Abstract
Purpose
Although there is a high co-occurrence of insomnia and obstructive sleep apnea (OSA), the administration of sedative hypnotics in patients with OSA is still inconsistent. The aim is to study the effect of non-benzodiazepine hypnotics (non-BZDs) on sleep quality and severity in patients with OSA.
Methods
We conducted a systemic search for controlled clinical trials in multiple databases and pooled analysis of the impact of non-BZDs on objective sleep quality and the severity of OSA, including the apnea-hypopnea index (AHI) and mean and nadir arterial oxygen saturation (SaO2) in patients with OSA. Sensitivity analysis was carried out to explore the robustness of results.
Results
Eight relevant placebo-controlled clinical trials involving 448 patients were included. Objective sleep quality, including sleep latency, sleep efficiency, and wake time after sleep onset, was significantly improved in patients taking non-BZDs compared with those taking placebo (p < 0.01). The weighted estimate indicated that the administration of non-BZDs prior to bedtime had no significant effect on AHI or SaO2 in OSA patients (p > 0.05).
Conclusions
The administration of non-BZDs at the commonly recommended dose has been shown to improve objective sleep quality in OSA patients without worsening sleep apnea. It suggests that OSA patients with a complaint of insomnia symptoms may benefit from taking non-BZDs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) and insomnia are common disorders that often co-occur in clinical settings. The prevalence of insomnia symptoms in OSA patients reaches up to 39–58 %, and 29~67 % of patients with insomnia have an apnea-hypopnea index (AHI) of greater than 5 [1]. Both insomnia and OSA result in similar consequences, such as increasing risk of cognitive-emotional complaints, cardiovascular diseases, increasing incidence of car accidents, sick leave and work disability, and decreasing health-related quality of life [2, 3]. These patients were more susceptible to psychiatric disorders and cognitive-emotional symptoms than those with OSA alone. An effective treatment for both OSA and insomnia is crucial for these patients.
Studies have revealed that insomnia symptoms affect the efficiency of polysomonography (PSG) and continuous positive airway pressure (CPAP) in patients with OSA. The proper diagnosis of OSA requires at least a 6.5-h recording of PSG, including at least 3 h of sleep [4]. The insomnia symptoms may cause failure of PSG diagnosis due to insufficient sleep duration. CPAP is considered as the first choice for the treatment of OSA in most cases. However, low adherence to CPAP limits its use and affects the benefits to patients. It has been reported that insomnia was associated with low adherence to CPAP in OSA patients [1, 5]. Good sleep quality has been shown to improve long-term compliance with CPAP [6]. Thus, the administration of sedative hypnotics in OSA patients may improve the diagnosis and CPAP therapy for OSA.
Sedative hypnotics are recommended as the first-line pharmacotherapy for insomnia [7]. However, the use of these drugs in patients with insomnia and concurrent OSA has been challenging [1]. The administration of benzodiazepine hypnotics is considered to be inappropriate in OSA patients due to potential adverse effects, such as decrease in wakefulness, airway muscle tone, and ventilation response to hypoxemia [8]. These effects prone to worsen OSA presented as increased AHI or decreased SaO2. Some benzodiazepine hypnotics, such as flurazepam and midazolam, have been reported to increase the severity of OSA [9] and may even cause life-threatening sleep in OSA patients [10]. Taking the possibility of respiratory suppression into account, benzodiazepine hypnotics are generally not recommended for patients with concurrent OSA [8].
Non-benzodiazepine hypnotics (non-BZDs), mainly including zaleplon, zolpidem, and eszopiclone, play an important role in the treatment of insomnia due to their efficacy and safety. Compared with benzodiazepines, non-BZDs are of higher level of recommendation for the treatment of insomnia in the latest guideline [7]. Although there were studies about the use of non-BZDs in OSA patients [11–18], it has not reached consensus yet that non-BZDs improve sleep quality without worsening the severity of OSA, and thus, a meta-analysis is warranted.
Methods
Searching strategy and selection criteria
We conducted a systematic search through PubMed, Medline, the Cochrane Library, EMBASE, China Biomedical Literature Database, and Chinese Medical Association Journals databases (all searched from inception to 15 July 2013). The cross combinations of the following terms were used as key words: “hypnotics or sedatives” and “sleep apnea or sleep apnea hypopnea or sleep-disordered breathing”. The publication types were limited to clinical trial, clinical conference, and letter. The reference lists on relevant reviews and studies were scanned by hand. If any of the data was absent or unclear, we attempted to contact authors.
