Abstract
Ramelteon is a selective MT1/MT2-receptor agonist indicated for insomnia treatment. Because it has no depressant effects on the nervous system, it is not expected to affect the control of breathing. The potential effects of ramelteon on apneic and hypopneic events and arterial oxygen saturation (SaO2) in individuals with obstructive sleep apnea were assessed. In this double-blind, randomized, crossover study, 26 adults with mild to moderate obstructive sleep apnea received ramelteon 16 mg and placebo for one night each, with a 5- to 12-day washout period between treatments. Treatments were administered 30 min before habitual bedtime. Respiratory effort was monitored using respiratory inductance plethysmography, SaO2 was measured by pulse oximetry, and sleep onset and duration were measured by polysomnography and post-sleep questionnaire. Post-sleep questionnaire also measured next-day residual effects. The primary measure was apnea–hypopnea index. Apnea–hypopnea index was similar in ramelteon and placebo groups (11.4 vs 11.1, respectively; CI = −2.1, 2.6, P = 0.812). Ramelteon had no effect on the number of central, obstructive, or mixed apnea episodes. No significant differences were observed in SaO2 for the entire night between ramelteon and placebo (95.1 vs 94.7%; P = 0.070). Ramelteon did not meaningfully affect sleep when evaluated by polysomnography and post-sleep questionnaire. Compared with placebo, ramelteon had no significant effect on next-day residual effects. Adverse events were reported by three subjects in the ramelteon group: headache (n = 2) and urinary tract infection (n = 1). No adverse events were reported with placebo. Ramelteon was well-tolerated and, as expected, did not worsen sleep apnea when administered to subjects with mild to moderate obstructive sleep apnea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep apnea and insomnia are commonly found as comorbid conditions. Recent studies have shown that as many as 50% of subjects referred to a sleep clinic for sleep apnea have insomnia symptoms [1, 2]. This is especially common in women with sleep apnea, who present with complaints of insomnia more often than men with sleep apnea [3]. Although sedative hypnotics represent the main pharmacologic therapy of insomnia, there are concerns about administering these medications in subjects who might have sleep apnea. Traditional sedative hypnotics that bind to the benzodiazepine receptor at the GABA-A-complex (e.g., triazolam, flurazepam) are known to be associated with depression of central respiratory drive, blunting of the arousal response to hypoxia, and decreases in muscle tone in the upper airways [4–6]. Limited data are available regarding the use of the newer benzodiazepine receptor agonists (e.g., zolpidem, zaleplon, zopiclone, and eszopiclone) in subjects with sleep apnea, and the use of these sedative hypnotics is not recommended in subjects with compromised respiratory function. Given the concurrence of insomnia and sleep apnea in many subjects and the potential for hypnotic agents to negatively affect respiration, it is important to examine the safety of any new hypnotic agent.
Ramelteon is a nonsedating chronohypnotic indicated for the treatment of insomnia. Unlike benzodiazepine receptor agonists, which act as broad CNS depressants and are sedative, ramelteon is highly selective for MT1 and MT2 receptors, which are located primarily in the suprachiasmatic nucleus of the hypothalamus, and does not produce general sedative effects [7–9]. In the present study, the safety of ramelteon was evaluated in subjects with mild to moderate obstructive sleep apnea.
Materials and methods
Experimental design
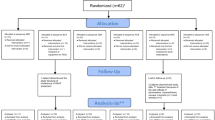
This double-blind, randomized, placebo-controlled, crossover study was conducted at five sleep centers. This study included a polysomnography screening period and two treatment periods of one night each, with a 5- to 12-day washout period between treatments.
The Institutional Review Board at each study site approved the study procedures and informed consent forms, and each subject provided consent before any study-related procedures were performed. Subjects were paid for their participation. The study was conducted according to applicable Food and Drug Administration laws and regulations, the World Medical Association Declaration of Helsinki (1989), and the International Conference for Harmonisation Harmonised Tripartite Guideline for Good Clinical Practice.
Subject eligibility and screening
Subjects eligible for study inclusion were men and nonpregnant, nonlactating women aged 21 to 64 years with a diagnosis of mild [apnea–hypopnea index (AHI) ≥5 and <10] or moderate (AHI ≥10 and ≤20) obstructive or mixed sleep apnea and a habitual bedtime between 8:30 p.m. and 12:00 a.m. and who reported sleeping more than 4 h per night. Insomnia was not required for eligibility. Subjects eligible for randomization had a confirmatory AHI of ≥5 and ≤20 per hour of sleep and an arterial blood oxygen saturation (SaO2) >80% at all times during the screening night, and did not have periodic leg movements with an arousal index of >20 per hour of sleep during the screening night.
