Abstract
Abrupt change in local pressure within a liquid medium is susceptible to break the liquid molecules cohesion, particularly when a gas is dissolved in the liquid. The created “cavity”, filled of gaseous/vaporous content is known as Cavitation. When the pressure perturbation is induced by an acoustic source, namely ultrasound, the phenomenon is then called Acoustic Cavitation. Although occurring at microscopic scale, the repletion of acoustic cavitation events presents a broad range of applications owing to the physical effects directly related to the oscillation of the bubbles, but also to their indirect chemical consequences. Nowadays, the acoustic cavitation is not only an attractive topic for fundamental research in physics and chemistry, but is recognized as a promising up-scalable solution in several fields, including agri-food.
The present chapter sheds light on the applications of ultrasound and acoustic cavitation in the agri-food domain, by linking the fundamental aspects of this non-thermal process, ranging from physical to chemical effects, to the intended applications in terms of extraction, pasteurization, crystallization, synthesis and oxidation. The chapter discusses the mechanisms of action of ultrasound leading to the aforementioned applications, and emphasizes on the green and sustainable features characterizing their pathways.
At the end of the present chapter, several case studies are reported from the literature in order to highlight the most promising agri-food applications harnessing the principle of acoustic cavitation, and exhibit their advantages relatively to conventional techniques, but also the challenges still facing their large-scale adoption, especially from a technological point of view.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Food processing can be simply defined as the effective conversion of agricultural products into more appealing, shelf-stable, compact and value-added consumer ready to use products (Feng et al. 2011).
Conserving the natural flavor and taste of foods and reducing or eliminating the use of chemical additives and preservatives increased the interest in the use of non-thermal technologies in food processing. Ultrasound assisted techniques have emerged as one of the promising non thermal solutions in agri-food sector involving less time, less water and less energy than conventional techniques. These techniques in the field of food industry are well known for significantly influencing the processing rate of different used processes (Bhargava et al. 2021; Dastkhoon et al. 2018). Sono-food processing has been a subject of research and development for many years (Gogate and Pandit 2011; Demirdöven and Baysal 2009; Mason et al. 1996), and is still in the surge of technologists’ attention owing to the variety of possible applications ranging from cutting, dehydration, extraction, freezing, crystallization, filtration to preservation and more.
The ultrasound assisted techniques in food processing rely principally on the mechanical effects induced by the propagation of ultrasonic waves in fluids, but also all the physical and chemical effects induced by acoustic cavitation bubble. In the present chapter, the fundamentals of acoustic cavitation and its physical and chemical effects will be presented, with the objective of linking the microscopic events occurring when irradiating a fluid with ultrasound, to the macroscopic applications (Bratsikhin et al. 2019) of ultrasound and acoustic cavitation in food industry.
The chapter will also cover selected applications of ultrasound in food-processing, with reference to experimental and theoretical works retrieved in the literature and dealing with those application at laboratory and industrial scales.
Finally, some perspectives are given to sono-processing and ultrasound assisted technologies in agri-food sector, while highlighting the main challenges facing this field.
2 Fundamentals of Acoustic Cavitation Bubble
2.1 Bubble Inception, Growth and Dynamics of Oscillation
An acoustic wave is a propagation of pressure oscillation with sound velocity in a medium such as liquid, gas, or solid. The acoustic pressure amplitude Pa is defined as the amplitude for pressure oscillation. The wavelength λ of an acoustic wave is defined as the length for one pressure oscillation. The acoustic period T is defined as time for one pressure oscillation. The frequency f of an acoustic wave is defined as the number of pressure oscillations per unit time and is then the reverse of the period. The sound velocity (or sound speed) c is defined as the distance for a pressure disturbance propagating per unit time. The sound velocity in liquid water at room temperature is about 1500 m/s, i.e., almost 4.5-fold higher than in dry air, where it is estimated at 340 m/s. The sound velocity in liquid water increases with the increase of the emperature and has a maximum value of about 1555 m/s at around 74 °C.
Owing to the high oscillating frequency of liquid molecules in an ultrasonic field and the high viscosity of the liquid medium compared with a gas, liquid molecules absorb a large amount of energy from the propagation of ultrasonic wave. The high energy absorption of liquid molecules and high acoustic pressure in the presence of gaseous germs make it possible for ultrasonic field to overcome intermolecular interactions in a solvent, and produce numerous cavities, which is referred to as acoustic cavitation.
In practice, weaknesses in liquid medium can typically occur in two forms. (i) Homogeneous nucleation, induced by the thermal motions within the liquid, which form temporary microscopic voids that constitute nuclei. (ii) Heterogeneous nucleation that explains the major weaknesses occurring in liquid medium, and manifesting at the boundary between the liquid and a solid phase or between the liquid and small particles suspended in the liquid (Jena 1965).
2.1.1 Homogeneous Nucleation Theory
We present here a brief and simplified version of homogeneous nucleation theory. Acoustic cavitation, concisely defined as the formation, growth and collapse of microbubbles within an aqueous solution, results from pressure fluctuations that occur in the liquid medium irradiated by an acoustic field in the range of ultrasound, with appropriate value of frequency and amplitude. The formed cavities drain and accumulate ultrasonic energy, and explosively release their energy by the collapse of cavities (Luo et al. 2015). The event of a collapsing bubble is a microscopic implosion accompanied of a significant increase of temperature and pressure of up to thousand Kelvin and hundred Bar (Yasui et al. 2007; Kerboua and Hamdaoui 2017), a release of heat energy and high local turbulence. The elevated temperatures in the vicinity of collapsing bubble, known as “hot spots”, can activate chemical mechanisms particularly characterized by the formation of free radicals (Leong et al. 2011; Kerboua and Hamdaoui 2019a), while the local turbulence and the abrupt release of concentrated localized energy induces several physical effects of mechanical nature such as mass transfer and surface erosion (Fu et al. 2020).
The ultrasonic wave propagates through ultrasonic generator, transducer, horn and radiation rod to liquid, causing medium molecules vibration. The average distance between molecules decreases in the acoustic compression phase, while increases in the rarefaction phase. In pure liquid, cavitation will occur when the liquid additional negative pressure reaches a critical value, whose magnitude is called cavitation threshold (Ye and Zhu 2017). In fact, cavities are formed when adequately large negative pressure is applied to the liquid so that the average distance between the molecules exceeds the critical molecular distance required to hold the liquid intact. However, in order to attain this critical distance, a huge energy is needed, to illustrate, the creation of a microscopic cavity-void with a radius of barely 0.4 nm in water, the minimum required negative pressure is estimated at 1400 atm.
However, what makes it easier in the case of acoustic cavitation is the presence of gaseous impurities, which dramatically reduces the tensile strength and consequently the required negative pressure. This has been proved by experiments on tensile strength (Brennen 2013).
2.1.2 Heterogeneous Nucleation Theory
Acoustic cavitation is observed with a pressure amplitude of about 1 atm, implying that pre-existing nuclei or sites for nucleation assist the growth of acoustic cavitation bubbles. Acoustic cavitation event is then explained by a sound wave imposing a sinusoidally varying pressure upon existing cavities in solution, namely gaseous impurities. In the rarefaction phase of the ultrasonic wave, instantaneous local pressures in liquid become negative when the acoustic pressure amplitude is larger than the ambient pressure. Negative pressure is the result of the “force” to expand a liquid element and is possible only in liquids or solids, and impossible in gases. Consequently, gases dissolved in the liquid appear as gas bubbles because gases can no longer be dissolved in the liquid under negative pressures (Yasui 2018). During the negative pressure cycle, the liquid is pulled apart at sites containing such a gaseous impurity, which are known as “weak spots” in the fluid. The number of bubbles that are produced during this rarefaction cycle is proportional to the density of such weak spots present in the fluid (Leong et al. 2011).
2.1.3 Cavitation Types and Expected Effects
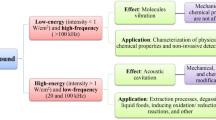
During its lifetime, acoustic cavitation bubble can be either vaporous or gaseous. Vapor of liquid is introduced to the bulk of the bubble through the non-equilibrium of evaporation and condensation process (Fujikawa and Akamatsu 1980; Yasui 1998; Kerboua and Hamdaoui 2018a), while gases are introduced through a diffusion process, much slower than the evaporation process. Physical effects are more pronounced when acoustic cavitation bubbles are of vaporous nature (Yasui 2018). The reason is that acoustic cavitation bubbles under low frequency ultrasound tend to grow to larger size than under mid to high frequencies (Carrillo-Lopez et al. 2017). During this growth process, vapors of the liquid accumulate in the volume of the bubble and give it a vaporous nature. These bubbles, of bigger size, are characterized by a harsh collapse accompanied of strong cavitation microstreaming, shock waves and microjets. This observation is summarized in Table 6.1.
2.1.4 Growth and Oscillation of Acoustic Cavitation Bubble
There are two mechanisms in growth of a bubble in acoustic cavitation. The first mechanism is coalescence of bubbles. The coalescence of bubbles is driven by the two processes. One is the attractive radiation force between bubbles called secondary Bjerknes force. The other is the radiation force called the primary Bjerknes force which drives active bubbles to the pressure antinode of a standing wave field (Sunartio et al. 2007).
The second mechanism for bubble growth is known as rectified diffusion and is defined as the gas diffusion into a bubble due to the area and shell effects. The bubble growth rate due to rectified diffusion strongly depends on acoustic conditions. This rate decreases as ultrasonic frequency increases for the same acoustic pressure amplitude (Crum 1980; Lee et al. 2005).
At the initial development of acoustic cavitation, rectified diffusion may be the main mechanism of bubble growth, whilst when acoustic cavitation is fully started, coalescence of bubbles may be the main mechanism of the bubble growth as the rate of coalescence is proportional to the square of the number density of bubbles which should be small at the initial stage of acoustic cavitation (Pankaj and Ashokkumar 2011).
Within the bubble population, if the bubbles are small and occur below the cavitation threshold, they will simply dissolve away, they are known as dissolving bubbles (Yasui 2002; Kerboua et al. 2021a).
Bubbles formed through cavitation will begin to expand and collapse under the influence of the acoustic field, if the expansion-collapse cycle is sinusoidal, mimicking that of the acoustic wave, the bubbles are qualified as linearly oscillating bubbles (Louisnard and González-García 2011).
Alternatively, for certain bubble sizes and acoustic pressures, the bubble expansion phase is extended and is followed by a violent collapse back to a smaller bubble size. Bubbles occurring such oscillation dynamics are known as inertial cavitation (Johansen et al. 2017).
