Abstract
Historically, the surgical access for neck endocrine diseases has been performed using a traditional Kocher access implying a wide skin incision, which results in a visible, large scar in the neck [1]. During the last two decades, the introduction of minimally invasive surgery interested all the field of general surgery and endocrine surgery was not an exception [2]. Indeed, after the description of the first successful case of endoscopic parathyroidectomy by Gagner [3] in 1996, several minimally invasive approaches for thyroidectomy and parathyroidectomy were developed [1, 4–11]. The different approach described for thyroidectomy include endoscopic [1, 5, 6, 9, 10] and video-assisted procedure and mini-access, non-endoscopic operations [11], this last implying a smaller incision without the use of endoscope and thus without the advantages of the endoscopic magnification for adequate visualization of neck structures. The approach with the endoscope can be classified respectively in purely endoscopic [3, 5, 9, 12] and video-assisted procedures [6, 7, 13], depending on the need or not of CO2 insufflation. The totally endoscopic techniques include procedures with a cervical access [1, 9] and procedures with an extra-cervical access [5, 12], which require the use of external devices (retractors) in order to create and to maintain the working space for the dissection and trocar positioning [9].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Video-assisted thyroidectomy (VAT)
- Minimally invasive thyroidectomy
- Papillary thyroid carcinoma
- Oncologic outcome
- Complications rate
- Cosmetic results
Introduction
Historically, surgical access for neck endocrine diseases has been performed using a traditional Kocher access, implying a wide skin incision, which results in a visible large scar in the neck [1]. During the last two decades, the introduction of minimally invasive surgery interested all the fields of general surgery, and endocrine surgery was not an exception [2]. Indeed, after the description of the first successful case of endoscopic parathyroidectomy by Gagner [3] in 1996, several minimally invasive approaches for thyroidectomy and parathyroidectomy were developed [1, 4,5,6,7,8,9,10,11]. The different approaches described for thyroidectomy include the endoscopic technique [1, 5, 6, 9, 10], video-assisted procedure and mini-access, nonendoscopic operations [11], the last implying a smaller incision without the use of an endoscope and thus without the advantages of endoscopic magnification for adequate visualization of neck structures. The approach with the endoscope can be classified respectively in purely endoscopic [3, 5, 9, 12] and video-assisted procedures [6, 7, 13], depending on the need or not of CO2 insufflation. The totally endoscopic techniques include procedures with cervical access [1, 9] and procedures with extracervical access [5, 12], which require the use of external devices (retractors) in order to create and maintain the working space for dissection and trocar positioning [9].
Video-assisted thyroidectomy (VAT) is a central, completely gasless approach that reproduces all the steps of conventional surgery, with the endoscope becoming a tool that allows the surgeon to perform the same operation through a smaller skin incision [6]. The approach is similar to the procedure that Miccoli and coworkers initially described for parathyroidectomy and successfully adopted for thyroidectomy [7, 14].
Twenty years after its first description, VAT is now one of the most broadly diffused minimally invasive approaches to thyroidectomy [15,16,17].
The explanation of the success of VAT resides in its safety and reproducibility in different clinical settings. Moreover, the similarity of VAT to conventional thyroidectomy allows one to exploit the typical advantages of minimally invasive procedures in terms of cosmetic results and postoperative course, without relevant changes in surgical technique [18,19,20,21]. After its initial descriptions, VAT has been evolving [22]. Indeed, the procedure initially limited to selected benign thyroid pathologies has now become a notable procedure in the surgical armamentarium for the treatment of thyroid surgical diseases, within the boundaries of its selection criteria [17, 20].
Being video assisted, VAT is performed under both direct and endoscopic vision [6], gaining the advantages of magnified vision for most critical steps of the surgical procedure. Indeed, thanks to endoscope magnification, the recurrent laryngeal nerve (RLN) and parathyroid glands can be adequately identified and preserved [23, 24]. Moreover, the endoscope allows for easy visualization of the external branch of the superior laryngeal nerve (EBSLN) in most of the cases of VAT [25].
