Abstract
In the present study, the larvicidal efficacy of mycogenic zinc oxide nanoparticles (ZONPs) were tested against white grubs, a potent pest of sugarcane in western Uttar Pradesh (India). The ZONPs were synthesized using Aspergillus niger biomass and characterized using UV–Vis spectroscopy, field emission scanning electron microscopy (FESEM), energy dispersive X-ray (EDX), dynamic light scattering (DLS) and Fourier transform infrared (FTIR). the parts per million (ppm) concentration of synthesized ZONPs was established by the inductively coupled plasma mass spectrometry (ICPMS) technique and several ppm dilutions were prepared to determine 50% lethal dose (LD50). The UV–Vis spectroscopy showed peaks at 240, 290, 340, and 380 nm, corresponding to ZONPs. The FESEM results also confirmed the synthesis of nano-sized particles. EDX analysis result showed the optical absorption peaks specific to ZONPs. The DLS result confirmed the synthesis of ZONPs with sizes ranging from 76.2 to 183.8 nm. The FTIR spectrum analysis confirmed the presence of various functional group interactions in the nanoparticle sample. The ZONPs were tested against the first instar larvae of white grubs. The LD50 was calculated to be 12.63 ppm which still needs to be validated for significance. In the near future, we are planning to establish the minimal lethal dosage of ZONPs to prepare effective larvicidal formulations against white grub infection with minimal toxicity to the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
White grubs (genus—Holotrichia; subfamily—Melolonthinae; family—Scarabaeidae; order—Coleoptera) are potent pests of various plants that feed on the roots of the plants and cause yield loss up to 40–70% [61]. The stalks of the infected plant fall down and ultimately dry up [11]. Some majorly affected plants include Acacia nilotica (gum arabic tree), Arachis hypogaea (groundnut), Azadirachta indica (neem tree), Butea monosperma (flame of the forest), Cajanus cajan (pigeon pea), Capsicum annuum (bell pepper), Delonix regia (flamboyant), Glycine max (soybean), Hevea brasiliensis (rubber), Nicotiana tabacum (tobacco), Oryza sativa (rice), Pennisetum glaucum (pearl millet), Saccharum officinarum (sugarcane), Schleichera oleosa (Macassar oil tree), Solanum lycopersicum (tomato), Solanum tuberosum (potato), Sorghum bicolor (sorghum), Tectona grandis (teak), Triticum aestivum (wheat), Vigna radiata (mung bean), Withania somnifera (poisonous gooseberry), Zea mays (maize), and Ziziphus mauritiana (jujube). However, the most susceptible crops include sugarcane, maize, ground nut, soybean, etc. [3, 43, 49, 56].
The life cycle of white grubs varies from 1 to 3 years from species to species, which includes three larval stages [57]. The larvae continue to feed on the roots until these attain a fully grown third instar stage, after which these stop feeding and dig deep to move down in the soil to change to pupae, which later develop into adult beetles [7]. Another factor that triggers the downward movement of white grubs is soil temperature; as winter approaches and soil temperature drops, the white grubs burrow deep into the soil to survive, and thereafter move upward at the onset of spring and restore feeding on the roots [44].
Chlorantraniliprole, clothianidin, imidacloprid, thiamethoxam, or a combination of clothianidin and bifenthrin or imidacloprid and bifenthrin are some good examples of chemical pesticides which are generally used to control white grub infections [41]. The major disadvantage associated with the use of chemical pesticides is that these bioaccumulate, which upon consumption may cause hazardous impact (neurological, psychological and behavioral dysfunctions; hormonal imbalances, leading to infertility, breast pain; immune system dysfunction; reproductive system defects; cancers; genotoxicity; blood disorders) on humans [33]. An attractive alternative to chemical pesticides is microbial control agents (MCAs), especially entomopathogenic fungi (EPF). Two major entomopathogenic fungi belonging to Clavicipitaceae, are Metarhizium anisopliae and Beauveria bassiana, have been widely studied and applied as biopesticides against white grub infection in many crops [13, 15, 23, 35]. The commercial formulations of M. anisopliae- and B. bassiana-based biopesticides are manufactured by various biotechnology industries. Some popular biopesticides used in Uttar Pradesh (India) for white grub control are M. anisopliae-based (Kalichakra, Green Meta, Metarhizium etc.) and B. bassiana-based (Bio-Powder, Daman, Basicon, Beauveria). Although the biopesticides are considered to be safe for the environment and non-targeted pest, there are several disadvantages associated with the use of biopesticides such as high selectivity or host specificity, the requirement of additional control measures, delayed effect or mortality, storage problem, the difficulty of culturing in large quantities, short residual effectiveness, soil quality dependency, and slow activity [8].
