Abstract
Nanotechnology is a new approach for the production of particles with unique features at the nanoscale dimensions. Among the various routes available for the synthesis of these nanoparticles, biogenic synthesis is a simple, low-cost, and eco-friendly method. The biosynthesis of gold nanoparticles is provided by various natural sources including plants, fungi, bacteria, actinomycetes, yeasts, and algae. Gold nanoparticles of various shapes and sizes are synthesized using biomass and/or extract of the organism. Enzymes secreted by microorganisms and metabolites of plants act as reducing, stabilizing, and capping agents for the production of the nanoparticles. The gold nanoparticles have antibacterial/antifungal properties that can be used to protect plants against pathogens. In addition, they can be applied for pesticide identification and water purification. This chapter focuses on the biosynthesis of gold nanoparticles, their characterization, and application in agriculture.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
In recent years, nanotechnology has development as an effective field in biology and material science. Nanotechnology is a technology at nanometer scale (1–100 nm) that controls the shape and size of particles. The nanoparticles have unique properties that are related to the very small size of particles and the increase of the surface to volume ratio (Ochekpe et al. 2009; Khadem Moghadam et al. 2019; Maghsoodi et al. 2019).
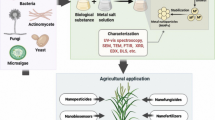
Nanoparticles are produced by different approaches, including physical, chemical, and biological methods. Biogenic synthesis of nanoparticles is a process that utilizes the biological agents such as plants, bacteria, fungi, etc. to produce nanoparticles (Fig. 11.1). The biological synthesis of nanoparticles is important because of its environment-friendly approach.
In recent years, the biosynthesis of noble metal nanoparticles (gold, silver, palladium, and platinum) has been considered due to the development of eco-friendly technologies in material synthesis (Chandran et al. 2006; Aromal and Philip 2012; Jia et al. 2009; Song et al. 2010). Among metal nanoparticles, gold is a very popular element due to being chemically inert and non-toxic (Connor et al. 2005). The gold nanoparticles are most stable and resistant to oxidation (Daniel and Astruc 2004). They are used in a variety of fields, including catalysis, gene expression, nonlinear optics, and delivery systems. The biosynthesis of metal nanoparticles is carried out using the “bottom-up” approach of nanotechnology (Golinska et al. 2014). In this method, the nanoparticles are formed through the growth or assembly of atoms or molecules that are the building units.
Biosynthesis of nanoparticles is formed through reduction/oxidation reactions of metal. In biogenic synthesis of metal nanoparticles, enzymes secreted by microbial agents and metabolites of plants are responsible for the occurrence of these reactions (Prabhu and Poulose 2012).
11.2 Biosynthesis of Nanoparticles
11.2.1 Synthesis of Gold Nanoparticles Using Plant
The use of plant extracts is preferred to produce metal nanoparticles compared with the use of microorganisms. The synthesis rate of nanoparticles using plants is faster than microbial agents, and nanoparticles obtained are more stable (Iravani 2011). In addition, plants are known as an important source of various metabolites, and they have the potential for synthesis of metal nanoparticles in large scale (Jha et al. 2009). However, reaction time required for biogenic synthesis methods is longer than chemical methods for the production of nanoparticles (Song and Kim 2009). Biomass and extract of different parts of plants such as leaf, root, flower, seed, stem, and fruit are used for the biosynthesis of gold nanoparticles. Extract of plants can act as a stabilizing, reducing, and capping agent for the synthesis of nanoparticles (Sharma et al. 2015).
11.2.1.1 Plant Biomass
The presence of metal elements, especially in drinking water, is a serious concern for global health. The use of plant biomass for the removal of heavy metals from aqueous solutions can be valuable as an eco-friendly method and also because of their potential application in removing contaminants from industrial wastewater in the future.
For this reason, many researchers have studied the role of plants in the absorption and accumulation of metal nanoparticles. The formation of gold nanoparticles from living plants was first reported by Gardea-Torresdey et al. (2002). The gold nanoparticles are synthesized inside live alfalfa plants (Medicago sativa) by gold ion uptake from the AuCl4-rich agar solid media. The absorption and formation of gold nanoparticles within the plant were confirmed by X-ray absorption spectroscopy (XAS) and transmission electron microscopy (TEM). TEM images showed that gold nanoparticles were in crystalline state, but also twinned crystal structures and icosahedral nanoparticles were found.
In another study, Armendariz et al. (2004a) reported the synthesis of gold nanoparticles using oat (Avena sativa) biomass. The binding trend of Au(III) to oat and the possible formation of gold nanoparticles were studied at different pH values (pH 2–6). The size of the nanoparticles produced by oat biomass was dependent on the pH of the solution, while the shape of the nanoparticles was not significantly affected by the different pH values. Similar results have been reported for gold nanoparticles formed by wheat biomass (Armendariz et al. 2004b).
11.2.1.2 Plant Extracts
The synthesis of gold nanoparticles using plant leaf extracts has been demonstrated by many researchers (Table 11.1). Dubey et al. (2010a) reported the rapid synthesis of gold nanoparticles using leaf extract of Rosa rugosa within 10 min. In addition, they evaluated the effect of leaf extract quantity and concentration of metal solution (auric acid) in order to optimize the synthesis route of the metal nanoparticles. The formation and stability of the biosynthesized gold nanoparticles was confirmed using spectroscopic characterizations of UV-Vis, TEM, FTIR (Fourier transform infrared spectroscopy), and zeta potential. The sharpness, shape, size, and rate of formation of gold nanoparticles depend on the concentrations of leaf extract and metal ion. Sharp and symmetrical nanoparticles were formed at higher concentrations of leaf extract. Comparatively larger size of gold nanoparticles (50–250 nm) was found at higher gold ion concentration, and the rate of formation of the nanoparticles was slower at lowest concentration.