Early after the first experiences with minimally invasive thyroidectomy, several small single institution reports [1, 4,5,6, 12, 13, 26], two multicenter studies [19, 27], and several large retrospective studies [16, 28,29,30,31] have been published confirming the reproducibility and safety of VAT. Specifically addressed advantages of VAT over conventional thyroidectomy are better cosmetic results and lower postoperative pain [32, 33]. These results have been further validated in two well-designed randomized controlled trials by Gal et al. [34] and El Labban [35], showing the superiority of VAT over conventional surgery in terms of cosmetic results and reduced postoperative distress.
Noteworthy to mention is that the additional advantage of VAT is the reduced incidence and severity of early voice and swallowing postthyroidectomy symptoms compared to conventional surgery [36].
Furthermore, a recent retrospective cost analysis shows that the cost of VAT appears to be equal to that of conventional thyroidectomy [37].
Finally, a recent meta-analysis demonstrated that VAT reduced immunosuppression, which can be considered proof of its minimally invasive nature [38].
Low invasiveness and similarity with the conventional procedure of VAT render this approach feasible and particularly suitable, in experienced hands, under locoregional anesthesia (cervical block) [39], showing the best results in patients with relative contraindications for general anesthesia.
In this chapter, we describe the surgical technique of minimally invasive video-assisted thyroidectomy, which has been routinely performed at our department since 1998 [6], addressing the specific indication of the technique.
Indications
An accurate patient selection plays a key role in successful VAT accomplishment. In the early experience of the technique, indications were quite limited (single nodule <3 cm in largest diameter, thyroid estimate volume ≤20 mL, small nodules with suspicious or indeterminate cytology, small pretoxic or toxic adenoma); contraindications at the beginning of the experience included thyroiditis and prior neck surgery [20]. With increasing experience, the selection criteria for VAT have been widened. Extended indications included thyroiditis patients who have undergone video-assisted thyroid lobectomy needing completion thyroidectomy and selected cases of Graves’ disease, where VAT can be performed safely with results comparable with conventional surgery [40].
The extensive use of fine-needle aspiration biopsy in clinical practice has increased the diagnosis of small thyroid nodules with indeterminate or suspicious cytology. This group of patients probably represents the ideal candidates for VAT. Indeed, the initial concerns regarding the adequacy of the resection of VAT in the treatment of papillary thyroid carcinoma (PTC) were overcome by several studies demonstrating its safety in selected cases of PTC [41,42,43]. In 2005, we demonstrated that thyroid gland manipulation is not significantly different between VAT and conventional thyroidectomy, without additional risks of thyroid capsule rupture and thyroid cell seeding related to the surgical technique [21].
Moreover, the completeness of the resection achieved with VAT resulted in a similar way to that of conventional surgery in selected cases of PTC with a comparable rate of recurrence in the short-to-medium term follow-up [43, 44]. These results have been further confirmed in a recent series of patients who underwent VAT for PTC followed up for a time period longer than 10 years [17], in which a very low recurrence rate was observed, comparable to that of conventional surgery.
After acquiring adequate experience with the technique and supported by the previous optimal results in terms of safety and completeness of the surgical resection, we standardized a video-assisted lymph-node dissection of the central compartment [43, 45].
From a purely technical point of view, it should be considered that the endoscope shows specific advantages. Indeed, it allows a meticulous exploration of the central compartment and enables the identification of even slightly enlarged lymph nodes that might be overlooked at open surgery.
Video-assisted approach with central access allows to performing a formal central neck compartment dissection when needed, providing optimal results in terms of the completeness of the oncological resection and the number of removed nodes, with an overall outcome comparable to that of the open counterpart [43, 46, 47]. However, the preoperative evidence of central neck lymph node metastases still represents a contraindication for the video-assisted method [46, 47].
Patients carrying RET oncogene mutation for familial forms of medullary thyroid carcinoma but not even expressing the disease (absence of detectable nodules and basal/stimulated calcitonin in the normal range) are excellent candidates for VAT [48].