Nanotechnology is a fast emerging science with enormous applications in various fields of technology; however, its use in crop protection is just in its infancy [47]. It involves the synthesis of nanoparticles (NPs) with most significant sizes ranging between 1 and 100 nm, which have several unique properties due to their very small structures, shapes, and high surface area to volume ratio [10]. NPs can be synthesized by physical, chemical, and biological methods, but the biological method of NP synthesis is much cost-effective, eco-friendly, and easier [54]. Also, their characterization is simple and reliable. The biosynthesis of NPs is based on the use of microbial extracts or plant extracts which contain various reducing agents that reduce the metal compounds to elemental metal NPs or metal oxide NPs [14]. There are various NPs that are prepared as their oxides, viz., including titanium dioxide (TiO2), indium (III) oxide (In2O3), zinc oxide (ZONPs), tin (IV) oxide (SnO2), and silicon dioxide (SiO2) [60]. ZONPs exhibit unique properties including semiconductor, a wide range of radiation absorption, piezoelectric, pyroelectric, and high catalytic activities [67]. Also, various evidences have proven that ZONPs could be applied as a potential antimicrobial agent against various bacteria [62, 65] and fungi [4, 32]. ZONPs have been studied as a potential insecticide as well and have found shown significant effect against various animal host pests, viz., Trialeurodesva porariorum [29], Culex quiquefasciatus [30], Rhipicephalus microplus [6], Anopheles stephensi [39], Pediculus humanus capitis [19], and Aedes aegypti [1]. Very limited studies have been conducted to evaluate the efficacy of ZONPs or any other nanoparticles against plant pests. The present study was conducted to establish the pesticidal activity of ZONPs against white grub pests of plants.
Materials and methods
Culturing of Aspergillus niger
The pure culture of Aspergillus niger was obtained from the Department of Biotechnology, C.C.S. University, Meerut, and sub-cultured on potato dextrose agar (PDA) media (to preserve) and malt glucose yeast peptone (MGYP) media (to generate biomass for nanoparticle synthesis).
Biosynthesis of zinc oxide nanoparticles by A. niger biomass
Two millimolar (2 mM) aqueous solution of zinc nitrate hexahydrate [Zn(NO3)2 6H2O] was prepared for the synthesis of zinc oxide nanoparticles (ZONPs). One gram of wet fungal biomass was taken and suspended in 100 ml of the 2 mM aqueous Zn(NO3)2 6H2O solution in 250 ml Erlenmeyer flasks for reduction of Zn(NO3)2.6H2O into ZONPs (in triplicate). Fungal biomass with water was kept as control. The control and test flasks were placed in an incubator shaker at 30 °C (at 150 rpm) and the reaction was carried out for 120 h. The biotransformation was routinely monitored visually after time intervals (0 h, 4 h, 12 h, 24 h, 48 h, 72 h, 96 h, and 120 h). The biomass was separated by filtration using Whatman No. 1 filter paper, followed by syringe filter (pore size 0.45 m).
Characterization of ZONPs
The synthesized ZONPs were characterized by UV–visible spectrophotometer (in the Department of Genetics and Plant Breeding, CCSU, Meerut), FESEM (facilitated from Indian Institute of Technology, Kanpur), EDX (facilitated from Indian Institute of Technology, Kanpur), FTIR (facilitated from SAIF, Indian Institute of Technology, Bombay), DLS (facilitated from Motilal Nehru National Institute of Technology, Allahabad) and ICPMS (facilitated from Indian Institute of Technology, New Delhi).
Larvicidal activity of ZONPs against white grub larva
The first instar white grubs were collected from the agricultural fields of Meerut (Uttar Pradesh, India). After the harvest of sugarcane crops, the agricultural fields were dug deeply (10–30 inches) and larvae of different instar levels were collected. Due to the long life cycle (1–3 years) of different Holotrichia sp., field collection was preferred over artificial rearing. The collected grubs were preserved in the laboratory in pots containing humus. The in vitro insecticidal activity of ZONPs against white grubs was determined by the method adapted and standardized from Kheswa [27]. The synthesized ZONPs mixture was directly applied on single first instar larva and another larva was treated with fungal extract used for nanoparticle synthesis (control). Further, the parts per million (ppm) concentration of ZONPs was determined by ICPMS technique availed from Indian Institute of Technology (IIT), New Delhi, and ppm dilutions (3 ppm, 5 ppm, 10 ppm, 15 ppm, and 20 ppm) were prepared to test for the mortality percentage and establishment of lethal dose (LD50). The experiment was conducted twice at an interval of 9 days, and ten first instar white grubs larvae were taken in duplicate in Petri dishes for each concentration both times (as collected larvae were not sufficient to make four replicates at one time). The control plate was kept without nanoparticle treatment. Larvae were kept at starvation during treatment and the data were analyzed by Probit analysis using Microsoft Excel 2010 [2].
