Abstract
The nature of continuous bone remodeling can be determined, thanks to the measurement of molecules released by osteoblasts and osteoclasts in the bone formation and resorption processes. These bone turnover markers (BTMs) can be measured in blood or urine. When on a diagnostic imaging test such as an x-ray, it is observed that the cortical bone is thinner and the trabeculae are less numerous and thinner, it is affirmed that the bone is osteoporotic. There is a more objective definition that differentiates between the concepts of osteoporosis and osteopenia based on the bone mineral density (BMD) T-score. This definition, however, is very controversial.
BTMs are powerful research tools for epidemiologists who study populations’ fracture risks, but not for their routine use for identifying individual patients who would optimally benefit from pharmaceutical therapies for osteoporosis. Nevertheless, unlike the limitations of the use of BTMs to identify patients at risk for rapid bone loss, their use in guiding osteoporosis therapy has a clearer potential utility. The pattern of change in BTMs in response to treatment is well-described. BTMs may be useful also for patients with “secondary” bone loss due to hyperparathyroidism, hyperthyroidism, vitamin D deficiency, and paraproteinemia.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The human skeleton comprises bones, which are organs that are connected by joints. These joints allow the bones—with the exception of the cranium bones—to move, thanks to the contractions of the muscles inserted on them. This muscle action is regulated by the peripheral nerves, which conduct electrical impulses that originate in the spinal cord. This set of elements is called the locomotor system. It makes it possible to move the lower limbs in order to travel and the upper limbs in order to grasp objects. An adequately developed musculature of the lower limbs allows for normal standing and walking while minimizing falls and the upper limbs also play a role in both actions by providing stability.
There are other soft tissues in this system, including several varieties of connective tissue as well as the skin, which serves as a covering. The entirety of the system is supplied with blood by the vascular system. In addition to movement, the skeleton serves to protect the organs and is also involved in cellular regulation of the hematopoietic system, which is contained within it, and mineral metabolism.
Bone tissue, the substrate that forms the skeleton, is organized into a hierarchical structure called building blocks (BB) [1], which consist of collagen fibers and other proteins configured in intertwined lamellae, osteons, and trabecular as well as cortical bone. This structure is interconnected by molecular links that mechanically join the BBs. Significant changes in bone quantity and quality occur in the structure throughout the lifespan, leading to a decrease in both.
The bone, as an organ, is composed of a compact external structure called cortical bone which encloses another less compact, spongier structure called cancellous bone. The cortical bone is responsible for 80% of the organ's weight. Its function is fundamentally mechanical and protective, although it also plays a role in regulating mineral metabolism when there is a prolonged severe deficiency, as it is affected by the hormonal changes that reach it through the blood supply. For example, in cases of postmenopausal osteoporosis, the cortical bone grows thinner with the passing of the years, especially in the long bones (Fig. 3.1).
Trabecular bone forms both ends of the organ in long bones and is surrounded by cortical bone that is thinner than diaphyseal cortical bone. While trabecular bone is much more active in metabolic processes than cortical bone, it also plays a role in mechanical support, though not for its hardness but rather for the architectural arrangement of its trabeculae. This is much more important in short bones, like the vertebrae. However, with age and especially after menopause, bone trabeculae grow fewer and thinner (Fig. 3.2) [2, 3]; as such, the trabeculae structure is less resistant to mechanical stress. This can lead to vertebral fractures due to a low-energy mechanism. These are most common in postmenopausal ages, as the vertebral bodies have a very thin cortical layer, and the trabeculae, due to their decreasing number and thinning, are not able to maintain the height of the vertebrae (Fig. 3.3).
With age, bone trabeculae are thinner and fewer in number, making the trabecular structure less resistant to mechanical stress. (a) Image of a bone from a patient with coxarthrosis (obtained via 4× optical microscopy). (b) Image of a bone from a patient with osteoporosis (obtained via 4× optical microscopy)
When on a diagnostic imaging test such as an x-ray, it is observed that the cortical bone is thinner and the trabeculae are less numerous and thinner, it is affirmed that the bone is osteoporotic. Although this is a judgment based on anatomical pathology, it is the image of the bone which indicates porosis. That is to say, the pores in the trabeculae are observed to be larger on the diagnostic test. The bone tissue whose image is used to diagnose osteoporosis would have osteopenia, if its matrix is diminished, or osteomalacia, if its matrix is less calcified. If the density analysis is performed via x-ray—on which a loss of 15-30% of bone mass is necessary for the image to be significantly different—osteoporosis will be diagnosed later than if the analyses were performed via a biopsy [4].
Therefore, the concept of osteoporosis is quantitative and based on an image of osteopenia or osteomalacia. Thus, the term osteoporotic fracture is erroneous because osteoporosis is a variable defined based on the image of an osteopenic or osteomalacic bone. Indeed, other variables such as age or sarcopenia, which are also independent variables and which cause falls, do not define a fracture and the use of the terms sarcopenic or senile fracture is not common [4,5,6]. It would therefore be more correct to use the term fracture in an osteopenic or osteomalacic bone. A fracture in an osteopenic bone thus includes everything from fractures in children who have osteogenesis imperfecta to those in the elderly who have “osteoporosis.”
However, usage has made it so that the visual opinion of the anatomy-pathology is inferred and it is understood that osteoporosis is characterized by loss of bone mass, changes in trabecular microstructure, and, as a consequence, skeletal fragility. This leads to a greater risk of fracture as a result of low-energy trauma and greater difficulty in achieving stable osteosynthesis (Fig. 3.4). Nevertheless, there is a more objective definition that differentiates between the concepts of osteoporosis and osteopenia based on the bone mineral density (BMD) T-score [7]. This definition, however, is very controversial [8,9,10,11,12,13].
A BMD T-score that is equal to or less than 2.5 standard deviations (SD), after having ruled out other causes of low BMD, is defined as osteoporosis. When the T-score is less than 1-2.5 SD, it is defined as osteopenia. When it is within 1 SD of the value for young adults, BMD is considered normal. Although values below 2.5 SDs tend to indicate a greater risk of fracture, these are more frequent in the 1-2.5 SD range due to the greater number of people in this category. This WHO categorization [7], which has been adopted by patients’ associations, has been called into question [9,10,11, 13] and systematically distorts both the evidence and the evidence-based medicine and indications [8, 12]. As a result, osteopathies that present with fragility are divided into different types, including non-osteopenic (normal bone mass), simple osteopenia (decreased bone mass), or osteopenic disorders that lead to fragility, such as osteoporosis. In clinical practice, however, this classification is not as categorical.
2 Bone Cells
Bone tissue is composed of a calcified protein matrix and the cells that regulate it: osteoblasts synthesize the matrix and osteoclasts digest it. In addition, it includes the precursor cells of both as well as other cells related to hematopoiesis and the immune system that are precursors to osteoclasts. When osteoblasts are surrounded by the protein matrix, they differentiate into osteocytes and their functions shift more toward the regulation of bone metabolism than the synthesis of the osteoid matrix.
2.1 Osteoblasts
Osteoblasts are cells that secrete the bone matrix protein that is later mineralized. They arise from the differentiation of multipotent mesenchymal stem cells (MSCs) [14] located in the bone marrow, such as stromal cells or pericytes, which are the MSCs adhered to the vascular endothelium and are also fundamental to the formation of blood vessels [15, 16].
The Runx2 protein, also known as core-binding factor alpha-1 (CBFA1), a member of the runt homology domain transcription factor family, is fundamental to the differentiation of MSCs into osteoblasts [17]. Runx2 is the earliest differentiation marker of osteogenic lineage and, along with Runx3, acts in the maturation of hypertrophic chondrocytes [18]. The Sp7 transcription factor (Osterix) and a zinc-finger protein act after Rnx2 and are responsible for the specialization of osteoprogenitor cells into preosteoblasts. The nuclear receptor peroxisome proliferator-activated receptor (PPAR)-gamma also acts, spurring the commitment process of multipotent osteoprogenitor cells [19, 20]. The differentiated osteoblasts express genes for these three proteins as well as for others such as osteopontin, or bone sialoprotein [21, 22]. Runx2 inhibits osteocalcin, halting the cells in differentiation [23]. Osterix controls the transcription of specific genes of osteoblasts, such as osteocalcin, osteopontin, and type I collagen [19].