In summary, today, in our experience, the eligibility criteria for VAT include thyroid nodules ≤35 mm in diameter, estimated thyroid volume ≤30 ml, selected cases of PTC, RET gene mutation carriers, concomitant thyroiditis, and selected cases of Graves’ disease [20].
Operative Technique
Patient and Surgical Team Positions
The patient is positioned supine on the operating table with the neck in slight extension and the arms tucked on each side. The less neck extension may contribute to the decreased postoperative pain observed in VAT with respect to conventional surgery. The skin is prepped from the lower lip to the nipples in a fashion similar to conventional thyroidectomy. However, the area of the surgical incision may also be covered by a transparent dressing (Tegaderm, for example) to protect the skin edges.
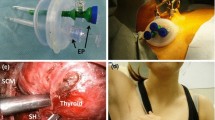
The surgical team is composed of the surgeon and two assistants, of which one handles the endoscope (Fig. 12.1). The monitor is placed in front of the surgeon, who is positioned on the right side of the patient. A second additional monitor is usually placed in front of the assistants, who are on the left side of the patient. The absence of any external support allows modulating the position of the endoscope in relation to the different steps of the dissection. This represents an important advantage of the video-assisted procedure over purely endoscopic techniques. The tip of the endoscope is usually oriented toward the patient’s head, but it can be changed to explore the upper mediastinum when a concomitant central compartment lymphadenectomy is required.
(a) Operating room setting and position of the surgical team. (From Raffaelli et al. [50]; with permission). (b) Position of the surgical team in VAT using the Nanoscope™ System Arthrex, a small (1.9 mm) single-use camera system that combines imaging sensors, LED lights, and image and operative room integration and control. (Unpublished personal experience)
Anesthesia
Thyroid surgery and VAT are usually performed under general anesthesia with endotracheal intubation. However, with the increasing experience, the feasibility of VAT under local anesthesia (LA-VAT) with a superficial cervical block (Fig. 12.2) has been demonstrated [39]. In our experience, besides the patients showing specific contraindications for general anesthesia, the indications to LA-VAT are mainly based on the patient’s and surgeon’s preference.
Surgical Instruments
Most of the surgical instruments necessary for VAT are usually available in operating theaters, and it is not a source of additional costs. The only instruments not used in conventional thyroidectomy are small, special spatulas and spatula-shaped aspirators (2–3 mm in diameter), which are useful in dissection. They come from ear, nose and throat and plastic surgery and are reusable (Fig. 12.3). Sealing systems (LigaSure Precise™, Harmonic Focus Plus®, Thunderbeat Open Fine Jaw (OFJ)®, Caiman®) have been proved to be effective during VAT since they allow the surgeon to reduce the operative time [49].
From a technical point of view, considering the small size of the skin incision, probably the ideal sealing systems would be those endowed with a long, freely rolling stem, similar to the CS-14 Ultracision Harmonic Scalpel® model, unfortunately out of production.