Result and discussion
Biosynthesis of ZONPs using Aspergillus niger
As the fungal biomass was mixed with an aqueous solution of Zn(NO3)2 6H2O, it started to appear pale yellow after 24 h in the present case, which indicated the formation of ZONPs. A number of the workers in the past have reported the synthesis of extracellular ZONPs with the help of fungal biomass. Aspergillus niger has been used for ZONPs synthesis in various researches in the past [24, 26, 46]. Ghareib et al. [21] reported the synthesis of ZONPs by about 20 fungal species including Aspergillus flavus, Aspergillus carneus, Corynoascus sepedonium, Penicillium aurantiogriseum, Alternaria alternata, A. aureoterreus, A. carneus, and A. sydowii. Yusof et al. [67] suggested the role of fungal extracellular reductase enzyme in the bioreduction of zinc precursors and synthesis of ZONPs.
UV–Vis analysis of ZONPs
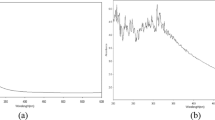
The ZONPs were characterized by UV–Vis double beam spectrophotometer (Lasany LI-295). All spectra were recorded at room temperature, in a quartz cell with 1 cm optical path, to know the kinetic behavior of ZONPs. The scanning range for the samples was 200–800 nm. The spectrophotometer was equipped with “UV prov software” to record and analyze the data. The baseline correction of the spectrophotometer was carried out by using a blank reference. The samples were analyzed at 0, 4, 12, 24, 48, 72, 96, and 120 h. Zinc nanoparticles generally show a broad peak in the UV–visible spectrum in the range of 230–330 nm [48]. In the present study, optical transitions were observed at 240, 290, 340, and 380 nm (Fig. 1). The reaction stabilized after 96 h. Umar et al. [64] reported the absorption peak for the synthesized ZNOPs at 368 nm. Fakhari et al. [18] reported green synthesis of ZONPs with UV–visible absorption spectrum peak centered around 350 nm. Al-Dhabi and Valan Arasu [1] observed the highest absorption of ZONPs at 274 nm. Kalpana et al. [26] reported the absorbance peak of ZONPs at 320 nm. Santhoskumar et al. [50] reported that the UV spectrum range of ZONPs was measured at 380 nm. Ezealisiji et al. [17] observed the peak of ZONPs at 359 nm.
Analysis of ZONPs by FESEM
The ZONPs dried samples were prepared by placing two drops (200 µl) of ZONPs solution on aluminum foil and allowing to air dry, followed by placing it in a hot air oven at 50 °C for 24 h. The FESEM facility was availed from Advance Imaging Centre, Indian Institute of Technology (IIT), Kanpur (UP, India). The image taken indicated that nanoparticles are well distributed with the lowest agglomeration of nanoparticles (Fig. 2). The particles were discrete, non-smooth, spherical in nature, and polydispersed. Studies of SEM micrograph also revealed nanoparticles with a few monoclinic non-spherical structures. Meruvu et al. [36] reported that after SEM analysis, the zinc oxide nanoparticles were found to be spherical in shape and their sizes were about 30–63 nm. Kumar et al. [31] reported SEM images of much less agglomerated ZONPs. Mohan and Renjanadevi [37] in their study found that the ZONPs were agglomerated and complete separation had not occurred. Getie et al. [20] reported the synthesis of ZONPs by using zinc nitrate hexahydrate [Zn(NO3)2 6H2O] and zinc acetate dehydrate [Zn(CH3COO)2 2H2O] as precursors. Zinc oxide nanoparticles synthesized from Zn(NO3)2 6H2O had grainy morphology, while zinc oxide nanoparticles synthesized from Zn(CH3COO)2 2H2O were nanorod and spherical types. Chikkanna et al. [9] reported ZONP SEM images which clearly emphasized that the obtained particles were spherical, nearly spongy, and had flower-like structures.
Analysis of ZONPs by EDX
This facility was also availed from Advance Imaging Centre, IIT, Kanpur (UP, India). The EDX report shows the EDX spectrum of ZONPs (Fig. 3). EDX spectrum shows four peaks which are identified as zinc (Zn), oxygen (O), and aluminum (Al). Zn and O elements confirm the existence of ZONPs. The peak corresponding to aluminum is obvious, as the sample smear was prepared on the aluminum foil base. The weight percentage of Zn, O, and Al were found to be 41.24%, 44.36%, and 14.4%, respectively.
A similar observation of EDX analysis of ZONPs has been reported by Patil and Raut [45]. In EDX analysis of ZONPs reported by Chikkanna et al. [9], the weight percentage of Zn and O elements was found to be 55.35 and 10.10 respectively.
Analysis of ZONPs by DLS
Dynamic light scattering (DLS) which is based on the laser diffraction method with multiple scattering techniques was employed to study the average particle size of ZONPs. The samples were sent to the Centre for Interdisciplinary Research (CIR), Motilal Nehru National Institute of Technology (MNNIT), Allahabad (U.P.), India. The aqueous sample was ultrasonicated before processing under DLS. The DLS size distribution image of biosynthesized ZONPs is shown in Fig. 4. It showed that the size of ZONPs ranged from 76.2 to 183.8 nm with an average size peak at 104.5 nm.
