Abstract
Co-Morbid Insomnia and Sleep Apnea (COMISA) is a common but complex sleep disorder requiring a multidisciplinary treatment approach. Cognitive behavioral therapy for insomnia (CBT-I) is first-line therapy for insomnia as well as for COMISA, administered prior to or contemporaneously with continuous positive airway pressure (CPAP) therapy or treatment of sleep apnea. However, given the complexity of the disorder and practical issues such as CBT-I availability, sedative/ hypnotics may need to be considered. Given their adverse event profile, benzodiazepines are not ideal agents for treatment of insomnia in general or in COMISA. Non-benzodiazepines appear to be safe and effective overall for COMISA with randomized, double-blind, placebo-controlled trials supporting eszopiclone not exacerbating obstructive sleep apnea (OSA), possibly improving apnea-hypopnea index (AHI) in untreated and treated patients along with improving compliance. Dual orexin receptor antagonists (DORAs) appear promising as recent randomized, double-blind, placebo-controlled, two-period crossover trials demonstrated respiratory safety of lemborexant at 10 mg for adults and elderly with mild, moderate and severe untreated OSA. Comorbidities needing antidepressants or benzodiazepines may be needed with consideration of their differential impact on sleep apnea. More research is needed into this complex area given the multiple neurotransmitter systems involved and agents with different mechanisms of actions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Co-Morbid Insomnia and Sleep Apnea (COMISA)
- Benzodiazepines (BZDs)
- Non-benzodiazepines (Non-BZDs)
- Dual orexin receptor antagonists (DORAs)
- Continuous positive airway pressure (CPAP)
- Apnea-hypopnea index (AHI)
1 Co-Morbid Insomnia and Sleep Apnea (COMISA)
Despite the frequent presentation of both sleep apnea and insomnia in the same patient, it was not immediately recognized that their combined presence could exacerbate and intensify the symptoms of each. COMISA was first identified by Guilleminault, Eldridge, and Dement [1, 2]. The article alerted clinicians to the possibility that patients with insomnia might be unaware of their sleep apnea symptoms and thus of the consequent risk of misdiagnosing the condition as a case of uncomplicated insomnia. The article had a very limited impact over the next 30 years until the publication of findings in 1999 and 2001 showing that insomnia and obstructive sleep apnea (OSA) have 30% to 50% comorbidity rates [3,4,5,6]. Since 1999 a substantial number of studies have further documented the considerable overlap and bidirectional relationship of comorbid insomnia and OSA [6].
COMISA has remained a persistent challenge to treat well. This observation emphasizes the importance of screening for insomnia in a “sleep apnea clinic,” most expeditiously by providing the patient with a screening questionnaire prior to the interview. When compared to patients with OSA who do not have insomnia, COMISA patients frequently show poor adherence with using continuous positive airway pressure (CPAP) therapy [6,7,8,9,10,11,12,13]. A major part of the challenge of dealing with this patient group thus relates to how to increase the initial acceptance of and subsequent use of CPAP therapy. This has led to advocacy of the importance of treating insomnia disorder prior to or concurrently with initiating CPAP treatment [6,7,8,9,10,11,12,13].
First-line treatment of chronic insomnia is cognitive behavioral therapy for insomnia (CBT-I). Chapter 17 Part 3 in this book describe the use and efficacy of CBT-I in COMISA patients. When CBT-I is not available, hypnotic agents may need to be considered.
2 Sedative/Hypnotic Agents in Patients with OSA
2.1 Overall Effects
A literature review of studies by Mason et al. contained in the Cochrane Airways Group Specialized Register of Trials found that a wide range of medications, including remifentanil 0.75 mcg/kg/hr (infused opioid), eszopiclone 3 mg, zolpidem 10 and 20 mg, brotizolam 0.25 mg, flurazepam 30 mg, nitrazepam 10 mg to 15 mg, temazepam 10 mg, triazolam 0.25 mg, ramelteon 8 mg and 16 mg, and sodium oxybate 4.5 g and 9 g, did not produce a deleterious effect on OSA severity as measured by change in apnea-hypopnea index (AHI) or oxygen desaturation index (ODI) [14,15,16,17,18,19,20,21,22,23]. Zolpidem at 20 mg, flurazepam 30 mg, remifentanil infusion, and triazolam 0.25 mg, however, did result in significant oxygen desaturations statistically and clinically suggesting caution with these agents at these doses. In two clinical trials, eszopiclone 3 mg and sodium oxybate 4.5 g decreased AHI (compared to placebo) [14]. The reviewers concluded that no evidence existed that these pharmacological compounds produced significant adverse changes in the AHI or ODI and thus did not severely effect OSA severity [14]. Caution was still suggested because the studies reviewed were small and short duration, and there are instances where oxygen desaturation could occur with particular agents at certain doses, such as zolpidem at higher than therapeutically recommended doses, which should not be used. The authors suggested that further investigation of agents which decreased AHI as a therapeutic option may be worthwhile for a subgroup of OSA patients [14].
2.2 Benzodiazepines (BZDs)
Efforts to treat insomnia in the context of obstructive sleep apnea used to be focused on benzodiazepines (BZDs) before the advent of non-benzodiazepine hypnotics. There are many concerns with BZD’s treatment for insomnia in general which include abuse, dependence, addiction, withdrawal, rebound insomnia, falls, cognitive impairment, and adverse changes in sleep architecture such as promoting light sleep while reducing deep and REM sleep [24, 25]. In addition to the overall adverse central nervous system (CNS) depressant effect of these agents, concerns for COMISA patients also include the decreased ventilatory response to hypoxia and reduced upper airway muscle tone as well as any other mechanisms exacerbating sleep disordered breathing [26,27,28,29,30].