Superficial cervical block. The cervical block is performed with the patient in the operating room. A mixture of bupivacaine 0.25% and carbocaine 0.5% is used as locoregional anesthetic. A 4-cm, 23-gauge needle is inserted at the midpoint along the posterior border of the sternocleidomastoid muscle on the affected side, and the tip is directed anteriorly. After having aspirated first, a 10-mL bolus of the bupivacaine-carbocaine mixture is injected. The needle is then directed toward the midline, and an additional 5 mL bolus of the anesthetic mixture is injected while the needle is withdrawn. Then a bolus of 5-mL of the anesthetic is administered along the incision line at an intermediate level between the sternal notch and the cricoid cartilage in the midline
Surgical Technique
A small (1.5–2 cm), central skin incision (Fig. 12.4) is performed between the cricoid cartilage and the sternal notch (Fig. 12.5). The skin incision is usually higher than in conventional cervicotomy and can also be modulated according to the neck conformation and thyroid position. However, the skin incision is usually performed just below (1 cm) the cricoid cartilage in order to obtain an optimal exposure and safe control of the upper thyroid vascular pedicle. The skin incision would be ideally placed in an existing skin wrinkle to optimize the aesthetic result. After incising the platysma muscle (Fig. 12.6) and preparing the upper and lower flaps (Fig. 12.7), the cervical linea alba is opened as far as possible (Fig. 12.8), taking care to avoid any minimal bleeding. The thyroid lobe (on the affected side) is then separated from the strap muscles by means of small conventional retractors (Farabeuf retractors), which are also used to maintain open operative space (Fig. 12.9). The thyroid lobe is medially retracted, while the strap muscles are laterally retracted using the two Farabeufs. At this point, the endoscope (5 mm – 30°) and the dedicated small surgical instruments (2 mm in diameter) are introduced through the single skin incision. The first step of the procedure consists in completely freeing the thyroid gland in order to have good exposure of the prevertebral fascia, which represents the posterior aspect of the dissection. The middle thyroid vein, if present, is sectioned to gain complete access to the prevertebral fascia. The medial and lateral edges of the dissection are, respectively, the tracheoesophageal groove and the medial aspect of the common carotid artery. If intraoperative neuromonitoring is used, the identification and stimulation of the vagus nerve are then performed as the first step of the surgical procedure (Fig. 12.10a). If continuous intraoperative nerve monitoring is used, the automated periodic stimulation (APS) electrode is placed at the level of the vagus nerve, prepared for about 1 cm (Fig. 12.10b).
The dissection is carried out by a blunt technique using two dedicated instruments (spatulas); one of the instruments is connected to an aspiration system. After its complete separation from the muscles, the thyroid lobe is retracted downward, in order to expose the upper pole vessels; these are dissected using the spatula and the spatula-shaped aspirator (Fig. 12.11). During this phase, it is usually possible to identify the EBSLN, thanks to the magnification of the endoscope (Fig. 12.12). The upper pole vessels are then either selectively clipped and cut or directly cut using a sealing system (Fig. 12.13). During this step, special attention should be paid to controlling the tip of the energy device in order to avoid pharynx or larynx thermal injury. After complete dissection of the upper pole, the thyroid lobe is retracted medially by means of the Farabeuf retractor to identify the RLN under endoscopic vision (Fig. 12.14). The magnification (two- to three fold) of the endoscope allows a straightforward identification of the RLN. Gentle medial traction on the thyroid lobe, in order to avoid any inadvertent stretch injury to the RLN, and lateral traction on the strap muscles allow to improve the exposition of the inferior thyroid artery, thus facilitating the identification of the RLN, typically found where it crosses the inferior thyroid artery. The Zuckerkandl tubercle can be another useful landmark for the identification of the RLN, as in conventional procedure. If intraoperative neuromonitoring is used, once the RLN is identified, it is stimulated in order to check the predissection function (Figs. 12.15 and 12.16). Thus, the RLN is prepared and bluntly dissected (Fig. 12.17), under endoscopic vision, with an upward direction from the mediastinum toward its entry point in the larynx. At this point, the superior parathyroid gland is identified at the level of the posterior aspect of the thyroid lobe (Fig. 12.18).
The parathyroid glands are identified and preserved with adequate vascularization supply, facilitated by the assistance of the endoscopic magnification.
The thyroid lobe is then extracted (Fig. 12.19) from the skin incision, and the procedure is accomplished under endoscopic and direct vision (Fig. 12.20). During the maneuver of thyroid lobe extraction, it is of utmost importance to avoid excessive traction, which can result in inadvertent stretch injury to the nerve.
After identifying and preserving the inferior parathyroid gland (Fig. 12.21), the lower vascular pole is selectively dissected by means of a conventional tie, clip, or energy device. It is important to carry out a completely bloodless dissection facilitated by using a spatula-shaped aspirator. At this step of the procedure, it is of the utmost importance to check again the RLN and the parathyroid glands. Although the separation of the thyroid from the trachea is usually carried out using a sealing system (Fig. 12.22), dissection close to the nerve can be accomplished by means of a titanium clip or conventional ligature in order to avoid the risk of a thermal injury to the RLN. At the end of the thyroid lobe resection, the RLN and the ipsilateral vagus nerve are stimulated (postdissection stimulation).