Among the techniques of nanoparticle characterization, DLS is the most commonly used [25, 28, 68]. The theory and mathematical basics of the DLS technique are already well known [16]. It measures the light scattered from the laser that passes through a colloid solution. Further, the scattered light intensity is analyzed as a function of time and the size of particles in the solution can be determined [22]. The mean diameter of NPs can also be determined by this technique. Tso et al. [63] investigated the stability and morphology of three metal oxide nanoparticles (TiO2, ZnO, and SiO2) in aqueous solutions using DLS technique; they found that ZONPs could not remain stable in suspensions, presumably due to the dissolution of particles to form a high concentration of ions, resulting in enhanced aggregation of particles. Shi et al. [53] reported sunscreen nanoparticle preparation with a size range between 80 and 200 nm as analyzed by the DLS technique. Nagarajan and Kuppusamy [40] reported ZONPs with average 36 nm particle size as analyzed by the DLS technique; however, there were many deviations in the size ranges as compared with other analysis reports of TEM, SEM and AFM (atomic focal microscopy). Shaheen et al. [51] compared the size results of ZONPs obtained from transmission electron microscopy (TEM) and DLS; they reported that the measured average sizes from DLS technique, Zetasizer, were much larger than that of TEM reports and suggested that this may be attributed to the fact that TEM and X-ray diffraction (XRD) are performed in the dry state, while that DLS uses the aqueous medium, which leads to swelling of the ZONPs nanoparticles.
Analysis of ZONPs by FTIR
The FTIR facility was availed from Sophisticated Analytical Instrument Facility (SAIF), Indian Institute of Technology (IIT), Bombay, to recognize the organic, inorganic, biomolecule residues along with nanoparticle formation, which may come along via reducing agent on to the surface of ZONPs (Fig. 5). Absorption bands for ZONPs were found to be at 419.51 cm−1, 494.34 cm−1, 601.14 cm−1, 1024.78 cm−1, 1153.08 cm−1, 1218.09 cm−1, 1543.81 cm−1, 1636.42 cm−1, 2922.70 cm−1, 3421.30 cm−1, 3615.99 cm−1, 3666.86 cm−1, 3742.79 cm−1, and 3858.01 cm−1. The intense bands at 3421.30 cm−1, 3615.99 cm−1,and 3666.86 cm−1correspond to O–H stretching [42]. The peak at 2922.70 cm−1 indicates the presence of the C–H bond. The peak at 1636.42 cm−1 corresponds to the C = C stretch in the aromatic ring. A band at 1543.81 cm−1indicates the asymmetric stretching of the N–O bond [12]. The peak at 1218.09 cm−1 indicates the significant presence of C–O bending vibration. Peaks at 1153.08 cm−1 and 601.14 cm−1 show the presence of alkyl halides. A peak at 1024.78 cm−1 corresponds to the C–O stretch [55]. The peaks at 419.51 cm−1 and 494.34 cm−1 indicate the stretching vibrations of zinc and oxygen bonds, which show the formation of ZONPs [9].
Larvicidal activity of ZONPs
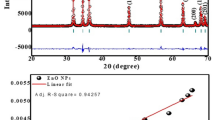
It was observed that the larvae died within 12 h in the ZONPs-treated plate. The larval mortality per ppm concentration is given in Table 1. The LD50 was calculated to be 12.63 ppm with lower and upper confidence levels 9.75 ppm and 15.50 ppm, respectively, at 95% confidence level; further validation and significance study (Chi-square at 5% significance) of the same would be done in the near future as soon as sufficient grubs would be available.
Most of the studies of larvicidal properties of zinc oxide nanoparticles or other metal-based nanoparticles have been done on animal pests. Shanmugasundaram and Balagurunathan [52] had established LC50 of silver nanoparticles (AgNPs) against Culex quinquefasciatus and Aedes aegypti. Similar studies were performed by Morejón et al. [38] and Sutthanont et al. [59]. Al-Dhabi and Valan Arasu [1] studied the larvicidal activity of ZONPs against Aedes aegypti larvae and eggs, giving a significant LC50 value of 34.04 ppm. Many researchers have reported the efficacy of nanoparticles against food storage pests. Yang et al. [66] examined the pesticidal efficacy of polyethylene glycol (PEG)-coated nanoparticles with garlic oils against adults of food storage pest Tribolium castaneum and observed 80% control in the pest population. Stadler et al. [58] reported the insecticidal activity of nano-alumina against food storage pests Sitophilus oryzae and Rhyzopertha dominica and found that both species experienced significant mortality after 3 days of continuous exposure and LD50 was calculated to be 235 mg per kg after the 9th day of exposure. Babu et al. [5] reported the synthesis of silver nanoparticles from bacteria Shewanella algae and its application as pesticide on third instar larva of white grubs (Lepidiota mansueta). Malaikozhundan and Vinodhini [34] synthesized biogenic zinc oxide nanoparticles using leaf extract of Pongamia pinnata, evaluated its insecticidal activity against pulse storage pest Callosobruchus maculatus and observed a significant delay in the total life cycle of C. maculatus and 100% mortality at 25 ppm concentration of zinc oxide nanoparticles with LC50 value of 10.85 ppm.
