Abstract
Nutrient fertilization and use of pesticides in agriculture aid in the improvement of crop productivity and quality. However, their use may be harmful to environmental health. It is then needed an innovative alternative in agricultural cultivation, increasing fertilizers and pesticides’ effectiveness, reducing its environmental impact, and improving food production. In particular, nanotechnology is emerging as a promising alternative. Inorganic nanoparticles can be used in association with active organic ingredients or as active ingredients. While nanofertilizers offer benefits in nutrition management, nanopesticides can increase environmental safety achieving better pest control. To that end, this chapter presents an overview of these materials’ use and their beneficial and damage effects in relation to conventional compounds. It describes the main types of nanofertilizers and nanopesticides (such as nanoparticles of essential elements and polymeric nanoparticles containing these elements), giving examples of products and their applications in plants compared to conventional chemicals. In contrast, despite the advantages of using nanotechnology in agriculture, it is necessary to consider its limitations and understand its environmental behavior. The internalization and subsequent toxicity of inorganic nanoparticles in the environment depend on their physical–chemical characteristics. It is essential to understand the biological responses to their exposure in nontarget organisms at various trophic levels, which may pose a risk to human health. In conclusion, although use of inorganic nanoparticles in agriculture offer opportunities to improve crop yields, it is mandatory to make a risk prognosis due to their use before their market entrance to make decisions of agricultural practices.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Nanomaterials (NMs) can improve crop productivity as fertilizers and pesticides. These materials can promote nutrient uptake by plants and suppress crop diseases by directly acting on pathogens through various mechanisms. Efficient use of NMs may complement or replace conventional fertilizers and pesticides, subsequently reducing the environmental impact of agricultural practices.
The nanotechnology uses for agri-food purposes are broadly conceived as a sustainable approach that is safer for human and animal consumption and for the environment, in addition to enhancing agricultural productivity. This technology will be a driving economic force to change the current agriculture practices. Novel delivery systems for crop improvement and productivity can decrease the use of bulk agrochemicals and provide more affordable solutions in the agriculture sector (Acharya & Pal, 2020). In the work of Kah et al. (2018), the authors make a critical assessment comparing nanopesticides and nanofertilizers against their conventional analogs. According to the authors, nanopesticides are more than 30% more efficient than nonnano analogs. However, the authors reinforce that biological and toxicological efficacy have not been confirmed for different target organisms/plants in many studies, which does not guarantee that this will be repeated in the field.
Before commercializing NMs used as fertilizers, phytological testing in both in vitro and in vivo setup must be carried out to ensure nutrient use efficiency with no or minimum material toxicity. Some NMs might be detrimental when applied directly and/or indirectly to the plants since they can sometimes readily aggregate or dissolute free ions in the immediate vicinity, which can cause tissue injury. The toxicity of nanoparticles (NPs) is dose, particle size, host plant, and plant growth-stage dependent. At higher doses, metal oxide NPs aggregate on root/seed surface due to physical attachment, electrostatic attraction, and hydrophobic interactions, causing local accumulation of ions released from the NPs to toxic levels. In this context, studies on uptake, translocation, internalization, and nutritional quality assessment must be carried out to understand NM–plant interactions (Pradhan & Mailapalli, 2017; Achari & Kowshik, 2018). Saleeb et al. (2019) found that the soil sorption of silver nanoparticles (Ag NPs) was significantly greater than Ag+. According to them, the environmental impact of the citrate-coated Ag NP release may be determined mainly by the equivalent mass concentration of Ag+. There is a considerable variation between plant species like spinach and silverbeet in Ag uptake that can accumulate sufficient Ag to pose a risk to human health.
Many NMs proposed for use in agriculture are made from metals known to be antimicrobial (Cu and Zn), photoactive (TiO2), or redox-active (CeO2). Their agriculture applications on a large scale may lead to toxicity risks that are not well understood. The impacts caused by these exposures can be the promotion of resistance in soil microbiome, bioaccumulation in plants and crops, and persistence in the environment, among others. The fate and subsequent consumption of NMs can cause human toxicity by ingesting an edible part of a crop where NM was translocated (Gilbertson et al., 2020). Understanding the potential toxicity and environmental impact of NPs requires that researchers study them at environmentally-relevant concentrations in complex, real-world systems. However, high metal concentrations of interest are present in every environmental compartment as well as many organisms. The successful development and application of various techniques that enable experimental designs reflecting the real environment will allow the determination of their toxicity mechanisms (Deline & Nason, 2019).
However, the synthesis protocols greatly influence the NM toxicity, and the use of toxic elements during the chemical synthesis process can lead to various health implications and environmental concerns. Hence, nowadays, there are efforts to synthesize NMs based on green principles by employing biogenic sources, as mentioned earlier (Baker et al., 2017). Once NPs are dispersed in the different environmental compartments (air, water, and soil), they suffer modifications through various physical, chemical, and biological transformation processes. Understanding the relationship between NM and critical ecosystem components as plants, pests, microbiomes, and livestock is essential. The agronomic and socioeconomic context and geographical differences that lead to some food deficit and an environmental impact should be considered to support the development of more viable and sustainable nano-innovations in agriculture (Kah & Kookana, 2020).
Nanotechnology offers potential solutions for sustainable agriculture, including increased nutrient utilization efficiency, improved pest management efficacy, mitigation of the impacts of climate change, and reduction of adverse environmental impacts of agricultural food production. However, for this technology adoption, it is necessary to use data and models that include sensitive endpoints for regulatory and safety concerns (Hofmann et al., 2020).
A significant challenge in nanotoxicology is establishing a comprehensive risk assessment framework for these materials since, after entering the environment, NMs can rapidly undergo surface modifications and chemical speciation changes. It is then necessary to assess potential environmental and human-exposure risks from NM fate, transport, and toxicity in environmental systems (soil and plants) and conditions relevant to agriculture fields (ultraviolet light, temperature, pH, and organic matter). In this scenario, this chapter examines the benefits of NMs used as pesticides and fertilizers and highlights critical challenges regarding their ecotoxicity, risk analysis, and regulatory issues to ensure safe application in agriculture viewing to achieve global food security.
2 Benefits of Inorganic Nanoparticles to Agriculture
2.1 Nanopesticides
Population growth, combined with environmental conditions changes, has put pressure on agriculture to increase food production (Bruinsma, 2017). Over time, agriculture has undergone countless revolutions, one of which is the so-called “green revolution.” It was based mainly on the extensive use of pesticides and fertilizers and the mechanization of production (Shiva, 2016). It is noteworthy that these facts brought about a significant change in the agricultural sector, allowing greater productivity. However, over time, several organisms have developed resistance to pesticides. Numerous environmental problems have also emerged, such as contamination of soils, surface, and underground water, in addition to the damage to nontarget organisms (pollinators, among others) and agricultural producers (Shiva, 2016).
In this context, there has been a growing concern to protect crops from pest attack and reconcile environmental gains. In this way, numerous technological approaches have been explored. Nanotechnology has proven to be an important platform to achieve a dynamic balance between agricultural production and environmental sustainability. Advances in this area have allowed developing different systems based on NPs for agricultural applications, the so-called nanopesticides (Usman et al., 2020). Nanopesticides are generally based on organic molecular active ingredients, encapsulated in nanocarriers of different matrices, as well as nanoscale inorganic active ingredients complexed or not with organic carriers. Regardless of the type of formulation, nanopesticides aim to i) increase the solubility and stability of the active compounds; ii) release them slowly; iii) protect them against premature degradation caused by environmental factors; and iv) target the active ingredients more effectively, promoting a reduction in the amount of active ingredient used (Parisi et al., 2015). Therefore, these systems cause the active compounds to remain in an effective concentration range, thus increasing their efficiency and decreasing the toxicity and possible environmental contamination (He et al., 2019).
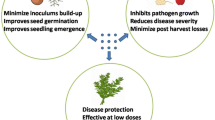
Concerning inorganic nanopesticides, these agents can act both in pest control and fighting diseases, such as those caused by viruses, bacteria, and fungi (Fig. 1). In the following subsections, we present some of the prominent examples in more detail, with Table 1 summarizing the literature’s works.
Application of different inorganic nanoparticles, which include metal nanoparticles, silicon nanoparticles, and C-based nanoparticles and nanocomposites in crop protection. Such formulations have shown biological effectiveness against different agricultural pests (insects, bacteria, fungi, and viruses)
2.1.1 Silicon Nanoparticles
Silicon (Si) is one of the most abundant metalloids on Earth. These compounds are characterized by their intermediate physical and chemical properties compared to metals and nonmetals (Blumenthal et al., 2018). Even though it is not considered an essential element, studies have described the application of Si in plants since it contributes to acclimation to different conditions of environmental stress (Abdel-Haliem et al., 2017; Cui et al., 2017). When on the nanoscale, this material has different properties compared to the bulk material; this is mainly due to its smaller size and surface area. Among the most commonly found compounds is silicon dioxide (SiO2), also known as silica (Bera, 2019).
These Si-based NMs have been investigated for use in agriculture as nanopesticides and carrier agents for active biomolecules, such as organic pesticides, nucleotides, and proteins (Jeelani et al., 2020). El-Naggar et al. (2020) evaluated the insecticidal effect of silica nanoparticles (SiO2 NPs) against four important pests that infect stored corn (Sitophilus oryzae, Rhizopertha dominica, Tribolium castaneum, and Orizaephilus surinamenisis). The results revealed that, when 0.25–2.0 g of SiO2 NPs were applied per kilo of seeds, O. surinamenisis, R. dominica, and T. castaneum exhibited 100% mortality, while S. oryzae was more resistant and exhibited 93.3% mortality. Therefore, SiO2 NPs have emerged as a promising insecticide during corn storage, with a minimal dose. In another study, Haroun et al. (2020) evaluated the conjugated effect of zinc oxide nanoparticles (ZnO NPs) and hydrophilic SiO2 NPs against important storage pests (S. oryzae, T. castaneum, and Callosobruchus maculatus). The systems exhibited a significant toxic effect against S. oryzae and C. maculatus in the highest concentration (8 g/kg seed), while T. castaneum showed high resistance. The insects also suffered a reduction in the F1 progeny, indicating the system as a potential protective alternative for stored seeds.
As previously described, SiO2 NPs are also commonly used as carrier agents for biomolecules. Bapat et al. (2020) have functionalized SiO2 NPs with the soybean trypsin inhibiting protein (STI) for smart delivery in tomato plants. The systems were synthesized in different sizes (20 and 100 nm), with no toxicity to plants. The functionalized NPs were absorbed by the plants through the roots and also through the leaf surfaces. The authors observed in in vitro tests that the NP-bound STI inhibited proteinase activity by 50% in the midgut of Helicoverpa armigera loopers. In addition, the second instar looper that ingested the systems (incorporated in artificial diet or leaves) showed significant growth retardation. Thus, the system proved to be a promising vehicle for the distribution of biomolecules to plants.
In another interesting work, a nanocarrier for the temperature-responsive insecticide imidacloprid was synthesized using mesoporous SiO2 NPs. The system had approximately 100 nm diameter and had an ordered hexagonal mesoporous structure with a surface coating of approximately 6 nm. In vitro tests showed sustained release that was sensitive to temperature. Also, biological tests in Aphis craccivora showed that the insecticidal activity increased significantly with the increase in temperature, directly linked to the release of the insecticide (Yao et al., 2020).
2.1.2 Metallic Nanoparticles
Nanotechnology has helped in the development of different materials for agricultural applications, including the synthesis of metallic NPs. Concerning these inorganic NMs, the biological effect against pests and pathogens is directly related to their synthesis route and the material origin (Singh et al., 2018). There are different methods for synthesizing these NPs: biological, chemical, and physical methods. However, chemical and physical methods often do not have an attractive cost–benefit and often require toxic products for synthesis, bringing deleterious impacts on human and environmental health (Gouda et al., 2019).
On the other hand, biological methods have shown a lower cost and reduced toxicity. Besides, NPs synthesized through green routes can have different properties since biomolecules (proteins, peptides, amino acids, etc.) that act as reducing agents influence the characteristics of NMs such as size, polydispersity, and shape. Among the main biological sources for synthesizing these types of particles are plants, algae, and microorganisms (Chhipa, 2019; Akther & Hemalatha, 2019).
In recent work, Vargas-Hernandez et al. (2020) described the potential of metallic NPs to control viral diseases that affect agriculture. The authors carried out an exhaustive analysis of the characteristics of different metal oxide NPs and related these properties to the possible beneficial effects on plants and combat these pathogens.
Ag NPs were synthesized by chemical reduction and had an average size of 27 nm. Different bioassays were carried out with T. castaneum, including mortality tests, anti-feeding tests, oviposition deterrence, and repellent activity. The authors observed that the NPs showed significant activity in all parameters analyzed, and the joint use with the chemical insecticide malathion contributed to decreasing the resistance to the synthetic insecticide. (Alif Alisha & Thangapandiyan, 2019). In another recent study, Jameel et al. (2020) prepared and characterized a nanocomposite based on ZnO NPs and the insecticide thiamethoxam. The synthesized nanocomposite had an average size of 34 nm, and castor leaves impregnated with different concentrations (10–90 mg/L) were provided for fourth instar larvae of Spodoptera litura. The results of biological activity demonstrated an increase in larval mortality, in addition to malformation in pupae and adults, late emergence, and reduced fertility.
As previously mentioned, the biogenic synthesis of metallic NPs has also gained prominence. In the work of Alam et al. (2019), nanoparticles of iron oxide (FeO2 NPs) were synthesized using the Skimmia laureola leaf extract. The NPs had sizes ranging from 56 nm to 350 nm. Biological tests showed that in vitro NPs (6 mg/mL) drastically inhibited the growth of the bacteria Ralstonia solanacearum. When the in-plant test was carried out, the severity of the disease was effectively reduced by treating the root zone with the same concentration of NPs. Sahayaraj et al. (2020) evaluated in laboratory conditions the antifungal activity of Ag NP prepared through the aqueous extract of dry leaves of Pongamia glabra against Rhizopus nigricans. The NPs had an average size of 29 nm, being able to drastically reduce the weight of the R. nigricans mycelia and the number of spores compared only to the crude extract.
In a recent chapter, Graily-Moradi et al. (2020) addressed the biosynthesis of gold nanoparticles (Au NPs) through different natural sources (plants, fungi, bacteria, actinomycetes, yeasts, and algae). The authors pointed out that Au NPs have different shapes and sizes and that enzymes secreted by microorganisms and plant metabolites act as reducing and stabilizing agents. Several works that show the potential agricultural applications of these systems have been published (Graily-Moradi et al., 2020).
The applicability of nanocomposites of inorganic NPs with different biopolymers (e.g., chitosan, gums) has been demonstrated. Ammar and Abd-ElAzeem (2020) synthesized copper oxide nanoparticles (CuO NPs) through fungal filtrates of Aspergillus wentii, which were then mixed in a polymeric gelatin matrix. The treatment with the conjugate allowed to reach higher values of larval and pupal mortality. Also, there was a significant decrease in the hatchability percentage and number of eggs. In a review article, Chouhan and Mandal (2020) addressed the use of hydrophilic polysaccharide chitosan in strategies for the synthesis of nanocomposites containing metallic NPs (silver, copper, zinc, iron, and nickel, among others). According to the authors, these systems are highly compatible, and chitosan has no toxic effects on the agricultural system. Several studies highlighting the applicability of these systems in the control of pests and pathogens of agricultural interest have been presented (Chouhan & Mandal, 2020).
2.2 Nanofertilizers
Many types of NPs have been developed aiming at agricultural applications, including those related to the supply of nutrients to plants (Fraceto et al., 2016). Nanofertilizers are structures in nanometric scale composed of or loaded with essential elements for plant development (Marchiol et al., 2019; Raliya et al., 2018). They are an efficient strategy for the delivery of nutrients directly to plants, allowing the reduction of the applied amount of fertilizers. In some cases, a gain of 100% can be achieved compared to conventional fertilizers, with positive impacts on crop growth, yield, and quality (Kalra et al., 2020; Nibin & Ushakumari, 2019; Qureshi et al., 2018).
The improved efficiency of nanofertilizers can be related to the gradual release of nutrient ions as well as to the enhanced dissolution in water or soil solution due to the high reactivity that results from the small particle size and the high superficial area (Liu & Lal, 2015; Kalra et al., 2020). In addition, nutrient availability may be increased due to the penetration of NPs through plant structures (e.g., stomata, trichomes, hydathodes, and cell pores), which improves nutrient uptake and reduces losses to the environment (Liu & Lal, 2015; Kalra et al., 2020; Nibin & Ushakumari, 2019; Mahil & Kumar, 2019; Ruttkay-Nedecky et al., 2017; Yaseen et al., 2020). In contrast, conventional fertilizers usually have a very low absorption efficiency resulting from processes like surface runoff, lixiviation, evaporation, hydrolysis, and microbiological degradation (Kalra et al., 2020; Marchiol et al., 2019; Preetha & Balakrishnan, 2017; Raliya et al., 2018). Thus, the production of nanofertilizers is an important alternative for sustainable agricultural production, as it could allow the increase of yield with reduced environmental impact (Yaseen et al., 2020).
According to Kah et al. (2018), nanofertilizers can be classified as macronutrient-based nanofertilizers, micronutrient-based nanofertilizers, and nutrient-carrier NPs. Liu and Lal (2015) also recognize as nanofertilizers plant growth-promoting NMs (i.e., elements that do not have a nutrient effect but promote plant growth by improving the use of nutrients or other physiological processes). Macronutrient nanofertilizers are composed of one or more essential elements that are required by plants in large amounts, like nitrogen (N), phosphorus (P), potassium (K), magnesium (Mg), and calcium (Ca). Micronutrient nanofertilizers are composed of those essential elements that are required in small amounts, like zinc (Zn), iron (Fe), copper (Cu), molybdenum (Mo), and manganese (Mn). Both macro and micronutrients can be encapsulated into polymeric NPs (Fig. 2). The applications of these three groups of nanofertilizers are summarized in Table 2 and presented in more detail in the following subsections.
