Abstract
Seaweeds or macroalgae are of extreme relevance in the scientific community as this biomass offers un-parallel opportunities in the discovery of new molecules and compounds including mainly carbohydrates, proteins, and lipids, as well as other minor compounds, such as minerals,vitamins and pigments. Most of these compounds have promising potential and demonstrated health benefits in in vivo and/or in vitro models, including antioxidant, anti-tumor, and antibacterial activities. Thus, the interest of several industries in seaweeds has been renewed as not only a source of biomass for basic nutrients but as promising “multi-factories” able to produce huge amounts of compounds with food/feed, pharmaceutical, and cosmeceutical applications. This chapter summarizes the current studies exploring the composition of seaweeds, emphasizing the most promising applications and/or biological activities of their main compounds from a food industry perspective; as well as current challenges for their exploitation including the extraction of the compounds and the sustainability issues associated to this growing industry from both an Irish and global perspectives.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Seaweeds have been commercially harvested for thousands of years in more than 50 countries, in waters ranging from cold to tropical. The global market of seaweeds, with a current annual growth rate of 8.9%, it is estimated to reach a value of around USD 22.13 billion by 2024 (Zhu et al. 2021a). In 2016, all the seaweeds harvested in Ireland summed up to 29,500 tons of biomass, accounting for 11% of the overall European seaweed market, making Ireland the third most productive country in Europe, right behind Norway and France (Table 10.1) (FAO 2018). Seaweed species can be classified within 3 phyla namelyRhodophyta (red algae), Phaeophyta (brown algae), and Chlorophyta (green algae). Amongst all the seaweed species available, the Irish industry identified 9 of them (Ascophyllum nodosum, Laminaria digitata, Laminaria hyperborea, Palmaria palmata, Chondrus crispus, Himanthalia elongata, Fucus serratus, Lithothamnion corallioides, and Ulva lactuca) as priorities regarding further exploitation opportunities of the biomass as a source of high-value molecules with added health benefits or bioactive compounds (Troy et al. 2017).
Seaweeds are traditional low caloric foods and their composition includes mainly polysaccharides and dietary fibres, proteins, polyunsaturated fatty acids (PUFA), and other compounds including pigments, vitamins, minerals and phenolic compounds. The industrial use of this biomass has shifted over the years. At all stages, the ‘potential’ of the algal industry has been viewed as being larger than its actual scale (Hafting et al. 2015). Moreover, the huge amount of bioactive compounds discovered in seaweeds anticipates promising prospects for the seaweed industry in Ireland and globally in the near future. These algal compounds can be used as functional products, such as value-added ingredients for food and feed (Fleurence 2016; Shama et al. 2019), cosmaceuticals (Wijesinghe and Jeon 2011), nutraceuticals (Shannon and Abu-Ghannam 2019), pharmaceuticals (Moualee and Pradhanang 2019; Rajauria et al. 2016), as well as low value applications, such as bioenergy,ensuring the full utilization of the biomass (Fernand et al. 2017). Currently, seaweed biorefinery has been proposed and multiple exploitation models are currently being developed aiming to minimize waste and environmental impacts of the seaweed industry while utilizing fully these natural resources. To be able to develop these exploitation models, a deep knowledge of seaweed composition, potential health benefits of seaweed compounds, as well as extraction technologies available for the recovery of these molecules, will be relevant in determining the future application of the biomass and derivedcompounds as summarized below.
2 Seaweeds as a Source of Major Nutritional Compounds
Seaweeds or macroalgae are a large and diverse group of organisms with over 10,000 species identified worldwide, being only 5% of them currently exploited for food or animal feed applications (Chojnacka et al. 2012). The consumption of seaweeds has a long tradition in many Asian countries, with a documented use of whole or processed algae over millennia (Fleurence et al. 2012). In Western countries, such as coastal areas of Ireland, Brittany, and Iceland, there was a discontinued tradition for eating seaweeds or incorporating them as ingredients or additives to improve the sensory attributes of foods, such as bread (Holdt and Kraan 2011; Mouritsen et al. 2013). The recent change of consumers’ perception of seaweeds as healthy, nutritious, and tasty food commodities has increased the demand of food products and dietary supplements containing algae in recent decades (Mouritsen et al. 2013; Roleda et al. 2019; Lafarga et al. 2021).
In terms of their composition, overall, the scientific literature describes the macroalgal biomass is rich in carbohydrates (up to 60%), with medium or high amounts of proteins (10–47%), low in lipids (1–3%), and variable contents of minerals (7–38%) and pigments (Dominguez and Loret 2019; Kraan 2013). Moreover, seaweeds can adapt to the rapid changes of the marine environmental conditions, i.e. changes in temperature, solar radiation, by producing unique secondary metabolites including polysaccharides, proteins, lipids, and phenolic compounds (Collins et al. 2016; García-Vaquero et al. 2017). Prolonged exposure of macroalgae to environmental stressors, such as fluctuations in water level, solar radiation and temperature, can lead to the formation of reactive oxygen species and other free radicals in the biomass. As a defense mechanism, the stressed macroalgal biomass produces high amounts of antioxidant compounds, such as phenolic compounds and sulphated polysaccharides amongst others, trying to maintain the integrity of the cellular structures (Roleda et al. 2019). Thus, this macroalgal biomass enriched in antioxidant compounds could be incorporated as food ingredients or supplements, providing additional health benefits to those of basic nutrition namely nutraceuticals or functional foods (Garcia-Vaquero and Hayes 2016) that could help in the prevention of chronic diseases, such as cancer, cardiovascular diseases, obesity and diabetes (Déléris et al. 2016; Lordan et al. 2011).
Understanding the composition of the major macronutrients of the macroalgal biomass (carbohydrates, proteins, lipids), as well as other minor compounds and micronutrients produced by macroalgae, will help to understand and elucidate the future utilization of this biomass and their derived compounds and associated health benefit for food and nutraceutical applications.
2.1 Carbohydrates
Carbohydrates are one of the major components of macroalgae, with overall contents ranging from 4–76% of the dry weight (DW) of seaweeds (Holdt and Kraan 2011). Significant variations in the overall amount of carbohydrates in seaweeds have been described depending on the species, the season of collection, and even parts of the biomass sampled (Kim 2012; Men’shova et al. 2012; Skriptsova et al. 2012). The main type of carbohydrates described in each seaweed class (red, brown or green macroalgae) together with their biological functions in the biomass and the future uses or applications of these compounds in the food industry are summarized in Table 10.2.