Gold nanoparticles were formed when the leaves of Pelargonium graveolens were exposed to aqueous chloroaurate ions. The rapid bioreduction of metal ions led to the formation of stable gold nanoparticles of different sizes. The size of the nanoparticles was in the range of 20–40 nm, and their shape was mainly decahedral and icosahedral (Shankar et al. 2003).
Shankar et al. (2004) reported the synthesis of pure metallic silver and gold nanoparticles and bimetallic Au core-Ag shell nanoparticles using the broth of neem leaves (Azadirachta indica). They proposed that the presence of reducing sugars and/or terpenoids in the broth can possibly facilitate the reduction of metal ions. The time of reduction of Au+ ion (2 h) by neem leaf extract was faster than that observed for Ag+ ion (4 h).
The biological synthesis of gold nanoparticles using olive leaf extracts has been reported (Khalil et al. 2012). The characterization of gold nanoparticles exhibited that the morphology of the gold nanoparticles depends on the extract concentration and the solution pH. The nanoparticles formed at lower concentrations of leaf broth were mainly triangular in shape, while spherical shaped nanoparticles were obtained at higher concentrations of leaf broth. The increase of pH also results in the production of smaller nanoparticles.
Green synthesis of gold nanoparticles using fruit extracts has been demonstrated by some researchers. For instance, Ankamwar et al. (2005) used Emblica officinalis (amla) fruit extract to produce gold nanoparticles. Chloroauric acid solution was treated with amla fruit extract (as the reducing agent), which results in the formation of highly stable gold nanoparticles. The size of the nanoparticles produced was in the range of 15–25 nm.
The effect of pH on the morphology of gold nanoparticles prepared from pear fruit extract has been investigated (Ghodake et al. 2010). According to the results of the investigation, gold nanostructures produced in an alkaline condition were very efficient and provide an optimal quantity of pure nanomaterial (Fig. 11.2b). The triangular and hexagonal nanoplates were formed in the range of 200–500 nm in size, depending on the shape (Fig. 11.2c, d). It was suggested that the mechanism of induction of these nanostructures is alkaline-responsive phytochemicals, such as organic acids, amino acids, peptides, and/or proteins. Gold nanoparticles obtained under the normal conditions showed plate-like morphologies with low production efficiency (Fig. 11.2a).
HR-TEM micrographs of the gold nanoparticles formed from pear fruit extract under the normal (a) and alkaline (b) conditions and HR-TEM micrographs of a gold nanohexagon and nanotriangle formed under alkaline conditions (c and d) (Ghodake et al. 2010)
Biosynthesis of gold nanoparticles using tansy fruit extract (Tanacetum vulgare) has also been reported (Dubey et al. 2010b). Zeta potential is an index of surface charge of the nanoparticles that is used to predict the stability of colloidal particles (Heurtault et al. 2003). The effect of pH on zeta potential of the nanoparticles produced by tansy fruit extract indicates that the zeta potential value of nanoparticles depends on the pH of the solution. The zeta potential value of gold nanoparticles in alkaline pH was slightly higher than that of acidic pH. Furthermore, size of the particles produced was increased by decreasing the pH.
In another study, gold nanoparticles have been fabricated by treatment of the HAuCl4 solution with Prunus domestica (plum) fruit extracts (Dauthal and Mukhopadhyay 2012). The catalytic activity of gold nanoparticles dispersed in the fruit extract was studied for 4-nitrophenol reduction to 4-aminophenol. FTIR analysis suggested that the water-soluble polyols like flavanols, glycosides, and phenols were responsible for the reduction of Au3+ ions. Biosynthesized gold nanoparticles showed dose-dependent catalytic activity for 4-NP reduction. The catalytic activity of 4-nitrophenol increased with increasing dosage of colloidal gold nanoparticles.
Flower extract of the plant Nyctanthes arbor-tristis has been used as the reducing and capping agent for the synthesis of gold nanoparticles (Das et al. 2011). TEM images of the nanoparticles showed a mixture of different shapes (triangular, pentagonal, rod shaped, and spherical) with an average size of 19.8 ± 5.0 nm. Sneha et al. (2011) also reported the formation of gold nanoparticles using cumin seeds (Cuminum cyminum). They stated that the particles were predominately monodispersed at higher pH and polydispersed particles formed at lower pH. Table 11.1 summarizes the important examples of gold nanoparticles synthesized by plants.
11.2.2 Synthesis of Gold Nanoparticles Using Bacteria
Many microorganisms can produce various biomolecules either intracellularly or extracellularly. In synthesis of nanoparticles outside the cell, extracellularly, the enzymes secreted by microorganism play an important role in the bioreduction of metal ions. In synthesis of nanoparticles inside the cell, intracellularly, the enzymes present in the cell wall of the microorganisms involve in the reduction of metal ions to metal nanoparticles (Hulkoti and Taranath 2014). The nanoparticles produced inside the organism can have a smaller size than extracellularly formed nanoparticles (Narayanan and Sakthivel 2010).
Beveridge and Murray (1980) synthesized the gold nanoparticles using the cell wall of Bacillus subtilis. They were chemically modified amine and carboxyl groups of the cell wall of B. subtilis to determine their contribution to the metal uptake values. Their results indicated that chemical modifications of amine functions did not decrease the metal uptake values, whereas alteration of carboxyl groups was severely restricted metal deposition of most of the metals tested.