Subjects were excluded from the study if they had a history of surgical intervention for sleep apnea or had used a continuous positive airway pressure device or dental appliance for sleep apnea within the preceding 30 days. Other exclusion criteria included known hypersensitivity to ramelteon or related compounds; recent participation in an investigational study or a study involving ramelteon; a recent acute, clinically significant illness or hospitalization; uncontrolled systemic illness; hepatitis; recent use of sleep medications or supplements, CNS medications, or drugs known to alter sleep; recent sleep schedule changes; a recent history of psychiatric disorder or drug or alcohol abuse; a history of seizure, chronic obstructive pulmonary disease, restless legs syndrome, periodic limb movement disorder, or other known sleep disorders; or other clinically important abnormal findings.
Study procedures
Subjects were asked to report to the sleep center 2 to 2.5 h before their habitual bedtime. Ramelteon 16 mg (twice the dose indicated for the management of insomnia) or a matching placebo was administered 30 min before habitual bedtime and the start of overnight monitoring with polysomnography, respiratory inductance plethysmography (RIP), and pulse oximetry. Subjects were awakened 8 h later and completed a post-sleep questionnaire upon waking. After a 5- to 12-day washout period, subjects returned to the sleep center and received the alternate treatment.
Outcome measures
The primary outcome measure was AHI, defined as the sum of the apnea and hypopnea indices. An apnea was defined as >50% reduction of airflow or tidal volume, and a hypopnea was defined as 25 to 50% reduction. The apnea index was determined by the total number of apneas divided by total sleep time. The hypopnea index was determined by the total number of hypopneas divided by total sleep time. Other measures included the BapC (number of central apneas per hour), BapM (number of mixed apneas per hour), and BapO (number of obstructive apneas per hour). Secondary outcome measures included mean SaO2 during the entire night, SaO2 during sleep stages (awake, REM, and NREM), and percentage of the night when SaO2 was less than 80%. In addition, sleep measures were assessed objectively by polysomnography [i.e., latency to persistent sleep (LPS), total sleep time (TST), sleep efficiency, wake time after sleep onset, and number of awakenings]. The percentages of TST in Stage 1, Stage 2, Stage 3/4, and REM sleep were also measured by polysomnography. Patient estimates of sleep were measured by post-sleep questionnaire (i.e., subjective sleep latency, subjective total sleep time, sleep quality, and awake time). The post-sleep questionnaire also assessed subjective level of alertness and ability to concentrate using a seven-point Likert scale on which 1 = excellent and 7 = extremely poor. Pulse oximetry and RIP was performed using the LifeShirt System (VivoMetrics, Ventura, CA). Polysomnographic records were scored by a central reader unaware of the study condition (Sleep Disorders and Research Center, Henry Ford Health System, Detroit, MI). Safety assessments included adverse events and vital signs evaluated at each visit and electrocardiogram and clinical laboratory tests performed at screening and on the morning after study drug administration.
Data analysis
All analyses were performed using SAS®, version 8.2. All analyses were two-sided and were performed at the 0.050 significance level. An initial analysis used AHI results to select a model for all crossover analyses. The difference between ramelteon and placebo [with 95% confidence interval (CI)] was obtained using the least-squares means from the analysis of variance (ANOVA) mixed model that included period and treatment condition as fixed effects and subject within sequence as a random effect. If the coefficient for “sequence” was not statistically significant at the 0.100 level, the mixed model procedure was applied with period and treatment as fixed effects and subject within sequence as a random effect. Alternatively, if the carryover effect was significant at the 0.100 level, comparisons of ramelteon and placebo were conducted with the carryover as a fixed effect. A nonparametric analysis (Wilcoxon rank sum test) of the primary outcome variable was performed as a confirmatory analysis. Whichever model was used for the primary outcome analysis was employed for subsequent secondary analyses.
The planned sample size for this study was 24 randomized subjects. For AHI, with an assumed between-subject standard deviation of 8.0 apnea episodes per hour, a significance level of 0.05 for a two-sided paired t test and 80% power, the sample size of 24 subjects was able to detect a difference between treatment conditions of approximately five apnea episodes per hour. The null hypothesis could not be rejected if the upper limit of the 95% CI for the difference between ramelteon and placebo was below five apnea/hypopnea episodes per hour (defined as a clinically relevant difference).