In general, and based on their functions in seaweeds, polysaccharides can be classified as (1) structural carbohydrates, involved in the maintenance of the structural integrity of the macroalgal cell walls; or (2) energy reserve polysaccharides, intracellular polysaccharides present inside reserve vacuoles and functions related to energy storage within the algal cells (García-Vaquero et al. 2017; Kumar et al. 2008; Ojima 2013). Once these compounds are extracted from their original matrices these carbohydrates can (1) exert biological benefits, and thus be used as bioactive compounds; (2) possess relevant physical properties that allow their use as stabilizers, thickeners, and emulsifiers; or (3) both, being valued compounds for both their physical and biological attributes when included in food (Lafarga et al. 2020).In fact, most macroalgal polysaccharides currently used as hydrocolloids, mainly alginate, agar, and carrageenan, have shown also promising biological properties (Gomez et al. 2020) expanding their range of applications to multiple industries including food, pharmaceutical, and cosmaceuticals. Other polysaccharides, such as fucoidans and laminarins, have recently gained momentum in the scientific literature due to their wide range and promising biological activities including anti-inflammatory, anticoagulant, antioxidant, antiviral, antitumor, antiapoptosis, antiproliferative, and immunostimulatory when assayed in vitro and/or in vivo models (García-Vaquero et al. 2017).
The industrial application of these carbohydrates in the food industry relies on the extraction of these high-value compounds from the seaweed biomass.These extraction processes should ideally be selective, achieve high yields of compounds, minimize the generation of waste and reduce the use of solvents and energy during the extraction process. Thus, increased research activity and industry interest has focused in recent years on using innovative technologies, including enzyme-assisted extraction, ultrasound-assisted extraction, microwave-assisted extraction, supercritical fluid extraction, ultra-high pressure extraction, and pressurized fluid extraction (Gomez et al. 2020). Moreover, recent technological trends in the sector include the use of a combination of technologies, applying multiple extraction forces in the biomass either sequentially or simultaneously aiming to increase the yields of polysaccharides extracted from the biomass. Garcia-Vaquero et al. (2020) explored ultrasounds, microwaves, and the simultaneous application of both extraction forces to extract fucoidan from A. nodosum. The authors demonstrated that both innovative technologies alone had a significant influence on the extraction of fucoidan, however, the yields of fucoidan extracted from this brown macroalgae using a simultaneous application of both ultrasounds and microwaves achieved yields of fucoidan of approximately 10 and three fold compared to the use of ultrasounds and microwaves alone, respectively (Garcia-Vaquero et al. 2020).
2.2 Proteins
Seaweeds have been increasingly studied as a source of proteins. Proteins are essential nutrients in both animal and human nutrition, as well as one of the most expensive ingredients in the diet. Thus, increased research interest has focused on proteins from seaweeds as novel and cheap alternative sources of protein (Nunes et al. 2014; Fleurence et al. 2018). However, the exploitation of seaweeds as a source of protein still offers challenges, mainly related to the wide ranges of protein content described in different seaweed species, ranging between 3 and 47% (Harnedy and Fitzgerald 2013); as well as the influences of external factors, such as season of harvest and place of collection (Zhu et al. 2021a), on the protein contents of the biomass.
Moreover, the methodology used to determine the levels of protein in seaweeds also represents a huge variation in the protein levels reported in the scientific literature. Commonly, the protein contents of bseaweeds have been assessed by measuring the nitrogen content of the biomass and then multiplying it by specific conversion factors. In terms of seaweeds, these conversion factors range from 3.75 to 5.72 (Lourenço et al. 2002). However, as some of the seaweeds are regarded as edible green food, many studies are using 6.25, the general nitrogen-to-protein factor, in their studies. Macroalgae may have non-protein nitrogen, including free amino acids, chlorophyll, nitrates, ammonium ions, and nucleic acids, and thus, the protein content calculation with 6.25 often results in an over estimation of algal protein (Makkar et al. 2016). Table 10.3 summarizes total protein concentrations (% DW) in selected brown, red, and green seaweed species. Generally, the protein content decreases in the order of seaweed group: red>green>brown (Černá 2011).
The most promising seaweed protein families,from a biotechnological point of view,are lectins and phycobilli-proteins. Lectins are carbohydrate binding proteins contributing in cell communication, including recognition of foreign or cancerous cells (Ziółkowska and Wlodawer 2006). Some red algal lectins have painkilling effects, anti-inflammatory, anti-cancer properties, and the potential to develop anti-viral agents with activity against HIV and coronavirus (Singh and Walia 2018).
Other compounds derived from algal proteins, such as amino acids (AA) and bioactive peptides, are being studied in recent years due to their promising biological benefits when included in foods, but also as cosmetic, pharmaceutical, and other applications in the biotechnologicalindustry (Harnedy and Fitzgerald 2011). Macroalgae contain all AA (Matanjun et al. 2009) and are regarded as an excellent source of essential amino acids (EAA),especially red and green species (Organization 1991; Wong and Cheung 2000). Contradictory results have been reported on the levels of particular AA between seaweed species. Methionine and cysteine were described to be at higher levels in red seaweeds compared to green and brown seaweeds (Qasim 1991), but an opposite behavior was reported by Gressler et al. (2011). Gaillard et al. (2018) studied the amino acid content in 9 seaweed species and determined that glutamine was the most abundant AA followed by Asp and Ala. In general, the seaweeds were rich in threonine, serine, glycine, valine, leucine, lysine, and arginine (on average 4.58 g/16 g N, ranging from 4.06 to 4.99 g/16 g N), and low in cysteine, histidine, and methionine (on average 1.70 g/16 g N, rangingfrom 1.24 to 2.28 g/16 g N).The AA concentrations of crude protein varied with the seasons, species, and methods of AA analysis used.
Moreover, seaweeds as well as other protein-rich products can be ideal candidates for the generation of bioactive peptides or cryptides (Garcia-Vaquero et al. 2019). Bioactive peptides are sequences of 2 to 30 amino acids in length that have hormone-like beneficial properties once these compounds are released from their original or parent proteins through several hydrolytic processes (Garcia-Vaquero et al. 2019). In Japan, a number of functional foods have been already commercialized as permitted by the Japanese Ministry of Health and Welfare (Arai 2000). Although there is a growing trend in isolating proteinand bioactive peptides from marine algae, the number of biologically active compounds generated from seaweed is still limited. Most seaweed bioactive compounds, including protein, are contained inside the algal cells by a highly rigid and structurally complex cell wall and thus, this has been described as one of the major obstaclesfor an efficient utilizationand digestibility of algal proteins (Fleurence et al. 2012; Harnedy and Fitzgerald 2011). There is a need to extract these compounds from seaweed, optimizing these processes in terms of resources (i.e. chemicals, solvents, energy…) consumed during the extraction, while maintaining or increasing the yields of protein achieved. Some protein extraction processes extracted from the recent scientific literature are summarized in Table 10.3. Some of the potential technologies with promising applications improving the extraction of protein from seaweeds include the use of ultrasound (Kadam et al. 2017), microwaves (Chemat 2012), supercritical fluid extraction (Liang and Fan 2013) and ultrahigh-pressure extraction (Xi et al. 2013) amongst others.