Wnt proteins are also important. They form part of a group of signaling molecules for skeletal and bone mass development and are mobilized through stimulation of genetic expression of Runx2. Activation of the canonical Wnt pathway gives rise to the formation of a complex of Wnt proteins, low-density lipoprotein receptor-related protein 5 (LRP5), or LRP6, which leads to the phosphorylation and inactivation of glycogen synthase kinase (GSK)-3 beta, inhibition of beta-catenin degradation, and the subsequent accumulation of this metabolite in the osteoblast nucleus [24, 25]. Nuclear beta-catenin binds to the family of TCF/LEF transcription factors and induces expression in the target genes [26]. Therefore, beta-catenin is essential for the differentiation of precursor cells into osteoblasts, preventing differentiation into chondrocytes or adipocytes. The action of beta-catenin in later stages can eliminate or activate osteoclastogenesis through regulation of osteoprotegerin as well as abnormalities in Wnt signaling, which may lead to defects in skeletal homeostasis that can lead to early-onset hereditary osteoporosis or osteogenesis imperfecta, as also occurs with loss of function or mutation in LRP5 [27, 28].
Osteoblasts are arranged lengthwise, increasing their surface area in order to deposit the secreted matrix protein (Fig. 3.5).
2.2 Osteocytes
When osteoblasts are surrounded by osteoid, they differentiate into osteocytes, transforming their phenotype through the development of long cytoplasmic extensions that connect to other osteocytes, surrounded by a gelatinous matrix linked through the bone tissue canaliculi (Fig. 3.6). Osteocytes are the last step in the cellular differentiation of MSCs into the osteogenic line and, therefore, as they are highly differentiated cells, they do not multiply [29]. Many osteoblasts do not differentiate into osteocytes, but rather die by apoptosis [30]. This differentiation is an active process in which the cell develops long cytoplasmic extensions thanks to the action of a protein called podoplanin [31]. Mature osteocytes also express high levels of SOST, an inhibitor of the canonical Wnt/β-catenin pathway. They also intervene in bone regulation through the secretion of sclerostin, a product of the SOST gene, which can antagonize LRP5 and LRP6. In fact, the absence of the SOST gene leads to a pathological increase in bone mass [27].
Osteocytes express different proteins related to mineral metabolism. These proteins include fibroblast growth factor (FGF), which regulates renal excretion of phosphorus, or matrix extracellular phosphoglycoprotein (MEPE), which inhibits mineralization [32].
Osteocytes, through their cytoplasmic extensions, play a very active role in bone mechanics. The transport of solutes through the canaliculi system that the osteocytic cytoplasmic extensions pass through is regulated by blood pressure and diffusion and convection induced by mechanical stress. This physical and chemical process is called mechanotransduction, a phenomenon by which mechanical stimuli are translated into molecular variations that lead to changes in cellular multiplication and differentiation [33, 34]. Cytoplasmic extensions bind the osteocytes to the collagen, which allows them to note changes in fluids, modulating secretion of sclerostin in order to stimulate bone formation or absorption [35, 36]. This distinctive response of the connective tissue to mechanical stimuli (mechanotransduction) characterizes Wolff’s law [37]. This is the reason why physical exercise is so important before and after menopause in order to preserve bone and muscle mass.
2.3 Osteoclasts
Osteoclasts are multinucleated cells formed by the fusion of monocytes from the monocyte-macrophage lineage which dissolves bone and produce resorption [38, 39]. The macrophages come from the hematopoietic lineage and have a function in inflammation, although they are now known to also have a role in bone metabolism itself.
Osteoclasts are stimulated by two cytokines: receptor activator of nuclear factor-kappa B ligand (RANKL) and macrophage colony-stimulating factor (M-CSF). They are differentiated from monocytes by the nuclear factor of activated T cells 1 (NAFATc1), the master regulator transcription factor responsible for this differentiation [40,41,42,43]. Osteoblasts and osteocytes, whether apoptotic or alive, are the main source of RANKL and osteoprotegerin, the signaling proteins which stimulate bone resorption in osteoclasts, although they are also produced by other cells, such as T lymphocytes [44,45,46].
The RANKL protein interacts with an osteoclast precursor cell receptor called RANK, which is identical to that of the T-cells and dendritic cells [42]. NFATc1 is induced by RANKL and coactivated by immunoglobulin-like receptors [39, 42, 47]. RANKL also binds to osteoprotegerin or osteoclastogenesis inhibitory factor [39, 42, 47]; as such, when osteoprotegerin or a RANKL antibody is administered to postmenopausal women, bone turnover markers (BTMs) reduce drastically, indicating increasing bone mass.
Bone resorption is carried out by osteoclasts, which have phosphatase acid in their cellular membrane and other hydrolytic enzymes that act on the calcified osteoid, releasing collagen fragments and minerals deposited in the reticular structure that collagen forms together with pyridinoline and deoxypyridinoline. These molecules are also released, circulating freely in the blood until they are excreted in urine [48]. Some of these molecules are digested incompletely and circulate, such as pyridinoline cross-links bound to alpha-1 and alpha-2 chains, which also circulate and are excreted in the same manner [49]. Some diseases, such as diabetes, can interfere with this metabolism [50, 51].
Both acid phosphatase and alkaline phosphate activity take place in other locations, but activities that occur there are fundamentally different from what occur in bone cells in regards to insensitivity to tartrate-tartrate-resistant acid phosphate (TRAP) inhibition, in the case of osteoclastic activity [52], and in regards to hepatic and pancreatic antigenic characterization, in the case of osteoblastic activity.
Therefore, osteocytes, their precursor osteoblasts, osteoclasts, and monocyte-origin cells are molecularly connected so that the bone formation-resorption balance is appropriate [45, 46, 53, 54].
3 Osteogenesis and Mineralization
3.1 Osteoid Synthesis
Bone formation is initiated by osteoblasts, which synthesize the triple-helix type I collagen of the bone tissue [55, 56] as well as other proteins—including osteocalcin—which combine extracellularly to form the osteoid on which mineralization occurs [57, 58].
This collagen is deposited in layers and strengthened by multiple intra- and intermolecular cross-links, interconnected with an alpha 2 polypeptide chain with two alpha 1 chains. This structure, known as procollagen, goes through a cleavage process in its aminoterminal and carboxyterminal peptides in order to form tropocollagen. In addition to a helicoidal structure, it also has a nonhelicoidal area in the aforementioned terminal peptides called N-telopeptide (NTX) and C-telopeptide (CTX), respectively. [59, 60].
The hydroxylysine side chains of different tropocollagen molecules condense to form a pyridinium ring, thus creating the pyridinoline cross-links that connect three different tropocollagen molecules. A deoxypyridinoline (D-PYR) cross-link is a variant of a pyridinoline cross-link that is formed when two hydroxylysine side chains condense with a lysine side chain. Pyridinoline cross-links are also present in many types of collagen in other tissues, except for in the skin [59, 61, 62]. There are three types of pyridinoline cross-links that are characteristic of bone collagen: D-PYR, which is only found in large amounts in the bone and dentin; N-telopeptide, which is the pyridinoline cross-link in the N-telopeptide region that binds to the alpha 1 and alpha 2 chains; and C-telopeptide, which is a fragment of alpha 1 peptide with an isomerized bond between the aspartate and the glycine of the C-telopeptide region [63, 64].
Immature collagen fibers do not have the necessary tensile strength until they are connected by these covalent bonds, which are resistant to degradation. Noncollagenous proteins in the bone matrix are fundamental in regulating mineralization and strengthening the collagen structure, forming a protein lattice which calcium and phosphate are deposited on in the form of hydroxyapatite crystals [60, 65, 66] (Fig. 3.7).
Microscopic images. (a) bone trabeculae of a larger size in a patient with coxarthrosis. Picrosirius staining that allows for identification of fibrillar collagens in red on bright-field microscopy. The same staining observed via polarized light microscopy in image (b) shows zones in which collagen has a parallel structure, which is seen with positive birefringence (between green, orange, and red). 40×
3.2 Mineralization
Noncollagenous proteins that bind calcium include vitamin K-dependent carboxylation/gamma-carboxyglutamic (Gla) proteins—including osteocalcin, which is secreted by osteoblasts—which contain gamma-carboxyglutamic acid and, like many coagulation factors, are vitamin K-dependent [67,68,69]. Some of these proteins, such as the calcification-inhibiting matrix Gla protein (MGP), can delay mineralization and allow for the bone matrix to mature. In this manner, secondary bone mineralization in humans does not cease suddenly, but rather slowly continues until a calcium content of around 30% of the bone’s weight is reached [70].