Results
All 26 enrolled subjects completed the study. Demographic and baseline (screening) characteristics of subjects are presented in Table 1. Fifteen subjects had mild obstructive sleep apnea, and 11 subjects had moderate obstructive sleep apnea. The subject demographics in this study were consistent with the expected profile of subjects with apnea; the average body mass index was 30.16 kg/m2, and approximately 30% of subjects were taking antihypertensive medications. Three subjects reported insomnia. No significant differences were observed between treatment sequences for any baseline demographic characteristic or for history of tobacco, alcohol, or caffeine. No significant differences were observed between treatment sequences for SaO2 or polysomnographic parameters at screening.
Respiratory assessment
Table 2 compares the effects of ramelteon and placebo on nighttime respiratory measures. Ramelteon had no statistically significant effect on mean AHI compared with placebo (P = 0.812). The minimum and maximum values of mean AHI were similar in the ramelteon and placebo groups (1.4 to 49.0, 0.5 to 33.8, respectively). The 95% CI for the treatment effect (−2.1, 2.6) indicated no clinically meaningful difference between treatments.
Ramelteon had no statistically significant effect on the other RIP measures vs placebo, including the apnea index (3.7 vs 4.4, respectively, P = 0.248), hypopnea index (7.7 vs 6.7, P = 0.296), BapC (0.0 vs 0.0, P = 0.802), BapM (0.6 vs 0.6, P = 0.980), or BapO (3.0 vs 3.8, P = 0.141).
There were no statistically significant differences in mean SaO2 percentages for the entire night between the ramelteon and placebo groups (P = 0.070), and the minimum and maximum values of mean SaO2 were similar for both treatments (93.0 to 97.0%, 92.0 to 98.0%, respectively). Ramelteon showed a significantly higher SaO2 than placebo (P = 0.036) during REM sleep and a trend toward a higher SaO2 than placebo (P = 0.051) during the awake stage. No statistically significant differences between treatments were observed for SaO2 during NREM sleep. The percentages of the night during which SaO2 was less than 80% was 0.0% in the ramelteon group and 0.2% in the placebo group (P = 0.406).
Sleep assessment
Table 3 summarizes the sleep results. There were no statistically significant differences in any parameters measured by polysomnography or post-sleep questionnaire.
Sleep architecture
No statistically significant differences in percentage of TST spent in Stage 1 or REM sleep were observed (Table 4). The percentage of TST spent in Stage 2 sleep was statistically significantly higher with ramelteon compared to placebo (57 vs 53%, P = 0.011); in Stage 3/4 sleep, TST was statistically significantly lower with ramelteon vs placebo (11.3 vs 14.2%, P = 0.014).
Next-morning residual effects
As determined by post-sleep questionnaire, there were no statistically significant differences between the ramelteon and placebo group for level of alertness (3.4 vs 3.3, P = 0.633) and ability to concentrate (3.1 vs 3.0, P = 0.920), indicating no next-morning residual effects.
Safety
Three adverse events were reported in the ramelteon group, and none were reported in the placebo group. The three adverse events consisted of two headaches, considered possibly related to the study drug, and one urinary tract infection that was considered unrelated to the study drug.
The urinary tract infection resolved spontaneously, and both cases of headache resolved after administration of acetaminophen. There were no consistent or meaningful changes in clinical laboratory values, vital signs, physical examination findings, or electrocardiogram results.
Discussion
Ramelteon’s non-depressant effect on the nervous system would suggest that it should have little effect on the control of breathing; this double-blind, placebo-controlled crossover trial showed that ramelteon 16 mg (twice the recommended therapeutic dose) had no significant effect on frequency of abnormal respiratory events (AHI values) or SaO2 levels across the night. This lack of effect indicates that ramelteon does not worsen sleep apnea in subjects with mild to moderate obstructive sleep apnea. Although the sample size was small (26 subjects), the study was designed to have sufficient statistical power to detect a clinically meaningful change in AHI of five events per hour. The mean difference in AHI between ramelteon and placebo treatments was less than 0.5; thus, the difference was neither statistically significant nor clinically meaningful.
A variety of sleep parameters were evaluated as secondary end point. A statistically significant effect on sleep onset was not observed with ramelteon compared with placebo, and this is largely due to the subjects’ relatively normal sleep latency at baseline as only 3 of 26 subjects reported insomnia (mean LPS=24.4 min). Additionally, given the small sample size, this study lacks the statistical power to detect a treatment effect on sleep parameters. A larger study with statistical power to detect differences on these parameters would be needed to demonstrate efficacy in this population.
The distribution of sleep stages was also evaluated as a secondary end points. Differences in Stage 2 and 3/4 NREM sleep were statistically significant, although small in magnitude. Given the size of the change, it is unlikely that these differences are of clinical importance.