Some studies have found adverse changes with some BZDs such as flurazepam and triazolam while others not, such as with temazepam in mild OSA and nitrazepam in mild to mod OSA patients [16,17,18,19]. A study by Berry et al. focused on the effects of triazolam (0.25 mg) in 12 patients with severe sleep apnea in a randomized crossover study [19]. Measurements of sleep were determined by polysomnography. Triazolam was found to increase the arousal threshold to airway occlusion, and this produced only a modest prolongation in the duration of events in this patient group.
There is a body of evidence against the use of BZDs in COMISA patients. For example, a recent study by Wang et al. performed a retrospective case review using the Taiwan National Health Insurance Database from 1996 to 2013 with the purpose of quantifying the extent of acute respiratory events among COMISA patients who were users of hypnotics [31]. The case group included 216 hypnotic users who were diagnosed as having experienced acute respiratory events, including pneumonia and respiratory failure. The hypnotics used included both benzodiazepines (BZDs) and non-benzodiazepines (non-BZDs). Following an adjusted multivariate analysis, the authors concluded that long-term BZD use may increase the risk of acute respiratory failure in OSA patients.
Although the effects of BZDs on OSA may be modest, BZDs are not ideally recommended for the treatment of insomnia in general or to patients with COMISA. There may however be instances when they may need to be prescribed like when there are severe comorbid refractory disorders of anxiety or PTSD with COMISA, although further studies are needed.
2.3 Non-benzodiazepines (Non-BZDs)
Non-benzodiazepines are a better treatment choice than BZDs for treatment of insomnia in general and in particular for COMISA patients.
Various studies have shown that non-benzodiazepines compared to BZDs can increase total sleep time, improve sleep continuity along with sleep architecture, and have fewer adverse effects and fewer interactions [32,33,34,35,36,37,38,39].
Given the limitations of BZDs, various studies have been carried out concerning the safety and efficacy of non-BZDs for insomnia in the context of sleep disordered breathing or obstructive sleep apnea. GABAergic non-benzodiazepine agents, zolpidem, zaleplon, and eszopiclone, have been investigated in OSA patients. There is evidence that non-BZDs are a better alternative to BZDs and may improve sleep without causing respiratory depression. The advantage of these agents are that they may have only limited muscle relaxant effects, which is a benefit to treating the core breathing problems of OSA.
Further, there is some evidence that these agents may not worsen sleep apnea and may alternatively decrease AHI in certain populations with potential to improve tolerance and adherence to CPAP therapy [40,41,42,43,44,45,46,47,48,49,50,51,52,53].
A meta-analysis by Nigram et al. of published studies over the 30-year period between 1988 and 2017 evaluated the efficacy and safety of non-benzodiazepine sedative hypnotics (NBSHs), included agents such as zolpidem, zaleplon, and eszopiclone [54]. The meta-analysis, comprising data from a total of 2099 patients, found that the NBSH drugs did not increase AHI, regardless of the baseline AHI values (mild, moderate, severe, or no OSA). The AHI was found to improve minimally with use of NBSH drugs, but eszopiclone showed the greatest difference, having an MD of −5.73 events/h.
Another literature review identified eight controlled clinical trials (with 448 patients) on the effect of non-BZDs on sleep quality and severity of OSA symptoms, including the AHI index and the nadir of arterial oxygen saturation (SaO2) [55]. The review by Zhang et al. supported the conclusion that non-BZDs in typically recommended doses improved sleep quality without worsening sleep apnea in OSA patients.
2.4 Non-benzodiazepines for OSA Without CPAP
A small pilot study (n = 22) by Rosenberg et al. was conducted prior to larger OSA/COMISA studies for eszopiclone [56]. The objective of the study was to evaluate the effect of eszopiclone 3 mg on respiration, sleep, and safety in mild-moderate OSA patients who were withdrawn from CPAP. The study was a double-blind, randomized crossover design with patients (35–64 years) receiving eszopiclone 3 mg or placebo on two consecutive nights in the sleep laboratory. There was a 5–7 day washout between the two treatments. Eszopiclone administration without CPAP did not worsen AHI and was found to improve sleep maintenance and efficiency.
An open label trial investigated the effect of zolpidem 10 mg over a period of 9 weeks on 20 patients who were suffering from idiopathic central sleep apnea [57]. Although three patients experienced significant increases in obstructive events, the majority of patients showed decreases in central apnea/hypopneas and associated symptoms with zolpidem. They also had improved sleep continuity and decreased subjective daytime sleepiness.
2.5 Non-benzodiazepines for OSA Treated with CPAP
The first large (n = 226), randomized, double-blind, placebo-controlled study of eszopiclone in OSA patients receiving diagnostic polysomnography (PSG) or CPAP titration investigated the effects of eszopiclone 3 mg on various parameters of sleep quality among the 226 patients to evaluate whether such treatment would improve the quality of diagnostic PSG and CPAP titration studies [58]. Either eszopiclone or placebo was administered once on the night of testing, just before polysomnography. Compared to placebo, pretreatment with eszopiclone improved CPAP titrations and produced fewer residual events/h (5.7 vs. 11.9) and fewer incomplete titrations (31.1% vs. 48.0%). There was also a trend for more non-usable studies with placebo than with eszopiclone (7.1% vs. 2.7%) with the authors concluding routine use of non-benzodiazepines as premedication for PSG should be considered.
