate that the perceived vibrations caused an unspecific disturbance. In absence of such controls, we cannot be certain that leaf-cutting ant foragers perceive substrate-borne vibrations as an indicator of an actual rain, as argued by the authors. Leaf-cutting ants do indeed behave anticipatory to rainfalls, for instance by increasing their foraging rates, yet as a direct response to a decrease in barometric pressure, which often drops before rains (Sujimoto et al. 2019).
In trying to outline the scenario in which the use of stridulation may have evolved, we had postulated that stridulation in leaf-cutting ants first evolved to mechanically support leaf-cutting, and subsequently as a communication signal (Tautz et al. 1995). Such a hypothesis was particularly tempting, yet like many evolutionary hypotheses, it was based on a plausible correlation rather than experimental proof. If we argue that the use of stridulation as a mechanical aid has been favored during evolution, stridulations should provide benefits for instance as a reduction of the time- and/or energy-costs of cutting, since leaf cutting is an energetically very expensive behavior. Even though the mandibular vibrations generated by stridulation reduce force fluctuations during the cut of tender leaves, the total force employed remained the same with or without vibrations, and stridulating ants did not cut leaves for instance faster than non-stridulating ants (Tautz et al. 1995). In addition, the metabolic rate of workers cutting tender leaves was similar for stridulating and non-stridulating ants (Roces and Hölldobler 1996). It is therefore unlikely that any potential energetic improvement (due to stridulation) on cutting mechanics would significantly decrease the impressive costs of leaf-cutting. The metabolic rate of the mandibular muscles measured during leaf cutting is extremely high (leaf-cutting costs are 31-times higher than costs of basal metabolism), and it approaches that for the insect flight muscle, the metabolically most active animal tissue known (Roces and Lighton 1995). Based on these arguments and experimental evidence, we later rejected our original hypothesis that stridulatory communication signals derived from a vibratory mechanism that mechanically aided the process of leaf-cutting, and hypothesized that ants stridulate to attract nestmates, with the mechanical facilitation of cutting being a byproduct of recruitment communication (Roces and Hölldobler 1996).
Stridulation in leaf-cutting ants is employed in a number of different contexts, yet the evolution of its function remains elusive. The early discovery that buried Atta workers stridulate and attract nestmates for rescue digging when part of the colony is confined by a cave-in of the nest (Markl 1965) led to the arguments that vibrational signals used in alarm and defense may have later acquired a function as a recruitment signal in the foraging context. Considering that leaf-cutting ants also stridulate during nest excavation (Pielström and Roces 2012), equally likely would be the hypothesis that the original function of stridulation was a communicative one to organize underground nest-building activities. Interestingly, the idea that stridulation may be fundamental to organizing collective ant behaviors inside the nest was advanced roughly 120 years ago by Wheeler (1903), who argued: “Even more remarkable is the stridulation in a colony of Atta fervens (= texana), the Texan’ leaf-cutting ant.” And further added: “The contact-odor sense, important as it undoubtedly is, must obviously have its limitations in the dark subterranean cavities in which the ants spend so much of their time, especially when the nests are very extensive like those of Atta. Under such conditions stridulation and hearing must be of great service in maintaining the integrity of the colony and its excavations.” Hopefully, additional studies on the use of stridulatory vibrations in leaf-cutting and other ants may uncover additional contexts in which these rather elementary communication signals are used, explore their use as modulatory signals that lower the response threshold for other stimuli, and outline a scenario to understand their evolution.