Although osteocalcin is the most specific protein product of osteoblasts, eliminating the osteocalcin gene does not alter growth or skeletal mineralization [68, 71] due to the concurrence of other proteins. Osteopontin—bone sialoprotein—binds both to calcium and to collagen and can also play a role in the adherence of osteoclasts to the bone surface [22, 72].
Phosphorylated osteopontin (OPN) inhibits the formation of hydroxyapatite crystals, whereas bone alkaline phosphatase (BALP) promotes extracellular mineralization through the release of inorganic phosphate from inorganic pyrophosphate (PPi), which inhibits mineralization. Tartrate-resistant acid phosphatase (TRAP) produced by osteoclasts, osteoblasts, and osteocytes exhibits potent phosphate activity toward osteopontin, though its potential effect on mineralization regulation is unknown. Therefore, osteopontin is important for mineralization inhibition regulated by TRAP, but not by BALP. In conclusion, BALP and TRAP appear to be able to improve the effect of osteopontin on mineralization, suggesting a potential role of TRAP in skeletal mineralization [52, 73].
Crystallized hydroxyapatite that is deposited on the aforementioned protein lattice—collagen or noncollagenous—represents approximately one-fourth of the volume and half of the mass of normal adult bones. The Ca and P (inorganic phosphate) components of these crystals are produced from blood plasma and, in turn, from nutritional sources. Amorphous Ca phosphate matures through various intermediate stages in order to form hydroxyapatite, with the vitamin D metabolites acting as important mediators of Ca regulation. Therefore, vitamin D deficiency will lead to the depletion of bone minerals [74,75,76]. Likewise, insufficient intake of Ca and P will lead to mineralization defects. Hydroxyapatite crystals may also contain carbonate, fluoride, and a variety of trace minerals, depending on the environment in which the skeleton grows. These crystals are relatively small, which is appropriate for a structure which may be subjected to tension, and thus suffer minor microdamage. However, despite the plasmatic and nutritional origin of the Ca and P that form hydroxyapatite crystals, in a study on bone extracted from the metaphysis of the proximal end of the femur in patients with hip fracture treated surgically with arthroplasty, our group found lower levels of Ca, P, and vitamin D in the blood, but not in bone concentration, when compared to a control group of patients without hip fracture, despite the fact that the patients with hip fracture were malnourished [3]. All results of the samples from both groups were calculated according to the weight of Ca and P [77]. The differences were not statistically significant for Ca, P, or the Ca:P ratio, revealing that bone mineral composition, measured by quantitative microanalysis of trabecular bone obtained from patients with hip fracture, is similar to the bone of patients with hip osteoarthrosis. This finding, associated with abnormal serum Ca and P concentrations (serum/bone levels with a correlation coefficient of −0.197 for Ca and −0.274 for P), refutes the idea of increasing Ca intake or administering medications to increase mineralization in patients with osteoporosis with the objective of preventing hip fractures. Therefore, it is to follow that some authors recommend measuring Ca and P fractions in BMD measurements in order to improve the evaluation of fracture risk and determine more specific therapies [78].
In the literature, there is little evidence of a relationship between bone density and calcium intake, but there is evidence of the occurrence of adverse effects such as gastrointestinal problems, kidney stones, or even cardiovascular problems [79]. Therefore, treatment of osteoporosis with Ca and vitamin D does not seem to be appropriate if there is no hypovitaminosis or hypocalcemia, as many authors have asserted [79,80,81,82,83]. On the other hand, extrapolating the results of research on vitamin D in animals to humans must be done cautiously, given that there are differences depending on the species. For example, whereas vitamin D stimulates mineralization in humans, it inhibits it in rodents [76].
3.3 Distribution of the Mineral Phase
In addition to the mineral composition of bone, the geographical distribution of mineralization within the proximal end of the femur is also important. In studies of the nanostructure, composition, and microarchitecture of the superolateral area of the femoral neck in elderly patients with hip fracture compared to healthy control subjects, it was observed that mineral crystals on the external cortical bone surfaces of the fracture group were larger and had a greater mineral content and a more homogeneous mineralization profile. Samples from the patients with hip fracture showed cortical porosity values that were nearly 35% higher [84]. In general, the Ca:P ratio did not appear to differ between the hypermineralized osteocytic lacunae (micropetrosis) and the bone matrix in the osteoporosis and osteoarthrosis groups, though the micropetrosis was greater in the group of patients with hip fractures [84]. Although the role of hypermineralized osteocyte lacunae in bone remodeling and the biomechanical properties of the bone requires more research, these findings are very interesting in regard to the relationship between hypermineralization and susceptibility to femoral neck fracture [85].
4 Contribution to Biochemical Homeostasis of the Mineral Phase
4.1 Calcium
In addition to its biomechanical function, the mineral fraction of the bone also plays an important role in the regulation of mineral metabolism in the human body. More than 98% of the body’s Ca is found in the bone, where in addition to acting as a mechanical support, it serves as an endogenous reservoir. One percent of bone Ca is exchangeable with extracellular fluid in order to maintain a stable Ca equilibrium. The Ca in the extracellular fluid, which, in turn, is 1% of total Ca, is found in various forms: as free ions (active form), ions bound to plasma proteins (predominantly albumin), and in compounds (phosphate, sulfate) [86]. Intestinal absorption of Ca is poor (<50%) and decreases in the elderly [87]. It is eliminated in urine, sweat, and feces. Kidney losses vary little even if the quantity consumed varies greatly. In cases of negative Ca balances, with greater losses than the Ca absorbed in the intestines, calcium levels will remain within normal ranges as a result of reabsorption of bone Ca. Normal total plasma Ca values in healthy adults range from 8.8 to 10.4 mg/dl [88]. Serum calcium levels in patients with hip fracture are lower with respect to patients with coxarthrosis, although this could be an effect of the malnutrition that the majority of these patients present with [3].
4.2 Phosphorus
The most important location of phosphorus is in the bone, where 80–85% of phosphorus in the human body is found. The remaining phosphorus is distributed in extracellular fluid and soft tissues. Phosphorus intervenes in a multitude of metabolic processes as an energy store. It acts as a cellular intermediary in membrane transport and is a component of ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) [86]. Normal plasma concentration of phosphorus in an adult is between 2.5 and 4.5 mg/dl. This range is maintained thanks to intestinal absorption, renal tube reabsorption, and intracellular and bone exchanges [88]. Our group found phosphorus levels within normal range in both the group of patients with hip fracture and the control group. However, in the case of the fracture group, phosphorus levels were on the lower limit of normal [3].
4.3 Other Ions
Sodium balance regulation within the human body is very complex. Appropriate sodium content in the body is necessary in order to maintain central blood volume and renal perfusion. Therefore, it is closely regulated by homeostatic defense mechanisms mediated by the renin-angiotensin-aldosterone (RAAS) system [89]. The role of elevated sodium intake in health problems has been the subject of controversy [90, 91]. The World Health Organization recommends limiting sodium intake to less than 2 g per day [92]. In the United States of America, it is recommended that sodium intake should not exceed 2300 mg per day or 1500 mg per day or less for certain populations. Sodium increases calcium excretion, which is associated with lower BMD that, in turn, is a predictor of bone fragility risk [89]. Consequently, a hypothesis has been posed that high sodium intake may also be a risk factor for developing osteoporosis [93, 94]. Our group found that the patients with hip fracture presented with lower serum sodium levels than the patients with coxarthrosis [3]. These low serum sodium levels must theoretically be a “protective” mechanism against calcium deficit, but serum calcium was also lower in this group of patients.
A high intake of potassium increases the absorption of calcium, but studies in this regard are not unanimous. Therefore, this could mean that the relationship between sodium intake and osteoporosis may depend on calcium and potassium intake [89, 95]. Our group found that potassium levels in the group of patients with fractures were significantly lower than in the coxarthrosis group [3].
5 Age- and Disease-Related Changes in Mineralization
There are other interactions in bone mineralization that in large part are related to age and diseases [62, 96,97,98].
Whereas gender and bone mass are not associated with bone mineralization, age is indeed related to the populations’ average increased calcium concentration spikes, the percentage of highly mineralized bone areas, and mean bone calcium content. Both the bone volume fraction and trabecular thickness are inversely correlated with mean calcium. Trabecular thickness is associated with calcium spikes, high calcium levels, and the quantity of poorly mineralized bone. It is the only structural parameter which can predict bone mineralization independently of age. Variables associated with the osteoid correlate with mineralization parameters and are the only predictor of its heterogeneity. Although elevated trabecular mineralization correlates with age and bone loss, these associations are attributed to the thinning of the bone trabeculae which occurs with high mineralization due to the loss of poorly mineralized bone surfaces. Therefore, it appears that the degree of bone mineral reabsorption is primarily associated with the quantity of osteoid that is physiologically present and the thickness of the mineralized trabecular bone [99].