Conclusion
The present study was performed to demonstrate the larvicidal effects of zinc oxide myconanoparticles against white grubs. This study primarily confirms the efficacy of synthetic ZONPs as effective pest control agents against white grubs, which can lead to the replacement of harmful chemical pesticides soon in the future. The most crucial and labor-demanding aspect of this study is the collection of white grubs, as these keep feeding on the roots during the crop season but dig deep down in the soil after the harvest of the crop. The collection of the grubs during the crop season would damage the crop; hence, only post-harvest collection is preferred.
It is possible that by adding ZONPs to formulations of pesticides, the toxicity of chemical pesticides for humans and other non-targeted organisms would be mitigated. In the near future, we are looking forward to establishing a minimum lethal dosage and effective dosage of ZONPs to effectively kill this pest. Further study will need to focus on methods to increase the stability and physiological mechanisms of nanoparticles to increase their effects in integrated pest management programs at large greenhouse and field levels. There have been relatively few studies on the applicability of ZONPs to control plant insects. Further studies are needed in the future to investigate whether the application of ZONPs into the soil might cause unwanted damages to useful flora and fauna. Also, lethal dosage against pests and formulation of effective dosage needs to be done, to minimize the chances of harm to humans and animals due to the bioaccumulation of ZONPs.
References
Al-Dhabi, N., Valan Arasu, M.: Environmentally-friendly green approach for the production of zinc oxide nanoparticles and their anti-fungal, ovicidal, and larvicidal properties. Nanomaterials 8(7), 500 (2018)
Andrade-Ochoa, S., Sánchez-Aldana, D., Chacón-Vargas, K.F., Rivera-Chavira, B.E., Sánchez-Torres, L.E., Camacho, A.D., Nogueda-Torres, B., Nevárez-Moorillón, G.V.: Oviposition deterrent and larvicidal and pupaecidal activity of seven essential oils and their major components against Culex quinquefasciatus say (Diptera: Culicidae): synergism–antagonism effects. Insects 9(1), 25 (2018)
Anitha, V., Wightman, J., Rogers, D.J.: Management of white grubs (Coleoptera: Scarabaeidae) on groundnut in southern India. Int. J. Pest Manag. 51(4), 313–320 (2005)
Arciniegas-Grijalba, P.A., Patiño-Portela, M.C., Mosquera-Sánchez, L.P., Guerrero-Vargas, J.A., Rodríguez-Páez, J.E.: ZnO nanoparticles (ZnO-NPs) and their antifungal activity against coffee fungus Erythricium salmonicolor. Appl. Nanosci. 7(5), 225–241 (2017)
Babu, M.Y., Janaki Devi, V., Ramakritinan, C.M., Umarani, R., Taredahalli, N., Kumaraguru, A.K.: Application of biosynthesized silver nanoparticles in agricultural and marine pest control. Curr. Nanosci. 10(3), 374–381 (2014)
Banumathi, B., Malaikozhundan, B., Vaseeharan, B.: Invitro acaricidal activity of ethnoveterinary plants and green synthesis of zinc oxide nanoparticles against Rhipicephalus (Boophilus) microplus. Vet. Parasitol. 216, 93–100 (2016)
Bhawane, G.P., Gaikwad, S.M., Mamlayya, A.B., Aland, S.R.: Life cycle of Holotrichia karschi Brenske (Coleoptera: Scarabaeidae: Melolonthinae). Bioscan 6(3), 471–474 (2011)
Chandler, D., Bailey, A.S., Tatchell, G.M., Davidson, G., Greaves, J., Grant, W.P.: The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. B 366(1573), 1987–1998 (2011)
Chikkanna, M.M., Neelagund, S.E., Rajashekarappa, K.K.: Green synthesis of Zinc oxide nanoparticles (ZnO NPs) and their biological activity. SN Appl. Sci. 1(1), 117 (2019)
Christian, P., Von der Kammer, F., Baalousha, M., Hofmann, T.: Nanoparticles: structure, properties, preparation and behaviour in environmental media. Ecotoxicology 17(5), 326–343 (2008)
Dadmal, S.M., Khadakkar, S.: Revision of Holotrichia hope (Scarabaeidae: Melolonthinae) in different agro–climatic zones of Maharashtra (India). J. Ent. Zool. Study 2(3), 50–58 (2014)