In another placebo-controlled study, the 16 participants with severe OSA and on CPAP therapy for at least 6 months received zolpidem 10 mg [59]. All patients were tested during one night of CPAP use. Sleep architecture, AHI, and arterial oxygen saturation showed no differences between zolpidem and placebo.
3 Hypnotic Agents and CPAP Adherence
3.1 Hypnotic Medications (Type Not Specified)
In a retrospective chart review of short-term CPAP therapy among 400 consecutive patients, only age and one-time sedative/hypnotic use during titration polysomnography were found to correlate with short-term compliance [48].
3.2 Non-benzodiazepines
Bradshaw et al. in 2006 investigated the effects of zolpidem, placebo, or standard care on compliance with CPAP therapy among 72 male patients who had been referred for CPAP treatment [47]. The duration of the study period was 14 days. Among this group of new CPAP users, those who had been given zolpidem did not show increased compliance to CPAP therapy when compared to the placebo or standard care group. Similarly, Park et al. studied 134 patients undergoing their first night of CPAP therapy [60]. The investigators sought to determine the effects on CPAP compliance of a single dose of zaleplon 10 mg among 73 patients. It was found that, at 1 month, zaleplon improved sleep latency and had beneficial effects on sleep quality on self-report inventories, when compared to placebo, but did not improve adherence to CPAP therapy.
However, Lettieri et al. (2009), in a second double-blind, randomized, placebo-controlled trial of eszopiclone, compared the effect of eszopiclone 3 mg with a matching placebo in 117 participants (of whom 98 completed the study) prior to CPAP titration polysomnography with respect to short-term CPAP compliance [49]. Compared to placebo, eszopiclone 3 mg improved residual obstructive events at the final CPAP pressure (eszopiclone, 6.4 events/ h vs. placebo, 12.8 events/ h) and improved short-term CPAP compliance during the first 4 to 6 weeks of therapy. Eszopiclone was found to improve mean sleep efficiency to a greater extent over placebo (87.8%vs. 80.1%).
An additional double-blind, randomized, placebo-controlled trial by Lettieri et al. was conducted to determine if a short course of eszopiclone 3 mg during the first 2 weeks of CPAP therapy, compared to placebo, would improve long-term adherence to CPAP in 160 adults who had severe OSA (mean AHI, 36.9 events/h) [50]. Adherence to CPAP in the eszopiclone group was found to be superior to the placebo group, in which patients used CPAP for 20.8% more nights. Further, eszopiclone patients used CPAP for 1.1 hours more than the placebo group over the course of 6 months.
These findings suggest that although there may be differences among the non-benzodiazepines in terms of effect and compliance in COMISA patients, eszopiclone therapy may potentially improve more consistently response, short- and longer-term compliance with CPAP therapy [47,48,49,50,51,52,53].
3.3 New Dual Orexin Receptor Antagonists (DORAs, Suvorexant, Lemborexant)
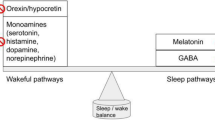
Orexin receptor antagonists appear to facilitate sleep by acting selectively on and blocking the wake system of the brain, which is mediated by orexin receptors. These receptors originate in the lateral hypothalamus and project throughout the brain, including to respiratory centers in the brainstem. It has thus been hypothesized that orexin receptors are involved in the cardiorespiratory response to acute stressors [61].
One study showed that single-use suvorexant was a safe and effective hypnotic for 84 patients with suspected OSA and who experienced insomnia during overnight PSG by Matsumura et al. [62, 63]. Patients who had difficulty falling asleep were permitted to take suvorexant and if they continued to experience insomnia (greater than one hour to fall asleep) were optionally permitted to take zolpidem. The resultant groupings were 44 achieved sufficient sleep with single-use suvorexant alone and 40 who needed suvorexant plus zolpidem. PSG results of 144 patients with AHI >= 5 events/h, revealed 63.1% in the insomnia group had severe OSA versus 70.8% in the non-insomnia comparison group. When the insomnia and non-insomnia groups were compared, there were no differences found in terms of subjectively assessed sleep time or morning mood. The results were interpreted to support the conclusion that single-use suvorexant is a safe and effective hypnotic for laboratory PSG in suspected OSA patients who suffer from insomnia.
Cheng et al. carried out a randomized, double-blind, placebo-controlled, two-period crossover study to examine respiratory safety parameters of lemborexant 10 mg (a DORA with more OX2 orexin receptor blocking affinity than OX1) in 39 individuals who had mild OSA [64]. The subjects were assigned to one of two treatment conditions to receive either lemborexant or placebo and continued on this for 8 days. This was followed by a washout period of 14 days, after which the subjects crossed over to receive the comparison agent. Lemborexant was not found to worsen mean AHI index nor reduce mean oxygen saturation following single or multiple doses when compared to placebo. These findings supported the conclusion that lemborexant at the 10 mg dose demonstrated respiratory safety in this adult and elderly mild OSA study population.