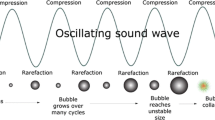
If inertial acoustic cavitation bubbles persist for many hundreds of acoustic cycles, they are referred to as stable, or repetitive transient cavitation (Kerboua and Hamdaoui 2018b). Furthermore, if stable or repetitive transient cavitation are active in sonochemistry, they are qualified as high energy stable cavitation (Yasui 2011; Kerboua et al. 2021b). An example of the dynamics of oscillation of a repetitive transient acoustic cavitation bubble is given in Fig. 6.1.
Alternatively, if the acoustic amplitude is higher, the inertial acoustic cavitation bubble will grow and collapse spectacularly within a very few acoustic cycles and the collapsed bubble then disintegrates into a mass of smaller daughter bubbles, which are often small and themselves collapse in chain so that complete annihilation of the original bubble occurs. These bubbles are known as unstable or transient cavitation (Yasui 2011; Kerboua and Hamdaoui 2020). An example of the dynamics of a transient bubble oscillation is illustrated in Fig. 6.2.
Finally, it is worthy to mention that higher yield of gases diffuses into the bubble during the expansion phase than leaks out during collapse. This causes larger bubbles to grow by rectified diffusion over a very large number of acoustic cycles. Large bubbles also tend to form coalesced bubbles. By both mechanisms, the formed big bubbles will attain a size where they will simply float away to the liquid surface. These bubbles are known as degassing bubbles (Leighton 1995).
2.2 Physical Effects of Acoustic Cavitation Bubble
This overview focuses on the physical effects induced by the passage of ultrasound through a liquid medium and the formed transient acoustic cavitation bubble. The physical effects may vary from the mechanical turbulence created due to the passage of ultrasound through water, namely vibration and acoustic streaming, to the physical consequences of acoustic cavitation, namely streamers, cavitation microstreaming, microjets and shock waves (Yusof et al. 2016). All these effects are reported in Fig. 6.3.
2.2.1 Acoustic Streaming
When a liquid medium is irradiated with ultrasound, the immediate consequence is acoustic streaming. This large-scale streaming refers to the regular oscillatory motion of the fluid volume elements with a time independent flow velocity due to the second-order nonlinear propagation of the acoustic wave occurring when the acoustic wave propagates through the liquid medium. Acoustic streaming can be viewed as physical forces of the sound waves that provide a driving force capable of displacing ions and small molecules (Marshall and Wu 2015). Acoustic streaming can have extremely complex pattern, with linear and nonlinear interactions between the cavitation bubbles and with the microstreaming associated with the microbubbles occurring at the small scale. Wu (2018) exposed in a recent review of acoustic streaming and its applications the detailed theoretical background behind the acoustic streaming.
There are mainly two types of acoustic streaming, namely, Eckart streaming and Rayleigh streaming (Luo et al. 2018). One is accelerating direct current fluid flow in the direction of the acoustic traveling wave propagation. This is caused by the attenuation of the acoustic traveling wave mainly resulting of the viscosity of the fluid. Due to attenuation, an unbalance of radiation forces is created since the radiation pressure pushing a fluid parcel becomes stronger than that pulling it. This causes the accelerating fluid flow called Eckart streaming (Bjørnø 2017). The second type of acoustic streaming is a vortex-like streaming caused by viscous stress at the boundary layer near a wall or an object, regardless of the situation of a traveling or standing wave. This type is known as Rayleigh streaming (Yasui 2017).
Large scale acoustic streaming plays a significant role in enhancing heat and mass transfer processes in the liquid but also around solid surfaces. Indeed, the acoustic streaming patterns around solid objects are believed to be the strongest within a few millimeters of the solid surface. This boundary layer effect around a solid surface is important and quite interesting in several applications, because it is usually such boundary layers that offer the greatest resistance to both heat and mass transfer. The surface effects of large-scale acoustic streaming are also important in emulsification, where interfacial turbulence is associated with droplet formation and nebulization. The acoustic streaming effects cause a fountain structure to form at the air/water interface from which microdroplets are ejected (Luo 2020).
2.2.2 Microstreamers
Acoustic standing waves can result from the reflection of sound from a solid surface or an air–liquid interface back into the solution at the same time that a wave is generated at the transducer. At the pressure antinode of such a standing wave pattern, the pressure fluctuates from a maximum to a minimum amplitude with time. Conversely, at the pressure node, the acoustic pressure is invariant and close to zero.
A phenomenon referred to as Bjerknes forces causes smaller bubbles to accumulate at the antinode, while larger bubbles accumulate at the node. In moving to the antinodes, the cavitation bubbles travel in ribbon-like structures referred to as “streamers” coalescing as they collide (Bai et al. 2014). A filamentary structure, referred to as an acoustic “Lichtenberg” figure is then created (Parlitz et al. 1999).
To illustrate, at 20 kHz, the bubbles forming the streamers are typically less than 10 μm in size, about a millimeter apart and traveling at less than 1 μm/s. This bubble translation is known to dislodge particles from fouled surfaces, in cases where the surface itself acts as the pressure antinode. Movement of bubbles toward a solid surface acting as a pressure antinode within an acoustic standing wave pattern results in increased turbulence within this same zone (Kentish and Ashokkumar 2010).
2.2.3 Cavitation Microstreaming
Acoustic cavitation microstreaming refers to the microscopic streaming most often created by a microbubble. Acoustic microstreaming do not rely at all on the presence of microbubbles, it can also occur around a solid particle. However, it is much more significant around a bubble because the speed of microstreaming is proportional to the square of vibration speed of an object. The speed of microstreaming around a pulsating acoustic cavitation bubble is 102 to 106 times larger than that around a solid particle. Thus, the term “microstreaming” is usually used for liquid streaming around a pulsating bubble and is preferentially called “cavitation microstreaming”. This is consistent with the early observations of Kolb and Nyborg (1956), who allowed cavitation bubbles to form under the influence of various frequencies in the audible range up to 11.4 kHz. They observed that streaming is most pronounced when the bubble is oscillating in its volumetric mode or when the bubble is on a solid boundary.
Cavitation microstreaming was noted to have detrimental biological effects. For instance, ultrasonic destruction of Paramecium cells was observed to be caused specifically by microstreaming flows. Marmottant and Hilgenfeldt (2003) also reported that microstreaming from a microbubble was also observed to disrupt an artificial vesicle, which is a model biological cell.
2.2.4 Microjets
Microjetting is a microscopic flow of liquid emitted by the bubble and occurring during asymmetric bubble collapse. Near an extended solid surface, the collapse of an acoustic cavitation bubble is non-spherical and drives high-speed jet of liquid to the surface known as microjet. This microjet is directed toward the solid surface and this can lead to pitting and erosion. The surface action can also dislodge particles attached to the surface and break down large aggregates into smaller particles (Yuan and Prosperetti 1997; Peters et al. 2015). The mechanism of microjets is attributed to the difference in pressures applied on the bubble wall from the solid and liquid sides, respectively. Just before the bubble collapses near a solid surface, the liquid pressure on the bubble surface near a solid boundary becomes much lower than that on the other side of the bubble surface. As a result, a jet penetrates into the bubble and finally hits the solid surface.
However, jet penetration also occurs when two neighboring bubbles collapse simultaneously. Another situation of jet penetration into a bubble can be encountered when the bubble collapses in a traveling ultrasonic wave (Yasui 2018).
Kornfeld and Suvorov (1944) were the first researchers to propose the microjet theory, they indicated that when a bubble collapses near a solid wall, the bubble deforms into flat-shape or ingot-shape and finally collapses producing a microscopic jet flow impingement on that wall. Crum (1995) and Gregorčič et al. (2007) subsequently observed the microjet impact produced by bubble collapse on a wall, using high-speed photography. Plesset and Chapman (1970) reported that the microjet velocity is about 130 m/s when the bubble collapses attached to the wall and is about 170 m/s when the bubble collapses near the wall. This result is coherent with the findings of Brujan (2004) who observed that microjet velocity produced by the collapse of a bubble having a maximum radius of 150 μm ranges from 80 to 130 m/s. Sato et al. (2016) used the impact effect of bubble collapse to deal with the surface of a hard aluminum alloy plate and found that the fatigue of the material increased more than 10 times (Yasui 2018), microjets are then believed to be the key phenomenon responsible of material erosion under the effect of ultrasound and acoustic cavitation.
2.2.5 Shock Wave
The collapse of a bubble during transient or repetitive transient cavitation induces extremely high pressures that result in outward propagating shock waves. This suddenly released pressure into the liquid medium generates shock waves that are helpful to enhance mass transfer and shear induced processes. This cataclysmic event, i.e., shock waves, can also cause severe turbulence within the immediate surroundings and produce high velocity interparticle collisions, the impact of which is sufficient to melt most metals (Jena 1965), break polymer chains or destroy the cell walls of plant and animals. The shock wave approach considers bubbles to remain spherical during collapse, and the collapse causes a shock wave with an order of magnitude of several megapascals at a final stage.
The energy released from a single transient collapse is extremely small, but millions of bubbles collapse every second and the cumulative effect is large. Fortes Patella and Reboud (Fortes Patella and Reboud 2004) investigated numerically the interaction between the shock wave emitted by a collapsing acoustic cavitation bubble and the material, they concluded that cavitation damage is directly related to the shock wave and material characteristics. Brujan (Brujan 2004) reported that at a distance of 68 μm from the bubble wall, the shock wave pressure is 1.3 ± 0.3 GPa (Ye and Zhu 2017).
2.3 Chemical Effects of Acoustic Cavitation Bubble
The violent collapse events that occur during transient and repetitive transient cavitation can also generate enormous temperatures at a localized level, exceeding 5000 K (Kerboua and Hamdaoui 2018c). These high temperatures and the violent pressure changes occurring simultaneously can cause a number of chemical changes to occur within both the gaseous phase inside the cavitation bubble and in the immediate fluid surrounding it (Pankaj and Ashokkumar 2011).
Primary radicals are formed as a direct result of the high temperatures inside a collapsing bubble. In the heated interior of the bubble, water vapor and gas molecules, if reactive, are dissociated and oxidants such as OH radicals, H2O2, O atoms, and O3 are formed. Table 6.2 illustrates the possible reactions occurring in the bulk volume of the bubble under oxygen atmosphere.
The oxidants diffuse out of the bulk volume of the bubble, represented in Fig. 6.4, into the surrounding liquid, and chemically react with solutes if present. Such chemical reactions are called sonochemical reactions, and chemistry associated with acoustic cavitation is called sonochemistry.