2.3 Lipids
Lipids are essential macronutrients in the diet as a source of energy and other antioxidants, such as tocopherols and carotenoids (Bialek et al. 2017). Lipids in seaweeds are present at relatively low levels (1–5% of DW), and thus, fresh seaweeds can be considered as low-energy foods. The health benefits of any source of dietary fat are linked to the fatty acid composition of the lipids, especially the content of PUFA. PUFA, such as eicosapentaenoic acid and arachidonic acid, are proved to be beneficial in controlling blood pressure and blood clotting and reducing the risk of cardiovascular diseases, osteoporosis, and diabetes (Maeda et al. 2008). The optimum dietary ratio of PUFAs (omega-6 to omega-3) of 5:1 is recommended by the European Nutritional Society, however, most of the European diets failed to meet these recommendations (Holdt and Kraan 2011). Fish oils have been utilized as the source of omega-3 or n-3 PUFAs (Rubio-Rodríguez et al. 2010). However, due to the environmental, economic, sustainable and food safety concerns, novel sources of PUFAs are currently required (Vannuccini et al. 2019).
As shown in Table 10.4, total PUFAs in selected seaweeds range from 16.64 to 23.8% of the total amount of fatty acids.Undaria pinnatifida has eicosapentaenoic acid at concentrations of up to 0.14 g per 100 g DW of seaweeds’ thallus (Khan et al. 2007). Gosch et al. (2012) reported that the green seaweed Derbesia tenuissima had high levels of fatty acids (39.58 mg/g DW) with a high proportion of PUFA (n-3) (31% of total fatty acid), which are suitable as nutraceuticals or fish oil replacers.Regarding biofuel production, Spatoglossum macrodontum had the highest fatty acid contents (57.40 mg/g DW) with a high content of C18:1, which is suitable as a biofuel feedstock (Gosch et al. 2012).
Generally, macro- and micronutrients, the lipid content and fatty acid composition in seaweeds are influenced by their intrinsic traits andalso climatological conditions (light intensity, seawater salinity, and temperature). Generally, red and brown seaweeds are rich in eicosapentaenoic and arachidonic acids, and green seaweeds like Ulva pertusa predominantly contain hexadecatetraenoic, oleic, and palmitic acids (Ortiz et al. 2006). Regarding the environmental conditions affecting the biomass, high light intensity and low salinity results in a decreased level of total fatty acids within the same species (Floreto and Teshima 1998). Furthermore, Ortiz et al. (2006) reported a strong influence of the temperature of the water (particularly low temperatures) on the fatty acid levels produced in different seaweed species.
There is an increased interest in the extraction of lipids from seaweed biomass. Traditionally the recovery of lipophilic compounds has been achieved by using a Soxhlet apparatus or soaking the biomass in organic solvents (Dickson et al. 2020). Recent developments in lipid extraction include the use of supercritical fluid extraction,as well as the application of ultrasounds and microwaves (Cravotto et al. 2008).
3 Macroalgae as a Source of Other Minor Compounds
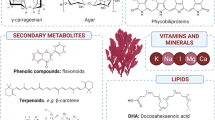
Apart from the aforementioned dominant macronutrients, minerals, vitamins, and other pigments are also highly valued compounds present in seaweeds. Seaweeds contain significant amounts of minerals (I, Ca, Fe, Zn, Mn, and Cu), which perform many important biological functions, including assisting in cell transportation and other metabolic processes (Mišurcová et al. 2011). Seaweeds can absorb inorganic substances from the environment due to the interactions of these compounds with cell surface polysaccharides (Yoshioka et al. 2007). Similar to the levels of macronutrients, the mineral profiles of seaweeds are also really variable depending on the type of seaweeds, season and place of collection (Teas et al. 2004). High contents of minerals have been reported in the literature in certain seaweed species. I.e. in Ulva clathrata biomass collected from Mexico the mineral contents of this seaweed represented approximately 49.6% of the dry matter of the biomass (Peña-Rodríguez et al. 2011). Mišurcová et al. (2011) summarized the mineral contents in selected marine algae and claimed that many of the seaweed species can be excellent contributors of these compounds based on the reference daily intakes (RDIs) in EU countries, United States, Australia, and Asia, especially when seaweeds are used as sources of I and Fe (Mišurcová et al. 2011).
Other micronutrients of nutritional relevance produced by seaweeds are vitamins. Vitamins are essential catalysts in human body that must be obtained from the diet, as they can only be synthesized to a limited extent. Certain vitamins from seaweeds have antioxidant activity, while other vitamins may contribute to maintaining and promoting health by providing additional health benefits, such as decreasing blood pressure, prevention of cardiovascular diseases, or reducing the risk of cancer (Škrovánková 2011). Ortiz et al. (2006) claimed that 100 g of seaweed provides more than the recommended daily requirements of vitamin A, B2, B12, and two-thirds of the recommended vitamin C levels (Ortiz et al. 2006). Many vitamins have been detected and measured in seaweeds, including L-ascorbic acid (vitamin C), thiamine (vitamin B1), riboflavin (vitamin B2), cobalamin (vitamin B12), folic acid and its derivatives, tocopherols (vitamin E), and carotenoids (Mišurcová 2012). Generally, red and green seaweeds are rich in vitamin C ranging from 500 mg/kg to 3000 mg/kg DW. Levels of up to 2000 mg/kg DW have been reported from the red seaweed Eucheuma denticulatum and 3000 mg/kg DW in the green seaweed Enteromorpha flexuosa (Mcdermid and Stuercke 2003). Other red seaweeds, such as Palmaria and Porphyra spp. contain a large amount of vitamins of the B group (Mabeau and Fleurence 1993). Although lipid content in seaweed is low, seaweed fats contain high levels of vitamin E. Generally, brown seaweeds contain more α-tocopherol (also β- and γ-tocopherols) than red and green algae which contain only α-tocopherol. Ortiz et al. (2009) reported a high amount of vitamin E detected in kelp Macrocystis pyrifera with 132.77 mg/100 g fat of α-tocopherol from total tocol content of 145.72 mg/100 g fat. Regarding the vitamin content in seaweeds, it also varies depending on the seaweed species and the aforementioned environmental factors. I.e., the highest levels of vitamin E in Eisenia arboreacollected in Mexico were appreciated during the month of September (Hernández-Carmona et al. 2009). Furthermore, other factors affecting the biomass including the concentration of certain compounds in the sea, the depth of growth of the biomass or the sunlight intensity at particular sea depths in which the biomass is growing will affect the vitamin content of the biomass even within the same species (Smith et al. 2007).
Other minor compounds from seaweeds include pigments such as chlorophylls, carotenoids and xanthophylls with promising potential applications in the food, cosmetics and pharmaceutical industries. Algal pigments can be applied in foods to impart the desired sensorial properties in food products, as well as for their health benefits including antioxidant, antidiabetic, immune-modulatory, and anti-angiogenic (Manivasagan et al. 2018). In order to meet the industry demands of algal pigments, many novel extraction techniques have been developed in recent years. Supercritical fluids were employed to obtain carotenoids, fucoxanthin, and phlorotannins from Saccharina japonica (Saravana et al. 2017). Microwave, ultrasound, and pulsed electric fields were reported to increase the extraction yields and reduce processing time to get carotenoids from Undaria pinnatifida (Zhu et al. 2017).