Recently, Moline et al. conducted a multi-centre, randomized, double-blind, placebo-controlled, two-period crossover study on the effects of lemborexant in 33 subjects with untreated moderate to severe OSA; data from the cohort of patients showed no increase in AHI or decrease in peripheral capillary oxygen saturation following single dose or 8 nights of treatment [65]. Given these safety findings and that lemborexant has been shown to significantly increase REM sleep compared to placebo in early trials, there is a suggestion it could play a significant role improving CPAP compliance by alleviating middle and late insomnia in particular, lessening REM-related obstructive sleep apnea through better compliance, possibly helping decrease REM-related OSA’s cardiovascular risk and complications in those predisposed, although more studies are needed [66, 67]. Positive long term 12 month sleep data for initial, middle and late insomnia along with positive early phase multiple PSG data (7 PSGs in Sunrise 1) with lemborexant suggests a potential long-term CPAP compliance study would be feasible and informative [68,69,70,71].
3.4 Antidepressants and OSA (Trazodone and Mirtazapine)
In various studies of both clinical and community-based populations, high rates of depression have been found in individuals diagnosed with OSA [72, 73]. While hypotheses for the linkage between the two conditions have been advanced, the exact mechanisms underlying the association have not been established [72, 73]. Additionally, the effect of antidepressants on OSA has only been minimally studied, possibly due to conceptual concerns that sedatives might worsen OSA in some patients. Nevertheless, some preliminary efforts have examined how trazodone might alter the arousal threshold and OSA severity in patients with OSA. Trazodone is a widely prescribed antidepressant which possesses hypnotic properties with associated increases in the arousal threshold.
Limited work in animal models and small-sample size clinical studies suggested to Smales et al. that these effects would not alter upper airway muscular activity and thus that the agent would have potential for reducing symptoms of OSA [74]. The investigators thus studied the effect of 1 week of trazodone or placebo administration in 15 OSA patients in a randomized crossover design study. Compared to placebo, trazodone was found to reduce the AHI index without worsening oxygen saturation or respiratory event duration. Eckert et al. studied the effects of trazodone in seven patients with OSA who had a low arousal threshold using a within subjects crossover design [75]. Trazodone was found to increase the respiratory threshold, but did not alter the AHI index, nor did it affect dilator muscle activity. This improvement in arousal threshold was not sufficient however to overcome the restrictive upper airway anatomy of these patients.
Similar to trazodone, mirtazapine has been the focus of relatively few studies regarding its efficacy for the treatment of sleep disordered breathing or OSA. Carley et al. studied the effect of mirtazapine on OSA symptoms in 12 newly diagnosed OSA patients [76]. The patients self-administered mirtazapine, either 4.5 mg or 15 mg, or placebo each night for three consecutive 7-day treatment periods. The order of treatments was randomized for all patients. While both dosages of mirtazapine were found to be superior to placebo, the 15 mg dosage was better than 4.5 mg for reducing the AHI, while only the 15 mg dosage reduced the degree of sleep fragmentation. The investigators concluded that in spite of these improvements, they could not offer an unqualified endorsement of mirtazapine in view of side effects of sedation and weight gain. In a follow-up study, Marshall et al. extended the basic experimental design of the Carley et al. (2007) investigation but increased the number of dosage regimens [77]. In the first component of the study, a three-way crossover design was applied: 20 OSA patients were asked to self-administer 7.5, 15, 30, and/or 45 mg or placebo before going to bed for 2 weeks at each dose. In a second parallel study, 65 OSA patients were asked to self-administer mirtazapine 15 mg or mirtazapine 15 mg plus compound CD0012 or placebo for 4 weeks. The investigators were unable to find any improvement in measures of sleep apnea following any of the dosage courses of mirtazapine and thus were not able to recommend the drug for treating OSA symptoms.
In a randomized, double blind crossover study on venlafaxine by Schmickl et al., it was found that AHI improved by 19% in patients with high arousal threshold (−10.9 events/h) but tended to increase in patients with a low arousal threshold (+7 events/ h) with other predictors including elevated AHI and less collapsible upper airway at baseline, concluding that venlafaxine simultaneously worsened and improved various pathophysiological traits, resulting in a zero net effect, and that careful patient selection based on pathophysiologic traits or combination therapy with drugs countering its alerting effects may produce a more robust response [78].
4 Medications for COMISA Conclusion
COMISA is a complex but common sleep disorder, which can result in increased morbidity and mortality more so than if either insomnia or obstructive sleep apnea were present alone. The presence of both disorders can make clinical diagnosis and treatment of each more difficult. Given the bidirectional nature of the disorder, optimal treatment of COMISA is multidisciplinary. CBT-I is not only first line for treatment of insomnia but also for insomnia of COMISA with the addition of CPAP contemporaneously or after, which may also improve CPAP compliance.
However, given the complexity, severity, chronicity, and refractoriness of the disorder along with comorbidities and the practical issues of CBT-I availability, sedative/hypnotics may be needed. The role of non-benzodiazepines for treatment of insomnia may need to be considered, and preliminary evidence suggests certain ones such as eszopiclone and zolpidem may be safe and effective at proper therapeutic doses and duration, in untreated and treated patients on CPAP, with eszopiclone possibly improving CPAP compliance and lowering AHI more in untreated patients. This may give relief to initial prescribers when insomnia severity warrants it and sleep apnea is not completely known waiting for a PSG, although caution is always exercised when starting any sedative/hypnotic agent.