There are three sites for chemical reactions for a cavitation bubble. (i) The interior of the bubble, where volatile solutes which evaporate into this region are dissociated by high temperature. (ii) The gas-liquid interface region, where the temperature dramatically increases due to the thermal conduction from the heated interior of a bubble where radicals with a relatively short lifetime such as OH and O react with solutes or radicals themselves and surfactants adsorbed at the bubble surface can dissociate at the interface region due to both heat and radical attack. (iii) The liquid region outside the interface region which is at the ambient temperature and where chemical species with a relatively long lifetime such as H2O2 diffusing out of the interface region chemically react with solutes (Jena 1965).
Sonochemistry is rigorously concerned by multibubble system, which is a complex system owing to bubble-bubble interactions, changes in local acoustic field due to attenuation and scattering by moving bubbles. Moreover, bubbles frequently coalesce each other, fragment into daughter bubbles, grow by rectified diffusion, shrink by gas diffusion, and spatially move in a complex pattern due to radiation forces. In addition, new bubbles are permanently created and the bubble pulsation as well as the acoustic wave propagation are strongly nonlinear. For all these reasons, most of researchers consider first a simpler system, which is the single acoustic cavitation bubble.
When a single bubble is considered, the number of generated free radicals abruptly increases when the temperature inside the collapsing bubble is at its maximum. This temperature can be increased by increasing the sonication power, increasing the static pressure, or decreasing the liquid temperature. The nature of the saturating gas plays a role as well, the heavier inert gases have a lower thermal conductivity and hence are less efficient at transferring heat away from the bubble to the surrounding fluid, they then increase the temperature at the bulk volume of the bubble. The amount of generated heat depends also on the size of the cavitation bubble, the bigger the transient cavitation, the higher the amount of heat.
In a multibubble field, the total yield of generated free radicals is governed not only by the bubble temperature, but also by the number of active bubbles. In fact, it has been shown that the number of bubbles generated is the predominant factor in controlling the radical yield. Thus, for a given liquid volume and acoustic power, a greater number of radicals are generated at higher frequencies (Kerboua and Hamdaoui 2019b) and this can dominate over the radical production per bubble. For this reason, sonochemical effects are generally the most dominant at intermediate frequencies generally ranging from 200 to 500 kHz (Kerboua et al. 2021c; Bermúdez-aguirre et al. 2011).
The sonochemical effect accompanying the oscillation of an acoustic cavitation bubble, when submitted to appropriate acoustic conditions, knows several applications in wastewater treatment, food and beverages industry, and biological processes (Leong et al. 2011; Yusof et al. 2016).
3 Sono-processing in Agri-food Applications
The effects induced by ultrasound and acoustic cavitation bubbles, particularly those of physical nature, know several applications in agri-food sector. Table 6.3 reports most of the applications involving ultrasound and/or acoustic cavitation bubble in food processing. Some of these applications are discussed in detail in the next section.
3.1 Sono-extraction
Extraction is a major step in the development of natural functional food. The conventional extraction technique is solvent extraction that has been associated with a large amount of organic solvent and heating. The development of extraction technologies has placed the ultrasound-assisted process as an emerging solution for improved recovery of food compounds with higher quality, lower extraction times, lower temperatures, less solvent volumes and lower energy consumption (Fu et al. 2020).
Sono-extraction is mainly explained by cell disruption caused by the implosion of acoustic cavitation bubble. The increase of the number of disrupted cells participate in weakening the limitation of cell structure on mass transfer from the solid matrix to the liquid phase (Yang et al. 2017).
Microstreaming accompanying the collapse of acoustic cavitation bubble causes violent turbulence that disrupts the surfaces of solid foodstuffs and facilitates the mixing of food materials and components in the medium. This also decreases diffusion boundary layers (Bhaskaracharya et al. 2009), which consequently accelerates mass and heat transfers (Tao and Sun 2015). The oscillation of the acoustic cavitation bubble, ending by its implosion can induce shear forces, fragmentation, sonoporation erosion, capillary effect, turbulence and mixing, which also contribute to mass-transfer enhancement (Rodrigues and Fernandes 2017).
Ultrasound also contributes to the reduction of the size of solid materials, which increases the contact surface area between the solid phase and the solvent. Many microscopic channels in tissue can appear as well due to alternative compression and rarefaction of the ultrasonic wave, inducing the so called “sponge effect”. This effect facilitates the penetration of the solvent into the solid matrix (Fernandes et al. 2008; Fernandes et al. 2009; Nowacka et al. 2014).
Literature counts a significant number of works that investigated the efficiency of sono-extraction in food processing. For instance, Vilkhu et al. (2008) studied the sono-extraction of bioactives such as polyphenols, anthocyanins and aroma compounds.
Panchev et al. (1988) examined the extraction of pectin from apple pomace in concentrated nitric acid using 22 kHz intermittent ultrasonication during 10–60 min at 1–1.2 W/cm2 followed by coagulation with 95% ethanol and separation by filtration. Panchev et al. (1988) revealed that ultrasound-assisted extraction reduced the extraction time by more than 33% for similar yields and improved the pectin yield by 22% for similar time.
Tang (2007) performed ultrasound assisted extraction of solids from buckwheat flour at 15 kHz and 500 W for 30 min. They demonstrated that the technique allowed the extraction of lipids and proteins in 87.5% shorter time compared to conventional method. This observation was explained by increased mass transfer.
Albu et al. (2004) carried out the extraction of an antioxidant, namely carnosic acid, from dried and fresh leaves of rosemary, they demonstrated improved yields and higher extraction rates using ultrasound and solvent at 50 °C.
Other researchers examined the possibility of coupling ultrasound with other conventional and innovative techniques in order to achieve synergetic effects. For instance, Lianfu and Zelong (2008) optimized the extraction of lycopene from fresh tomatoes at 86.4 °C to theoretical values of 97% using coupled ultrasound and microwave. At this temperature, cavitation would be unlikely to occur, which explains the ultrasound effect by acoustic streaming. Another example is that of Cravotto et al. (2008) who extracted vegetable oils from soybean germ and sea weed using a solvent with a combination of ultrasound and microwave. The innovative extraction technique allowed the reduction of the extraction time by 8-fold compared to separated techniques.
Some other studies combined Soxhlet extraction (Alara et al. 2018; Luque de Castro and Priego-Capote 2010; López-Bascón-Bascon and Luque de Castro 2019) with ultrasound to quicken the extraction of fat and oil (Chemat et al. 2017). This strategy has been adopted for instance by Djenni et al. (2013) who developed a novel design of sono-Soxhlet extraction of oils from crushed dried olives using an ultrasonic horn in situ to provide rapid and complete recovery of analytes from the matrix. The authors proved through physicochemical characterization and gravimetric analysis that extraction time was substantially reduced compared to conventional Soxhlet extraction, without affecting the composition and quality of target extracts. Luque de Castro and coworkers (2004) used similar procedure as Djenni et al. (2013) and proved that ultrasound boosts mass transfer, the specimen is then collected completely, effortlessly and precisely.
3.2 Sono-emulsification
An emulsion consist of two naturally immiscible liquids and is thermodynamically unstable due to the interfacial tension between the continuous and dispersed phases (Ashokkumar et al. 2010). Ultrasound has been introduced in the last decades as efficient emulsification technique, particularly in food industry.
Two mechanisms occurring in parallel explain the ultrasound-assisted emulsification, namely the development of instabilities at the oil/water interface and the turbulence induced by acoustic cavitation bubble (Bhaskaracharya et al. 2009).
low frequency and high intensity ultrasound is usually used in the food sector for the preparation of mayonnaise, emulsions fruit juices, ketchup, and homogenized milk (Hongyu et al. 2000). Several research groups studied the ultrasound assisted emulsification of protein. For instance, Li et al. (2020) used ultrasound at 20 kHz and 450 W to improve the emulsification of chicken myofibrillar protein. Their experiments showed that better oil/water emulsion gel elasticity was obtained after 6 min of sonication.
3.3 Sono-homogenization
Sono-homogenization is particularly applied in the dairy industry. Indeed, milk is an oil in water emulsion. During storage, in order to avoid the creaming of the natural full cream milk, the size milk fat particles should be reduced below 0.8 µm (Silva and Chandrapala 2021; Villamiel and De Jong 2000). Ultrasound is considered as an alternative method for reducing the size of fat globules and can be applied effectively to homogenize milk (Bosiljkov et al. 2011). For instance, Vercet et al. (2002) demonstrated that the firmness and texture of a yogurt made from milk exposed to sonication are considerably improved.
Karlović (2015) exposed milk to high pressure ultrasound of up to 600 MPa and treatment time up to 9 min. They reported an improvement in the homogenization of milk fat globules, which were significantly reduced in size by the high pressure (Carrillo-Lopez et al. 2017).
Bermúdez-Aguirre et al. (2008) exposed whole milk to thermosonication treatment of 24 kHz and 400 W over a duration of 30 min and studied the changes to the microstructure of fat globules. The sonication of whole milk at 63 °C resulted in fat globules of less than 1 μm with more binding sites on the fat globule membrane enhancing the amalgamation of casein and serum proteins, which produces an ideal ingredient for cheese making. The authors attributed the observed effect to cavitation since heat treatment alone did not show similar changes to the fat globules (Ashokkumar et al. 2010).
Ertugay et al. (2004) proved that the size range of fat globules in ultrasonically homogenized milk samples at 20 kHz was much smaller than that obtained with conventional homogenizer (2–5 μm). The mean size and size distribution of the fat globules were dependent on the ultrasonic power and duration of sonication. The authors explained the observed effect by the physical effects generated during acoustic cavitation.
In addition to the decrease of the diameter of fat globules (Nguyen and Anema 2010), several authors demonstrated that high pressure ultrasound induces alterations in the secondary structure of proteins in milk, aggregation of protein particles, and denaturation (Chandrapala et al. 2011) depolarizes the particles of gamma-carrageenan and reduces their size, allowing for better homogenization of nanoparticles in a dispersion mixed with beta-lactoglobulin. Consequently, ultrasound participate significantly in the enrichment of acidified milk drinks (Hosseini et al. 2013). Power ultrasound is also proved to cause alterations in the composition and structure of the membrane of fat globules, which improves the efficiency of homogenization in casein gel (Chandrapala et al. 2013).
3.4 Sono-foaming/defoaming
The formation of foams, which passes through three main steps (i) transportation (ii) penetration and (iii) reorganization of the molecules at the gas-liquid interface, is influenced by multiple factors such as particle size, structural flexibility of surfactant molecules and surface hydrophobicity (Wilde and Clark 1993). Application of ultrasound technologies in foaming of food processes has emerged significantly in dairy industry. For instance, Jambrak et al. (2008) sonicated whey protein isolate, whey protein concentrate and whey protein hydrolysate solutions using low-intensity ultrasound through sonication bath at 500 kHz and high-intensity ultrasound through sonication probe at 20 kHz and sonication bath at 40 kHz. Significant increase in foaming capacities and foam stabilities were observed at 20 and 40 kHz. This observation was explained by the homogenization effect of ultrasound which disperses the protein and fat particles enhancing the foaming properties, and eventually by the partial unfolding of proteins during sonication.
