Abstract
In recent years, power ultrasound (PU) has attracted considerable interest in food science and technology due to its promising applications in food processing and preservation. It is well known to have significant effects on the rate of various processes in the food industry, and it is recognized as innovative technology for achieving the objective of sustainable “green” chemistry and industrialization. Using ultrasound, food processes can be completed in seconds or minutes with higher reproducibility, lower processing costs, easier operation, higher product purity, elimination of post-treatment of waste water, and lower energy requirements than traditional processes. Several processes such as extraction, degradation, sterilization, and enzyme modification have been applied efficiently in the food industry. Food processes performed under ultrasound treatment will be affected by cavitation phenomena and mass transfer processes. This chapter presents the current knowledge on the applications of ultrasound in food processing.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Sound waves with a frequency that exceeds the upper limit of human hearing (~20 kHz) are classified as ultrasound, and they can be divided into different frequency ranges. Until recently, most applications of ultrasound in food technology involved a nondestructive analysis that is particularly useful for quality assessments; such applications use high-frequency ultrasound (100 kHz–1 MHz) with low power (typically <1 W cm−2). On the other hand, the power levels used in low frequency (20–100 kHz) applications are so high (typically in the range of 10–1000 W cm−2) that they have ability to change the physical or chemical properties of food (McClements 1995). This kind of ultrasound is usually referred to as power ultrasound (PU).
In recent years, PU has attracted considerable interest in food science and technology owing to its promising effects for food processing and preservation. It is recognized as an innovate technology for achieving sustainable “green” chemistry and industrialization (Chemat et al. 2017). As an innovative food technology, PU can be applied to develop gentle but targeted processes to improve the quality and safety of processed foods, and to offer the potential to improve existing processes, as well as develop new processing options (Knorr et al. 2004).
7.2 Principles of Power Ultrasound in Food Processing
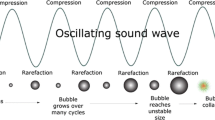
The effects of ultrasound on liquid systems are mainly related to the phenomenon of cavitation (Fig. 7.1). Ultrasound is propagated via a series of compression and rarefaction waves induced on the molecules of the medium it passes through (Mason et al. 2005). At a sufficiently high power, the rarefaction cycle may exceed the attractive forces of the liquid molecules and cavitation bubbles from gas nuclei will emerge within the liquid. These bubbles, distributed throughout the liquid, grow over the period of a few cycles to a critical size until they become unstable and violently collapse (Barbosa-Canovas and Rodriguez 2002). For food processing purposes, it is important to address the generation of heat due to ultrasound and the related implosion of cavitation bubbles that can cause rapid changes in temperature, up to 5500 °C, and pressure, up to 50 Mpa, which produces extremely high-shear energy waves and turbulence in the cavitation zone. These combined effects have a variety of applications (Knorr et al. 2004). The extent of cavitation is determined by many factors, including ultrasound intensity (W cm−2), medium viscosity, surface tension, vapor pressure, nature and concentration of dissolved gases, the presence of solid particles, temperature, and pressure of the treatment (Soria and Villamiel 2010). When liquid processing is designed for large-scale systems, the ultrasonic density (W cm−3) should be considered (Patist and Bates 2008).
Another phenomenon, resulting from the variations in bubble size and subsequent collapse, is the development of strong microstreaming currents, associated with high-velocity gradients and shear stresses that alter the characteristics of the media. Moreover, part of the acoustic energy can be absorbed as heat. However, depending on the operating conditions and substrates, the temperatures are usually lower than 70 °C (Villamiel et al. 2000). Another important effect is that water molecules can be converted into highly reactive-free radicals (H2O → H + OH) that can react with other molecules (Riesz and Kondo 1992). These mechanisms may induce physical and chemical effects with potential applications in the food industry.
7.3 Applications of Power Ultrasound in Food Processing
Power ultrasound has been used as an alternative to conventional food processing operations for sonocrystallization, emulsification, defoaming, modification of the functional properties of food proteins, inactivation or enhancement of enzymatic activity for improvement of shelf life and quality, microbial inactivation, freezing, thawing, freeze drying and concentration, and drying, as well as facilitating the extraction of various foods and bioactive components (Awad et al. 2012).
7.3.1 Ultrasound-Assisted Extraction (UAE)
Ultrasound-assisted extraction (UAE) is an emerging technology that can facilitate the release of extractable compounds and enhance mass transport by disrupting plant cell walls and other cells more easily. UAE is a “green” method that avoids the use of large quantities of organic solvents and reduces the working time. Several food components such as aromas, pigments, antioxidants, and other organic and mineral compounds have been extracted efficiently from a variety of matrices (mainly animal tissues, and plant materials) (Vilkhu et al. 2008). The UAE process can be completed in a few minutes with high reproducibility, and it reduces the consumption of solvent, simplifies manipulation, and produces higher purity products compared with conventional extraction techniques such as Soxhlet extraction, maceration, and Clevenger distillation.
7.3.1.1 Mechanisms of UAE
During the UAE process, the cavitation phenomena cause high-shear forces in the media. The implosion of cavitation bubbles on the product’s surface results in micro-jetting, which can generate effects such as surface peeling, erosion, and particle breakdown. Additionally, implosion of cavitation bubbles within a liquid medium leads to macro-turbulences and micro-mixing. To understand the effects of ultrasound on a vegetal matrix during UAE, experimental results show that there are several different combined mechanisms that must be understood, such as fragmentation, erosion, capillarity, detexturation, and sonoporation.
During the application of ultrasound to a liquid media containing raw materials, rapid fragmentation of the raw materials is observed. The impact of fragmentation induced by ultrasound was illustrated throughout the example of chlorophyll extraction from spinach leaves (Chemat et al. 2017). The effects were examined using an ultrasound probe (20 kHz, UIP1000 HdT, Hielscher). During the processing, the quick fragmentation of the spinach leaves happened in the first minutes of sonication, whereas the leaves did not change during conventional extraction performed by maceration. Comparing the extraction rates of chlorophyll from UAE and maceration, a linear increase was observed at the beginning of UAE, corresponding to the direct solubilization of chlorophylls. The effects were most probably due to the reductions in particle size during the application of ultrasound. Fragmentation of friable solids caused from ultrasonic cavitation has been identified by several authors (Suslick and Price 1999). The fragmentation process was resulted from interparticle collisions, and shockwaves through collapsing cavitation bubbles in the liquid. A direct consequence of the reductions in particle size was the increase in the surface area of the solid, resulting in greater mass transfer and higher extraction rates and yields.
Some studies have already noticed the erosion of raw plant materials when treated by ultrasound. For example, the UAE of Boldo leaves was studied by Petigny et al. (2013) using an ultrasonic probe (20 kHz). The results showed that the extraction yields were enhanced by 25% with UAE treatments. From scanning electron microscope (SEM) observations on the leaf surfaces before and after UAE treatments, the results showed that leaves were not fragmented, but localized effects were observed. Boldo leaves possess trichomes on the surface of leaves, which seemed to be specifically impacted by ultrasound. This erosion enhanced the ability of water to access the leaf for further extraction. Another possible mechanism for extraction enhancement could be the implosion of cavitation bubbles on the leaf surface to induce the erosion of plant structures, and to speed up the release of target components from the extraction medium. The erosion could be used for several purposes such as cleaning or sonochemical reactions with metals (Chemat et al. 2017).
Ultrasonic capillary effect (UCE) refers to an increase in the depth and velocity by which a liquid can penetrate into canals and pores under sonication (Mason 2015). These ultrasounds induced effects were experimentally demonstrated with molten aluminum by Tzanakis et al. (2015). Although the mechanism of UCE is not fully understood, a relationship between cavitation and UCE has been established. UCE could also be one of the mechanisms that increases extraction yields. In the study by Pingret et al. (2012), the recovery of total polyphenols from apple pomaces was performed using ultrasound and the extraction kinetics improved under sonication. The impact of UCE on extraction was proposed by Vinatoru (2001). The author identified that the swelling index of several vegetable matrixes increased from 5% to 10% when using ultrasound. By increasing the swelling and rehydration of vegetable tissue, ultrasound impacts positively on the extraction, desorption, and diffusion of a solute out of the vegetable structure. UCE can directly improve the mass transfer process. The improvement in the water holding capacity of the matrix under ultrasound applied to meat brining or curing has also been investigated by several authors (Carcel et al. 2007; McDonnell et al. 2014; Ozuna et al. 2013; Siro et al. 2009; Stadnik et al. 2008). They reported the modification of meat structures after ultrasound treatment as well as an enhancement of salt and moisture transfer processing. The ultrasonic intensity is considered to be an important factor with great influence on the results (e.g., between 40 and 50 W cm−2 is optimal for the ultrasound-assisted brining of pork loins) (Carcel et al. 2007).
The sonoporation effect of ultrasound is well known in the field of biology, and it is applied when permeability of cell membranes is desired. Sonoporation has been used in vitro for uptake of molecules by cells, e.g., drugs, genes (reversible sonoporation), or for cell destruction (irreversible sonoporation). For these cases, high ultrasound frequencies greater than 500 kHz were applied (Karshafian et al. 2009). However, a few studies have focused on the use of low frequencies (20 kHz) for cell wall permeabilization (Miller et al. 2002), or bacteria inactivation (Ugarte-Romero et al. 2006). In the field of extractions, sonoporation can be used to create reversible or irreversible cell membrane pores, which result in the release of the cellular contents into the extractive medium. The study performed by Meullemiestre et al. (2016) on the processing of wet yeast (Yarrowia lipolityca) for the recovery of oil in yeast cells, used ultrasound at 20 kHz. The ultrasound-treated yeasts exhibited a high impact on the surface and perforations of the visible membranes.
In an ultrasound-treated solid–liquid mixture, shear forces were generated within the liquid and at the vicinity of the solid materials, resulting from the evolution (oscillation and collapse) of cavitation bubbles within the fluid. The streaming and acoustic microstreaming effects are of interest for mixing and emulsification applications (Veillet et al. 2010). In the case of leaves, the oil glands would explode after ultrasound irradiation. It could be hypothesized that the shear forces generated by the collapse of cavitation bubbles close to the oil glands, is what caused them rupture. Another hypothesis may be that there was a pressure build-up within the glands, or cavitation inside of the glands (Vinatoru et al. 1997).
Ultrasound extraction causes the destruction or detexturation of plant structures, which has been observed when obtaining essential oil from caraway seeds (Chemat et al. 2004). The total oil yields of conventional extraction (reflux extraction with hexane) and UAE (ultrasound probe, 20 kHz) were similar; however, a higher selectivity towards terpenes was noted for UAE. Distinguishable physical changes to the caraways seeds were noticed following the extraction process. After ultrasound, the gradual degradation of cell walls was observed: after 30 min, the cell walls were affected by varying degrees; by 60 min, the cell structures were totally broken and converted to undefined shapes. It can be assumed that this cell disruption improves the accessibility of the solvents (Chemat et al. 2004).
In summary, several mechanisms of UAE have been proposed, such as fragmentation, erosion, sonocapillary effect, sonoporation, local shear stress, destruction, and detexturation of plant structures. However, during UAE treatments, a combination of effects is likely to occur. These effects are sequential during the extraction processes. The intense mixing effects generated by the propagation of ultrasound through a liquid medium can enhance the mass transfer rates. The mixing effects at a macroscopic scale are due to acoustic streaming, especially microstreaming occurring at a local level. The combined mixing and physical effects of ultrasound on the raw materials may have applications that can enhance the performance of UAE.
Ultrasound can facilitate extraction from dried materials using a two-stage process: (i) vegetal materials are steeped in solvents to facilitate the swelling and hydration processes; (ii) diffusion and osmotic processes drive the mass transfer of soluble constituents from the materials into the solvents. Toma et al. (2001) monitored the effects of ultrasound on crushed fennel seeds, hop strobiles, pot marigold, peganum seeds, mint leaves, lime flowers, and elecampane root in terms of the swelling index (SI) and extractive value (EV) in the ultrasonic bath (33 kHz), as measured using a microscope. The data shows that all of the sonicated tissues absorbed extra water, and the EV of many species increased with a short sonication time. This indicates that low frequency indirect sonication has a significant influence on the swelling process of dried plants. Microscopic examination of vegetal materials revealed that sonication can significantly affect the structures of vegetal tissues. Xu et al. (2014) investigated the effects of ultrasound on the yield and swelling behavior of pectin extraction from grapefruit peel (Citrus paradisi Macf. cv. Changshanhuyou). The results showed that the SI presented similar trends to the pectin yields. The results of these studies provide strong evidence to support that a mechanism of UAE is a hydration process, which occurs simultaneously with vegetal material fragmentation. Petrovic et al. (2012) analyzed the kinetics and mass transfer phenomena for different extraction processes from thyme leaves (Thymus vulgaris L.) using ethanol. These processes included Soxhlet extraction and ultrasound-assisted batch extraction at the laboratory scale, as well as a pilot plant batch extraction with mixing. The results show that ultrasound contributed most to the increase of mass transfer rates, increasing the rate by ten times compared to Soxhlet extraction during the period of slow extraction.
7.3.1.2 Influencing Parameters of UAE
The sonochemical effects of ultrasound in a liquid are attributed to acoustic cavitation phenomena. As ultrasound is a mechanical wave, its characteristics such as frequency, wavelength, and amplitude can affect the acoustic cavitation and extraction. In addition to the input power, the reactor design and probe shape can influence the process (Chemat et al. 2017).
-
Frequency
Ultrasound frequency can affect the cavitation effect. The most commonly used frequencies in UAE processing are between 20 and 100 kHz. Toma et al. (2001) noticed a reduction in the physical impact on the structure of marigold petals when applying higher frequencies (500 kHz) compared to 20 kHz. Chukwumah et al. (2009) reported selective extraction of some phenolics from peanuts at frequencies of 25 kHz (higher extraction of daidzein and genistein) and 80 kHz (biochanin A and trans-resveratrol). However, longer extraction durations were required at 80 kHz. González-Centeno et al. (2014) evaluated three frequencies (40, 80, and 120 kHz) for the extraction of phenolics from grape pomace. Using the surface response methodology to study the influencing parameters, the authors highlighted that 40 kHz was the most effective.
As the ultrasound frequency increases, the production and intensity of cavitation in the liquid decreases. At higher frequencies, acoustic cavitation is more difficult to induce since the cavitation bubbles need a delay to be initiated during the rarefaction cycle. The cycles of compression and rarefaction might be too short to allow the growth of the cavitation bubbles. The length of the rarefaction phase (for the growth of cavitation bubbles) is inversely proportional to the ultrasonic frequency. Therefore, larger amplitudes and intensities are required to generate cavitation at high frequencies (Chemat et al. 2017).
At lower frequencies, there are fewer transient cavitation bubbles, but they have larger diameters that show the physical effects (Leong et al. 2011; Mason et al. 2011). The effects of frequency may be linked not only to the cavitation bubble size, but also to its influence on the resistance to mass transfer (Esclapez et al. 2011).