References
Autrum H (1936) Über Lautäusserungen und Schallwahrnehmung bei Arthropoden 1. Untersuchungen an Ameisen. Eine allgemeine Theorie der Schallwahrnehmung bei Arthropoden. Z vergl Phys 23:332–373
Baroni-Urbani C, Buser MW, Schilliger E (1988) Substrate vibration during recruitment in ant social organization. Insect Soc 35:241–250
Blum MS, Padovani F, Amante E (1968) Alkanones and terpenes in the mandibular glands of Atta species (hymenoptera: Formicidae). Comp Biochem Physiol 26:291–299
Bollazzi M, Roces F (2007) To build or not to build: circulating dry air organizes building responses for climate control in the leaf-cutting ant Acromyrmex ambiguus. Anim Behav 74:1349–1355
Bollazzi M, Roces F (2010) Control of nest water losses through building behavior in leaf-cutting ants (Acromyrmex heyeri). Insect Soc 57:267–273
Bollazzi M, Forti L, Roces F (2012) Ventilation of the giant nests of Atta leaf-cutting ants: does underground circulating air enter the fungus chambers? Insect Soc 59:487–498
Carlos AA, Barbero F, Casacci LO, Bonelli S, Bueno OC (2014) Bioacoustics of Trachymyrmex fuscus, Trachymyrmex tucumanus, and Atta sexdens rubropilosa (hymenoptera: Formicidae). J Acoust Soc Am 136:2074–2074
Carlos AA, Diniz EA, Verza da Silva S, Bueno OC (2018) Vibration of the plant substrate generated by workers’ stridulation during fungus garden cultivation in Atta laevigata (smith) (hymenoptera: Formicidae). EntomoBrasilis 11:107–112
Cherrett JM (1972) Chemical aspects of plant attack by leaf-cutting ants. In: Harborne JB (ed) Phytochemical ecology. Academic Press, London, pp 13–24
Cosarinsky MI, Roces F (2012) The construction of turrets for nest ventilation in the grass-cutting ant Atta vollenweideri: import and assembly of building materials. J Insect Behav 25:222–241
Diehl-Fleig E, Lucchese MEP (1992) Nest foundation by Acromyrmex striatus (hymenoptera, Formicidae). In: Billen J (ed) Biology and evolution of social insects. Leuven University Press, Leuven, pp 51–54
Düdükcü B (2018) Wie graben Blattschneiderameisen ihre Kammern? Untersuchungen zum kontextabhängigen Grabeverhalten. Master Thesis, University of Würzburg, Germany
Eibl-Eibesfeldt I, Eibl-Eibesfeldt E (1967) Das Parasitenabwehren der Minima-Arbeiterinnen der Blattschneider-Ameise (Atta cephalotes). Z Tierpsych 24:278–281
Farji-Brener AG, Dalton MC, Balza U, Courtis A, Lemus-Domínguez I, Fernández-Hilario R (2018) Working in the rain? Why leaf-cutting ants stop foraging when it’s raining. Insect Soc 65:233–239
Feener DH Jr, Moss KAG (1990) Defense against parasites by hitchhikers in leaf-cutting ants: a quantitative assessment. Behav Ecol Sociobiol 26:17–29
Fielde AM, Parker GH (1904) The reactions of ants to material vibrations. Proc Acad Nat Sci Phila 56:642–650
Forbes HO (1881) Sound-producing ants. Nature 24:101–102
Fröhle K, Roces F (2009) Underground agriculture: the control of nest size in fungus-growing ants. In: Theraulaz G, Solé R, Kuntz P (eds) From insect nests to human architecture – workshop on engineering principles of innovation in swarm-made architectures. European Centre for Living Technology, Venice, pp 95–104
Golden TMJ, Hill PSM (2016) The evolution of stridulatory communication in ants, revisited. Insect Soc 63:309–319
Graber V (1881) Die chordotonalen Sinnesorgane und das Gehör der Insecten. Arch Mikrosk Anat 20:506–640
Grassé P-P (1959) La reconstruction du nid et les coordinations interindividuelles chez Bellicositermes natalensis et Cubitermes sp. La théorie de la stigmergy: Essai d’interprétation du comportement des termites constructeurs. Insect Soc 6:41–84
Hager FA, Kirchner L, Kirchner WH (2017) Directional vibration sensing in the leafcutter ant Atta sexdens. Biol Open 6:1949–1952
Hahn M, Maschwitz U (1985) Foraging strategies and recruitment behaviour in the European harvester ant Messor rufitarsis (F.). Oecologia 68:45–51