In the other hand, Tan et al. (2015) observed that the foaming properties of whey protein concentrates improved with increasing ultrasound amplitude and time. To illustrate, at 60% amplitude and 25 min treatment time, foam drainage decreased by 35% while foaming capacity, viscosity and consistency increased significantly. In another study, Tan et al. (2016) demonstrated that sonication of whey protein suspensions at 20%, 40% and 60% amplitude, 20 kHz and 400 W for 5, 15 and 25 min significantly enhanced the foaming properties. Better foaming properties can then be obtained using higher ultrasound amplitudes and longer treatment times. The observed effect was attributed to the acoustic cavitation that induces the fracturing of bubbles in the protein suspension leading to the formation of small-sized bubbles. Due to the denaturation of proteins, gel film is created on the surface of bubbles which further improve the stability.
Intensive foaming in several technological food processes negatively impacts the useful volume of the technological equipment and the conditions of sterility in biotechnological processes (Chemat et al. 2011). Conventional techniques rely on mechanical breakers, decrease of temperature and addition of anti-foaming chemical reagents in order to control excessive foaming (Gallo et al. 2018; Morey et al. 1999). Ultrasonic defoaming has been developed and successfully applied to control the excessive foaming, particularly in beverages industry (Riera et al. 2006).
Rodríguez et al. (2010) achieved the scale-up of ultrasonic defoaming systems by mounting a focused airborne ultrasonic emitter on an electronically controlled rotating system. When the ultrasonic transducer rotates, it creates a complex movement covering a large defoaming area at different rotation speeds for sufficient duration. The authors demonstrated that most of the bubbles break almost instantaneously under the acoustic beam.
3.5 Ultrasound-assisted Fermentation
Ultrasound has been introduced in food processing, particularly in dairy industry, as an accelerator for the fermentation process. Low intensity ultrasound is believed to increase mass transfer into fermentation chamber, which enhances fermentation rate. It was proved by Sakakibara et al. (1994) that ultrasound accelerates lactose hydrolysis in milk at starter Lactobacillus delbrueckii and could promote the fermentation process. Several researchers demonstrated through experimental studies that fermentation time for milk can be significantly decreased, along with the syneresis reduction and viscous increasing of yogurt because of enzyme activity enhancement under ultrasonic treatment (Bratsikhin et al. 2019). Indeed, Vercet et al. (2002) studied the manothermosonication at 20 kHz and 40 °C and demonstrated that it produces low syneresis in yoghurts prepared from cow milk (Ashokkumar et al. 2010). Moreover, Riener et al. (2009) experienced the thermosonication of milk at 25 kHz and 45 or 75 °C and proved that the obtained yoghurts had higher water holding capacities and viscosities. Obtained yoghurts also displayed improvements in microstructural properties containing honeycomb-like network with a more porous nature. Nguyen and Anema (2010) showed that yoghurt prepared from skim milk treated with ultrasound at 22 kHz and 50 W and up to 30 min had higher viscosity. In the other hand, Masuzawa and Ohdaira (2002) proved that ultrasonic treatment at 20 kHz reduced ripening time for yogurt production (Ashokkumar et al. 2010). Sfakianakis et al. (2015) demonstrated significant improvements in water holding capacity and viscosity of yoghurts with reduced syneresis can be achieved using ultrasound treatment and the effect has been shown to be more pronounced with the increases in the ultrasound amplitude. Hence, high intensity ultrasound provides several benefits over conventional fermentation in milk gels and yoghurts. All of the mentioned effects were justified by water binding capacity enhancing because of fat globe surface enlargement (Ashokkumar et al. 2010; Hongyu et al. 2000).
3.6 Sono-crystallization
In order to obtain appropriate food structure, texture and consumer appeal, crystallization is a key factor to control in the food industry. With conventional techniques of crystallization, it is difficult to get a uniform crystal size due to non-uniform nucleation, inefficient cooling caused by surface encrustation of cooling coils and non-uniform growth of crystals due to uneven mixing. With ultrasound, it is possible to initiate the nucleation process at a higher temperature or in a shorter time, smaller and more uniform crystals are then obtained (Mason 2007). The use of power ultrasound provides a useful approach to producing crystals with desired properties. Sono-crystallization facilitates process control by modulating crystal size distribution and morphology for several food products such as chocolate, honey, fats and frozen foods (Deora et al. 2013).
Bund and Pandit (2007) examined the sonication of a lactose solution using a 22 kHz ultrasonic bath, with 85% v/v ethanol as an anti-solvent. They demonstrated that the ultrasound assisted process led to 92% recovery of lactose as compared to 15% recovery for mechanically stirred samples. They also proved that the size and shape characteristics of the lactose crystals were enhanced. The observed improvements were explained by rapid mixing of the anti-solvent into the solution and eventually by either cavitation bubbles acting as crystal nucleation sites or solvent depletion in the zone of a cavitation bubble. These hypotheses are confirmed by the observations of Chow et al. (2005) who confirmed through direct imaging of a 15% sucrose solution that cavitational micro-bubbles can indeed act as nucleation sites. Kougoulos et al. (2010) employed power ultrasound to crystallize lactose and showed that a longer duration time resulted in a smaller crystal size, the minimum crystal size can reach 10 μm. They also demonstrated that ultrasound plays a positive role in increasing nucleation rate and yield.
In the other hand, Luque De Castro and Priego-Capote (2007) indicated that the results obtained so far in the literature make foreseeable that crystal size distribution and crystal shape can be tailored by appropriate selection of the sonication conditions. Patrick et al. (2004) indicated that the optimum acoustic conditions in order to obtain small crystals in the shortest time should be selected just below the cavitation intensity threshold.
3.7 Sono-osmo-dehydration
Osmo-dehydration is a technique used for dehydration of fruits and vegetables. Employing ultrasound with osmotic dehydration results in higher wastage of water and solute at a lesser temperature of the solution, in addition to protecting the attributes like flavor, color and temperature-sensitive nutrients (Rahaman et al. 2019; Prithani and Dash 2020). Deng and Zhao (2008a) studied the osmo-dehydration and rehydration of apple cylinders using an ultrasonic bath at 50/60 kHz and a pulsed vacuum chamber with 13 MPa vacuum applied twice for 5 min intervals. They demonstrated that ultrasound treatment increased the mass transfer of water from the substrate without heating the fruit pieces to very high temperatures. Such mass transfer enhancement is attributed to the turbulence and physical shear induced both by the acoustic field and by the acoustic cavitating bubbles (Bhaskaracharya et al. 2009). In this context, Simal et al. (1998) also affirmed that the sono-osmo-dehydratation to dry out the apples has been found to accelerate the mass transfer rate of “water out” and “solute in”. Cárcel et al. (2007) indicated that diffusion coefficients of water and solute were found to increase around 117% and 137% separately due to ultrasound.
Similar observations were made by Stojanovic and Silva when treating blue berries with ultrasound (Stojanovic and Silva 2006), they showed that the diffusion rates of moisture throughout osmotic dehydration of rabbit eye blueberries are notably enhanced with application of ultrasound though, this is attributed to the loss of anthocyanins and phenolics. Sánchez et al. (1999) also confirmed the increase of the rate of expulsion of water and sodium chloride in the case of cheese brining, when ultrasound are used.
Moreover, Bchir et al. (2020) showed that ultrasound pretreatment of the pomegranate seeds induces a reduction in the osmotic solution temperature and leads to more hardness of the samples microstructure compared to the fresh and osmotic dehydrated samples. Deng and Zhao (2008a) compared ultrasound assisted dehydratation with pulsed vacuum technique, and proved that ultrasound treatment resulted in higher glass transition temperature, lower water activity, reduction in rehydration rate and water content, a more extreme rupturing of the structure, and fewer crevices and calcium take-up, as compared to pulsed vacuum. However, it is important to notice that the same research group (Deng and Zhao 2008b) observed that rehydration rates were slower for apples pretreated with ultrasound, which would be explained by cell deformation and severe tissue damage at the surface. Deng and Zhao reported an increased hardness and crispness of apples pre-treated with osmo-dehydration assisted by ultrasound upon air drying or freeze drying.
3.8 Ultrasound Assisted Freezing
Freezing is an important unit operation in food industry intended to produce food products in frozen state, which extends their shelf life. Freezing passes generally by two phases: (i) initial ice nucleation and (ii) crystal growth towards the liquid phase (Tao and Sun 2015). The speed of ice nucleation is often slower than the growth of ice crystals. In conventional freezing, the crystals will rapidly grow to large size which may destroy the cell tissue instead of forming small and uniform ice crystals.
When using ultrasound, acoustic cavitation bubbles constitute nucleation sites for ice, which results in generating a mass of ice nuclei. Simultaneously, heat and mass transfer are enhanced due to the turbulence created by acoustic waves and acoustic cavitation (Dalvi-Isfahan et al. 2017). During primary nucleation, it is believed that the extreme conditions of pressure created during the collapse of acoustic cavitation bubble contribute to the decrease of the supercooling degree driving the process of nucleation (Kiani et al. 2011). During secondary nucleation, the collapse of cavitation bubbles and the shear forces accompanying it can break up the dendrites of the pre-existing crystal ice into many smaller fragments, producing more nucleation sites (Fu et al. 2020).
Delgado et al. (2009) performed the ultrasound assisted freezing of apple cylinders. They demonstrated that the application of ultrasonic irradiation using ultrasonic bath working intermittently at 40 kHz and 131.3 W at 0 or −1 °C for 120 s with 30 s intervals highly improved the freezing rate. Besides, Islam et al. (2014) studied the ultrasound-assisted freezing of three types of mushroom, namely Lentinula edodes, Agaricus bisporus and Pleurotus eryngii. The times for nucleation formation during sonication for Lentinula edodes, Agaricus bisporus and Pleurotus eryngii were reduced by 24%, 53%, and 34%, respectively, while the drip loss during the thawing process was decreased by an order of 10%. Power ultrasound proved to inhibit polyphenol oxidase and peroxidase enzyme activities by generating fine crystals with even distribution, which leads to high quality of mushrooms during long term storage. It is worthy to mention that power ultrasound results in the sponge effect which retains some residual water, the microstructures of frozen samples were better in comparison with samples obtained using conventional methods.