DORAs look promising for treating COMISA, as studies for lemborexant 10 mg single use and up to 8 nights did not worsen AHI or lower mean oxygen saturation in adult or elderly untreated patients with mild, moderate or severe sleep apnea. Even suvorexant in combination with zolpidem was found to be safe and effective in a single use PSG laboratory context.
Given the many neurotransmitter systems involved in the sleep-wake cycle and sedative/hypnotics with different mechanisms of action, more research is certainly needed in this complex area such as which agent, class, or combination is optimal, the dosing, timing, and duration of treatment in relation to patient insomnia type, severity, complexity, and duration, along with whether CPAP or other therapy is present or not. Effects on compliance and overall treatment response will also need to be examined with the various treatment agents, comparing different classes and particular agents.
References
Guilleminault C, Eldridge FL, Dement WC. Insomnia with sleep apnea: a new syndrome. Science. 1973;181(4102):856–8. https://doi.org/10.1126/science.181.4102.856. PMID: 4353301.
Guilleminault C, Davis K, Huynh NT. Prospective randomized study of patients with insomnia and mild sleep disordered breathing. Sleep. 2008;31(11):1527–33. https://doi.org/10.1093/sleep/31.11.1527. PMID: 19014072.
Lichstein KL, Riedel BW, Lester KW, et al. Occult sleep apnea in a recruited sample of older adults with insomnia. J Consult Clin Psychol. 1999;67(3):405–10. PubMed:10369061.
Krakow B, Melendez D, Ferreira E, et al. Prevalence of insomnia symptoms in patients with sleep-disordered breathing. Chest. 2001;120(6):1923–9. PubMed: 11742923.
Krakow B, Melendrez D, Lee SA, Warner TD, Clark JO, Sklar D. (2004). Refractory insomnia and sleep-disordered breathing: a pilot study. Sleep Breath. 2004;8(1):15–29. https://doi.org/10.1007/s11325-004-0015-5. PMID: 150269350.
Ong JC, Crawford MR. Insomnia and obstructive sleep apnea. Sleep Med Clin. 2013;8(3):389–98. https://doi.org/10.1016/j.jsmc.2013.04.004.
Sweetman A, Lack L, Bastien C. Co-Morbid Insomnia and Sleep Apnea (COMISA): prevalence, consequences, methodological considerations, and recent randomized controlled trials. Brain Sci. 2019;9(12):371. https://doi.org/10.3390/brainsci9120371. PMID: 31842520.
Sweetman A, Lack L, Catcheside PG, Antic NA, Smith S, Chai-Coetzer CL, Douglas J, O'grady A, Dunn N, Robinson J, Paul D, Williamson P, McEvoy RD. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. https://doi.org/10.1093/sleep/zsz178. PMID: 31403168.
Sweetman A, Lack L, Lambert S, Gradisar M, Harris J. Does comorbid obstructive sleep apnea impair the effectiveness of cognitive and behavioral therapy for insomnia? Sleep Med. 2017;39:38–46. https://doi.org/10.1016/j.sleep.2017.09.003. Epub 2017 Sep 22. PMID: 29157586.
Sweetman A, Lack L, McEvoy RD, Antic NA, Smith S, Chai-Coetzer CL, Douglas J, O'Grady A, Dunn N, Robinson J, Paul D, Eckert D, Catcheside PG. Cognitive behavioural therapy for insomnia reduces sleep apnoea severity: a randomised controlled trial. ERJ Open Res. 2020;6(2):00161–2020. https://doi.org/10.1183/23120541.00161-2020. eCollection 2020 Apr. PMID: 32440518.
Sweetman AM, Lack LC, Catcheside PG, Antic NA, Chai-Coetzer CL, Smith SS, Douglas JA, McEvoy RD. Developing a successful treatment for co-morbid insomnia and sleep apnoea. Sleep Med Rev. 2017;33:28–38. https://doi.org/10.1016/j.smrv.2016.04.004. Epub 2016 May 6. PMID: 27401786.
Bahr K, Carmara RJ, Gouveris H, Tuin I. Current treatment of comorbid insomnia and obstructive sleep apnea with CBTI and PAP-therapy: a systematic review. Front Neurol. 2018;9:804. https://doi.org/10.3389/fneur.2018.00804. PMID: 30420826.
Crawford MR, Turner AD, Wyatt JK, Fogg LF, Ong JC. Evaluating the treatment of obstructive sleep apnea comorbid with insomnia disorder using an incomplete factorial design. Contemp Clin Trials. 2016;47:146–52. https://doi.org/10.1016/j.cct.2015.12.017. Epub 2015 Dec 28. PMID: 26733360.
Mason M, Cates CJ, Smith I. Effects of opioid, hypnotic and sedating medications on sleep-disordered breathing in adults with obstructive sleep apnoea. Cochrane Database Syst Rev. 2015;(7):CD011090. https://doi.org/10.1002/14651858.CD011090.pub2. PMID: 26171909.
Cirignotta F, Mondini S, Gerardi R, Zucconi M. Effect of brotizolam on sleep-disordered breathing in heavy snorers with obstructive apnea. Curr Therap Res Clin Exp. 1992;51(3):360–6.
Dolly FR, Block AJ. Effects of flurazepam on sleep-disordered breathing and nocturnal oxygen desaturation in asymptomatic subjects. Am J Med. 1982;73:239–43.
Höijer U, Hedner J, Ejnell H, Grunstein R, Odelberg E, Elam M. Nitrazepam in patients with sleep apnoea: a double-blind placebo-controlled study. Eur Respir J. 1994;7(11):2011–5.