The isthmus is sectioned using a sealing device in case of thyroid lobectomy. If total thyroidectomy is planned, the same steps are performed on the contralateral side. In the case of total thyroidectomy, before proceeding to the contralateral lobe dissection, the dissected lobe is repositioned in its original cervical space in order to optimize the surgical working space. After checking the hemostasis, the strap muscles are sutured along the midline, and the same is done for the platysma. The skin is closed by means of a nonresorbable subcuticular running suture (Fig. 12.23) or by a skin sealant. Generally, no position of drain required. Hemostatic agents can be used and placed in the operating field, such as the Tabotamp Fibrillar® (Ethicon).
Blunt dissection frees the thyroid lobe from the strap muscle. Then the operative space is created by two conventional Farabeuf divaricators that retract the thyroid lobe medially and the strap muscles laterally, maintaining an adequate working space. To note, the lateral limit of the operative space is the common carotid artery, easily exposed by means of the lateral Farabeuf. After creating the operative space, the 5-mm-30° endoscope and the 2-mm dedicated instruments are introduced through the same central access. In cases in which intraoperative neuromonitoring is used, predissection vagus nerve stimulation can easily be accomplished as the very first step of the surgical procedure
Conclusions
From our experience of more than 20 years, we can reaffirm the concept that VAT is a safe procedure that is not burdened by an increase of complication rate or additional costs. Furthermore, this technique offers advantages in terms of cosmetic results and postoperative pain. VAT represents a valid option also in cases of “low-risk” papillary thyroid carcinomas with excellent oncologic outcomes.
References
Yeung GH. Endoscopic surgery of the neck: a new frontier. Surg Laparosc Endosc. 1998;8(3):227–32. http://www.ncbi.nlm.nih.gov/pubmed/9649050. Accessed 11 Dec 2018.
Duh Q-Y. Presidential address: minimally invasive endocrine surgery--standard of treatment or hype? Surgery. 2003;134(6):849–57. https://doi.org/10.1016/S0039.
Gagner M. Endoscopic subtotal parathyroidectomy in patients with primary hyperparathyroidism. Br J Surg. 1996;83(6):875. http://www.ncbi.nlm.nih.gov/pubmed/8696772. Accessed 11 Dec 2018.
Inabnet WB III, Jacob BP, Gagner M. Minimally invasive endoscopic thyroidectomy by a cervical approach. Surg Endosc. 2003;17(11):1808–11. https://doi.org/10.1007/s00464-002-8760-7.
Ikeda Y, Takami H, Tajima G, et al. Total endoscopic thyroidectomy: axillary or anterior chest approach. Biomed Pharmacother. 2002;56 Suppl 1:72s–8s. http://www.ncbi.nlm.nih.gov/pubmed/12487257. Accessed 11 Dec 2018.
Bellantone R, Lombardi CP, Raffaelli M, Rubino F, Boscherini M, Perilli W. Minimally invasive, totally gasless video-assisted thyroid lobectomy. Am J Surg. 1999;177(4):342–3. http://www.ncbi.nlm.nih.gov/pubmed/10326857. Accessed 11 Dec 2018.
Miccoli P, Berti P, Conte M, Bendinelli C, Marcocci C. Minimally invasive surgery for thyroid small nodules: preliminary report. J Endocrinol Investig. 1999;22(11):849–51. https://doi.org/10.1007/BF03343657.
Mourad M, Ngongang C, Saab N, et al. Video-assisted neck exploration for primary and secondary hyperparathyroidism. Surg Endosc. 2001;15(10):1112–5. https://doi.org/10.1007/s004640090017.