In larger trials designed to study efficacy in subjects with insomnia, ramelteon demonstrated significant sleep-promoting effects [10–12]. Importantly, ramelteon was not associated with next-day psychomotor or memory effects, withdrawal symptoms, rebound insomnia, or abuse potential [10–14].
The activity of ramelteon at MT1/MT2 receptors is believed to mediate its sleep-promoting properties, as these receptors are thought to be involved in the circadian rhythm underlying the normal sleep–wake cycle [8, 9, 15, 16]. Receptor-binding studies have demonstrated that ramelteon has no appreciable affinity for a large number of CNS receptors, transporters, and ion channels [7]. Ramelteon’s negligible affinity to GABA, serotonin, acetylcholine, glutamate, noradrenaline, opioid, histamine, and dopamine receptors is noteworthy, as ancillary activity at these receptors is not directly related to sleep and may result in unwanted side effects. The results of this study support the notion that ramelteon does not adversely affect respiration.
Sleep apnea is common in the general population [17] and one of its clinical manifestations is insomnia [3]. Based on the findings of the present study, ramelteon is unlikely to worsen sleep apnea in insomnia patients. Nevertheless, all insomnia patients should be questioned about features of sleep apnea (e.g., snoring, observed apnea, and daytime sleepiness) to ensure proper treatment.
In summary, the nonsedating chronohypnotic ramelteon was well-tolerated and did not worsen sleep-disordered breathing when administered to subjects with mild to moderate obstructive sleep apnea.
References
Smith S, Sullivan K, Hopkins W, Douglas J (2004) Frequency of insomnia reports in patients with obstructive sleep apnoea hypopnea syndrome (OSAHS). Sleep Med 5:449–456
Krakow B, Melendrez D, Ferreira E, Clark J, Warner TD, Sisley B, Sklar D (2001) Prevalence of insomnia symptoms in patients with sleep-disordered breathing. Chest 120:1923–1929
Shepertycky MR, Banno K, Kryger MH (2005) Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep 28;309–314
George CF (2000) Perspectives on the management of insomnia in patients with chronic respiratory disorders. Sleep 23(Suppl 1):S31–S35
Guilleminault C (1990) Benzodiazepines, breathing, and sleep. Am J Med 88:25S–28S
Berry RB, Kouchi K, Bower J, Prosise G, Light RW (1995) Triazolam in patients with obstructive sleep apnea. Am J Respir Crit Care Med 151:450–454
Kato K, Hirai K, Nishiyama K, Uchikawa O, Fukatsu K, Ohkawa S, Kawamata Y, Hinuma S, Miyamoto M (2005) Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacology 48:301–310
Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK, Reppert SM (1997) Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 19:91–102
von Gall C, Stehle JH, Weaver DR (2002) Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res 309:151–162
Erman M, Seiden D, Zammit G, Sainati S, Zhang J (2006) An efficacy, safety, and dose–response study of ramelteon in patients with chronic primary insomnia. Sleep Med 7:17–24
Zammit G, Roth T, Erman M, Sainati S, Weigand S, Zhang J (2005) Double-blind, placebo-controlled polysomnography and outpatient trial to evaluate the efficacy and safety of ramelteon in adult patients with chronic insomnia. Sleep 28:A228–A229
Roth T, Seiden D, Sainati S, Wang-Weigand S, Zhang J, Zee P (2006) Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med 7:312–318
Roth T, Stubbs C, Walsh J (2005) Ramelteon (TAK-375), a selective MT1/MT2-receptor agonist, reduces latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep 28:303–307
Johnson M, Suess P, Griffiths RR (2006) Ramelteon: a novel hypnotic lacking abuse liability and sedative side effects. Arch Gen Psychiatry 63:1149–1157
Dubocovich ML, Yun K, Al-Ghoul WM, Benloucif S, Masana MI (1998) Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J 12:1211–1220
Jin X, von Gall C, Pieschl RL, Gribkoff VK, Stehle JH, Reppert SM, Weaver DR (2003) Targeted disruption of the mouse Mel(1b) melatonin receptor. Mol Cell Biol 23:1054–1060
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328:1230–1235
Acknowledgements
This study was funded by Takeda Pharmaceutical Company Limited.
Author Financial Disclosure
Meir Kryger, MD: Consultant, Takeda Pharmaceuticals North America, Inc.
Thomas Roth, PhD: Grants and Consultant, Takeda Pharmaceuticals North America, Inc.
Sherry Wang-Weigand, MD, PhD: Employee, Takeda Global Research & Development Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kryger, M., Wang-Weigand, S. & Roth, T. Safety of ramelteon in individuals with mild to moderate obstructive sleep apnea. Sleep Breath 11, 159–164 (2007). https://doi.org/10.1007/s11325-006-0096-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-006-0096-4