-
Intensity
The measurement of the actual applied acoustic power in a sonochemical process has not been reported in details, although some physical methods can directly or indirectly measure the applied energy. These methods estimate the transferred energy by measuring either chemical or physical changes in the medium when ultrasound is applied. The most common physical methods are the measurement of acoustic pressure using hydrophones or optical microscopes, the aluminum foil method, and the calorimetric method (Chivate and Pandit 1995; Margulis and Margulis 2003). Among the chemical methods, the indirect measurement of ·OH radicals formed by sonoluminescence, or chemical dosimeters have been used (Suslick et al. 2011). As an example, to calculate the power by calorimetry, the actual input power from the device is converted to heat, which is dissipated in the medium. The effective ultrasound power (P) is calculated according to Eq. 7.1 (Contamine et al. 1995; Toma et al. 2011).
where Cp is the heat capacity of the solvent at constant pressure (J g−1 C−1), m is the mass of solvent (g), and dT/dt is the temperature rise per second.
The ultrasound intensity (UI) is expressed as the energy transmitted per second and per square meter of emitting surface (Tiwari 2015). This parameter is directly correlated to the amplitude of the transducer, and consequently to the pressure amplitude of the sound wave. As the pressure increases, the collapse of the bubbles will be more violent. To achieve the cavitation threshold, a minimum value of ultrasound intensity is required. Regarding extraction, UI is a relevant input value that strongly affects extraction efficiency. UI is calculated using the calculated power delivered to the media, as shown in Eq. 7.2 (Tiwari 2015).
where UI is the ultrasonic intensity (W cm−2), P is the ultrasound power (W) as calculated by Eq. (7.1), and S is the emitting surface of the transducer.
In some studies, UI is calculated according to Eq. 7.3 It better demonstrates that the ultrasound power transmitted into the processed system is related to volume
where UI is the ultrasonic intensity (W mL−1), P is the ultrasound power (W) as calculated by Eq. (7.1), and V is the volume of liquid in the system (mL).
An increase of UI generally results in an increase of sonochemical effects. However, it is worth noting that a high UI can lead to rapid deterioration of the ultrasonic transducer, resulting in liquid agitation instead of cavitation, and in poor transmission of the ultrasound through the liquid media. However, the amplitude should be increased when working with high viscosity liquids such as oils (Chemat et al. 2017).
The effects of UI were evaluated at 16.4, 20.9, and 47.6 W cm−2 at 20 kHz for soybean oil extraction by Li et al. (2004). The study showed increases in yields up to 20.9 W cm−2, after which no further increases were observed. A similar tendency was noted by Wang et al. (2015), whose study on the UAE of pectin at 20 kHz indicated that the UI (varied between 10.18 and 14.26 W cm−2) should be subjected to an optimization, since the highest value of UI would not lead to the highest yields.
Xu et al. (2014) investigated effects of ultrasound and/or heating on the swelling behavior of the material, and the yield and kinetics of pectin extracted from grapefruit (Citrus paradisi Macf. cv. Changshanhuyou) peel. For ultrasound-assisted heating extraction (UAHE), the collapse of cavitation bubbles became more violent as the amplitude or power increased, since the resonant bubble size was proportional to the amplitude of the ultrasonic wave (Brotchie et al. 2009; Merouani et al. 2013). However, when the power density became higher than 0.40 W mL−1, a significant decrease in pectin yields occurred (p < 0.05). This was probably because cavitation was reduced at high bubble volume concentrations. A cloud of cavitation bubbles produced around the probe tip could screen and reduce the energy transmission into the reaction medium; this is called the “saturation effect” (Contamine et al. 1995). An increase in interbubble impacts would increase the probability of the bubbles deformation collapse in a nonspherical manner, thus decreasing the energy efficiency of the collapse. Ultrasonic degradation of the extracted pectin would also be responsible for yield decrease because the degradation effect on pectin increases with rising ultrasound intensity (Zhang et al. 2013a).
-
Medium Parameters
Solvent choice in UAE is driven by the solubility of the target metabolites but also by physical parameters such as the viscosity, surface tension, and vapor pressure of the solvent. These physical parameters will affect the acoustic cavitation phenomenon, and specifically, the cavitation threshold. The initiation of cavitation in a liquid requires negative pressure during the rarefaction cycle to overcome the cohesive forces between the molecules composing the liquid. The amplitude of the ultrasound should be increased when working with samples of high viscosity, because as the viscosity of the sample increases, the resistance of the sample to the movement of the ultrasonic device also increases. Therefore, a high intensity (or high amplitude) is advised in order to obtain the necessary mechanical vibrations to develop cavitation. A solvent with low vapor pressure is preferred for UAE, as the collapse of the cavitation bubble is more intense compared to solvents with high vapor pressure (Flannigan and Suslick 2010). Moreover, vapor pressure depends on the temperature of the liquid medium.
Temperature has a strong effect on the efficiency of extractions. In UAE, some authors have reported a beneficial effect of a temperature increase from 20 to 70 °C (Shirsath et al. 2012). This effect has been justified by an increase in the number of cavitation bubbles and a larger solid-solvent contact area, as well as by the enhancement of solvent diffusivity with consequent enhancement of desorption and solubility of the target compounds. However, this effect decreases when the temperature is near the solvent’s boiling point. Thus, some authors have reported the beneficial effects of low temperatures (below 30 °C) (Esclapez et al. 2011; Palma and Barroso 2002; Zhang et al. 2008). It is important to optimize extraction temperatures to obtain the highest yield of the target compounds without degradation, because this parameter can vary depending on the type of product.
The cavitation bubbles are formed from gas (vapors) dissolved in the liquid (Chemat et al. 2017). Gases dissolved in the solvents would act as nuclei for new cavitation bubbles (Leong et al. 2011). When external pressure is increased, a greater acoustic pressure is required to induce cavitation; however, once the cavitation threshold is reached under external pressure (>1 atm), a higher intensity of cavitation bubble collapse is attained than at normal pressure, hence increasing the cavitation effect (Leong et al. 2011).
The plant matrix that can be used could either be fresh (e.g., algae, yeast) or dry (e.g., herbs, oleaginous seeds). Since the extractive systems are heterogeneous complex porous media, the size of the cavitation bubbles has an effect on the efficiency of the extractions. The extraction yields may also vary due to the plant material’s structure, plasticity, or compositional differences that result in different impacts from cavitation effects (Chemat et al. 2017).
-
Shape and Size of Ultrasonic Reactors
Since ultrasound waves are reflected by solid surfaces, the shape of the reaction vessel is critical. The best choice would be a vessel with a flat bottom, such as a conical flask, in order to attain minimal reflected waves (Chemat et al. 2017). The thickness of the vessel should also be kept to the minimum to reduce attenuation. It is necessary to calculate the optimum reactor dimensions and the position of emitter in relation to the transducer to maximize the energy transferred to the medium (Sun et al. 2010). Further advances have been made by accounting for the lack of homogeneity of the pressure field in the reactor in order to optimize the process efficiency (Esclapez et al. 2010, 2011).
7.3.1.3 Applications of the UAE Process
Hammi et al. (2015) demonstrated the efficacy and absence of degradation for the UAE of antioxidants from Zyzyphus lotus fruit. The use of ultrasound was also proved to be a promising technology for the extraction on carotenoids from tomato byproducts (skin, seeds, and part of the pulp) (Luengo et al. 2014). Ultrasound significantly increased the extraction yield, 143% compared with conventional extraction, and did not cause any degradation of the carotenoids. Sivakumar et al. (2011) reported significant improvement (13%–100%) in the extraction yield of natural colors obtained from different plant materials. Kimbaris et al. (2006) demonstrated that the use of ultrasound for essential oil extraction from garlic reduced the degradation of thermal-sensitive molecules, compared with hydro-distillation.
Wang et al. (2016) compared the properties of pectin from grapefruit (Citrus paradisi Macf. cv. Changshanhuyou) peel extracted by ultrasound-assisted extraction (UP), and by the conventional heating extraction (CP). Results showed that UAE caused more severe degradation than heating extraction, and the degree of acetylation of UP was slightly higher than that of CP. UAE did give rise to changes to the physico-chemical properties of UP compared with CP. The results proved that ultrasound irradiation has more significant degradation effects on the main chains, and less on the side chains, of pectin compared with thermal and acidic degradation. The UAE process with a shorter extraction time and lower temperature was supposed to protect pectin side chains from further degradation.
7.3.2 Ultrasound Degradation
7.3.2.1 Applications of Ultrasound Degradation
The physico-chemical properties of polysaccharides treated with ultrasound have been extensively investigated (Ebringerova and Hromadkova 1997; Koda et al. 2011; Wang et al. 2010). As supported by Baxter et al. (2005), the sonication of chitosan led to a decrease in the intrinsic viscosity while maintaining the acetylation degree. In a study by Cheng et al. (2010), the sonication of starch resulted in a drastic decrease in viscosity and a distinct increase in solubility. Huang et al. (2007) found that there was almost no change in the crystalline structure of ultrasound-treated corn starch granules, while the amorphous area was slightly destroyed and pores or channels were detected.
Zhang et al. (2013a) investigated the effects of ultrasound on the molecular weight and structures of the apple pectin. The results indicate that the average molecular weight of apple pectin decreased significantly after ultrasound treatment and the molecular weight of degraded products had a uniform and narrow distribution. The ultrasound intensity and temperature play an important role in the degradation reaction. A degradation kinetics model of apple pectin fitted to the second-order kinetics model from 5 to 45 °C. The degree of methylation of the apple pectin reduced when ultrasound was applied. Ultrasound treatment could not alter the primary structure of apple pectin according to the results determined by high-performance liquid chromatography (HPLC), IR, and nuclear magnetic resonance (NMR). Meanwhile, the viscosity of untreated apple pectin was 103 times larger than the ultrasound-treated apple pectin, which showed predominantly viscous responses (Gʹ < Gʺ) over the same frequency range. These results suggest that ultrasound can provide a viable alternative method for the modification of pectin (Zhang et al. 2013a, b, 2015).
González-Centeno (2014) studied the effects of ultrasound on the molecular weight, structure, and antioxidant potential of a fucoidan found in Isostichopus badionotus. The antioxidant activity assay showed that the antioxidant activity of ultrasound-treated fucoindan was slightly improved. Therefore, ultrasound is considered to have a unique effect on the degradation and modification of polysaccharides by decreasing the molecular weight while improving their bioactivities (Zhou et al. 2008).
7.3.2.2 Combined Technologies
Although recently reported studies have shown that sonochemical degradation of various polymers and organic pollutants can be achieved, sonolysis has drawbacks including energy consumption and a longer reaction time. Further, they are still not used at an industrial scale owing to economic viability (Rayaroth et al. 2016). In this case, ultrasound is often used in conjunction with conventional techniques, such as biological, UV, Fenton, ozonation, and electrochemical methods, in order to increase the effectiveness and reduce chemical or energy usage.
Yue et al. (2008) investigated the effects of ozone in combination with ultrasonic and UV irradiation on the degradation of chitosan, and reported that the use of ozone in 35, 50, and (65 ± 5) mg min−1 doses, and ultrasound treatment at 550 W and 40 kHz resulted in the reduction of the viscosity-average molecular weight by 31.18%, 37.03%, 41.38%, and 12.74%, respectively. The combined operation resulted in a significant reduction by 90.25%, 95.52%, and 96.82%, respectively.
Prajapat and Gogate (2015) studied the degradation of guar gum using ultrasound, UV, and ozone. The use of UV + H2O2 (0.1% loading) resulted in approximately 99.1% degradation in 20 min. Whereas, UV + KPS (0.1% loading), ultrasound + O3 (100 mg h−1), and UV + O3 (100 mg h−1) resulted in the reduction of intrinsic viscosity by approximately 98.3%, 99.1%, and 98.3% in 90, 30, and 35 min, respectively. In scaled up studies (7 L capacity), a maximum degradation of 98.2% was achieved in 150 min using an ultrasound bath + UV + H2O2 (0.03% loading). The structure of the treated guar gum was also characterized by Fourier transform infrared spectroscopy (FTIR) and it was established there were no significant changes to the chemical structure as a result of the treatment.
Zhi et al. (2017) applied a combination of ultrasound and a Fenton system (US-Fenton) to pectin degradation. This combination significantly accelerated the degradation process and also greatly improved the degradation efficiency, as demonstrated by the appearance of much smaller (5.2 kDa) products within 60 min. The ultrasound accelerated Fenton process degrades pectin by functioning as a catalyst for the generation of free radicals. The RG-I domain, the most elective portion of natural pectin, was well preserved and highly enriched.
7.3.3 Ultrasound Sterilization
Ultrasound alone is not very effective at killing bacteria in food (Piyasena et al. 2003); however, the use of ultrasound coupled with pressure and/or heat is promising. Thermosonic (heat plus sonication), manosonic (pressure plus sonication), and manothermosonic (heat and pressure plus sonication) treatments are some of the proposed methods, as they are more energy-efficient in the reduction of microbial and enzymatic activity in comparison to conventional heat treatment (Demirdoeven and Baysal 2009; Piyasena et al. 2003).
7.3.3.1 Mechanisms of Microorganism Inactivation
A frequency of 20 kHz has been used, which is available commercially, and this has proved quite satisfactory in microbial inactivation. The variable parameters are temperature, treatment time, and acoustic power (Demirdoeven and Baysal 2009), while the effects of ultrasound in liquid media depends on many variables, such as the characteristics of the treatment medium (viscosity, surface tension, vapor pressure, nature and concentration of dissolved gases, and the presence of solid particles), treatment parameters (pressure, and temperature), ultrasound generator performance (frequency, and power input), and size and geometry of the treatment vessel (Berlan and Mason 1992). The resistance spores, Gram-positive, and coccal cells to ultrasound treatment is higher than for vegetative cells, Gram-negative, and rod-shaped bacteria (Feng et al. 2008). It has also been shown that the mortality rate varies among different strains, for example, Escherichia coli and Saccharomyces cerevisiae, were reduced by more than 99% after ultrasonication, whereas Lactobacillus acidophilus was reduced by 72% and 84% depending on the media used (Cameron et al. 2008).
When ultrasound is applied to a liquid or slurry, it achieves both physical and chemical effects. This occurs through the formation and collapse (cavitation) of high-energy micro-bubbles (Krefting et al. 2004; Leighton 2007; Maisonhaute et al. 2002). Research has been conducted to understand the mechanism played by ultrasound on the disruption of microorganisms, which has been explained by acoustic cavitation and its physical, mechanical, and chemical effects that inactivate bacteria, and de-agglomerate bacterial clusters or flocs (Joyce et al. 2003). The cause of microbial death is mainly thinning of cell membranes, localized heating, and the production of free radicals (Butz and Tauscher 2002).
-
Physical Effects of Ultrasound
During the ultrasound process, longitudinal waves are created where a sonic wave meets a liquid medium, thereby creating regions of alternating compression and expansion (Sala Trepat 1995). These regions of pressure change cause cavitation to occur, and gas bubbles are formed in the medium. At the point where cavitation occurs the condensed molecules collide violently, creating shock waves (Piyasena et al. 2003) which create regions of very high temperature and pressure in very short periods of time; the order of temperature variation is 109 °C s−1 (Demirdoeven and Baysal 2009). The high-shear energy wave can travel at 570 km h−1 at the surface of solid boundaries. The hot zones can kill some bacteria, but they are localized and do not affect large enough areas (Piyasena et al. 2003).