Menopause is a physiological phenomenon in women that begins at varying ages which tend to range from 45 to 55 years. In menopause, in addition to various clinical symptoms due to hormones, some histological changes in bone also occur; these changes are generally asymptomatic. The mineralized bone matrix appears to be preserved during the first year post-menopause; its density does not change [100]. In young postmenopausal women with vitamin D deficiency, isolated supplementation with 1000 IU of vitamin D3 for 9 months is associated with a reduction in BTMs. However, no differences in BTMs were observed between the group that was supplemented with vitamin D and the group that was not [101].
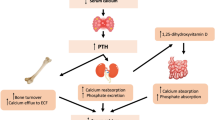
Inorganic calcium and phosphate are also critically important for many body functions. Consequently, regulation of their plasma concentration is strictly controlled by renal absorption-reabsorption, intestinal absorption, and bone exchange, as bone is a reservoir of calcium and phosphate. Parathyroid hormone and 1,25-dihydroxyvitamin D control calcium homeostasis, whereas these hormones and FGF 23 are derived from bone control phosphate homeostasis [74, 75, 102,103,104,105]. As a result, hypoparathyroidism can cause hypocalcemia and hyperphosphatasemia, whereas deficient vitamin D action can cause osteomalacia in adults and rickets in children. On the contrary, hyperparathyroidism can cause hypercalcemia and hypophosphatasemia. In order to diagnose these abnormalities associated with calcium and phosphate metabolism, a laboratory diagnostic test for calcium, phosphate, PTH, and 25-hydroxyvitamin D is very important [74, 75, 102,103,104,105].
On the other hand, the combination of elevated mean calcium concentration in the bone and low mineralization heterogeneity in adults with type 2 diabetes can have detrimental effects on the biomechanical properties of the bone. These microscopic abnormalities in bone mineralization, which may be obstructed by suppression of bone remodeling, provoke a higher risk of fracture in adults with type 2 diabetes [50].
6 Bone Turnover Markers
Continuous bone remodeling is important because it allows for a bone to adapt to physical requirements, such as load, through the formation of more bone (mechanotransduction) or as a result of chemical stimulants produced by signaling molecules that are released in fractures [33, 34, 37]. Modulation of sclerotin secretion is important in order to stimulate bone formation or bone absorption that occurs based on the cytoplasmic extensions of osteocytes into the collagen, which capture changes [35, 36].
The nature of this continuous replacement of bone tissue can be determined thanks to the measurement of molecules released by osteoblasts and osteoclasts in the bone formation and resorption processes. These BTMs can be measured in blood or urine.
The amino acids that form the cross-link between collagen molecules are released during bone resorption as free forms or as peptides that can be measured in serum or urine. Although cross-links are not exclusive to the bone, given that bone tissue is the largest reservoir of type 1 collagen in the entire human body and is remodeled more quickly than the rest of the connective tissues, it is believed that the majority of cross-links present in the urine of an adult come from the bone resorption process. In this process, collagen begins to break down, releasing free forms of cross-links (40%) and peptide-bound cross-links (60%), both of which are excreted in the urine. Measurement of BTMs is very useful for detecting bone metabolism abnormalities [98].
BMTs are predictive of loss of bone mass and in some studies are used as a fracture risk test [106,107,108,109]. However, they must be measured over time because a single measurement is meaningless. Measured over time, progressive bone mass loss does seem to correlate to fracture risk. Therefore, although BMTs are useful when they are measured over time, their variability makes it so that they do not form part of the majority of osteoporosis diagnostic and treatment guidelines, despite their usefulness in detecting lack of response to treatment.
For bone formation, serum measurements of bone-specific alkaline phosphatase (BSAP)—which requires normal liver functioning, given that on the contrary, they may appear abnormal—, osteocalcin, and aminoterminal propeptide of type I procollagen (PINP) are highly clinically useful. The serum concentration of BSAP and osteocalcin shows osteoblastic activity [71]. The serum concentration of carboxyterminal and aminoterminal propeptides of type I collagen (PICP and PINP, respectively) shows changes in the synthesis of new collagen; measuring PINP is more specific than measuring PICP. For bone resorption, the N-telopeptide (NTX) cross-link in urine and the C-terminal telopeptide of type I collagen (ICTX) and the pyridinoline cross-link can be measured in blood [63, 97].
Urine and serum measurements of collagen cross-link concentrations show bone resorption. Therefore, these substances are better indicators of bone resorption than calcium in urine or excretion of hydroxyproline. What is more, as D-PYR and the peptide binding alpha 1 to alpha 2 NTX and ICTX are almost exclusively derived from bone collagen, measurement of these substances specifically shows bone resorption.
The measurement of these metabolites (BTMs) can vary depending on the measurement method as well as patient variables. The circadian rhythm, which peaks at dawn and decreases in the afternoon; a high body mass index; tobacco use; ovulation; and the first 4–6 months following a fracture increase BTMs. Use of contraception, the postprandial period, and physical exercise decrease BTMs. Therefore, urine collection must always be done at the same time mid-morning and in the same laboratory. Likewise, dietary intake also influences these measurements [110].
The validity of BTM measurements must comply with some requirements. Changes in the metabolite must correspond to real changes in turnover measured by means of histomorphometry and calcium kinematics. Serum and urine concentrations of these metabolites must correspond to the appearance of metabolic bone diseases such as those related to the thyroids and parathyroids or to the administration of certain drugs.
Nevertheless, although these markers are useful for understanding a drug’s mechanism of action, their role in each patient is unclear; indeed, they are not important for the selection of candidates for osteoporosis treatment. In addition, there is significant variability between individuals, which in some instances may lead to poor clinical interpretation. Furthermore, it is important to note that the predictive validity of variations in BTM values varies according to which BTM is measured. For example, a variation by a factor of more than 2.8 is considered abnormal, whereas for a lower value of NTX to be predictive of improvement in mineral density and decrease of fracture risk, it must be 50% or 30% of serum values of ICTX, PINP, or BSAP [97, 111,112,113]. (Table 3.1)
7 Osteoporosis
Osteoporosis is a generalized skeletal disease classified as an osteopenic fragilizing osteopathy that predisposes individuals to a greater risk of fracture. Therefore, it is called fragilizing because fracture can occur with low-energy trauma. One of the etiopathogenic problems related to these fractures is establishing the limit from which trauma is considered of low- or high-energy. Though it seems clear that vertebral fractures spontaneously appear in patients with osteoporosis, hip fractures require a fall, even if it is from standing, for them to be considered significant trauma. Even a young individual who is not wearing protective gear whose trochanteric area impacts directly on the ground has a high probability of fracturing the hip [114]. However, unlike an elderly person, a young person has reflexes and the protection of the upper limbs to avoid this impact.
Generally speaking, the literature offers diverse classifications of osteoporosis. Five types can be distinguished: Type I: primary or due to a decrease in estrogen; type 2: due to aging; type 3: secondary or due to a genetic or acquired disease excluding menopause or aging, most often metabolic or rheumatic diseases; type IV: idiopathic juvenile; type V: regional due to immobility.
7.1 Symptoms and Diagnosis of Postmenopausal Osteoporosis
The majority of postmenopausal women present with osteoporosis due to estrogen deficiency. Medical records show the osteoporosis risk-related medical history, and thus, the indication for the imaging and laboratory tests that should be performed (Table 3.2). Early diagnosis offers the possibility of slowing disease progression, especially in cases of osteoporosis secondary to endocrinologic or rheumatic disease.
In general, there are no symptoms of osteoporosis unless a fracture occurs which reveals the disease. This, among other circumstances, differentiates it from osteomalacia, in which there is pain even if a fracture does not occur. Therefore, it is very common to incidentally observe vertebral fractures as well as a progressive decrease in height in osteoporotic women.
A diagnosis of osteoporosis is usually made when a fracture occurs. These fractures are generally located in the vertebral body, hip, wrist, humerus, rib, or pelvis. Many of them, except for hip and wrist fractures, occur without clinical episodes of pain or trauma. Therefore, the concept of clinical onset of osteoporosis is related to the occurrence of a fracture [2, 87, 114, 115].