Other minor constituents from seaweeds with health benefits, such as anti-diabetic, anti-inflammatory and anti-microbial, are phenolic compounds (Generalić Mekinić et al. 2019; Nwosu et al. 2011; Lopes et al. 2012). Phenolic compounds encompass a widely diverse chemical group of compounds including phlorotannins, bromophenols, flavonoids, and terpenoids produced by seaweeds. Phlorotannins are the main phenolic compounds present in brown seaweeds. The highest total phenolic content of 47.16 mg galic acid equivalent/g dry Ascophyllum nodosum after post-harvest treatments was reported by Zhu et al. (2021b). Other large proportions of phenolic compounds detected in green and red seaweeds are bromophenols, flavonoids, phenolics acids, and terpenoids (Wells et al. 2017). As an example of the chemical diversity of these compounds, the chemical structures of the main phlorotannins produced by brown macroalgae are represented in Fig. 10.1.
Chemical structures of phlorotannins as described by Lopes et al. (2012) originally published by PLOS ONE. Numbers inside the image represent: phloroglucinol (1), tetrafucol A (2), tetraphlorethol B (3), fucodiphlorethol A (4), tetrafuhalol A (5), tetraisofuhalol (6), and phlorofucofuroeckol (7)
4 Prospects of the Seaweed Industry
Public awareness towards sustainability hasrecently increased in response to current global challenges, such as climate change, intensive production, poor waste management, and overpopulation, among others. To reduce the negative impact of these challenges in the environment, population, and economy, new alternatives and production systems are currently being considered in replacement of conventional processes and products. In the past decades, seaweed production and cultivation have increased the interest of the scientific and industrial communities as a more sustainable system compared to traditional agricultural practices. Moreover, seaweedshave been demonstrated as excellent source of nutrients and have a multipurpose nature (e.g. animal feed, biofuel, biomass) (Tiwari and Troy 2015; Mohammad et al. 2019; Hessami et al. 2019). Additionally, the global market of seaweed aquaculture and wild capture has been increasing with an annual production of 31.1 million tonnes at the global level. This rising trend is explained by the increased consumer acceptance of seaweeds as food and the broad applicability of these raw materials and theirby-products in biotechnological, pharmaceutical, and chemical industries (Kraan 2020).
The cultivation of seaweeds is now facing the challenge of variability and availability of biomass. In general, aquaculture of seaweeds usually enhances the content of protein and also decreases the pressure of harvesting large quantities of biomass from wild populations. However, in Europe, more than 97% of seaweed production is harvested from the wild in Norway, France, and Ireland (Grote 2019). This current scenario represents a challenge in utilizing seaweed protein, but can also be an opportunity to develop the seaweed industry further. Eco-intensification and sustainability of seaweed cultivation can be improved through seaweed permaculture by recreating seaweed forest habitat and other ecosystems water environments. For example, Rhodymenia pseudopalmata showed an increase of total protein content after 3 days of cultivation (18.7% DW) in fishpond effluents rich in ammonium (NH4+) than it harvested from the wild (9.4% DW) (Pliego-Cortés et al. 2019). Seaweed permaculture allows seaweeds to absorb the NH4+ that is produced by seafood production system, and meanwhile, reduces the negative impact on the ecosystems due to the capacity of seaweeds to absorb inorganic nutrients (Grote 2019). The high protein content of seaweed biomass can be recycled as feed. U. lactuca (37.4% DW) and Gracilaria conferta (29.4% DW) produced by seaweed permaculture were used to feed sea urchins as a sole feed. Compared with traditional fish diet, U. lactuca enhanced somatic and gonad growth, and improved the protein assimilation efficiency at levels of 81% compared to urchins receiving formulated diets (59%) (Shpigel et al. 2018).
Seaweed cultivation is commonly carried out in open water and requires a prior optimisation to assure the productivity of the process and the continuous monitoring of different cultivation factors (e.g. seawater temperature, available nutrients, water movement and quality) to maintain its commercial quality. The physiological processes of the seaweed can affect the biomass production in the seaweed aquaculture and therefore, a deeper understanding of the physiological and metabolic mechanisms of seaweed could improve its productivity (Hayashi et al. 2020). Additionally, controlling environmental and process parameters of the seaweed cultivation in open water can be difficult due to climate conditions and negative actions of wild animals and marine microbiota. In this way, cultivation in open water could be replaced by an intensive system in tanks or ponds which facilitates the monitoring of the biomass and prevents unnecessary depreciation of the production as a result of natural causes. Nevertheless, the maintenance of seaweed on tanks is expensive and can lead to the stress of cultivated seaweeds and thereby, a previous selection of the seaweed species is required (Hayashi et al. 2020; Kim et al. 2017). Another strategy that could be applied in seaweed cultivation is the implementation of an integrated multi-trophic aquaculture (IMTA) in a closed environment like ponds, tanks, bays, and coastal lagoons. IMTA consist of farming fish or seafoodin a shared environment with other living individuals (e.g. macroalgae, mussels, sea-cucumber) to increase the productivity of the cultivation system, enhancing the sustainability of the process and product, and generate enriched products and/or species with high commercial value. In open-water systems, IMTA cultivation has been also contemplated; however, the impact of this type of cultivation in the environment and the final product has been difficult to measure due to the unpredictability of nutrients and high dilution (Ramli et al. 2020).
The harvesting of wild seaweeds is also being currently carried out.Small countries with a long harvesting tradition like Ireland are the focus of international and large-scale companies. Ireland’s shores meet suitable conditions for the proliferation of a large range of seaweed species. In addition, the cultivation of seaweeds is limited as a result of the important tradition of harvesting wild species and the pristine state of the Irish’s shores. A growth rate in seaweed cultivation together with an increase in automation and scaling up processes are expected in many of these countries which would lead to an increase in intensive production (Monagail and Morrison 2020).
In general, the cultivation of seaweeds has been demonstrated to be a sustainable system beneficial for the ecosystems.Some of these positive effects of seaweed proliferation in the environment relate to water purification,production of nutrients and oxygen, and the reduction of greenhouse gas emissions and the eutrophication phenomenon (Buschmann et al. 2017). Although seaweed cultivation has been considered as a potential strategy to reduce anthropogenic CO2, a portion of this materialized gas into the organic matter has been observed to be easily decomposed back to CO2 gas and therefore, further research is necessary to understand the underlying mechanisms of CO2 assimilation by seaweeds. Other concerns have been raised in the last few years with regard to seaweed aquaculture. Global environmental changes,such as increased atmospheric CO2 and temperature, and intensification of ultraviolet light levels and ocean acidification are likely to affect the productivity of seaweed cultivation worldwide (Chung et al. 2017). Furthermore, the intensive cultivation of seaweed could also entailsevere repercussions like the hybridism between wild and domesticated seaweed strains and the proliferation of parasites or pathogens that could lead to disease outbreaks within the seaweed industry (Buschmann et al. 2017; Hayashi et al. 2020). The implementation of IMTA could reduce some effects of global climate change and the selection of seaweed species could prevent the spread of infectious diseases (Buschmann and Camus 2019). Thus, there is a need for strategies or alternatives to be applied in the seaweed cultivation that could mitigate the already mentioned negative impacts.