-
Chemical Effects of Ultrasound
The lethal effect of ultrasound on some microorganisms was first demonstrated by Kaloyereas (1955); thus, ultrasound has been proposed as a means of sterilization for liquid food (Pagan et al. 1999a). Most applications will use a combination of ultrasound and other preservation methods (Raso et al. 1998a, b).
Ultrasound is known to disrupt biological structures, and potentially cause cell death when applied with sufficient intensity and these bactericidal are attributed to intracellular cavitation. That is, micromechanical shocks disrupt structural and functional components of the cells up to the point of cell lysis (Hughes and Nyborg 1962; Lopez-Malo et al. 2005; Williams et al. 1970).
Li et al. (2016) investigated the ultrasound-induced damage to Escherichia coli and Staphylococcus aureus. The results show that the damage was independent of the initial bacterial concentrations, while the mechanism of cellular damage differed according to the bacterial species. For the Gram-negative bacterium E. coli, ultrasound worked first on the outer membrane rather than the cytoplasmic membrane.
7.3.3.2 Combined Technologies
Research activities now focus on the combination of ultrasound with other preservation processes (e.g., heat and mild pressure) which appear to have the greatest potential for industrial applications (Butz and Tauscher 2002), such as reduction in processing times and increased efficiency at the industrial level.
-
Thermosonication
The combination of ultrasound and heat treatment is able to reduce the operating requirements (e.g., temperature levels and processing times) while achieving a microbial inactivation similar to conventional heat treatments (Villamiel et al. 1999). Reduction of the temperature and processing time should result in improved food quality. These combined treatments have been reported to lower the maximum processing temperatures by 25%–50% (Demirdoeven and Baysal 2009).
The heat resistance of Bacillus cereus, Bacillus licheniformis, and thermoduric streptococci decreased following ultrasonication treatment at 20 kHz (Betts et al. 1999; Burgos et al. 1972; Garcia-Graells et al. 1998; Piyasena et al. 2003). The food properties, such as shelf life and surface color in orange juice, could be improved (Zenker et al. 2003), while organoleptic characteristics could also be enhanced (Lopez-Malo et al. 2005). The effects on microbial destruction, of the combined treatment in a continuous process, were demonstrated by the comparison of the integrated time-temperature intensity (F value) of each treatment (Demirdoeven and Baysal 2009). The continuous (24 kHz, 400 W, 120 μm) and pulsing ultrasound treatments at 60 °C over 10 min were applied to different juices (i.e., pineapple, cranberry, and grape) (Bermudez-Aguirre and Barbosa-Canovas 2012).
During thermosonication the heat contributes to the mechanical disruption of cells, making them more susceptible to cavitation (Chandrapala et al. 2012); however, some studies (Lopez-Malo et al. 1999; Raso et al. 1998b) have shown that the effectiveness of the cavitation phenomena could decrease with increasing temperatures. At high temperatures, vapor pressure is higher and the viscosity is lower, reducing the energy release during the bubble implosion (Guerrero et al. 2001). Herceg et al. (2012) applied ultrasound at different temperatures (20 and 60 °C), and reported that the maximum inactivations of Staphylococcus aureus were achieved when milk was treated in a thermosonication process at 20 kHz, 600 W, 120 μm, and 60 °C for 12 min. Herceg et al. (2012) also reported that the antagonistic effect of temperature was not detected due to the presence of solid elements in the milk suspensions, which increased the cavitation phenomena. Wordon et al. (2012) reported that the synergistic effects between ultrasound and heat could also be linked to the ability of ultrasound to produce nonlethal intracellular injuries, resulting in more vulnerable cells and increasing their disruption rates from the heat treatment. Li et al. (2017) investigated the combined effects of ultrasound and mild heat on the viability of S. aureus in association with cell membrane integrity, and intracellular enzyme activity. The antibacterial value of thermosonication was greater than the sum of the individual treatments; when treating suspensions containing solid particles the enhancement of the sonication effect indicates that liquid food products such as milk and juice are most suitable for thermosonication (Sango et al. 2014).
-
Manosonication
The simultaneous application of ultrasound and external hydrostatic pressure up to 600 kPa (manosonication, MS) increases the lethality of treatment substantially (Demirdoeven and Baysal 2009). This is due to an increase in free radical production (Vercet et al. 1998) and higher bubble implosion (Whillock and Harvey 1997). It was reported that the D-value of Listeria monocytogenes in a citrate phosphate buffer decreased from 5.70 to 2.50 min when the pressure was raised from 0 to 200 kPa in combination with ultrasound at 20 kHz and 90 μm (Manas et al. 2000). Another study showed a reduction in D-values from 1.52 to 0.28 min in Yersinia enterocolitica in a citrate phosphate buffer when there was an increase in pressure from 0 to 300 kPa with ultrasound at 150 μm and 20 kHz (Raso et al. 1998b). This phenomenon can be attributed to the fact that hydrostatic pressure can enhance some effects such as sonoluminescence (emission of short bursts of light from imploding cavitation bubbles in a liquid), and free radical production (Manas et al. 2000).
The following relationships were developed with respect to amplitude on the manosonication inactivation rate (Pagan et al. 1999c):
where Dms is the decimal reduction time (min) for each manosonication treatment, D0 is the control decimal reduction time (min) for a manosonication treatment at an amplitude of 62 μm, and A is the ultrasonic wave amplitude (μm). With respect to pressure on the manosonication, the inactivation rate was related to pressure as
where D0 (min) is defined at an amplitude of 117 μm, 40 °C, and ambient pressure, and p is the static relative pressure (kPa).
Above this pressure, there is a decrease in effectiveness, associated with a decrease in cavitation, because ultrasound waves are unable to overcome the combined cohesive forces of pressure and the cohesive force of the liquid molecules (Condón et al. 2004).
-
Manothermosonication
Combinations of heat, pressure, and ultrasound can be applied to achieve a higher microbial inactivation, called manothermosonication (MTS). While in most vegetative cells the lethal effect of MTS was additive, on Enterococcus faecium, Bacillus subtilis, Bacillus coagulans, Bacillus cereus, Bacillus sterothermophilus, Saccharomyces cerevisiae, and Aeromonas hydrophila, a synergistic effect was observed (Pagan et al. 1999c; Raso et al. 1998b). For example, the D-value of tomato pectin methylesterase (PME) at 62.5 °C was reduced, from 45 min in thermal treatments to 0.85 min by MTS (Lopez et al. 1998). The effectiveness of this treatment can be related to two mechanisms acting independently, i.e., heat treatment and manosonication (Raso et al. 1998b). The lethality of ultrasound under pressure is almost not modified by an increase in temperature unless lethal temperatures are reached (MTS treatments), in which an additive lethal effect is generally attained (Alvarez et al. 2003; Pagan et al. 1999a, b).
Application of ultrasound at 117 μm, 20 kHz, ambient temperature, and low pressure can produce a D-value for L. monocytogenes of 4.30 min. The D-values decreased significantly when higher temperatures were applied, compared with cases where temperature and pressure were applied alone (e.g. the D-value for manothermosonication was 1 min, and the D-value without the use of ultrasound was 2.37 min, at 55 °C and 200 kPa) (Pagan et al. 1999b). Alvarez et al. (2006) found that ultrasound at 117 μm, and 20 kHz, with pressure of 175 kPa, and temperature of 35 °C reduced the D-value of Salmonella Senftenberg in McIlvaine’s citrate-phosphate buffer until 1.71 min. When the temperature was raised to 67 °C, the D-value decreased to 0.02 min. The same synergetic effect of temperature was also described by Lee et al. (2009). The identification of the synergistic effect of temperature on microbial inactivation is a key point for optimizing this technology (Sango et al. 2014).
7.3.3.3 Applications of Ultrasound Sterilization in the Food Industry
Ultrasound is a nonthermal technology which contributes to the increase of microbial safety and prolongs shelf life, especially in food with heat-sensitive, nutritional, sensory, and functional characteristics.
-
Fruit and Vegetable Industry
For decontamination lettuce, spinach, shredded carrot, truffles, cherry tomatoes, and strawberries, high-power ultrasound with low frequencies between 20 and 45 kHz, and short treatment times of 1–10 min were generally applied. For different applications and combinations of the parameters (such as power, frequency, temperature, and time), the microbial reduction with ultrasound varies between 0.5 and 1.98 log CFU g−1 (Alegria et al. 2009; Alexandre et al. 2012, 2013; Brilhante Sã José et al. 2012; Cao et al. 2010; Chen and Zhu 2011; Elizaquivel et al. 2012; Huang et al. 2006; Sagong et al. 2011; Rivera et al. 2011; Zhou et al. 2009). The single and combined effects of ultrasound with some chemicals such as organic acids, acidified sodium chloride, ethanol, chlorine dioxide, and peracetic acid on microbial inactivation in some fruit and vegetables were extensively studied (Brilhante Sao Jose and Dantas Vanetti 2012; Huang et al. 2006; Sagong et al. 2011; Susana Rivera et al. 2011; Zhou et al. 2009).
-
Meat Industry
High power ultrasound is a potential tool for the reduction of microorganisms in poultry (Haughton et al. 2012; Kordowska-Wiater and Stasiak 2011; Loretz et al. 2010), pork, and other meat (Birk and Knochel 2009; Morild et al. 2011). Several researchers have demonstrated that the antimicrobial effects of ultrasound are enhanced when used in combination with other decontamination/preservation techniques such as hypochlorite, mild heat, pressure, steam, or organic acid (Arroyo et al. 2011; Bilek and Turantas 2013; Piyasena et al. 2003).
Ultrasound has been used in beef, chicken, and pork, however, the combination of parameters (frequencies and treatment times between 24 and 45 kHz and 2–120 min, respectively) is applied for improving the general quality of the meat (Dickens et al. 1991; Leal-Ramos et al. 2011; Ozuna et al. 2013; Xiong et al. 2012). High power ultrasound, with low frequencies and treatment times (20 kHz–47 kHz and 2–1800 s, respectively), is generally used for antimicrobial purposes.
7.3.4 Ultrasound Applied in the Inactivation and Activation of Enzymes
7.3.4.1 Inactivation of Enzymes
-
Mechanisms
The inactivation of enzymes by ultrasound is mainly the result of protein denaturation, either by shear forces from the formation and collapse of cavitation bubbles, or by the free radicals produced from the sonolysis of water molecules. Ultrasound makes stable cavitatin bubbles vibrate, creating shock waves which cause strong shear, and microstream in the adjacent liquid. Under these extreme conditions, sonication could cause the breakdown of hydrogen bonding and van der Waals interactions in the polypeptide chains, leading to the modification of the secondary and tertiary structure of the protein. With such changes, the biological activity of the enzyme is usually lost. The extreme localized increase in pressure and temperature also leads to homolytic water molecule cleavage, generating high-energy intermediates such as hydroxyl, and hydrogen-free radicals.
The formation of free radicals due to the sonolysis of water is another mechanism by which the inactivation of enzymes takes place. During sonication, the formation of free radicals is the most widely reported mechanism of enzyme inactivation, which can be measured by the rate of hydrogen peroxide (H2O2) generation. The free radicals interact with the amino acid residues of the enzymes that participate in structural stability, substrate binding, or catalytic functions, and thus affect the enzyme activity. Barteri et al. (2004) studied the inactivation of fumarase by ultrasound. They concluded that the inactivation of the enzyme was due to the formation of disulfide-linked aggregates during sonication. The effects of free radicals on the inactivation of trypsin resulted indirectly through the strong protective effect of mannitol against ultrasound inactivation, with the presence of polypeptide fragments following sonication (Tian et al. 2004). Aggregation of laccase was observed following sonication at different frequencies (20, 50 and 500 kHz). The role of free radicals on the ultrasound inactivation of enzymes was indirectly confirmed through the effect of free radical scavenging solutes on horseradish peroxidase (Grintsevich et al. 2001), catalase (Potapovich et al. 2003), glucose-6-phosphate dehydrogenase (G-6-PDH) (Karaseva and Metelitza 2006), and urease (Tarun et al. 2003). Sonication-induced aggregation was observed in α-amylase, while no such aggregation was observed in β-amylase, which showed that the free-radical-induced oxidation of amino acid residues is dependent on the structure of the protein (Liu et al. 2003).
-
Influencing Factors
Ultrasonic inactivation of enzymes depends on ultrasound-related parameters such as frequency and ultrasonic power, and enzyme-related factors such as enzyme type, concentration, pH of the medium, and temperature (Tarun et al. 2006). The ultrasonic inactivation of different types of enzymes has been reported.
The effect of sonication on laccase from Trametes villosa has been studied at different ultrasonic powers and frequencies. The inactivation kinetics for all the treatments increased in comparison with heat treatments at 50 °C. Sonication, carried out at 72 W and 150 kHz, promoted the formation of protein (laccase) aggregates, which became more evident with increasing treatment times (up to 4 h), and led to inactivation by hindering the active sites. Half-life (time required to reduce the activity to a half of the initial value) was found to decrease by up to 80%–82% when using ultrasound treatment (Basto et al. 2007b).
Grintsevich and Metelitsa (2002) investigated the inactivation kinetics of horseradish peroxidase (POD) in a 0.01 M phosphate citrate buffer (pH 5.4) by applying high-frequency (2.64 MHz) ultrasound (1 W cm−2, at 35.5–55 °C for 1–2 h). At pH less than 5, the free radicals generated during ultrasonic cavitation reacted rapidly with the functionally important amino acid residues at the active sites of POD. Lopez and Burgos (1995) showed a similar pH dependence (D-value increased with decreasing pH) in both thermal and manothermosonication inactivations of peroxidase.
The effects of ultrasound on the activity of lactoperoxidase (LPO) were studied by Ertugay et al. (2003), using different power levels (90–360 W at 20 kHz), times (up to 120 s), and temperatures (20 and 40 °C). The higher ultrasonic amplitudes showed three times as many inactivations of LPO enzymes at 40 °C. Additionally, an increase in temperature and sonication time showed a synergistic inactivation effect. Gebicka and Gebicki (1997) studied the effect of cavitating ultrasound (22 kHz) on peroxidases, horseradish peroxidase, and lactoperoxidase; they found that the activity of peroxidases decreased because of conformational changes upon sonolysis when the sonication time increased.
The inactivation of Bacillus amyloliquefaciens α-amylase type II (A 6380) by ultrasound has been studied at a frequency of 30 kHz (Kadkhodaee and Povey 2008). The sonication was carried out in a thermostated water bath over a temperature range from 20 to 80 °C. Thermosonication greatly enhanced the efficacy of enzyme inactivation (by more than 50%), and also lowered the activation energy (19.29 kJ mol−1 K−1) compared with thermal inactivation (109 kJ mol−1 K−1). Ultrasound strongly decreases the energy barrier required for enzyme inactivation. Manas et al. (2006) studied the inactivation of egg white lysozyme by manothermosonication (117 μm, 200 kPa, 70 °C) where an increase of the implosion intensity of bubbles undergoing cavitation occurred due to the effect of pressure on the efficacy of ultrasound.