Nevertheless, it is believed that a more objective diagnosis of osteoporosis, especially if there has not been a fracture due to fragility, would be when the T-score is less than or equal to 2.5 SD of BMD measured via dual-energy x-ray absorciometry (DXA) and after ruling out other causes of low BMD. The T-score is a comparison of a patient’s mean bone density with that of a healthy 30-year-old person of the same sex and ethnicity. This value is used in men and postmenopausal women older than 50 years of age as it better predicts the risk of future fractures. Another measurement, the Z-score, is the number of SD of a patient with a mean bone density different from the mean bone density that corresponds to a person of their age, sex, and ethnicity. This value is used in premenopausal women, men younger than 50 years of age, and children. It also serves to establish whether a patient has a mean bone density that is so low with respect to his/her age group that it leads the physician to suspect a secondary cause [7].
According to the pharmaceutical industry, all people who present with these abnormalities must have pharmaceutical treatment, even if the clinical situation does not indicate illness [7]. Moreover, the industry claims that people older than an unspecified age should receive pharmaceuticals to increase and preserve their bone mass. Consequently, to the majority of physicians, the elderly population is in large part undertreated. Nevertheless, there is not enough evidence to support these assertions, according to reports from assessment agencies [5, 10, 11, 116]. It is also important to highlight that although all elderly people present with osteopenia, only a small percentage suffer a fall and less than half sustain a lesion as a consequence of trauma. People older than 65 years of age who suffer a fall may have another within 1 year without this necessarily entailing a fracture [117].
Various epidemiological studies have attempted to identify osteoporosis early in order to prevent complex fracture patterns. However, only a better understanding of the molecular pathways, gene expression regulators, and gene expression profiles related to osteoporosis can allow for personalized treatments to be introduced [118,119,120]. Given that osteoporosis is caused by changes in the number or activities of osteoblasts and osteoclasts, by monitoring the biomarkers of these cells’ activities, trends in osteoporosis risk can be identified. However, the majority of osteoporotic fractures occur not in individuals with osteoporosis, but rather in individuals with osteopenic BMD. While osteopenic patients (T-score of BMD −1 to −2.5 SD according to DXA testing) have an individual risk of fracture that is lower than osteoporotic patients (T-score <−2.5 SD), the larger overall number of osteopenic patients means that the majority of fractures will occur in this subset of the total population [121].
Therefore, the positive predictive value of abnormal BTM levels for accelerated bone loss in elderly white women is modest [122]. Due to the low efficiency and cost-effectiveness of detection programs, use of BTMs as a public health measure for identifying patients at increased risk of rapid bone loss is not currently recommendable [116].
The bone equilibrium index is a creative solution to this problem. It is based on a regression to determine the relative quantities of osteocalcin (OC) versus urine NTX observed in a cohort of patients with stable bone mass [123]. Patients are then evaluated in relation to this regression standard in order to determine if their quantity of NTX in relation to osteocalcin is greater than or less than the expected quantity that corresponds to stable bone mass.
It is necessary to distinguish between the capacity of BTMs to predict bone loss, as discussed above, and their capacity to predict fracture risk, as patients may have markedly different fracture risks but the same general level of bone mass due to demographic variations, clinical factors, and bone microarchitecture.
In general, prospective studies that analyze the relationship between bone formation markers and posterior fracture risk have not demonstrated a clear utility for anabolic BTMs for this purpose [122]. On the contrary, many studies have demonstrated that an increase in bone resorption markers is predictive of fracture due to posterior fragility [124].
Comorbid clinical conditions can alter the relationship among BTMs for predicting fracture risk. One of the better studied examples is that measurements of BMD underestimate fracture risk in people with diabetes [125]. ROC analyses have not been able to demonstrate that a combination of low BMD and an increase in BTMs detects more women at risk of fracture than low BMD alone [126].
In conclusion, though BTMs are powerful research tools for epidemiologists who study populations’ fracture risks, the current evidence is insufficient for recommending their routine use for identifying individual patients who would optimally benefit from pharmaceutical therapies for osteoporosis. However, a distinction must be made for patients with “secondary” bone loss for reasons such as hyperparathyroidism, hyperthyroidism, vitamin D deficiency, and paraproteinemia, as BTMs may be useful for these subgroups of higher-risk patients.
Furthermore, unlike the limitations of the use of BTMs to identify patients at risk for rapid bone loss, their use in guiding osteoporosis therapy has a clearer potential utility. The pattern of change in BTMs in response to treatment is well-described. These changes have been used to predict both increases in bone density and therapeutic efficiency for reducing fracture risk.
References
Bala Y, Seeman E. Bone’s material constituents and their contribution to bone strength in health, disease, and treatment. Calcif Tissue Int. 2015;97:308–26. https://doi.org/10.1007/s00223-015-9971-y.
Guerado E, Cruz E, Cano JR, Crespo PV, Alaminos M, del Carmen Sánchez-Quevedo M, et al. Bone mineral density aspects in the femoral neck of hip fracture patients. Injury. 2016;47(Suppl. 1):S21–4. https://doi.org/10.1016/S0020-1383(16)30005-5.
Cano JR, Crespo PV, Cruz E, Rivas-Ruiz F, Sanchez-Quevedo MC, Guerado E, Campos A. In patients with hip fracture, is the bone tissue of the femoral neck demineralised? Injury. 2019; In editorial process
Etxebarria-Foronda I, Caeiro-Rey JR, Larrainzar-Garijo R, Vaquero-Cervino E, Roca-Ruiz L, Mesa-Ramos M, et al. SECOT-GEIOS guidelines in osteoporosis and fragility fracture. An update. Rev Esp Cir Ortop Traumatol. 2015;59:373–93. https://doi.org/10.1016/j.recot.2015.05.007.
Guerado E, Sandalio RM, Caracuel Z, Caso E. Understanding the pathogenesis of hip fracture in the elderly, osteoporotic theory is not reflected in the outcome of prevention programmes. World J Orthop. 2016;7:218. https://doi.org/10.5312/wjo.v7.i4.218.
Chenbhanich J, Thongprayoon C, Atsawarungruangkit A, Phupitakphol T, Cheungpasitporn W. Osteoporosis and bone mineral density in patients with Wilson’s disease: a systematic review and meta-analysis. Osteoporos Int. 2018;29(2):315–22. https://doi.org/10.1007/s00198-017-4295-6.
The World Health Organization (WHO). Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Se. 1994;843:1–129.
Upshur REG, Tracy CS. Is evidence-based medicine overrated in family medicine? Yes. Can Fam Physician. 2013;59:1160–1.
Tiner R. The pharmaceutical industry and disease mongering. The industry works to develop drugs, not diseases. BMJ. 2002;325:216. author reply 216
Moynihan R, Cassels A. Selling sickness: how the world’s biggest pharmaceutical companies are turning us all into patients. New York, NY: NationBooks; 2005.
Gotzsche PC. Deadly medicines and organised crime. how big pharma has corrupted healthcare. London: Radcliffe Publishing; 2013.
Spencer D. Evidence based medicine is broken. Br Med J. 2014;348:g22.
Morgan DJ, Wright SM, Dhruva S. Update on medical overuse. JAMA Intern Med. 2015;175:120–4. https://doi.org/10.1001/jamainternmed.2014.5444.
Shyh-Chang N, Ng HH. The metabolic programming of stem cells. Genes Dev. 2017;31(4):336–46. https://doi.org/10.1101/gad.293167.116. Epub 2017 Mar 17
Thomas H, Cowin AJ, Mills SJ. The importance of pericytes in healing: wounds and other pathologies. Int J Mol Sci. 2017;18(6) pii: E1129 https://doi.org/10.3390/ijms18061129.
Fang J, Hirschi K. Molecular regulation of arteriovenous endothelial cell specification. F1000Res. 2019;8. pii: F1000 Faculty Rev-1208; eCollection 2019 https://doi.org/10.12688/f1000research.16701.1.
Komori T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2010;339:189–95. https://doi.org/10.1007/s00441-009-0832-8.
Balogh P, Adelman ER, Pluvinage JV, Capaldo BJ, Freeman KC, Singh S, et al. RUNX3 levels in human hematopoietic progenitors are regulated by aging and dictate erythroid-myeloid balance. Haematologica. 2019. pii: haematol.2018.208918 [Epub ahead of print]; https://doi.org/10.3324/haematol.2018.208918.
Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. https://doi.org/10.1016/s0092-8674(01)00622-5.
Seenprachawong K, Tawornsawutruk T, Nantasenamat C, Nuchnoi P, Hongeng S, Supokawej A. miR-130a and miR-27b Enhance osteogenesis in human bone marrow mesenchymal stem cells via specific down-regulation of peroxisome proliferator-activated receptor γ. Front Genet. 2018;9:543. https://doi.org/10.3389/fgene.2018.00543. eCollection 2018
Hoac B, Susan-Resiga D, Essalmani R, Marcinkiweicz E, Seidah NG, McKee MD. Osteopontin as a novel substrate for the proprotein convertase 5/6 (PCSK5) in bone. Bone. 2018;107:45–55. https://doi.org/10.1016/j.bone.2017.11.002. Epub 2017 Nov 7
Singh A, Gill G, Kaur H, Amhmed M, Jakhu H. Role of osteopontin in bone remodeling and orthodontic tooth movement: a review. Prog Orthod. 2018;19(1):18. https://doi.org/10.1186/s40510-018-0216-2.
Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–54. https://doi.org/10.1016/s0092-8674(00)80257-3.
Xia Z, Wu S, Wei X, Liao Y, Yi P, Liu Y, et al. Hypoxic ER stress suppresses beta-catenin expression and promotes cooperation between the transcription factors XBP1 and HIF1alpha for cell survival. J Biol Chem. 2019;294:13811–21. pii: jbc.RA119.008353. https://doi.org/10.1074/jbc.RA119.008353.
Wang J, Yang J, Cheng X, Yin F, Zhao Y, Zhu Y, et al. Influence of calcium supplementation against fluoride-mediated osteoblast impairment in vitro: involvement of the canonical Wnt/beta-catenin signaling pathway. J Agric Food Chem. 2019;67:10285–95. https://doi.org/10.1021/acs.jafc.9b03835.
Salazar VS, Zarkadis N, Huang L, Watkins M, Kading J, Bonar S, et al. Postnatal ablation of osteoblast Smad4 enhances proliferative responses to canonical Wnt signaling through interactions with beta-catenin. J Cell Sci. 2013;126:5598–609. https://doi.org/10.1242/jcs.132233.
Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179–92. https://doi.org/10.1038/nm.3074.
Laine CM, Joeng KS, Campeau PM, Kiviranta R, Tarkkonen K, Grover M, et al. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N Engl J Med. 2013;368:1809–16. https://doi.org/10.1056/NEJMoa1215458.
Balaian E, Wobus M, Weidner H, Baschant U, Stiehler M, Ehninger G, et al. Erythropoietin inhibits osteoblast function in myelodysplastic syndromes via the canonical Wnt pathway. Haematologica. 2018;103(1):61–8. https://doi.org/10.3324/haematol.2017.172726.
Manolagas SC, Parfitt AM. What old means to bone. Trends Endocrinol Metab. 2010;21:369–74. https://doi.org/10.1016/j.tem.2010.01.010.
Zhang K, Barragan-Adjemian C, Ye L, Kotha S, Dallas M, Lu Y, et al. E11/gp38 Selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol Cell Biol. 2006;26:4539–52. https://doi.org/10.1128/mcb.02120-05.
Plotkin LI, Bellido T. Osteocytic signalling pathways as therapeutic targets for bone fragility. Nat Rev Endocrinol. 2016;12:593–605. https://doi.org/10.1038/nrendo.2016.71.
Guerado E, Andrist T, Andrades JA, Santos L, Cervan A, Guerado G, et al. Spinal arthrodesis. basic science. Rev Esp Cir Ortop Traumatol. 2012;56:227–44. https://doi.org/10.1016/j.recot.2012.01.003.
Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22:6267–76. https://doi.org/10.1093/emboj/cdg599.
Ellies DL, Viviano B, McCarthy J, Rey J, Itasaki N, Saunders S, et al. Bone density ligand, sclerostin, directly interacts with LRP5 but Not LRP5G171V to modulate Wnt activity. J Bone Miner Res. 2006;21:1738–49. https://doi.org/10.1359/jbmr.060810.
Delgado-Calle J, Sato AY, Bellido T. Role and mechanism of action of sclerostin in bone. Bone. 2017;96:29–37. https://doi.org/10.1016/j.bone.2016.10.007.
Frost HM. Wolff’s Law and bone’s structural adaptations to mechanical usage: an overview for clinicians. Angle Orthod. 1994;64:175–88. https://doi.org/10.1043/0003-3219(1994)064<0175:WLABSA>2.0.CO;2.
Miyamoto T. Regulators of osteoclast differentiation and cell-cell fusion. Keio J Med. 2011;60:101–5. https://doi.org/10.2302/kjm.60.101.
Ikeda K, Takeshita S. The role of osteoclast differentiation and function in skeletal homeostasis. J Biochem. 2016 Jan;159(1):1–8. https://doi.org/10.1093/jb/mvv112.
Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–42. https://doi.org/10.1038/nature01658.
Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–8. https://doi.org/10.1126/science.289.5484.1504.
Park JH, Lee NK, Lee SY. Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol Cells. 2017;40(10):706–13. https://doi.org/10.14348/molcells.2017.0225.
Tanaka S, Tanaka Y, Ishiguro N, Yamanaka H, Takeuchi T. RANKL: a therapeutic target for bone destruction in rheumatoid arthritis. Mod Rheumatol. 2018 Jan;28(1):9–16. https://doi.org/10.1080/14397595.2017.1369491.
Boyce BF. Advances in the regulation of osteoclasts and osteoclast functions. J Dent Res. 2013;92(10):860–77. https://doi.org/10.1177/0022034513500306.
Drissi H, Sanjay A. The multifaceted osteoclast; far and beyond bone resorption. J Cell Biochem. 2016 Aug;117(8):1753–6. https://doi.org/10.1002/jcb.25560.
Chen X, Wang Z, Duan N, Zhu G, Schwarz EM, Xie C. Osteoblast–osteoclast interactions. Connect Tissue Res. 2018 Mar;59(2):99–107. https://doi.org/10.1080/03008207.2017.1290085.
Takahashi N, Maeda K, Ishihara A, Uehara S, Kobayashi Y. Regulatory mechanism of osteoclastogenesis by RANKL and Wnt signals. Front Biosci (Landmark Ed.). 2011 Jan;1(16):21–30. https://doi.org/10.2741/3673.
Naffa R, Watanabe S, Zhang W, Maidment C, Singh P, Chamber P, et al. Rapid analysis of pyridinoline and deoxypyridinoline in biological samples by liquid chromatography with mass spectrometry and a silica hydride column. J Sep Sci. 2019 Apr;42(8):1482–8. https://doi.org/10.1002/jssc.201801292.
Camozzi V, Tossi A, Simoni E, Pagani F, Francucci CM, Moro L. Role of biochemical markers of bone remodeling in clinical practice. J Endocrinol Invest. 2007;30(6 suppl):13–7.
Pritchard JM, Papaioannou A, Tomowich C, Giangregorio LM, Atkinson SA, Beattie KA, et al. Bone mineralization is elevated and less heterogeneous in adults with type 2 diabetes and osteoarthritis compared to controls with osteoarthritis alone. Bone. 2013;54(1):76–82. https://doi.org/10.1016/j.bone.2013.01.032.
Sav NM, Kendirci M, Akin L, Kurtoglu S. Urinary levels of pyridinoline and deoxypyridinoline and bone mineral density in children with type 1 diabetes mellitus. Endocr Res. 2017 Nov;42(4):281–6. https://doi.org/10.1080/07435800.2017.1295982.
Halling Linder C, Ek-Rylander B, Krumpel M, Norgård M, Narisawa S, Millán JL, Andersson G, Magnusson P. Bone alkaline phosphatase and tartrate-resistant acid phosphatase: potential co-regulators of bone mineralization. Calcif Tissue Int. 2017 Jul;101(1):92–101. https://doi.org/10.1007/s00223-017-0259-2.
Charles JF, Aliprantis AO. Osteoclasts: more than “bone eaters”. Trends Mol Med. 2014 Aug;20(8):449–59. https://doi.org/10.1016/j.molmed.2014.06.001.
Kylmaoja E, Nakamura M, Tuukkanen J. Osteoclasts and remodeling based bone formation. Curr Stem Cell Res Ther. 2016;11(8):626–33. https://doi.org/10.2174/1574888x10666151019115724.