Gagner M, Inabnet WB. Endoscopic thyroidectomy for solitary thyroid nodules. Thyroid. 2001;11(2):161–3. https://doi.org/10.1089/105072501300042848.
Miccoli P, Pinchera A, Cecchini G, et al. Minimally invasive, video-assisted parathyroid surgery for primary hyperparathyroidism. J Endocrinol Investig. 1997;20(7):429–30. https://doi.org/10.1007/BF03347996.
Ferzli GS, Sayad P, Abdo Z, Cacchione RN. Minimally invasive, nonendoscopic thyroid surgery. J Am Coll Surg. 2001;192(5):665–8. http://www.ncbi.nlm.nih.gov/pubmed/11333106. Accessed 11 Dec 2018.
Ohgami M, Ishii S, Arisawa Y, et al. Scarless endoscopic thyroidectomy: breast approach for better cosmesis. Surg Laparosc Endosc Percutan Tech. 2000;10(1):1–4. http://www.ncbi.nlm.nih.gov/pubmed/10872517. Accessed 19 Dec 2018.
Mourad M, Saab N, Malaise J, et al. Minimally invasive video-assisted approach for partial and total thyroidectomy. Surg Endosc. 2001;15(10):1108–11. https://doi.org/10.1007/s004640090018.
Miccoli P, Bendinelli C, Conte M, Pinchera A, Marcocci C. Endoscopic parathyroidectomy by a gasless approach. J Laparoendosc Adv Surg Tech – Part A. 1998;8(4):189–94. https://doi.org/10.1089/lap.1998.8.189.
Miccoli P, Biricotti M, Matteucci V, Ambrosini CE, Wu J, Materazzi G. Minimally invasive video-assisted thyroidectomy: reflections after more than 2400 cases performed. Surg Endosc. 2016;30(6):2489–95. https://doi.org/10.1007/s00464-015-4503-4.
Lombardi CP, Raffaelli M, De Crea C, D’Amore A, Bellantone R. Video-assisted thyroidectomy: lessons learned after more than one decade. Acta Otorhinolaryngol Ital. 2009;29(6):317–20. http://www.ncbi.nlm.nih.gov/pubmed/20463836. Accessed 15 Dec 2018.
Bellantone R, Raffaelli M, De Crea C, et al. Video-assisted thyroidectomy for papillary thyroid carcinoma: oncologic outcome in patients with follow-up ≥ 10 years. World J Surg. 2018;42(2):402–8. https://doi.org/10.1007/s00268-017-4392-x.
Bellantone R, Lombardi CP, Bossola M, et al. Video-assisted vs conventional thyroid lobectomy: a randomized trial. Arch Surg. 2002;137(3):301–4; discussion 305. http://www.ncbi.nlm.nih.gov/pubmed/11888453. Accessed 13 Dec 2018.
Miccoli P, Bellantone R, Mourad M, Walz M, Raffaelli M, Berti P. Minimally invasive video-assisted thyroidectomy: multiinstitutional experience. World J Surg. 2002;26(8):972–5. https://doi.org/10.1007/s00268-002-6627-7.
Sessa L, Lombardi CP, De Crea C, Raffaelli M, Bellantone R. Video-assisted endocrine neck surgery: state of the art. Updat Surg. 2017;69(2):199–204. https://doi.org/10.1007/s13304-017-0467-3.
Lombardi CP, Raffaelli M, Princi P, et al. Safety of video-assisted thyroidectomy versus conventional surgery. Head Neck. 2005;27(1):58–64. https://doi.org/10.1002/hed.20118.
Bakkar S, Materazzi G, Biricotti M, et al. Minimally invasive video-assisted thyroidectomy (MIVAT) from A to Z. Surg Today. 2016;46(2):255–9. https://doi.org/10.1007/s00595-015-1241-0.
Bellantone R, Lombardi CP, Raffaelli M, Boscherini M, De Crea C, Traini E. Video-assisted thyroidectomy. J Am Coll Surg. 2002;194(5):610–4. http://www.ncbi.nlm.nih.gov/pubmed/12022601. Accessed 11 Dec 2018.