Deplanche and Macaskie (2008) demonstrated microbial reduction of gold using Escherichia coli and Desulfovibrio desulfuricans and determined the location and size of the formed gold particles. According to their report, hydrogenases are responsible in the bacteria-mediated reduction of the gold ions. The size and shape of the gold nanoparticles produced depend on the solution pH and the location of the formation of the nanoparticles. The nanoparticles ranged from 5 to 50 nm and located in the periplasmic space and on the cell surface as well as intracellularly.
The extracellular synthesis of gold nanoparticles using the gram-negative soil bacterium Pseudomonas fluorescens has been proven (Rajasree and Suman 2012). In a recent study, a human pathogenic bacterium Salmonella enterica subsp. enterica serovar Typhi isolated from blood and stool specimens of patients provided the biogenic synthesis of gold nanoparticles (Mortazavi et al. 2017). Characterizations of some gold nanoparticles synthesized by bacteria are enlisted in Table 11.2.
11.2.3 Synthesis of Gold Nanoparticles Using Actinomycetes, Algae and Yeast
Actinomycetes are a group of gram-positive bacteria that have the characteristics of fungi. They produce various biomolecules including proteins, enzymes, antibiotics, and vitamins. Actinomycetes can be used as stabilizing and capping agents for the synthesis of metal nanoparticles, including gold. Among the bioactive agents secreted by actinomycetes, proteins play an important role in the synthesis of the nanoparticles. It is proven that free amine groups or cysteine residues in the proteins can bind to gold nanoparticles (Gole et al. 2001).
The alkalothermophilic actinomycete Thermomonospora sp. has been explored for extracellular synthesis of stable gold nanoparticles (Ahmad et al. 2003a). Intracellular synthesis of gold nanoparticles has been provided by Rhodococcus sp. actinomycetes (Ahmad et al. 2003b). Spherical nanoparticles with size range of 5–15 nm were achieved.
Algae are photosynthetic eukaryotic microorganisms used for synthesis of gold nanoparticles. Cell walls of algae contain biomolecules, including polysaccharides, proteins, and enzymes which act as reducing agents for the reduction of gold ions (Sharma et al. 2015).
The powder and the ethanolic extract of marine red alga Galaxaura elongate have been used for synthesis of gold nanoparticles. The formation of gold nanoparticles by powder (3 h) was faster than alcoholic extract (2–5 min) (Abdel-Raouf et al. 2017).
Yeasts are single-cell eukaryotic microorganisms that are classified in the fungus kingdom. The biogenic synthesis of gold nanoparticles by the non-conventional yeasts Yarrowia lipolytica and Magnusiomyces ingens is described (Agnihotri et al. 2009; Zhang et al. 2016). X-ray diffraction (XRD) data and TEM images of Y. lipolytica showed that the nanoparticles are synthesized with a size of 15 nm and located on the wall of the cells (Agnihotri et al. 2009). TEM images and dynamic light scattering (DLS) data of M. ingens indicated that the average size of gold nanoparticles was 80.1 ± 9.8 and 137.8 ± 4.6 nm, respectively. According to the results of the investigation, some biomolecules were absorbed on the surface of the nanoparticles, which can act as organic ligands in the formation of gold nanoparticles (Zhang et al. 2016). Important examples of biosynthesis of gold nanoparticles by actinomycetes, algae, and yeasts are summarized in Table 11.2.
11.2.4 Synthesis of Gold Nanoparticles Using Fungi
The biosynthesis of gold nanoparticles using fungi has been demonstrated (Table 11.3). Among the microorganisms used for the synthesis of metal nanoparticles, fungi are a suitable candidate for the production of different enzymes, which have high growth capacity and are easy to handle.
The exact mechanism of synthesis of nanoparticles using biological agents is still unknown, but it has been demonstrated that different biomolecules have a significant role in the synthesis of nanoparticles. It has been revealed that the enzyme nitrate reductase is involved in biosynthesis of nanoparticles by fungi (Kumar et al. 2007a, b). The mechanisms for intracellular and extracellular synthesis of nanoparticles are different. Moreover, the shape and size of nanoparticles produced can be affected by enzymes and mechanisms involved in the synthesis of nanoparticles.
Mukherjee et al. (2001) reported intracellular synthesis of gold nanoparticles by bioreduction of aqueous AuCl4− ions using the fungus Verticillium sp. TEM image of a single cell showed that the gold nanoparticles were formed on both the cell wall (outer boundary) and the cytoplasmic membrane (inner boundary). The number of gold nanoparticles on the cytoplasmic membrane was more than on the cell wall. The shape of gold nanoparticles was mostly spherical, although a few triangular and hexagonal particles were observed.
In addition, they reported extracellular synthesis of gold nanoparticles by a eukaryotic system such as fungi for the first time (Mukherjee et al. 2002). The nanoparticles were synthesized by treatment of the fungus Fusarium oxysporum with AuCl4− solution. TEM pictures showed that gold particles have spherical and triangular morphology with a size range of 20–40 nm. Indeed, the gold nanoparticle formed by reaction of gold ions with extracellular secreted enzymes by the fungus. Thakker et al. (2013) synthesized gold nanoparticles using a plant pathogenic fungus F. oxysporum f. sp. cubense and reported their antibacterial activity against Pseudomonas sp.
The microbial synthesis of gold nanoparticles has been investigated using the fungus Rhizopus oryzae to remove different organophosphorus pesticides (model) from water along with some microorganisms (Das et al. 2009). The gold nanoparticles were formed on the surface of R. oryzae and were stable even up to 6 months. FTIR spectra after treatment of R. oryzae with HAuCl4− revealed the presence of amide I, II, and III groups and the disappearance of carboxyl groups present in mycelia. Based on the FTIR results, it was suggested that polypeptides/proteins are involved in the reduction of gold ions. Indeed, the gold nanoparticles are formed by surface-bound protein molecules that act as both reducing and stabilizing agents.