Loro E, Ramaswamy G, Chandra A, Tseng W-J, Mishra MK, Shore EM, et al. IL15RA is required for osteoblast function and bone mineralization. Bone. 2017 Oct;103:20–30. https://doi.org/10.1016/j.bone.2017.06.003.
Unal M, Creecy A, Nyman JS. The role of matrix composition in the mechanical behavior of bone. Curr Osteoporos Rep. 2018 Jun;16(3):205–15. https://doi.org/10.1007/s11914-018-0433-0.
Wei J, Karsenty G. An overview of the metabolic functions of osteocalcin. Rev Endocr Metab Disord. 2015 Jun;16(2):93–8. https://doi.org/10.1007/s11154-014-9307-7.
Moser SC, van der Eerden BCJ. Osteocalcin—a versatile bone-derived hormone. Front Endocrinol (Lausanne). 9:794. https://doi.org/10.3389/fendo.2018.00794.
Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int. 2010;21(2):195–214. https://doi.org/10.1007/s00198-009-1066-z.
Oftadeh R, Perez-Viloria M, Villa-Camacho JC, Vaziri A, Nazarian A. Biomechanics and mechanobiology of trabecular bone: a review. J Biomech Eng. 2015;137:1–15. https://doi.org/10.1115/1.4029176.
Knott L, Bailey AJ. Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance. Bone. 1998;22(3):181–7. https://doi.org/10.1016/s8756-3282(97)00279-2.
Shankar S, Hosking DJ. Biochemical assessment of Paget’s disease of bone. J Bone Miner Res. 2006;21(Suppl. 2):P22–7. https://doi.org/10.1359/JBMR.06S204.
Hanson DA, Weis MA, Bollen AM, Maslan SL, Singer FR, Eyre DR. A specific immunoassay for monitoring human bone resorption: quantitation of type I collagen cross-linked N-telopeptides in urine. J Bone Miner Res. 1992;7:1251–8. https://doi.org/10.1002/jbmr.5650071119.
Enciso R, Keaton J, Saleh N, Ahmadieh A, Clark GT, Sedghizadeh PP. Assessing the utility of serum C-telopeptide cross-link of type 1 collagen as a predictor of bisphosphonate-related osteonecrosis of the jaw: a systematic review and meta-analysis. J Am Dent Assoc. 2016;147(7):551–560.e11. https://doi.org/10.1016/j.adaj.2016.02.011.
Fonseca H, Moreira-Goncalves D, Coriolano H-JA, Duarte JA. Bone quality: the determinants of bone strength and fragility. Sports Med. 2014;44(1):37–53. https://doi.org/10.1007/s40279-013-0100-7.
Main RP. Osteocytes and the bone lacunar-canalicular system: insights into bone biology and skeletal function using bone tissue microstructure. Int J Paleopathol. 2017;18:44–6. https://doi.org/10.1016/j.ijpp.2017.05.002.
Villa JKD, Diaz MAN, Pizziolo VR, Martino HSD. Effect of vitamin K in bone metabolism and vascular calcification: a review of mechanisms of action and evidences. Crit Rev Food Sci Nutr. 2017;57(18):3959–70. https://doi.org/10.1080/10408398.2016.1211616.
Mizokami A, Kawakubo-Yasukochi T, Hirata M. Osteocalcin and its endocrine functions. Biochem Pharmacol. 2017;132:1–8. https://doi.org/10.1016/j.bcp.2017.02.001.
Myneni VD, Mezey E. Regulation of bone remodeling by vitamin K2. Oral Dis. 2017;23(8):1021–8. https://doi.org/10.1111/odi.12624.
Buenzli PR, Lerebours C, Roschger A, Roschger P, Weinkamer R. Late stages of mineralization and their signature on the bone mineral density distribution. Connect Tissue Res. 2018 Dec;59(Suppl. 1):74–80. https://doi.org/10.1080/03008207.2018.1424149.
Karsenty G. Update on the biology of osteocalcin. Endocr Pract. 2017;23(10):1270–4. https://doi.org/10.4158/EP171966.RA.
Hao C, Cui Y, Owen S, Li W, Cheng S, Jiang WG. Human osteopontin: potential clinical applications in cancer (review). Int J Mol Med. 2017;39(6):1327–37. https://doi.org/10.3892/ijmm.2017.2964.
Hayman AR. Tartrate-resistant acid phosphatase (TRAP) and the osteoclast/immune cell dichotomy. Autoimmunity. 2008;41(3):218–23. https://doi.org/10.1080/08916930701694667.
Priemel M, Von Domarus C, Klatte TO, Kessler S, Schlie J, Meier S, et al. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-Hydroxyvitamin D in 675 patients. J Bone Miner Res. 2010 Feb;25(2):305–12. https://doi.org/10.1359/jbmr.090728.
Fogelman I, Van Der Wall H, Gnanasegaran G. Radionuclide and hybrid bone imaging. Radionucl Hybrid Bone Imaging. 2012;9783642024:1–1046. https://doi.org/10.1007/978-3-642-02400-9.
Van Driel M, Van Leeuwen JPTM. Vitamin D endocrinology of bone mineralization. Mol Cell Endocrinol. 2017 Sep;15(453):46–51. https://doi.org/10.1016/j.mce.2017.06.008.
Sanchez-Quevedo MC, Nieto-Albano OH, Garcia JM, Gomez de Ferraris ME, Campos A. Electron probe microanalysis of permanent human enamel and dentine. A methodological and quantitative study. Histol Histopathol. 1998;13:109–13. https://doi.org/10.14670/HH-13.109.
Zoehrer R, Perilli E, Kuliwaba JS, Shapter JG, Fazzalari NL, Voelcker NH. Human bone material characterization: integrated imaging surface investigation of male fragility fractures. Osteoporos Int. 2012;23:1297–309. https://doi.org/10.1007/s00198-011-1688-9.
Reid IR, Bristow SM, Bolland MJ. Calcium supplements: benefits and risks. J Intern Med. 2015;278:354–68. https://doi.org/10.1111/joim.12394.
Winzenberg T, Powell S, Shaw KA, Jones G. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254. https://doi.org/10.1136/bmj.c7254.
Bolland MJ, Grey A, Reid IR. Should we prescribe calcium or vitamin D supplements to treat or prevent osteoporosis? Climacteric. 2015;18 https://doi.org/10.3109/13697137.2015.1098266.
Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet. 2014;383:146–55. https://doi.org/10.1016/S0140-6736(13)61647-5.
Bolland MJ, Leung W, Tai V, Bastin S, Gamble GD, Grey A, et al. Calcium intake and risk of fracture: systematic review. BMJ. 2015;351:h4580. https://doi.org/10.1136/bmj.h4580.
Milovanovic P, Rakocevic Z, Djonic D, Zivkovic V, Hahn M, Nikolic S, et al. Nano-structural, compositional and micro-architectural signs of cortical bone fragility at the superolateral femoral neck in elderly hip fracture patients vs. healthy aged controls. Exp Gerontol. 2014;55:19–28. https://doi.org/10.1016/j.exger.2014.03.001.
Carpentier VT, Wong J, Yeap Y, Gan C, Sutton-Smith P, Badiei A, et al. Increased proportion of hypermineralized osteocyte lacunae in osteoporotic and osteoarthritic human trabecular bone: implications for bone remodeling. Bone. 2012;50:688–94. https://doi.org/10.1016/j.bone.2011.11.021.
Cabezas Agrícola JM. Hipocalcemia. Med Programa Form Médica Contin Acreditado. 2004;9:1045–54. https://doi.org/10.1016/S0211-3449(04)70157-5.
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, T. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–95.
Domínguez López O, De La M, Almazán EM, Muñoz C, Araceli R, Martín Á, et al. Intepretación de las pruebas del metabolismo calcio-fósforo y magnesio. Tratado Geriatr Para Resid. 2014:615–26.
Carbone L, Johnson KC, Huang Y, Pettinger M, Thomas F, Cauley J, et al. Sodium intake and osteoporosis. J Clin Endocrinol Metab. 2016;101:1414–21. https://doi.org/10.1210/jc.2015-4017.
Mente A, O’Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med. 2014;371:601–11. https://doi.org/10.1056/NEJMoa1311989.
O’Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612–23. https://doi.org/10.1056/NEJMoa1311889.
Buring JE, Hennekens CH. Prevention of cardiovascular disease. J Gen Intern Med. 2007;5:S54–7. https://doi.org/10.1007/bf02600843.