Two investigators independently reviewed titles and abstracts and retrieved related studies according to the following criteria: adult patients with AHI ≥ 5 measured by PSG; at least one of the non-BZDs (zaleplon, zolpidem, and eszopiclone) was studied; the design of study had to be a controlled clinical trial; at least one of the following outcome measurements was included—severity of OSA, such as AHI, nadir or average saturation of arterial oxygen (SaO2), objective sleep quality, including sleep latency, sleep efficiency, and wake after sleep onset (WASO); and the study was published in Chinese or English. We did not exclude abstracts or letters if we could identify the needed information. Any disagreement was resolved by consensus. The methodological quality of each study was identified by analyzing the level of blinding, methods of randomization, dropout rate, etc.
The meta-analysis
Review Manager Software (version 5.0) was used to analyze the statistics. As the included studies were cross-over and parallel designs with random distribution, the baseline measurements were considered to have no statistical difference and the final outcome values were analyzed. In one study [11], means were reported without variances, and thus, estimates were obtained from studies with similar methodology and sample size. The values of the I 2 test meant the percentage of observed variability due to heterogeneity rather than chance. I 2 values ranging from <25 to 50 and to 75 % were considered to represent low, moderate, and high heterogeneity, respectively. If the I 2 value was more than 75 %, the studies could not be combined directly, and then the source of heterogeneity needed to be further explored. As the included studies differed in experimental protocols and characteristics, a random effects model was applied to study the combined effect in accordance with the majority of meta-analyses. The inverse variance method was used for weighting combined trials. The measurements in this analysis were continuous data, and thus, the results were described as mean and mean difference (MD) with 95 % confidence interval (CI). By removing one study at a time, sensitivity analysis was performed to test the robustness of results.
Results
Identification and description of included studies
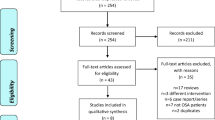
The flow diagram of identifying studies is shown in Fig. 1. According to the systemic search, all investigators agreed that eight trials involving 448 subjects were suitable for inclusion in this meta-analysis [11–18]. Design characteristics of each included study are shown in Table 1. The involved subjects were all diagnosed as having OSA by PSG prior to study, but in Cirignotta’s study, the scoring criteria was different from the rest, which was according to the criteria of Lugaresi et al. [19]. Six studies were cross-over designs and the other two were parallel designs, and that may explain the discrepancy between the total number of subjects (n = 448) and the number of subjects taking non-BZDs (n = 286) and placebo (n = 285). All of the eight studies observed the effects of non-BZDs on AHI, among which four studies were performed under CPAP treatment. The authors’ discretion as to the quality of each study is summarized in Table 2. The results of Coyle’s study were published in a letter without details [11]. In order to decrease the possibility of publication bias, the values of measurements in this study were also included, although it was difficult to assess its quality.
Meta-analyses: AHI and nadir and mean SaO2
Totally, eight studies reported the effect of non-BZDs on AHI, one of which included subgroups with or without CPAP. Five studies (172 subjects) without CPAP treatment had moderate heterogeneity (I 2 = 33 %), and four studies (278 subjects) performed under CPAP were highly heterogeneous (I 2 = 62 %). It seemed that non-BZDs decreased AHI of OSA patients under CPAP treatment (p = 0.03) (as shown in Fig. 2), but the sensitivity analysis indicated that the result was not robust. When one study [13] was excluded, the change of AHI was mild in OSA patients under CPAP (p = 0.12, Fig. 3). Overall, it was shown that the administration of non-BZDs had no significant effect on AHI in OSA patients, regardless of CPAP treatment.
Five cross-over studies (82 subjects) reported the change of nadir SaO2, among which two were on the night with CPAP treatment. The result indicated that the non-BZDs had no significant effect on nadir SaO2 in OSA patients, even in those without CPAP treatment (−1.10 favoring non-BZDs, 95 % CI −2.45 to 0.24, p = 0.11, random effects model, Fig. 4). Four studies reporting the effect of non-BZDs on mean SaO2 were highly heterogeneous (I 2 = 78 %). As was described above, one study was removed at a time to find the source of heterogeneity. When Cirignotta’s study [11] was excluded, the other three studies were considerably homogeneous (I 2 = 19 %). In this study, zolpidem was administered at a dose of 20 mg, twice the common dose, which might account for the significant decrease in mean SaO2. Finally, meta-analysis of three studies showed that the administration of the commonly used dose of non-BZDs had no significant effect on mean SaO2 in OSA patients (0.02 favoring non-BZDs, 95 % CI −0.7 to 0.74, p = 0.96, random effects model, Fig. 5).