In another study, the use of a marine-derived fungus Aspergillus sydowii resulted in the formation of spherical gold nanoparticles with an average size of 10 nm. The fungus could synthesize gold nanoparticles extra-/intracellularly depending on the applied gold ion concentration (Vala 2015). Fungus-mediated synthesis of gold nanoparticles by Penicillium aurantiogriseum, P. citrinum, and P. waksmanii has been demonstrated (Honary et al. 2013).
11.3 Characterization of Gold Nanoparticles
The characteristics of gold nanoparticles are determined using various techniques such as scanning electron microscopy (SEM), atomic force microscopy (AFM), TEM, DLS, FTIR, XRD, and UV-Vis spectroscopy. The shape and size of nanoparticles are determined by TEM, SEM, and AFM. DLS is also used for determination of size, dispersity, and zeta potential of nanoparticles. Furthermore, FTIR and XRD are applied for the determination of structural characteristics and crystallinity of formed particles.
In the biogenic synthesis of gold nanoparticles, the change of color of the reaction mixture from pale yellow to dark purple/deep red reflects the formation of gold particles. The different colors of gold nanoparticle solution are due to their surface plasmon resonance properties (He et al. 2007). Generally, UV-visible spectroscopy is utilized to confirm formation of metal nanoparticles including gold. The UV-visible spectrum of the reaction mixtures (organism-gold ions) represents the formation of a gold surface plasmon resonance (detection of gold nanoparticles) that ranged from 500 to 600 nm (Deplanche and Macaskie 2008).
11.4 Applications of Gold Nanoparticles in Agriculture
In recent years, the use of nanotechnology in various fields, including pharmaceuticals, engineering, and agriculture, has been developed. The application of nanotechnology in the agricultural sector has improved, especially in the area of food industry and plant protection. Gold nanoparticles have many potential applications in agriculture due to their antimicrobial activity and unique optical property.
Gold nanoparticles can be applied as a sensor in a series of colorimetric assays. In this assay, the interaction between the analyte and the gold nanoparticles can induce the aggregation of gold nanoparticles and consequently solution color changes from red to purple. This feature can be used to identify different molecules, including pesticides. For instance, Bai et al. (2010) have studied gold nanoparticles as colorimetric probes for screening insecticide pymetrozine. It has been demonstrated that compounds containing nitrogen heterocycles and amine groups can be bound to the surface of metal nanoparticles and induce the accumulation of the nanoparticles (Gittins and Caruso 2001; Ai et al. 2009). Chemical structure of pymetrozine contains multiple binding sites including one exocyclic secondary amine and four-nitrogen hybrid ring. Indeed, the color change and aggregation of gold nanoparticles can be attributed to the specific interactions between the functional groups of gold nanoparticle and pymetrozine.
In addition, gold nanoparticle-based sensors can be utilized to determine the residue of different pesticides in plants and food products. For example, Bai and his colleagues (2010) determined the concentration of pymetrozine with the low detection limit (1 × 10 −6 M) and reported the high sensitivity of this method for pymetrozine compared with other 11 pesticides. The detection sensitivity of this system could be increased by adding salt and reducing the pH. The use of bacterial-derived gold nanoparticles to detect organophosphorus pesticide residues in fruits and vegetables has been proven (Malarkodi et al. 2017).
Gold nanoparticles can also be useful in water purification. For instance, Zhang et al. (2014) fabricated imidazole ionic liquid functionalized gold nanoparticles for the recognition of imidacloprid. The researchers suggested that the detection system could be used to determine and remove imidacloprid in different water samples based on the aggregation phenomena of gold nanoparticles. The application of fungus-mediated synthesized gold nanoparticles to remove pesticides and pathogens from water has been reported (Das et al. 2009).
Gold nanoparticles have antibacterial and antifungal properties that can be used in plant disease management, food safety, and medical applications. Jayaseelan et al. (2013) synthesized gold nanoparticles using seed aqueous extract of Abelmoschus esculentus and posed antifungal activity of the nanoparticles against Candida albicans, Aspergillus niger, Aspergillus flavus, and Puccinia graminis tritci. The antibacterial activity of the biosynthesized gold nanoparticles against Klebsiella pneumoniae, Bacillus subtilis, Pseudomonas aeruginosa, and Escherichia coli has been reported (Annamalai et al. 2013; Muthuvel et al. 2014).
The mycelial growth inhibition of Phomopsis theae by Trichoderma atroviride-mediated biosynthesized gold nanoparticles has been demonstrated (Ponmurugan 2016). Field experiments conducted with soil application and wound dressing of the nanoparticles confirmed the efficacy of the nanoparticles for control of Phomopsis canker disease in tea plants.
It has been revealed that biosynthesized gold nanoparticles can be useful to control pests in agriculture and public health (Thakur et al. 2018; Sundararajan and Kumari 2017). Thakur et al. (2018) studied the effect of biosynthesized gold nanoparticles on root-knot nematodes (Meloidogyne incognita) in tomato crop. The nanoparticles showed suitable nematicidal effect and had no negative impact on plant growth. All articles on the insecticidal activity of gold nanoparticles focused on mosquito species of medical and veterinary importance.