Jones G, Beard T, Parameswaran V, Greenaway T, von Witt R. A population-based study of the relationship between salt intake, bone resorption and bone mass. Eur J Clin Nutr. 1997;51:561–5.
Heaney RP. Role of dietary sodium in osteoporosis. J Am Coll Nutr. 2006;25:271S–6S.
Kim Y, Kim H-Y, Kim JH. Associations between reported dietary sodium intake and osteoporosis in Korean Postmenopausal Women: the 2008–2011 Korea National Health and Nutrition Examination Survey. Asia-Pacific J Public Heal. 2017;29:430–9. https://doi.org/10.1177/1010539517712759.
D’Oronzo S, Brown J, Coleman R. The role of biomarkers in the management of bone-homing malignancies. J Bone Oncol. 2017 Sep;11(9):1–9. https://doi.org/10.1016/j.jbo.2017.09.001.
Greenblatt MB, Tsai JN, Wein MN. Bone turnover markers in the diagnosis and monitoring of metabolic bone disease. Clin Chem. 2017 Feb;63(2):464–74. https://doi.org/10.1373/clinchem.2016.259085.
Vlot MC, den Heijer M, de Jongh RT, Vervloet MG, Lems WF, de Jonge R, et al. Clinical utility of bone markers in various diseases. Bone. 2018;114:215–25. https://doi.org/10.1016/j.bone.2018.06.011.
Koehne T, Vettorazzi E, Kusters N, Luneburg R, Kahl-Nieke B, Puschel K, et al. Trends in trabecular architecture and bone mineral density distribution in 152 individuals aged 30–90 years. Bone. 2014;66:31–8. https://doi.org/10.1016/j.bone.2014.05.010.
Misof BM, Roschger P, Blouin S, Recker R, Klaushofer K. Bone matrix mineralization is preserved during early perimenopausal stage in healthy women: a paired biopsy study. Osteoporos Int. 2016;27(5):1795–803. https://doi.org/10.1007/s00198-015-3446-x.
Nahas-Neto J, Cangussu LM, Orsatti CL, Bueloni-Dias FN, Poloni PF, Schmitt EB, et al. Effect of isolated vitamin D supplementation on bone turnover markers in younger postmenopausal women: a randomized, double-blind, placebo-controlled trial. Osteoporos Int. 2018;29(5):1125–33. https://doi.org/10.1007/s00198-018-4395-y.
Silva BC, Bilezikian JP. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr Opin Pharmacol. 2015;22:41–50. https://doi.org/10.1016/j.coph.2015.03.005.
Khundmiri SJ, Murray RD, Lederer E. PTH and vitamin D. Compr Physiol. 2016;6(2):561–601. https://doi.org/10.1002/cphy.c140071.
Van Driel M, Van Leeuwen JP. Vitamin D endocrine system and osteoblasts. Bonekey Rep. 2014;3:493. https://doi.org/10.1038/bonekey.2013.227. PMID: 24605210; PMCID: PMC3944124
Corbetta S, Mantovani G, Spada A. Metabolic syndrome in parathyroid diseases. Front Horm Res. 2018;49:67–84. https://doi.org/10.1159/000486003.
Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res. 1996;11:337–49. https://doi.org/10.1002/jbmr.5650110307.
Garnero P. Biomarkers for osteoporosis management: utility in diagnosis, fracture risk prediction and therapy monitoring. Mol Diagn Ther. 2008;12:157–70. https://doi.org/10.1007/BF03256280.
Bauer DC, Sklarin PM, Stone KL, Black DM, Nevitt MC, Ensrud KE, et al. Biochemical markers of bone turnover and prediction of hip bone loss in older women: the study of osteoporotic fractures. J Bone Miner Res. 1999;14:1404–10. https://doi.org/10.1359/jbmr.1999.14.8.1404.
Mohamed Y, Haifa H, Datel O, Fadoua HN, Smeh BH, Mahbouba J, et al. The role of biochemical markers of bone turnover in the diagnosis of osteoporosis and predicting fracture risk. Tunis Med. 2014;92:304–10.
Song L. Calcium and bone metabolism indices. Adv Clin Chem. 2017;82:1–46. https://doi.org/10.1016/bs.acc.2017.06.005.
Christgau S, Rosenquist C, Alexandersen P, Bjarnason NH, Ravn P, Fledelius C, et al. Clinical evaluation of the Serum CrossLaps One Step ELISA, a new assay measuring the serum concentration of bone-derived degradation products of type I collagen C-telopeptides. Clin Chem. 1998;44:2290–300.
Bauer DC, Black DM, Garnero P, Hochberg M, Ott S, Orloff J, et al. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res. 2004;19:1250–8. https://doi.org/10.1359/JBMR.040512.
Rosen HN. Use of biochemical markers of bone turnover in osteoporosis UpToDate.www.uptodate.com 2019. Last Accessed June 2019.
Guerado E, Cruz E, Cano JR, Crespo PV, Alaminos M, Sánchez-Quevedo MC, Campos A. Mineralization: effectson hip fractures. Injury. 2015;47(Suppl. 1):S21–4.
Rachner T, Khosla S, Hofbauer L, Manuscript A. New horizons in osteoporosis. Lancet. 2011;377:1276–87. https://doi.org/10.1016/S0140-6736(10)62349-5.New.
Burch J, Rice S, Yang H, Neilson A, Stirk L, Francis R, et al. Systematic review of the use of bone turnover markers for monitoring the response to osteoporosis treatment: The secondary prevention of fractures, and primary prevention of fractures in high-risk groups. Health Technol Assess (Rockv). 2014;18:1–180. https://doi.org/10.3310/hta18110.
To KG, Meuleners LB, Fraser ML, Van DD, Van DD, Huynh V-AN, et al. Prevalence and visual risk factors for falls in bilateral cataract patients in Ho Chi Minh City, Vietnam. Ophthalmic Epidemiol. 2014;21:79–85. https://doi.org/10.3109/09286586.2014.885058.
Rivadeneira F, Makitie O. Osteoporosis and bone mass disorders: from gene pathways to treatments. Trends Endocrinol Metab. 2016;27:262–81. https://doi.org/10.1016/j.tem.2016.03.006.
Nishi E, Masuda K, Arakawa M, Kawame H, Kosho T, Kitahara M, et al. Exome sequencing-based identification of mutations in non-syndromic genes among individuals with apparently syndromic features. Am J Med Genet A. 2016;170:2889–94. https://doi.org/10.1002/ajmg.a.37826.
Zheng H-F, Forgetta V, Hsu Y-H, Estrada K, Rosello-Diez A, Leo PJ, et al. Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature. 2015;526:112–7. https://doi.org/10.1038/nature14878.
Sornay-Rendu E, Munoz F, Garnero P, Duboeuf F, Delmas PD. Identification of osteopenic women at high risk of fracture: The OFELY study. J Bone Miner Res. 2005;20:1813–9. https://doi.org/10.1359/JBMR.050609.
Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grandjean H, Muller C, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996;11:1531–8. https://doi.org/10.1002/jbmr.5650111021.
Shieh A, Han W, Ishii S, Greendale GA, Crandall CJ, Karlamangla AS. Quantifying the balance between total bone formation and total bone resorption: an index of net bone formation. J Clin Endocrinol Metab. 2016;101:2802–9. https://doi.org/10.1210/jc.2015-4262.
Gerdhem P, Ivaska KK, Alatalo SL, Halleen JM, Hellman J, Isaksson A, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2003;19:386–93. https://doi.org/10.1359/JBMR.0301244.
Vestergaard P, Rejnmark L, Mosekilde L. Diabetes and its complications and their relationship with risk of fractures in type 1 and 2 diabetes. Calcif Tissue Int. 2009;84:45–55. https://doi.org/10.1007/s00223-008-9195-5.
Szulc P, Bauer DC. Biochemical markers of bone turnover in osteoporosis. In: Marcus R, Feldman D, Dempster DW, Luckey M, Cauley JA, editors. Osteoporosis, vol. 4. San Diego (CA): Academic Press; 2013. p. 1573–610.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Guerado, E., Cano, J.R., Crespo, V., Campos, A. (2022). Bone Mineralization and Osteoporotic Changes. In: Pape, HC., Kates, S.L., Hierholzer, C., Bischoff-Ferrari, H.A. (eds) Senior Trauma Patients . Springer, Cham. https://doi.org/10.1007/978-3-030-91483-7_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-91483-7_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-91482-0
Online ISBN: 978-3-030-91483-7
eBook Packages: MedicineMedicine (R0)