Kiani and Sun (2018) were able to accurately predict the ultrasound-assisted freezing of various shapes of potatoes and suggested the existence of optimum condition of ultrasonic intensity to reduce freezing time. The same research group (Kiani et al. 2015) assessed the ultrasound-assisted freezing of potato spheres using experimental, numerical and analytical approaches aiming to predict temperature distribution, phase ratios and process time. The author demonstrated that the shortest freezing time could be achieved only within the range of 30–70% duty cycles. This was attributed to enhanced heat transfer and the thermal effect at the sample surface. Numerical simulations proposed by Kiani et al. (2015) provided the temperature and water fraction profiles in potatoes under different conditions.
Cheng et al. (2014) also employed ultrasound to assist the immersion freezing of strawberries. The authors proved that usage of ultrasound resulted in nucleation at a lower supercooling degree, they also revealed a linear correlation of the supercooling with the ultrasonic temperature.
Comandini et al. (2013) investigated the ultrasound-assisted freezing of potato cubes using a sonotrode at 35 kHz. The sonication was activated when the temperature of the geometrical center of the sonotrode was within the range of −0.1 to −3 °C. The authors reported that nucleation occurred significantly earlier when applying ultrasound. However, the authors mentioned that a significant reduction in freezing time was observed only when ultrasound was applied at −2 °C.
3.9 Ultrasound Assisted Filtration
The ultrasound-assisted filtration depends on several factors such as feed solution characteristics, cleaning solution characteristics, membrane properties and ultrasound parameters (Ashokkumar et al. 2010). The technique takes advantage of four mechanisms induced by ultrasound, namely (i) the bulk water movement toward and away from the membrane cake layer due to turbulence generated by the acoustic streaming, which removes particles from the membrane surface. (ii) the mechanical vibrational energy generated through sonication, which keeps the particles partly suspended in the solution and provides more free channels for the solvent elution, (iii) the cleaning of the membrane crevices and pores by acoustic cavitation bubbles, and (iv) the agglomeration of fine particles and thereby the reduction of the pore blockage of the membranes and compaction of cake on the membrane (Muthukumaran et al. 2005a, b).
Koh et al. (2014) observed a small but significant decrease in membrane fouling at solid concentrations higher than 10% w/w when applying ultrasound irradiation at 20 kHz to the whey protein concentration solution prior to filtration. This observation is explained by the ultrasound induced reduction of the solution viscosity and the size of the aggregates leading to the reduced cake growth and pore blockage.
3.10 Sono-preservation (Enzyme Inactivation)
Ultrasound can be used for food preservation in combination with other treatments by improving its inactivation efficacy. There have been many studies combining ultrasound with either pressure, temperature, or pressure and temperature (Ercan and Soysal 2012). When ultrasound is used alone, long time of ultrasonic treatment is required to achieve efficient inactivation of enzymes and microorganisms (Zheng and Sun 2006). When ultrasonic irradiated is combined to moderated heating, the technique is known as thermosonication. If thermosonication is employed for pasteurization or sterilization purpose, it is observed that lower process temperatures and times are required to achieve the same lethality values as compared to conventional processes (Mason et al. 1996). If pressure and sonication are combined, the technique is then called manosonication, its use in enzyme inactivation showed higher efficiency than ultrasound alone at the same temperature (Demirdöven and Baysal 2009). Finally, if ultrasound is coupled to both temperature and pressure effects, it results in manothermosonication. This technique is believed to inactivate more enzymes at lower temperatures and in a shorter time than thermal treatments at the same temperatures (Chemat et al. 2011).
First enzyme inactivation by ultrasound was applied to pure pepsin almost 60 years ago (Ercan and Soysal 2012) and since then, several researchers performed enzyme inactivation using ultrasound alone or with heating and pressure. For instance, Raso and Barbosa-Cánovas (2003) studied the ultrasound-assisted inactivation of polyphenol oxidase. Ercan and Soysal (2011) performed the therrmosonication technique to inactivate tomato peroxidase, while De Gennaro et al. (1999) explored the inactivation of peroxidase by thermosonication. Besides, Guiseppi-Elie et al. (2009) inspected the effect of ultrasonic processing on enzymatic activity of glucose oxidase. Raviyan et al. (2005) carried out both ultrasonication and thermosonication to inactivate tomato pectin methyl esterase, they examined the effect of cavitation intensity and temperature. Vercet et al. (2001) also studied the inactivation of proteases and lipases using ultrasound, while Cruz et al. (2006) investigated the effect of thermal and thermosonication treatments on inactivation kinetics of watercress peroxidase.
3.11 Sono-pasteurization (Microbial Inactivation)
Conventional thermal techniques of pasteurization result in unwanted flavors and loss of nutrients. These undesirable effects can be overcome using ultrasound (Ercan and Soysal 2012). Ultrasound is believed to inactivate microbial activity in liquid food. The ultrasound-assisted inactivation of microbial activity is explained by four mechanisms. (i) Ultrasound induces intracellular cavitation in the bacterial cells, which destroys the structural and functional components of the bacterial cells up to cell lysis. (ii) The collapse of acoustic cavitation bubbles on hydrophobic surfaces of micro-organism results in severe damage to the microbial cell walls. (iii) the microstreaming generated by the passage of the ultrasonic wave causes thinning of the cell membranes. (iv) The generated hot spot and the formation of free radicals can damage the DNA of the microorganism (Ashokkumar et al. 2010).
Cameron et al. (2009) studied the ultrasound assisted pasteurization of milk and proved that ultrasound eliminates spoilage and potential pathogens to zero or to levels acceptable by legislation, even when initial inoculum loads were 5 times higher than permitted. According to their study, viable cell counts of E. coli were reduced by 100% after 10 min of ultrasonication, while viable counts of Pseudomonas fluorescens were reduced by 100% after 6 min and Listeria monocytogenes were reduced by 99% after 10 min.
Besides, Saikia et al. (2016) performed ultrasound pasteurization of juices and demonstrated that sonication has a positive effect on the total flavonoid content in carambola, black jamun, watermelon, pineapple and litchi juice samples followed by microwave treatment with exceptions in some cases.
Salleh-Mack and Roberts (2007) carried out a parametric study of ultrasound pasteurization by examining the effects of temperature, sugar, organic acids and pH on ultrasound inactivation of Escherichia coli ATCC 25922. Overall, ultrasound increased the sensitivity of Escherichia coli to thermal inactivation. The presence of soluble solids had a protective effect where the sonication time requirement increased. Similar to heat sensitivity, the lower pH environment resulted in Escherichia coli having less resistance to sonication. In another study, Wordon et al. (2012) examined the effect of the acoustic frequency and suggested that high frequency ultrasound was more effective in inactivation of microorganisms.
4 Ultrasound in Agri-food Application: Challenges
Although ultrasound assisted processes in food industry have known a rapid development and even actual implementation at large scale, the literature and industrial documentation seriously lack standardized documentation of methodology and control parameters until the widespread implementation of the technology. The inexistence of such documentation prevents reproducibility of results and questions the liability of the use of ultrasound assisted processes in large-scale applications (Rastogi 2011).
Joint efforts of fundamental studies and technical research should be gathered in order to define parametric standards in terms of sono-food processing technologies. Efforts should also be invested in the security of ultrasound technologies applied to food processing, notably in order to prevent undesirable effects reported by some researchers at laboratory scale and design reliable and viable industrial solutions (Singla and Sit 2021).
References
Alara OR, Abdurahman NH, Ukaegbu CI (2018) Soxhlet extraction of phenolic compounds from Vernonia cinerea leaves and its antioxidant activity. J Appl Res Med Aromat Plants 11:12–17. https://doi.org/10.1016/j.jarmap.2018.07.003
Albu S, Joyce E, Paniwnyk L, Lorimer JP, Mason TJ (2004) Potential for the use of ultrasound in the extraction of antioxidants from Rosmarinus officinalis for the food and pharmaceutical industry. Ultrason Sonochem 11:261–265. https://doi.org/10.1016/j.ultsonch.2004.01.015
Ashokkumar M, Bhaskaracharya R, Kentish S, Lee J, Palmer M, Zisu B (2010) The ultrasonic processing of dairy products – an overview, dairy. Sci Technol 90:147–168. https://doi.org/10.1051/dst/2009044
Bai L, Xu W, Deng J, Li C, Xu D, Gao Y (2014) Generation and control of acoustic cavitation structure. Ultrason Sonochem 21:1696–1706. https://doi.org/10.1016/j.ultsonch.2014.02.027