Miccoli P, Fregoli L, Rossi L, et al. Minimally invasive video-assisted thyroidectomy (MIVAT). Gland Surg. 2020;9(Suppl 1):S1–5. https://doi.org/10.21037/gs.2019.12.05.
Berti P, Materazzi G, Conte M, Galleri D, Miccoli P. Visualization of the external branch of the superior laryngeal nerve during video-assisted thyroidectomy. J Am Coll Surg. 2002;195(4):573–4. https://doi.org/10.1016/S1072-7515(02)01338-8.
Wilhelm T, Metzig A. Endoscopic minimally invasive thyroidectomy: first clinical experience. Surg Endosc. 2010;24(7):1757–8. https://doi.org/10.1007/s00464-009-0820-9.
Terris DJ, Angelos P, Steward DL, Simental AA. Minimally invasive video-assisted thyroidectomy: a multi-institutional North American experience. Arch Otolaryngol Head Neck Surg. 2008;134(1):81–4. https://doi.org/10.1001/archoto.2007.22.
Miccoli P, Berti P, Frustaci GL, Ambrosini CE, Materazzi G. Video-assisted thyroidectomy: indications and results. Langenbeck's Arch Surg. 2006;391(2):68–71. https://doi.org/10.1007/s00423-006-0027-7.
Lombardi CP, Raffaelli M, Princi P, De Crea C, Bellantone R. Video-assisted thyroidectomy: report of a 7-year experience in Rome. Langenbeck's Arch Surg. 2006;391(3):174–7. https://doi.org/10.1007/s00423-006-0023-y.
Lombardi CP, Raffaelli M, Princi P, De Crea C, Bellantone R. Video-assisted thyroidectomy: report on the experience of a single center in more than four hundred cases. World J Surg. 2006;30(5):794–800; discussion 801. https://doi.org/10.1007/s00268-005-0390-5.
Minuto MN, Berti P, Miccoli M, et al. Minimally invasive video-assisted thyroidectomy: an analysis of results and a revision of indications. Surg Endosc. 2012;26(3):818–22. https://doi.org/10.1007/s00464-011-1958-9.
Miccoli P, Berti P, Raffaelli M, Materazzi G, Baldacci S, Rossi G. Comparison between minimally invasive video-assisted thyroidectomy and conventional thyroidectomy: a prospective randomized study. Surgery. 2001;130(6):1039–43. https://doi.org/10.1067/msy.2001.118264.
Miccoli P, Rago R, Massi M, et al. Standard versus video-assisted thyroidectomy: objective postoperative pain evaluation. Surg Endosc. 2010;24(10):2415–7. https://doi.org/10.1007/s00464-010-0964-7.
Gal I, Solymosi T, Szabo Z, Balint A, Bolgar G. Minimally invasive video-assisted thyroidectomy and conventional thyroidectomy: a prospective randomized study. Surg Endosc. 2008;22(11):2445–9. https://doi.org/10.1007/s00464-008-9806-2.
El-Labban GM. Minimally invasive video-assisted thyroidectomy versus conventional thyroidectomy: a single-blinded, randomized controlled clinical trial. J Minim Access Surg. 2009;5(4):97–102. https://doi.org/10.4103/0972-9941.59307.
Lombardi CP, Raffaelli M, De Crea C, et al. Long-term outcome of functional post-thyroidectomy voice and swallowing symptoms. Surgery. 2009;146(6):1174–81. https://doi.org/10.1016/j.surg.2009.09.010.
Byrd JK, Nguyen SA, Ketcham A, Hornig J, Gillespie MB, Lentsch E. Minimally invasive video-assisted thyroidectomy versus conventional thyroidectomy: a cost-effective analysis. Otolaryngol Head Neck Surg. 2010;143(6):789–94. https://doi.org/10.1016/j.otohns.2010.08.002.