The nutrients can be supplied to plants by metallic nanoparticles (Me), metal-oxide nanoparticles (MeO), polymeric nanoparticles (e.g., chitosan) loaded with nutrients allowing their gradual release, or inorganic nanoparticles composed of macronutrients (e.g., hydroxyapatite, composed of calcium and phosphorus), which can carry other nutrients (e.g., nitrogen in the form of urea)
Despite the benefits involved in the use of nanofertilizers, some factors can interfere with their efficiency, such as the method of application and characteristics of the plant that alter its interaction with the NMs (Raliya et al., 2018). The foliar treatment seems to result in a more effective uptake of the NPs than the soil treatments (Alidoust & Isoda, 2013; Raliya et al., 2015), as several soil properties can alter the nutrient availability to the plants (e.g., texture, pH, salt content) (Kalra et al., 2020). Even when applied directly to the leaves, some problems might occur, including specific leaf characteristics, stomatal behavior, and potential phytotoxicity (Kalra et al., 2020). For the uptake and translocation of NPs by the plants, they can enter through different structures (e.g., stomata, cuticle, hydathodes, trichomes, lenticels, wounds, root junctions) with the need to surpass many barriers (Rastogi et al., 2017; Ruttkay-Nedecky et al., 2017). Thus, studies are necessary to improve the knowledge regarding the interactions of different types of nanofertilizers with plants, which would bring valuable information about the mechanisms involved in the nutrient delivery by these systems and allow the development of more efficient nanoformulations.
2.2.1 Micronutrient Nanoparticles
Although required by plants in small amounts, micronutrients play essential roles in plant metabolism (Bisquera et al., 2017). They are usually applied to crop fields in the form of salts, a significant part of which is not used by the plants, thereby contaminating the environment (Deshpande et al., 2017). Many metals have been manipulated in nanoscale to act as nanofertilizers (Yaseen et al., 2020). Metallic or metal-oxide NPs show physicochemical properties that differ from the bulk materials, showing improved efficiency (Rastogi et al., 2017).
Zn, both in ionic or oxide (ZnO) forms, has been widely used in the last decades for the development of NPs (Liu & Lal, 2014). This micronutrient is essential for membrane integrity, seed development, and plant reproduction (Sturikovaa et al., 2018; Deshpande et al., 2017). Zn-based nanofertilizers show greater and faster dissolution than bulk materials, allowing lower dosages (Milani et al., 2012). Moreover, they have limited mobility in the leaves and are kept attached to the leaf surface, where Zn ions are gradually released and then translocated, improving the use of this nutrient by the plant (Kopittke et al., 2019; Rossi et al., 2019). The positive effects of Zn and ZnO NPs have been reported to occur when applied to plants in different developmental stages, leading to the improvement of biomass accumulation, crop yield, and seed quality (Bisquera et al., 2017; Lawre & Raskar, 2014; Mahdieh et al., 2018; Rossi et al., 2019; Song & Kim, 2020; Subbaiah et al., 2016; Yusefi-Tanha et al., 2020). The biological effects of Zn and ZnO NPs depend on their size, morphology, and concentration, as observed by Yusefi-Tanha et al. (2020) in soybean plants. It is also noteworthy that Zn phytotoxicity is lower when this element is applied as NPs compared to the ionic form.
Cu is another metal with several agricultural applications, as it is a constituent of many plant enzymes (Adhikari et al., 2016; Rastogi et al., 2017; Ruttkay-Nedecky et al., 2017). In the soil, CuO NPs can provide this micronutrient to the roots in a slow and sustained manner (Spielman-Sun et al., 2018). CuO NPs have also been shown to improve plant growth, regulate enzymatic activity, and have antifungal properties (Adhikari et al., 2016; Ruttkay-Nedecky et al., 2017).
Many plant metabolism processes require Fe, including chlorophyll biosynthesis, nitrogen fixation/assimilation, and redox reactions (Drostkar et al., 2016). Most studies applying iron nanoparticles (Fe NPs) have reported the increment of chlorophyll levels and photosynthetic activity, with the consequent increase of plant growth and yield (Alidoust & Isoda, 2013; Bakhtiari et al., 2015; Drostkar et al., 2016; Ghafariyan et al., 2013; Moghadam et al., 2012; Raju et al., 2016; Rui et al., 2016). Also, the application of iron oxide nanoparticles (FeO NPs) has been considered a strategy for food biofortification (Siva & Benita, 2016).
Other micronutrient-based NPs (as Mn, MnO, and Mo) have been shown to benefit plant growth and physiology, with the improvement of photosynthesis and nitrogen fixation (Ghassemi-Golezani & Afkhami, 2018; Pradhan et al., 2013; Pradhan et al., 2014; Taran et al., 2014).
2.2.2 Macronutrient Nanoparticles
P-based nanofertilizers have been developed aiming at the promotion of the controlled ion release and at the increase of P mobility in the soil, which would allow an improved uptake and usage of this macronutrient by the plants (Kopittke et al., 2019). Hydroxyapatite [(Ca10(PO4)6(OH)2] nanoparticles (HA NPs) have been considered the main alternative to conventional P fertilization (Kottegoda et al., 2017). In addition to providing Ca, they efficiently deliver P to plants, thus reducing eutrophication risk. The beneficial effects of HA NPs have been attributed to their higher and more persistent availability in the soil than conventional P ions, which are rapidly adsorbed to soil colloids (Liu & Lal, 2014; Maghsoodi et al., 2020). Moreover, HA NPs did not induce phytotoxic effects on the germination and initial development of tomato seedlings (Marchiol et al., 2019).
Due to its low efficiency and high production cost, N fertilization has also arisen great interest in the development of nanotechnology-based solutions. Urea can be coated to HA NPs, as the large surface area of this NM allows the binding of many urea molecules (Kottegoda et al., 2017; Gunaratne et al., 2016; Kottegoda et al., 2011). This association decreases urea solubility (that is very high), yielding a slower N release. Another multinutrient nanofertilizer, composed of amorphous calcium phosphate, K, and N (nitrate and urea), was recently formulated (Ramírez-Rodríguez et al., 2020). Due to the gradual nutrient release, this nanofertilizer avoided losses to the environment and decreased by 40% the applied amount of nutrients compared to conventional fertilizer. Another advantage of this nano-NPK was the presence of two N forms with different release kinetics in its composition. Magnesium nanoparticles (Mg NPs) have also been developed and shown to promote the growth of maize plants, which was related to the increment of chlorophyll content (Shinde et al., 2020).
2.2.3 Nutrient-Loaded Polymeric Nanoparticles
The use of polymeric NPs as nutrient carrier systems can provide a safe strategy for the delivery of fertilizers to the plants, decreasing the environmental impacts. Moreover, the nanoformulations can be adjusted to allow a gradual nutrient release, which improves the nutrient availability and its use efficiency by the plants (Chen et al., 2013; Guo et al., 2018). A variety of polymeric matrixes have been used to prepare NPs, including chitosan, a chitin-derived polysaccharide that can promote per se benefits to plants (Chen et al., 2013). For example, the treatment with chitosan oligomers induced nutrient uptake, the biosynthesis of photosynthetic pigments, and the growth of coffee plants (Dzung et al., 2011). Chitosan nanoparticles (CS NPs) have been demonstrated as an excellent alternative for the nanoencapsulation of both micro and macronutrients, as they show characteristics as biocompatibility, biodegradability, low phytotoxicity, high adsorption, gradual nutrient release, and protection of biomolecules against adverse environmental conditions (pH, light, temperature) (Chen et al., 2013; Kashyapa et al., 2015; Mujtaba et al., 2020).
In association with Zn2+, CS NPs stimulated the germination, initial growth, and defense system of maize plants, as well as increased the yield and promoted the biofortification of wheat and maize grains (Choudhary et al., 2019; Deshpande et al., 2017). As Cu2+-carrier systems, CS NPs induced α-amylase activity and storage mobilization, yielding improved germination and growth of maize and tomato seedlings (Saharan et al., 2015; Saharan et al., 2016).
In addition to micronutrients, CS NPs have been used to encapsulate NPK fertilizers, enhancing the growth of potato and coffee plants (Elshamy et al., 2019; Ha et al., 2019) and wheat yield (Abdel-Aziz et al., 2016; Abdel-Aziz et al., 2018). However, the mechanisms involved in the positive effects of NPK-loaded CS NPs have not been completely elucidated, as they can be related to the gradual nutrient release or the direct internalization of the NPs by the plant, followed by the posterior release (Guo et al., 2018).
3 Adverse Ecotoxicological Impacts of Inorganic Nanoparticles
The small size of NPs, which gives immense benefit for their use, also contributes to their toxicity issues with several adverse effects. NPs react with various environmental components due to their high surface area. They are highly dynamic and reactive; various physical, chemical, or biological transformations may occur in the environment. Then, the use of nanoproducts in pest control is subjected to various environmental risks. These effects range from environmental hazards to human and animal health in general. The toxicity and responses of materials used in the delivery system may be species-dependent driven by a series of factors, including the NM itself and the environmental and physiological conditions on which they are applied (Vega-Vásquez et al., 2020). NM-induced toxicity could be changed by environmental factors such as sunlight irradiation, natural organic matter, and mineral particles. Because of the uncertainties on environmental concentrations and ecotoxicity, there are significant challenges in understanding the environmental risks of NMs (Zhao et al., 2020a).
Engineered NMs may adversely impact human health and environmental safety by nano–bio–eco interactions not fully understood. Their interactions with biotic and abiotic environments are varied and complicated, ranging from individual species to entire ecosystems. Biological, chemical, and physical dimension properties, the so-called multidimensional characterization, determine interactions. Intermediate species generated in the dynamic process of NM transformation increase the complexity of assessing nanotoxicity (He et al., 2018). Dispersion and dosing of NMs are critical aspects of nanosafety studies since the environmental concentration is the potential dose to that an organism can be exposed. Also, the fate and behavior of NMs are determined by transformations during and following their dispersion in biological and environmental media. In complex environmental media, where natural nanoscale particles and colloids with plenty of positive and negative charged moieties are present, NM heteroagglomeration is the dominant process. Thus, NM heteroagglomeration rather than homoagglomeration or freely dispersed NMs are expected under environmentally relevant conditions (Wigger et al., 2020).
The physicochemical transformations suffered by NM can result in different characteristics leading to the formation of transformed NM functional fate groups. Transformation, especially speciation changes, results in reduced potency. Further reactions at the surface, such as ecocorona formation and heteroagglomeration, may also reduce NM potency. Different NMs that suffered transformation in the environment may have their hazard reduced in the same way, leading to similar actual hazards under realistic exposure conditions (Spurgeon et al., 2020).
Bio–nano interactions between proteins and NMs lead to the formation of the protein corona. Corona formation has proven to be critical for cellular uptake, intracellular localization, and toxicity arising from NMs. Even if the aquatic factors remain consistent, the intrinsic physicochemical properties of multifarious NMs (e.g., metallic and polymeric NPs) may produce unique characteristics in their acquired coronas. The most altered environmental corona interactions appear to be membrane adhesion, membrane damage, cellular internalization, and oxidative stress responses induced by NMs. When natural organic matter (NOM) or expanded polystyrene (EPS)-coated NMs enter the organisms or cells, the macromolecules in the surrounding medium will change into proteins, lipids, and nucleic acids. However, it is not clearly understood whether the adsorbed NOM or EPS macromolecules will be covered or replaced by other biomolecules and form an evolutional corona inside cells or organisms (Xu et al., 2020).
Biomolecule affinities for NM surfaces can change the corona composition. It was recently shown that the chronic (reproductive) ecotoxicity of Ag and TiO2 NPs to Daphnia magna is reduced by environmental aging of the NPs in media of different ionic strengths and natural organic matter contents (Ellis & Lynch, 2020). Then, corona determines how organisms’ cells interact with NMs, and its proteins confer a biological identity to NMs, influencing the uptake by cells. However, the role of metabolite corona is not fully understood. Metabolites are orders of magnitude smaller than proteins (typically below 1000 Da), whereas proteins are measured on the kDa scale, and metabolites are typically reactants, intermediaries, and products of enzymatic activity. These coronal metabolites are beginning to gain interest since they influence NM impacts on molecular signaling and adverse outcome pathways (Chetwynd & Lynch, 2020).
Consequently, these processes change the properties of NMs, thereby affecting transport in soil, uptake, and translocation in the plant, and their toxicity to organisms (Fig. 3). The released metal ions can be accumulated by the plant directly or as complexes with other components from the environment. Also, aggregation and agglomeration may occur, modifying NM surface charge and chemistry and influencing subsequent behavior and bioavailability. The various kinds of nanopesticides, from emulsion to nanodispersion, have diverse environmental interactions due to the difference in the chemical components and preparation method. Thus, the safety evaluation of the developed NMs has increasingly become important. A clear understanding of the environmental safety and fate of nanopesticides and their active ingredients is mandatory before commercial application (Acharya & Pal, 2020; Zhang et al., 2020a).
Coatings on NP surfaces play a crucial role in dictating their behavior in the environment. The fate of NPs as ligand displacement reactions will modify the stability of these NPs during their transport in the environment, NP agglomeration, and their interactions with biological systems. Corona formation of environmental or biological molecules on the surface of these NMs could occur, which either accelerates or slows the dissolution. For metal oxide NPs, the physicochemical processes of dissolution, aggregation, and reactivity are all impacted by surface coatings. The relative binding affinity to the surface depends on the ability of different functional groups to interact with the surface and through nonspecific surface interactions that become important for species with higher molar mass (Wu et al., 2019). So, physicochemical parameters for NP–protein corona formation are frequently derived from protein corona fingerprints, and NPs and protein can suffer aggregation or disaggregation (Falahati et al., 2019).
3.1 Interactions of Nanoproducts and Ecosystem
Current agricultural practices pose unintentional and adverse effects on environmental health, highlighting the need for more sustainable agriculture strategies. Excessive use of conventional chemical fertilizers and pesticides has been increasing toxicity in ground and surface water reservoirs, which has adverse effects on environmental and human health. Some of these agricultural practices can humiliate soil quality and is responsible for the eutrophication of water bodies. Although nanotechnology is of significance for different agricultural applications, further research is needed to explore their applications’ effects. Thus, nanotechnology use risks should be carefully examined to guarantee a correct and safe application of NMs in agriculture (Yadav et al., 2020).
NP properties and environmental conditions govern environmental transformation processes and ultimately alter their fate and behavior. Environmental fate assessment remains a critical aspect of studies to understand NM behavior in the environment and the nature and concentrations of the materials that do not damage human and environmental species. Environmental factors such as pH, ionic strength, salts, and sunlight can play a role in the degree of toxicity, and effects resulting from a combination of these factors will undoubtedly be dynamic and complex.
In the aquatic environment, NM agglomeration trends in aqueous systems are controlled by the water chemical properties, most importantly, ionic strength, the valence of the electrolytes, and pH. These parameters largely determine the surface charges/zeta potential of the particles. Then, aggregation refers to strongly bonded or fused particles where the resulting external surface area is significantly smaller than the sum of the individual components’ surface areas. In contrast, agglomerates refer to weakly or medium strongly bound particles where the resulting external surface area is similar to the sum of the individual components’ surface areas. Thus, NM agglomeration and the formation of a surface coating are closely linked and depend on the surrounding matrices (Wigger et al., 2020).
A major concern arises when commercialized metal-based NMs come into contact with the aquatic ecosystem since their ion dissolution mechanisms and release kinetics into the water are highly unpredictable. Because NMs can readily dissolute and aggregate in many cases, the released ions can be potentially harmful to living systems (Pradhan & Mailapalli, 2017). The fate of nano-TiO2 in the aquatic environment depends on their aggregation and sedimentation rates, transport in water and sediments, and interactions with the living and nonliving components of the ecosystem (Luo et al., 2020). Also, irradiation by ultraviolet (UV) light is a factor that is of particular concern for photocatalytically active metal oxides such as TiO2 NPs and ZnO NPs. Under these conditions, there is reactive oxygen species (ROS) formation. Then, the illumination of these NMs in surface waters results in the formation of reactive intermediates, consequently altering the ecotoxicological potential of co-occurring organic micropollutants, including pesticides, due to catalytic degradation (Lüderwald et al., 2020).
Clemente et al. (2013, 2014) showed the importance of considering the experimental conditions in nanoecotoxicological tests. They evaluated the effects on fish exposed to different TiO2 NP concentrations and illumination conditions by observing the organisms’ survival, together with biomarkers of biochemical and genetic alterations. Also, prolonged fish exposure (21 days) to two different TiO2 NP crystal phases (anatase and a mixture of anatase 80% and rutile 20%) were evaluated at the same light conditions. Similarly, the occurrence of sublethal effects was influenced by the TiO2 NP crystal phase and illumination condition. Pure anatase caused more oxidative damage without co-exposure to UV, while the mixture anatase:rutile caused more sublethal effects when exposure occurred under UV (Clemente et al., 2015). Nowadays, it is well known that light conditions play an essential role in the dissolution processes of NPs as Ag NPs and ZnO NPs (Odzak et al., 2017). Besides, the behavior of Ag NPs is influenced by environmental factors (including pH, dissolved oxygen, sunlight, temperature, and NOM), which alter their bioaccumulation and toxicity. There are driving processes and potential sources that show correlations between Ag NPs concentrations and biogeochemical parameters, like dissolved organic carbon concentration and divalent cation concentrations. The trace element dissolved in environmental compartments should be considered in material flow analysis and toxicity models since it is the most reactive (Wang et al., 2020a).