5 Conclusions and Future Perspectives
Seaweeds , either from wild or aquaculture, are valuable resources. During their cultivation, seaweeds can efficiently absorb CO2 from the seawater and thus, have the potential to neutralize greenhouse gas emissions. Moreover, seaweeds produce a wide range of bioactives mainly carbohydrates, proteins and lipids, as well as other minor compounds that have expanded the interest of this biomass for multiple applications as pharmaceuticals, cosmetics, functional foods, fertilizers, animal feeds and biofuels. Currently Ireland has a long coastline, a wide variety of seaweed species and over 97% of Irish seaweeds are wildly harvested, thus, the development of seaweed farms or permaculture systems are promising businesses in the near future. However, there are still many challenges remaining with respect to the use of seaweeds for the production of high value compounds. These challenges refer to (1) the raw biomass, including aspects of biomass production, availability and chemical or compositional stability of the raw biomass for future industrial use; (2) industrial utilization of these natural resources by developing biorefinery exploitation models, including the use of pre-treatments and novel or disruptive technologies aiming to utilise the full biomass. Moreover, these new seaweed exploitation models should be evaluated from economic, social, and environmental perspectives to contribute to the circular economy of Ireland, addressing current challenges, such as the recycling of nutrients,while alleviating current and upcoming environmental challenges related to greenhouse emissions.
References
Arai S (2000) Functional food science in Japan: state of the art. Biofactors 12:13–16
Bialek A, Bialek M, Jelinska M, Tokarz A (2017) Fatty acid composition and oxidative characteristics of novel edible oils in Poland. CyTA-J Food 15:1–8
Biancarosa I, Espe M, Bruckner CG, Heesch S, Liland N, Waagbø R, Torstensen B, Lock EJ (2017) Amino acid composition, protein content, and nitrogen-to-protein conversion factors of 21 seaweed species from Norwegian waters. J Appl Phycol 29:1001–1009
Buschmann AH, Camus C (2019) An introduction to farming and biomass utilisation of marine macroalgae. Phycologia 58:443–445
Buschmann AH, Camus C, Infante J, Neori A, Israel Á, Hernández-González MC, Pereda SV, Gomez-Pinchetti JL, Golberg A, Tadmor-Shalev N, Critchley AT (2017) Seaweed production: overview of the global state of exploitation, farming and emerging research activity. Eur J Phycol 52:391–406
Černá M (2011) Seaweed proteins and amino acids as nutraceuticals. Adv Food Nutr Res 64:297–312
Chan PT, Matanjun P (2017) Chemical composition and physicochemical properties of tropical red seaweed, Gracilaria changii. Food Chem 221:302–310
Chemat F (2012) Microwave-assisted extraction for bioactive compounds. Springer
Chiellini F, Morelli A (2011) Ulvan: a versatile platform of biomaterials from renewable resources. Biomater Phys Chem:75–98
Chojnacka K, Saeid A, Witowska Z, Tuhy L (2012) Biologically active compounds in seaweed extracts-the prospects for the application. The Open Conference Proceedings J
Chung IK, Sondak CFA, Beardall J (2017) The future of seaweed aquaculture in a rapidly changing world. Eur J Phycol 52:495–505
Collins KG, Fitzgerald GF, Stanton C, Ross RP (2016) Looking beyond the terrestrial: the potential of seaweed derived bioactives to treat non-communicable diseases. Mar Drugs 14:60
Cravotto G, Boffa L, Mantegna S, Perego P, Avogadro M, Cintas P (2008) Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason Sonochem 15:898–902
de Jesus Raposo MF, De Morais AMB, De Morais RMSC (2015) Marine polysaccharides from algae with potential biomedical applications. Mar Drugs 13:2967–3028
Delattre C, Michaud P, Courtois B, Courtois J (2005) Oligosaccharides engineering from plants and algae: applications in biotechnology and therapeutics. Minerva Biotecnologica 17:107
Deleris P, Nazih H, Bard JM (2016) Seaweeds in human health; In: Fleurence J and Levine I (Eds.) Seaweed in health and disease prevention. PP: 319-367, Academic Press, USA.
Dickson R, Brigljevic B, Lim H, Liu J (2020) Maximizing the sustainability of a macroalgae biorefinery: a superstructure optimization of a volatile fatty acid platform. Green Chem 22:4174–4186
Dominguez H, Loret EP (2019) Ulva lactuca, a source of troubles and potential riches. Mar Drugs 17:357
FAO (2018) FAO yearbook: Fishery and aquaculture statistics. 2016
Fernand F, Israel A, Skjermo J, Wichard T, Timmermans KR, Golberg A (2017) Offshore macroalgae biomass for bioenergy production: environmental aspects, technological achievements and challenges. Renew Sust Energ Rev 75:35–45
Fleurence J (1999) Seaweed proteins: biochemical, nutritional aspects and potential uses. Trends Food Sci Technol 10:25–28
Fleurence J (2016) Seaweeds as food. In: Seaweed in health and disease prevention, pp 149–167
Fleurence J, Morançais M, Dumay J (2018) Seaweed proteins. Elsevier, Proteins in food processing
Fleurence J, Morançais M, Dumay J, Decottignies P, Turpin V, Munier M, Garcia-Bueno N, Jaouen P (2012) What are the prospects for using seaweed in human nutrition and for marine animals raised through aquaculture? Trends Food Sci Technol 27:57–61
Floreto E, Teshima S (1998) The fatty acid composition of seaweeds exposed to different levels of light intensity and salinity. Bot Mar 41:467–482
Gaillard C, Bhatti HS, Novoa-Garrido M, Lind V, Roleda MY, Weisbjerg MR (2018) Amino acid profiles of nine seaweed species and their in situ degradability in dairy cows. Anim Feed Sci Technol 241:210–222
Galland-Irmouli AV, Fleurence J, Lamghari R, Luçon M, Rouxel C, Barbaroux O, Bronowicki JP, Villaume C, Guéant JL (1999) Nutritional value of proteins from edible seaweed Palmaria palmata (Dulse). J Nutr Biochem 10:353–359
Ganesan AR, Shanmugam M, Bhat R (2018) Producing novel edible films from semi refined carrageenan (SRC) and ulvan polysaccharides for potential food applications. Int J Biol Macromol 112:1164–1170
Garcia-Vaquero M, Hayes M (2016) Red and green macroalgae for fish and animal feed and human functional food development. Food Rev Intl 32:15–45
Garcia-Vaquero M, Mora L, Hayes M (2019) In vitro and in silico approaches to generating and identifying angiotensin-converting enzyme I inhibitory peptides from green macroalga Ulva lactuca. Mar Drugs 17:204
García-Vaquero M, Rajauria G, O’Doherty JV, Sweeney T (2017) Polysaccharides from macroalgae: recent advances, innovative technologies and challenges in extraction and purification. Food Res Int 99:1011–1020
Garcia-Vaquero M, Ummat V, Tiwari B, Rajauria G (2020) Exploring ultrasound, microwave and ultrasound–microwave assisted extraction technologies to increase the extraction of bioactive compounds and antioxidants from brown macroalgae. Mar Drugs 18:172
Generalić Mekinić I, Skroza D, Šimat V, Hamed I, Čagalj M, Popović Perković Z (2019) Phenolic content of brown algae (Pheophyceae) species: Extraction, identification, and quantification. Biomolecules 9:244
Getachew AT, Saravana PS, Cho YJ, Woo HC, Chun BS (2018) Concurrent extraction of oil from roasted coffee (Coffea arabica) and fucoxanthin from brown seaweed (Saccharina japonica) using supercritical carbon dioxide. J CO2 Utilizat 25:137–146
Gomez LP, Alvarez C, Zhao M, Tiwari U, Curtin J, Garcia-Vaquero M, Tiwari BK (2020) Innovative processing strategies and technologies to obtain hydrocolloids from macroalgae for food applications. Carbohydr Polym 248:116784
Gosch BJ, Magnusson M, Paul NA, de Nys R (2012) Total lipid and fatty acid composition of seaweeds for the selection of species for oil-based biofuel and bioproducts. Gcb Bioenergy 4:919–930
Goyanes NS, D` Accorso NB (2017) Industrial applications of renewable biomass products- Past, Present and Future. Springer International Publishing AG, Springer, Cham. PP: 332.