The effects of ultrasonic power (100–500 W at 20 kHz) and duration on the function and structures of trypsin have been studied by Tian et al. (2004). The activity of trypsin decreased gradually with the increase of ultrasonic power up to 400 W, with a larger decrease from 400 to 500 W. Combined treatments (manothermosonication and thermosonication) accelerated trypsin inactivation only at low temperatures (Vercet et al. 2001). The effect of ultrasound (26.4 kHz, 26 W cm−2) on the activation of a mixture of chymotrypsinogen and trypsinogen has been studied, and the results show a significant decrease in proteolytic activity at 26.4 W cm−2 (Ovsianko et al. 2005).
-
Applications
Juice
Pectinmethylesterase (PME), a ubiquitous enzyme found in plants, can hydrolyze pectin resulting in a decrease of cloud stability, and a reduction of viscosity due to pectin chain degradation. Ultrasound was reported to inactivate PME in tomato juice and orange juice (O’Donnell et al. 2010) in combination with heat and/or pressure. Wu et al. (2008) reported a reduction in the D-value for PME inactivation at 60 and 65 °C, compared to thermal inactivation.
Raviyan et al. (2005) reported increased inactivation of PME in sonicated tomato juice for a temperature range of 50–72 °C, dependent on cavitational intensity. The reduction of PME activity in sonicated lemon juice resulted in enhanced cloud stability during storage for 18 days at 4 °C compared to thermally processed lemon juice (Knorr et al. 2004). The improved cloud stability observed during storage could be due to the mechanical damage of the PME protein structure during sonication.
Polyphenoloxidase (PPO) is a copper-containing enzyme that causes enzymatic browning in fresh fruit and vegetable products such as juices. Enzymatic browning is a problem during the processing of fruit and vegetables (Yemenicioglu and Cemeroglu 2003). Cheng et al. (2007) reported changes in PPO in sonicated (35 kHz, for 30 min) guava juice. The low power levels can induce the stimulation of enzymes, whereas the higher power levels inactivate enzymes by denaturing them.
Peroxidase (POD) is a heme-containing enzyme which can be used to evaluate the efficiency of vegetable blanching because of its relatively high thermal stability. Thermosonication has been reported to reduce the blanching time required for inactivation of POD in watercress; for example, to obtain 90% POD inactivation at 90 °C, a thermal treatment time of 70 s is necessary compared to 5 s when combined with thermosonication treatment at the same temperature (Cruz et al. 2006). The results show an increase in POD activity during the blanching of watercress for thermosonication in a temperature range of 40–80 °C, and a decrease in enzymatic activity at a higher temperature range of 82.5–92.5 °C. De Gennaro et al. (1999) reported first order inactivation kinetics for POD during sonication.
Dairy
Applications of ultrasound in the dairy industry have been reviewed by Villamiel et al. (1999). Thermoresistant enzymes can reduce the quality and shelf life of heat-treated milk and other dairy products. The simultaneous applications of heat and ultrasound under pressure (manothermosonication) have been found to be more effective than the heat treatment alone in the inactivation of the heat-resistant protease and lipase secreted by Pseudomonas fluorescens (Vercet et al. 1997). Sala et al. (1995) has reported that enzyme inactivation increases with an increase in the content of solids, and decreases with an increase in enzyme concentration.
Villamiel and de Jong (2000) outlined the effect of ultrasound on native milk enzymes. No effect on milk enzymes was observed when the ultrasound was applied without thermal treatment; however, inactivation effects were reported when the sonication was carried out above 61 °C. In skimmed milk, the concentration of solids is lower than in whole milk, resulting in a reduced ultrasonic effect. However, the concentration of enzymes in skimmed milk (alkaline phosphatase, AP and γ-glutamyltranspeptidase, GGTP) is also lower than that in whole milk leading to a more pronounced effect, as these enzymes are linked to fat globules and can be liberated by the ultrasound effect to the serum phase; on the other hand, lactoperoxidase (LPO) is located in the whey. The main cause of the larger decrease in enzyme activity in whole milk compared to skimmed milk by ultrasound and heat (75.5 °C; 102.3 s) could be the higher concentration of solids in whole milk (Villamiel and de Jong 2000).
7.3.4.2 Activation of Enzymes
-
Mechanisms
Using ultrasound treatments at appropriate frequencies and intensity levels can lead to an increase of enzyme activity due to several different effects. These effects can be subdivided into physical and biochemical effects.
Physical effects: There are a number of effects enhancing enzyme activity based on pure physical effects elaborated in the following parts.
Mass transfer and micro-mixing: Enzymatic reactions are often limited by a lack of substrate due to the structural configuration of the substrate (Cadoret et al. 2002) or restricted diffusion of the substrate to enzymes or vice versa (Francis et al. 1995). Ultrasound waves at different frequencies can overcome this limitation by inducing fluid motion and increasing mass transfer, ensuring substrate availability at the enzyme and the removal of products from the enzyme (Sinisterra 1992). This increase in mass transfer is obtained either at lower frequencies by the generation of cavitation, or at higher frequencies through the generation of turbulent microstreams and the induction of a turbulent channel flow in narrow porous structures (Bengtsson and Laurell 2004; Francis et al. 1995). Starting in the early 1990s, a number of workgroups investigated the effect of synergistically enhancing enzyme activities for different application. Typically, systems that are involved solid–liquid or liquid–liquid interfaces were investigated. Such studies included: enzyme scouring of cotton (Basto et al. 2007a, b; Yachmenev et al. 2004), paper recycling (Xie et al. 2002), cellulose hydrolysis (Aliyu and Hepher 2000; Barton et al. 1996; Li et al. 2005), sucrose hydrolysis (Sakakibara et al. 1996), esterifications (Xiao et al. 2005), hydrolysis of esters (Lie Ken Jie and Syed-Rahmatullah 1995), hydrolysis of phenolic compounds in wastes and bleaching (Entezari et al. 2006; Entezari and Petrier 2004; Tauber et al. 2005), and biofilm removal (Oulahal-Lagsir et al. 2003).
Enzyme release: Ultrasound waves at low frequencies and high intensities can induce cell break-up leading to a discharge of the cell contents including enzymes (Farkade et al. 2006; Persike et al. 2002; Vargas et al. 2004). However, using ultrasound at lower intensities can also release enzymes from cells while causing little damage to cell membranes (Roncales et al. 1993). These applications allow the enhancement of enzyme activity by overcoming the limitations of inter-membrane transport and improving enzyme extraction from cellular material.
Immobilized enzymes: The effects of increasing the activity of immobilized enzymes by using ultrasound are described by mass transfer and micro-mixing, and increasing substrate availability by enhanced mass transfer to and through the carrier material (Ma et al. 2017). The frequencies used were in the range of 1 MHz or greater, allowing turbulent microstreams to be generated.
Pretreatment procedures leading to enhanced enzyme activity: Not only can the combined processes of enzymatic reactions and ultrasonication lead to an improvement in enzyme activity, but a substrate or immobilization carrier pretreatment with ultrasound irradiation can also significantly enhance substrate conversion. The enhancement occurs through an increase in the available substrate surface, e.g. degradation of the substrate masking substances such as lignin on cellulose (Entezari and Petrier 2005; Li et al. 2005; Wood et al. 1997), grafting of a polymeric surface on which enzymes are immobilized (Jiang and Xiang 1992; Popa et al. 1994), or the size reduction of oil droplets by means of ultrasonic emulsification (Ramachandran et al. 2006).
Biochemical effects: Ultrasound waves can also induce biochemical effects in living cells, leading to an increase of the production of certain enzymes (Wu and Ge 2004; Wu and Lin 2003). The tissue reverts to normal metabolism if there is no further ultrasonic stimulation (Wu and Lin 2002).
-
Applications
Ultrasound activation of enzymes
Polygalacturonase (PG) is one of the most commonly used enzymes in fruit and vegetable processing. Ultrasound has the potential to enhance enzyme activity, modify the PG enzyme, and enlarge its application range. The maximum activity of PG was observed at 4.5 W mL−1 intensity with an ultrasound duration of 15 min, under which the enzyme activity increased by 20.98% over the control. The results of degradation kinetics and the thermodynamics of hydrolysis reactions catalyzed by PG certified that ultrasound treatment could make PG exhibit higher reaction ability. After ultrasound treatment, the value of Vmax for the enzymatic reaction increased, whereas Km decreased in comparison to the control. These results demonstrate that the substrate was converted into the product at a higher rate and efficiency, and that the enzyme displayed better affinity to the substrate. Ultrasound improved the temperature stability of PG and prolonged its lifetime without affecting its optimum temperature. Fluorescence spectra and far-UV CD spectra revealed that ultrasound treatment irreversibly decreased the amount of tryptophan on the PG surface, but increased the β-sheet in PG secondary conformation, possibly by the exposure of more active sites (Ma et al. 2015).
Subhedar and Gogate (2014) investigated the effect of low intensity ultrasonic irradiation on the activity of cellulase. The results show that ultrasound has a positive effect on the activity of cellulase. The maximum cellulase activity was observed at an intensity of 17.33 W cm−2 and ultrasonic treatment times of 30 min, under which the enzyme activity increased by approximately 25% over the untreated enzyme. A significant reduction in the thermodynamic parameters was observed after ultrasonic irradiation.
The effects of energy-gathered ultrasound on the activity, kinetics, thermodynamics, and molecular structure of alcalase were explored by Ma et al. (2011). The results show that the highest alcalase activity was achieved when the sample was treated with energy-gathered ultrasound at 80 W for 4 min, under which the enzyme activity increased by 5.8% over the control. Fluorescence and CD spectra revealed that the ultrasonic treatment had increased the number of tryptophan on the alcalase surface slightly, increased the number of α-helices by 5.2%, and reduced the number of random coils by 13.6%. The changes in enzyme conformation induced by ultrasound may lead to the increase of enzymatic activity.
A study by Jadhav and Gogate (2014) shows that lipase had a maximum activity at an ultrasound intensity of 12.22 W cm−2 and an optimized sonication time of 9 min. The maximum increase in the activity of the enzyme was twofold. Immobilization of the enzyme was achieved after sonication at the optimized parameters while retaining its activity, which gave retention of 47.9% in the activity of the tributyrin hydrolysis reaction. The use of ultrasound certainly provided some permanent intensification in the activity of the enzyme.
Comparison of the impact of ultrasound on free and immobilized cellulase shows that the highest activity of the free biocatalyst was achieved when the sample was treated with ultrasound at a frequency of 24 kHz and an intensity of 15 W for 10 min, under which the enzyme activity increased by 18.17% over the control (Wang et al. 2012). The highest activity of immobilized cellulase was achieved when the sample was treated with 24 kHz ultrasound at 60 W for 10 min, under which the activity of the enzyme increased by 24.67% over the control (Wang et al. 2012).
Introduced during the immobilization process, ultrasound at an intensity of 9 W mL−1 for 20 min increased the immobilization yield by 92.28% more than the control. Higher Vmax and lower Km were obtained after ultrasound treatment, indicating the increased catalytic efficiency, and the enhanced affinity of immobilized pectinase (Ma et al. 2017). For the increase in activity of commercial lipase immobilized on beads of a macroporous acrylic resin, when low intensity ultrasound was used after being used up to four times (Batistella et al. 2012), and even up to 8 (Zheng et al. 2013) or 10 times (Liu et al. 2015).
Ultrasound-assisted enzymatic hydrolysis
Comparative studies on lipase-catalyzed hydrolysis of soy oil in solvent-free systems were carried out in a shaking ultrasound bath (ultrasonic intensity 1.64 W cm−2 and frequency of 28 kHz). Under the optimum conditions, the overall enzymatic hydrolysis assisted by ultrasound was two times higher than that assisted by shaking (Liu et al. 2008).
Xenobiotics, such as anabolic steroids in urine, have also been deconjugated with the help of β-glucuronidase and an ultrasound probe in only 10 min. The experimental data suggests that the reaction followed Michaelis–Menten kinetics and that ultrasound affected the initial reaction rate, which was higher, compared to the classical method of incubation at 55 °C. Also, the values of Vmax and kcat were higher for the ultrasonic assay, whilst the Michaelis–Menten constant obtained from both methods showed similar values. Deactivation of the enzyme was also observed under ultrasound treatment, which was particularly evident for experimental conditions with an excess of substrate (Galesio et al. 2012).
The production of bio-ethanol from lignocellulosic materials is currently impeded by the high cost and low efficiency of enzymatic hydrolysis and plant cellulase activity. This can be partially overcome by the introduction of low energy ultrasound (using a 50 kHz ultrasound hexagon reactor system; at 50 °C) during the enzymatic hydrolysis of corn stover and sugarcane bagasse where the enzyme efficiency was greatly improved (Yachmenev et al. 2009). Another study was carried out by using cassava chip slurry as feedstock, the reducing sugar release from the slurry samples with enzyme addition during sonication was as high as 180% of the control samples. Heat generated during sonication did not account for the increased reducing sugar release (Nitayavardhana et al. 2008). The enzymatic degradation of lignocellulose was improved through the use of ultrasonic treatment at a frequency between 2 and 200 kHz and 30–48 °C. Continuous irradiation can result in a decrease in hydrolysis compared to discontinuous application, probably due to the fact that constant mixing does not allow the cellulase to rebind to cellulose for catalysis to occur (Ingram and Wood 2001).
The ultrasonic intensification of enzymatic depolymerization of aqueous guar gum solution has been reported by Prajapat et al. (2016). The kinetic rate constant was found to increase with an increase in temperature and cellulase loading. In the presence of cellulase, the maximum extent of depolymerization of guar gum has been observed at an ultrasonic power of 60 W, and a treatment time of 30 min. The results reveal that enzymatic depolymerization of guar gum results in a polysaccharide with a low degree of polymerization, viscosity, and consistency index, without any change in the core chemical structure which could make it useful for incorporation in food products.
The factors that affect the efficiency of the enzymatic hydrolysis of cellulose with low frequency ultrasound have been considered by Szabo and Csiszar (2017). The optimal operating conditions were reached at 60% amplitude and 9 mm. The yield depended mainly on important factors such as amplitude, the presence of a reflector, distance from the horn, and the form of the substrate.
Ma et al. (2016a) have investigated the synergistic effects of ultrasound and pectinase on pectin hydrolysis. The hydrolysis rate of pectin achieved maximum value with ultrasound treatment at 4.5 W mL−1 intensity and a treatment time of 10 min, resulting in an increase of 32.59% over the control. The optimum temperature for the hydrolysis reaction was 50 °C. The value of Vmax increased whereas Km decreased in the sonoenzymolysis reaction compared with the routine enzymolysis reaction, which indicates that pectin was hydrolyzed at an elevated rate, and that the pectinase exhibited a stronger affinity to the substrate with ultrasound. The degree of methoxylation (DM) of sonoenzymolysis pectin significantly decreased, whereas the degree of acetylation (DAc) remained unchanged compared to the original and enzymolysis pectin (Ma et al. 2016b).
7.3.5 Other Applications
7.3.5.1 Emulsification
Ultrasonic emulsification is primarily driven by cavitation, wherein bubbles collapse at the oil-water interface causing disruption that result in the formation of very fine emulsions.