Consequently, their bioavailability and potential ecotoxicity are associated with these environmental factors, and Ag NPs can exert different toxic effects depending on the environment and the surface properties (Yang et al., 2018a; Zhang et al., 2018a; Zhang et al., 2019). Moreover, Ag NPs can interact with metal and metal oxide particles/NPs, and their biological effects may not only be limited by NP concentration or particle size but also on the amount and species of products yielded from chemical interactions between Ag NPs and other variables (Sharma et al., 2019).
Similarly, the interaction of NPs with NOM alters the NPs’ persistence and toxicity (Abbas et al., 2020). The NOM levels found in most natural waters have been reported to influence the fate and transport of NMs (De Marchi et al., 2018). NOM adsorbed onto NM surfaces alters their surface properties. Humic acid can increase the suspension stability of TiO2 NPs, diminishing the bioavailability (Luo et al., 2020). More than that, humic acid in a concentration of 20 mg/L (realistic for surface waters) was able to disperse NPs during periods of 24 h or more (Pradhan et al., 2018). Different aquatic sources of NOM can result in differential toxicity, and different concentrations of humic acid can affect aggregation state and toxicity (Ong et al., 2017). However, the combined impacts of UVA, photoactive NMs such as TiO2 NPs, and NOM on co-occurring pollutants toxicity seem not easily predictable (Lüderwald et al., 2020).
Moreover, NMs can suffer transformations by environmental factors such as climate change and soil moisture. Interactions between nano-sized chemicals and the various climatic stresses in the agro-ecosystem are possible and may result in synergistic, antagonistic, or susceptibility to adverse environmental effects and their combinations. The evaluation of environmental fate, uptake by plants, aquatic and terrestrial ecosystems, and changes in test methodology should form research priorities. Therefore, the ideal situation is analysis of nanopesticides for some of the fundamental molecular and physicochemical aspects that determine their efficacy, stability, and environmental and/or human safety (Kranjc & Drobne, 2019; Gahukar & Das, 2020).
Terrestrial environments are expected to be the largest repository for environmentally released NMs from agriculture and facilitate NM exposure of soil microorganisms, such as plant growth-promoting rhizobacteria. In the soil, NMs can interact with microorganisms and compounds, facilitating or hampering their absorption. NMs can lead to severe effects on soil microbial communities and diversities, soil enzyme activities, carbon and nitrogen cycling, etc., depending on the soil physicochemical spatial heterogeneity at different microenvironments in areas such as the rhizosphere (Zhang et al., 2020a). For example, metal NP nanopesticides can target pathogens through several mechanisms such as the generation of ROS, binding to metabolites, and penetration of cells and spores. The NPs of plant essential and nonessential elements act by diverse mechanisms to elicit beneficial activity to plants in microbes. In its turn, plant beneficial microbes participate in NP transformations in rhizosphere/soil and mitigate toxic effects on plants of specific NPs. However, this NP action is nonspecific and can also benefit pathogenic microbes in the plant rhizosphere (Achari & Kowshik, 2018).
The toxicity of NMs to various soil bacteria has been investigated using various toxicity end-points and experimental procedures. NP toxic effects are due to their uptake by the microbial cells, their chemical nature and concentration in the soil and within the plant roots, ions released interactions between NPs and cellular biomolecules, protein expression, and cell membrane stability alterations, among others (Achari & Kowshik, 2018). The employment of microbial ecoreceptors can highlight NM–bacteria interactions in complex, environmentally relevant media in the future and contribute to nanotoxicological research (Lewis et al., 2019).
The microbial composition and enzyme activities show great potential to indicate NP environmental risks since the soil is an essential sink for NMs due to applications of nanoagrochemicals. Some critical pathways implicating soil enzymes are good indicators of the quality of the soil ecosystem and are likely to be affected by NPs. For example, environmental concentrations of Ag NPs affected microbial biomass but had little impact on microbial diversity and may have little effect on the soil biogeochemical cycles mediated by extracellular enzyme activities (Oca-Vasquez et al., 2020). Functional properties of antioxidant enzymes may affect the stability of NPs and vice versa and that NPs could affect the enzymes’ reactivity (Liu et al., 2020). Then, NMs may affect agricultural systems through modifications in nutrient cycling and soil fertility. However, whereas soil enzyme activity measurements are likely to provide critical information on NP effects on soil function in a risk evaluation, there is a need to further research to validate their use as an internationally accepted environmental indicator (Galhardi et al., 2020; Zhang et al., 2020b). An application of the nanoinformatics approach can help understand NM complex transformation processes in the soil–plant environment (Zhang et al., 2020a).
After NM exposure, soil organic matter (SOM) and exudates from roots or rhizosphere microbes can interact with the surface of NMs and change their physicochemical characteristics as hydrophobicity and charge. Soil organic matter may exhibit contradictory effects on the mobility and stability of NMs depending upon their nature. Soil colloids and minerals, mainly clay and Fe minerals, are considered an important sink for NMs. Thus, the surface coating can increase the bioavailability of NMs by decreasing the heteroaggregation of NMs with soil particles and increasing the interaction between NMs and plants. Dissolved organic carbon concentration may control dissolved metal concentration as Cu from CuO NPs in calcareous soil pore waters varying in organic matter concentration. Also, exudates from the root and microorganisms in the rhizosphere can affect physicochemical processes such as the NM heteroaggregation and dissolution in the soil. Root exudate in the rhizosphere could assist the dissolution of metal species as Cu and increase the contact possibility between particle surfaces and plant cells, both likely resulting in higher toxicity of CuO NPs to plants. Besides, the activities of soil fauna could also modify the physical and biochemical environment of rhizosphere soils. Earthworms can also increase the bioavailability of NMs, influencing the physical, chemical, and biological soil environment (Shang et al., 2019; Hortin et al., 2020; Usman et al., 2020; Wang et al., 2020b). Considering all environmental interferences, an in-depth evaluation of the effect of nanoagrochemicals in soils with different physicochemical properties is necessary to recommend a specific one for a specific crop and soil type (Zulfiqar et al., 2019). In this regard, a deeper understanding of the interactions between root exudates and NPs can enhance our knowledge on NP toxicity to plants and promote the effective and safe use of NPs as antimicrobial agents in agriculture.
Furthermore, NPs have their entrance into the environment facilitated by plant functions as a significant route for the bioaccumulation of the NPs into the food chain. The physicochemical properties of NPs and plant physiology significantly contribute to the interaction between NPs and plants, as well as the application method. Several tissues and barriers must be crossed before reaching the vascular tissues, depending on the entry point (roots or leaves). The cell wall barrier mostly restricts the access of NPs in the plant body. Plant cells can either enlarge the pore diameter or generate new pores in the cell wall to enhance NP uptake. Also, NP can enter the cell, crossing the membrane via transport carrier proteins or ion channel mechanisms. NMs can move up and down the plant (Pérez-de-Luque, 2017; Acharya & Pal, 2020).
In the aquatic environment, invertebrates serve as food for higher trophic level organisms, such as fish. Fish are broadly used to assess the strength and health of aquatic environments. For example, TiO2 NPs are released into the aquatic environment from multiple sources and can promote cytogenetic and hematological alterations in African catfish Clarias gariepinus and are relevant to biodiversity and aquatic health management (Ogunsuyi et al., 2020).
NPs that reach the aquatic environment will likely accumulate in sediment where they may be available for uptake by invertebrates (Kim et al., 2016). CuO NPs associated with sediment can enter the aquatic food web, and their chemical and biological processes can result in NP transformation. Depending on the organisms studied, the uptake, fate, and biological effects of CuO NPs and dissolved Cu are different. In this way, transfer of CuO NPs from benthic invertebrates (Tubifex tubifex) that serve as food for higher trophic level organisms as fish (Gasterosteus aculeatus) may be limited compared to dissolved Cu (Lombi et al., 2019). Also, different NP uptake mechanisms take place in oysters. Ingestion of particles dominated the uptake of 60-nm Ag NPs, whereas dermal uptake and ingestion contributed equally to 15-nm Ag NPs (Shao & Wang, 2020).
Depending on the environmental fate of NMs, feeding groups may be differentially exposed to NMs. For water exposures of single-celled and small multicellular species suspended, it is necessary to separate the suspended NMs from small organisms not to overestimate bioaccumulation. It is important for multicellular organisms to distinguish between the NM adsorbed by external surfaces or by the digestive tract and the amount absorbed by the epithelium. As for multicellular plants, the main considerations include the interactions between the route of exposure and the effect of the rhizosphere on measuring its absorption. Invertebrates can potentially accumulate NMs actively via ingestion and consecutive uptake across the epithelium in the body and to a lesser extent by anal uptake, or passively via uptake through body surfaces or body openings. Then, quantifying uptake and elimination bioaccumulation of NMs is a step toward understanding the potential for NM trophic transfer and biomagnification, both of which are essential concerns in ecotoxicology (Petersen et al., 2019). However, very little is known about the accumulation capacity and coping mechanisms of organisms in NM-contaminated soil due to its release in the terrestrial environment. In this way, Courtois et al. (2020) observed that Eisenia fetida bioaccumulates Ag but in a limited way. The Ag location in the organism, the competition between Ag and Cu, and the speciation of internal Ag suggest a link between Ag and metallothioneins, which are key proteins in the sequestration and detoxification of metals.
Consequently, there is a need to characterize actual exposure and quantification of NP bioaccumulation and toxicokinetics to understand toxicological effects. Despite that, tissue concentrations were generally quantified as the total metal content (NP and ions). Since dissolution is considered a crucial reaction for the study of the toxicity of metal NPs, more studies are needed to confirm it as an essential paradigm for assessing metal NP uptake in soil organisms. This understanding is vital to a more accurate risk assessment of NMs (Baccaro et al., 2018).
In aquatic environments, suspension feeders will be exposed predominantly to waterborne NMs, while deposit feeders will be exposed mainly to NMs following sedimentation. Once taken up by organisms, NMs can be retained in the body or excreted. Accumulation of NMs in organisms depends on their availability in the exposure medium and on the physiological traits of the species evaluated. The kinetics of uptake and elimination of metal-based NMs, or derived metal ions, vary among organisms and determine their accumulation patterns. Besides, uptake and elimination kinetics of metal NMs may also be form-dependent; the same organism can use different uptake and depuration pathways for NMs and ions. The fate of NMs in the body will depend on the NM manufactured material and their transformations while aging. For metal-containing NMs that dissolve, it is possible for the free metal ion to be taken up and subsequently incorporated into a metal storage granule inside the organism. The organism’s physiology influences the metals and NM elimination rate from organisms, beyond other parameters such as medium, NM characteristics, and the exposure route. NM elimination may involve several different processes among aquatic and terrestrial invertebrates (van den Brink et al., 2019). Also, fish developmental stage-dependent toxicity can affect the profiles of metal oxide NPs as seen in the zebrafish embryo and larvae that emphasize the importance of considering developmental stage differences when evaluating safety assessment of NPs when using living organisms (Peng et al., 2018).
Thus far, with the increasing application of metal NPs, metal ions will accumulate in the environment to threaten the ecosystem (Wang et al., 2020b). Although TiO2 NPs were initially classified as a biologically inert material, there is growing evidence of toxicity to humans and nontarget organisms requiring further research and improved regulatory practices. Mechanical stress due to the interactions of cells with TiO2 NPs can impair the cell membrane integrity and affect ion homeostasis and activity of the membrane-associated receptors and enzymes. Intracellular accumulation of TiO2 NPs leads to DNA damage, whereas altered gene expression affects the induced oxidative stress and inflammation (Luo et al., 2020).
Concerning Ag NPs, sodium (Na) ion channels are involved in the uptake of ionic Ag in freshwater fish rainbow trout. Primarily intact NPs enter tissues through the endocytosis pathway in respiratory or digestive system epithelial tissue. Ions released as a result of NP dissolution are internalized in the cell through transporter proteins or ion channels. Primary NP toxicity induction modes include the release of ions with particle dissolution, oxidative stress, cellular protein injury, and membrane and DNA damage, among others. Also, physicochemical characteristics of NPs such as shape, size, charge, crystalline phase, and coating materials could influence their bioactivity and toxicity (Abbas et al., 2020). In addition to particle size, surface area, and charge, NP surface coating or intentional surface modification are essential determinants to NP translocation in organisms. However, the age of the healthy animal seems not to affect it. The particle properties may also affect the time-course of translocation and clearance mechanisms (Raftis & Miller, 2019).
In addition, bioaccumulation of chemical compounds is the first step toward inducing toxic effects in aquatic organisms. The bioaccumulation kinetics and tissue distribution of Ag NPs in aquatic organisms are affected by NOM since NOM molecules are adsorbed on the surface of Ag NP. This fact increases the particle sizes and negative charges and suppresses the dissolution of Ag NP. As a result, the uptake by zebrafish via dissolved Ag and ingestion of Ag NPs was reduced. Also, NOM inhibited the cell membrane crossing by Ag NPs and promoted the depuration of Ag NP from the fish body, alleviating the bioaccumulation of Ag NPs in zebrafish (Xiao et al., 2020).
Surface chemistry can be used to alter multifunctional properties in metal oxide NPs, leading to broader use of NPs in agriculture, for example, as adjuvants for agrochemicals. Any use evaluation of NMs must address the diverse nature of their shapes (size, shape, organic coating), states (free versus embedded in the matrix, monodispersed versus clustered), and behavior (dynamic transformations that affect shape and state) immediately before entering the environment and after a while (Svendsen et al., 2020; Zhao et al., 2020b).
In their turn, the dissolution of ceria NPs at the nano–bio interface can lead to cytotoxicity as other easily ionized NPs. For that, NPs could bypass the cellular membrane and release high levels of toxic ions in cells after their internalization (Xie et al., 2019). NM biotransformations result from NM–biota interactions and alter the behavior and fate of engineered nanomaterials (ENMs) in the environment. NM biotransformations include dissolution, redox reactions, and chemical reactions with surrounding molecules. NM dissolution appears to be a significant driver of toxicity due to the increased bioavailability of ions, and biotransformation of undissolved NMs does not appear to occur (Kranjc & Drobne, 2019).
Whereas ions released by dissolution can diffuse more freely toward biological receptors and transfer across cellular boundaries, the NM arrival in organisms may be limited by transformations or attachment to other surfaces in the environment. NM heteroagglomeration and dissolution and subsequent chemical speciation in organisms are extremely important in studying their exposure since they affect their uptake. Indeed, during laboratory tests, the attachment efficiency of NMs to organisms is a good predictor of their uptake potential and subsequent toxicity (Klaessig, 2018; Svendsen et al., 2020).
The heteroaggregation between Ag NPs and other particles, such as microbial colloids and mineral particles, can reduce effective Ag NP exposure. Hence, it is essential to study the interactions between ions and solid environmental matrices to predict Ag NPs’ fate and risk in the environments. Dong and Zhou (2020) observed distinct mechanisms in heteroaggregation of Ag NPs with mineral and organic particles. While metal ions enhance the attachment of Ag NPs to kaolin, humic acid prevents Ag NP–kaolin attachment at low concentrations. In contrast, lowering pH or adding metal ions inhibited Ag NP–cell attachment associated with the solubility product of metal salts. Although humic acid has little impact on Ag NP–cell attachment, it may complex with metal ions and reduce their effective solution concentrations. As a consequence, metal ion’s competition for Ag NP adsorption by bacterial cells can be mitigated. Besides, chronic exposures to NMs may allow vertebrate microbiota to adapt to the xenobiotic presence, resulting in the development of a new bacterial community with a modified composition, which may change microbiota–host signaling and physiological regulation (Zhang et al., 2020c).
As seen, NMs that enter into the environment are often harmful to the living systems. So, safer NP development is essential to cope with the need for more secure and safe NMs. Due to their toxic effects, metal NPs should be given proper care in the production and application process, mostly the chemically synthesized metal NPs. An ideal nanodevice for use in agriculture should be nontoxic and environmentally safe and avoid further contamination problems and a negative perception of consumers. Besides, its synthesis and production must be easily up-scaled, involve low-cost materials, and be affordable to farmers. The establishment of collaborative and interdisciplinary research could assess NM risks and benefits, allowing for better exploration of their potential (Vurro et al., 2019).
NP shape-based toxicity differences could be due to increased uptake of NP of specific shapes by plants and differences in their stability or dissolution patterns in soil (Achari & Kowshik, 2018). Nevertheless, little information is available on the role of properties such as shape and charge of NPs in bringing about beneficial or toxic effects in plant systems (Achari & Kowshik, 2018). In a safer-by-design perspective, the environmental risk related to NMs may be mitigated by lowering the hazard or the exposure potential. Controlling the shape of NMs, as their surface reactivities, could be an option to increase their applicative potential while reducing their potentially harmful effects once released in the environment. Indeed, it was observed an Ag NP shape-dependent impact under such environmentally relevant exposure conditions. From an environmental risk perspective, Ag NP shape can predict which ecological niches of a lotic ecosystem would be more impacted since it was observed a dependent biological response by this characteristic (Auffan et al., 2020). Also, NP aggregates with larger sizes may not be taken up, eliminating the toxicity, or restricting it to the root surface (Achari & Kowshik, 2018).