Zheng C, Liu S, Geng P, et al. Minimally invasive video-assisted versus conventional open thyroidectomy on immune response: a meta analysis. Int J Clin Exp Med. 2015;8(2):2593–9.
Lombardi CP, Raffaelli M, Modesti C, Boscherini M, Bellantone R. Video-assisted thyroidectomy under local anesthesia. Am J Surg. 2004;187(4):515–8. https://doi.org/10.1016/j.amjsurg.2003.12.030.
Berti P, Materazzi G, Galleri D, Donatini G, Minuto M, Miccoli P. Video-assisted thyroidectomy for Graves? Disease: report of a preliminary experience. Surg Endosc. 2004;18(8):1208–10. https://doi.org/10.1007/s00464-003-9225-3.
Miccoli P, Elisei R, Materazzi G, et al. Minimally invasive video-assisted thyroidectomy for papillary carcinoma: a prospective study of its completeness. Surgery. 2002;132(6):1070–4. https://doi.org/10.1067/msy.2002.128694.
Bellantone R, Lombardi CP, Raffaelli M, et al. Video-assisted thyroidectomy for papillary thyroid carcinoma. Surg Endosc. 2003;17(10):1604–8. https://doi.org/10.1007/s00464-002-9220-0.
Lombardi CP, Raffaelli M, de Crea C, et al. Report on 8 years of experience with video-assisted thyroidectomy for papillary thyroid carcinoma. Surgery. 2007;142(6):944–51. https://doi.org/10.1016/j.surg.2007.09.022.
Miccoli P, Pinchera A, Materazzi G, et al. Surgical treatment of low- and intermediate-risk papillary thyroid cancer with minimally invasive video-assisted thyroidectomy. J Clin Endocrinol Metab. 2009;94(5):1618–22. https://doi.org/10.1210/jc.2008-1418.
Lombardi CP, Raffaelli M, De Crea C, Sessa L, Rampulla V, Bellantone R. Video-assisted versus conventional total thyroidectomy and central compartment neck dissection for papillary thyroid carcinoma. World J Surg. 2012;36(6):1225–30. https://doi.org/10.1007/s00268-012-1439-x.
Bellantone R, Lombardi CP, Raffaelli M, Boscherini M, Alesina PF, Princi P. Central neck lymph node removal during minimally invasive video-assisted thyroidectomy for thyroid carcinoma: a feasible and safe procedure. J Laparoendosc Adv Surg Tech – Part A. 2002;12(3):181–5. https://doi.org/10.1089/10926420260188074.
Miccoli P, Elisei R, Donatini G, Materazzi G, Berti P. Video-assisted central compartment lymphadenectomy in a patient with a positive RET oncogene: initial experience. Surg Endosc Other Interv Tech. 2007;21(1):120–3. https://doi.org/10.1007/s00464-005-0642-3.
Miccoli P, Elisei R, Berti P, et al. Video assisted prophylactic thyroidectomy and central compartment nodes clearance in two RET gene mutation adult carriers. J Endocrinol Investig. 2004;27(6):557–61. https://doi.org/10.1007/BF03347478.
Miccoli P, Berti P, Raffaelli M, Materazzi G, Conte M, Galleri D. Impact of Harmonic Scalpel on operative time during video-assisted thyroidectomy. Surg Endosc. 2002;16(4):663–6. https://doi.org/10.1007/s00464-001-9117-3.
Raffaelli M, Traini E, Lombardi CP, Bellantone R. Minimally invasive video-assisted parathyroidectomy: how to correctly approach of the adenoma. In: Shifrin A, editor. Atlas of parathyroid surgery. New York: Springer; 2020.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Raffaelli, M., De Crea, C., Pennestrì, F., Lombardi, C.P., Bellantone, R. (2022). Video-Assisted Thyroidectomy. In: Shifrin, A. (eds) Atlas of Thyroid Surgery . Springer, Cham. https://doi.org/10.1007/978-3-030-93673-0_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-93673-0_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-93672-3
Online ISBN: 978-3-030-93673-0
eBook Packages: MedicineMedicine (R0)