Halboth F, Roces F (2017) The construction of ventilation turrets in Atta vollenweideri leaf-cutting ants: carbon dioxide levels in the nest tunnels, but not airflow or air humidity, influence turret structure. PLoS One 12:e0188162
Haskins CP, Enzmann EV (1938) Studies of certain sociological and physiological features in the Formicidae. Ann N Y Acad Sci 37:97–162
Hickling R, Brown RL (2000) Analysis of acoustic communication by ants. J Acoust Soc Am 108:1920–1929
Hill PSM, Wessel A (2016) Primer – Biotremology. Curr Biol 26:R187–R191
Hölldobler B (1999) Multimodal signals in ant communication. J Comp Phys A 184:129–141
Hölldobler B, Roces F (2001) The behavioral ecology of stridulatory communication in leafcutting ants. In: Dugatkin LS (ed) Model Systems in Behavioral Ecology - integrating conceptual, theoretical, and empirical approaches. Princeton University Press, Princeton, pp 92–109
Hölldobler B, Wilson EO (1990) The ants. Belknap Press, Harvard University Press, Cambridge
Hölldobler B, Wilson EO (2011) The leafcutter ants – civilization by instinct. Norton & Company, New York
Hunt JH, Richard FJ (2013) Intracolony vibroacoustic communication in social insects. Insect Soc 60:403–417
Jonkman JCM (1980) The external and internal structure and growth of nests of the leaf-cutting ant Atta vollenweideri Forel, 1893 (Hym.: Formicidae). Part II. The internal nest structure and growth. Z ang Ent 89:217–246
Karlson P, Butenandt A (1959) Pheromones (ectohormones) in insects. Annu Rev Entomol 4:39–58
Kermarrec A, Mauléon H, Antun AA (1976) La stridulation de Acromyrmex octospinosus Reich. (Formicidae, Attini): biométrie de l’appareil stridulateur et analyse du signal produit. Insect Soc 23:29–47
Kleineidam C, Ernst R, Roces F (2001) Wind-induced ventilation in the giant nests of the leaf-cutting ant Atta vollenweideri. Naturwissenschaften 88:301–305
Kleineidam C, Rössler W, Hölldobler B, Roces F (2007) Perceptual differences in trail-following leaf-cutting ants relate to body size. J Insect Physiol 53:1233–1249
LaPolla JS, Mueller UG, Mueller M, Seid M, Cover S (2002) Predation by the army ant Neivamyrmex rugulosus on the fungus-growing ant Trachymyrmex arizonensis. Insect Soc 49:251–256
Lubbock J (1877) On some points in the anatomy of ants. Mon Microsc J 18:121–142
Markl H (1965) Stridulation in leaf-cutting ants. Science 149:1392–1393
Markl H (1967) Die Verständigung durch Stridulationssignale bei Blattschneiderameisen. I. Die biologische Bedeutung der Stridulation. Z vergl Physiol 57:299–330
Markl H (1968) Die Verständigung durch Stridulationssignale bei Blattschneiderameisen. II. Erzeugung und Eigenschaften der Signale. Z vergl Physiol 60:103–150
Markl H (1970) Die Verständigung durch Stridulationssignale bei Blattschneiderameisen. III. Die Empfindlichkeit für Substratvibrationen. Z vergl Physiol 69:6–37
Markl H (1973) The evolution of stridulatory communication in ants. In: Proceedings VII Congress IUSSI, London, pp 258–265
Markl H, Fuchs S (1972) Klopfsignale mit Alarmfunktion bei Rossameisen (Camponotus, Formicidae, hymenoptera). Z vergl Physiol 76:204–255
Markl H, Hölldobler B (1978) Recruitment and food-retrieving behavior in Novomessor (Formicidae, hymenoptera). II: vibration signals. Behav Ecol Sociobiol 4:183–216
Masoni A, Frizzi F, Nieri R, Casacci LP, Mazzoni V, Turrilazzi S, Santini G (2021) Ants modulate stridulatory signals depending on the behavioral context. Sci Rep 11:5933
Masters WM (1979) Insect disturbance stridulation: its defensive role. Behav Ecol Sociobiol 5:187–200
Masters WM, Tautz J, Fletcher NH, Markl H (1983) Body vibration and sound production in an insect (Atta sexdens) without specialized radiating structures. J Comp Physiol A 150:239–249
Moreira AA, Forti LC, de Andrade APP, Boaretto MAC, Lopes JFS (2004) Nest architecture of Atta laevigata (F. smith, 1858) (hymenoptera: Formicidae). Stud Neotropical Fauna Environ 39:109–116
Morgan ED (2008) Chemical sorcery for sociality: exocrine secretions of ants (hymenoptera: Formicidae). Myrmecol News 11:79–90