Devi, T.R., Gayathri, S.: FTIR and FT-Raman spectral analysis of paclitaxel drugs. Int. J. Pharm. Sci. Rev. Res. 2(2), 106–110 (2010)
Dhoj, G.C.Y.: Microbial control of white grubs in Nepal: the way forward. J. Agric. Environ. 10, 134–142 (2009)
Dorcheh, S.K., Vahabi, K.: Biosynthesis of nanoparticles by fungi: large-scale production. Fungal Metabolites, pp. 1–20. Springer, Berlin (2016)
Erler, F., Ates, A.O.: Potential of two entomopathogenic fungi, Beauveria bassiana and Metarhizium anisopliae (Coleoptera: Scarabaeidae), as biological control agents against the June beetle. J. Insect Sci. 15(1), 44 (2015)
Evanoff Jr., D.D., Chumanov, G.: Synthesis and optical properties of silver nanoparticles and arrays. ChemPhysChem 6(7), 1221–1231 (2005)
Ezealisiji, K.M., Siwe-Noundou, X., Maduelosi, B., Nwachukwu, N., Krause, R.W.M.: Green synthesis of zinc oxide nanoparticles using Solanum torvum (L.) leaf extract and evaluation of the toxicological profile of the ZnO nanoparticles–hydrogel composite in Wistar albino rats. Int. Nano Lett. 9(2), 99–107 (2019)
Fakhari, S., Jamzad, M., Kabiri Fard, H.: Green synthesis of zinc oxide nanoparticles: a comparison. Green Chem. Lett. Rev. 12(1), 19–24 (2019)
Gandhi, P.R., Jayaseelan, C., Mary, R.R., Mathivanan, D., Suseem, S.R.: Acaricidal, pediculicidal and larvicidal activity of synthesized ZnO nanoparticles using Momordica charantia leaf extract against blood feeding parasites. Exp. Parasitol. 181, 47–56 (2017)
Getie, S., Belay, A., Chandra Reddy, A.R., Belay, Z.: Synthesis and characterizations of zinc oxide nanoparticles for antibacterial applications. J. Nanomedic. Nanotechnol. 8, 2 (2017)
Ghareib, M., Abdallah, W.E., Tahon, M.A., Hussein, M.: Eco-friendly approach for biosynthesis of zinc oxide nanoparticles using some soil fungi. Nano Sci. 7(3), 108–118 (2018)
Hao, E., Schatz, G.C.: Electromagnetic fields around silver nanoparticles and dimers. J. Chem. Phys. 120(1), 357–366 (2004)
Inglis, G.D., Goettel, M.S., Butt, T.M., Strasser, H.: Use of hyphomycetous fungi for managing insect pests. Fungi as biocontrol agents, pp. 23–69. Springer, Berlin (2001)
Jacob, S.P., Bharathkumar, R., Ashwathram, G.: Aspergillus niger mediated synthesis of ZnO nanoparticles and their antimicrobial and in vitro anticancerous activity. World J. Pharm. Res. 3(2), 3044–3054 (2014)
Jans, H., Liu, X., Austin, L., Maes, G., Huo, Q.: Dynamic light scattering as a powerful tool for gold nanoparticle bioconjugation and biomolecular binding studies. Anal. Chem. 81(22), 9425–9432 (2009)
Kalpana, V.N., Kataru, B.A.S., Sravani, N., Vigneshwari, T., Panneerselvam, A., Rajeswari, V.D.: Biosynthesis of zinc oxide nanoparticles using culture filtrates of aspergillus niger: antimicrobial textiles and dye degradation studies. OpenNano 3, 48–55 (2018)
Kheswa, N.: Development of beauveria brongniartii as a bio-Insecticide to control white grub (coleoptera: scarabaeidae) species attacking sugarcane in South Africa (Doctoral dissertation) (2016)
Khlebtsov, N., Dykman, L.: Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem. Soc. Rev. 40(3), 1647–1671 (2011)
Khooshe-Bast, Z., Sahebzadeh, N., Ghaffari-Moghaddam, M., Mirshekar, A.: Insecticidal effects of zinc oxide nanoparticles and Beauveria bassiana TS11 on Trialeurodes vaporariorum (Westwood, 1856) (Hemiptera: Aleyrodidae). Acta Agric. Slov. 107(2), 299–309 (2016)
Kirthi, A.V., Rahuman, A.A., Rajakumar, G., Marimuthu, S., Santhoshkumar, T., Jayaseelan, C., Velayutham, K.: Acaricidal, pediculocidal and larvicidal activity of synthesized ZnO nanoparticles using wet chemical route against blood feeding parasites. Parasitol. Res. 109(2), 461–472 (2011)
Kumar, S.S., Venkateswarlu, P., Rao, V.R., Rao, G.N.: Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int. Nano Lett. 3(1), 30 (2013)
la Rosa-García, D., Susana, C., Martínez-Torres, P., Gómez-Cornelio, S., Corral-Aguado, M.A., Quintana, P., Gómez-Ortíz, N.M.: Antifungal activity of ZnO and MgO nanomaterials and their mixtures against Colletotrichum gloeosporioides strains from tropical fruit. J. Nanomater. (2018). https://doi.org/10.1155/2018/3498527