Many researchers have demonstrated that gold nanoparticles induce cell division, seed and pollen germination, and plant growth (Arora et al. 2012; Gopinath et al. 2013; Mahakham et al. 2016; Thakur et al. 2018). Therefore, application of gold nanoparticles in agriculture and plant sciences could be beneficial to increase the plant growth and crop yield like several types of engineered nanomaterials (Baiazidi-Aghdam et al. 2016; Ghorbanpour and Hadian 2015; Hatami et al. 2013, 2016, 2017, 2019; Ghorbanpour et al. 2015, 2018; Ghorbanpour and Hatami 2014, 2015; Ghorbanpour 2015; Ghorbanpour and Hadian 2017; Ghorbanpour and Fahimirad 2017; Hatami et al. 2014; Hatami and Ghorbanpour 2013, 2014; Hatami 2017; Chegini et al. 2017; Mohammadi et al. 2018; Tian et al. 2018; Ahmadi et al. 2018).
11.5 Conclusions
The main goal of most nanotechnology research is to design and produce nanoparticles with new features. Compared with the chemical method, the biological synthesis of gold nanoparticles by organisms is an environmentally friendly and reliable method. The gold nanoparticles of a variety of shapes and sizes can be easily synthesized from different types of plants and microbes. The synthesis of gold nanoparticles depends on various factors including the concentration of plant extract/biomass and metal salt, pH of the solution, temperature, reaction time, and the location of nanoparticle formation (extracellular/intracellular). Applications of such eco-friendly nanoparticles in agriculture to purify rivers and lakes from pesticides can reduce the harmful impacts on nontarget organisms. Biosynthesized gold nanoparticles can be effective to protect the various crop plants against plant pathogens and can be a suitable alternative to chemical pesticides that are toxic to human and the environment.
References
Abdel-Raouf N, Al-Enazi NM, Ibraheem IB (2017) Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab J Chem 10:S3029–S3039
Agnihotri M, Joshi S, Kumar AR, Zinjarde S, Kulkarni S (2009) Biosynthesis of gold nanoparticles by the tropical marine yeast Yarrowia lipolytica NCIM 3589. Mater Lett 63:1231–1234
Ahmad A, Senapati S, Khan MI, Kumar R, Sastry M (2003a) Extracellular biosynthesis of monodisperse gold nanoparticles by a novel extremophilic actinomycete, Thermomonospora sp. Langmuir 19:3550–3553
Ahmad A, Senapati S, Khan MI, Kumar R, Ramani R, Srinivas V, Sastry M (2003b) Intracellular synthesis of gold nanoparticles by a novel alkalo tolerant actinomycete, Rhodococcus species. Nanotechnology 14:824
Ahmadi SZ, Ghorbanpour M, Hadian J, Salehi-Arjmand H (2018) Impact of foliar spray of spherical Nano-carbon and Salicylic acid on physiological traits and Parthenolide content in two feverfew cultivars (Tanacetum parthenium Linn. cv. Pharmasaat and Jelitto). J Med Plant 17(4):82–98
Ai K, Liu Y, Lu L (2009) Hydrogen-bonding recognition-induced color change of gold nanoparticles for visual detection of melamine in raw milk and infant formula. J Am Chem Soc 131:9496–9497
Ankamwar B, Damle C, Ahmad A, Sastry M (2005) Biosynthesis of gold and silver nanoparticles using Emblica officinalis fruit extract, their phase transfer and transmetallation in an organic solution. J Nanosci Nanotechnol 5:1665–1671
Annamalai A, Christina VLP, Sudha D, Kalpana M, Lakshmi PTV (2013) Green synthesis, characterization and antimicrobial activity of Au NPs using Euphorbia hirta L. leaf extract. Colloids Surf B Biointerfaces 108:60–65
Armendariz V, Herrera I, Jose-yacaman M, Troiani H, Santiago P, Gardea-Torresdey JL (2004a) Size controlled gold nanoparticle formation by Avena sativa biomass: use of plants in nanobiotechnology. J Nanopart Res 6:377–382
Armendariz V, Jose-Yacaman M, Duarte Moller A, Peralta-Videa JR, Troiani H, Herrera I, Gardea-Torresdey JL (2004b) HRTEM characterization of gold nanoparticles produced by wheat biomass. Revista Mexicana de Fisica Supplement 50:7–11
Aromal SA, Philip D (2012) Green synthesis of gold nanoparticles using Trigonellafoenum-graecum and its size-dependent catalytic activity. Spectrochim Acta A Mol Biomol Spectrosc 97:1–5
Arora S, Sharma P, Kumar S, Nayan R, Khanna PK, Zaidi MGH (2012) Gold-nanoparticle induced enhancement in growth and seed yield of Brassica juncea. Plant Growth Regul 66:303–310
Bai LY, Zhang YP, Chen J, Zhou XM, Hu LF (2010) Rapid, sensitive and selective detection of pymetrozine using gold nanoparticles as colorimetric probes. Micro Nano Lett 5:304–308
Baiazidi-Aghdam MT, Mohammadi H, Ghorbanpour M (2016) Effects of nanoparticulate anatase titanium dioxide on physiological and biochemical performance of Linum usitatissimum (Linaceae) under well watered and drought stress conditions. Braz J Bot 39:139–146
Barabadi H, Honary S, Mohammadi MA, Ahmadpour E, Rahimi MT, Alizadeh A, Naghibi F, Saravanan M (2017) Green chemical synthesis of gold nanoparticles by using Penicillium aculeatum and their scolicidal activity against hydatid cyst protoscolices of Echinococcus granulosus. Environ Sci Pollut Res 24:5800–5810
Beveridge TJ, Murray RG (1980) Sites of metal deposition in the cell wall of Bacillus subtilis. J Bacteriol 141:876–887
Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M (2006) Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog 22:577–583
Chegini E, Ghorbanpour M, Hatam M, Taghizadeh M (2017) Effect of multi-walled carbon nanotubes on physiological traits, phenolic contents and antioxidant capacity of Salvia mirzayanii Rech. f & Esfandunder drought stress. J Med Plant 16(2):191–207
Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD (2005) Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 1:325–327
Daniel MC, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104:293–346
Das SK, Das AR, Guha AK (2009) Gold nanoparticles: microbial synthesis and application in water hygiene management. Langmuir 25:8192–8199
Das RK, Gogoi N, Bora U (2011) Green synthesis of gold nanoparticles using Nyctanthesarbortristis flower extract. Bioprocess Biosyst Eng 34:615–619
Dauthal P, Mukhopadhyay M (2012) Prunus domestica fruit extract-mediated synthesis of gold nanoparticles and its catalytic activity for 4-nitrophenol reduction. Ind Eng Chem Res 51:13014–13020
Deplanche K, Macaskie LE (2008) Biorecovery of gold by Escherichia coli and Desulfovibrio desulfuricans. Biotechnol Bioeng 99:1055–1064
Dhanasekar NN, Rahul GR, Narayanan KB, Raman G, Sakthivel N (2015) Green chemistry approach for the synthesis of gold nanoparticles using the fungus Alternaria sp. J Microbiol Biotechnol 25:1129–1135
Dubey SP, Lahtinen M, Sillanpää M (2010a) Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa. Colloids Surf A Physicochem Eng Asp 364:34–41
Dubey SP, Lahtinen M, Sillanpää M (2010b) Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem 45:1065–1071
El-Batal AI, ElKenawy NM, Yassin AS, Amin MA (2015) Laccase production by Pleurotus ostreatus and its application in synthesis of gold nanoparticles. Biotechnol Reps 5:31–39
Gardea-Torresdey JL, Parsons JG, Gomez E, Peralta-Videa J, Troiani HE, Santiago P, Yacaman MJ (2002) Formation and growth of Au nanoparticles inside live alfalfa plants. Nano Lett 2:397–401
Ghodake GS, Deshpande NG, Lee YP, Jin ES (2010) Pear fruit extract-assisted room-temperature biosynthesis of gold nanoplates. Colloids Surf B Biointerfaces 75:584–589
Ghorbanpour M (2015) Major essential oil constituents, total phenolics and flavonoids content and antioxidant activity of Salvia officinalis plant in response to nano-titanium dioxide. Ind J Plant Physiol 20(3):249–256
Ghorbanpour M, Fahimirad SH (2017) Plant nanobionics a novel approach to overcome the environmental challenges. In: Ghorbanpour M, Varma A (eds) Medicinal plants and environmental. Springer. https://doi.org/10.1007/978-3-319-68717-9_14
Ghorbanpour M, Hadian J (2015) Multi-walled carbon nanotubes stimulate callus induction, secondary metabolites biosynthesis and antioxidant capacity in medicinal plant Satureja khuzestanica grown in vitro. Carbon 94:749–759
Ghorbanpour M, Hadian (2017) Engineered nanomaterials and their interactions with plant cells: injury indices and detoxification pathways. In: Ghorbanpour M et al (eds) Nanoscience and plant–soil systems, Soil biology 48. https://doi.org/10.1007/978-3-319-46835-8_13
Ghorbanpour M, Hatami M (2014) Spray treatment with silver nanoparticles plus thidiazuron increases anti-oxidant enzyme activities and reduces petal and leaf abscission in four cultivars of geranium (Pelargonium zonale) during storage in the dark. J Hortic Sci Biotechnol 89(6):712–718
Ghorbanpour M, Hatami H (2015) Changes in growth, antioxidant defense system and major essential oils constituents of Pelargonium graveolens plant exposed to nano-scale silver and thidiazuron. Ind J Plant Physiol 20(2):116–123
Ghorbanpour M, Hatami M, Hatami M (2015) Activating antioxidant enzymes, hyoscyamine and scopolamine biosynthesis of Hyoscyamus niger L. plants with nano-sized titanium dioxide and bulk application. Acta Agric Slov 105:23–32
Ghorbanpour M, Khaltabadi Farahani AH, Hadian J (2018) Potential toxicity of nano-graphene oxide on callus cell of Plantago major L. under polyethylene glycol-induced dehydration. Ecotoxicol Environ Saf 148:910–922
Gittins DI, Caruso F (2001) Spontaneous phase transfer of nanoparticulate metals from organic to aqueous media. Angew Chem Int Ed 40:3001–3004
Gole A, Dash C, Ramakrishnan V, Sainkar SR, Mandale AB, Rao M, Sastry M (2001) Pepsin- gold colloid conjugates: preparation, characterization, and enzymatic activity. Langmuir 17:1674–1679
Golinska P, Wypij M, Ingle AP, Gupta I, Dahm H, Rai M (2014) Biogenic synthesis of metal nanoparticles from actinomycetes: biomedical applications and cytotoxicity. Appl Microbiol Biotechnol 98:8083–8097
Gopinath K, Venkatesh KS, Ilangovan R, Sankaranarayanan K, Arumugam A (2013) Green synthesis of gold nanoparticles from leaf extract of Terminalia arjuna, for the enhanced mitotic cell division and pollen germination activity. Ind Crop Prod 50:737–742
Hatami M (2017) Stimulatory and inhibitory effects of Nanoparticulates on seed germination and seedling vigor indices. In: Ghorbanpour M et al (eds) Nanoscience and plant–soil systems, Soil biology 48. https://doi.org/10.1007/978-3-319-46835-8_13