Ultrasonic emulsification offers several benefits over conventional methods such as use of mechanical shaking, colloid mills, high- or ultra high-pressure homogenizers, and microfluidizers. For example, the energy required to produce an emulsion by ultrasound is less than that needed for conventional methods. Also, emulsions generated by ultrasound are more stable, require minimal surfactant, and have a sub-micron size and an extremely narrow size distribution. Ultrasonic emulsification has attracted much interest for the homogenization of milk (Wu et al. 2001; Bosiljkov et al. 2011; Windhab et al. 2005), aroma encapsulation (Mongenot et al. 2000), and online processing of tomato sauces, fruit juices, mayonnaise, and other similar blended food products. Several parameters affect the emulsification process including hydrostatic pressure, gas content (Behrend and Schubert 2001), pre-emulsification (Jafari et al. 2007), viscosity of the continuous phase (Behrend et al. 2000), oil:water ratio, surfactant concentration (Abismail et al. 1999; Jafari et al. 2006), position of the ultrasonic probe in relation to the liquid–liquid interface (Cucheval and Chow 2008), ultrasonic power, and exposure time (Jafari et al. 2006; 2007).
7.3.5.2 Filtration
Membrane technology is extensively used in the food and dairy industry, for water purification and treatment of liquid effluent streams (Maskooki et al. 2010). One of the critical issues in filtration is the decline in permeate flux as a result of both concentration polarization and membrane fouling. The application of ultrasound has been proven to be an effective approach for enhancing the flux in ultrafiltration or microfiltration processes, and to improve the cleaning of fouled membranes (Muthukumaran et al. 2005a, b, 2007).
7.3.5.3 Viscosity Modification
Controlling the viscosity of food systems by ultrasound is one of the most promising processes. The elucidated merits of the ultrasonic process are that: no chemicals or additives are required; it is cost effective; and there are no large changes in the chemical structure. The ultrasonic process has been confirmed to be applicable for many kinds of starches (corn, potato, tapioca, and sweet potato) and polysaccharides. Jambrak et al. (2010) used an ultrasound probe at different intensities (34, 55, 73 W cm−2) and treatment times (15 and 30 min), and an ultrasound bath at an intensity of 2 W cm−2 with treatment times of 15 and 30 min, for corn starch suspensions. The results show that ultrasound treatment of corn starch distorted the crystalline region in the starch granules prior to a reversible hydration of the amorphous phase, which resulted in the destruction of the granular structure.
7.3.5.4 Tenderization
The effects of ultrasound on meat texture are predominantly from cavitation effects, as the samples are chilled immediately after ultrasound treatment. Ultrastructural changes in the muscle, either by physical disruption of collagen or myofibrils, or by increased enzymatic degradation of the muscle through increased availability of calcium ions for the calcium-mediated calpain system, increased the ability of ultrasound to rapidly tenderize meat (Jayasooriya et al. 2007).
7.3.5.5 Defoaming
PU in pulsed operation (1 s/1 s) has been described as an effective procedure to remove foam and dissolved oxygen (80% of foam reduction) with very low energy consumption (40 kJ L−1) in super-saturated milk (Villamiel et al. 1999). Recently, a stepped-plate air-borne ultrasound defoamer was developed and commercially applied to control the excess foam produced during the filling operation of bottles and cans on high-speed canning lines, in fermenting vessels, and other reactors (Gallego-Juarez et al. 2010).
7.3.5.6 Crystallization
Sonocrystallisation is the application of ultrasound energy to control the nucleation of a crystallization process. The use of the power ultrasound provides a useful approach to produce crystals with desired properties. Sonocrystallisation facilitates process control by modulating crystal size distribution and morphology (Deora et al. 2013).
7.3.5.7 Freezing
Ultrasound has gained considerable interest in food processing and preservation due to its ability to control and modify nucleation and crystal growth (Knorr et al. 2004; Li and Sun 2002a; Sanz et al. 1999; Mason et al. 1996). Several studies have indicated the potential of using ultrasound to accelerate freezing rates and to improve the quality of frozen plant foods, such as potatoes (Li and Sun 2002b; Sun and Li 2003). Ultrasound-treated frozen potatoes exhibited a better cellular structure with fewer extracellular voids and less cell disruption/ breakage than those without acoustic treatment (Sun and Li 2003). The most important effect of power ultrasound in food freezing is due to acoustic cavitation, which not only promotes ice nucleation by micro-bubbles, but also enhances the heat and mass transfer due to the violent agitation created by the acoustic microstreaming (Zheng and Sun 2006). Cavitation bubbles arising from sonication benefit the freezing process by reducing the resistance to both heat and mass transport at the ice/liquid interface, thereby increasing the freezing rate. The random motion of the cavitation bubbles is also suspected to break down any ice dendrites as they form (Chow et al. 2004).
7.3.5.8 Thawing of Frozen Foods
More studies have been carried out to investigate the effectiveness of PU for thawing frozen foods by varying ultrasound parameters such as frequency and power (Kissam 1985; Kissam et al. 1982; Brody and Antonevich 1959). Miles et al. (1999) reported that overheating occurred near the surface of frozen foods at high intensities, as well as at high and low frequencies, due to the increase in attenuation with frequency, and the onset of cavitation at low frequencies. They were able to overcome this problem by adjusting the frequency (500 kHz) and intensity (0.5 W cm−2) for frozen beef, pork, and cod, which were thawed to a depth of 7.6 cm within about 2.5 h. In other work, a block of frozen Pacific cod was exposed to 1500 Hz acoustic energy and up to 60 W continuous input to the transducer (Kissam et al. 1982). The block thawed in 71% less time than the water-only controls, and the acoustic waves did not alter the quality of the flesh.
7.3.5.9 Fermentation
Ultrasound can influence the course of fermentation by improving mass transfer and cell permeability leading to improved process efficiency and production rates. These methods produce no degradation or chemical alterations in the fermentation media (Henning and Rautenberg 2006). The velocity of an ultrasonic wave traveling through a fermentation tank can also be used to infer the concentration of alcohol and sugars during the fermentation process (Resa et al. 2005). Studies have shown that empirical relationships can be developed between the ultrasonic parameters and the concentration of alcohol and soluble solids in wine (Winder et al. 1970) as well as the density of beer (Becker et al. 2001) while fermentation is ongoing.
7.3.5.10 Drying
Ultrasonic dehydration can be utilized at low temperatures, which prevents the degradation of the nutritional components of food at high temperatures (Kumar et al. 2014; Lechtanska et al. 2015; Schoessler et al. 2012), it can also improve color, antioxidant volume, hardness, etc. (Bantle and Eikevik 2011; Kowalski et al. 2015; Santacatalina et al. 2016). The PU improves heat and mass transfer phenomena in drying processes. It can be assumed that supplementary ultrasonic effects, such as moisture and vapor migration improvement, boundary layer reduction, evaporation and sublimation improvement, and changes in structure or properties (density, viscosity, etc.) of the material, have contributed to the drying process (Musielak et al. 2016).
7.3.5.11 Freeze Concentration
Due to the ability to initiate nucleation at lower degrees of supercooling, PU can be an effective tool for controlling the size of ice crystals in freeze concentrated products (Zheng and Sun 2006). Botsaris and Qian (1999) utilized an ultrasound-assisted freeze concentration system and used it to induce ice nucleation at low degrees of supercooling.
7.4 Concluding Remarks
The high-power (low frequency) ultrasound modifies the properties of food by inducing mechanical, physical, and chemical/biochemical changes through cavitation, which reduces reaction time and increases yield under mild conditions compared to conventional procedures. Power ultrasound is considered to be a green technology with many promising applications in the food industry. Despite the potential applications of ultrasound in food processing, there are some limits for extensive applications such as the cost of probes, portability, and the ability to modify it in industrial applications. Therefore, extensive research is necessary to integrate fully automated ultrasound systems for food production lines, reduce cost, save energy, and ensure high food quality, and safety.
References
Abismail, B., J.P. Canselier, A.M. Wilhelm, et al. 1999. Emulsification by ultrasound: Drop size distribution and stability. Ultrasonics Sonochemistry 6 (1–2): 75–83.
Alegria, C., J. Pinheiro, E.M. Goncalves, et al. 2009. Quality attributes of shredded carrot (Daucus carota L. cv. Nantes) as affected by alternative decontamination processes to chlorine. Innovative Food Science and Emerging Technologies 10 (1): 1–69.
Alexandre, E.M.C., T.R.S. Brandao, and C.L.M. Silva. 2012. Efficacy of non-thermal technologies and sanitizer solutions on microbial load reduction and quality retention of strawberries. Journal of Food Engineering 108 (3): 417–426.
Alexandre, E.M.C., T.R.S. Brandao, and C.L.M. Silva. 2013. Impact of non-thermal technologies and sanitizer solutions on microbial load reduction and quality factor retention of frozen red bell peppers. Innovative Food Science and Emerging Technologies 17: 99–105.
Aliyu, M., and M.J. Hepher. 2000. Effects of ultrasound energy on degradation of cellulose material. Ultrasonics Sonochemistry 7 (4): 265–268.
Alvarez, I., P. Manas, F.J. Sala, et al. 2003. Inactivation of Salmonella enterica serovar enteritidis by ultrasonic waves under pressure at different water activities. Applied and Environmental Microbiology 69 (1): 668–672.
Alvarez, I., P. Manas, R. Virto, et al. 2006. Inactivation of Salmonella senftenberg 775 W by ultrasonic waves under pressure at different water activities. International Journal of Food Microbiology 108 (2): 218–225.
Arroyo, C., G. Cebrian, R. Pagan, et al. 2011. Inactivation of Cronobacter sakazakii by manothermosonication in buffer and milk. International Journal of Food Microbiology 151 (1): 21–28.
Awad, T.S., H.A. Moharram, O.E. Shaltout, et al. 2012. Applications of ultrasound in analysis, processing and quality control of food: A review. Food Research International 48 (2): 410–427.
Bantle, M., and T.M. Eikevik. 2011. Parametric study of high-intensity ultrasound in the atmospheric freeze drying of peas. Drying Technology 29 (10): 1230–1239.
Barbosa-Canovas, G.V., and J.J. Rodriguez. 2002. Update on nonthermal food processing technologies: Pulsed electric field, high hydrostatic pressure, irradiation and ultrasound. Food Australia 54 (11): 513–520.
Barteri, M., M. Diociaiuti, A. Pala, et al. 2004. Low frequency ultrasound induces aggregation of porcine furnarase by free radicals production. Biophysical Chemistry 111 (1): 35–42.
Barton, S., C. Bullock, and D. Weir. 1996. The effects of ultrasound on the activities of some glycosidase enzymes of industrial importance. Enzyme and Microbial Technology 18 (3): 190–194.
Basto, C., C.J. Silva, G. Guebitz, et al. 2007a. Stability and decolourization ability of Trametes villosa laccase in liquid ultrasonic fields. Ultrasonics Sonochemistry 14 (3): 355–362.
Basto, C., T. Tzanov, and A. Cavaco-Paulo. 2007b. Combined ultrasound-laccase assisted bleaching of cotton. Ultrasonics Sonochemistry 14 (3): 350–354.
Batistella, L., L.A. Lerin, P. Brugnerotto, et al. 2012. Ultrasound-assisted lipase-catalyzed transesterification of soybean oil in organic solvent system. Ultrasonics Sonochemistry 19 (3): 452–458.
Baxter, S., S. Zivanovic, and J. Weiss. 2005. Molecular weight and degree of acetylation of high-intensity ultrasonicated chitosan. Food Hydrocolloids 19 (5): 821–830.
Becker, T., M. Mitzscherling, and A. Delgado. 2001. Ultrasonic velocity — A noninvasive method for the determination of density during beer fermentation. Chemical Engineering and Technology 24 (8): 61A–67A.
Behrend, O., K. Ax, and H. Schubert. 2000. Influence of continuous phase viscosity on emulsification by ultrasound. Ultrasonics Sonochemistry 7 (2): 77–85.
Behrend, O., and H. Schubert. 2001. Influence of hydrostatic pressure and gas content on continuous ultrasound emulsification. Ultrasonics Sonochemistry 8 (3): 271–276.
Bengtsson, M., and T. Laurell. 2004. Ultrasonic agitation in microchannels. Analytical and Bioanalytical Chemistry 378 (7): 1716–1721.
Berlan, J., and T.J. Mason. 1992. Sonochemistry-from research laboratories to industrial-plants. Ultrasonics 30 (4): 203–212.
Bermudez-Aguirre, D., and G.V. Barbosa-Canovas. 2012. Inactivation of Saccharomyces cerevisiae in pineapple, grape and cranberry juices under pulsed and continuous thermo-sonication treatments. Journal of Food Engineering 108 (3): 383–392.
Betts, G. D., A. Williams, and R.M. Oakley. 1999. Ultrasonic standing waves: Inactivation of food-borne microorganisms using power ultrasound. In Encyclopedia of food microbiology, 2202–2208.
Bilek, S.E., and F. Turantaş. 2013. Decontamination efficiency of high power ultrasound in the fruit and vegetable industry, a review. International Journal of Food Microbiology 166 (1): 155–162.
Birk, T., and S. Knochel. 2009. Fate of food-associated bacteria in pork as affected by marinade, temperature, and ultrasound. Journal of Food Protection 72 (3): 549–555.
Bosiljkov, T., B. Tripalo, M. Brncic, et al. 2011. Influence of high intensity ultrasound with different probe diameter on the degree of homogenization (variance) and physical properties of cow milk. African Journal of Biotechnology 10 (1): 34–41.
Botsaris, G.D., and R.Y. Qian. 1999. Process and system for freeze concentration using ultrasonic nucleation useful in effluent processing. Washington, DC, 1999, US Patents, No. 5.966.966.
Brilhante Sã José, J.F., and M.C. Dantas Vanetti. 2012. Effect of ultrasound and commercial sanitizers in removing natural contaminants and Salmonella enterica Typhimurium on cherry tomatoes. Food Control, 24 (1–2): 95-99.
Brody, A.L., and J.N. Antonevich. 1959. Ultrasonic defrosting of frozen foods. Food Technology 13 (2): 109–112.
Brotchie, A., F. Grieser, and M. Ashokkumar. 2009. Effect of power and frequency on bubble-size distributions in acoustic cavitation. Physical Review Letters 102 (0843028).
Burgos, J., J.A. Ordonez, and F. Sala. 1972. Effect of ultrasonic-waves on heat resistance of Bacillus cereus and Bacillus licheniformis spores. Applied Microbiology 24 (3): 497.
Butz, P., and B. Tauscher. 2002. Emerging technologies: Chemical aspects. Food Research International 35 (2–3): 279–284. https://doi.org/10.1016/S0963-9969(01)00197-1.
Cadoret, A., A. Conrad, and J.C. Block. 2002. Availability of low and high molecular weight substrates to extracellular enzymes in whole and dispersed activated sludges. Enzyme and Microbial Technology 31 (1-2): 179–186. https://doi.org/10.1016/S0141-0229(02)00097-2.
Cameron, M., L.D. McMaster, and T.J. Britz. 2008. Electron microscopic analysis of dairy microbes inactivated by ultrasound. Ultrasonics Sonochemistry 15 (6): 960–964.
Cao, S.F., Z.C. Hu, B. Pang, et al. 2010. Effect of ultrasound treatment on fruit decay and quality maintenance in strawberry after harvest. Food Control 21 (4): 529–532.