Bchir B, Bouaziz MA, Ettaib R, Sebii H, Danthine S, Blecker C, Besbes S, Attia H (2020) Optimization of ultrasound-assisted osmotic dehydration of pomegranate seeds (Punica granatum L.) using response surface methodology. J Food Process Preserv 44:1–17. https://doi.org/10.1111/jfpp.14657
Bermúdez-Aguirre D, Mawson R, Barbosa-Cánovas GV (2008) Microstructure of fat globules in whole milk after thermosonication treatment. J Food Sci 73:325–332. https://doi.org/10.1111/j.1750-3841.2008.00875.x
Bermúdez-aguirre D, Mobbs T, Barbosa-cánovas GV (2011) Ultrasound technologies for food and bioprocessing. https://doi.org/10.1007/978-1-4419-7472-3
Bhargava N, Mor RS, Kumar K, Sharanagat VS (2021) Advances in application of ultrasound in food processing: a review. Ultrason Sonochem 70:105293. https://doi.org/10.1016/j.ultsonch.2020.105293
Bhaskaracharya RK, Kentish S, Ashokkumar M (2009) Selected applications of ultrasonics in food processing. Food Eng Rev 1:31–49. https://doi.org/10.1007/s12393-009-9003-7
Bjørnø L (2017) Finite-amplitude waves. In: Applied underwater acoustics. Elsevier, pp 857–888. https://doi.org/10.1016/B978-0-12-811240-3.00013-8
Bosiljkov T, Tripalo B, Brnčić M, Ježek D, Karlovi S, Jagušt I (2011) Influence of high intensity ultrasound with different probe diameter on the degree of homogenization (variance) and physical properties of cow milk. Afr J Biotechnol 10:34–41. https://doi.org/10.4314/ajb.v10i1
Bratsikhin A, Leschenko E, Kostenko K (2019) Influence of cavitation disintegration on dairy foods production. J Hyg Eng Des 27:173–177
Brennen CE (2013) Cavitation and bubble dynamics. https://doi.org/10.1017/CBO9781107338760
Brujan EA (2004) The role of cavitation microjets in the therapeutic applications of ultrasound. Ultrasound Med Biol 30:381–387. https://doi.org/10.1016/j.ultrasmedbio.2003.10.019
Bund RK, Pandit AB (2007) Sonocrystallization: effect on lactose recovery and crystal habit. Ultrason Sonochem 14:143–152. https://doi.org/10.1016/j.ultsonch.2006.06.003
Cameron M, McMaster LD, Britz TJ (2009) Impact of ultrasound on dairy spoilage microbes and milk components. Dairy Sci Technol 89:83–98. https://doi.org/10.1051/dst/2008037
Cárcel JA, Benedito J, Bon J, Mulet A (2007) High intensity ultrasound effects on meat brining. Meat Sci 76:611–619. https://doi.org/10.1016/j.meatsci.2007.01.022
Carrillo-Lopez LM, Alarcon-Rojo AD, Luna-Rodriguez L, Reyes-Villagrana R (2017) Modification of food systems by ultrasound. J Food Qual 2017. https://doi.org/10.1155/2017/5794931
Chandrapala J, Zisu B, Palmer M, Kentish S, Ashokkumar M (2011) Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason Sonochem 18:951–957. https://doi.org/10.1016/j.ultsonch.2010.12.016
Chandrapala J, Zisu B, Kentish S, Ashokkumar M (2013) Influence of ultrasound on chemically induced gelation of micellar casein systems. J Dairy Res 80:138–143. https://doi.org/10.1017/S0022029912000696
Chemat F, Zill-E-Huma, Khan MK (2011) Applications of ultrasound in food technology: processing, preservation and extraction. Ultrason Sonochem 18:813–835. https://doi.org/10.1016/j.ultsonch.2010.11.023
Chemat F, Rombaut N, Sicaire AG, Meullemiestre A, Fabiano-Tixier AS, Abert-Vian M (2017) Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason Sonochem 34:540–560. https://doi.org/10.1016/j.ultsonch.2016.06.035
Cheng XF, Zhang M, Adhikari B, Islam MN, Xu BG (2014) Effect of ultrasound irradiation on some freezing parameters of ultrasound-assisted immersion freezing of strawberries. Int J Refrig 44:49–55. https://doi.org/10.1016/j.ijrefrig.2014.04.017
Chow R, Blindt R, Chivers R, Povey M (2005) A study on the primary and secondary nucleation of ice by power ultrasound. Ultrasonics 43:227–230. https://doi.org/10.1016/j.ultras.2004.06.006
Comandini P, Blanda G, Soto-Caballero MC, Sala V, Tylewicz U, Mujica-Paz H, Valdez Fragoso A, Gallina Toschi T (2013) Effects of power ultrasound on immersion freezing parameters of potatoes. Innov Food Sci Emerg Technol 18:120–125. https://doi.org/10.1016/j.ifset.2013.01.009
Cravotto G, Boffa L, Mantegna S, Perego P, Avogadro M, Cintas P (2008) Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason Sonochem 15:898–902. https://doi.org/10.1016/j.ultsonch.2007.10.009
Crum LA (1980) Measurements of the growth of air bubbles by rectified diffusion. J Acoust Soc Am 68:203–211. https://doi.org/10.1121/1.384624
Crum LA (1995) Comments on the evolving field of sonochemistry by a cavitation physicist. Ultrason Sonochem 2:147–152. https://doi.org/10.1016/1350-4177(95)00018-2
Cruz RMS, Vieira MC, Silva CLM (2006) Effect of heat and thermosonication treatments on peroxidase inactivation kinetics in watercress (Nasturtium officinale). J Food Eng 72:8–15. https://doi.org/10.1016/j.jfoodeng.2004.11.007
Dalvi-Isfahan M, Hamdami N, Xanthakis E, Le-Bail A (2017) Review on the control of ice nucleation by ultrasound waves, electric and magnetic fields. J Food Eng 195:222–234. https://doi.org/10.1016/j.jfoodeng.2016.10.001
Dastkhoon M, Ghaedi M, Asfaram A, Jannesar R, Sadeghfar F (2018) Magnetic based nanocomposite sorbent combination with ultrasound assisted for solid-phase microextraction of Azure II in water samples prior to its determination spectrophotometric. J Colloid Interface Sci 513:240–250. https://doi.org/10.1016/j.jcis.2017.11.031
De Gennaro L, Cavella S, Romano R, Masi P (1999) Use of ultrasound in food technology I: inactivation of peroxidase by thermosonication. J Food Eng 39:401–407. https://doi.org/10.1016/S0260-8774(99)00028-X
Delgado AE, Zheng L, Sun DW (2009) Influence of ultrasound on freezing rate of immersion-frozen apples. Food Bioprocess Technol 2:263–270. https://doi.org/10.1007/s11947-008-0111-9
Demirdöven A, Baysal T (2009) The use of ultrasound and combined technologies in food preservation. Food Rev Int 25:1–11. https://doi.org/10.1080/87559120802306157
Deng Y, Zhao Y (2008a) Effects of pulsed-vacuum and ultrasound on the osmodehydration kinetics and microstructure of apples (Fuji). J Food Eng 85:84–93. https://doi.org/10.1016/j.jfoodeng.2007.07.016
Deng Y, Zhao Y (2008b) Effect of pulsed vacuum and ultrasound osmopretreatments on glass transition temperature, texture, microstructure and calcium penetration of dried apples (Fuji). LWT – Food Sci Technol 41:1575–1585. https://doi.org/10.1016/j.lwt.2007.10.018
Deora NS, Misra NN, Deswal A, Mishra HN, Cullen PJ, Tiwari BK (2013) Ultrasound for improved crystallisation in food processing. Food Eng Rev 5:36–44. https://doi.org/10.1007/s12393-012-9061-0
Djenni Z, Pingret D, Mason TJ, Chemat F (2013) Sono-soxhlet: in situ ultrasound-assisted extraction of food products. Food Anal Methods 6:1229–1233. https://doi.org/10.1007/s12161-012-9531-2
Ercan SŞ, Soysal Ç (2011) Effect of ultrasound and temperature on tomato peroxidase. Ultrason Sonochem 18:689–695. https://doi.org/10.1016/j.ultsonch.2010.09.014
Ercan SŞ, Soysal Ç (2012) Ultrasound in food preservation. Nat Sci 5:251–262. https://doi.org/10.1007/978-1-4614-2038-5_11
Ertugay MF, Şengül M, Şengül M (2004) Effect of ultrasound treatment on milk homogenisation and particle size distribution of fat. Turk J Vet Anim Sci 28:303–308
Feng H, Barbosa-Cánovas GV, Weiss J (2011) Ultrasound technologies for food and bioprocessing. Springer, New York
Fernandes FAN, Gallão MI, Rodrigues S (2008) Effect of osmotic dehydration and ultrasound pre-treatment on cell structure: melon dehydration. LWT – Food Sci Technol 41:604–610. https://doi.org/10.1016/j.lwt.2007.05.007
Fernandes FAN, Gallão MI, Rodrigues S (2009) Effect of osmosis and ultrasound on pineapple cell tissue structure during dehydration. J Food Eng 90:186–190. https://doi.org/10.1016/j.jfoodeng.2008.06.021
Fortes Patella R, Reboud J-L, Briancon Marjollet L (2004) A phenomenological and numerical model for scaling the flow agressiveness in cavitation erosion. In: EROCAV workshop. https://www.researchgate.net/publication/281921326
Fu X, Belwal T, Cravotto G, Luo Z (2020) Sono-physical and sono-chemical effects of ultrasound: primary applications in extraction and freezing operations and influence on food components. Ultrason Sonochem 60:104726. https://doi.org/10.1016/j.ultsonch.2019.104726
Fujikawa S, Akamatsu T (1980) Effects of the non-equilibrium condensation of vapour on the pressure wave produced by the collapse of a bubble in a liquid. J Fluid Mech 97:481–512. https://doi.org/10.1017/S0022112080002662
Gallo M, Ferrara L, Naviglio D (2018) Application of ultrasound in food science and technology: a perspective. Foods 7:1–19. https://doi.org/10.3390/foods7100164
Gogate PR, Pandit AB (2011) Sonocrystallization and its application in food and bioprocessing. In: Ultrasound technologies for food and bioprocessing, pp 65–105. https://doi.org/10.1007/978-1-4419-7472-3
Gregorčič P, Petkovšek R, Možina J (2007) Investigation of a cavitation bubble between a rigid boundary and a free surface. J Appl Phys 102. https://doi.org/10.1063/1.2805645
Guiseppi-Elie A, Choi SH, Geckeler KE (2009) Ultrasonic processing of enzymes: effect on enzymatic activity of glucose oxidase. J Mol Catal B Enzym 58:118–123. https://doi.org/10.1016/j.molcatb.2008.12.005
Hongyu W, Hulbert GJ, Mount JR (2000) Effects of ultrasound on milk homogenization and fermentation with yogurt starter. Innov Food Sci Emerg Technol 1:211–218. https://doi.org/10.1016/S1466-8564(00)00020-5
Hosseini SMH, Emam-Djomeh Z, Razavi SH, Moosavi-Movahedi AA, Saboury AA, Mohammadifar MA, Farahnaky A, Atri MS, Van Der Meeren P (2013) Complex coacervation of β-lactoglobulin - κ-carrageenan aqueous mixtures as affected by polysaccharide sonication. Food Chem 141:215–222. https://doi.org/10.1016/j.foodchem.2013.02.090