Peal SE (1881) Sound-producing ants. Nature 24:484
Pielström S, Roces F (2012) Vibrational communication in the spatial organization of collective digging in the leaf-cutting ant Atta vollenweideri. Anim Behav 84:743–752
Pielström S, Roces F (2014) Soil moisture and excavation behaviour in the Chaco leaf-cutting ant (Atta vollenweideri): digging performance and prevention of water inflow into the nest. PLoS One 9(4):e95658
Qiao F, Roces F, Schilling C, Wurmus H (2002) Cutting with flexible ultrasound transmission for minimally invasive surgery with biological inspiration. In: Actuator 2002. Bremer Actuator, Bremen, pp 668–671
Rauth SJ, Vinson SB (2006) Colony wide behavioral contexts of stridulation in imported fire ants (Solenopsis invicta Buren). J Insect Behav 19:293–304
Robinson SW, Moser JC, Blum MS, Amante E (1974) Laboratory investigations of the trail-following responses of four species of leaf-cutting ants with notes on the specificity of a trail pheromone of Atta texana (Buckley). Insect Soc 21:87–94
Roces F (1990) Leaf-cutting ants cut fragment sizes in relation to the distance from the nest. Anim Behav 40:1181–1183
Roces F (1993) Both evaluation of resource quality and speed of recruited leaf-cutting ants (Acromyrmex lundi) depend on their motivational state. Behav Ecol Sociobiol 33:183–189
Roces F (1994) Odour learning and decision-making during food collection in the leaf-cutting ant Acromyrmex lundi. Insect Soc 41:235–239
Roces F, Bollazzi M (2009) Information transfer and the organization of foraging in grass- and leaf-cutting ants. In: Jarau S, Hrncir M (eds) Food exploitation by social insects: ecological, behavioral, and theoretical approaches. CRC Press: Contemporary Topics in Entomology Series, Boca Raton, FL, pp 261–275
Roces F, Hölldobler B (1994) Leaf density and a trade-off between load-size selection and recruitment behavior in the ant Atta cephalotes. Oecologia 97:1–8
Roces F, Hölldobler B (1995) Vibrational communication between hitchhikers and foragers in leaf-cutting ants (Atta cephalotes). Behav Ecol Sociobiol 37:297–302
Roces F, Hölldobler B (1996) Use of stridulation in foraging leaf-cutting ants: mechanical support during cutting or short-range recruitment signal? Behav Ecol Sociobiol 39:293–299
Roces F, Lighton JRB (1995) Larger bites of leaf-cutting ants. Nature 373:392–393
Roces F, Manrique G (1996) Different stridulatory vibrations during sexual behaviour and disturbance in the blood-sucking bug Triatoma infestans (Hemiptera: Reduviidae). J Insect Physiol 42:231–238
Roces F, Núñez JA (1993) Information about food quality influences load-size selection in recruited leaf-cutting ants. Anim Behav 45:135–143
Roces F, Tautz J (2001) Ants are deaf. J Acoust Soc Am 109:3080–3082
Roces F, Tautz J, Hölldobler B (1993) Stridulation in leaf-cutting ants: short-range recruitment through plant-borne vibrations. Naturwissenschaften 80:521–524
Rohe W, Rupprecht R (2001) Klopfen und Kratzen als Alarmsignale bei westmalayischen Drüsenameisen (Hymenoptera: Formicidae: Dolochoderinae). Entomol Gen 25:81–96
Römer D, Roces F (2014) Nest enlargement in leaf-cutting ants: relocated brood and fungus trigger the excavation of new chambers. PLoS One 9(5):e97872
Römer D, Roces F (2015) Available space, symbiotic fungus and colony brood influence excavation and lead to the adjustment of nest enlargement in leaf-cutting ants. Insect Soc 62:401–413
Römer D, Roces F (2019) Waste deposition in leaf-cutting ants is guided by olfactory cues from waste. Sci Nat 106:3
Römer D, Bollazzi M, Roces F (2019) Leaf-cutting ants use relative humidity and temperature but not CO2 levels as cues for the selection of an underground dumpsite. Ecol Entomol 44:502–511
Röschard J, Roces F (2003a) Fragment-size determination and size-matching in the grass-cutting ant Atta vollenweideri depend on the distance from the nest. J Trop Ecol 19:647–653