Furthermore, atrazine (ATZ) and atrazine-loaded poly-ɛ-caprolactone nanocapsules (ATZ NP) have distinct adverse effects on the nontarget rhizosphere bacterial communities of plants after long-term exposure. Long-term exposure to high concentrations of ATZ NPs was found to act more effectively and gave more microbial community impacts (decreased the community metabolic capacity and shifted the community structure and composition to a greater extent) compared to the same amount of ATZ. The ATZ NP surface modification may solve this effect and promote benefits from other promising properties of these materials (Monikh et al., 2020).
Falinski et al. (2018) proposed a framework for sustainable NM selection and design based on performance, hazard, and economic considerations. This framework’s development and implementation can facilitate promising applications, prevent unintended consequences, and support a proactive regulatory action. The final goal is to contribute to nanotechnology governance, having faster, cheaper, effective, and safer nanoproducts on the market for users and the environment (Kraegeloh et al., 2018). The collaboration between regulatory risk assessors and academia helps regulators keep up with novel materials and techniques and support regulatory preparedness (Soeteman-Hernández et al., 2020). Regulatory barriers to the use of nanotechnology in agriculture require careful selection of starting materials, as well as a comprehensive and holistic analysis of the associated risks, fate, and impacts. In a recent publication, Hofmann et al. (2020) explored these barriers: efficient delivery on a field scale, regulatory and safety issues, and consumer acceptance. These authors also proposed ways to overcome these barriers and develop effective, safe, and acceptable nanotechnologies for agriculture. A network of sentinel sites can generate the data needed to understand any associated risks, and more advanced analytical tools are needed to identify and quantify these NMs in natural environments (Hofmann et al., 2020).
Bringing this awareness, biological methods may be the safer, cost-effective, and eco-friendly option than chemical synthesis and allow the synthesis of NPs at physiological pH, temperature, and pressure (Chaudhry et al., 2018; Souza et al., 2019). Some studies have indicated that NP containing Ca, Mo, Mg, and mineral nanoconjugates of chitosan exhibited limited adverse effects on plants after soil application (Achari & Kowshik, 2018). Biogenic NPs are comparatively safer and less toxic than the chemically synthesized ones (Girilal et al., 2015). Although green synthesized NPs can induce harmful effects as oxidative stress, they are milder than the chemically synthesized ones (Krishnaraj et al., 2016; Shobana et al., 2018; Yaqub et al., 2019). Due to the lack of toxic chemicals during their synthesis and their high adaptability, green NMs have a vast application domain (Bartolucci et al., 2020). In this context, nanotechnology interest in agriculture use is today mainly turned to green production of NMs, slow and sustained delivery of nutrients from nanofertilizers, and active ingredient delivery from nanopesticides. For example, contrary to chemically synthesized Ag NPs, biogenic Ag NPs at lower concentrations can be a promising option for many applications in both industrial and environmental areas. However, it is still crucial to understand the interaction between these Ag NPs with living organisms and their potential environmental toxicity (Ottoni et al., 2020).
3.2 Bioaccumulation and Trophic Transfer of NPs
Another critical issue to consider is the bioavailability of the accumulated NPs to the next trophic level since NPs can reach different environmental compartments and their organisms. Chae et al. (2016) showed that the transfer of NPs through a model terrestrial food chain consisting of the yeast, the collembolan, and the pill bug indicated the potential hazards of released NPs for organisms at different trophic levels. Furthermore, Skjolding et al. (2014) observed the trophic transfer of ZnO NPs from daphnids (Daphnia magna) to zebrafish (Danio rerio). Nemati et al. (2019) found that CuO NPs can be transferred from one trophic level to the next level, as verified after diet-borne exposure of Amatitlania nigrofasciata larvae for 21 days to Artemia salina nauplii pre-exposed.
NM trophic transfer to the next level depends upon NM stability and surface properties (Pradhan & Mailapalli, 2017). Tangaa et al. (2016) defined four processes that influence the trophic transfer of metal NPs: environmental transformations of metal NPs, uptake and accumulation in the prey organism, internal fate and localization in the prey, and the digestive physiology of the predator. Additionally, in aquatic food webs, they suggest that the NP association with sediments may be a process that results in the transfer of intact particles. However, other possible co-existing effects of contaminants may also interfere with nano-toxicity. There are some potential routes for NP increasing bioaccumulation of co-exposure contaminants. Then, NP can absorb other contaminants, serve as carriers for the contaminants, bind with contaminants, facilitate the formation of more reactive metabolites, and cause cellular damage. Also, few studies have investigated the joint toxicity of NP mixture. These studies focused on mixtures of metal-based NP as plant fertilizers, ZnO and CuO NPs, since there may be effects of interactions between dissolved ions, dissolved and particulate NPs, and particulate NPs (Du et al., 2018).
Several organic and inorganic contaminants are distributed in the natural environment, and NPs act as carriers to transport these environmental contaminants into the cells of living organisms due to their enormous sorption capacity. NP surface can adsorb contaminants that have synergistic or antagonistic effects on the toxicity of them to different organisms depending on the contaminant surface charge and NPs’ zeta potential (Abbas et al., 2020). For example, a mixture of NPs and metals can lead to decreased ingestion and filtration rates of copepods leading to an alteration of their metabolic responses. Then, combined lead (Pb) and TiO2 NPs exposure may negatively impact the physiology of aquatic biodiversity and food chain dynamics in freshwater ecosystems (Matouke & Mustapha, 2018). Also, Yang et al. (2018b) observed that the increased transfer of algae by the food chain to A. salina of arsenic (As) in the presence of nano-TiO2 can be explained by adsorption of As onto nano-TiO2 in contaminated food (algae).
Indeed, there is limited information regarding what extent metal NPs could accumulate in biota and magnify along the food chain in real natural aquatic environments. Baudrimont et al. (2018) verified some effects of Au NPs from periphytic biofilms to the crustacean Gammarus fossarum due to transfer and bioaccumulation of Au NPs along with the food web. Moreover, Ag NPs and TiO2 NPs may endanger phytoplankton via inducing oxidative stress and compromising photosynthetic activities. For invertebrates, sediment served as the main reservoir and a vital exposure source of Ag NPs and TiO2 NPs. Chironomid larvae, which are associated with benthic substrates and link primary producers to secondary consumers, can be considered the entry point for the Ag transference to the higher trophic levels. Also, chironomids seem to play a critical role in enhancing Ag bioaccumulation due to their feeding habits in macrophytic zones (Williams et al., 2018). In turn, the potential great bioaccumulation and biomagnification of Ag NPs in benthic invertebrates (e.g., shrimp, shellfish) and fish species highlight the risks of aquatic food product consumption. However, the potential of metal NP accumulation in organisms depends on the material. For instance, Ag NPs showed stronger bioaccumulation than TiO2 NPs and biomagnified in fish food webs (Xiao et al., 2019).
In addition, NP interaction with biota at one trophic level may alter the biological response at the next trophic level in a way that is dependent on the delivery scenario (Fig. 4). That is, direct exposure to CuO NPs can cause significantly higher Daphnia magna mortality relative to feeding exposure, whereas neonate production from adult daphnids exposed indirectly to CuO NPs was significantly reduced. Besides, exposure to Cu(OH)2 nanopesticides showed a significant effect on the expression of genes related to detoxification and the reproductive system in D. magna. Short-term (24 h) exposure to the nanopesticide reduced the expression of genes associated with detoxification, but its expression increased significantly after 48 h of exposure. The expression of genes related to the reproductive system changed with concentration and time-dependent manner. These results show the role of genes related to detoxification and the reproductive system in response to Cu(OH)2 nanopesticides. These facts show the importance of evaluating potential ecological impacts of NMs in more relevant, complex exposure scenarios and stress the importance of considering dietary uptake as a pathway for NP exposure (Majumdar et al., 2016; Wu et al., 2017; Aksakal & Arslan, 2020).
Only a few studies evaluated the NM transfer along food chains, including predatory fish as a secondary consumer. TiO2 NPs are among the most studied. For example, Wang et al. (2016a) studied the trophic transfer of TiO2 NPs in a marine benthic food chain from clamworm to juvenile turbot. The authors reported trophic transfer but no biomagnification of TiO2 NPs between trophic levels. Also, only a few studies are assessing the dietary uptake of nanoparticulate Cu in fish. However, some information on NP transfer from invertebrate prey organisms to fish can be inferred from studies that examined intestinal uptake and accumulation of metal oxide NPs from artificial diets (Lammel et al., 2020).
Two arthropod species with different exposure routes to soil contaminants (isopod Porcellio scaber and springtail Folsomia candida) accumulated Ag when exposed to pristine Ag NPs, suggesting a risk for food-chain Ag accumulation. In contrast, no Ag bioaccumulation was detected in the case of the poorly soluble Ag2S NPs, which is the more environmentally relevant form of Ag NPs. From this study, it is verified that soil pH and soil texture are the strongest predictors of Ag bioavailability, respectively, to isopods and springtails and is evidenced the dominant role of dissolution in Ag NP bioavailability (Talaber et al., 2020).
Given that, NP adverse effects, including its transfer through the food chain risks, have to be studied to ensure both the safe use and social acceptance of nanotechnology. In the heterogeneous environment, NP ecotoxicity monitoring is a challenging task as this process is considered dependent on both abiotic and biotic factors. Mammals, including human beings, are the ultimate recipient of the NPs through dermal absorption, inhalation, or ingestion of contaminated food (Abbas et al., 2020). So, the use of more complex experimental systems may evidence routes of exposure that are poorly or not estimated in classical standardized tests based on single-species assessments (Wang et al., 2016b).
4 Risk Analysis and Legislation
Nature-derived biopolymeric NPs such as chitosan and cellulose can be safely incorporated into the food matrix without affecting their sensory properties (Valencia et al., 2019). Therefore, the production of nanofertilizers should focus on the slow release of mineral ions entrapped in NPs of biodegradable, natural polymeric materials, such as chitosan, carboxymethylcellulose, hydroxyapatite, mesoporous silica, etc. Biopolymer–mineral nanoconjugates can be formulated with greater stability, biodegradability, and reduced toxicity (Achari & Kowshik, 2018). Biocompatibility, biodegradability, and low toxicity make chitosan an effective nano-delivery system since it is stable, has low toxicity, and requires simple preparative methods, which make it a versatile and user-friendly drug delivery agent (Chandra et al., 2015).
In the agricultural sector, polymer-based NPs help the local delivery of fertilizers and pesticides without polluting soil and air. Polymers are widely employed for the nanoencapsulation of pesticides. Several studies have also demonstrated the benefits of polymeric nanocarriers to reduce the toxicity of synthetic pesticides toward nontarget crop species. The significant advantage of natural polymers is that they can be degraded by soil microorganisms resulting in environmentally nontoxic products compared to their nondegradable synthetic counterparts. However, the potential ecological and safety benefits of nano-formulations conferred through the reduction in cytotoxicity or ecotoxicity of the active ingredient or reduced proliferation of antibiotic-resistant organisms should also be considered (Siracusa, 2019; Shakiba et al., 2020; Zhao et al., 2020b).
Although polymeric NPs can minimize ecological impacts, vital information on the toxicity of inorganic NMs like TiO2, ZnO, and SiO2 and organic NMs like carbon nanostructures are still lacking. From a safety and regulatory standpoint, proper legislation has to go through more studies and improvements. On the other hand, exposure to NMs may be harmful to the consumer and the environment and might increase risk potential. Risk assessment of NMs is still a controversial and extensive topic because of the lack of sufficient scientific data. The properties, physiological and chemical interactions, and toxicity of NPs under different environments are important considerations before they are commercialized for use in the market. Quality control is also an essential factor to be considered, and product shelf life and stability are important aspects. The cost would be another mitigating factor (Shakiba et al., 2020; Svendsen et al., 2020). Furthermore, products should be tested under relevant field conditions, mainly if they aim to improve production in regions where practices are inadequate and where pedo-climatic conditions are unfavorable and variable. Also, both technological development and improvement of agronomic practices should be considered concurrently, aiming at the reduction of currently used agrochemicals that have lower reliance (Kah & Kookana, 2020).
There is a need to develop proper methods to quantify NMs worldwide since the detection and identification of NMs is very challenging. Furthermore, a reasonable correlation between nanocompounds and toxicology is not yet well explored. For risk management, we should take a systems innovation approach for scaling up from laboratory to industrial level, which is not merely about changes in technical products but also about policy, user practices, infrastructure, and industry structure (Liu et al., 2018). Indeed, there is a lack of scientific data for different regulatory agencies to assess and provide risk management guidelines. It is needed to enhance the knowledge and awareness of nanotechnology applications in agriculture. Advances in these directions will contribute to the fast nanotechnology expansion.
Additionally, more research is needed to apply nanotechnology in different environmental systems and their interaction with organisms and biomolecules (Dasgupta et al., 2015; Abbas et al., 2020). No method dominates in applicability and use over the others, within all contexts. One option is governance using holistic, multi-criteria approaches, which comparatively review risks, benefits, and other implications of nano-enabled products against conventional alternatives (Trump et al., 2018).
The development of standardized testing protocols is needed to allow stakeholders to efficiently and consistently parameterize exposure models (Singh et al., 2019; Svendsen et al., 2020; Xiarchos et al., 2020). As an alternative to analytical methods, the potential NM environmental concentration in a given region can be estimated by in silico modeling approaches (Wigger et al., 2020). Although traditional risk management frameworks for agriculture have largely been deemed adequate for the task, there are several characteristics unique to nanotechnologies that need attention as physical, chemical, and biological properties of NMs that may differ in important ways from the properties of single atoms, molecules, or bulk materials. These proprieties interfere in identifying any direct, indirect, and/or cumulative impacts of NMs and nanotechnologies. Besides, some concerns related to subtle changes in the method of preparation can lead to significant alterations in the physicochemical properties and morphologies of the resulting NPs (Mitter & Hussey, 2019).
For this reason, evaluation of the potential risks resulting from the interaction of NMs with biological systems, humans, and the environment need more studies before commercialization (Sadeghi et al., 2017). Consumer acceptance of foods produced using nanotechnologies is essential for their widespread adoption, and public attitudes toward nano-enabled agriculture would likely vary by area of application. Consumer perception and acceptance will then decide the success or failure of nanotechnologies in agriculture (Hofmann et al., 2020).
Further research on socioeconomic aspects would be ideal while recommending nanopesticides in crops and stored grains. Thus, the commercial use of NMs requires thorough investigations into the screening and optimization of the NMs for different plant species (Usman et al., 2020). The need for adequate regulation to support nanosecurity is critical as its continued advances are quickly translated into new commercial products. Consequently, the lack of validated protocols and a need for regulatory approval before using any new technology have led to a delay in its adoption (Lombi et al., 2019).
The agricultural applications of nanotechnology are affected by several factors, including technological feasibility, cost-effectiveness, regulatory requirements, and consumer acceptance. Since agriculture is, and always has been, a socioecological system, the assessment of new technologies entering it requires integrating different forms of knowledge. To overcome any agri-nanotechnology doubts, it is vital to perform comparative toxicological studies, engage the public and stakeholders in research and innovation, and contribute to developing a transdisciplinary risk governance framework for nanotechnology (Lombi et al., 2019).
To be safely introduced to the market, the risk assessment of these nanoproducts demands establishing the proposed use pattern (Walker et al., 2018). For nanotechnology implementation in agricultural practices, it is necessary to evaluate changes of NM properties in the environment and make an ecotoxicological risks diagnosis due to their use. As a result, nanotoxicology has become a significant concern for all areas. The information obtained may be used by regulatory agencies to assess the potential NM risks throughout different stages of the product life cycle. The effects of using ENMs in agricultural practices cascade throughout their life cycle and include effects from upstream-embodied resources and emissions from ENM production as well as their potential downstream environmental implications. These analyses are important for the agriculture sector due to the relationship between food production, global health, and prosperity (Gilbertson et al., 2020).
Nanoformulations are challenging to implement due to their production costs, legislative uncertainties, and public opinion challenges (Nehra et al., 2021). From the perspective of researchers and stakeholders in agriculture, public understanding can lead to greater security to decide which technological solutions are a priority. Public perception of safety and regulatory concerns surrounding the use of engineered NMs in food production must be addressed to ensure safety and assist the acceptance and adoption of plant nanobiotechnology approaches (Lowry et al., 2019).
5 Conclusion and Perspective
NM applications raise some concerns about their impact on human health and the environment. These concerns emerge because a reliable risk assessment in nanotechnology is yet to be achieved. The reasons for such a shortcoming are the inherent difficulties in characterizing NMs properties (Xiarchos et al., 2020). Understanding NM environmental behavior and the time needed to track them in natural systems is challenging (Wigger et al., 2020). There are uncertainties concerning the use of NMs appropriately in an ethical way to preserve the sustainability of the environment. Nanotechnologies should be considered to ensure intergenerational and ecological equity. Ethics plays a role in protecting our environment from the NM risks and involves identifying and assessing potential risks in the environment. For that, values and actions need to be considered to protect ecological systems (Besha et al., 2020). The incorporation of ethics into a scientific decision support framework for risk governance of NMs is essential.
On the other hand, there is no platform where all stakeholders can meet and discuss these issues. Ethical dilemmas cannot easily be accommodated in an appropriate balance between precaution and innovation as it depends on cultural differences. However, it is important to consider conflicting values and worldviews and place them in historical contexts (Malsch et al., 2020). There is a long way to be covered to produce commercially successful, eco-friendly, and safe nanopesticides. Further studies on environmental fate and bioaccumulation of nanoformulations are still required to develop environmentally friendly and sustainable methods to avoid the excessive use of pesticides (Nehra et al., 2021).