Islam MN, Zhang M, Adhikari B, Xinfeng C, Xu BG (2014) The effect of ultrasound-assisted immersion freezing on selected physicochemical properties of mushrooms. Int J Refrig 42:121–133. https://doi.org/10.1016/j.ijrefrig.2014.02.012
Jambrak AR, Mason TJ, Lelas V, Herceg Z, Herceg IL (2008) Effect of ultrasound treatment on solubility and foaming properties of whey protein suspensions. J Food Eng 86:281–287. https://doi.org/10.1016/j.jfoodeng.2007.10.004
Jena F (1965) Sonochemistry of selected hydrocarbons, sulfur-containing and nitrogen-containing organic compounds in aqueous solutions and nonaqueous liquids. Dissertation
Johansen K, Song JH, Prentice P (2017) Validity of the Keller-Miksis equation for “non-stable” cavitation and the acoustic emissions generated. In: 2017 IEEE International Ultrasonics Symposium (IUS). https://doi.org/10.1109/ULTSYM.2017.8091852
Karlović S (2015) Reducing fat globules particle-size in goat milk: ultrasound and high hydrostatic pressures approach. Chem Biochem Eng Q J 28:499–507. https://doi.org/10.15255/cabeq.2014.19400
Kentish S, Ashokkumar M (2010) The physical and chemical effects of ultrasound. In: Ultrasound technologies for food and bioprocessing. Springer, New York, pp 1–19
Kerboua K, Hamdaoui O (2017) Computational study of state equation effect on single acoustic cavitation bubble’s phenomenon. Ultrason Sonochem 38. https://doi.org/10.1016/j.ultsonch.2017.03.005
Kerboua K, Hamdaoui O (2018a) Ultrasonic waveform upshot on mass variation within single cavitation bubble: investigation of physical and chemical transformations. Ultrason Sonochem 42:508–516. https://doi.org/10.1016/j.ultsonch.2017.12.015
Kerboua K, Hamdaoui O (2018b) Numerical investigation of the effect of dual frequency sonication on stable bubble dynamics. Ultrason Sonochem 49. https://doi.org/10.1016/j.ultsonch.2018.08.025
Kerboua K, Hamdaoui O (2018c) Influence of reactions heats on variation of radius, temperature, pressure and chemical species amounts within a single acoustic cavitation bubble. Ultrason Sonochem 41. https://doi.org/10.1016/j.ultsonch.2017.10.001
Kerboua K, Hamdaoui O (2019a) Oxidants emergence under dual-frequency sonication within single acoustic bubble: effects of frequency combinations. Iran J Chem Chem Eng. https://doi.org/10.30492/ijcce.2019.36705
Kerboua K, Hamdaoui O (2019b) Void fraction, number density of acoustic cavitation bubbles, and acoustic frequency: a numerical investigation. J Acoust Soc Am 146. https://doi.org/10.1121/1.5126865
Kerboua K, Hamdaoui O (2020) Sonochemistry in green processes: modeling, experiments, and technology. In: Sustainable green chemical processes and their allied applications, p 603. https://doi.org/10.1007/978-3-030-42284-4
Kerboua K, Merouani S, Hamdaoui O, Alghyamah A, Islam MH, Hansen HE, Pollet BG (2021a) How do dissolved gases affect the sonochemical process of hydrogen production?An overview of thermodynamic and mechanistic effects – on the “hot spot theory”. Ultrason Sonochem 72:105422. https://doi.org/10.1016/j.ultsonch.2020.105422
Kerboua K, Hamdaoui O, Alghyamah A (2021b) Energy balance of high-energy stable acoustic cavitation within dual-frequency sonochemical reactor. Ultrason Sonochem 73. https://doi.org/10.1016/j.ultsonch.2021.105471
Kerboua K, Hamdaoui O, Alghyamah A (2021c) Acoustic frequency and optimum sonochemical production at single and multi-bubble scales: a modeling answer to the scaling dilemma. Ultrason Sonochem 70:105341. https://doi.org/10.1016/j.ultsonch.2020.105341
Kiani H, Sun DW (2018) Numerical simulation of heat transfer and phase change during freezing of potatoes with different shapes at the presence or absence of ultrasound irradiation. Heat Mass Transf Und Stoffuebertragung 54:885–894. https://doi.org/10.1007/s00231-017-2190-5
Kiani H, Zhang Z, Delgado A, Sun DW (2011) Ultrasound assisted nucleation of some liquid and solid model foods during freezing. Food Res Int 44:2915–2921. https://doi.org/10.1016/j.foodres.2011.06.051
Kiani H, Zhang Z, Sun DW (2015) Experimental analysis and modeling of ultrasound assisted freezing of potato spheres. Ultrason Sonochem 26:321–331. https://doi.org/10.1016/j.ultsonch.2015.02.015
Koh LLA, Nguyen HTH, Chandrapala J, Zisu B, Ashokkumar M, Kentish SE (2014) The use of ultrasonic feed pre-treatment to reduce membrane fouling in whey ultrafiltration. J Memb Sci 453:230–239. https://doi.org/10.1016/j.memsci.2013.11.006
Kolb J, Nyborg WL (1956) Small-scale acoustic streaming in liquids. J Acoust Soc Am 28:1237–1242
Kornfeld M, Suvorov L (1944) On the destructive action of cavitation. J Appl Phys 15:495–506. https://doi.org/10.1063/1.1707461
Kougoulos E, Marziano I, Miller PR (2010) Lactose particle engineering: influence of ultrasound and anti-solvent on crystal habit and particle size. J Cryst Growth 312:3509–3520. https://doi.org/10.1016/j.jcrysgro.2010.09.022
Lee J, Kentish S, Ashokkumar M (2005) Effect of surfactants on the rate of growth of an air bubble by rectified diffusion. J Phys Chem B 109:14595–14598. https://doi.org/10.1021/jp051758d
Leighton TG (1995) Bubble population phenomena in acoustic cavitation. Ultrason Sonochem 2:123–136. https://doi.org/10.1016/1350-4177(95)00021-W
Leong T, Ashokkumar M, Sandra K (2011) The fundamentals of power ultrasound – a review. Acoust Aust 39:54–63
Li K, Fu L, Zhao YY, Xue SW, Wang P, Xu XL, Bai YH (2020) Use of high-intensity ultrasound to improve emulsifying properties of chicken myofibrillar protein and enhance the rheological properties and stability of the emulsion. Food Hydrocoll 98. https://doi.org/10.1016/j.foodhyd.2019.105275
Lianfu Z, Zelong L (2008) Optimization and comparison of ultrasound/microwave assisted extraction (UMAE) and ultrasonic assisted extraction (UAE) of lycopene from tomatoes. Ultrason Sonochem 15:731–737. https://doi.org/10.1016/j.ultsonch.2007.12.001
López-Bascón-Bascon MA, Luque de Castro MD (2019) Soxhlet extraction. Liq Extr:327–354. https://doi.org/10.1016/B978-0-12-816911-7.00011-6
Louisnard O, González-García J (2011) Acoustic cavitation. Food Eng Ser:13–64. https://doi.org/10.1007/978-1-4419-7472-3_2
Luo MR (2020) Encyclopedia of color science and technology. https://doi.org/10.1007/978-3-642-27851-8
Luo J, Fang Z, Smith RL, Qi X (2015) Fundamentals of acoustic cavitation in sonochemistry. https://doi.org/10.1007/978-94-017-9624-8_1
Luo X, Cao J, Gong H, Yan H, He L (2018) Phase separation technology based on ultrasonic standing waves: a review. Ultrason Sonochem 48:287–298. https://doi.org/10.1016/j.ultsonch.2018.06.006
Luque de Castro MD, Priego-Capote F (2007) Ultrasound-assisted crystallization (sonocrystallization). Ultrason Sonochem 14:717–724. https://doi.org/10.1016/j.ultsonch.2006.12.004
Luque de Castro MD, Priego-Capote F (2010) Soxhlet extraction: past and present panacea. J Chromatogr A 1217:2383–2389. https://doi.org/10.1016/j.chroma.2009.11.027
Luque-García JL, Luque De Castro MD (2004) Ultrasound-assisted Soxhlet extraction: an expeditive approach for solid sample treatment – application to the extraction of total fat from oleaginous seeds. J Chromatogr A 1034:237–242. https://doi.org/10.1016/j.chroma.2004.02.020
Marmottant P, Hilgenfeldt S (2003) Controlled vesicle deformation and lysis by single oscillating bubbles. Nature 423:153–156. https://doi.org/10.1038/nature01613
Marshall JS, Wu J (2015) Acoustic streaming, fluid mixing, and particle transport by a Gaussian ultrasound beam in a cylindrical container. Phys Fluids 27. https://doi.org/10.1063/1.4932232
Mason TJ (2007) Developments in ultrasound-non-medical. Prog Biophys Mol Biol 93:166–175. https://doi.org/10.1016/j.pbiomolbio.2006.07.007
Mason TJ, Paniwnyk L, Lorimer JP (1996) The uses of ultrasound in food technology. Ultrason Sonochem 3. https://doi.org/10.1016/S1350-4177(96)00034-X
Masuzawa N, Ohdaira E (2002) Attempts to shorten the time of lactic fermentation by ultrasonic irradiation. Jpn J Appl Physics Part 1 Regul Pap Short Notes Rev Pap 41:3277–3278. https://doi.org/10.1143/jjap.41.3277
Milton RBC, Plesset S, Office of Naval Research (1970) Collapse of an initially spherical vapor cavity in the neighborhood of a solid boundary. J Fluid Mech 47:49–85
Morey MD, Deshpande NS, Barigou M (1999) Foam destabilization by mechanical and ultrasonic vibrations. J Colloid Interface Sci 219:90–98. https://doi.org/10.1006/jcis.1999.6451
Muthukumaran S, Kentish S, Lalchandani S, Ashokkumar M, Mawson R, Stevens GW, Grieser F (2005a) The optimisation of ultrasonic cleaning procedures for dairy fouled ultrafiltration membranes. Ultrason Sonochem 12:29–35. https://doi.org/10.1016/j.ultsonch.2004.05.007
Muthukumaran S, Kentish SE, Ashokkumar M, Stevens GW (2005b) Mechanisms for the ultrasonic enhancement of dairy whey ultrafiltration. J Memb Sci 258:106–114. https://doi.org/10.1016/j.memsci.2005.03.001
Nguyen NHA, Anema SG (2010) Effect of ultrasonication on the properties of skim milk used in the formation of acid gels. Innov Food Sci Emerg Technol 11:616–622. https://doi.org/10.1016/j.ifset.2010.05.006
Nowacka M, Tylewicz U, Laghi L, Dalla Rosa M, Witrowa-Rajchert D (2014) Effect of ultrasound treatment on the water state in kiwifruit during osmotic dehydration. Food Chem 144:18–25. https://doi.org/10.1016/j.foodchem.2013.05.129
Panchev I, Kirchev N, Kratchanov C (1988) Improving pectin technology. II. Extraction using ultrasonic treatment. Int J Food Sci Technol 23:337–341. https://doi.org/10.1111/j.1365-2621.1988.tb00587.x
Pankaj, Ashokkumar M (2011) Theoretical and experimental sonochemistry involving inorganic systems. Theor Exp Sonochem Involv Inorg Syst:1–404. https://doi.org/10.1007/978-90-481-3887-6
Parlitz U, Mettin R, Luther S, Akhatov I, Voss M, Lauterborn W (1999) Spatio-temporal dynamics of acoustic cavitation bubble clouds. Philos Trans R Soc A Math Phys Eng Sci 357:313–334. https://doi.org/10.1098/rsta.1999.0329