Meta-analyses: sleep efficiency, sleep latency, and wake time after sleep onset
In total, three cross-over studies and two parallel studies (396 subjects) were included to analyze the effect of non-BZDs on sleep efficiency in patients with OSA. The results indicated that the administration of non-BZDs in OSA patients significantly increased sleep efficiency, with a mean improvement of 7.3 % (7.30 favoring non-BZDs, 95 % CI 3.86 to 10.73, p < 0.0001, random effects model, Fig. 6).
According to the meta-analysis of six studies (391 subjects), sleep latency was significantly reduced in OSA patients prescribed with non-BZDs (−7.59 favoring non-BZDs, 95 % CI −11.09 to −4.08, p < 0.0001, random effects model, Fig. 7). Similarly, the administration of non-BZDs significantly decreased WASO in OSA patients (−22.71 favoring non-BZDs, 95 % CI −28.55 to −16.87, p < 0.00001, random effects model, Fig. 8), according to the meta-analysis of six studies (416 subjects).
Evidence of publication reporting bias
To minimize publication bias, the meta-analysis included results from one study published as a letter. As shown in Fig. 9, the funnel plot of AHI was roughly symmetric, suggesting no evidence of publication bias. One trial [20] published as abstract was excluded for inadequate information, and another was excluded for inappropriate expression in median with interquartile range [21], which was not suitable for meta-analysis. However, their results were consistent with our finding that the administration of non-BZDs significantly improved objective sleep quality without worsening sleep apnea in OSA patients [20, 21].
Discussion
The key finding of this meta-analysis is that the administration of commonly used dosage of non-BZDs in OSA patients is effective in improving objective sleep quality without adverse effect of worsening sleep apnea. The results demonstrate that sleep efficiency increases and sleep latency and WASO decrease significantly in OSA patients taking non-BZDs. It supports the use of non-BZDs in OSA patients when needed, especially in those with a complaint of insomnia symptoms. Though the included OSA patients are not assessed for insomnia symptoms, it can be assumed that the improvement of sleep quality may be more prominent in patients with insomnia symptoms. Furthermore, the use of non-BZDs in OSA patients is safe without the adverse effect of worsening the severity of OSA. In this meta-analysis, the AHI and mean and nadir SaO2 have no significant change in OSA patients taking non-BZDs. It has been reported that the administration of non-BZDs may benefit the diagnosis and therapeutic efficiency of OSA. The temporary administration of zolpidem or eszopiclone prior to PSG monitoring is an independent predictor of better CPAP compliance [22]. The increased CPAP adherence may be associated with the improvement of sleep quality in subjects taking non-BZDs. However, limited studies about the effect of non-BZDs on CPAP adherence are inconsistent [13, 21, 23], and thus, more researches are needed, especially in patients with insomnia symptoms.
It is quite common to observe the co-occurrence of OSA and insomnia symptoms in clinical settings, which is largely due to the high prevalence of these two disorders. As early as 1973, the association between insomnia and OSA was described by Guilleminault, and it was called sleep-insomnia apnea syndrome [24]. In OSA patients, the prevalence of insomnia symptoms should not be ignored: 33.8 % having difficulty in maintaining sleep and 33.4 % in initiating sleep, and 31.4 % having early morning awakenings [3]. Difficulties in maintaining sleep were more common among OSA patients compared with the general population [25]. Besides, OSA patients often have a complaint of difficulty in initiating sleep, which can be associated with the unfamiliar environment of the sleep center, the manipulation of PSG test, the noise from CPAP machine, the compression of mask and the discomfort caused by airflow, etc. In addition, the key pathologic processes of OSA, including apnea/hypopnea events and sequential oxygen desaturation, usually contribute to sleep fragmentations and awakenings. On the other hand, sleep fragmentation may lead to higher collapsibility of the upper airway, which may further increase OSA severity [26]. It is presumed that the effective relief of insomnia symptoms benefits the clinical outcomes of OSA, especially when co-existing with insomnia.