Carcel, J.A., J. Benedito, J. Bon, et al. 2007. High intensity ultrasound effects on meat brining. Meat Science 76 (4): 611–619.
Chandrapala, J., C. Oliver, S. Kentish, et al. 2012. Ultrasonics in food processing—Food quality assurance and food safety. Trends in Food Science and Technology 26 (2): 88–98.
Chemat, S., A. Lagha, H. AitAmar, et al. 2004. Comparison of conventional and ultrasound-assisted extraction of carvone and limonene from caraway seeds. Flavour and Fragrance Journal 19 (3): 188–195.
Chemat, F., N. Rombaut, A. Sicaire, et al. 2017. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrasonics Sonochemistry 34: 540–560.
Chen, Z., and C.H. Zhu. 2011. Combined effects of aqueous chlorine dioxide and ultrasonic treatments on postharvest storage quality of plum fruit (Prunus salicina L.). Postharvest Biology and Technology 61 (2–3): 117–123.
Cheng, L.H., C.Y. Soh, S.C. Liew, et al. 2007. Effects of sonication and carbonation on guava juice quality. Food Chemistry 104 (4): 1396–1401.
Cheng, W.J., J.C. Chen, D.H. Liu, et al. 2010. Impact of ultrasonic treatment on properties of starch film-forming dispersion and the resulting films. Carbohydrate Polymers 81 (3): 707–711.
Chivate, M.M., and A.B. Pandit. 1995. Quantification of cavitation intensity in fluid bulk. Ultrasonics Sonochemistry 2 (1): S19–S25.
Chow, R., R. Blindt, A. Kamp, et al. 2004. The microscopic visualisation of the sonocrystallisation of ice using a novel ultrasonic cold stage. Ultrasonics Sonochemistry 11 (3–4): 245–250.
Chukwumah, Y.C., L.T. Walker, M. Verghese, et al. 2009. Effect of frequency and duration of ultrasonication on the extraction efficiency of selected isoflavones and trans-resveratrol from peanuts (Arachis hypogaea). Ultrasonics Sonochemistry 16 (2): 293–299.
Condón, S., J. Raso, R. Pagán, et al. 2004. Microbial inactivation by ultrasound. In Novel Food Processing Technologies, ed. G.V. Barbosa-Canovas, M.S. Tapia, and M.P. Cano. Boca Raton, USA: CRC Press.
Contamine, R.F., A.M. Wilhelm, J. Berlan, et al. 1995. Power measurement in sonochemistry. Ultrasonics Sonochemistry 2 (1): S43–S47.
Cruz, R., M.C. Vieira, and C. Silva. 2006. Effect of heat and thermosonication treatments on peroxidase inactivation kinetics in watercress (Nasturtium officinale). Journal of Food Engineering 72 (1): 8–15.
Cucheval, A., and R.C.Y. Chow. 2008. A study on the emulsification of oil by power ultrasound. Ultrasonics Sonochemistry 15 (5): 916–920.
Demirdoeven, A., and T. Baysal. 2009. The use of ultrasound and combined technologies in food preservation. Food Reviews International 25 (1): 1–11.
Deora, N.S., N.N. Misra, A. Deswal, et al. 2013. Ultrasound for improved crystallisation in food processing. Food Engineering Reviews 5 (1): 36–44.
Dickens, J.A., C.E. Lyon, and R.L. Wilson. 1991. Effect of ultrasonic radiation on some physical characteristics of broiler breast muscle and cooked meat. Poultry Science 70 (2): 389–396.
Ebringerova, A., and Z. Hromadkova. 1997. The effect of ultrasound on the structure and properties of the water-soluble corn hull heteroxylan. Ultrasonics Sonochemistry 4 (4): 305–309.
Elizaquivel, P., G. Sanchez, M.V. Selma, et al. 2012. Application of propidium monoazide-qPCR to evaluate the ultrasonic inactivation of Escherichia coli O157:H7 in fresh-cut vegetable wash water. Food Microbiology 30 (1): 316–320.
Entezari, M.H., M. Mostafai, and A. Sarafraz-yazdi. 2006. A combination of ultrasound and a bio-catalyst: removal of 2-chlorophenol from aqueous solution. Ultrasonics Sonochemistry 13 (1): 37–41.
Entezari, M.H., and C. Petrier. 2004. A combination of ultrasound and oxidative enzyme: sono-biodegradation of phenol. Applied Catalysis B-Environmental 53 (4): 257–263.
Entezari, M.H., and C. Petrier. 2005. A combination of ultrasound and oxidative enzyme: Sono-enzyme degradation of phenols in a mixture. Ultrasonics Sonochemistry 12 (4): 283–288.
Ertugay, M.F., Y. Yuksel, and M. Sengul. 2003. The effect of ultrasound on lactoperoxidase and alkaline phosphatase enzymes from milk. Milchwissenschaft-Milk Science International 58 (11–12): 593–595.
Esclapez, M.D., J.V. Garcia-Perez, A. Mulet, et al. 2011. Ultrasound-assisted extraction of natural products. Food Engineering Reviews 3 (2): 108–120.
Esclapez, M.D., V. Saez, D. Milan-Yanez, et al. 2010. Sonoelectrochemical treatment of water polluted with trichloroacetic acid: From sonovoltammetry to pre-pilot plant scale. Ultrasonics Sonochemistry 17 (6SI): 1010–1020.
Farkade, V.D., S.T.L. Harrison, and A.B. Pandit. 2006. Improved cavitational cell disruption following pH pretreatment for the extraction of beta-galactosidase from Kluveromyces lactis. Biochemical Engineering Journal 31 (1): 25–30.
Feng, H., W. Yang, and T. Hielscher. 2008. Power Ultrasound. Food Science and Technology International 14 (5): 433–436.
Flannigan, D.J., and K.S. Suslick. 2010. Inertially confined plasma in an imploding bubble. Nature Physics 6 (8): 598–601.
Francis, C.W., A. Blinc, S. Lee, et al. 1995. Ultrasound accelerates transport of recombinant tissue-plasminogen activator into clots. Ultrasound in Medicine and Biology 21 (3): 419–424.
Galesio, M., J. Lourenco, D. Madeira, et al. 2012. Unravelling the role of ultrasonic energy in the enhancement of enzymatic kinetics. Journal of Molecular Catalysis B-Enzymatic 74 (1–2): 9–15.
Gallego-Juarez, J.A., G. Rodriguez, V. Acosta, et al. 2010. Power ultrasonic transducers with extensive radiators for industrial processing. Ultrasonics Sonochemistry 17 (6SI): 953–964.
Garcia-Graells, C., E. Hauben, and C.W. Michiels. 1998. High-pressure inactivation and sublethal injury of pressure-resistant Escherichia coli mutants in fruit juices. Applied and Environmental Microbiology 64 (4): 1566–1568.
Gebicka, L., and J.L. Gebicki. 1997. The effect of ultrasound on heme enzymes in aqueous solution. Journal of Enzyme Inhibition 12 (2): 133–141.
Gennaro, L., S. Cavella, R. Romano, et al. 1999. The use of ultrasound in food technology. I. Inactivation of peroxidase by thermosonication. Journal of Food Engineering 39 (4): 401–407.
González-Centeno, M.R., K. Knoerzer, H. Sabarez, et al. 2014. Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.)—A response surface approach. Ultrasonics Sonochemistry 21 (6SI): 2176–2184.
Grintsevich, E.E., I.E. Adzerikho, A.G. Mrochek, et al. 2001. Polydisulfides of substituted phenols as effective protectors of peroxidase against inactivation by ultrasonic cavitation. Biochemistry-Moscow 66 (7): 740–746.
Grintsevich, E.E., and D.I. Metelitsa. 2002. Kinetics of ultrasonic inactivation of peroxidase in aqueous solutions. Russian Journal of Physical Chemistry 76 (8): 1368–1373.
Guerrero, S., A. Lopez-Malo, and S.M. Alzamora. 2001. Effect of ultrasound on the survival of Saccharomycescerevisiae: Influence of temperature, pH and amplitude. Innovative Food Science and Emerging Technologies 2 (1): 31–39.
Hammi, K.M., A. Jdey, C. Abdelly, et al. 2015. Optimization of ultrasound-assisted extraction of antioxidant compounds from Tunisian Zizyphus lotus fruits using response surface methodology. Food Chemistry 184: 80–89.
Haughton, P.N., J.G. Lyng, D.J. Morgan, et al. 2012. An evaluation of the potential of high-intensity ultrasound for improving the microbial safety of poultry. Food and Bioprocess Technology 5 (3): 992–998.
Henning, B., and J. Rautenberg. 2006. Process monitoring using ultrasonic sensor systems. Ultrasonics 441: E1395–E1399.
Herceg, Z., A.R. Jambrak, V. Lelas, et al. 2012. The effect of high intensity ultrasound treatment on the amount of Staphylococcus aureus and Escherichia coli in milk. Food Technology and Biotechnology 50 (1): 46–52.
Huang, Q., L. Li, and X. Fu. 2007. Ultrasound effects on the structure and chemical reactivity of cornstarch granules. Starch-Starke 59 (8): 371–378.
Huang, T., C. Xu, K. Walker, et al. 2006. Decontamination efficacy of combined chlorine dioxide with ultrasonication on apples and lettuce. Journal of Food Science 71 (4): M134–M139.
Hughes, D.E., and W.L. Nyborg. 1962. Cell disruption by ultrasound. Science 138 (3537): 108.
Ingram, L. O., and B.E. Wood. 2001. Ethanol production from lignocellulose. US Patent 6 (333): 81.
Jadhav, S.H., and P.R. Gogate. 2014. Intensification in the activity of lipase enzyme using ultrasonic irradiation and stability studies. Industrial and Engineering Chemistry Research 53 (4): 1377–1385.
Jafari, S.M., Y.H. He, and B. Bhandari. 2006. Nano-emulsion production by sonication and microfluidization—A comparison. International Journal of Food Properties 9 (3): 475–485.
Jafari, S.M., Y. He, and B. Bhandari. 2007. Production of sub-micron emulsions by ultrasound and microfluidization techniques. Journal of Food Engineering 82 (4): 478–488.
Jambrak, A.R., Z. Herceg, D. Subaric, et al. 2010. Ultrasound effect on physical properties of corn starch. Carbohydrate Polymers 79 (1): 91–100.
Jayasooriya, S.D., P.J. Torley, B.R. D’Arcy, et al. 2007. Effect of high power ultrasound and ageing on the physical properties of bovine semitendinosus and longissimus muscles. Meat Science 75 (4): 628–639.
Jiang, B., and M. Xiang. 1992. Immobilization of enzymes on polymers modified by ultrasonic irradiation. European Polymer Journal 28 (7): 827–830.
Joyce, E., S.S. Phull, J.P. Lorimer, et al. 2003. The development and evaluation of ultrasound for the treatment of bacterial suspensions. A study of frequency, power and sonication time on cultured Bacillus species. Ultrasonics Sonochemistry 10 (6): 315–318.
Kadkhodaee, R., and M.J.W. Povey. 2008. Ultrasonic inactivation of Bacillus alpha-amylase. I. Effect of gas content and emitting face of probe. Ultrasonics Sonochemistry 15 (2): 133–142.
Kaloyereas, S.A. 1955. Preliminary report on the effect of ultrasonic waves on the crystallization of honey. Science 121 (3140): 339–340.
Karaseva, E.I., and D.I. Metelitza. 2006. Stabilization of glucoso-6-phosphate dehydrogenase by its substrate and cofactor in an ultrasonic field. Russian Journal of Bioorganic Chemistry 32 (5): 485–493.
Karshafian, R., P.D. Bevan, R. Williams, et al. 2009. Sonoporation by ultrasound-activated microbubble contrast agents: Effect of acoustic exposure parameters on cell membrane permeability and cell viability. Ultrasound in Medicine and Biology 35 (5): 847–860.
Kimbaris, A.C., N.G. Siatis, D.J. Daferera, et al. 2006. Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum). Ultrasonics Sonochemistry 13 (1): 54–60.
Kissam, A.D. 1985. Acoustic thawing of frozen food. Acoustical Society of America 78 (2): 819–819.
Kissam, A.D., R.W. Nelson, J. Ngao, et al. 1982. Water-thawing of fish using low-frequency acoustics. Journal of Food Science 47 (1): 71–75.
Knorr, D., M. Zenker, V. Heinz, et al. 2004. Applications and ultrasonics in food potential of processing. Trends in Food Science and Technology 15 (5): 261–266.
Koda, S., K. Taguchi, and K. Futamura. 2011. Effects of frequency and a radical scavenger on ultrasonic degradation of water-soluble polymers. Ultrasonics Sonochemistry 18 (1): 276–281.
Kordowska-Wiater, M., and D.M. Stasiak. 2011. Effect of ultrasound on survival of gram-negative bacteria on chicken skin surface. Bulletin of the Veterinary Institute in Pulawy 55 (2): 207–210.
Kowalski, S.J., J. Szadzinska, and A. Pawlowski. 2015. Ultrasonic-assisted osmotic dehydration of carrot followed by convective drying with continuous and intermittent heating. Drying Technology 33 (13SI1): 1570–1580.
Krefting, D., R. Mettin, and W. Lauterborn. 2004. High-speed observation of acoustic cavitation erosion in multibubble systems. Ultrasonics Sonochemistry 11 (3–4): 119–123.
Kumar, C., M.A. Karim, and M.U.H. Joardder. 2014. Intermittent drying of food products: A critical review. Journal of Food Engineering 121: 48–57.
Leal-Ramos, M.Y., A.D. Alarcon-Rojo, T.J. Mason, et al. 2011. Ultrasound-enhanced mass transfer in Halal compared with non-Halal chicken. Journal of the Science of Food and Agriculture 91 (1): 130–133.
Lechtanska, J.M., J. Szadzinska, and S.J. Kowalski. 2015. Microwave- and infrared-assisted convective drying of green pepper: Quality and energy considerations. Chemical Engineering and Processing 98: 155–164.
Lee, H., B. Zhou, W. Liang, et al. 2009. Inactivation of Escherichia coli cells with sonication, manosonication, thermosonication, and manothermosonication: Microbial responses and kinetics modeling. Journal of Food Engineering 93 (3): 354–364.
Leighton, T. 2007. What is ultrasound? Progress in Biophysics and Molecular Biology 93 (1–3): 3–83.
Leong, T., M. Ashokkumar, and S. Kentish. 2011. The fundamentals of power ultrasound—A review. Acoustics Australia 39 (2): 54–63.
Li, B., and D.W. Sun. 2002a. Novel methods for rapid freezing and thawing of foods—A review. Journal of Food Engineering 54 (3): 175–182. https://doi.org/10.1016/S0260-8774(01)00209-6.
Li, B., and D.W. Sun. 2002b. Effect of power ultrasound on freezing rate during immersion freezing of potatoes. Journal of Food Engineering 55 (3): 277–282. https://doi.org/10.1016/S0260-8774(02)00102-4.
Li, C.Z., M. Yoshimoto, H. Ogata, et al. 2005. Effects of ultrasonic intensity and reactor scale on kinetics of enzymatic saccharification of various waste papers in continuously irradiated stirred tanks. Ultrasonics Sonochemistry 12 (5): 373–384.