Gressler V, Fujii MT, Martins AP, Colepicolo P, Mancini-Filho J, Pinto E (2011) Biochemical composition of two red seaweed species grown on the Brazilian coast. J Sci Food Agric 91:1687–1692
Grote B (2019) Recent developments in aquaculture of Palmaria palmata (Linnaeus)(Weber & Mohr 1805): cultivation and uses. Rev Aquac 11:25–41
Hafting JT, Craigie JS, Stengel DB, Loureiro RR, Buschmann AH, Yarish C, Edwards MD, Critchley AT (2015) Prospects and challenges for industrial production of seaweed bioactives. J Phycol 51:821–837
Harnedy PA, Fitzgerald RJ (2011) Bioactive proteins, peptides, and amino acids from macroalgae 1. J Phycol 47:218–232
Harnedy PA, Fitzgerald RJ (2013) Extraction of protein from the macroalga Palmaria palmata. LWT-Food Sci Technol 51:375–382
Harrysson H, Hayes M, Eimer F, Carlsson NG, Toth GB, Undeland I (2018) Production of protein extracts from Swedish red, green, and brown seaweeds, Porphyra umbilicalis Kützing, Ulva lactuca Linnaeus, and Saccharina latissima (Linnaeus) JV Lamouroux using three different methods. J Appl Phycol 30:3565–3580
Hayashi L, Cantarino SDJ, Critchley AT (2020) Challenges to the future domestication of seaweeds as cultivated species: understanding their physiological processes for large-scale production. Seaweeds Around the World, State of Art and Perspectives
Hernández-Carmona G, Carrillo-Domínguez S, Arvizu-Higuera DL, Rodríguez-Montesinos YE, Murillo-Álvarez JI, Muñoz-Ochoa M, Castillo-Domínguez RM (2009) Monthly variation in the chemical composition of Eisenia arborea JE Areschoug. J Appl Phycol 21:607–616
Hernandez-Carmona G, Freile-Pelegrín Y, Hernández-Garibay E (2013) Conventional and alternative technologies for the extraction of algal polysaccharides. Elsevier, Functional ingredients from algae for foods and nutraceuticals
Hessami MJ, Cheng SF, Ranga Rao A, Yin YH, Phang SM (2019) Bioethanol production from agarophyte red seaweed, Gelidium elegans using a novel sample preparation method for analysing bioethanol content by gas chromatography. 3-Biotech J 9(1):25
Hlima HB, Dammak M, Karkouch N, Hentati F, Laroche C, Michaud P, Fendri I, Abdelkafi S (2019) Optimal cultivation towards enhanced biomass and floridean starch production by Porphyridium marinum. Int J Biol Macromol 129:152–161
Holdt S, Kraan S (2011) Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol 23:543–597
Hou X, Hansen JH, Bjerre A-B (2015) Integrated bioethanol and protein production from brown seaweed Laminaria digitata. Bioresour Technol 197:310–317
Husni A (2018) Therapeutic potential of seaweed polysaccharides for diabetes mellitus. Seaweed Biomaterials. IntechOpen, Riejeka, pp 27–45
Kadam SU, Álvarez C, Tiwari BK, O’Donnell CP (2017) Extraction and characterization of protein from Irish brown seaweed Ascophyllum nodosum. Food Res Int 99:1021–1027
Kamble P, Cheriyamundath S, Lopus M, Sirisha VL (2018) Chemical characteristics, antioxidant and anticancer potential of sulfated polysaccharides from Chlamydomonas reinhardtii. J Appl Phycol 30:1641–1653
Kazir M, Abuhassira Y, Robin A, Nahor O, Luo J, Israel A, Golberg A, Livney YD (2019) Extraction of proteins from two marine macroalgae, Ulva sp. and Gracilaria sp., for food application, and evaluating digestibility, amino acid composition and antioxidant properties of the protein concentrates. Food Hydrocoll 87:194–203
Khan MNA, Cho JY, Lee MC, Kang JY, Park NG, Fujii H, Hong YK (2007) Isolation of two anti-inflammatory and one pro-inflammatory polyunsaturated fatty acids from the brown seaweed Undaria pinnatifida. J Agric Food Chem 55:6984–6988
Kim JK, Yarish C, Hwang EK, Park M, Kim Y (2017) Seaweed aquaculture: cultivation technologies, challenges and its ecosystem services. Algae 32:1–13
Kim KT (2012) Seasonal variation of seaweed components and novel biological function of fucoidan extracted from brown algae in Quebec Ph.D thesis, Universite Laval, Quebec City, Canada
Kraan S (2012) Algal polysaccharides, novel applications and outlook. Carbohydrates-comprehensive studies on glycobiology and glycotechnology, IntechOpen
Kraan S (2013) Pigments and minor compounds in algae. Elsevier, Functional ingredients from algae for foods and nutraceuticals
Kraan S (2020) Seaweed resources, collection, and cultivation with respect to sustainability. Sustainable Seaweed Technologies
Kumar CS, Ganesan P, Suresh PV, Bhaskar N (2008) Seaweeds as a source of nutritionally beneficial compounds-a review. J Food Sci Technol 45:1
Lafarga T, Acién-Fernández FG, Garcia-Vaquero M (2020) Bioactive peptides and carbohydrates from seaweed for food applications: natural occurrence, isolation, purification, and identification. Algal Res 48:101909
Lafarga T, Rodríguez-Bermúdez R, Morillas-España A, Villaró S, García-Vaquero M, Morán L, Sánchez-Zurano A, González-López CV, Acién-Fernández FG (2021) Consumer knowledge and attitudes towards microalgae as food: the case of Spain. Algal Res 54:102174
Lakshmi DS, Trivedi N, Reddy C (2017) Synthesis and characterization of seaweed cellulose derived carboxymethyl cellulose. Carbohydr Polym 157:1604–1610
Liang X, Fan Q (2013) Application of sub-critical water extraction in pharmaceutical industry. J Mat Sci Chem Eng 1:1
Lopes G, Sousa C, Silva LR, Pinto E, Andrade PB, Bernardo J, Mouga T, Valentão P (2012) Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS One 7:e31145
Lorbeer AJ, Lahnstein J, Fincher GB, Su P, Zhang W (2015) Kinetics of conventional and microwave-assisted fucoidan extractions from the brown alga, Ecklonia radiata. J Appl Phycol 27:2079–2087