Röschard J, Roces F (2003b) Cutters, carriers and transport chains: distance-dependent foraging strategies in the grass-cutting ant Atta vollenweideri. Insect Soc 50:237–244
Sendova-Franks A (2012) Vibrational communication in digging ants – in focus. Anim Behav 84:739–741
Sharp D (1893) On stridulation in ants. Trans Entomol Soc London 1893:199–213
Spangler HG (1967) Ant stridulations and their synchronization with abdominal movement. Science 155:1687–1689
Spangler HG (1973) Vibration aids soil manipulation in hymenoptera. J Kansas Entomol Soc 46:157–160
Sujimoto FR, Costa CM, Zitelli CHL, Bento JMS (2019) Foraging activity of leaf-cutter ants is affected by barometric pressure. Ethology 126:290–296
Tautz J, Roces F, Hölldobler B (1995) Use of a sound-based vibratome by leaf-cutting ants. Science 267:84–87
Tumlinson JH, Silverstein RM, Moser JC, Brownlee RG, Ruth JM (1971) Identification of the trail pheromone of a leaf-cutting ant, Atta texana. Nature 234:348–349
Weber NA (1972) Gardening ants - the Attines. The American Philosophical Society, Philadelphia
Wheeler WM (1903) Ethological observations on an American ant (Leptothorax emersoni Wheeler). J Psychol Neurol 2:64–78
Wilson EO, Bossert WH (1963) Chemical communication among animals. Recent Prog Hormone Res 19:673–716
Yao G, Feng L, Zhang D, Jiang X (2018) Morphology and mechanical properties of vibratory organs in the leaf-cutting ant (Atta cephalotes). J Bionic Engin 15:722–730
Acknowledgments
I initiated my studies on vibrational communication in leaf-cutting ants in 1992 as a postdoc fellow at the Department of Behavioral Physiology, Biocenter, University of Würzburg. I am deeply indebted to Bert Hölldobler for generous support, encouragement, and discussions over the years, and for having established a very active, diverse, and highly reputed department focusing on social insect research, in which I was free to develop my own projects as a young researcher. I also thank Jürgen Tautz for having introduced me to the methods of bioacoustics. I am particularly indebted to my former Ph.D. student Steffen Pielström, who discovered and explored the amazing use of vibrational signals during the organization of collective nest digging in leaf-cutting ants, and for his comments on the manuscript. I also thank Baris Düdükcü, who discovered the significance of stridulations by digging workers for the spatial organization of underground waste disposal, and for his comments. Thanks are also due to Annette Laudahn and Adrienne Gerber-Kurz for maintenance of the laboratory colonies, to my sister Griselda Roces for the beautiful drawings presented in the first two figures of this chapter, to Rainer Christian Rosenbaum for helping with the layout of the first figure, and to Malu Obermayer for the nice ant drawing of Fig. 17.3. Our own field work reported here was performed at the Reserva Ecológica El Bagual (Alparamis SA - Aves Argentinas) in eastern Chaco, Province of Formosa, Argentina. I am very much indebted to the ornithologist Alejandro G. Di Giacomo, his field assistants, and especially the Götz family for providing facilities at the Biological Station, daily help during our stays and invaluable logistical support throughout years of ant research. Financial support was provided by the DAAD and the DFG, Germany.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Roces, F. (2022). Vibrational Communication Outside and Inside the Nest in Leaf-Cutting Ants. In: Hill, P.S.M., Mazzoni, V., Stritih-Peljhan, N., Virant-Doberlet, M., Wessel, A. (eds) Biotremology: Physiology, Ecology, and Evolution. Animal Signals and Communication, vol 8. Springer, Cham. https://doi.org/10.1007/978-3-030-97419-0_17
Download citation
DOI: https://doi.org/10.1007/978-3-030-97419-0_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-97418-3
Online ISBN: 978-3-030-97419-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)