The administration of hypnotics for insomnia symptoms in OSA patients is contradictory with regard to some serious side effects. Benzodiazepine hypnotics are not recommended in patients with concurrent OSA, as some studies have shown their significant suppression on respiratory function [9, 10]. The increased collapsibility of the upper airway and the unstable respiratory control during sleep are regarded as the main causes of repetitive apnea/hypopnea and desaturation/re-saturation of oxygen among OSA patients. Benzodiazepine hypnotics act as hypnotic agents by activating the alpha1 receptor of alpha1 γ-aminobutyric acid A (GABAA) receptors. However, the co-activation of alpha-2 or alpha-3 GABAA receptors by benzodiazepines may cause myo-relaxation effects [27], which is associated with the increased collapsibility of the upper airway. Additionally, benzodiazepine hypnotics are likely to worsen OSA by suppressing the function of respiratory centers [8]. Some benzodiazepine hypnotics, such as flurazepam and midazolam, have been found to increase apnea/hypopnea events and lower SaO2 in OSA patients [9, 10].
Compared with benzodiazepines, non-BZDs are the newer generation of hypnotics and have shown more selective profiles and fewer side effects. These drugs, including zolpidem, eszopiclone, and zaleplon, were structurally different but all act through binding to BZD/GABAA receptors to induce sleep. Research data has demonstrated that the long-term use of non-BZDs is safe with minimal side effects and no tolerance, dependence, withdrawal, or rebound insomnia [28–30]. Besides, these drugs also have shown no respiratory depressant effect in patients at high altitude [31], with chronic obstructive pulmonary disease [32] or with idiopathic central sleep apnea [33]. In our meta-analysis, the result suggests that the administration of non-BZDs is safe without the adverse effect of worsening OSA severity (AHI, mean and nadir SaO2) in patients with OSA, and it supports the use of non-BZDs in OSA patients when needed.
OSA has been thought to be a REM-associated disorder, as the collapsibility of the upper airway muscle is prone to increase during the stage of REM sleep. Non-BZDs are mainly with a short half-life time, and that may be associated with their little adverse effect on sleep architecture. Research data has demonstrated that non-BZDs have little effect on the time spent on different stages of sleep [34]. The percentage of time spent on REM sleep has been found to decrease significantly in subjects taking zaleplon[35], and this effect is considered as a protection factor against sleep apnea. Besides, non-BZDs have shown a high affinity to the BZ/GABAA receptor containing the alpha-1 subunit [36]. With less affinity to alpha-2 or alpha-3 subunits of the BZ/GABAA receptor, they have little effects of myo-relaxation or central respiratory suppression. Additionally, the difference between sedative and muscle-relaxant doses allows non-BZDs to improve sleep without respiratory depression [37]. However, in Cirignotta’s study, the nadir SaO2 during sleep apnea-hypopnea and the mean SaO2 decreased significantly in 12 subjects taking 20 mg of zolpidem [11]. In contrast, 10 mg of zolpidem shows no significant effect on respiratory parameters in 42 of OSA patients in George’s study [15]. These contradictory findings may result from the small sample size of former studies or the different doses used in the two studies. In one study, AHI decreased significantly in the eszopiclone arm compared with the placebo arm (24 ± 4 vs 31 ± 5, p < 0.05) [14]. This may be explained by the difference of patient selection criteria, as patients with marked oxygen desaturation were excluded in this study. The result of this study indicated that non-BZDs may decrease the severity of OSA in a specific subgroup of OSA patients. This effect could be associated with the increase of arousal threshold which resulted from the shift of sleep from stage 1 to stage 2 [16]. It has been reported that the higher arousal threshold is associated with the decrease in sleep apnea severity [38], which may account for the decrease of AHI. However, the sedative hypnotics may increase the duration of sleep apnea in patients with poor muscle responsiveness during sleep.
There are some limitations in our meta-analysis which need to be noted. As is known, meta-analysis is based on the results of prior clinical studies. The various methodologies may result in heterogeneity of studies. To minimize this effect, we used the random effects model in each meta-analysis. One study published as a letter was also included to decrease the publication bias. Though it was hard to evaluate the quality of this study, the sensitivity analysis indicated that the result was stable when this study was excluded. Clinical studies in this area are still limited; therefore, larger scale, randomized, control clinical trials are needed to support our findings.