Camacho ME, Morin CM. The effect of Temazepam on respiration in elderly insomniacs with mild sleep apnea. Sleep. 1995;18:644–5. https://doi.org/10.1093/sleep/18.8.644.
Berry RB, Kouchi K, Bower J, Prosise G, Light RW. Triazolam in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151(2 Pt 1):450–4. https://doi.org/10.1164/ajrccm.151.2.7842205. PMID: 7842205.
Kryger M, Wang-Weigand S, Roth T. Safety of ramelteon in individuals with mild to moderate obstructive sleep apnea. Sleep Breath. 2007;11:159–64.
Gooneratne NS, Gehrman P, Gurubhagavatula I, Al-Shehabi E, Marie E, Schwab R. Effectiveness of ramelteon for insomnia symptoms in older adults with obstructive sleep apnea: a randomized placebo-controlled pilot study. J Clin Sleep Med. 2010;6(6):572–80.
George CFB, Feldman N, Zheng Y, Steininger TL, Grzeschik SM, Lai C, Inhaber N. A 2-week, polysomnographic, safety study of sodium oxybate in obstructive sleep apnea syndrome. Published online: 18 January 2010. This article is published with open access at Springerlink.com.
George CFP, Feldman N, Inhaber N, Steininger TL, Grzeschik SM, Lai C, Zheng Y. A safety trial of sodium oxybate in patients with obstructive sleep apnea: acute effects on sleep-disordered breathing. Sleep Med. 2010;11(1):38–42. https://doi.org/10.1016/j.sleep.2009.06.006. Epub 2009 Nov 7.
Janhsen K, Roser P, Hoffmann K. The problems of long-term treatment with benzodiazepines and related substances. Dtsch Arztebl Int. 2015;112(1–2):1–7. https://doi.org/10.3238/arztebl.2015.0001. PMID: 25613443.
Riemann D, Perlis ML. The treatments of chronic insomnia: a review of benzodiazepine receptor agonists and psychological and behavior therapies. Sleep Med Rev. 2009;13(3):205–14. https://doi.org/10.1016/j.smrv.2008.06.001.
Bonora M, St John WM, Bledsoe TA. Differential elevation by protriptyline and depression by diazepam of upper airway respiratory motor activity. Am Rev Respir Dis. 1985;131:41–5.
Leiter JC, Knuth SL, Krol RC, Bartlett D Jr. The effect of diazepam on genioglossal muscle activity in normal human subjects. Am Rev Respir Dis. 1985;132:216–9.
Hanly P, Powles P. Hypnotics should never be used in patients with sleep apnea. J Psychosom Res. 1993;37:59–65.
Lu B, Budhiraja R, Parthasarathy S. Sedating medications and undiagnosed obstructive sleep apnea: physician determinants and patient consequences. J Clin Sleep Med. 2005;1:367–71.
Luyster FS, Buysse DJ, Strollo PJ Jr. Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research. J Clin Sleep Med. 2010;6(2):196–204. PMID: 20411700.
Wang SH, Chen WS, Tang SE, Lin HC, Peng CK, Chu HT, Kao CH. Benzodiazepines associated with acute respiratory failure in patients with obstructive sleep apnea. Front Pharmacol. 2019;9:1513. https://doi.org/10.3389/fphar.2018.01513. eCollection 2018. PMID: 30666205.
Nutt DJ, Stahl SM. Searching for perfect sleep: the continuing evolution of GABAa receptor modulators as hypnotics. J Psychopharmacol. 24(11):1601–12. https://doi.org/10.1177/0269881109106927.
Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD, Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–33. https://doi.org/10.7326/M15-2175. Epub 2016 May 3. PMID: 27136449.
Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(2):307–49. https://doi.org/10.5664/jcsm.6470. PMID: 27998379.
MacFarlane J. Taking control of acute insomnia- restoring healthy sleep patterns. The Canadian Sleep Society, Insomnia Rounds. 2012;1(2).
MacFarlane J. The effects of psychotropic and neurotropic medications on sleep. Sleepreviewmag.com. 2019, Aug/Sep, 22–24.
NIH State of the Science Conference Statement on manifestations and management of chronic insomnia in adults statement. J Sleep Med. 2005; 1(4):412–421. PMID 17308547 https://consensus.nih.gov/2005/insomniastatement.htm.
Pagel JF, Pandi-Perumal SR, Monti JM. Review: treating insomnia with medications. Sleep Sci Pract. 2018;2:5.BMC. https://doi.org/10.1186/s41606-018-0025-z.
Janseen HCJP, Venekamp LN, Peeters GAM, Pijpers A, Pevernagie AA. Management of insomnia in sleep disordered breathing. Eur Respir Rev. 2019;28:190080. https://doi.org/10.1183/16000617.0080-2019.
Eckert DJ, Owens RL, Kehlmann GB, Wellman A, Rahangdale S, Yim-Yeh S, White DP, Malhotra A. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond). 2011;120(12):505–14. https://doi.org/10.1042/CS20100588.
Smith PR, Sheikh KL, Costan-Toth C, Forsthoefel D, Bridges E, Andrada TF, Holley AB. Eszopiclone and zolpidem do not affect the prevalence of the low arousal threshold phenotype. J Clin Sleep Med. 13(1):115–9. https://doi.org/10.5664/jcsm.6402.
Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:144–53.
Eckert DJ, Sweetman A. Impaired central control of sleep depth propensity as a common mechanism for excessive overnight wake time: implications for sleep apnea, insomnia and beyond. J Clin Sleep Med. 2020;16(3):341–3. https://doi.org/10.5664/jcsm.8268. Epub 2020 Jan 14. PMID: 32003739.
Hagen C, Patel A, McCall WV. Prevalence of insomnia symptoms in sleep laboratory patients with and without sleep apnea. Psychiatry Res. 2009;170(2–3):276–7. https://doi.org/10.1016/j.psychres.2009.02.001. Epub 2009 Nov 6. PMID: 19896722.
Lofaso F, Goldenberg F, Thebault C, Janus C, Harf A. Effect of zopiclone on sleep, night-time ventilation, and daytime vigilance in upper airway resistance syndrome. Eur Respir J. 1997;10:2573–7.
Cirignotta F, Mondini S, Zucconi M, Gerardi R, Farolfi A, Lugaresi E. Zolpidem-polysomnographic study of the effect of a new hypnotic drug in sleep apnea syndrome. Pharmacol Biochem Behav. 1988;29(4):807–9. https://doi.org/10.1016/0091-3057(88)90212-2. PMID: 3413202.
Bradshaw DA, Ruff GA, Murphy DP. An oral hypnotic medication does not improve continuous positive airway pressure compliance in men with obstructive sleep apnea. Chest. 2006;130(5):1369–76. https://doi.org/10.1378/chest.130.5.1369. PMID: 17099012.
Collen J, Lettieri C, Kelly W, Roop S. Clinical and polysomnographic predictors of short-term continuous positive airway pressure compliance. Chest. 2009;135(3):704–9. https://doi.org/10.1378/chest.08-2182. Epub 2008 Nov 18. PMID: 19017888.
Lettieri CJ, Collen JF, Eliasson AH, Quast TM. Sedative use during continuous positive airway pressure titration improves subsequent compliance: a randomized, double-blind, placebo-controlled trial. Chest. 2009;136(5):1263–8. https://doi.org/10.1378/chest.09-0811. Epub 2009 Jun 30. PMID: 19567493.
Lettieri CJ, Shah AA, Holley AB, Kelly WF, Chang AS, Roop SA. Effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized tria. Ann Intern Med. 2009;151(10):696–702. https://doi.org/10.7326/0003-4819-151-10-200911170-00006. PMID: 19920270.
Nguyên XL, Chaskalovic J, Rakotonanahary D, Fleury B. Insomnia symptoms and CPAP compliance in OSAS patients: a descriptive study using data mining methods. Sleep Med. 2010;11(8):777–84. https://doi.org/10.1016/j.sleep.2010.04.008. Epub 2010 Jul 6. PMID: 20599419.
Pieh C, Bach M, Popp R, Jara C, Crönlein T, Hajak G, Geisler P. Insomnia symptoms influence CPAP compliance. Sleep Breath. 2013;17(1):99–104. https://doi.org/10.1007/s11325-012-0655-9. Epub 2012 Feb 4. PMID: 22311553.
Wallace DM, Vargas SS, Schwartz SJ, Aloia MS, Shafazand S. Determinants of continuous positive airway pressure adherence in a sleep clinic cohort of South Florida Hispanic veterans. Sleep Breath. 2013;17(1):351–63. https://doi.org/10.1007/s11325-012-0702-6. Epub 2012 Apr 17. PMID: 22528953.
Nigam G, Camacho M, Riaz M. The effect of nonbenzodiazepines sedative hypnotics on apnea-hypopnea index: a meta-analysis. Ann Thorac Med. 2019;14(1):49–55. https://doi.org/10.4103/atm.ATM_198_18. PMID: 30745935.
Zhang XJ, Li QY, Wang Y, Xu HJ, Lin YN. The effect of non-benzodiazepine hypnotics on sleep quality and severity in patients with OSA: a meta-analysis. Sleep Breath. 2014;18(4):781–9. https://doi.org/10.1007/s11325-014-0943-7. Epub 2014 Jan 29. PMID: 24474447.
Rosenberg R, Roach JM, Scharf M, Amato DA. A pilot study evaluating acute use of eszopiclone in patients with mild to moderate obstructive sleep apnea syndrome. Sleep Med. 2007;8(5):464–70. https://doi.org/10.1016/j.sleep.2006.10.007. Epub 2007 May 18. PMID: 17512799.
Quadri S, Drake C, Hudgel DW. Improvement of idiopathic central sleep apnea with zolpidem. J Clin Sleep Med. 2009;5(2):122–9. PMID: 19968044.
Lettieri CJ, Quast TN, Eliasson AH, Andrada T. Eszopiclone improves overnight polysomnography and continuous positive airway pressure titration: a prospective, randomized, placebo-controlled trial. Sleep. 2008;31(9):1310–6. PMID: 18788656.
Berry RB, Patel PB. Effect of zolpidem on the efficacy of continuous positive airway pressure as treatment for obstructive sleep apnea. Sleep. 2006;29(8):1052–6. https://doi.org/10.1093/sleep/29.8.1052. PMID: 16944674.
Park JG, Olson EJ, Morgenthaler TI. Impact of Zaleplon on continuous positive airway pressure therapy compliance. J Clin Sleep Med. 2013;9(5):439–44. https://doi.org/10.5664/jcsm.2660. PMID: 23674934.