Environmental risks of NMs have mainly focused on the characterization and quantification of their hazards, using standard toxicity assays or slightly adapted procedures to cope with the unique properties of NMs. Dose–response relationships may be derived from nominal exposure concentrations. However, the use of measured concentrations is difficult to obtain with the present methods, and the biological matrices present many challenges to NM detection inside organisms (van den Brink et al., 2019). Then, additional studies are needed for investigating transformation and its related toxicity at environmentally relevant concentrations. Further research is needed to elucidate the influence of transformation processes on NM toxicity and their transformed products (Zhang et al., 2018b). In the agriculture sector, the adoption of a technology is commonly driven by favorable economic trade-offs. Targeted applications, as a soil amendment, seed coating, or foliar spray, will prevent the excessive release of NM to the environment, which will reduce costs to promote crop production and the potential adverse environmental implications as the fate and subsequent consumer exposure potential of NMs (Gilbertson et al., 2020).
As demonstrated throughout this chapter, nanopesticide and nanofertilizer research and development can provide new tools that support the sustainable growth of agriculture, directly impacting the present scenario as in the coming decades. However, despite these advances, it is still necessary to overcome some barriers to the consolidation of these materials. Among these barriers, we can highlight the lack of more specific regulatory protocols for these compounds and the intensification of studies on the fate and behavior of these NMs in the environment. Overcoming these barriers will allow a better understanding of these materials’ effects on nontarget organisms, leading to greater security.
Therefore, more effective collaboration among universities, companies, and government agencies will be needed in order to strengthen and secure these products on the market. In addition, research will be required under more realistic conditions and on larger scales to provide important data for the real assessment of the advantages of these systems. Future research priorities may include developing methods to detect and characterize NMs in complex matrices and determine their transformations in such environments. Furthermore, to assess NM nanosafety, the experimental design must also consider adequate calibration, method validation, accurate dosimetry, and the availability of reference materials (Johnston et al., 2020). More strategic and interdisciplinary research is thus urgently needed to support technological innovation that will help achieve more environmentally sustainable food production (Kah & Kookana, 2020) and reduce the NM input per agricultural area. Biosynthesized NP-based fertilizers and pesticides should be explored further as a promising technology to improve yields while achieving sustainability.
References
Alif Alisha, A. S., & Thangapandiyan, S. A. (2019). Comparative bioassay of silver nanoparticles and malathion on infestation of red flour beetle, Tribolium castaneum. The Journal of Basic Applied Zoology, 80(1), 55. https://doi.org/10.1186/s41936-019-0124-0
Abbas, Q., Yousaf, B., Ullah, H., Ali, M. U., Ok, Y. S., & Rinklebe, J. (2020). Environmental transformation and nano-toxicity of engineered nano-particles (ENPs) in aquatic and terrestrial organisms. Critical Reviews in Environmental Science and Technology, 50(23), 2523–2581. https://doi.org/10.1080/10643389.2019.1705721
Abdel-Aziz, H. M. M., Hasaneen, M. N. A., & Omer, A. M. (2016). Nano chitosan-NPK fertilizer enhances the growth and productivity of wheat plants grown in sandy soil. Dig Spanish Journal of Agricultural Research. https://doi.org/10.5424/sjar/2016141-8205
Abdel-Aziz, H. M. M., Hasaneen, M. N. A., & Omer, A. M. (2018). Foliar application of nano chitosan NPK fertilizer improves the yield of wheat plants grown on two different soils. Egyptian Journal of Experimental Biology (Bot), 14(1), 63–72. https://doi.org/10.5455/egyjebb.20180106032701
Abdel-Haliem, M. E. F., Hegazy, H. S., Hassan, N. S., & Naguib, D. M. (2017). Effect of silica ions and nano silica on rice plants under salinity stress. Ecological Engineering, 99, 282–289. https://doi.org/10.1016/j.ecoleng.2016.11.060
Achari, G. A., & Kowshik, M. (2018). Recent developments on nanotechnology in agriculture: Plant mineral nutrition, health, and interactions with soil microflora. Journal of Agricultural and Food Chemistry, 66, 8647–8661. https://doi.org/10.1021/acs.jafc.8b00691
Acharya, A., & Pal, P. K. (2020). Agriculture nanotechnology: Translating research outcome to field applications by influencing environmental sustainability. NanoImpact, 19, 100232. https://doi.org/10.1016/j.impact.2020.100232
Adhikari, T., Sarkar, D., Mashayekhi, H., et al. (2016). Growth and enzymatic activity of maize (Zea mays L.) plant: Solution culture test for copper dioxide nano particles. Journal of Plant Nutrition, 39(1), 99–115. https://doi.org/10.1080/01904167.2015.1044012
Aksakal, F. I., & Arslan, H. (2020). Detoxification and reproductive system-related gene expression following exposure to Cu(OH)2 nanopesticide in water flea (Daphnia magna Straus 1820). Environmental Science and Pollution Research, 27, 6103–6111. https://doi.org/10.1007/s11356-019-07414-x
Akther, T., & Hemalatha, S. (2019). Mycosilver nanoparticles: Synthesis, characterization and its efficacy against plant pathogenic fungi. BioNanoScience, 9(2), 296–301. https://doi.org/10.1007/s12668-019-0607-y
Alam, T., Khan, R. A. A., Ali, A., Sher, H., Ullah, Z., & Ali, M. (2019). Biogenic synthesis of iron oxide nanoparticles via Skimmia laureola and their antibacterial efficacy against bacterial wilt pathogen Ralstonia solanacearum. Materials Science and Engineering: C, 98, 101–108. https://doi.org/10.1016/j.msec.2018.12.117
Alidoust, D., & Isoda, A. (2013). Effect of γFe2O3 nanoparticles on photosynthetic characteristic of soybean (Glycine max (L.) Merr.): Foliar spray versus soil amendment. Acta Physiologiae Plantarum, 35, 3365–3375. https://doi.org/10.1007/s11738-013-1369-8
Ammar, H. A., & Abd-ElAzeem, E. M. (2020). Novel treatment of gelatin-copper bio-nanoparticles as a management method against the spiny bollworm, Earias insulana, (Boisd.) (Lepidoptera: Noctuidae) in comparison studies with the uncoated nanoparticles. Inorganic Nano-Metal Chemistry, 1–13. https://doi.org/10.1080/24701556.2020.1786403
Arumugam, G., Velayutham, V., Shanmugavel, S., & Sundaram, J. (2016). Efficacy of nanostructured silica as a stored pulse protector against the infestation of bruchid beetle, Callosobruchus maculatus (Coleoptera: Bruchidae). Applied Nanoscience, 6(3), 445–450. https://doi.org/10.1007/s13204-015-0446-2
Attia, R. G., Rizk, S. A., Hussein, M. A., Abdel Fattah, H. M., Khalil, M. M. H., & Mamoun, S. A. M. (2020). Effect of cinnamon oil encapsulated with silica nanoparticles on some biological and biochemical aspects of the rice moth, Corcyra cephalonica (Staint.). (Lepidoptera: Pyralidae). Annals of Agricultural Science, 65(1), 1–5. https://doi.org/10.1016/j.aoas.2020.05.003
Auffan, M., Santaella, C., Brousset, L., Tella, M., Morel, E., Ortet, P., Barakat, M., Chaneac, C., Issartel, J., Angeletti, B., Levard, C., Hazemann, J.-L., Wiesner, M., Rose, J., Thiery, A., & Bottero, J.-Y. (2020). The shape and speciation of ag nanoparticles drive their impacts on organisms in a lotic ecosystem. Environmental Science: Nano Advance Article. https://doi.org/10.1039/d0en00442a
Baccaro, M., Undas, A. K., de Vriendt, J., van den Berg, J. H. J., Peters, R. J. B., & van den Brink, N. W. (2018). Ageing, dissolution and biogenic formation of nanoparticles: How do these factors affect the uptake kinetics of silver nanoparticles in earthworms? Environmental Science: Nano, 5, 1107–1116. https://doi.org/10.1039/c7en01212h
Baker, S., Volova, T., Prudnikova, S. V., Satish, S., & Prasad, N. M. N. (2017). Nanoagroparticles emerging trends and future prospect in modern agriculture system. Environmental Toxicology and Pharmacology, 53, 10–17. https://doi.org/10.1016/j.etap.2017.04.012
Bakhtiari, M., Moaveni, P., & Sani, B. (2015). The effect of iron nanoparticles spraying time and concentration on wheat. Biological Forum, 7(1), 679–683.
Bapat, G., Zinjarde, S., & Tamhane, V. (2020). Evaluation of silica nanoparticle mediated delivery of protease inhibitor in tomato plants and its effect on insect pest Helicoverpa armigera. Colloids and Surfaces. B, Biointerfaces, 193, 111079. https://doi.org/10.1016/j.colsurfb.2020.111079
Bartolucci, C., Antonacci, A., Arduini, F., Moscone, D., Fraceto, L., Campos, E., Attaallah, R., Amine, A., Zanardi, C., Cubillana-Aguilera, L. M., Santander, J. M. P., & Scognamiglio, V. (2020). Green nanomaterials fostering agrifood sustainability. Trends in Analytical Chemistry, 125, 115840. https://doi.org/10.1016/j.trac.2020.115840
Baudrimont, M., Andrei, J., Mornet, S., Gonzalez, P., Mesmer-Dudons, N., Gourves, P.-Y., Jaffal, A., Dedourge-Geffard, O., Geffard, A., Geffard, O., Garric, J., & Feurtet-Mazel, A. (2018). Trophic transfer and effects of gold nanoparticles (AuNPs) in Gammarus fossarum from contaminated periphytic biofilm. Environmental Science and Pollution Research, 25, 11181–11191. https://doi.org/10.1007/s11356-017-8400-3
Bera, B. (2019). Particle size measurement of porous silicon particles: A short review. International Journal of Composit Constituent Materials, 5(1), 8–11.
Besha, A. T., Liu, Y., Bekele, D. N., Dong, Z., Naidu, R., & Gebremariam, G. N. (2020). Sustainability and environmental ethics for the application of engineered nanoparticles. Environmental Science and Policy, 103, 85–98. https://doi.org/10.1016/j.envsci.2019.10.013
Bisquera, K. P. P., Salazar, J. R., Romero, E. S., et al. (2017). Synthesis and characterization as zinc oxide nanoparticles as a source of zinc micronutrient in organic fertilizer. IJAT, 13(7.2), 1695–1706.
Blumenthal, D. J., Heideman, R., Geuzebroek, D., Leinse, A., & Roeloffzen, C. (2018). Silicon nitride in silicon photonics. Proceedings of the IEEE, 106(12), 2209–2231. https://doi.org/10.1109/JPROC.2018.2861576
Bramhanwade, K., Shende, S., Bonde, S., Gade, A., & Rai, M. (2016). Fungicidal activity of Cu nanoparticles against Fusarium causing crop diseases. Environmental Chemistry Letters, 14(2), 229–235. https://doi.org/10.1007/s10311-015-0543-1
Bruinsma, J. (2017). World agriculture: Towards 2015/2030: An FAO study. Routledge.
Chae, Y., Kim, S. W., & An, Y.-J. (2016). In vivo visual evaluation of nanoparticle transfer in a three-species terrestrial food chain. Chemosphere, 151, 101–107. https://doi.org/10.1016/j.chemosphere.2016.02.075
Chandra, S., Chakraborty, N., Dasgupta, A., Sarkar, J., Panda, K., & Acharya, K. (2015). Chitosan nanoparticles: A positive modulator of innate immune responses in plants. Scientific Reports, 5, 15195. https://doi.org/10.1038/srep15195
Chaudhry, N., Dwivedi, S., Chaudhry, V., Singh, A., Saquib, Q., Azam, A., & Musarrat, J. (2018). Bio-inspired nanomaterials in agriculture and food: Current status, foreseen applications and challenges. Microbial Pathogenesis, 123, 196–200. https://doi.org/10.1016/j.micpath.2018.07.013
Chen, C., Gao, Z., Qiu, X., & Hu, S. (2013). Enhancement of the controlled-release properties of chitosan membranes by crosslinking with suberoyl chloride. Molecules, 18, 7239–7252. https://doi.org/10.3390/molecules18067239
Chetwynd, A. J., & Lynch, I. (2020). The rise of the nanomaterial metabolite corona, and emergence of the complete corona. Environmental Science: Nano, 7, 1041. https://doi.org/10.1039/c9en00938h
Chhipa, H. (2019). Chapter 5—Mycosynthesis of nanoparticles for smart agricultural practice: A green and eco-friendly approach. In A. K. Shukla & S. Iravani (Eds.), Green synthesis, characterization and applications of nanoparticles (pp. 87–109). Elsevier.
Choudhary, R. C., Joshi, A., Kumari, S., et al. (2017). Preparation of cu-chitosan nanoparticle and its effect on growth and enzyme activity during seed germination in maize. Journal of Pharmacognosy Phytochemistry, 6(4), 669–673.
Choudhary, R. C., Kumaraswamy, R. V., Kumari, S., et al. (2019). Zinc encapsulated chitosan nanoparticle to promote maize crop yield. International Journal of Biological Macromolecules, 127, 126–135. https://doi.org/10.1016/j.ijbiomac.2018.12.274
Chouhan, D., & Mandal, P. (2020). Applications of chitosan and chitosan based metallic nanoparticles in agrosciences-a review. International Journal of Biological Macromolecules. https://doi.org/10.1016/j.ijbiomac.2020.11.035
Clemente, Z., Castro, V. L., Feitosa, L., Lima, R., Jonsson, C. M., Maia, A., & Fraceto, L. F. (2013). Fish exposure to nano-TiO2 under different experimental conditions: Methodological aspects for nanoecotoxicology investigations. Science of the Total Environment, 463–464, 647–656. https://doi.org/10.1016/j.scitotenv.2013.06.022
Clemente, Z., Castro, V. L., Feitosa, L. O., Lima, R., Jonsson, C. M., Maia, A. H. N., & Fraceto, L. F. (2015). Biomarker evaluation in fish after prolonged exposure to nano-TiO2: Influence of illumination conditions and crystal phase. Journal of Nanoscience and Nanotechnology, 15, 5424–5433. https://doi.org/10.1166/jnn.2015.10021
Clemente, Z., Castro, V. L., Moura, M. A. M., Jonsson, C. M., & Fraceto, L. F. (2014). Toxicity assessment of TiO2 nanoparticles in zebrafish embryos under different exposure conditions. Aquatic Toxicology, 147, 129–139. https://doi.org/10.1016/j.aquatox.2013.12.024
Courtois, P., Rorat, A., Lemiere, S., Levard, C., Chaurand, P., Grobelak, A., Lors, C., & Vandenbulcke, F. (2020). Accumulation, speciation and localization of silver nanoparticles in the earthworm Eisenia fetida. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-020-08548-z
Cui, J., Liu, T., Li, F., Yi, J., Liu, C., & Yu, H. (2017). Silica nanoparticles alleviate cadmium toxicity in rice cells: Mechanisms and size effects. Environmental Pollution Barking Essex, 1987(228), 363–369. https://doi.org/10.1016/j.envpol.2017.05.014
Das, S., Yadav, A., & Debnath, N. (2019). Entomotoxic efficacy of aluminium oxide, titanium dioxide and zinc oxide nanoparticles against Sitophilus oryzae (L.): A comparative analysis. Journal of Stored Products Research, 83, 92–96. https://doi.org/10.1016/j.jspr.2019.06.003
Dasgupta, N., Ranjan, S., Mundekkad, D., Ramalingam, C., Shanker, R., & Kumar, A. (2015). Nanotechnology in agro-food: From field to plate. Food Research International, 69, 381–400. https://doi.org/10.1016/j.foodres.2015.01.005
De Marchi, L., Pretti, C., Gabriel, B., Marques, P. A. A. P., Freitas, R., & Neto, V. (2018). An overview of graphene materials: Properties, applications and toxicity on aquatic environments. Science of the Total Environment, 631-632, 1440–1456. https://doi.org/10.1016/j.scitotenv.2018.03.132
Deline, A. R., & Nason, J. A. (2019). Evaluation of labeling methods used for investigating the environmental behavior and toxicity of metal oxide nanoparticles. Environmental Science: Nano, 6, 1043–1066. https://doi.org/10.1039/c8en01187g
Deshpande, P., Dapkekar, A., Oak, M. D., et al. (2017). Zinc complexed chitosan/TPP nanoparticles: A promising micronutrient nanocarrier suited for foliar application. Carbohydrate Polymers, 165, 394–401. https://doi.org/10.1016/j.carbpol.2017.02.061
Dong, F., & Zhou, Y. (2020). Distinct mechanisms in the heteroaggregation of silver nanoparticles with mineral and microbial colloids. Water Research, 170, 115332. https://doi.org/10.1016/j.watres.2019.115332
Drostkar, E., Talebi, R., & Kanouni, H. (2016). Foliar application of Fe, Zn and NPK nano-fertilizers on seed yield and morphological traits in chickpea under rainfed condition. Journal of Research in Ecology, 4(2), 221–228.
Du, W., Xu, Y., Yin, Y., Ji, R., & Guo, H. (2018). Risk assessment of engineered nanoparticles and other contaminants in terrestrial plants. Current Opinion in Environmental Science & Health, 6, 21–28. https://doi.org/10.1016/j.coesh.2018.07.010
Dzung, N. A., Khanh, V. T. P., & Dzung, T. T. (2011). Research on impact of chitosan oligomers on biophysical characteristics, growth, development and drought resistance of coffee. Carbohydrate Polymers, 84, 751–755. https://doi.org/10.1016/j.carbpol.2010.07.066
El-Helaly, A. A., Mak, E.-S., & S E. (2016). The silica-nano particles treatment of squash foliage and survival and development of Spodoptera littoralis (Bosid.) larvae. Journal of Entomology and Zoology Studies, 4(1), 175–180.