Maksymiv, I.: Pesticides: benefits and hazards. J. Vasyl Stefanyk Precarpathian Natl. Univ. 2(1), 70–76 (2015)
Malaikozhundan, B., Vinodhini, J.: Nanopesticidal effects of Pongamia pinnata leaf extract coated zinc oxide nanoparticle against the Pulse beetle, Callosobruchus maculatus. Mater. Today Commun. 14, 106–115 (2018)
Mane, P.B., Mohite, P.B.: Bioefficacy of different species of entomopathogenic fungi against white grub, Leucopholis lepidophora (Blanchard) infesting sugarcane in Maharashtra. Asian J. Bio. Sci. 9(2), 234–237 (2014)
Meruvu, H., Vangalapati, M., Chippada, S.C., Bammidi, S.R.: Synthesis and characterization of zinc oxide nanoparticles and its antimicrobial activity against Bacillus subtilis and Escherichia coli. J. Rasayan Chem 4(1), 217–222 (2011)
Mohan, A.C., Renjanadevi, B.: Preparation of zinc oxide nanoparticles and its characterization using scanning electron microscopy (SEM) and X-ray diffraction (XRD). Proced. Technol. 24, 761–766 (2016)
Morejón, B., Pilaquinga, F., Domenech, F., Ganchala, D., Debut, A., Neira, M.: Larvicidal activity of silver nanoparticles synthesized using extracts of Ambrosia arborescens (Asteraceae) to Control Aedes aegypti L. (Diptera: Culicidae). J. Nanotechnol. (2018). https://doi.org/10.1155/2018/6917938
Murugan, K., Roni, M., Panneerselvam, C., Suresh, U., Rajaganesh, R., Aruliah, R., Mahyoub, J.A., Trivedi, S., Rehman, H., Al-Aoh, H.A.N., Kumar, S.: Sargassum wightii-synthesized ZnO nanoparticles reduce the fitness and reproduction of the malaria vector Anopheles stephensi and cotton bollworm Helicoverpa armigera. Physiol. Mol. Plant Pathol. 101, 202–213 (2018)
Nagarajan, S., Kuppusamy, K.A.: Extracellular synthesis of zinc oxide nanoparticle using seaweeds of gulf of Mannar India. J. Nanobiotechnol. 11(1), 39 (2013)
Nataraja, M.V., Jadon, K.S., Dutta, R., Savalia, S.D.: White grubs and their management in groundnut. Web report, Indian Council of Agricultural Research
Oancea, A., Grasset, O., Le Menn, E., Bollengier, O., Bezacier, L., Le Mouélic, S., Tobie, G.: Laboratory infrared reflection spectrum of carbon dioxide clathrate hydrates for astrophysical remote sensing applications. Icarus 221(2), 900–910 (2012)
Oliveira, L.J., Hoffmann-Campo, C.B., Garcia, M.A.: Effect of soil management on the white grub population and damage in soybean. Pesquisa Agropecuária Brasileira 35(5), 887–894 (2000)
Pathania, M., Chandel, R.S., Verma, K.S., Mehta, P.K.: Seasonal life cycle of Holotrichia longipennis (Blanchard) (Coleoptera: Scarabaeidae: Melolonthinae): a serious foliage and root feeding pest in India. Phytoparasitica 44(5), 615–629 (2016)
Patil, S., Raut, S.J.: Synthesis and characterization of ZnO nanoparticles and 50% ZnO-bentonite nanocomposite. Int. J. Chem. Sci. 10(2), 1124–1132 (2012)
Rajan, A., Cherian, E., Baskar, G.: Biosynthesis of zinc oxide nanoparticles using Aspergillus fumigatus JCF and its antibacterial activity. Int. J. Mod. Sci. Technol. 1(2), 52–57 (2016)
Resham, S., Khalid, M., Kazi, A.G.: Nanobiotechnology in agricultural development. In: Barh, D., Khan, M., Davies, E. (eds.) PlantOmics: The Omics of Plant Science, pp. 683–698. Springer, New Delhi (2015)
Revina, A.A., Oksentyuk, E.V., Fenin, A.A.: Synthesis and properties of zinc nanoparticles: the role and prospects of radiation chemistry in the development of modern nanotechnology. Prot. Metal 4(3), 613–618 (2007)
Samson, P.R., Milner, R.J., Sander, E.D., Bullard, G.K.: Effect of fungicides and insecticides applied during planting of sugarcane on viability of Metarhizium anisopliae and its efficacy against white grubs. Biocontrol 50(1), 151–163 (2005)
Santhoshkumar, J., Kumar, S.V., Rajeshkumar, S.: Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resour. Effic. Technol. 3(4), 459–465 (2017)
Shaheen, T.I., El-Naggar, M.E., Abdelgawad, A.M., Hebeish, A.: Durable antibacterial and UV protections of in situ synthesized zinc oxide nanoparticles onto cotton fabrics. Int. J. Biol. Macromol. 83, 426–432 (2016)