Lordan S, Ross RP, Stanton C (2011) Marine bioactives as functional food ingredients: potential to reduce the incidence of chronic diseases. Mar Drugs 9:1056–1100
Lourenço SO, Barbarino E, De-Paula JC, Pereira LODS, Marquez UML (2002) Amino acid composition, protein content and calculation of nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycol Res 50:233–241
Mabeau S, Fleurence J (1993) Seaweed in food products: biochemical and nutritional aspects. Trends Food Sci Technol 4:103–107
Maeda H, Tsukui T, Sashima T, Hosokawa M, Miyashita K (2008) Seaweed carotenoid, fucoxanthin, as a multi-functional nutrient. Asia Pacific J Clin Nutrit 17
Makkar HP, Tran G, Heuzé V, Giger-Reverdin S, Lessire M, Lebas F, Ankers P (2016) Seaweeds for livestock diets: a review. Anim Feed Sci Technol 212:1–17
Manivasagan P, Bharathiraja S, Santha Moorthy M, Mondal S, Seo H, Dae Lee K, Oh J (2018) Marine natural pigments as potential sources for therapeutic applications. Crit Rev Biotechnol 38:745–761
Matanjun P, Mohamed S, Mustapha NM, Muhammad K (2009) Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J Appl Phycol 21:75–80
Mcdermid KJ, Stuercke B (2003) Nutritional composition of edible Hawaiian seaweeds. J Appl Phycol 15:513–524
Men’shova RV, Ermakova SP, Rachidi SM, Al-Hajje AH, Zvyagintseva TN, Kanaan HM (2012) Seasonal variations of the composition, structural features, and antitumor properties of polysaccharides from Padina pavonica (Lebanon) as a function of composition. Chem Nat Compounds 47:870–875
Mišurcová L (2012) Chemical composition of seaweeds. Wiley Online Library
Mišurcová L, Machů L, Orsavová J (2011) Seaweed minerals as nutraceuticals. Adv Food Nutr Res 64:371–390
Mohammad JH, Ranga Rao A, Ravishankar GA (2019) Opportunities and challenges in seaweeds as feed stock for biofuel production. In: Ravishankar GA, Rao AR (eds) Handbook of algal technologies and phytochemicals: volume-II Phycoremediation, biofuels and global biomass production, volume-II. CRC Press, USA, pp 39–50
Mols-Mortensen A, Jacobsen C, Holdt SL (2017) Variation in growth, yield and protein concentration in Saccharina latissima (Laminariales, Phaeophyceae) cultivated with different wave and current exposures in the Faroe Islands. J Appl Phycol 29:2277–2286
Monagail MM, Morrison L (2020) The seaweed resources of Ireland: a twenty-first century perspective. J Appl Phycol 32:1287–1300
Moualee, A. & Pradhanang, T. 2019. Seaweed consumption and its effect on breast cancer
Mouritsen OG, Dawczynski C, Duelund L, Jahreis G, Vetter W, Schröder M (2013) On the human consumption of the red seaweed dulse (Palmaria palmata (L.) Weber & Mohr). J Appl Phycol 25:1777–1791
Naseri A, Marinho GS, Holdt SL, Bartela JM, Jacobsen C (2020) Enzyme-assisted extraction and characterization of protein from red seaweed Palmaria palmata. Algal Res 47:101849
Nunes AJ, Sá MV, Browdy CL, Vazquez-Anon M (2014) Practical supplementation of shrimp and fish feeds with crystalline amino acids. Aquaculture 431:20–27
Nwosu F, Morris J, Lund VA, Stewart D, Ross HA, McDougall GJ (2011) Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem 126:1006–1012
Ojima T (2013) Polysaccharide-degrading enzymes from herbivorous marine invertebrates. Elsevier, Marine enzymes for biocatalysis
Ortiz J, Romero N, Robert P, Araya J, Lopez-Hernández J, Bozzo C, Navarrete E, Osorio A, Rios A (2006) Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea Antarctica. Food Chem 99:98–104
Ortiz J, Uquiche E, Robert P, Romero N, Quitral V, Llantén C (2009) Functional and nutritional value of the Chilean seaweeds Codium fragile, Gracilaria chilensis and Macrocystis pyrifera. Eur J Lipid Sci Technol 111:320–327
Øverland M, Mydland LT, Skrede A (2019) Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J Sci Food Agric 99:13–24
Peña-Rodríguez A, Mawhinney TP, Ricque-Marie D, Cruz-Suárez LE (2011) Chemical composition of cultivated seaweed Ulva clathrata (Roth) C. Agardh. Food Chem 129:491–498
Pliego-Cortés H, Bedoux G, Boulho R, Taupin L, Freile-Pelegrín Y, Bourgougnon N, Robledo D (2019) Stress tolerance and photoadaptation to solar radiation in Rhodymenia pseudopalmata (Rhodophyta) through mycosporine-like amino acids, phenolic compounds, and pigments in an integrated multi-trophic aquaculture system. Algal Res 41:101542
Qasim R (1991) Amino acid composition of some common seaweeds. Pak J Pharm Sci 4:49–54
Qin Y (2018) Applications of bioactive seaweed substances in functional food products. Bioactive seaweeds for food applications. Elsevier
Rajauria G, Foley B, Abu-Ghannam N (2016) Identification and characterization of phenolic antioxidant compounds from brown Irish seaweed Himanthalia elongata using LC-DAD–ESI-MS/MS. Innovative Food Sci Emerg Technol 37:261–268
Ramli NM, Verreth JAJ, Yusoff FM, Nurulhuda K, Nagao N, Verdegem MC (2020) Integration of algae to improve nitrogenous waste Management in Recirculating Aquaculture Systems: a review. Front Bioeng Biotechnol 8:1004
Robin A, Kazir M, Sack M, Israel A, Frey W, Mueller G, Livney YD, Golberg A (2018) Functional protein concentrates extracted from the green marine macroalga Ulva sp., by high voltage pulsed electric fields and mechanical press. ACS Sustain Chem Eng 6:13696–13705
Roleda MY, Marfaing H, Desnica N, Jónsdóttir R, Skjermo J, Rebours C, Nitschke U (2019) Variations in polyphenol and heavy metal contents of wild-harvested and cultivated seaweed bulk biomass: health risk assessment and implication for food applications. Food Control 95:121–134