Ellis, L.-J. A., & Lynch, I. (2020). Mechanistic insights into toxicity pathways induced by nanomaterials in Daphnia magna from analysis of the composition of the acquired protein corona. Environmental Science: Nano. https://doi.org/10.1039/d0en00625d
El-Naggar, M. E., Abdelsalam, N. R., Fouda, M. M. G., Mackled, M. I., Al-Jaddadi, M. A. M., Ali, H. M., Siddiqui, M. H., & Kandil, E. E. (2020). Soil application of nano silica on maize yield and its insecticidal activity against some stored insects after the post-harvest. Nanomaterials, 10(4), 739. https://doi.org/10.3390/nano10040739
El-Saadony, M. T., Abd El-Hack, M. E., Taha, A. E., Fouda, M. M. G., Ajarem, J. S., Maodaa, S. N., Allam, A. A., & Elshaer, N. (2020). Ecofriendly synthesis and insecticidal application of copper nanoparticles against the storage pest tribolium castaneum. Nanomaterials, 10(3), 587. https://doi.org/10.3390/nano10030587
Elshamy, M. T., ELKhallal, S. M., Husseiny, et al. (2019). Application of nano-chitosan NPK fertilizer on growth and productivity of potato plant. Journal of Scientific Research in Science, 36(1), 424–441. https://doi.org/10.21608/JSRS.2019.58522
Falahati, M., Attar, F., Sharifi, M., Haertlé, T., Berret, J.-F., Hasan, R., Ali, K., & Sabourye, A. (2019). A health concern regarding the protein corona, aggregation and disaggregation. BBA—General Subjects, 1863, 971–991. https://doi.org/10.1016/j.bbagen.2019.02.012
Falinski, M. M., Plata, D. L., Chopra, S. S., Theis, T. L., Gilbertson, L. M., & Zimmerman, J. B. (2018). A framework for sustainable nanomaterial selection and design based on performance, hazard, and economic considerations. Nature Nanotechnology, 13(8), 708–714. https://doi.org/10.1038/s41565-018-0120-4
Feng, J., Chen, W., Shen, Y., Chen, Q., Yang, J., Zhang, M., Yang, W., & Yuan, S. (2020). Fabrication of abamectin-loaded mesoporous silica nanoparticles by emulsion-solvent evaporation to improve photolysis stability and extend insecticidal activity. Nanotechnology, 31(34), 345705. https://doi.org/10.1088/1361-6528/ab91f0
Fraceto, L. F., Grillo, R., Medeiros, G. A., et al. (2016). Nanotechnology in agriculture: Which innovation potential does it have? Frontiers in Environmental Science. https://doi.org/10.3389/fenvs.2016.00020
Gahukar, R. T., & Das, R. K. (2020). Plant-derived nanopesticides for agricultural pest control: Challenges and prospects. Nanotechnology for Environmental Engineering, 5, 3. https://doi.org/10.1007/s41204-020-0066-2
Galhardi, J. A., Fraceto, L. F., Wilkinson, K. J., & Ghoshal, S. (2020). Soil enzyme activities as an integral part of the environmental risk assessment of nanopesticides. Journal of Agricultural and Food Chemistry, 68, 8514–8516. https://doi.org/10.1021/acs.jafc.0c04344
Gao, Y., Xiao, Y., Mao, K., Qin, X., Zhang, Y., Li, D., Zhang, Y., Li, J., Wan, H., & He, S. (2020). Thermoresponsive polymer-encapsulated hollow mesoporous silica nanoparticles and their application in insecticide delivery. Chemical Engineering Journal, 383, 123169. https://doi.org/10.1016/j.cej.2019.123169
Ghafariyan, M. H., Malakouti, M. J., Dadpour, M. R., et al. (2013). Effects of magnetite nanoparticles on soybean chlorophyll. Environmental Science & Technology, 47(18), 10645–10652. https://doi.org/10.1021/es402249b
Ghassemi-Golezani, K., & Afkhami, N. (2018). Changes in some morpho-physiological traits of safflower in response to water deficit and nano-fertilizers. Journal of Biological & Environmental Science, 12(3), 391–398.
Gilbertson, L. M., Pourzahedi, L., Laughton, S., Gao, X., Zimmerman, J. B., Theis, T. L., Westerhoff, P., & Lowry, G. (2020). Guiding the design space for nanotechnology to advance sustainable crop production. Nature Nanotechnology, 15(9), 801–810. https://doi.org/10.1038/s41565-020-0706-5
Girilal, M., Krishnakumar, V., Poornima, P., Fayaz, A. M., & Kalaichelvan, P. T. (2015). A comparative study on biologically and chemically synthesized silver nanoparticles induced heat shock proteins on fresh water fish Oreochromis niloticus. Chemosphere, 139, 461–468. https://doi.org/10.1016/j.chemosphere.2015.08.005
Gouda, S., George Kerry, R., Das, G., & Kumar Patra, J. (2019). Chapter 11—Synthesis of nanoparticles utilizing sources from the mangrove environment and their potential applications: An overview. In D. K. Tripathi, P. Ahmad, S. Sharma, D. K. Chauhan, & N. K. Dubey (Eds.), Nanomaterials in plants, algae and microorganisms (pp. 219–235). Academic Press.
Graily-Moradi, F., Maadani Mallak, A., & Ghorbanpour, M. (2020). Biogenic synthesis of gold nanoparticles and their potential application in agriculture. In M. Ghorbanpour, P. Bhargava, A. Varma, & D. K. Choudhary (Eds.), Biogenic nano-particles and their use in agro-ecosystems (pp. 187–204). Springer.
Gunaratne, G. P., Kottegoda, N., Madusanka, N., et al. (2016). Two new plant nutrient nanocomposites based on urea coated hydroxyapatite: Efficacy and plant uptake. Indian Journal of Agricultural Sciences, 86(4), 494–499.
Guo, H., White, J. C., Wang, Z., et al. (2018). Nano-enabled fertilizers to control the release and use efficiency of nutrients. Current Opinion in Environmental Science & Health, 6, 77–83. https://doi.org/10.1016/j.coesh.2018.07.009
Ha, N. M. C., Nguyen, T. H., Wang, S., et al. (2019). Preparation of NPK nanofertilizer based on chitosan nanoparticles and its effect on biophysical characteristics and growth of coffee in green house. Research on Chemical Intermediates, 45, 51–63. https://doi.org/10.1007/s11164-018-3630-7
Haroun, S. A., Elnaggar, M. E., Zein, D. M., & Gad, R. I. (2020). Insecticidal efficiency and safety of zinc oxide and hydrophilic silica nanoparticles against some stored seed insects. Journal of Plant Protection Research, 60(1), 77–85. https://doi.org/10.24425/jppr.2020.132211
He, X., Deng, H., & Hwang, H. (2019). The current application of nanotechnology in food and agriculture. Journal of Food and Drug Analysis, 27(1), 1–21. https://doi.org/10.1016/j.jfda.2018.12.002
He, X., Fu, P., Aker, W. G., & Hwang, H.-M. (2018). Toxicity of engineered nanomaterials mediated by nano–bio–eco interactions. Journal of Environmental Science and Health, Part C, 36, 21–42. https://doi.org/10.1080/10590501.2017.1418793
Hofmann, T., Lowry, G. V., Ghoshal, S., Tufenkji, N., Brambilla, D., Dutcher, J. R., Gilbertson, L. M., Giraldo, J. P., Kinsella, J. M., Landry, M. P., Lovell, W., Naccache, R., Paret, M., Pedersen, J. A., Unrine, J. M., White, J. C., & Wilkinson, K. J. (2020). Technology readiness and overcoming barriers to sustainably implement nanotechnology-enabled plant agriculture. Nature Food, 1, 416–425. https://doi.org/10.1038/s43016-020-0110-1
Hortin, J. M., Anderson, A. J., Britt, D. W., Jacobson, A. R., & McLean, J. E. (2020). Copper oxide nanoparticle dissolution at alkaline pH is controlled by dissolved organic matter: Influence of soil-derived organic matter, wheat, bacteria, and nanoparticle coating, Environmental Science: Nano. https://doi.org/10.1039/d0en00574f
Jameel, M., Shoeb, M., Khan, M. T., Ullah, R., Mobin, M., Farooqi, M. K., & Adnan, S. M. (2020). Enhanced insecticidal activity of thiamethoxam by zinc oxide nanoparticles: A novel nanotechnology approach for Pest control. ACS Omega, 5(3), 1607–1615. https://doi.org/10.1021/acsomega.9b03680
Jeelani, P. G., Mulay, P., Venkat, R., & Ramalingam, C. (2020). Multifaceted application of silica nanoparticles. A Review. Silicon, 12(6), 1337–1354. https://doi.org/10.1007/s12633-019-00229-y
Johnston, L. J., Gonzalez-Rojano, N., Wilkinson, K. J., & Xing, B. (2020). Key challenges for evaluation of the safety of engineered nanomaterials. NanoImpact, 18, 100219. https://doi.org/10.1016/j.impact.2020.100219
Kah, M., & Kookana, R. (2020). Emerging investigator series: Nanotechnology to develop novel agrochemicals: Critical issues to consider in the global agricultural context. Environmental Science: Nano, 7, 1867–1873. https://doi.org/10.1039/d0en00271b
Kah, M., Kookana, R. S., Gogos, A., & Bucheli, T. D. (2018). A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nature Nanotechnology, 13(8), 677. https://doi.org/10.1038/s41565-018-0131-1
Kalra, T., Tomar, P. C., & Arora, K. (2020). Micronutrient encapsulation using nanotechnology: Nanofertilizers. Plant Archives, 20(2), 1748–1753.
Kaman, P. K., & Dutta, P. (2019). Synthesis, characterization and antifungal activity of biosynthesized silver nanoparticle. Indian Phytopathology, 72(1), 79–88. https://doi.org/10.1007/s42360-018-0081-4
Kashyapa, P. L., Xiang, X., & Heiden, P. (2015). Chitosan nanoparticle based delivery systems for sustainable agriculture. International Journal of Biological Macromolecules, 77, 36–51. https://doi.org/10.1016/j.ijbiomac.2015.02.039
Kim, J. I., Park, H.-G., Chang, K.-H., Nam, D. H., & Yeo, M.-K. (2016). Trophic transfer of nano-TiO2 in a paddy microcosm: A comparison of single-dose versus sequential multi-dose exposures. Environmental Pollution, 212, 316–324. https://doi.org/10.1016/j.envpol.2016.01.076
Klaessig, F. C. (2018). Dissolution as a paradigm in regulating nanomaterials. Environmental Science: Nano, 5, 1070. https://doi.org/10.1039/c7en01130j
Kopittke, P. M., Lombi, E., Wang, P., et al. (2019). Nanomaterials as fertilizers for improving plant mineral nutrition and environmental outcomes. Environmental Science. Nano, 6, 3513–3524. https://doi.org/10.1039/C9EN00971J
Kora, A. J., Mounika, J., & Jagadeeshwar, R. (2020). Rice leaf extract synthesized silver nanoparticles: An in vitro fungicidal evaluation against Rhizoctonia solani, the causative agent of sheath blight disease in rice. Fungal Biology, 124(7), 671–681. https://doi.org/10.1016/j.funbio.2020.03.012
Kottegoda, N., Munaweera, I., Madusanka, N., et al. (2011). Agreen slow-release fertilizer composition based on urea-modified hydroxyapatite nanoparticles encapsulated wood. Current Science, 101, 73–78.
Kottegoda, N., Sandaruwan, C., Priyadarshana, G., et al. (2017). Urea-hydroxyapatite nanohybrids for slow release of nitrogen. ACS Nano, 11, 1214–1221. https://doi.org/10.1021/acsnano.6b07781
Kraegeloh, A., Suarez-Merino, B., Sluijters, T., & Micheletti, C. (2018). Implementation of safe-by-design for nanomaterial development and safe innovation: Why we need a comprehensive approach. Nanomaterials, 8(4), 239. https://doi.org/10.3390/nano8040239
Kranjc, E., & Drobne, D. (2019). Nanomaterials in plants: A review of hazard and applications in the agri-food sector. Nanomaterials (Basel), 9(8), 1094. https://doi.org/10.3390/nano9081094
Krishnaraj, C., Harper, S. L., & Yun, S. (2016). In vivo toxicological assessment of biologically synthesized silver nanoparticles in adult Zebrafish (Danio rerio). Journal of Hazardous Materials, 301, 480–491. https://doi.org/10.1016/j.jhazmat.2015.09.022
Kumari, M., Giri, V. P., Pandey, S., Kumar, M., Katiyar, R., Nautiyal, C. S., & Mishra, A. (2019). An insight into the mechanism of antifungal activity of biogenic nanoparticles than their chemical counterparts. Pesticide Biochemistry and Physiology, 157, 45–52. https://doi.org/10.1016/j.pestbp.2019.03.005
Lakshmi, S. J., Rs, R. B., & Nadagouda, S. (2020). Effect of zinc oxide nanoparticles on pulse beetle (Callosobruchus maculatus) (Col.: Chrysomelidae) in greengram. Journal of Entomological Zoology Studies, 4.
Lammel, T., Thit, A., Cui, X., Mouneyrac, C., Baun, A., Valsami-Jones, E., Sturve, J., & Selck, H. (2020). Trophic transfer of CuO NPs from sediment to worms (Tubifex tubifex) to fish (Gasterosteus aculeatus): A comparative study of dissolved Cu and NPs enriched with a stable isotope tracer (65Cu). Environmental Science: Nano, 7, 2360–2372. https://doi.org/10.1039/d0en00227e
Lawre, S. L., & Raskar, S. (2014). Influence of zinc oxide nanoparticles on growth, flowering and seed productivity in onion. International Journal of Current Microbiology and Applied Sciences, 3(7), 874–881.
Lewis, R. W., Bertsch, P. M., & McNear, D. H. (2019). Nanotoxicity of engineered nanomaterials (ENMs) to environmentally relevant beneficial soil bacteria - a critical review. Nanotoxicology, 13(3), 392–428. https://doi.org/10.1080/17435390.2018.1530391
Liu, R., & Lal, R. (2014). Synthetic apatite nanoparticles as a phosphorus fertilizer for soybean (Glycine max). Scientific Reports, 4. https://doi.org/10.1038/srep05686
Liu, R., & Lal, R. (2015). Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Science Total Environment, 514, 131–139. https://doi.org/10.1016/j.scitotenv.2015.01.104
Liu, S., Lu, Y., & Chen, W. (2018). Bridge knowledge gaps in environmental health and safety for sustainable development of nano-industries. Nano Today, 23, 11–15. https://doi.org/10.1016/j.nantod.2018.09.002
Liu, W., Worms, I., & Slaveykova, V. I. (2020). Interaction of silver nanoparticles with antioxidant enzymes. Environmental Science: Nano, 7, 1507–1517. https://doi.org/10.1039/c9en01284b
Lombi, E., Donner, E., Dusinska, M., & Wickson, F. (2019). A one health approach to managing the applications and implications of nanotechnologies in agriculture. Nature Nanotechnology, 14, 523–531. https://doi.org/10.1038/s41565-019-0460-8
Lowry, G. V., Avellan, A., & Gilbertson, L. M. (2019). Opportunities and challenges for nanotechnology in the Agri-tech revolution. Nature Nanotechnology, 14, 517–522. https://doi.org/10.1038/s41565-019-0461-7
Lüderwald, S., Meyer, F., Gerstle, V., Friedrichs, L., Rolfing, K., Schreiner, V. C., Bakanov, N., Schulz, R., & Bundschuh, M. (2020). Reduction of pesticide toxicity under field-relevant conditions? The interaction of titanium dioxide nanoparticles, ultraviolet, and natural organic matter. Environmental Toxicology and Chemistry. https://doi.org/10.1002/etc.4851
Luo, Z., Li, Z., Xie, Z., Sokolova, I. M., Song, L., Peijnenburg, W. J. G. M., Hu, M., & Wang, Y. (2020). Rethinking nano-TiO2 safety: Overview of toxic effects in humans and aquatic animals. Small, 16, 2002019. https://doi.org/10.1002/smll.202002019
Maghsoodi, M. R., Ghodszad, L., & Lajayer, B. A. (2020). Dilemma of hydroxyapatite nanoparticles as phosphorus fertilizer: Potentials, challenges and effects on plants. Environmental Technology and Innovation, 19, 100869. https://doi.org/10.1016/j.eti.2020.100869
Mahdieh, M., Sangi, M. R., Bamdad, F., et al. (2018). Effect of seed and foliar application of nano-zinc oxide, zinc chelate, and zinc sulphate rates on yield and growth of pinto bean (Phaseolus vulgaris) cultivars. Journal of Plant Nutrition, 41(18), 2401–2412. https://doi.org/10.1080/01904167.2018.1510517
Mahil, E. I. T., & Kumar, B. A. (2019). Foliar application of nanofertilizers in agricultural crops–a review. Journal of Farm Science, 32(3), 239–249.