Conclusions
The administration of non-BZDs has no significant adverse effect on AHI and mean and nadir SaO2 in OSA patients, even in those without the protection of CPAP. In addition, the administration of non-BZDs significantly improves sleep quality in OSA patients, which favors the administration of non-BZDs in OSA patients when needed.
References
Luyster FS, Buysse DJ, Strollo PJ Jr (2010) Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research. J Clin Sleep Med 6:196–204
Sivertsen B, Overland S, Glozier N, Bjorvatn B, Maeland JG, Mykletun A (2008) The effect of OSAS on sick leave and work disability. Eur Respir J 32:497–503
Krell SB, Kapur VK (2005) Insomnia complaints in patients evaluated for obstructive sleep apnea. Sleep Breath 9:104–110
Thurnheer R (2011) Diagnostic approach to sleep-disordered breathing. Expert Rev Respir Med 5:573–589
Drake CL, Day R, Hudgel D, Stefadu Y, Parks M, Syron ML et al (2003) Sleep during titration predicts continuous positive airway pressure compliance. Sleep 26:308–311
Lettieri CJ, Shah AA, Holley AB, Kelly WF, Chang AS, Roop SA et al (2009) Effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized trial. Ann Intern Med 151:696–702
Pinto LR Jr, Alves RC, Caixeta E, Fontenelle JA, Bacellar A, Poyares D et al (2010) New guidelines for diagnosis and treatment of insomnia. Arq Neuropsiquiatr 68:666–675
Hanly P, Powles P (1993) Hypnotics should never be used in patients with sleep apnea. J Psychosom Res 37(Suppl 1):59–65
Dolly FR, Block AJ (1982) Effect of flurazepam on sleep-disordered breathing and nocturnal oxygen desaturation in asymptomatic subjects. Am J Med 73:239–243
Bezel R, Russi E, Kronauer H, Mothersill I (1987) Life-threatening apnea after midazolam administration in a patient with obstructive sleep apnea syndrome. Schweiz Med Wochenschr 117:579–583
Cirignotta F, Mondini S, Zucconi M, Gerardi R, Farolfi A, Lugaresi E (1988) Zolpidem-polysomnographic study of the effect of a new hypnotic drug in sleep apnea syndrome. Pharmacol Biochem Behav 29:807–809
Coyle MA, Mendelson WB, Derchak PA, James SP, Wilson MG (2005) Ventilatory safety of zaleplon during sleep in patients with obstructive sleep apnea on continuous positive airway pressure [Letter]. J Clin Sleep Med 1:97
Lettieri CJ, Collen JF, Eliasson AH, Quast TM (2009) Sedative use during continuous positive airway pressure titration improves subsequent compliance: a randomized, double-blind, placebo-controlled trial. Chest 136:1263–1268
Eckert DJ, Owens RL, Kehlmann GB, Wellman A, Rahangdale S, Yim-Yeh S et al (2011) Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 120:505–514
George CF, Feldman N, Inhaber N, Steininger TL, Grzeschik SM, Lai C et al (2010) A safety trial of sodium oxybate in patients with obstructive sleep apnea: acute effects on sleep-disordered breathing. Sleep Med 11:38–42
Lettieri CJ, Quast TN, Eliasson AH, Andrada T (2008) Eszopiclone improves overnight polysomnography and continuous positive airway pressure titration: a prospective, randomized, placebo-controlled trial. Sleep 31:1310–1316
Rosenberg R, Roach JM, Scharf M, Amato DA (2007) A pilot study evaluating acute use of eszopiclone in patients with mild to moderate obstructive sleep apnea syndrome. Sleep Med 8:464–470
Berry RB, Patel PB (2006) Effect of zolpidem on the efficacy of continuous positive airway pressure as treatment for obstructive sleep apnea. Sleep 29:1052–1056
Zucconi M, Ferini-Strambi L, Palazzi S, Orena C, Zonta S, Smirne S (1992) Habitual snoring with and without obstructive sleep apnoea: the importance of cephalometric variables. Thorax 47:157–161
Sato R, Malish S, Dickel GD, Hungs M, Sassoon C (2010) Hypnotics fail to improve polysomnographic quality and efficacy of CPAP titration [Abstract]. Chest 138:701A
Park JG, Olson EJ, Morgenthaler TI (2013) Impact of zaleplon on continuous positive airway pressure therapy compliance. J Clin Sleep Med 9:439–444