Li, H.Z., L. Pordesimo, and J. Weiss. 2004. High intensity ultrasound-assisted extraction of oil from soybeans. Food Research International 37 (7): 731–738.
Li, J., J. Ahn, D.H. Liu, et al. 2016. Evaluation of ultrasound induced damage on Escherichia coli and Staphylococcus aureus by flow cytometry and transmission electron microscopy. Applied and Environmental Microbiology 82 (6): 1828.
Li, J., Y.J. Suo, X.Y. Liao, et al. 2017. Analysis of Staphylococcus aureus cell viability, sublethal injury and death induced by synergistic combination of ultrasound and mild heat. Ultrasonics Sonochemistry 39: 101–110.
Lie Ken Jie, M.S.F., and M.S.K. Syed-Rahmatullah. 1995. Enzymatic hydrolysis of long-chain N-heterocyclic fatty esters. Lipids 30 (11): 995–999.
Liu, H.L., W.J. Chen, and S.N. Chou. 2003. Mechanisms of aggregation of alpha- and beta-amylases in aqueous dispersions. Colloids and Surfaces B-Biointerfaces 28 (2–3): 215–225. https://doi.org/10.1016/S0927-7765(02)00142-X.
Liu, S.L., X.Y. Dong, F. Wei, et al. 2015. Ultrasonic pretreatment in lipase-catalyzed synthesis of structured lipids with high 1,3-dioleoyl-2-palmitoylglycerol content. Ultrasonics Sonochemistry 23: 100–108.
Liu, Y.X., Q.Z. Jin, L. Shan, et al. 2008. The effect of ultrasound on lipase-catalyzed hydrolysis of soy oil in solvent-free system. Ultrasonics Sonochemistry 15 (4): 402–407.
Lopez, P., and J. Burgos. 1995. Peroxidase stability and reactivation after heat-treatment and mano thermosonication. Journal of Food Science 60 (3): 451.
Lopez, P., A. Vercet, A.C. Sanchez, et al. 1998. Inactivation of tomato pectic enzymes by manothermosonication. European Food Research and Technology 207 (3): 249–252.
Lopez-Malo, A., S. Guerrero, and S.M. Alzamora. 1999. Saccharomyces cerevisiae thermal inactivation kinetics combined with ultrasound. Journal of Food Protection 62 (10): 1215–1217.
Lopez-Malo, A., E. Palou, M. Jimenez-Fernandez, et al. 2005. Multifactorial fungal inactivation combining thermosonication and antimicrobials. Journal of Food Engineering 67 (1–2): 87–93.
Loretz, M., R. Stephan, and C. Zweifel. 2010. Antimicrobial activity of decontamination treatments for poultry carcasses: A literature survey. Food Control 21 (6): 791–804.
Luengo, E., S. Condon-Abanto, S. Condon, et al. 2014. Improving the extraction of carotenoids from tomato waste by application of ultrasound under pressure. Separation and Purification Technology 136: 130–136.
Ma, H.L., L.R. Huang, J.Q. Jia, et al. 2011. Effect of energy-gathered ultrasound on alcalase. Ultrasonics Sonochemistry 18 (1): 419–424.
Ma, X.B., W.J. Wang, M.M. Zou, et al. 2015. Properties and structures of commercial polygalacturonase with ultrasound treatment: role of ultrasound in enzyme activation. RSC Advances 5 (130): 107591–107600.
Ma, X.B., W.J. Wang, D.L. Wang, et al. 2016a. Degradation kinetics and structural characteristics of pectin under simultaneous sonochemical-enzymatic functions. Carbohydrate Polymers 154: 176–185.
Ma, X.B., L.F. Zhang, W.J. Wang, et al. 2016b. Synergistic effect and mechanisms of combining ultrasound and pectinase on pectin hydrolysis. Food and Bioprocess Technology 9 (7): 1249–1257.
Ma, X.B., D.L. Wang, M. Yin, et al. 2017. Characteristics of pectinase treated with ultrasound both during and after the immobilization process. Ultrasonics Sonochemistry 36: 1–10.
Maisonhaute, E., C. Prado, P.C. White, et al. 2002. Surface acoustic cavitation understood via nanosecond electrochemistry. PartIII: Shear stress in ultrasonic cleaning. Ultrasonics Sonochemistry, 9 (6): 297–303. https://doi.org/10.1016/S1350-4177(02)00089-5.
Manas, P., B. Muñz, D. Sanz, et al. 2006. Inactivation of lysozyme by ultrasonic waves under pressure at different temperatures. Enzyme and Microbial Technology 39 (6): 1177–1182.
Manas, P., R. Pagán, and J. Raso. 2000. Predicting lethal effect of ultrasonic waves under pressure treatments on Listeria monocytogenes ATCC 15313 by power measurements. Journal of Food Science 65 (4): 663–667.
Margulis, M.A., and I.M. Margulis. 2003. Calorimetric method for measurement of acoustic power absorbed in a volume of a liquid. Ultrasonics Sonochemistry 10 (6): 343–345.
Maskooki, A., T. Kobayashi, S.A. Mortazavi, et al. 2010. Effect of low frequencies and mixed wave of ultrasound and EDTA on flux recovery and cleaning of microfiltration membranes. Separation and Purification Technology 59: 67–73.
Mason, T.J. 2015. Some neglected or rejected paths in sonochemistry: A very personal view. Ultrasonics Sonochemistry 25 (SI): 89–93.
Mason, T.J., A.J. Cobley, J.E. Graves, et al. 2011. New evidence for the inverse dependence of mechanical and chemical effects on the frequency of ultrasound. Ultrasonics Sonochemistry 18 (1): 226–230.
Mason, T.J., L. Paniwnyk, and J.P. Lorimer. 1996. The uses of ultrasound in food technology. Ultrasonics Sonochemistry 3 (3): S253–S260.
Mason, T., E. Riera, A. Vercet, et al. 2005. Aplications of ultrasound. In Emerging Technologies for Food Processing, ed. D. Sun, 323–350. California, USA: Elsevier Academic Press.
McClements, D.J. 1995. Advances in the application of ultrasound in food analysis and processing. Trends in Food Science and Technology 6 (9): 293–299.
McDonnell, C.K., P. Allen, C. Morin, et al. 2014. The effect of ultrasonic salting on protein and water-protein interactions in meat. Food Chemistry 147: 245–251.
Merouani, S., O. Hamdaoui, Y. Rezgui, et al. 2013. Effects of ultrasound frequency and acoustic amplitude on the size of sonochemically active bubbles: Theoretical study. Ultrasonics Sonochemistry 20 (3): 815–819.
Meullemiestre, A., C. Breil, M. Abert-Vian, et al. 2016. Microwave, ultrasound, thermal treatments, and bead milling as intensification techniques for extraction of lipids from oleaginous Yarrowia lipolytica yeast for a biojetfuel application. Bioresource Technology 211: 190–199.
Miller, D.L., S.V. Pislaru, and J.F. Greenleaf. 2002. Sonoporation: Mechanical DNA delivery by ultrasonic cavitation. Somatic Cell and Molecular Genetics 27 (1–6): 115–134.
Miles, C.A., M.J. Morley, and M. Rendell. 1999. High power ultrasonic thawing of frozen foods. Journal of Food Engineering 39 (2): 151–159.
Mongenot, N., S. Charrier, and P. Chalier. 2000. Effect of ultrasound emulsification on cheese aroma encapsulation by carbohydrates. Journal of Agricultural and Food Chemistry 48 (3): 861–867.
Morild, R.K., P. Christiansen, A.H. Sorensen, et al. 2011. Inactivation of pathogens on pork by steam-ultrasound treatment. Journal of Food Protection 74 (5): 769–775.
Musielak, G., D. Mierzwa, and J. Kroehnke. 2016. Food drying enhancement by ultrasound: A review. Trends in Food Science and Technology 56: 126–141.
Muthukumaran, S., S.E. Kentish, M. Ashokkumar, et al. 2005a. Mechanisms for the ultrasonic enhancement of dairy whey ultrafiltration. Journal of Membrane Science 258 (1–2): 106–114.
Muthukumaran, S., S. Kentish, S. Lalchandani, et al. 2005b. The optimisation of ultrasonic cleaning procedures for dairy fouled ultrafiltration membranes. Ultrasonics Sonochemistry 12 (1–2): 29–35.
Muthukumaran, S., S.E. Kentish, G.W. Stevens, et al. 2007. The application of ultrasound to dairy ultrafiltration: The influence of operating conditions. Journal of Food Engineering 81 (2): 364–373.
Nitayavardhana, S., S.K. Rakshit, D. Grewell, et al. 2008. Ultrasound pretreatment of cassava chip slurry to enhance sugar release for subsequent ethanol production. Biotechnology and Bioengineering 101 (3): 487–496.
O’Donnell, C.P., B.K. Tiwari, P. Bourke, et al. 2010. Effect of ultrasonic processing on food enzymes of industrial importance. Trends in Food Science and Technology 21 (7): 358–367.
Oulahal-Lagsir, N., A. Martial-Gros, M. Bonneau, et al. 2003. “Escherichia coli-milk” biofilm removal from stainless steel surfaces: Synergism between ultrasonic waves and enzymes. Biofouling 19 (3): 159–168.
Ovsianko, S.L., E.A. Chernyavsky, V.T. Minchenya, et al. 2005. Effect of ultrasound on activation of serine proteases precursors. Ultrasonics Sonochemistry 12 (3): 219–223.
Ozuna, C., A. Puig, J.V. Garcia-Perez, et al. 2013. Influence of high intensity ultrasound application on mass transport, microstructure and textural properties of pork meat (Longissimus dorsi) brined at different NaCl concentrations. Journal of Food Engineering 119 (1): 84–93.
Pagan, R., P. Manas, I. Alvarez, et al. 1999a. Resistance of Listeria monocytogenes to ultrasonic waves under pressure at sublethal (manosonication) and lethal (manothermosonication) temperatures. Food Microbiology 16 (2): 139–148.
Pagan, R., P. Manas, A. Palop, et al. 1999b. Resistance of heat-shocked cells of Listeria monocytogenes to mano-sonication and mano-thermo-sonication. Letters in Applied Microbiology 28 (1): 71–75.
Pagan, R., P. Manas, J. Raso, et al. 1999c. Bacterial resistance to ultrasonic waves under pressure at nonlethal (manosonication) and lethal (manothermosonication) temperatures. Applied and Environmental Microbiology 65 (1): 297–300.
Palma, M., and C.G. Barroso. 2002. Ultrasound-assisted extraction and determination of tartaric and malic acids from grapes and winemaking by-products. Analytica Chimica Acta, 458 (1): 119–130. https://doi.org/10.1016/S0003-2670(01)01527-6.
Patist, A., and D. Bates. 2008. Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innovative Food Science and Emerging Technologies 9 (2): 147–154.
Persike, D.S., T. Bonfim, M. Santos, et al. 2002. Invertase and urease activities in the carotenogenic yeast Xanthophyllomyces dendrorhous (formerly Phaffia rhodozyma). Bioresource Technology 82 (1): 79–85.
Petigny, L., S. Périno-Issartier, J. Wajsman, et al. 2013. Batch and continuous ultrasound assisted extraction of boldo leaves (Peumus boldus Mol.). International Journal of Molecular Sciences 14 (3): 5750–5764.
Petrovic, S.S., J. Ivanovic, S. Milovanovic, et al. 2012. Comparative analyses of the diffusion coefficients from thyme for different extraction processes. Journal of the Serbian Chemical Society 77 (6): 799–813.
Pingret, D., A. Fabiano-Tixier, C. Le Bourvellec, et al. 2012. Lab and pilot-scale ultrasound-assisted water extraction of polyphenols from apple pomace. Journal of Food Engineering 111 (1): 73–81.
Piyasena, P., E. Mohareb, and R.C. McKellar. 2003. Inactivation of microbes using ultrasound: A review. International Journal of Food Microbiology 87 (3): 207–216.
Popa, M., P.C. Ionescu, and C. Vasiliuoprea. 1994. Mechanochemical complexation of micronized cellulose with CO(3+) ions by ultrasonic treatment in order to obtain macromolecular carriers for invertase immobilization. Cellulose Chemistry and Technology 28 (2): 129–141.
Potapovich, M.V., A.N. Eremin, and D.I. Metelitza. 2003. Kinetics of catalase inactivation induced by ultrasonic cavitation. Applied Biochemistry and Microbiology 39 (2): 140–146.
Prajapat, A.L., P.B. Subhedar, and P.R. Gogate. 2016. Ultrasound assisted enzymatic depolymerization of aqueous guar gum solution. Ultrasonics Sonochemistry 29: 84–92.
Prajapat, A.L., and P.R. Gogate. 2015. Intensification of degradation of guar gum: Comparison of approaches based on ozone, ultraviolet and ultrasonic irradiations. Chemical Engineering and Processing 98: 165–173.
Ramachandran, K.B., S. Al-Zuhair, C.S. Fong, et al. 2006. Kinetic study on hydrolysis of oils by lipase with ultrasonic emulsification. Biochemical Engineering Journal 32 (1): 19–24.
Raso, J., R. Pagan, S. Condon, et al. 1998a. Influence of temperature and pressure on the lethality of ultrasound. Applied and Environmental Microbiology 64 (2): 465–471.
Raso, J., A. Palop, R. Pagan, et al. 1998b. Inactivation of Bacillus subtilis spores by combining ultrasonic waves under pressure and mild heat treatment. Journal of Applied Microbiology 85 (5): 849–854.
Raviyan, P., Z. Zhang, and H. Feng. 2005. Ultrasonication for tomato pectinmethylesterase inactivation: effect of cavitation intensity and temperature on inactivation. Journal of Food Engineering 70 (2): 189–196.
Rayaroth, M.P., U.K. Aravind, and C.T. Aravindakumar. 2016. Degradation of pharmaceuticals by ultrasound-based advanced oxidation process. Environmental Chemistry Letters 14 (3): 259–290.
Resa, P., L. Elvira, F.M. de Espinosa, et al. 2005. Ultrasonic velocity in water-ethanol-sucrose mixtures during alcoholic fernientation. Ultrasonics 43 (4): 247–252.
Riesz, P., and T. Kondo. 1992. Free-radical formation induced by ultrasound and its biological implications. Free Radical Biology and Medicine 13 (3): 247–270.
Rivera, C.S., M.E. Venturini, R. Oria, et al. 2011. Selection of a decontamination treatment for fresh Tuber aestivum and Tuber melanosporum truffles packaged in modified atmospheres. Food Control 22 (3–4): 626–632.
Roncales, P., P. Cena, J.A. Beltran, et al. 1993. Ultrasonication of lamb skeletal-muscle fibers enhances postmortem proteolysis. European Food Research and Technology 196 (4): 339–342.
Sagong, H., S. Lee, P. Chang, et al. 2011. Combined effect of ultrasound and organic acids to reduce Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh lettuce. International Journal of Food Microbiology 145 (1): 287–292.
Sakakibara, M., D. Wang, R. Takahashi, et al. 1996. Influence of ultrasound irradiation on hydrolysis of sucrose catalyzed by invertase. Enzyme and Microbial Technology 18 (6): 444–448.