Majumdar, S., Trujillo-Reyes, J., Hernandez-Viezcas, J. A., White, J. C., Peralta-Videa, J. R., & Gardea-Torresdey, J. L. (2016). Cerium biomagnification in a terrestrial food chain: Influence of particle size and growth stage. Environmental Science & Technology, 50, 6782–6792. https://doi.org/10.1021/acs.est.5b04784
Majumdar, T. D., Singh, M., Thapa, M., Dutta, M., Mukherjee, A., & Ghosh, C. K. (2019). Size-dependent antibacterial activity of copper nanoparticles against Xanthomonas oryzae pv. Oryzae – A synthetic and mechanistic approach. Colloid Interface. Science Communication, 32, 100190. https://doi.org/10.1016/j.colcom.2019.100190
Malsch, I., Isigonis, P., Dusinska, M., & Bouman, E. A. (2020). Embedding ethical impact assessment in nanosafety decision support. Small, 16, e2002901. https://doi.org/10.1002/smll.202002901
Marathe, K., Naik, J., & Maheshwari, V. (2020). Biogenic synthesis of silver nanoparticles using Streptomyces spp. and their antifungal activity against fusarium verticillioides. Journal of Cluster Science. https://doi.org/10.1007/s10876-020-01894-5
Marchiol, L., Filippi, A., Adamiano, A., et al. (2019). Influence of hydroxyapatite nanoparticles on germination and plant metabolism of tomato (Solanum lycopersicum L.): Preliminary evidence. Agronomy, 9, 4. https://doi.org/10.3390/agronomy9040161
Matouke, M. M., & Mustapha, M. (2018). Bioaccumulation and physiological effects of copepods sp. (Eucyclop sp.) fed Chlorella ellipsoides exposed to titanium dioxide (TiO2) nanoparticles and lead (Pb2+). Aquatic Toxicology, 198, 30–39. https://doi.org/10.1016/j.aquatox.2018.02.013
Milani, N., McLaughlin, M. J., Stacey, S. P., et al. (2012). Dissolution kinetics of macronutrient fertilizers coated with manufactured zinc oxide nanoparticles. Journal of Agricultural and Food Chemistry, 60(16), 3991–3998. https://doi.org/10.1021/jf205191y
Mitter, N., & Hussey, K. (2019). Moving policy and regulation forward for nanotechnology applications in agriculture. Nature Nanotechnology, 14, 508–514. https://doi.org/10.1038/s41565-019-0464-4
Moghadam, A. L., Vattani, H., Baghaei, N., et al. (2012). Effect of different levels of fertilizer nanoiron chelates on growth and yield characteristics of two varieties of spinach (Spinacia oleracea L.): Varamin 88 and viroflay. Research Journal of Applied Sciences, Engineering and Technology, 4(12), 4813–4818.
Monikh, F. A., Zhai, Y., Wu, J., Grillo, R., Arenas-Lago, D., Darbha, G. K., Vijver, M. G., & Peijnenburg, W. (2020). Interaction between a nano-formulation of atrazine and the rhizosphere bacterial community: Atrazine degradation and bacterial community alterations. Environmental Science: Nano Accepted Manuscript. https://doi.org/10.1039/D0EN00638F
Mujtaba, M., Khawar, K. M., Camara, M. C., et al. (2020). Chitosan-based delivery systems for plants: A brief overview of recent advances and future directions. International Journal of Biological Macromolecules, 154, 683–697. https://doi.org/10.1016/j.ijbiomac.2020.03.128
Nehra, M., Dilbaghi, N., Marrazza, G., Kaushik, A., Sonne, C., Kim, K.-H., & Kumar, S. (2021). Emerging nanobiotechnology in agriculture for the management of pesticide residues. Journal of Hazardous Materials, 401, 123369. https://doi.org/10.1016/j.jhazmat.2020.123369
Nemati, T., Sarkheil, M., & Johari, S. A. (2019). Trophic transfer of CuO nanoparticles from brine shrimp (Artemia salina) nauplii to convict cichlid (Amatitlania nigrofasciata) larvae: Uptake, accumulation and elimination. Environmental Science and Pollution Research, 26(10), 9610–9618. https://doi.org/10.1007/s11356-019-04263-6
Nibin, P. M., & Ushakumari. (2019). Organic nano NPK formulations for enhancing soil post-harvest nutrient status of bhindi. Journal of Pharmacognosy & Phytochemistry, SP2, 22–25.
Oca-Vasquez, G. M., Solano-Campos, F., Vega-Baudrit, J. R., Lopez-Mondejar, R., Odriozola, I., Vera, A., Moreno, J. L., & Bastida, F. (2020). Environmentally relevant concentrations of silver nanoparticles diminish soil microbial biomass but do not alter enzyme activities or microbial diversity. Journal of Hazardous Materials, 391, 122224. https://doi.org/10.1016/j.jhazmat.2020.122224
Odzak, N., Kistler, D., & Sigg, L. (2017). Influence of daylight on the fate of silver and zinc oxide nanoparticles in natural aquatic environments. Environmental Pollution, 226, 1–11. https://doi.org/10.1016/j.envpol.2017.04.006
Ogunsuyi, O. M., Adegoye, E. O., Ogunsuyi, O. I., Alabi, O. A., Alimba, C. G., & Bakare, A. A. (2020). Titanium dioxide nanoparticles-induced cytogenotoxicity and alterations in haematological indices of Clarias gariepinus (Burchell, 1822). Toxicology and Industrial Health, 36, 807. https://doi.org/10.1177/0748233720948682
Ong, K. J., Felix, L. C., Boyle, D., Ede, J. D., Ma, G., Veinot, J. G. C., & Goss, G. G. (2017). Humic acid ameliorates nanoparticle-induced developmental toxicity in zebrafish. Environmental Science: Nano, 4, 127. https://doi.org/10.1039/C6EN00408C
Ottoni, C. A., Lima Neto, M. C., Leo, P., Ortolan, B. D., Barbieri, E., & de Souza, A. O. (2020). Environmental impact of biogenic silver nanoparticles in soil and aquatic organisms. Chemosphere, 239, 124698. https://doi.org/10.1016/j.chemosphere.2019.124698
Parisi, C., Vigani, M., & Rodríguez-Cerezo, E. (2015). Agricultural nanotechnologies: What are the current possibilities? Nano Today, 10(2), 124–127. https://doi.org/10.1016/j.nantod.2014.09.009
Peng, G., He, Y., Zhao, M., Yu, T., Qin, Y., & Lin, S. (2018). Differential effects of metal oxide nanoparticles on zebrafish embryos and developing larvae. Environmental Science: Nano, 5, 1200. https://doi.org/10.1039/c8en00190a
Pérez-de-Luque, A. (2017). Interaction of nanomaterials with plants: What do we need for real applications in agriculture. Frontiers in Environmental Science, 5, 1–7. https://doi.org/10.3389/fenvs.2017.00012
Petersen, E. J., Mortimer, M., Burgess, R. M., Handy, R., Hanna, S., Ho, K. T., Johnson, M., Loureiro, S., Selck, H., Scott-Fordsmand, J. J., Spurgeon, D., Unrine, J., van den Brink, N. W., Wang, Y., White, J., & Holden, P. (2019). Strategies for robust and accurate experimental approaches to quantify nanomaterial bioaccumulation across a broad range of organisms. Environmental Science: Nano, 6, 1619–1656. https://doi.org/10.1039/C8EN01378K
Pradhan, S., Hedberg, J., Rosenqvist, J., Jonsson, C. M., Wold, S., Blomberg, E., & Wallinder, I. O. (2018). Influence of humic acid and dihydroxy benzoic acid on the agglomeration, adsorption, sedimentation and dissolution of copper, manganese, aluminum and silica nanoparticles - a tentative exposure scenario. PLoS One, 13(2), e0192553. https://doi.org/10.1371/journal.pone.0192553
Pradhan, S., & Mailapalli, D. R. (2017). Interaction of engineered nanoparticles with the Agri-environment. Journal of Agricultural and Food Chemistry, 65, 8279–8294. https://doi.org/10.1021/acs.jafc.7b02528
Pradhan, S., Patra, P., Das, S., et al. (2013). Photochemical modulation of biosafe manganese nanoparticles on Vigna radiata: A detailed molecular, biochemical, and biophysical study. Environmental Science & Technology, 47(22), 13122–13131. https://doi.org/10.1021/es402659t
Pradhan, S., Patra, P., Mitra, S., et al. (2014). Manganese nanoparticles: Impact on non-nodulated plant as a potent enhancer in nitrogen metabolism and toxicity study both in vivo and in vitro. Journal of Agricultural and Food Chemistry, 62(35), 8777–8785. https://doi.org/10.1021/jf502716c
Preetha, P. S., & Balakrishnan, N. (2017). A review of nano fertilizers and their use and functions in soil. International Journal of Current Microbiology and Applied Sciences, 6(12), 3117–3133. https://doi.org/10.20546/ijcmas.2017.612.364
Qureshi, A., Singh, D. K., & Dwivedi, S. (2018). Nano-fertilizers: A novel way for enhancing nutrient use efficiency and crop productivity. International Journal of Current Microbiology and Applied Sciences, 7(2), 3325–3335. https://doi.org/10.20546/ijcmas.2018.702.398
Raftis, J. B., & Miller, M. R. (2019). Nanoparticle translocation and multi-organ toxicity: A particularly small problem. Nano Today, 26, 8–12. https://doi.org/10.1016/j.nantod.2019.03.010
Rahman, M. A., Parvin, A., Khan, M. S. H., War, A. R., Lingaraju, K., Prasad, R., Das, S., Hussain, B., & Bhattacharyya, A. (2020). Efficacy of the green synthesized nickel-oxide nanoparticles against pulse beetle, Callosobruchus maculatus (F.) in black gram (Vigna mungo L.). International Journal of Pest Management, 1–9. https://doi.org/10.1080/09670874.2020.1773572
Raju, D., Mehta, U. J., & Beedu, S. R. (2016). Biogenic green synthesis of monodispersed gum kondagogu (Cochlospermum gossypium) iron nanocomposite material and its application in germination and growth of mung bean (Vigna radiata) as a plant model. IET Nanobiotechnology, 10(3), 141–146. https://doi.org/10.1049/iet-nbt.2015.0112
Raliya, R., Nair, R., Chavalmane, S., et al. (2015). Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics, 7, 1584–1594. https://doi.org/10.1039/C5MT00168D
Raliya, R., Saharan, V., Dimkpa, C., et al. (2018). Nanofertilizer for precision and sustainable agriculture: Current state and future perspectives. Journal of Agricultural and Food Chemistry, 66, 6487–6503. https://doi.org/10.1021/acs.jafc.7b02178
Ramírez-Rodríguez, G. B., Dal Sasso, G., Carmona, F. J., et al. (2020). Engineering biomimetic calcium phosphate nanoparticles: A green synthesis of slow-release multinutrient (NPK) Nanofertilizers. ACS Applied Bio Materials, 3(3), 1344–1353. https://doi.org/10.1021/acsabm.9b00937
Rastogi, A., Zivcak, M., Sytar, O., et al. (2017). Impact of metal and metal oxide nanoparticles on plant: A critical review. Frontiers in Chemistry, 5. https://doi.org/10.3389/fchem.2017.00078
Rossi, L., Fedenia, L. N., Sharifana, H., et al. (2019). Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee (Coffea arabica L.) plants. Plant Physiology and Biochemistry, 135, 160–166. https://doi.org/10.1016/j.plaphy.2018.12.005
Rui, M. M., Ma, C. X., Hao, Y., et al. (2016). Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Frontiers in Plant Science, 7. https://doi.org/10.3389/fpls.2016.00815
Ruttkay-Nedecky, B., Krystofova, O., Nejdl, L., et al. (2017). Nanoparticles based on essential metals and their phytotoxicity. Journal of Nanobiotechnology, 15, 33. https://doi.org/10.1186/s12951-017-0268-3
Sadeghi, R., Rodriguez, R. J., Yao, Y., & Kokini, J. L. (2017). Advances in nanotechnology as they pertain to food and agriculture: Benefits and risks. Annual Review of Food Science and Technology, 8, 467–492. https://doi.org/10.1146/annurev-food-041715-033338
Saharan, V., Kumaraswamy, R. V., Choudhary, R. C., et al. (2016). Cu-chitosan nanoparticle mediated sustainable approach to enhance seedling growth in maize by mobilizing reserved food. Journal of Agricultural and Food Chemistry, 64(31), 6148–6155. https://doi.org/10.1021/acs.jafc.6b02239
Saharan, V., Sharma, G., Yadav, M., et al. (2015). Synthesis and in vitro antifungal efficacy of cu–chitosan nanoparticles against pathogenic fungi of tomato. International Journal of Biological Macromolecules, 75, 346–353. https://doi.org/10.1016/j.ijbiomac.2015.01.027
Sahayaraj, K., Balasubramanyam, G., & Chavali, M. (2020). Green synthesis of silver nanoparticles using dry leaf aqueous extract of Pongamia glabra Vent (Fab.), characterization and phytofungicidal activity. Environmental Nanotechnology Monitoring & Management, 14, 100349. https://doi.org/10.1016/j.enmm.2020.100349
Saleeb, N., Gooneratne, R., Cavanagh, J., Bunt, C., Mofasser Hossain, A. K. M., Gaw, S., & Robinson, B. (2019). The mobility of silver nanoparticles and silver ions in the soil-plant system. Journal of Environmental Quality. https://doi.org/10.2134/jeq2019.03.0098
Santo-Orihuela, P. L., Foglia, M. L., Targovnik, A. M., Miranda, M. V., & Desimone, M. F. (2016). Nanotoxicological effects of SiO2 nanoparticles on Spodoptera frugiperda Sf9 cells. Current Pharmaceutical Biotechnology, 17(5), 465–470. https://doi.org/10.2174/138920101705160303165604
Saqib, S., Zaman, W., Ayaz, A., Habib, S., Bahadur, S., Hussain, S., Muhammad, S., & Ullah, F. (2020). Postharvest disease inhibition in fruit by synthesis and characterization of chitosan iron oxide nanoparticles. Biocatalysis and Agricultural Biotechnology, 28, 101729. https://doi.org/10.1016/j.bcab.2020.101729
Shakiba, S., Astete, C. E., Paudel, S., Sabliov, C. M., Rodrigues, D. F., & Louie, S. M. (2020). Emerging investigator series: Polymeric nanocarriers for agricultural applications: Synthesis, characterization, and environmental and biological interactions. Environmental Science: Nano, 7, 37–67. https://doi.org/10.1039/C9EN01127G
Shams, A. S., & Abbas, M. H. (2019). Can hydroxyapatite and boron oxide Nano-fertilizers substitute calcium superphosphate and boric acid for broccoli (Brassica oleracea var. italica) Grown on A Heavy Clay Soil? Egyptian Journal of Horticulture, 46(2), 215–234. https://doi.org/10.21608/ejoh.2019.16154.1113
Shan, Y., Cao, L., Xu, C., Zhao, P., Cao, C., Li, F., Xu, B., & Huang, Q. (2019). Sulfonate-functionalized mesoporous silica nanoparticles as carriers for controlled herbicide diquat dibromide release through electrostatic interaction. International Journal of Molecular Sciences, 20(6), 1330. https://doi.org/10.3390/ijms20061330
Shang, H., Guo, H., Ma, C., Li, C., Chefetz, B., Polubesova, T., & Xing, B. (2019). Maize (Zea mays L.) root exudates modify the surface chemistry of CuO nanoparticles: Altered aggregation, dissolution and toxicity. Science of the Total Environment, 690, 502–510. https://doi.org/10.1016/j.scitotenv.2019.07.017
Shao, Z., & Wang, W.-X. (2020). Biodynamics of silver nanoparticles in an estuarine oyster revealed by 110mAgNP tracing. Environmental Science & Technology, 54, 965–974. https://doi.org/10.1021/acs.est.9b04241
Sharma, V. K., Sayes, C. M., Guo, B., Pillai, S., Parsons, J. G., Wang, C., Yan, B., & Ma, X. (2019). Interactions between silver nanoparticles and other metal nanoparticles under environmentally relevant conditions: A review. Science of the Total Environment, 653, 1042–1051. https://doi.org/10.1016/j.scitotenv.2018.10.411
Shinde, S., Paralikar, P., Ingle, A. P., et al. (2020). Promotion of seed germination and seedling growth of Zea mays by magnesium hydroxide nanoparticles synthesized by the filtrate from aspergillus Niger. Arabian Journal of Chemistry, 13(1), 3172–3182. https://doi.org/10.1016/j.arabjc.2018.10.001
Shiva, V. (2016). The violence of the green revolution: Third world agriculture, ecology, and politics. University Press of Kentucky.
Shoaib, A., Elabasy, A., Waqas, M., Lin, L., Cheng, X., Zhang, Q., & Shi, Z. (2018). Entomotoxic effect of silicon dioxide nanoparticles on Plutella xylostella (L.) (Lepidoptera: Plutellidae) under laboratory conditions. Toxicological and Environmental Chemistry, 100(1), 80–91. https://doi.org/10.1080/02772248.2017.1387786
Shobana, C., Rangasamy, B., Poopal, R. K., Renuka, S., & Ramesh, M. (2018). Green synthesis of silver nanoparticles using Piper nigrum: Tissue-specific bioaccumulation, histopathology, and oxidative stress responses in Indian major carp Labeo rohita. Environmental Science and Pollution Research, 25, 11812–11832. https://doi.org/10.1007/s11356-018-1454-z
Shukla, G., Gaurav, S. S., & Singh, A. (2020). Synthesis of mycogenic zinc oxide nanoparticles and preliminary determination of its efficacy as a larvicide against white grubs (Holotrichia sp.). Int. Nano Letters, 10(2), 131–139. https://doi.org/10.1007/s40089-020-00302-0
Singh, A. V., Laux, P., Luch, A., Sudrik, C., Wiehr, S., Wild, A.-M., Santomauro, G., Bill, J., & Sitti, M. (2019). Review of emerging concepts in nanotoxicology: Opportunities and challenges for safer nanomaterial design. Toxicology Mechanisms and Methods, 9(5), 378–387. https://doi.org/10.1080/15376516.2019.1566425
Singh, P., Garg, A., Pandit, S., Mokkapati, V. R. S. S., & Mijakovic, I. (2018). Antimicrobial effects of biogenic nanoparticles. Nanomaterials, 8(12), 1009. https://doi.org/10.3390/nano8121009
Siracusa, V. (2019). Microbial degradation of synthetic biopolymers waste. Polymers, 11, 1066. https://doi.org/10.3390/polym11061066
Siva, G. V., & Benita, L. F. J. (2016). Synthesis, characterization of iron oxide nanoparticles and their applications as nano-fertilizers on some quality characters of ginger (Zingiber officinale Rosc.). IJSRST, 2(3), 11–18.