Shanmugasundaram, T., Balagurunathan, R.: Mosquito larvicidal activity of silver nanoparticles synthesised using actinobacterium, Streptomyces sp. M25 against Anopheles subpictus, Culex quinquefasciatus and Aedes aegypti. J. Parasit. Dis. 39(4), 677–684 (2015)
Shi, L., Shan, J., Ju, Y., Aikens, P., Prudhomme, R.K.: Nanoparticles as delivery vehicles for sunscreen agents. Colloids Surf. A 396, 122–129 (2012)
Shukla, G., Dixit, R., Kumar, A., Singh, R., Rani, A., Kumar, P.: Nanotechnology: an innovative approach for waste water treatment. Appl. Nanotechnol. Introd. (2017). https://doi.org/10.1007/978-3-030-02381-2_5
Singh, A.K., Talat, M., Singh, D.P., Srivastava, O.N.: Biosynthesis of gold and silver nanoparticles by natural precursor clove and their functionalization with amine group. J. Nanopart. Res. 12(5), 1667–1675 (2010)
Sreedevi, K., Tyagi, S.: Species diversity of white grubs associated with sugarcane ecosystem of western Uttar Pradesh. A case study. Curr. Biotica 8(4), 404–410 (2015)
Srivastava, A.S., Srivastava, K.M., Nigam, P.M.: On the life history of white grub, holotrichia consanguinea Blanch (Coleopt, Melolonthidae). Zeitschrift für Angewandte Entomologie 68(1–4), 154–157 (1971)
Stadler, T., Buteler, M., Weaver, D.K.: Novel use of nanostructured alumina as an insecticide. Pest Manag. Sci. 66(6), 577–579 (2010)
Sutthanont, N., Attrapadung, S., Nuchprayoon, S.: Larvicidal activity of synthesized silver nanoparticles from Curcuma zedoaria essential oil against Culex quinquefasciatus. Insects 10(1), 27 (2019)
Talebian, N., Nilforoushan, M.R.: Comparative study of the structural, optical and photocatalytic properties of semiconductor metal oxides toward degradation of methylene blue. Thin Solid Films 518(8), 2210–2215 (2010)
Theurkar, S.V., Ghadage, M.K., Patil, S.B.: New laboratory culture method for white grub national pest, India. Int. Res. J. Biol. Sci. 2(5), 83–85 (2013)
Tiwari, V., Mishra, N., Gadani, K., Solanki, P.S., Shah, N.A., Tiwari, M.: Mechanism of anti-bacterial activity of zinc oxide nanoparticle against carbapenem-resistant Acinetobacter baumannii. Front. Microbiol. 9, 1218 (2018)
Tso, C.P., Zhung, C.M., Shih, Y.H., Tseng, Y.M., Wu, S.C., Doong, R.A.: Stability of metal oxide nanoparticles in aqueous solutions. Water Sci. Technol. 61(1), 127–133 (2010)
Umar, H., Kavaz, D., Rizaner, N.: Biosynthesis of zinc oxide nanoparticles using Albizia lebbeck stem bark, and evaluation of its antimicrobial, antioxidant, and cytotoxic activities on human breast cancer cell lines. Int. J. Nanomed. 14, 87 (2019)
Xie, Y., He, Y., Irwin, P.L., Jin, T., Shi, X.: Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 77(7), 2325–2331 (2011)
Yang, F.L., Li, X.G., Zhu, F., Lei, C.L.: Structural characterization of nanoparticles loaded with garlic essential oil and their insecticidal activity against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Agric. Food Chem. 57, 10156–10162 (2009)
Yusof, H.M., Mohamad, R., Zaidan, U.H.: Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: a review. J. Anim. Sci. Biotechnol. 10(1), 57 (2019)
Zanetti-Ramos, B.G., Fritzen-Garcia, M.B., de Oliveira, C.S., Pasa, A.A., Soldi, V., Borsali, R., Creczynski-Pasa, T.B.: Dynamic light scattering and atomic force microscopy techniques for size determination of polyurethane nanoparticles. Mater. Sci. Eng. C 29(2), 638–640 (2009)
Author information
Authors and Affiliations
Contributions
GS: Designed and performed experiments, analysed data and co-wrote the paper. SSG: Supervised the work and conceived the original idea. AS: Helped in the field work as a part of this study and co-wrote the paper.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shukla, G., Gaurav, S.S. & Singh, A. Synthesis of mycogenic zinc oxide nanoparticles and preliminary determination of its efficacy as a larvicide against white grubs (Holotrichia sp.). Int Nano Lett 10, 131–139 (2020). https://doi.org/10.1007/s40089-020-00302-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40089-020-00302-0