Sango, D.M., D. Abela, A. Mcelhatton, et al. 2014. Assisted ultrasound applications for the production of safe foods. Journal of Applied Microbiology 116 (5): 1067–1083.
Sala Trepat, F.J. 1995. Combined processes for food preservation. Microbiologia 11 (1): 23–32.
Sala, F.J., J. Burgos, S. Condon, et al. 1995. Effect of heat and ultrasounds on microorganisms and enzymes. In New methods of food preservation, ed. G.W. Gould, 176–204. Glasgow, US: Springer.
Santacatalina, J.V., M. Contreras, S. Simal, et al. 2016. Impact of applied ultrasonic power on the low temperature drying of apple. Ultrasonics Sonochemistry 28: 100–109.
Sanz, P.D., C. de Elvira, M. Martino, et al. 1999. Freezing rate simulation as an aid to reducing crystallization damage in foods. Meat Science 52 (3): 275–278.
Schoessler, K., H. Jaeger, and D. Knorr. 2012. Novel contact ultrasound system for the accelerated freeze-drying of vegetables. Innovative Food Science and Emerging Technologies 16: 113–120.
Shirsath, S.R., S.H. Sonawane, and P.R. Gogate. 2012. Intensification of extraction of natural products using ultrasonic irradiations—A review of current status. Chemical Engineering and Processing 53: 10–23.
Sinisterra, J.V. 1992. Application of ultrasound to biotechnology—An overview. Ultrasonics 30 (3): 180–185.
Siro, I., C. Ven, C. Balla, et al. 2009. Application of an ultrasonic assisted curing technique for improving the diffusion of sodium chloride in porcine meat. Journal of Food Engineering 91 (2): 353–362.
Sivakumar, V., J. Vijaeeswarri, and J.L. Anna. 2011. Effective natural dye extraction from different plant materials using ultrasound. Industrial Crops and Products 33 (1): 116–122.
Soria, A.C., and M. Villamiel. 2010. Effect of ultrasound on the technological properties and bioactivity of food: a review. Trends in Food Science & Technology 21 (7): 0–331.
Stadnik, J., Z.J. Dolatowski, and H.M. Baranowska. 2008. Effect of ultrasound treatment on water holding properties and microstructure of beef (M. semimembranosus) during ageing. LWT-Food Science and Technology 41 (10): 2151–2158.
Subhedar, P.B., and P.R. Gogate. 2014. Enhancing the activity of cellulase enzyme using ultrasonic irradiations. Journal of Molecular Catalysis B-Enzymatic 101: 108–114.
Sun, D.W., and B. Li. 2003. Microstructural change of potato tissues frozen by ultrasound-assisted immersion freezing. Journal of Food Engineering 57(4): 337–345. https://doi.org/10.1016/S0260-8774(02)00354-0.
Sun, Y.J., G.P. Ma, X.Q. Ye, et al. 2010. Stability of all-trans-β-carotene under ultrasound treatment in a model system: Effects of different factors, kinetics and newly formed compounds. Ultrasonics Sonochemistry 17 (4): 654–661.
Suslick, K.S., N.C. Eddingsaas, D.J. Flannigan, et al. 2011. Extreme conditions during multibubble cavitation: Sono luminescence as a spectroscopic probe. Ultrasonics Sonochemistry 18 (4SI): 842–846.
Suslick, K.S., and G.J. Price. 1999. Applications of ultrasound to materials chemistry. Annual Review of Materials Science 29: 295–326.
Szabo, O.E., and E. Csiszar. 2017. Some factors affecting efficiency of the ultrasound-aided enzymatic hydrolysis of cotton cellulose. Carbohydrate Polymers 156: 357–363.
Tarun, E.I., I.E. Adzerikho, and D.I. Metelitsa. 2003. Inactivation of urease under the action of ultrasonically induced cavitation. Russian Journal of Physical Chemistry 77 (3): 468–476.
Tarun, E.I., V.P. Kurchenko, and D.I. Metelitza. 2006. Flavonoids as effective protectors of urease from ultrasonic inactivation in solutions. Russian Journal of Bioorganic Chemistry 32 (4): 391–398.
Tauber, M.M., G.M. Guebitz, and A. Rehorek. 2005. Degradation of azo dyes by laccase and ultrasound treatment. Applied and Environmental Microbiology 71 (5): 2600–2607.
Tian, Z.M., M.X. Wan, S.P. Wang, et al. 2004. Effects of ultrasound and additives on the function and structure of trypsin. Ultrasonics Sonochemistry 11 (6): 399–404.
Tiwari, B. K. 2015. Ultrasound: A clean, green extraction technology. TrAC-Trends in Analytical Chemistry, 71 (SI): 100–109.
Toma, M., S. Fukutomi, Y. Asakura, et al. 2011. A calorimetric study of energy conversion efficiency of a sonochemical reactor at 500 kHz for organic solvents. Ultrasonics Sonochemistry 18 (1): 197–208.
Toma, M., M. Vinatoru, L. Paniwnyk, et al. 2001. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrasonics Sonochemistry 8 (2): 137–142.
Tzanakis, I., W.W. Xu, D.G. Eskin, et al. 2015. In situ observation and analysis of ultrasonic capillary effect in molten aluminium. Ultrasonics Sonochemistry 27: 72–80.
Ugarte-Romero, E., H. Feng, S.E. Martin, et al. 2006. Inactivation of Escherichia coli with power ultrasound in apple cider. Journal of Food Science 71 (2): E102–E108.
Vargas, L., A. Piao, R.N. Domingos, et al. 2004. Ultrasound effects on invertase from Aspergillus niger. World Journal of Microbiology and Biotechnology 20 (2): 137–142.
Veillet, S., V. Tomao, and F. Chemat. 2010. Ultrasound assisted maceration: An original procedure for direct aromatisation of olive oil with basil. Food Chemistry 123 (3): 905–911.
Vercet, A., J. Burgos, S. Crelier, et al. 2001. Inactivation of proteases and lipases by ultrasound. Innovative Food Science and Emerging Technologies 2 (2): 139–150.
Vercet, A., P. Lopez, and J. Burgos. 1997. Inactivation of heat-resistant lipase and protease from Pseudomonas fluorescens by manothermosonication. Journal of Dairy Science 80 (1): 29–36.
Vercet, A., P. Lopez, and J. Burgos. 1998. Free radical production by manothermosonication. Ultrasonics 36 (1–5): 615–618.
Vilkhu, K., R. Mawson, L. Simons, et al. 2008. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innovative Food Science and Emerging Technologies 9 (2): 161–169.
Villamiel, M., and P. de Jong. 2000. Influence of high-intensity ultrasound and heat treatment in continuous flow on fat, proteins, and native enzymes of milk. Journal of Agricultural and Food Chemistry 48 (7): 3068.
Villamiel, M., E.H. van Hamersveld, and P. de Jong. 1999. Review: Effect of ultrasound processing on the quality of dairy products. Milchwissenschaft-Milk Science International 54 (2): 69–73.
Villamiel, M., R. Verdurmen, and P. de Jong. 2000. Degassing of milk by high-intensity ultrasound. Milchwissenschaft-Milk Science International 55 (3): 123–125.
Vinatoru, M. 2001. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrasonics Sonochemistry 8 (3): 303–313.
Vinatoru, M., M. Toma, O. Radu, et al. 1997. The use of ultrasound for the extraction of bioactive principles from plant materials. Ultrasonics Sonochemistry 4 (2): 135–139.
Wang, Z.M., Y.C. Cheung, P.H. Leung, et al. 2010. Ultrasonic treatment for improved solution properties of a high-molecular weight exopolysaccharide produced by a medicinal fungus. Bioresource Technology 101 (14): 5517–5522.
Wang, Z.B., X.M. Lin, P.P. Li, et al. 2012. Effects of low intensity ultrasound on cellulase pretreatment. Bioresource Technology 117: 222–227.
Wang, W.J., X.B. Ma, P. Jiang, et al. 2016. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocolloids 61: 730–739.
Wang, W.J., X.B. Ma, Y.T. Xu, et al. 2015. Ultrasound-assisted heating extraction of pectin from grapefruit peel: Optimization and comparison with the conventional method. Food Chemistry 178: 106–114.
Whillock, G., and B.F. Harvey. 1997. Ultrasonically enhanced corrosion of 304L stainless steel. 1. The effect of temperature and hydrostatic pressure. Ultrasonics Sonochemistry 4 (1): 23–31.
Williams, A.R., D.A. Stafford, A.G. Callely, et al. 1970. Ultrasonic dispersal of activated sludge flocs. Journal of Applied Bacteriology 33 (4): 656.
Winder, W.C., D.J. Aulik, and A.C. Rice. 1970. An ultrasonic method for direct and simultaneous determination of alcohol and extract content of wines. American Journal of Enology and Viticulture 21 (1): 1.
Windhab, E.J., M. Dressler, K. Feigl, et al. 2005. Emulsion processing—From single-drop deformation to design of complex processes and products. Chemical Engineering Science 60 (8–9): 2101–2113.
Wood, B.E., H.C. Aldrich, and L.O. Ingram. 1997. Ultrasound stimulates ethanol production during the simultaneous saccharification and fermentation of mixed waste office paper. Biotechnology Progress 13 (3): 232–237.
Wordon, B.A., B. Mortimer, and L.D. McMaster. 2012. Comparative real-time analysis of Saccharomyces cerevisiae cell viability, injury and death induced by ultrasound (20 kHz) and heat for the application of hurdle technology. Food Research International 47 (2SI): 134–139.
Wu, H., G.J. Hulbert, and J.R. Mount. 2001. Effects of ultrasound on milk homogenization and fermentation with yogurt starter. Innovative Food Science and Emerging Technology 1 (3): 211–218.
Wu, J.Y., and X.C. Ge. 2004. Oxidative burst, jasmonic acid biosynthesis, and taxol production induced by low-energy ultrasound in Taxus chinensis cell suspension cultures. Biotechnology and Bioengineering 85 (7): 714–721.
Wu, J.Y., and L.D. Lin. 2002. Ultrasound-induced stress responses of Panax ginseng cells: Enzymatic browning and phenolics production. Biotechnology Progress 18 (4): 862–866. https://doi.org/10.1021/bp0255210.
Wu, J., T.V. Gamage, K.S. Vilkhu, et al. 2008. Effect of thermosonication on quality improvement of tomato juice. Innovative Food Science and Emerging Technologies 9 (2): 186–195.
Wu, J., and L. Lin. 2003. Enhancement of taxol production and release in Taxus chinensis cell cultures by ultrasound, methyl jasmonate and in situ solvent extraction. Applied Microbiology and Biotechnology 62 (2–3): 151–155.
Xiao, Y.M., Q. Wu, Y. Cai, et al. 2005. Ultrasound-accelerated enzymatic synthesis of sugar esters in nonaqueous solvents. Carbohydrate Research 340 (13): 2097–2103.
Xie, Y.M., H. Wu, and Y.M. Lai. 2002. Deinking of colored offset newsprint with enzyme treatment in cooperation with ultrasonic wave. Cellulose Chemistry and Technology 36 (3–4): 285–293.
Xiong, G.Y., L.L. Zhang, W. Zhang, et al. 2012. Influence of ultrasound and proteolytic enzyme inhibitors on muscle degradation, tenderness, and cooking loss of Hens during aging. Czech Journal of Food Sciences 30 (3): 195–205.
Xu, Y.T., L.F. Zhang, Y. Bailina, et al. 2014. Effects of ultrasound and/or heating on the extraction of pectin from grapefruit peel. Journal of Food Engineering 126: 72–81.
Yachmenev, V.G., E.J. Blanchard, and A.H. Lambert. 2004. Use of ultrasonic energy for intensification of the bio-preparation of greige cotton. Ultrasonics 42 (1–9): 87–91.
Yachmenev, V., B. Condon, T. Klasson, et al. 2009. Acceleration of the enzymatic hydrolysis of corn stover and sugar cane bagasse celluloses by low intensity uniform ultrasound. Journal of Biobased Materials and Bioenergy 3 (1): 25–31.
Yemenicioglu, A., and B. Cemeroglu. 2003. Consistency of polyphenol oxidase (PPO) thermostability in ripening apricots (Prunus armeniaca L.): Evidence for the presence of thermostable PPO forming and destabilizing mechanisms in apricots. Journal of Agricultural and Food Chemistry 51 (8): 2371–2379.
Yue, W., P.J. Yao, Y.N. Wei, et al. 2008. Synergetic effect of ozone and ultrasonic radiation on degradation of chitosan. Polymer Degradation and Stability 93 (10): 1814–1821.
Zenker, M., V. Heinz, and D. Knorr. 2003. Application of ultrasound-assisted thermal processing for preservation and quality retention of liquid foods. Journal of Food Protection 66 (9): 1642–1649.
Zhang, L.F., X.Q. Ye, T. Ding, et al. 2013a. Ultrasound effects on the degradation kinetics, structure and rheological properties of apple pectin. Ultrasonics Sonochemistry 20 (1): 222–231.
Zhang, L.F., X.Q. Ye, S.J. Xue, et al. 2013b. Effect of high-intensity ultrasound on the physicochemical properties and nanostructure of citrus pectin. Journal of the Science of Food and Agriculture 93 (8): 2028–2036.
Zhang, L.F., X.Z. Zhang, D.H. Liu, et al. 2015. Effect of degradation methods on the structural properties of citrus pectin. LWT-Food Science and Technology 61 (2): 630–637.
Zhang, Z.S., L.J. Wang, D. Li, et al. 2008. Ultrasound-assisted extraction of oil from flaxseed. Separation and Purification Technology 62 (1): 192–198.
Zheng, L., and D.W. Sun. 2006. Innovative applications of power ultrasound during food freezing processes—A review. Trends in Food Science and Technology 17 (1): 16–23.
Zheng, M.M., L. Wang, F.H. Huang, et al. 2013. Ultrasound irradiation promoted lipase-catalyzed synthesis of flavonoid esters with unsaturated fatty acids. Journal of Molecular Catalysis B-Enzymatic 95: 82–88.
Zhi, Z.J., J.L. Chen, S. Li, et al. 2017. Fast preparation of RG-I enriched ultra-low molecular weight pectin by an ultrasound accelerated Fenton process. Scientific Reports 7: 541.
Zhou, B., H. Feng, and Y.G. Luo. 2009. Ultrasound enhanced sanitizer efficacy in reduction of escherichia coli O157:H7 population on spinach leaves. Journal of Food Science 74 (6): M308–M313.
Zhou, C.S., Y. Wang, H.L. Ma, et al. 2008. Effect of ultrasonic degradation on in vitro antioxidant activity of polysaccharides from Porphyra yezoensis (Rhodophyta). Food Science and Technology International 14 (6): 479–486.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd. and Zhejiang University Press, Hangzhou
About this chapter
Cite this chapter
Liu, D., Ma, X., Wang, W., Zou, M., Wang, D., Ling, J. (2019). Research Progress on Power Ultrasound Technology. In: Jia, J., Liu, D., Ma, H. (eds) Advances in Food Processing Technology. Springer, Singapore. https://doi.org/10.1007/978-981-13-6451-8_7
Download citation
DOI: https://doi.org/10.1007/978-981-13-6451-8_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-6450-1
Online ISBN: 978-981-13-6451-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)