Skjolding, L. M., Winther-Nielsen, M., & Baun, A. (2014). Trophic transfer of differently functionalized zinc oxide nanoparticlesfrom crustaceans (Daphnia magna) to zebrafish (Danio rerio). Aquatic Toxicology, 157, 101–108. https://doi.org/10.1016/j.aquatox.2014.10.005
Soeteman-Hernández, L. G., Blab, G. A., Carattino, A., Dekker, F., Dekkers, S., van der Linden, M., van Silfhout, A., & Noorlander, C. W. (2020). Challenges of implementing nano-specific safety and safe-by-design principles in academia. NanoImpact, 19, 100243. https://doi.org/10.1016/j.impact.2020.100243
Song, U., & Kim, J. (2020). Zinc oxide nanoparticles: A potential micronutrient fertilizer for horticultural crops with little toxicity. Horticulture, Environment and Biotechnology, 61, 625–631. https://doi.org/10.1007/s13580-020-00244-8
Souza, T. A. J. D., Souza, L. R. R., & Franchi, L. P. (2019). Silver nanoparticles: An integrated view of green synthesis methods, transformation in the environment, and toxicity. Ecotoxicology and Environmental Safety, 171, 691–700. https://doi.org/10.1016/j.ecoenv.2018.12.095
Spielman-Sun, E., Lombi, E., Donner, E., et al. (2018). Temporal evolution of copper distribution and speciation in roots of Triticum aestivum exposed to CuO, Cu(OH)2, and CuS nanoparticles. Environmental Science & Technology, 52(17), 9777–9784. https://doi.org/10.1021/acs.est.8b02111
Spurgeon, D. J., Lahive, E., & Schultz, C. L. (2020). Nanomaterial transformations in the environment: Effects of changing exposure forms on bioaccumulation and toxicity. Small. https://doi.org/10.1002/smll.202000618
Sturikovaa, H., Krystofovaa, O., Huskaa, D., et al. (2018). Zinc, zinc nanoparticles and plants. Journal of Hazardous Materials, 349(5), 101–110. https://doi.org/10.1016/j.jhazmat.2018.01.040
Subbaiah, L. V., Prasad, T. N. V. K. V., Krishna, T. G., et al. (2016). Novel effects of nanoparticulate delivery of zinc on growth, productivity, and zinc biofortification in maize (Zea mays L.). Journal of Agricultural and Food Chemistry, 64(19), 3778–3788. https://doi.org/10.1021/acs.jafc.6b00838
Svendsen, C., Walker, L. A., Matzke, M., Lahive, E., Harrison, S., Crossley, A., Park, B., Lofts, S., Lynch, I., Vázquez-Campos, S., Kaegi, R., Gogos, A., Asbach, C., Cornelis, G., von der Kammer, F., van den Brink, N. W., Mays, C., & Spurgeon, D. J. (2020). Key principles and operational practices for improved nanotechnology environmental exposure assessment. Nature Nanotechnology, 15, 731. https://doi.org/10.1038/s41565-020-0742-1
Swati, K. J., & Joshi, A. (2020). Cu-chitosan nanoparticle induced plant growth and antibacterial activity against bacterial pustule disease in soybean [Glycine max (L.)]. Journal Pharmacognosy Phytochemistry, 9(1), 450–455.
Talaber, I., Van Gestel, C. A. M., Jemec Kokalj, A., Marolt, G., Novak, S., Zidar, P., & Drobne, D. (2020). Comparative biokinetics of pristine and sulfidized ag nanoparticles in two arthropod species exposed to different field soils. Environmental Science: Nano, 7, 2735–2746. https://doi.org/10.1039/D0EN00291G
Tangaa, S. R., Selck, H., Winther-Nielsen, M., & Khan, F. R. (2016). Trophic transfer of metal-based nanoparticles in aquatic environments: A review and recommendations for future research focus. Environmental Science: Nano, 3, 966–981. https://doi.org/10.1039/C5EN00280J
Taran, N. Y., Gonchar, O. M., Lopatko, K. G., et al. (2014). The effect of colloidal solution of molybdenum nanoparticles on the microbial composition in rhizosphere of Cicer arietinum L. Nanoscale Research Letters, 9, 289. https://doi.org/10.1186/1556-276X-9-289
Trump, B. D., Hristozov, D., Malloy, T., & Linkov, I. (2018). Risk associated with engineered nanomaterials: Different tools for different ways to govern. Nano Today, 21, 9–13. https://doi.org/10.1016/j.nantod.2018.03.002
Usman, M., Farooq, M., Wakeel, A., Nawaz, A., Cheema, S. A., Rehman, H., Ashraf, I., & Sanaullah, M. (2020). Nanotechnology in agriculture: Current status, challenges and future opportunities. Science of the Total Environment, 721, 137778. https://doi.org/10.1016/j.scitotenv.2020.137778
Valencia, G. A., Zare, E. N., Makvandi, P., & Gutierrez, T. J. (2019). Self-assembled carbohydrate polymers for food applications: A review. Comprehensive Reviews in Food Science and Food Safety, 18, 2009–2024. https://doi.org/10.1111/1541-4337.12499
van den Brink, N. W., Kokalj, A. J., Silva, P. V., Lahive, E., Norrfors, K., Baccaro, M., Khodaparast, Z., Loureiro, S., Drobne, D., Cornelis, G., Lofts, S., Handy, R. D., Svendsen, C., Spurgeon, D., & van Gestel, C. A. M. (2019). Tools and rules for modelling uptake and bioaccumulation of nanomaterials in invertebrate organisms. Environmental Science: Nano, 6, 1985–2001. https://doi.org/10.1039/c8en01122b
Vanti, G. L., Masaphy, S., Kurjogi, M., Chakrasali, S., & Nargund, V. B. (2020). Synthesis and application of chitosan-copper nanoparticles on damping off causing plant pathogenic fungi. International Journal of Biological Macromolecules, 156, 1387–1395. https://doi.org/10.1016/j.ijbiomac.2019.11.179
Vargas-Hernandez, M., Macias-Bobadilla, I., Guevara-Gonzalez, R. G., Rico-Garcia, E., Ocampo-Velazquez, R. V., Avila-Juarez, L., & Torres-Pacheco, I. (2020). Nanoparticles as potential antivirals in agriculture. Agriculture, 10(10), 444. https://doi.org/10.3390/agriculture10100444
Vega-Vásquez, P., Mosier, N. S., & Irudayaraj, J. (2020). Nanoscale drug delivery systems: From medicine to agriculture. Frontiers in Bioengineering and Biotechnology, 8, 79. https://doi.org/10.3389/fbioe.2020.00079
Vurro, M., Miguel-Rojas, C., & Pérez-de-Luque, A. (2019). Safe nanotechnologies for increasing the effectiveness of environmentally friendly natural agrochemicals. Pest Management Science, 75, 2403–2412. https://doi.org/10.1002/ps.5348
Walker, G. W., Kookana, R. S., Smith, N. E., Kah, M., Doolette, C. L., Reeves, P. T., Lovell, W., Anderson, D. J., Turney, T. W., & Navarro, D. A. (2018). Ecological risk assessment of nano-enabled pesticides: A perspective on problem formulation. Journal of Agricultural and Food Chemistry, 66(26), 6480–6486. https://doi.org/10.1021/acs.jafc.7b02373
Wang, Z., Yin, L., Zhao, J., & Xing, B. (2016a). Trophic transfer and accumulation of TiO2 nanoparticles from clamworm (Perinereis aibuhitensis) to juvenile turbot (Scophthalmus maximus) along a marine benthic food chain. Water Research, 95, 250–259. https://doi.org/10.1016/j.watres.2016.03.027
Wang, P., Lombi, E., Zhao, F. J., & Kopittke, P. M. (2016b). Nanotechnology: A new opportunity in plant sciences. Trends in Plant Science, 21(8), 699–712. https://doi.org/10.1016/j.tplants.2016.04.005
Wang, J.-L., Alasonati, E., Tharaud, M., Gelabert, A., Fisicaro, P., & Benedetti, M. F. (2020a). Flow and fate of silver nanoparticles in small French catchments under different land-uses: The first one-year study. Water Research, 176, 115722. https://doi.org/10.1016/j.watres.2020.115722
Wang, Z., Yue, L., Dhankher, O. P., & Xing, B. (2020b). Nano-enabled improvements of growth and nutritional quality in food plants driven by rhizosphere processes. Environment International, 142, 105831. https://doi.org/10.1016/j.envint.2020.105831
Wigger, H., Kägi, R., Wiesner, M., & Nowack, B. (2020). Exposure and possible risks of engineered nanomaterials in the environment—Current knowledge and directions for the future. Reviews of Geophysics. https://doi.org/10.1029/2020RG000710
Williams, N., Rizzo, A., Arribére, M. A., Suárez, D. A., & Guevara, S. R. (2018). Silver bioaccumulation in chironomid larvae as a potential source for upper trophic levels: A study case from northern Patagonia. Environmental Science and Pollution Research International, 25(2), 1921–1932. https://doi.org/10.1007/s11356-017-0656-0
Wu, F., Bortvedt, A., Harper, B. J., Crandon, L. E., & Harper, S. L. (2017). Uptake and toxicity of CuO nanoparticles to Daphnia magna varies between indirect dietary and direct waterborne exposures. Aquatic Toxicology, 190, 78–86. https://doi.org/10.1016/j.aquatox.2017.06.021
Wu, H., Gonzales-Pech, N. I., & Grassian, V. H. (2019). Displacement reactions between environmentally and biologically relevant ligands on TiO2 nanoparticles: Insights into the aging of nanoparticles in the environment. Environmental Science: Nano, 6(2), 489–504. https://doi.org/10.1039/C8EN00780B
Xiao, B., Zhang, Y., Wang, X., Chen, M., Sun, B., Zhang, T., & Zhu, L. (2019). Occurrence and trophic transfer of nanoparticulate ag and Ti in the natural aquatic food web of Taihu Lake. China, Environmental Science: Nano, 6, 3431–3441. https://doi.org/10.1039/c9en00797k
Xiao, B., Wang, X., Yang, J., Wang, K., Zhang, Y., Sun, B., Zhang, T., & Zhu, L. (2020). Bioaccumulation kinetics and tissue distribution of silver nanoparticles in zebrafish: The mechanisms and influence of natural organic matter. Ecotoxicology and Environmental Safety, 194, 110454. https://doi.org/10.1016/j.ecoenv.2020.110454
Xiarchos, I., Morozinis, A. K., Kavouras, P., & Charitidis, C. A. (2020). Nanocharacterization, materials modeling, and research integrity as enablers of sound risk assessment: Designing responsible nanotechnology. Small, 2001590. https://doi.org/10.1002/smll.202001590
Xie, C., Zhang, J., Ma, Y., Ding, Y., Zhang, P., Zheng, L., Chai, Z., Zhao, Y., Zhang, Z., & He, X. (2019). Bacillus subtilis causes dissolution of ceria nanoparticles at the nano-bio interface. Environmental Science. Nano, 6, 216–223. https://doi.org/10.1039/C8EN01002A
Xu, L., Xu, M., Wang, R., Yin, Y., Lynch, I., & Liu, S. (2020). The crucial role of environmental coronas in determining the biological effects of engineered nanomaterials. Small, 2003691. https://doi.org/10.1002/smll.202003691
Yadav, R. K., Singh, N. B., Singh, A., Yadav, V., Bano, C., & Khare, S. (2020). Expanding the horizons of nanotechnology in agriculture: Recent advances, challenges and future perspectives. Vegetos, 33, 203–221. https://doi.org/10.1007/s42535-019-00090-9
Yang, Y., Xu, G., Xu, S., Chen, S., Xu, A., & Wu, L. (2018a). Effect of ionic strength on bioaccumulation and toxicity of silver nanoparticles in Caenorhabditis elegans. Ecotoxicology and Environmental Safety, 165, 291–298. https://doi.org/10.1016/j.ecoenv.2018.09.008
Yang, F., Zeng, L., Luo, Z., Wang, Z., Huang, F., Wang, Q., Drobne, D., & Yan, C. (2018b). Complex role of titanium dioxide nanoparticles in the trophic transfer of arsenic from Nannochloropsis maritima to Artemia salina nauplii. Aquatic Toxicology, 198, 231–239. https://doi.org/10.1016/j.aquatox.2018.03.009
Yao, P., Zou, A., Tian, Z., Meng, W., Fang, X., Wu, T., & Cheng, J. (2020). Construction and characterization of a temperature-responsive nanocarrier for imidacloprid based on mesoporous silica nanoparticles. Colloids and Surfaces. B, Biointerfaces, 111464. https://doi.org/10.1016/j.colsurfb.2020.111464
Yaqub, A., Ditta, S. A., Anjum, K. M., Tanvir, F., Malkani, N., & Yousaf, M. Z. (2019). Comparative analysis of toxicity induced by different synthetic silver nanoparticles in albino mice. BioNanoScience. https://doi.org/10.1007/s12668-019-00642-y
Yaseen, R., Ahmed, A. I. S., Omer, A. M., et al. (2020). Nano-fertilizers: Bio-fabrication, application and biosafety. Novel Research in Microbiology Journal, 4(4), 884–900. https://doi.org/10.21608/NRMJ.2020.107540
Yusefi-Tanha, E., Fallaha, S., Rostamnejadi, A., et al. (2020). Zinc oxide nanoparticles (ZnONPs) as a novel nanofertilizer: Influence on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar). Science of the Total Environment, 738. https://doi.org/10.1016/j.scitotenv.2020.140240
Zhang, W., Xiao, B., & Fang, T. (2018a). Chemical transformation of silver nanoparticles in aquatic environments: Mechanism, morphology and toxicity. Chemosphere, 191, 324–334. https://doi.org/10.1016/j.chemosphere.2017.10.016
Zhang, J., Guo, W., Li, Q., Wang, Z., & Liu, S. (2018b). The effects and the potential mechanism of environmental transformation of metal nanoparticles on their toxicity in organisms. Environmental Science: Nano, 5, 2482–2499. https://doi.org/10.1039/c8en00688a
Zhang, W., Ke, S., Sun, C., Xu, X., Chen, J., & Yao, L. (2019). Fate and toxicity of silver nanoparticles in freshwater from laboratory to realistic environments: A review. Environmental Science and Pollution Research, 26(8), 7390–7404. https://doi.org/10.1007/s11356-019-04150-0
Zhang, P., Guo, Z., Zhang, Z., Fu, H., White, J. C., & Lynch, I. (2020a). Nanomaterial transformation in the soil–plant system: Implications for food safety and application in agriculture. Small, 16, 2000705. https://doi.org/10.1002/smll.202000705
Zhang, X., Xu, Z., Qian, X., Lin, D., Zeng, T., Filser, J., Li, L., & Kah, M. (2020b). Assessing the impacts of cu(OH)2 nanopesticide and ionic copper on soil enzyme activity and bacterial community. Journal of Agricultural and Food Chemistry, 68(11), 3372–3381. https://doi.org/10.1021/acs.jafc.9b06325
Zhang, Y., Mortimer, M., Guo, L.-H. (2020c). Interplay between engineered nanomaterials and microbiota. Environmental Science: Nano. doi: https://doi.org/10.1039/d0en00557f.
Zhao, J., Lin, M., Wang, Z., Cao, X., & Xing, B. (2020a). Engineered nanomaterials in the environment: Are they safe? Critical Reviews in Environmental Science and Technology. https://doi.org/10.1080/10643389.2020.1764279
Zhao, L., Lu, L., Wang, A., Zhang, H., Huang, M., Wu, H., Xing, B., Wang, Z., & Ji, R. (2020b). Nano-biotechnology in agriculture: Use of nanomaterials to promote plant growth and stress tolerance. Journal of Agricultural and Food Chemistry, 68, 1935–1947. https://doi.org/10.1021/acs.jafc.9b06615
Ziaee, M., & Ganji, Z. (2016). Insecticidal efficacy of silica nanoparticles against Rhyzopertha dominica F. and Tribolium confusum Jacquelin du Val. Journal of Plant Protection Research, 56(3), 250–256. https://doi.org/10.1515/jppr-2016-0037
Zulfiqar, F., Navarro, M., Ashraf, M., Akram, N. A., & Munné-Bosch, S. (2019). Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Science, 289, 110270. https://doi.org/10.1016/j.plantsci.2019.110270
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
de Sousa, B.T., de Oliveira, J.L., Caixeta Oliveira, H., de Castro, V.L.S.S. (2022). Balancing the Benefits to Agriculture and Adverse Ecotoxicological Impacts of Inorganic Nanoparticles. In: Fernandes Fraceto, L., Pereira de Carvalho, H.W., de Lima, R., Ghoshal, S., Santaella, C. (eds) Inorganic Nanopesticides and Nanofertilizers. Springer, Cham. https://doi.org/10.1007/978-3-030-94155-0_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-94155-0_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-94154-3
Online ISBN: 978-3-030-94155-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)