Hatami M, Ghorbanpour M (2013) Effect of nanosilver on physiological performance of Pelargonium plants exposed to dark storage. J Hortic Res 21(1):15–20
Hatami M, Ghorbanpour M (2014) Defense enzymes activity and biochemical variations of Pelargonium zonale in response to nanosilver particles and dark storage. Turk J Biol 38:130–139
Hatami M, Hatamzadeh A, Ghasemnezhad M, Ghorbanpour M (2013) The comparison of antimicrobial effects of silver nanoparticles (SNP) and silver nitrate(AgNo3) to extend the vase life of ‘red ribbon’ cut rose flowers. Trakia J Sci 2:144–151
Hatami M, Ghorbanpour M, Salehiarjomand H (2014) Nano-anatase TiO2 modulates the germination behavior and seedling vigority of the five commercially important medicinal and aromatic plants. J Biol Environ Sci 8(22):53–59
Hatami M, Kariman K, Ghorbanpour M (2016) Engineered nanomaterial-mediated changes in the metabolism of terrestrial plants. Sci Total Environ 571:275–291
Hatami M, Hadian J, Ghorbanpour M (2017) Mechanisms underlying toxicity and stimulatory role of single-walled carbon nanotubes in Hyoscyamus niger during drought stress simulated by polyethylene glycol. J Hazard Mater 324:306–320
Hatami M, Hosseini SM, Ghorbanpour M, Kariman K (2019) Physiological and antioxidative responses to GO/PANI nanocomposite in intact and demucilaged seeds and young seedlings of Salvia mirzayanii. Chemosphere 233:920–935
He S, Guo Z, Zhang Y, Zhang S, Wang J, Gu N (2007) Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulata. Mater Lett 61:3984–3987
Heurtault B, Saulnier P, Pech B, Proust JE, Benoit JP (2003) Physico-chemical stability of colloidal lipid particles. Biomaterials 24:4283–4300
Honary S, Gharaei-Fathabad E, Barabadi H, Naghibi F (2013) Fungus-mediated synthesis of gold nanoparticles: a novel biological approach to nanoparticle synthesis. J Nanosci Nanotechnol 13:1427–1430
Hulkoti NI, Taranath TC (2014) Biosynthesis of nanoparticles using microbes-a review. Colloids Surf B Biointerfaces 121:474–483
Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13:2638–2650
Jayaseelan C, Ramkumar R, Rahuman AA, Perumal P (2013) Green synthesis of gold nanoparticles using seed aqueous extract of Abelmoschus esculentus and its antifungal activity. Ind Crop Prod 45:423–429
Jha AK, Prasad K, Prasad K, Kulkarni AR (2009) Plant system: nature’s nanofactory. Colloids Surf B Biointerfaces 73:219–223
Jia L, Zhang Q, Li Q, Song H (2009) The biosynthesis of palladium nanoparticles by antioxidants in Gardenia jasminoides Ellis: long lifetime nanocatalysts for p-nitrotoluene hydrogenation. Nanotechnology 20:385601
Khadem Moghadam N, Hatami M, Rezaei S, Bayat M, Asgari Lajayer B (2019) Induction of plant defense machinery against nanomaterials exposure. In: Ghorbanpour M, Wani SH (eds) Advances in phytonanotechnology: from synthesis to application. Elsevier, London
Khalil MH, Ismail EH, El-Magdoub F (2012) Biosynthesis of Au nanoparticles using olive leaf extract. Arab J Chem 5(4):431–437
Kumar SA, Abyaneh MK, Gosavi SW, Kulkarni SK, Pasricha R, Ahmad A, Khan MI (2007a) Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol Lett 29:439–445
Kumar SA, Ansary AA, Ahmad A, Khan MI (2007b) Extracellular biosynthesis of CdSe quantum dots by the fungus, Fusarium oxysporum. J Biomed Nanotechnol 3:190–194
Lengke MF, Fleet ME, Southam G (2006) Morphology of gold nanoparticles synthesized by filamentous cyanobacteria from gold (I)− thiosulfate and gold (III)− chloride complexes. Langmuir 22:2780–2787
Maghsoodi MR, AsgariLajayer B, Hatami M, Mirjalili MH (2019) Challenges and opportunities of nanotechnology in plants-soil mediated systems: beneficial role, phytotoxicity and phytoextraction. In: Ghorbanpour M, Wani SH (eds) Advances in phytonanotechnology: from synthesis to application, Elsevier, London
Mahakham W, Theerakulpisut P, Maensiri S, Phumying S, Sarmah AK (2016) Environmentally benign synthesis of phytochemicals-capped gold nanoparticles as nanopriming agent for promoting maize seed germination. Sci Total Environ 573:1089–1102
Malarkodi C, Rajeshkumar S, Annadurai G (2017) Detection of environmentally hazardous pesticide in fruit and vegetable samples using gold nanoparticles. Food Control 80:11–18
Mohammadi M, Hatami M, Feghezadeh K, Ghorbanpour M (2018) Mitigating effect of nano-zerovalent iron, iron sulfate and EDTA against oxidative stress induced by chromium in Helianthus annuus L. Acta Physiol Plant 40:69
Molnár Z, Bódai V, Szakacs G, Erdélyi B, Fogarassy Z, Sáfrán G, Varga T, Kónya Z, Tóth-Szeles E, Szűcs R, Lagzi I (2018) Green synthesis of gold nanoparticles by thermophilic filamentous fungi. Sci Rep 8:3943
Mortazavi SM, Khatami M, Sharifi I, Heli H, Kaykavousi K, Poor MHS, Kharazi S, Nobre MAL (2017) Bacterial biosynthesis of gold nanoparticles using Salmonella enterica subsp. enterica serovar Typhi isolated from blood and stool specimens of patients. J Clust Sci 28:2997–3007
Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI, Ramani R, Parischa R, Ajayakumar PV, Alam M, Sastry M, Kumar R (2001) Bioreduction of AuCl4− ions by the fungus, Verticillium sp. and surface trapping of the gold nanoparticles formed. Angew Chem Int Ed 40:3585–3588
Mukherjee P, Senapati S, Mandal D, Ahmad A, Khan MI, Kumar R, Sastry M (2002) Extracellular synthesis of gold nanoparticles by the fungus Fusarium oxysporum. Chembiochem 3:461–463
Muthuvel A, Adavallan K, Balamurugan K, Krishnakumar N (2014) Biosynthesis of gold nanoparticles using Solanum nigrum leaf extract and screening their free radical scavenging and antibacterial properties. Biomed Prev Nutr 4:325–332
Narayanan KB, Sakthivel N (2010) Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interf Sci 156:1–13
Ochekpe NA, Olorunfemi PO, Ngwuluka NC (2009) Nanotechnology and drug delivery part 2: nanostructures for drug delivery. Trop J Pharm Res 8(3):275–287
Pinto RJ, Lucas JM, Morais MP, Santos SA, Silvestre AJ, Marques PA, Freire CS (2017) Demystifying the morphology and size control on the biosynthesis of gold nanoparticles using Eucalyptus globulus bark extract. Ind Crop Prod 105:83–92
Ponmurugan P (2016) Biosynthesis of silver and gold nanoparticles using Trichoderma atroviride for the biological control of Phomopsis canker disease in tea plants. IET Nanobiotechnol 11:261–267
Prabhu S, Poulose EK (2012) Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett 2:1–10
Rajasree SR, Suman TY (2012) Extracellular biosynthesis of gold nanoparticles using a gram negative bacterium Pseudomonas fluorescens. Asian Pac J Trop Dis 2:S796–S799
Shankar SS, Ahmad A, Pasricha R, Sastry M (2003) Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J Mater Chem 13:1822–1826
Shankar SS, Rai A, Ahmad A, Sastry M (2004) Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using neem (Azadirachta indica) leaf broth. J Colloid Interface Sci 275:496–502
Sharma D, Kanchi S, Bisetty K (2015) Biogenic synthesis of nanoparticles: a review. Arab J Chem. https://doi.org/10.1016/j.arabjc.2015.11.002
Shen W, Qu Y, Pei X, Li S, You S, Wang J, Zhang Z, Zhou J (2017) Catalytic reduction of 4-nitrophenol using gold nanoparticles biosynthesized by cell-free extracts of Aspergillus sp. WL-Au. J Hazard Mater 321:299–306
Singaravelu G, Arockiamary JS, Kumar VG, Govindaraju K (2007) A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf B Biointerfaces 57:97–101
Sneha K, Sathishkumar M, Lee SY, Bae MA, Yun YS (2011) Biosynthesis of Au nanoparticles using cumin seed powder extract. J Nanosci Nanotechnol 11:1811–1814
Soltani Nejad M, Shahidi Bonjar GH, Khaleghi N (2015) Biosynthesis of gold nanoparticles using Streptomyces fulvissimus isolate. Nanomed J 2:153–159
Song JY, Kim BS (2009) Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng 32:79–84
Song JY, Kwon EY, Kim BS (2010) Biological synthesis of platinum nanoparticles using Diopyros kaki leaf extract. Bioprocess Biosyst Eng 33:159–164
Suman TY, Rajasree SR, Ramkumar R, Rajthilak C, Perumal P (2014) The green synthesis of gold nanoparticles using an aqueous root extract of Morinda citrifolia L. Spectrochim Acta A Mol Biomol Spectrosc 118:11–16
Sundararajan B, Kumari BR (2017) Novel synthesis of gold nanoparticles using Artemisia vulgaris L. leaf extract and their efficacy of larvicidal activity against dengue fever vector Aedes aegypti L. J Trace Elem Med Biol 43:187–196
Thakker JN, Dalwadi P, Dhandhukia PC (2013) Biosynthesis of gold nanoparticles using Fusarium oxysporum f. sp. cubense JT1, a plant pathogenic fungus. ISRN Biotechnol 2013:1–5
Thakur RK, Shirkot P, Dhirta B (2018) Studies on effect of gold nanoparticles on Meloidogyne incognita and tomato plants growth and development. Bio Rxiv 428144. https://doi.org/10.1101/428144
Tian H, Ghorbanpour M, Kariman K (2018) Manganese oxide nanoparticle-induced changes in growth, redox reactions and elicitation of antioxidant metabolites in deadly nightshade (Atropa belladonna L.). Ind Crop Prod 126:403–414
Vala AK (2015) Exploration on green synthesis of gold nanoparticles by a marine-derived fungus Aspergillus sydowii. Environm Prog Sustain Energy 34:194–197
Zhang X, Sun Z, Cui Z, Li H (2014) Ionic liquid functionalized gold nanoparticles: synthesis, rapid colorimetric detection of imidacloprid. Sensors Actuators B Chem 191:313–319
Zhang X, Qu Y, Shen W, Wang J, Li H, Zhang Z, Li S, Zhou J (2016) Biogenic synthesis of gold nanoparticles by yeast Magnusiomyces ingens LH-F1 for catalytic reduction of nitrophenols. Colloids Surf A Physicochem Eng Asp 497:280–285
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Graily-Moradi, F., Maadani Mallak, A., Ghorbanpour, M. (2020). Biogenic Synthesis of Gold Nanoparticles and Their Potential Application in Agriculture. In: Ghorbanpour, M., Bhargava, P., Varma, A., Choudhary, D. (eds) Biogenic Nano-Particles and their Use in Agro-ecosystems. Springer, Singapore. https://doi.org/10.1007/978-981-15-2985-6_11
Download citation
DOI: https://doi.org/10.1007/978-981-15-2985-6_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2984-9
Online ISBN: 978-981-15-2985-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)