Collen J, Lettieri C, Kelly W, Roop S (2009) Clinical and polysomnographic predictors of short-term continuous positive airway pressure compliance. Chest 135:704–709
Bradshaw DA, Ruff GA, Murphy DP (2006) An oral hypnotic medication does not improve continuous positive airway pressure compliance in men with obstructive sleep apnea. Chest 130:1369–1376
Guilleminault C, Eldridge FL, Dement WC (1973) Insomnia with sleep apnea: a new syndrome. Science 181:856–858
Björnsdóttir E, Janson C, Gíslason T, Sigurdsson JF, Pack AI, Gehrman P, Benediktsdóttir B (2012) Insomnia in untreated sleep apnea patients compared to controls. J Sleep Res 21:131–138
Sériès F, Roy N, Marc I (1994) Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med 150:481–485
Crestani F, Löw K, Keist R, Mandelli M, Möhler H, Rudolph U (2001) Molecular targets for the myorelaxant action of diazepam. Mol Pharmacol 59:442–445
Hajak G, Cluydts R, Declerck A, Estivill SE, Middleton A, Sonka K, Unden M (2001) Continuous versus non-nightly use of zolpidem in chronic insomnia: results of a large-scale, double-blind, randomized, outpatient study. Int Clin Psychopharmacol 17:9–17
Roth T, Walsh JK, Krystal A, Wessel T, Roehrs TA (2005) An evaluation of the efficacy and safety of eszopiclone over 12 months in patients with chronic primary insomnia. Sleep Med 6:487–495
Israel AG, Kramer JA (2002) Safety of zaleplon in the treatment of insomnia. Ann Pharmacother 36:852–859
Beaumont M, Batéjat D, Piérard C, Van Beers P, Philippe M, Léger D et al (2007) Zaleplon and zolpidem objectively alleviate sleep disturbances in mountaineers at a 3,613 meter altitude. Sleep 30:1527–1533
Girault C, Muir JF, Mihaltan F, Borderies P, Borderies P, De La Giclais B, Verdure A et al (1996) Effects of repeated administration of zolpidem on sleep, diurnal and nocturnal respiratory function, vigilance, and physical performance in patients with COPD. Chest 110:1203–1211
Quadri S, Drake C, Hudgel DW (2009) Improvement of idiopathic central sleep apnea with zolpidem. J Clin Sleep Med 5:122–129
Walsh JK, Pollak CP, Scharf MB, Schweitzer PK, Vogel GW (2000) Lack of residual sedation following middle-of-the-night zaleplon administration in sleep maintenance insomnia. Clin Neuropharmacol 23:17–21
Walsh JK, Vogel GW, Scharf M, Erman M, William Erwin C, Schweitzer PK et al (2000) A five week, polysomnographic assessment of zaleplon 10 mg for the treatment of primary insomnia. Sleep Med 1:41–49
Crestani F, Martin JR, Möhler H, Rudolph U (2000) Mechanism of action of the hypnotic zolpidem in vivo. Br J Pharmacol 131:1251–1254
Sanger DJ, Benavides J, Perrault G, Morel E, Cohen C, Joly D, Zivkovic B (1994) Recent developments in the behavioral pharmacology of benzodiazepine (omega) receptors: evidence for the functional significance of receptor subtypes. Neurosci Biobehav Rev 18:355–372
Ratnavadivel R, Chau N, Stadler D, Yeo A, McEvoy RD, Catcheside PG (2009) Marked reduction in obstructive sleep apnea severity in slow wave sleep. J Clin Sleep Med 5:519–524
Acknowledgments
The authors would like to thank Bai Song Wang for his help in statistical analysis.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Glossary
- AHI
-
Apnea-hypopnea index
- CI
-
Confidence interval
- CPAP
-
Continuous positive airway pressure
- γ-GABAA
-
Benzodiazepine/γ-aminobutyric acid A
- MD
-
Mean difference
- BZDs
-
Benzodiazepine hypnotics
- OSA
-
Obstructive sleep apnea
- PSG
-
Polysomnography
- SaO2
-
Arterial oxygen saturation
- WASO
-
Wake after sleep onset
Rights and permissions
About this article
Cite this article
Zhang, X.J., Li, Q.Y., Wang, Y. et al. The effect of non-benzodiazepine hypnotics on sleep quality and severity in patients with OSA: a meta-analysis. Sleep Breath 18, 781–789 (2014). https://doi.org/10.1007/s11325-014-0943-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-014-0943-7