Rubio-Rodríguez N, Beltrán S, Jaime I, Sara M, Sanz MT, Carballido JR (2010) Production of omega-3 polyunsaturated fatty acid concentrates: a review. Innovative Food Sci Emerg Technol 11:1–12
Santos JP, Guihéneuf F, Fleming G, Chow F, Stengel DB (2019) Temporal stability in lipid classes and fatty acid profiles of three seaweed species from the north-eastern coast of Brazil. Algal Res 41:101572
Saravana PS, Getachew AT, Cho YJ, Choi JH, Park YB, Woo HC, Chun BS (2017) Influence of co-solvents on fucoxanthin and phlorotannin recovery from brown seaweed using supercritical CO2. J Supercrit Fluids 120:295–303
Sedlmeyer F (2011) Xylan as by-product of biorefineries: characteristics and potential use for food applications. Food Hydrocoll 25:1891–1898
Shama A, Joyce SG, Mari FD, Ranga Rao A, Ravishankar GA, Hudaa N (2019) Macroalgae and microalgae: novel sources of functional food and feed. In: Ravishankar GA, Rao AR (eds) Handbook of algal technologies and phytochemicals: Volume-I: food, health and nutraceutical applications. CRC Press, USA, pp 207–219
Shannon E, Abu-Ghannam N (2019) Seaweeds as nutraceuticals for health and nutrition. Phycologia 58:563–577
Shpigel M, Shauli L, Odintsov V, Ashkenazi N, Ben-Ezra D (2018) Ulva lactuca biofilter from a land-based integrated multi trophic aquaculture (IMTA) system as a sole food source for the tropical sea urchin Tripneustes gratilla elatensis. Aquaculture 496:221–231
Singh RS, Walia AK (2018) Lectins from red algae and their biomedical potential. J Appl Phycol 30:1833–1858
Skriptsova AV, Shevchenko NM, Tarbeeva DV, Zvyagintseva TN (2012) Comparative study of polysaccharides from reproductive and sterile tissues of five brown seaweeds. Mar Biotechnol 14:304–311
Škrovánková S (2011) Seaweed vitamins as nutraceuticals. Adv Food Nutr Res 64:357–369
Smith AG, Croft MT, Moulin M, Webb ME (2007) Plants need their vitamins too. Curr Opin Plant Biol 10:266–275
Teas J, Pino S, Critchley A, Braverman LE (2004) Variability of iodine content in common commercially available edible seaweeds. Thyroid 14:836–841
Tiwari BK, Troy DJ (2015) Seaweed sustainability–food and nonfood applications. In: Seaweed sustainability. Academic press, pp 1–6
Troy DJ, Tiwari BK, Hayes M, Ross RP, Stanton C, Johnson M, Stengel D, O’Doherty JV, Fitzgerald RJ, McSorley E, Kerry JP (2017) Marine functional foods research initiative (NutraMara)
Vannuccini S, Kavallari A, Bellù LG, Müller M, Wisser D (2019) Understanding the impacts of climate change for fisheries and aquaculture: global and regional supply and demand trends and prospects. Impacts Climate Change on Fisheries and Aquaculture 41
Wells ML, Potin P, Craigie JS, Raven JA, Merchant SS, Helliwell KE, Smith AG, Camire ME, Brawley SH (2017) Algae as nutritional and functional food sources: revisiting our understanding. J Appl Phycol 29:949–982
Wijesinghe W, Jeon YJ (2011) Biological activities and potential cosmeceutical applications of bioactive components from brown seaweeds: a review. Phytochem Rev 10:431–443
Wolf B (2010) Polysaccharide functionality through extrusion processing. Curr Opin Colloid Interface Sci 15:50–54
Wong KH, Cheung PC (2000) Nutritional evaluation of some subtropical red and green seaweeds: part I—proximate composition, amino acid profiles and some physico-chemical properties. Food Chem 71:475–482
World Health Organization (1991) Protein Quality Evaluation: Report of the Joint FAO/WHO Expert Consultation, Bethesda, Md., USA 4–8 December 1989. Food & Agriculture Org
Xi J, Xue Y, Xu Y, Shen Y (2013) Artificial neural network modeling and optimization of ultrahigh pressure extraction of green tea polyphenols. Food Chem 141:320–326
Yoshioka Y, Satoh H, Mitani M (2007) Theoretical study on electronic structures of FeOO, FeOOH, FeO (H2O), and FeO in hemes: as intermediate models of dioxygen reduction in cytochrome c oxidase. J Inorg Biochem 101:1410–1427
Zhu X, Healy L, Zhang Z, Maguire J, Sun DW, Tiwari BK (2021a) Novel post-harvest processing strategies for value-added applications of marine algae. J Sci Food Agric
Zhu X, Zhang Z, Hinds LM, Sun DW, Tiwari BK (2021b) Applications of ultrasound to enhance fluidized bed drying of Ascophyllum Nodosum: drying kinetics and product quality assessment. Ultrason Sonochem 70:105298
Zhu Z, Wu Q, Di X, Li S, Barba FJ, Koubaa M, Roohinejad S, Xiong X, He J (2017) Multistage recovery process of seaweed pigments: investigation of ultrasound assisted extraction and ultra-filtration performances. Food Bioprod Process 104:40–47
Ziółkowska NE, Wlodawer A (2006) Structural studies of algal lectins with anti-HIV activity. Acta Biochim Pol 53:617–626
Acknowledgements
The authors would like to acknowledge UCD-CSC Scholarship Scheme supported by University College Dublin (UCD) and China Scholarship Council (CSC) for this study. The researchers also acknowledge funding from the transnational ERA-NET Co-fund on Blue Bioeconomy (BlueBio) and the Department of Agriculture Food and the Marine (DAFM) as part of the project BIOCARB-4-FOOD.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Zhu, X., Soro, A.B., Tiwari, B.K., Garcia-Vaquero, M. (2022). Seaweeds in Ireland: Main Components, Applications, and Industrial Prospects. In: Ranga Rao, A., Ravishankar, G.A. (eds) Sustainable Global Resources Of Seaweeds Volume 1. Springer, Cham. https://doi.org/10.1007/978-3-030-91955-9_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-91955-9_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-91954-2
Online ISBN: 978-3-030-91955-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)