Carrive P, Kuwaki T. Orexin and Central Modulation of Cardiovascular and Respiratory Function. Curr Top Behav Neurosci. 2017;33:157–196. https://doi.org/10.1007/7854_2016_46.
Matsumura T, Terada J, Yoshimura C, Koshikawa K, Kinoshita T, Yahaba M, Nagashima K, Sakao S, Tatsumi K. Single-use suvorexant for treating insomnia during overnight polysomnography in patients with suspected obstructive sleep apnea: a single-center experience. Drug Des Devel Ther. 2019;13:809–16. https://doi.org/10.2147/DDDT.S197237. eCollection 2019. PMID: 30880914.
Sun H, Palcza J, Card D, Gipson A, Rosenberg R, Kryger M, Lines C, Wagner JA, Troyer MD. Effects of suvorexant, an orexin receptor antagonist, on respiration during sleep in patients with obstructive sleep apnea. J Clin Sleep Med. 2016;12(1):9–17.
Cheng JY, Filippov G, Moline M, Zammit GZ, Bsharat M, Hall N. Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: a randomized, double blind, placebo-controlled, crossover study. J Sleep Res. 2020;29(4):e13021. https://doi.org/10.1111/jsr.13021.
Moline M, Cheng JY, Lorch D, Hall N, Shah D. Respiratory Safety of Lemborexant in Adult and Elderly Subjects with Moderate to Severe Obstructive Sleep Apnea. Poster presented at: American College of Neuropsychopharmacology Congress; Dec 5–8, 2021; San Juan, Puerto Rico.
Murphy PJ, Moline M, Pinner K, Hong Q, Yardley J, Zammit G, Satlin A. Effects of Lemborexant on sleep architecture in subjects with insomnia disorder. Poster session presented at: SLEEP 2016; Jun 11–15; Denver, CO, USA.
Murphy P, Kumar D, Zammit G, Rosenberg R, Moline M. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765–73.
Murphy P, Moline M, Mayleben D, et al. Lemborexant, a dual orexin receptor antagonist (DORA) for treatment for insomnia disorder: results from a Bayesian, adaptive, randomized, double-blind, placebo-controlled study. J Clin Sleep Med. 2017;13(11):1289–99. https://doi.org/10.5564/jcsm.6800. PMID: 29065953.
Yardley J, Mikko K, Inoue Y, Pinner K, Perdomo C, Ishikawa K, Filippov N, Moline M. Long-term effectiveness and safety of lemborexant in adults with insomnia disorder: results from a phase 3 randomized clinical trial. Sleep Med. 2021;80:333–42. https://doi.org/10.1016/j.sleep.2021.01.048.
Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2:e1918254. https://doi.org/10.1001/jamanetworkopen.2019.18254.
Karppa M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE-2. Sleep. 2020; https://doi.org/10.1093/sleep/zsaa123.
Harris M, Glozier N, Ratnavadivel R, Grunstein RR. Obstructive sleep apnea and depression. Sleep Med Rev. 2009;13(6):437–44. https://doi.org/10.1016/j.smrv.2009.04.001. Epub 2009 Jul 10. PMID: 19596599.
Ong JC, Gress JL, San Pedro-Salcedo MG, Manber R. Frequency and predictors of obstructive sleep apnea among individuals with major depressive disorder and insomnia. J Psychosom Res. 2009;67(2):135–41. https://doi.org/10.1016/j.jpsychores.2009.03.011. Epub 2009 Apr 25. PMID: 19616140.
Smales ET, Edwards BA, Deyoung PN, McSharry DG, Wellman A, Velasquez A, Owens R, Orr JE, Malhotra A. Trazodone effects on obstructive sleep apnea and non-REM arousal threshold. Ann Am Thorac Soc. 2015;12(5):758–64.
Eckert DJ, Malhotra A, Wellman A, White DP. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep. 2014;37(4):811–9. https://doi.org/10.5665/sleep.3596.
Carley DW, Olopade C, Ruigt GS, Radulovacki M. Efficacy of mirtazapine in obstructive sleep apnea syndrome. Sleep. 2007;30(1):35–41. https://doi.org/10.1093/sleep/30.1.35.
Marshall NS, Yee BJ, Desai AV, Buchanan PR, Wong KKH, Crompton R, et al. Two randomized placebo-controlled trials to evaluate the efficacy and tolerability of mirtazapine for the treatment obstructive sleep apnea. Sleep. 2008;31(6):824–31. https://doi.org/10.1093/sleep/31.6.824.
Schmickl CN, Yanru L, Orr JE, Jen R, Sands SA, Bradley EA, DeYoung P, Owens RL, Malhotra A. Effects of venlafaxine on apnea-hypopnea index in patients with sleep apnea: a randomized. Double-Blind Crossover Study Chest. 2020;158(2):765–75. https://doi.org/10.1016/j.chest.2020.02.074.Epub. 2020 Apr 9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lowe, A.D., Lowe, M.S. (2022). Overview of Medication Treatment for Co-Morbid Insomnia and Sleep Apnea (COMISA). In: Shapiro, C.M., Gupta, M., Zalai, D. (eds) CPAP Adherence. Springer, Cham. https://doi.org/10.1007/978-3-030-93146-9_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-93146-9_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-93144-5
Online ISBN: 978-3-030-93146-9
eBook Packages: MedicineMedicine (R0)