Patrick M, Blindt R, Janssen J (2004) The effect of ultrasonic intensity on the crystal structure of palm oil. Ultrason Sonochem 11:251–255. https://doi.org/10.1016/j.ultsonch.2004.01.017
Peters A, Sagar H, Lantermann U, el Moctar O (2015) Numerical modelling and prediction of cavitation erosion. Wear 338–339:189–201. https://doi.org/10.1016/j.wear.2015.06.009
Prithani R, Dash KK (2020) Mass transfer modelling in ultrasound assisted osmotic dehydration of kiwi fruit. Innov Food Sci Emerg Technol 64:102407. https://doi.org/10.1016/j.ifset.2020.102407
Rahaman A, Zeng XA, Kumari A, Rafiq M, Siddeeg A, Manzoor MF, Baloch Z, Ahmed Z (2019) Influence of ultrasound-assisted osmotic dehydration on texture, bioactive compounds and metabolites analysis of plum. Ultrason Sonochem 58. https://doi.org/10.1016/j.ultsonch.2019.104643
Raso J, Barbosa-Cánovas GV (2003) Nonthermal preservation of foods using combined processing techniques. Crit Rev Food Sci Nutr 43:265–285. https://doi.org/10.1080/10408690390826527
Rastogi NK (2011) Opportunities and challenges in application of ultrasound in food processing. Crit Rev Food Sci Nutr 51:705–722. https://doi.org/10.1080/10408391003770583
Raviyan P, Zhang Z, Feng H (2005) Ultrasonication for tomato pectinmethylesterase inactivation: effect of cavitation intensity and temperature on inactivation. J Food Eng 70:189–196. https://doi.org/10.1016/j.jfoodeng.2004.09.028
Riener J, Noci F, Cronin DA, Morgan DJ, Lyng JG (2009) The effect of thermosonication of milk on selected physicochemical and microstructural properties of yoghurt gels during fermentation. Food Chem 114:905–911. https://doi.org/10.1016/j.foodchem.2008.10.037
Riera E, Gallego-Juárez JA, Mason TJ (2006) Airborne ultrasound for the precipitation of smokes and powders and the destruction of foams. Ultrason Sonochem 13:107–116. https://doi.org/10.1016/j.ultsonch.2005.04.001
Rodrigues S, Fernandes FAN (2017) Extraction processes assisted by ultrasound. Elsevier Inc. https://doi.org/10.1016/B978-0-12-804581-7.00014-2
Rodríguez G, Riera E, Gallego-Juárez JA, Acosta VM, Pinto A, Martínez I, Blanco A (2010) Experimental study of defoaming by air-borne power ultrasonic technology. Phys Procedia 3:135–139. https://doi.org/10.1016/j.phpro.2010.01.019
Saikia S, Mahnot NK, Mahanta CL (2016) A comparative study on the effect of conventional thermal pasteurisation, microwave and ultrasound treatments on the antioxidant activity of five fruit juices. Food Sci Technol Int 22:288–301. https://doi.org/10.1177/1082013215596466
Sakakibara M, Wang D, Ikeda K, Suzuki K (1994) Effect of ultrasonic irradiation on production of fermented milk with Lactobacillus delbrueckii. Ultrason Sonochem 1:107–110. https://doi.org/10.1016/b0-12-227235-8/00243-1
Salleh-Mack SZ, Roberts JS (2007) Ultrasound pasteurization: the effects of temperature, soluble solids, organic acids and pH on the inactivation of Escherichia coli ATCC 25922. Ultrason Sonochem 14:323–329. https://doi.org/10.1016/j.ultsonch.2006.07.004
Sánchez ES, Simal S, Femenia A, Benedito J, Rosselló C (1999) Influence of ultrasound on mass transport during cheese brining. Eur Food Res Technol 209:215–219. https://doi.org/10.1007/s002170050483
Sato M, Takakuwa O, Nakai M, Niinomi M, Takeo F, Soyama H (2016) Using cavitation peening to improve the fatigue life of titanium alloy Ti-6Al-4V manufactured by electron beam melting. Mater Sci Appl 07:181–191. https://doi.org/10.4236/msa.2016.74018
Sfakianakis P, Topakas E, Tzia C (2015) Comparative study on high-intensity ultrasound and pressure milk homogenization: effect on the kinetics of yogurt fermentation process. Food Bioprocess Technol 8:548–557. https://doi.org/10.1007/s11947-014-1412-9
Silva M, Chandrapala J (2021) Ultrasound in dairy processing. In: Innovative food processing technologies a comprehensive review. Elsevier, pp 439–464. https://doi.org/10.1016/b978-0-08-100596-5.22955-7
Simal S, Benedito J, Sánchez ES, Rosselló C (1998) Use of ultrasound to increase mass transport rates during osmotic dehydration. J Food Eng 36:323–336. https://doi.org/10.1016/S0260-8774(98)00053-3
Singla M, Sit N (2021) Application of ultrasound in combination with other technologies in food processing : a review. Ultrason Sonochem 73:105506. https://doi.org/10.1016/j.ultsonch.2021.105506
Stojanovic J, Silva JL (2006) Influence of osmoconcentration, continuous high-frequency ultrasound and dehydration on properties and microstructure of rabbiteye blueberries. Dry Technol 24:165–171. https://doi.org/10.1080/07373930600558995
Sunartio D, Ashokkumar M, Grieser F (2007) Study of the coalescence of acoustic bubbles as a function of frequency, power, and water-soluble additives. J Am Chem Soc 129:6031–6036. https://doi.org/10.1021/ja068980w
Tan MC, Chin NL, Yusof YA, Taip FS, Abdullah J (2015) Characterisation of improved foam aeration and rheological properties of ultrasonically treated whey protein suspension. Int Dairy J 43:7–14. https://doi.org/10.1016/j.idairyj.2014.09.013
Tan MC, Chin NL, Yusof YA, Abdullah J (2016) Effect of high power ultrasonic treatment on whey protein foaming quality. Int J Food Sci Technol 51:617–624. https://doi.org/10.1111/ijfs.13013
Tang CH (2007) Functional properties and in vitro digestibility of buckwheat protein products: influence of processing. J Food Eng 82:568–576. https://doi.org/10.1016/j.jfoodeng.2007.01.029
Tao Y, Sun DW (2015) Enhancement of food processes by ultrasound: a review. Crit Rev Food Sci Nutr 55:570–594. https://doi.org/10.1080/10408398.2012.667849
Vercet A, Burgos J, Crelier S, Lopez-Buesa P (2001) Inactivation of proteases and lipases by ultrasound. Innov Food Sci Emerg Technol 2:139–150. https://doi.org/10.1016/S1466-8564(00)00037-0
Vercet A, Oria R, Marquina P, Crelier S, Lopez-Buesa P (2002) Rheological properties of yoghurt made with milk submitted to manothermosonication. J Agric Food Chem 50:6165–6171. https://doi.org/10.1021/jf0204654
Vilkhu K, Mawson R, Simons L, Bates D (2008) Applications and opportunities for ultrasound assisted extraction in the food industry – a review. Innov Food Sci Emerg Technol 9:161–169. https://doi.org/10.1016/j.ifset.2007.04.014
Villamiel M, De Jong P (2000) Influence of high-intensity ultrasound and heat treatment in continuous flow on fat, proteins, and native enzymes of milk. J Agric Food Chem 48:472–478. https://doi.org/10.1021/jf990181s
Wilde PJ, Clark DC (1993) The competitive displacement of β-lactoglobulin by tween 20 from oil-water and air-water interfaces. J Colloid Interface Sci 155:48–54. https://doi.org/10.1006/jcis.1993.1008
Wordon BA, Mortimer B, McMaster LD (2012) Comparative real-time analysis of Saccharomyces cerevisiae cell viability, injury and death induced by ultrasound (20 kHz) and heat for the application of hurdle technology. Food Res Int 47:134–139. https://doi.org/10.1016/j.foodres.2011.04.038
Wu J (2018) Acoustic streaming and its applications. Fluids 3. https://doi.org/10.3390/fluids3040108
Yang RF, Geng LL, Lu HQ, Fan XD (2017) Ultrasound-synergized electrostatic field extraction of total flavonoids from Hemerocallis citrina baroni. Ultrason Sonochem 34:571–579. https://doi.org/10.1016/j.ultsonch.2016.06.037
Yasui K (1997) Alternative model of single bubble sonoluminescence. Phys Rev E 56:6750–6760. https://doi.org/10.1103/PhysRevA.65.054304
Yasui K (1998) Effect of non-equilibrium evaporation and condensation on bubble dynamics near the sonoluminescence threshold. Ultrasonics 36:575–580. https://doi.org/10.1016/S0041-624X(97)00107-8
Yasui K (2002) Influence of ultrasonic frequency on multibubble sonoluminescence. J Acoust Soc Am 112:1405–1413. https://doi.org/10.1121/1.1502898
Yasui K (2011) Fundamentals of acoustic cavitation and sonochemistry. In: Theoretical and experimental sonochemistry involving inorganic systems. National Institute of Advanced Industrial Science and Technology, Anagahora-Japan, pp 1–29. https://doi.org/10.1007/978-90-481-3887-6
Yasui K (2017) Acoustic cavitation and bubble dynamics. Springer Briefs in Molecular Science, Nagoya
Yasui K (2018) Acoustic cavitation. In: Acoustic cavitation and bubble dynamics, pp 1–35, Springer, Cham, Switzerland
Yasui K, Tuziuti T, Kozuka T, Towata A, Iida Y (2007) Relationship between the bubble temperature and main oxidant created inside an air bubble under ultrasound. J Chem Phys 127:1–9. https://doi.org/10.1063/1.2790420
Ye L, Zhu X (2017) Analysis of the effect of impact of near-wall acoustic bubble collapse micro-jet on Al 1060. Ultrason Sonochem 36:507–516. https://doi.org/10.1016/j.ultsonch.2016.12.030
Yuan H, Prosperetti A (1997) Gas-liquid heat transfer in a bubble collapsing near a wall. Phys Fluids 9:127–142. https://doi.org/10.1063/1.869153
Yusof NSM, Babgi B, Alghamdi Y, Aksu M, Madhavan J, Ashokkumar M (2016) Physical and chemical effects of acoustic cavitation in selected ultrasonic cleaning applications. Ultrason Sonochem 29:568–576. https://doi.org/10.1016/j.ultsonch.2015.06.013
Zheng L, Sun DW (2006) Innovative applications of power ultrasound during food freezing processes – a review. Trends Food Sci Technol 17:16–23. https://doi.org/10.1016/j.tifs.2005.08.010
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kerboua, K., Mazouz, D., Hasaounia, I., Hamdaoui, O. (2022). Mechanical Technologies: Ultrasound and Cavitation in Food Processing. In: Režek Jambrak, A. (eds) Nonthermal Processing in Agri-Food-Bio Sciences. Food Engineering Series. Springer, Cham. https://doi.org/10.1007/978-3-030-92415-7_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-92415-7_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92414-0
Online ISBN: 978-3-030-92415-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)